1. Introduction
Flexible electronic devices, characterized by intrinsic flexibility and adaptability, adhere effortlessly to any bendable surface, ensuring highly consistent contact with attached objects [
1,
2,
3,
4]. This unique capability enables continuous and stable monitoring and transmission of sensor data without restricting dynamic activities. Such attributes are particularly crucial in wearable technology, where devices must closely conform to the human body, adjusting flexibly with movement and contours to maintain optimal functionality [
5,
6,
7]. Among various sensors, electrochemical sensors stand out for their ability to convert chemical information into electrical signals, facilitating rapid and precise detection of target analytes [
8,
9,
10,
11,
12]. These sensors exhibit high flexibility and bendability, seamlessly adapting to complex and irregular surfaces while enduring various mechanical stresses [
13,
14]. Consequently, electrochemical sensors are indispensable in wearable devices [
15,
16,
17], environmental monitoring [
18,
19,
20], and healthcare diagnostics [
21,
22,
23]. The sensitivity, stability, and selectivity of electrochemical sensors critically depend on the working electrode, a core component influencing sensor performance [
24,
25,
26,
27]. The rapid evolution of nanomaterials such as carbon nanotubes [
28,
29], semiconductor metal oxides [
30,
31], porous framework compounds [
32,
33], and conductive polymers [
34,
35] has significantly enhanced sensor sensitivity by facilitating electron transfer and signal response intensity. This advancement expands the applications of electrochemical sensors in biological detection, environmental monitoring, and medical diagnostics.
Metal-organic frameworks (MOFs) are crystalline materials formed by coordinating metal ions or clusters with organic ligands, renowned for their high surface area, tunable porosity, and multifunctional chemical properties [
36,
37,
38,
39]. These attributes make MOFs suitable for diverse applications including gas storage [
40,
41], catalysis [
42,
43], and sensing [
44,
45]. MOFs exhibit dynamic responses to physical and chemical stimuli, making them particularly attractive for integration into large-area flexible electronic devices that adapt to environmental changes, offering more accurate and reliable sensing capabilities [
46,
47]. However, traditional methods like pressing or drop-casting MOFs powders onto working electrodes suffer from issues such as uneven coating, poor continuity, and susceptibility to detachment, compromising electrode activity distribution and consequently lowering sensor sensitivity and accuracy, especially in detecting low-concentration analytes [
1,
48,
49,
50]. Moreover, discontinuous electrode coatings may render portions of the electrode surface inactive, further diminishing sensor performance. Addressing these challenges involves developing uniform and dense MOFs films electrode to enhance sensor performance by maximizing active site exposure and optimizing electron transfer efficiency within the film [
51,
52,
53].
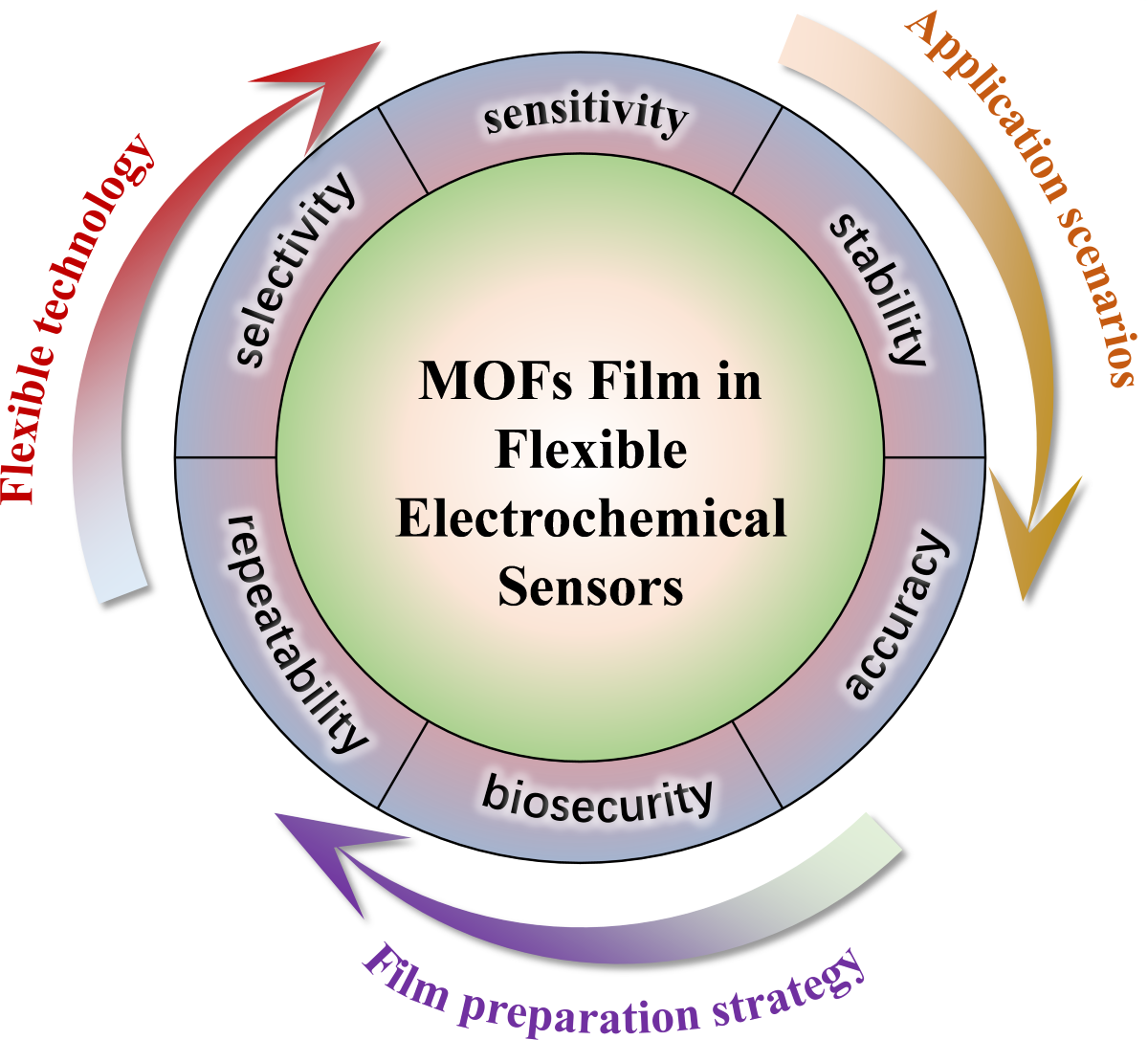
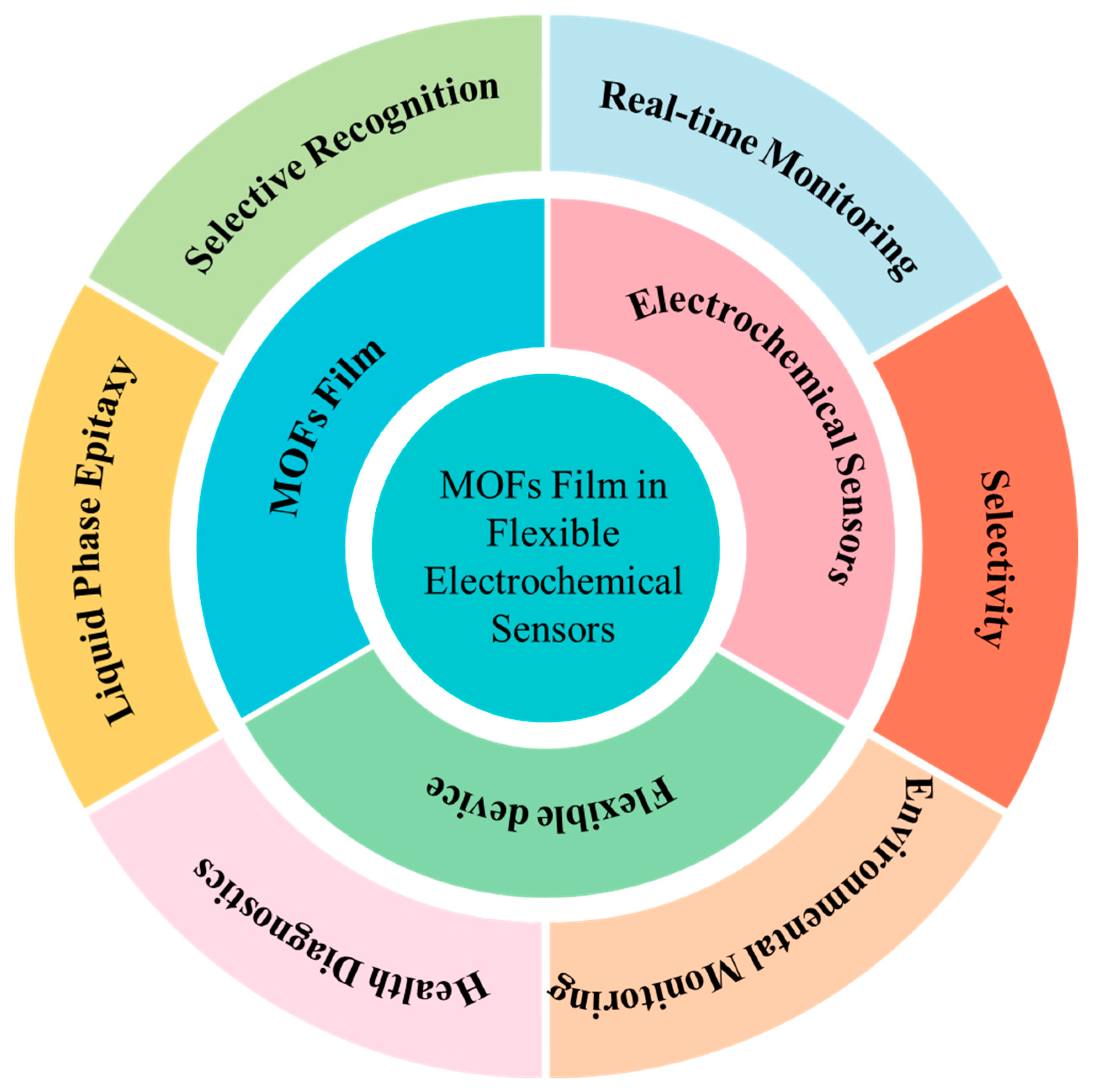
Integrating MOFs thin films into flexible electrochemical sensors represents a frontier in sensor technology, promising enhanced sensitivity, selectivity, and durability. This review summarizes recent advancements and challenges in MOFs thin films applied in flexible electrochemical sensors. By exploring the structure-performance relationships and assembly protocols of MOFs-based sensing electronic devices, this review emphasizes their multifunctionality and effectiveness in healthcare, environmental monitoring, and food safety applications. In environmental monitoring, MOFs-based flexible sensors demonstrate high sensitivity and specificity for detecting pollutants, toxins, and other harmful substances. In food safety, MOFs-based sensors ensure food safety and quality by detecting contaminants and indicators of spoilage. Furthermore, this review timely summarizes the latest progress achieved by MOFs-based flexible electronic technologies in monitoring vital signs, detecting biomarkers, and contributing real-time data for disease diagnosis and management in healthcare. Key challenges such as stability, reproducibility, and scalability are discussed, alongside future directions for MOFs-based flexible sensor development, ranging from advanced manufacturing techniques to personalized health monitoring and large-scale environmental sensing. This review aims to bridge the gap between MOFs research and their integration into flexible electronic sensing, providing valuable insights for developing high-performance flexible sensing devices through MOFs integration.
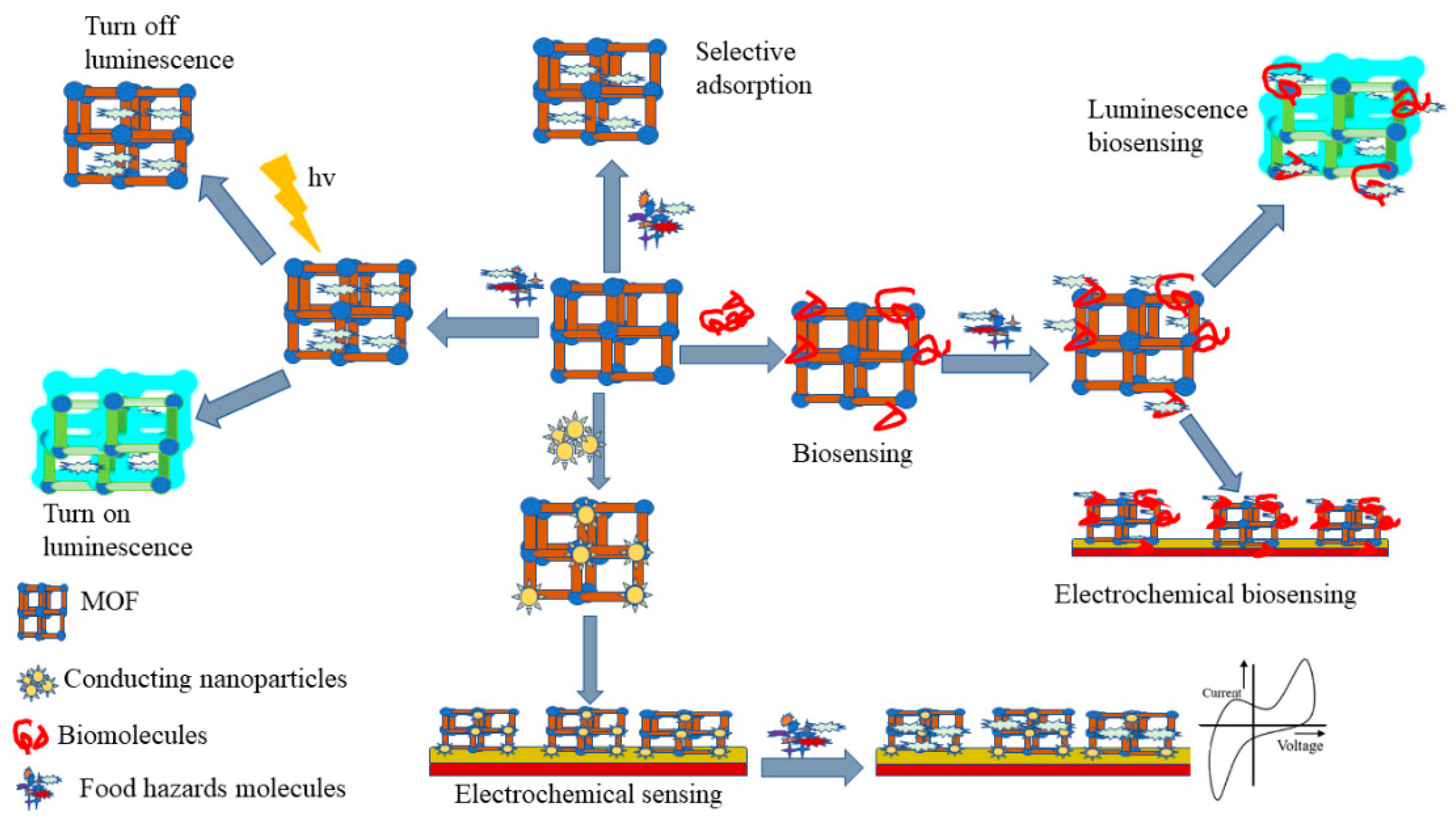
Figure 1.
Overview of MOFs film in flexible electrochemical sensors.
Figure 1.
Overview of MOFs film in flexible electrochemical sensors.
2. Flexible Electrochemical Sensors
2.1. Applications of Flexible Electrochemical Sensors
Flexible electrochemical sensors, a rapidly evolving field at the intersection of materials science, electronics, and analytical chemistry, are designed to bend, stretch, and conform to various shapes while maintaining functionality. These characteristics make them ideal for a wide range of applications, including wearable technology, medical diagnostics, environmental monitoring, and industrial uses. The development of flexible matrix materials has led to the emergence of flexible electrochemical sensors that align with these trends [
54,
55,
56]. Unlike traditional electrochemical sensors, which are typically rigid, incorporating flexible and stretchable materials extends their application scope. Flexible electrochemical sensors have the potential to revolutionize various fields with their unique combination of flexibility, sensitivity, and versatility [
57,
58,
59]. To meet the demands of emerging signals and specialized environments, sensor technology must continue to develop. This includes the development of new materials, innovative processes, and novel types of sensors, as well as advancements in sensor integration and intelligence. Flexible electrochemical sensors should also possess qualities such as transparency, flexibility, ductility, and the ability to bend or fold freely, making them portable and wearable [
60,
61,
62].
Flexible electrochemical sensors are indispensable in various aspects of life, such as monitoring physiological parameters to detect potential health issues at an early stage. Ongoing advancements in materials science, fabrication techniques, and electronic system integration will continue to enhance the capabilities and applications of flexible electrochemical sensors [
63,
64]. Flexible electrochemical sensors are poised to become essential tools for future technologies in health monitoring, environmental protection, and industrial process control. Despite operating on the same basic principles as traditional electrochemical sensors—utilizing the redox reactions of analytes at the sensor’s surface—the key difference lies in their ability to maintain performance while being deformed. In summary, flexible electrochemical sensors are crucial in various fields due to their sensitivity, selectivity, and real-time monitoring capabilities [
65,
66,
67]. Ongoing research focuses on enhancing sensor performance, expanding their range of applications, and addressing current challenges to meet the growing demand for reliable chemical detection technologies [
68,
69,
70].
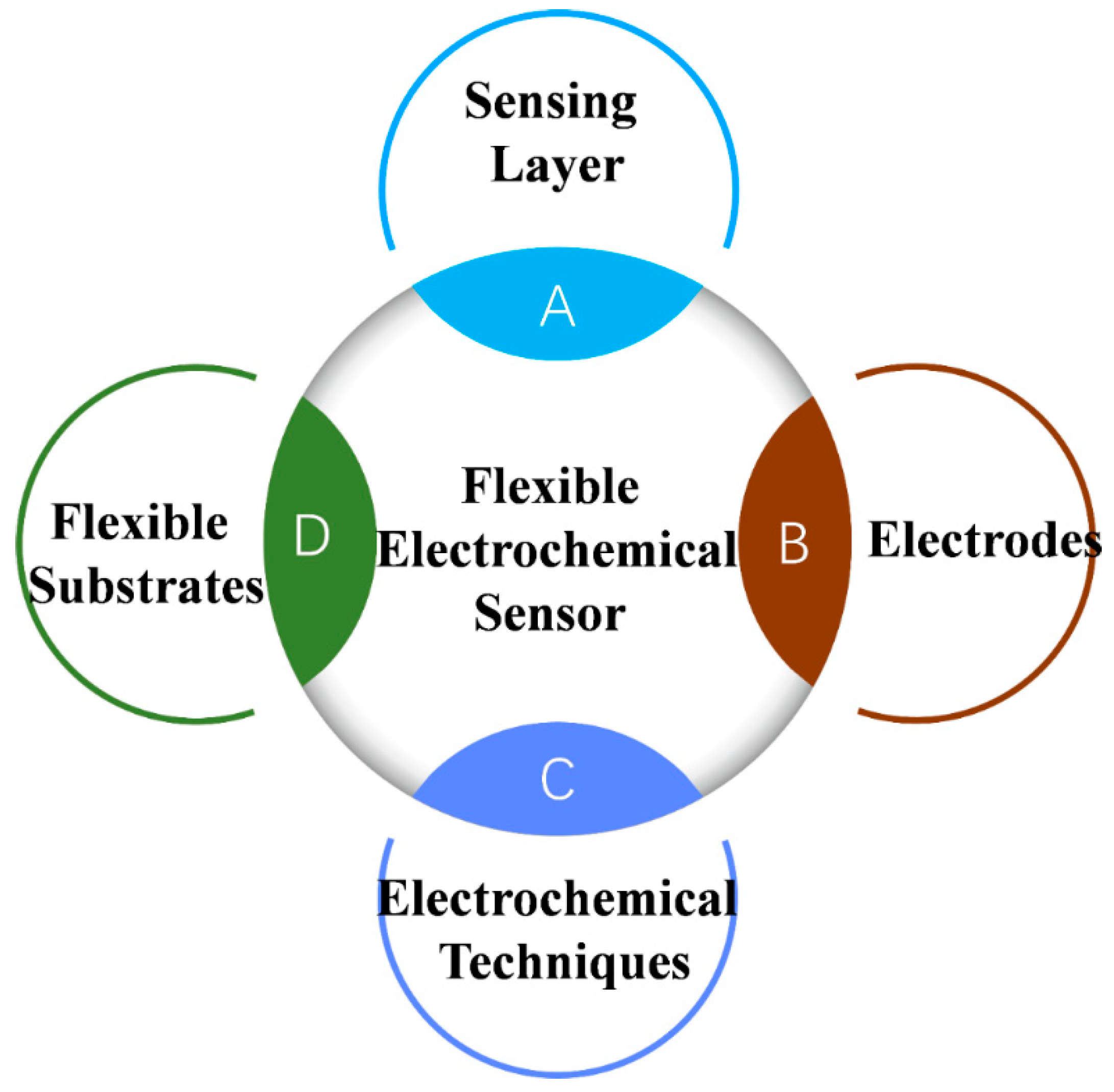
Figure 2.
Schematics illustrating a flexible electrochemical sensor composed of substrates, electrodes, electrochemical techniques and sensing layer.
Figure 2.
Schematics illustrating a flexible electrochemical sensor composed of substrates, electrodes, electrochemical techniques and sensing layer.
2.2. Materials Employed in Electrochemical Sensing Platforms
Electrochemical sensors operate based on the principle of electrochemistry, which involves the interaction between electrical energy and chemical reactions occurring at electrode surfaces. A typical flexible electrochemical sensing platform comprises several key components: the substrate, the active layer, and the interface layer. The selection of materials for these components is critical, as they must maintain their functional integrity under mechanical deformation, such as bending and stretching. For wearable applications, biocompatibility is essential to prevent adverse reactions when in contact with skin or other biological tissues [
71,
72,
73]. Additionally, the materials used must exhibit chemical and thermal stability to ensure consistent sensor performance over time and under various environmental conditions. These materials also need to be compatible with microfabrication techniques, allowing for seamless integration into wearable devices [
74,
75].
2.2.1. Substrate Layer
The substrate layer is a foundational component of flexible electrochemical sensors, providing structural support and mechanical flexibility. The substrate must withstand bending, stretching, and other forms of mechanical deformation without compromising its structural integrity or sensor performance. For wearable applications, the substrate must be biocompatible to ensure it does not cause adverse reactions when in contact with skin or other biological tissues. Additionally, the substrate should be chemically inert and stable under a range of environmental conditions to maintain sensor performance over time. It must also be suitable for advanced microfabrication processes, enabling the seamless integration of various sensor components and materials. For example, Avuthu et al. [
76] made a flexible electrochem sensor screen printed on polyethylene terephthalate selectivly detected heavy metal ions Hg
2+ and Pb
2+. Salem et al. [
77] reported a flexible poly(imide) films substrate applied to laser-induced graphene patterned electrode of a sensitive, selective and reproducible electrochem. sensor for detection nitrite in water. The choice of substrate material depends on the specific application requirements, including the desired flexibility, biocompatibility, and environmental stability needed for the sensor’s intended use. Flexible substrates are often made from materials like polyethylene terephthalate (PET), polyimide (PI), or polydimethylsiloxane (PDMS), as these materials provide the necessary flexibility and mechanical stability [
78,
79,
80,
81].
2.2.2. Electrode Layer
The electrode layer is a critical component of flexible electrochemical sensors, serving as the site for electrochemical reactions and signal transduction [
82,
83]. It typically consists of conductive materials that maintain their performance under mechanical deformation, such as bending and stretching. The electrode material must be chemically stable and resistant to corrosion to ensure long-term performance in diverse environmental conditions, while also possessing high electrical conductivity to facilitate efficient electron transfer during sensing. The active layer includes electrodes made from materials like gold, platinum, carbon-based materials (graphene, carbon nanotubes), or conducting polymers (PEDOT, polypyrrole (PPy)) [
26,
84,
85,
86,
87]. Electrochemical sensors typically incorporate working electrodes, reference electrodes, and sometimes auxiliary electrodes [
88]. The working electrode facilitates the chemical reaction of interest, where interactions with the target analyte lead to changes in electrochemical properties, such as potential or current. These changes translate into measurable electrical signals-current, potential (voltage), or impedance. Materials such as conductive polymers (e.g., polyaniline, PPy, PEDOT), carbon-based materials (e.g., graphene, CNTs, carbon black), metal nanoparticles and nanowires (e.g., gold, silver, platinum), and metal oxides [
89,
90,
91](e.g., ITO, ZnO) are chosen for their conductivity, flexibility, and compatibility with microfabrication techniques.
In summary, designing the electrode layer of flexible electrochemical sensors requires balancing conductivity, mechanical flexibility, biocompatibility, chemical stability, and integration capability to optimize performance in diverse applications, especially in wearable sensing devices.
2.2.3. Interface Layer
The interface layer in flexible electrochemical sensors serves a critical role in ensuring optimal performance and durability [
92,
93]. It acts as a bridge between the active electrode material and the external environment, influencing both the sensor’s sensitivity and its mechanical properties. This layer promotes strong adhesion between the active electrode material and the substrate, ensuring the sensor remains intact during mechanical deformation such as bending or stretching, which is crucial for maintaining electrical continuity and preventing delamination. Efficient charges transfer between the active electrode material and the analyte in the environment is facilitated, thereby enhancing the sensor’s sensitivity and response time. Optimization of the interface layer often involves adjusting its surface morphology and chemical composition. Additionally, the interface layer serves as a barrier, protecting the active electrode material from environmental factors such as moisture, contaminants, and chemical reactions that could degrade sensor performance over time [
94,
95].
For manufacturing flexibility, the interface layer must be compatible with microfabrication techniques, ensuring reliable and efficient deposition or patterning on flexible substrates. It can incorporate hydrogel coatings, ionic liquids, or nanostructured materials to improve sensor-biofluid interactions, enhancing sensitivity and selectivity by facilitating efficient mass transport and providing a biocompatible environment [
96,
97,
98,
99]. In summary, the interface layer in flexible electrochemical sensors plays a crucial role in optimizing performance, ensuring durability under mechanical stress, enhancing sensitivity, providing protection from environmental factors, and promoting biocompatibility for wearable applications.
2.2.4. Active Layer
The active layer in flexible electrochemical sensors plays a crucial role in facilitating electrochemical reactions for detecting and quantifying target analytes. This layer is composed of materials selected for their specific electrochemical properties, including conductivity, catalytic activity, and selectivity towards the analyte of interest. Popular materials include conductive polymers such as polyaniline, polypyrrole, and poly(3,4-ethylenedioxythiophene) (PEDOT), known for their excellent electrical conductivity and stability. Carbon-based materials like graphene and carbon nanotubes offer high surface area, conductivity, and chemical stability, enhancing sensitivity and response times. Metals such as gold, platinum, and silver nanoparticles provide catalytic activity, while metal oxides like tin dioxide (SnO
2), titanium dioxide (TiO
2), and indium oxide (In
2O
3) offer both conductivity and catalytic properties for diverse sensing applications [
100,
101,
102]. Hybrid materials combining these elements can further tailor properties like selectivity and stability to specific sensing needs. Deposition onto a flexible substrate, through techniques like spin-coating, inkjet printing, or vapor deposition, ensures uniform coverage and adherence [
103,
104,
105,
106,
107,
108]. The composition and structure of this layer are critical in determining sensor sensitivity, selectivity, and overall performance, making material selection and deposition methods crucial areas of research and development in flexible electrochemical sensor technology.
3. MOFs in Flexible Electrochemical Sensors
3.1. MOFs as a Tunable Platform for Flexible Electrochemical Sensors
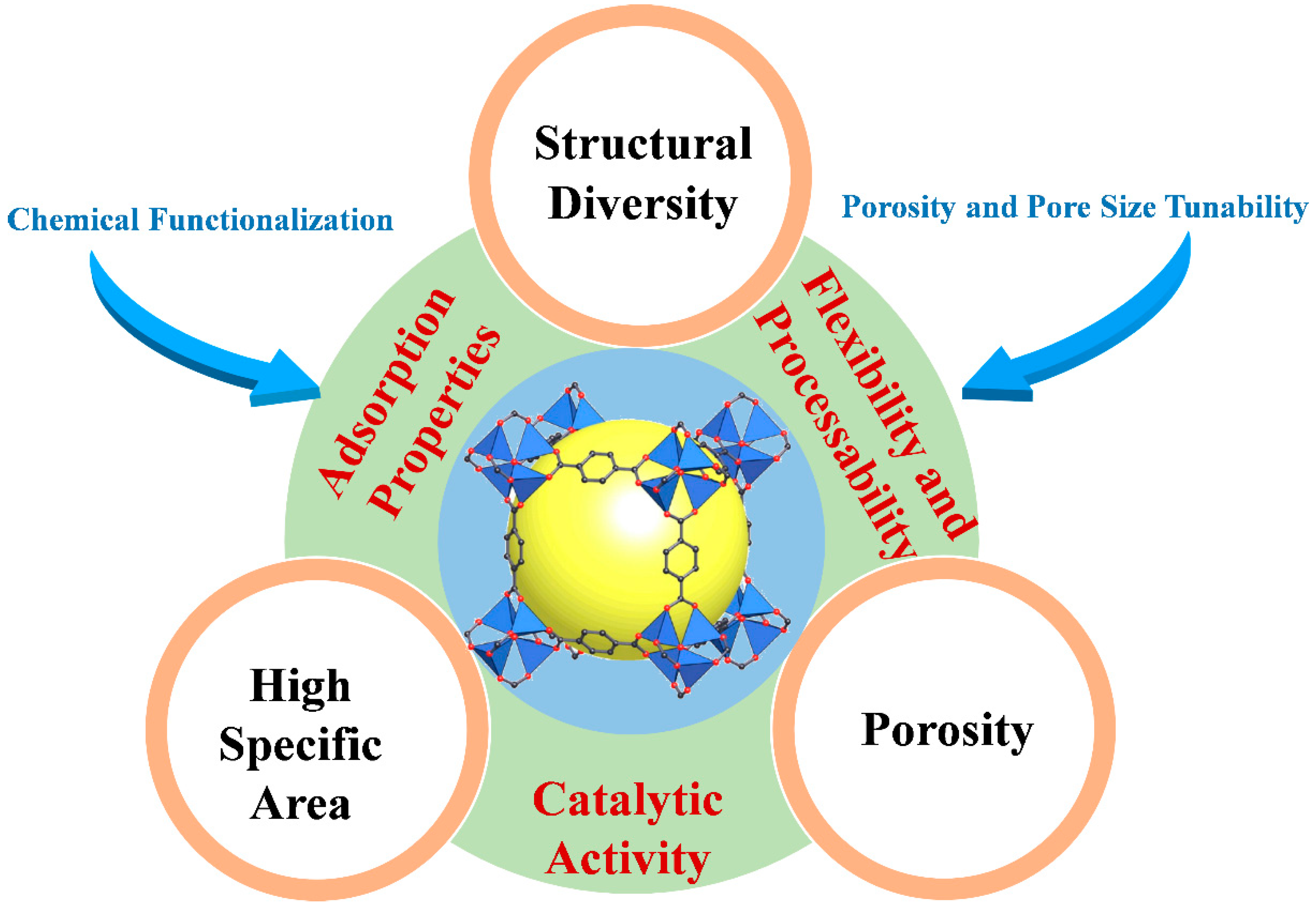
MOFs are an extraordinary class of porous materials that have garnered significant attention in past decades due to their unique and highly tunable properties. Key findings highlight the unique properties of MOFs, such as high surface area, tunable pore structures, and chemical functionality, which contribute to enhanced sensitivity, selectivity, and stability in electrochemical sensing applications [
109,
110,
111]. MOFs provide a large surface area per unit volume, maximizing the active sites available for electrochemical reactions, thereby enhancing sensor sensitivity. The porosity of MOFs can be finely tuned by selecting specific metal ions and organic ligands, allowing for precise control over analyte diffusion and interaction within the sensor. Furthermore, MOFs can be designed with different metal nodes and organic linkers, offering flexibility to optimize sensor selectivity and stability towards target analytes. They can also be functionalized with various functional groups, biomolecules, or nanoparticles to impart additional functionalities such as enhanced catalytic activity or specific analyte recognition [
112,
113,
114]. In addition to their functional versatility, some MOFs exhibit excellent chemical stability, which is crucial for sensor longevity and reliability, especially in dynamic environments like wearable sensors. Certain MOFs also possess inherent catalytic activity, enabling them to directly participate in electrochemical reactions or enhance the catalytic performance of other sensor materials. MOFs’ ability to integrate into flexible substrates, such as polymer matrices or films, further enables the fabrication of flexible and conformable sensors suitable for wearable applications. Researchers are actively exploring strategies to leverage these advantages of MOFs, such as hybridizing them with conductive polymers or incorporating them into nanocomposites, to significantly enhance sensor performance in terms of sensitivity, selectivity, and stability [
115,
116]. Ongoing research focuses on optimizing MOF properties, deposition techniques, and integration strategies to enhance sensor performance, broaden applications, and address challenges such as mechanical durability and biocompatibility for wearable sensor technologies [
83,
117].
Figure 3.
Fundamental MOFs properties related for sensing applications.
Figure 3.
Fundamental MOFs properties related for sensing applications.
3.2. Selective Recognition of MOFs for Electrochemical Sensing
Selective recognition refers to the ability of a material to specifically adsorb, detect, or react with a class of molecules or ions in a complex mixture environment. In sensing technology, selective recognition is particularly important because there are a lot of interferences in the actual environment. This ability not only helps quickly and accurately identify the target molecule or ion, but also avoids interference from other unrelated substances, ensuring the accuracy and reliability of the data. MOFs is a highly designable and functional material, and its selective recognition ability is a basic aspect of MOFs function [
118,
119]. This ability is mainly due to the MOFs high porosity, tunable structure and chemical versatility. These properties allow MOFs to interact strongly with specific molecules or ions, enabling efficient selective recognition.
The selective recognition ability of MOFs has shown great application potential in many fields. In the field of gas separation, MOFs can selectively adsorb and separate specific gas molecules, such as carbon dioxide and hydrogen [
120,
121]. In the field of sensing, the high surface area and adjustable pore size of MOFs enable them to effectively bind biomarkers such as uric acid, glucose and ascorbic acid, enabling rapid detection of these substances [
122,
123]. In the field of catalysis, the specific structure and chemical properties of MOFs enable them to selectively catalyze certain chemical reactions, improving reaction efficiency and product purity. In addition, MOFs have shown promising applications in environmental remediation, such as selective adsorption and removal of harmful substances from water [
124].
Taking the application of MOFs in the field of electrochemical sensors as an example, its wide surface area and porosity provide sufficient space for preferentially adsorbing neurotransmitters such as dopamine [
125]. Due to the differences in structure and properties between dopamine and other neurotransmitters, MOFs can selectively interact with dopamine to achieve efficient detection. In addition, the aperture of MOFs can be precisely controlled during synthesis to achieve size-selective identification of guest molecules of different sizes and shapes. Some MOFs also exhibit flexible frame structures that are able to accommodate guest molecules of different shapes and sizes, further improving their selective recognition ability [
126].
It is worth mentioning that the selective recognition ability of MOFs can be fine-tuned by adjusting their pore size and surface chemistry [
127,
128]. This tunability makes MOFs more flexible and adaptable in sensor applications. By precisely controlling the structure and chemistry of MOFs, we can achieve efficient selective identification of different target molecules, leading to more efficient and targeted applications in a variety of scientific and industrial fields. With the continuous development and progress of science and technology, the research on the selective recognition ability of MOF will continue to deepen. Future research will likely reveal more information about the selective recognition mechanism of MOFs and further optimize its structure and function for more efficient and accurate sensor applications. This will bring more convenience and possibilities to our lives and promote the development of science and technology to a higher level.
4. Fabrication of MOFs Film
In flexible electrochemical sensors, MOF films serve as the active layer where electrochemical reactions take place, detecting analytes through changes in electrical signals. MOFs films can be deposited onto flexible substrates using techniques like drop casting method, allowing for the fabrication of flexible sensors that can conform to irregular surfaces or be integrated into wearable devices [
129,
130]. Recently, there has been a growing emphasis on integrating high quality MOFs films with flexible substrates, which opens up new possibilities for flexible and wearable electronics, sensors, and other advanced applications. MOFs thin films possess a substantial specific surface area, abundant metal active sites, and an orderly and compact arrangement, rendering them highly promising for applications in thin film separation, sensors, and devices. Currently, various methods are available for synthesizing MOFs thin films including in-situ growth, interface assembly, thermal stamping, hydrothermal deposition, secondary growth, and electrochemical approaches. These techniques enable the preparation and functionalization of MOFs thin films while establishing a solid foundation for their utilization in thin film devices. However, controlling the thickness, surface morphology, and growth orientation of MOFs thin films remains challenging which restricts their application in high-quality demanding devices such as separation processes [
131]. Consequently, there is an urgent need to develop a method that can produce MOFs thin films with uniform flat surfaces and specific growth orientations. The insolubility of MOFs in any solvent makes the preparation of thin films still a great challenge
Flexible substrates, such as polymers (e.g., polyimide, PET), elastomers (e.g., PDMS), and even paper, offer unique advantages in terms of mechanical flexibility, lightweight nature, and conformability to irregular surfaces. The fabrication of MOFs on flexible substrates represents a promising frontier in materials science, offering new opportunities for flexible electronics, wearable sensors, and advanced functional materials. Continued advancements in fabrication techniques, materials design, and application-specific integration will further propel the field towards practical implementations in various industrial and consumer applications. As research progresses, the synergy between MOFs and flexible substrates is expected to drive innovations in flexible electronics and beyond, paving the way for transformative technologies in the coming years.
4.1. Substrate Treatment
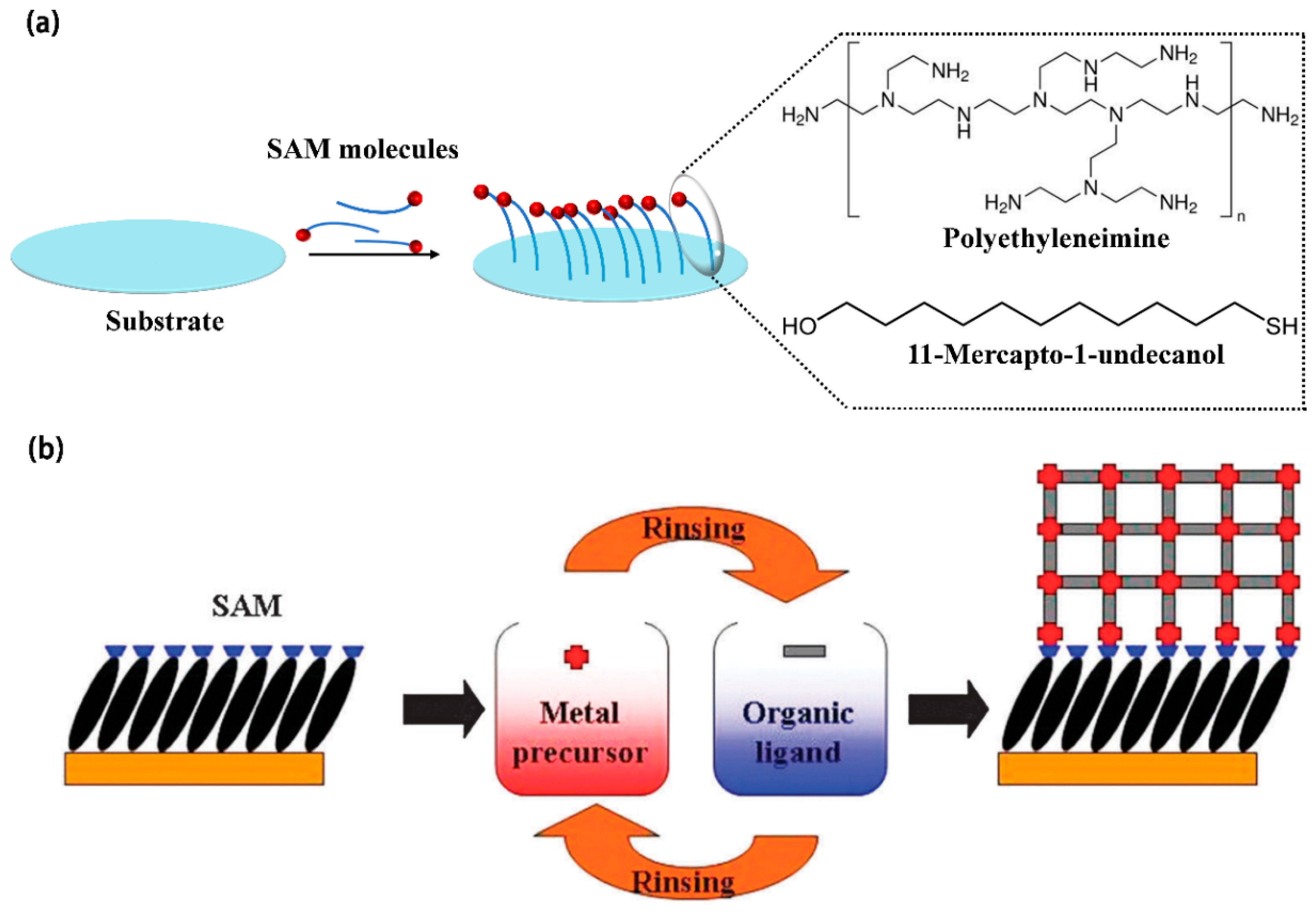
Ensuring a strong and reliable interface between the MOF film and flexible substrates is crucial for practical applications, as a mismatch in mechanical properties can lead to delamination or performance degradation [
132]. The surface of the substrate used for MOFs film preparation must be thoroughly cleaned to remove contaminants such as dust, grease, and organic residues. Common cleaning methods include solvent cleaning (using solvents like acetone, ethanol, or isopropyl alcohol) and ultrasonic cleaning to ensure a pristine surface. Additionally, to enhance adhesion between the MOF film and the substrate, the substrate surface needs to undergo hydrophilic treatment by introducing functional groups (
Figure 4). Self-assembled monolayers (SAMs) with specific functional groups are particularly beneficial as they can modify the chemical properties of the substrate surface, influencing the growth of MOF crystals and the properties of the resulting film. For conductive glass, silicon wafers, sheet metal and other planar substrates, this is typically achieved by treating them with a piranha solution (a mixture of concentrated sulfuric acid and 30% hydrogen peroxide in a volume ratio of 3:1) heated to 80-100 °C for at least half an hour. This treatment modifies the substrate surface with a layer of hydroxyl groups, facilitating the coordination of metal ions and thereby enhancing the adhesion between the MOFs film and the substrate. Therefore, select the appropriate substrate material, and through the steps of cleaning, treatment and surface modification, ensure the flatness of the substrate surface and appropriate surface chemical properties to promote the growth and adhesion of MOF films.
Figure 4.
(a) Hydrophilic treatment of the substrate. (b) A schematic diagram illustrating the step-by-step approach for growing MOFs on substrates functionalized with self-assembled monolayers (SAMs) (O. Shekhah, Layer-by-Layer method for the synthesis and growth of surface mounted metal–organic frameworks (SURMOFs). Reproduced with permission [
132].
Figure 4.
(a) Hydrophilic treatment of the substrate. (b) A schematic diagram illustrating the step-by-step approach for growing MOFs on substrates functionalized with self-assembled monolayers (SAMs) (O. Shekhah, Layer-by-Layer method for the synthesis and growth of surface mounted metal–organic frameworks (SURMOFs). Reproduced with permission [
132].
4.2. Liquid Phase Epitaxy
Liquid phase epitaxy (LPE) is a method used to sequentially assemble materials layer by layer on the surface of a substrate [
133]. High-quality MOF films produced by LPE on the substrate surface enable precise control over their crystal structure and thickness. Dip-coating, spin-coating, and layer-by-layer deposition are common techniques where flexible substrates are immersed or coated with MOF precursor solutions. Liquid phase epitaxy offers several advantages for preparing MOF films: it operates under relatively mild conditions, facilitates uniform growth of high-quality films over large substrate areas, and allows precise control over the crystal structure, pore size, and chemical functionality of MOFs. This capability is beneficial for maximizing the exposure of MOF active sites, thereby meeting specific application requirements for MOF materials.
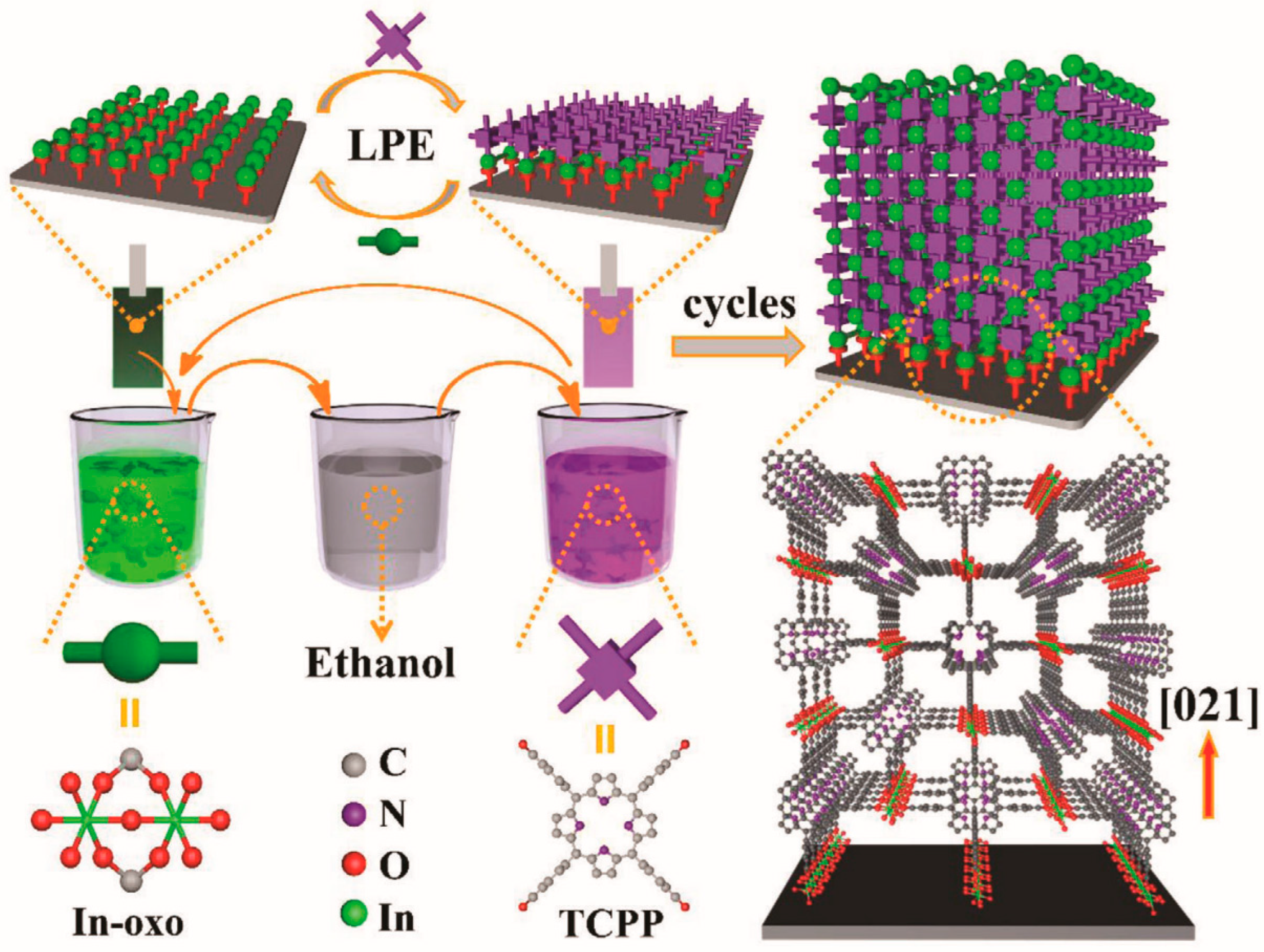
As shown in
Figure 5, LPE primarily includes the following steps: firstly, metal ions and organic ligands are fully dissolved in their respective solvents to form precursor solutions. The concentration of these solutions is typically adjusted to control the thickness of the desired MOF film during growth. Following substrate pretreatment, the precursor solution is applied to the substrate surface. By varying the concentration, temperature, and duration of exposure to metal ions and organic ligands in the solution, MOF crystals grow layer by layer from the substrate surface. After each immersion, the substrate is thoroughly cleaned with ethanol and dried with nitrogen to remove any residual reactants. Upon completion of MOF film growth, additional steps such as solvent removal, enhancement of crystallinity, or surface modification are performed to further refine the structure and properties of the MOF films.
Figure 5.
Schematic illustration of porphyrinic MOF thin film grown on functionalized substrate by using liquid phase epitaxy. [
133].
Figure 5.
Schematic illustration of porphyrinic MOF thin film grown on functionalized substrate by using liquid phase epitaxy. [
133].
4.3. In Situ Growth Methods
In situ growth methods involve direct synthesis of MOFs films on the relevant substrates, often using solvothermal or microwave-assisted methods to induce MOF formation directly on the substrate surface. The hydrothermal/solvothermal method is a significant approach for preparing MOF films [
134,
135]. This method involves exposing the substrate directly to a precursor sol or solution under either hydrothermal or solvothermal conditions, facilitating the nucleation and growth of MOF crystals directly on the substrate. However, this approach presents several challenges and limitations. To address issues such as uneven deposition or overly thick film layers, two strategies are commonly employed: orienting the substrate with the crystal growth face down or vertically immersing it into the reaction solution. Both methods help enhance the uniformity and control the thickness of MOF films to some extent. Nevertheless, despite these measures, MOF films obtained directly via hydrothermal/solvothermal methods often exhibit poor orientation and limited controllability over their properties. This instability can make it challenging to precisely regulate the film properties according to specific requirements. To improve the quality of MOF films, effective surface functionalization of the substrate material is crucial.
In addition to surface functionalization, another method involves depositing synthesized powdered MOFs directly onto the substrate using surface drop coating or spin coating with MOF particle suspensions or mixtures with solutions like Nafion [
136,
137]. This approach allows for rapid solvent evaporation to achieve precise control over film thickness and uniformity. It also enables adjustment of MOF particle concentration and type to tailor the film performance as needed. In conclusion, while the hydrothermal/solvothermal method is effective for MOF film preparation, careful attention is required to enhance film uniformity, thickness control, orientation, and overall controllability of film properties. Surface functionalization and the use of MOF particle suspensions offer promising avenues to further enhance the quality and performance of MOF films. In a study by Jeong Liu et al. [
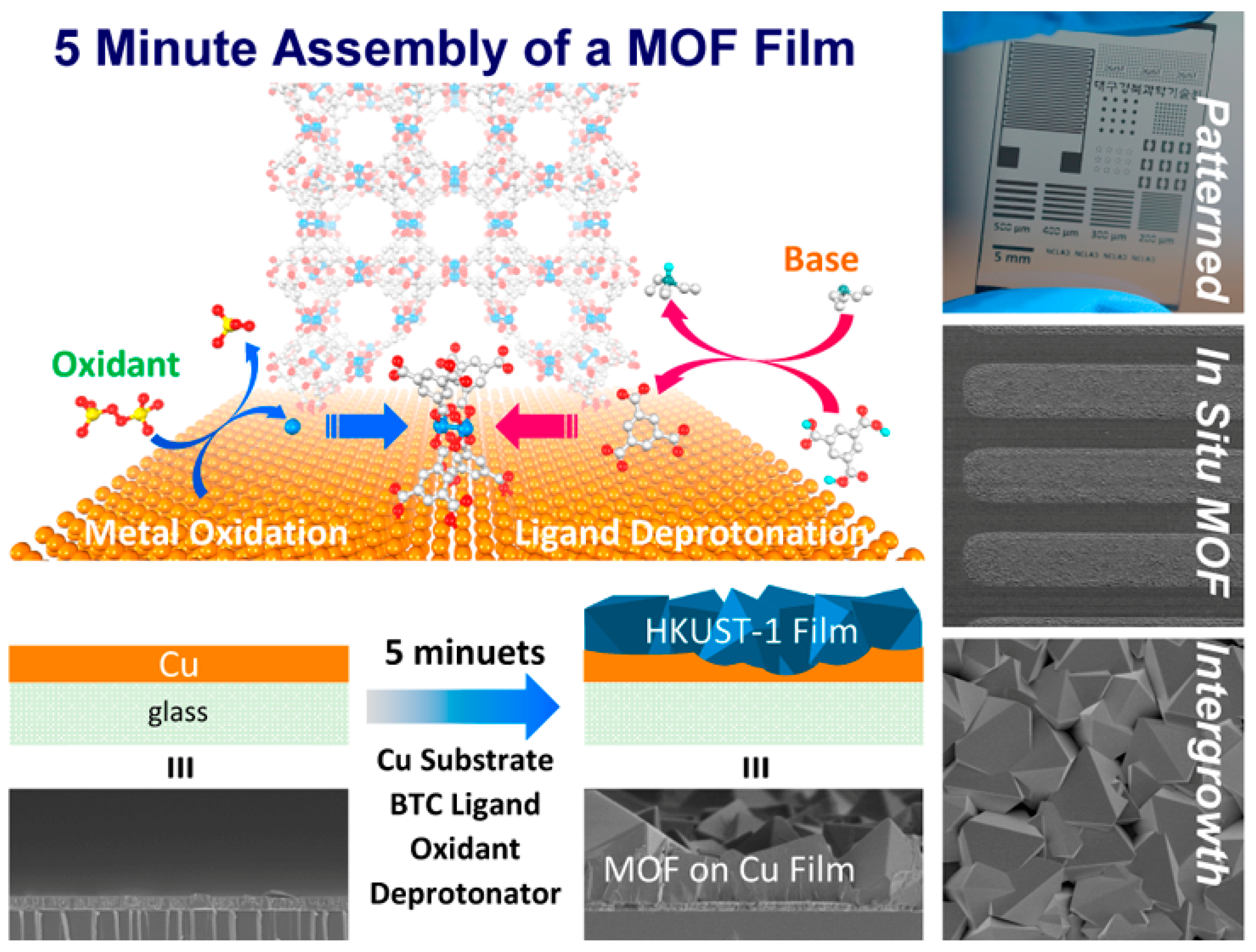
138], HKUST-1 films was rapidly grown on Cu substrates, where the Cu substrate acts both as a conducting substrate and a source of Cu
2+ ions during the synthesis of films.
Figure 6.
Rapid growth of HKUST-1 Films on Cu substrates. Reproduced with permission [
138].
Figure 6.
Rapid growth of HKUST-1 Films on Cu substrates. Reproduced with permission [
138].
MOF films not only retain the inherent characteristics of MOFs such as a large specific surface area, abundant metal active sites, and high porosity, but also exhibit significant potential applications in gas separation, sensors, heterogeneous catalysis, and other fields. However, the majority of MOFs exist in powder form and are insoluble in any solvent, posing challenges for their transformation into films. Despite successive research advancements in methods like in-situ growth, interface assembly, hot pressing, hydrothermal deposition, secondary growth, and electrochemical deposition for preparing MOF thin films, controlling the thickness, surface morphology, and growth orientation remains difficult. This limitation hinders their application in high-quality devices and separation technologies. Hence, there is an urgent need to develop methods that can produce MOF films with uniform surfaces and specific orientations.
4.4. Electrophoretic Deposition Method
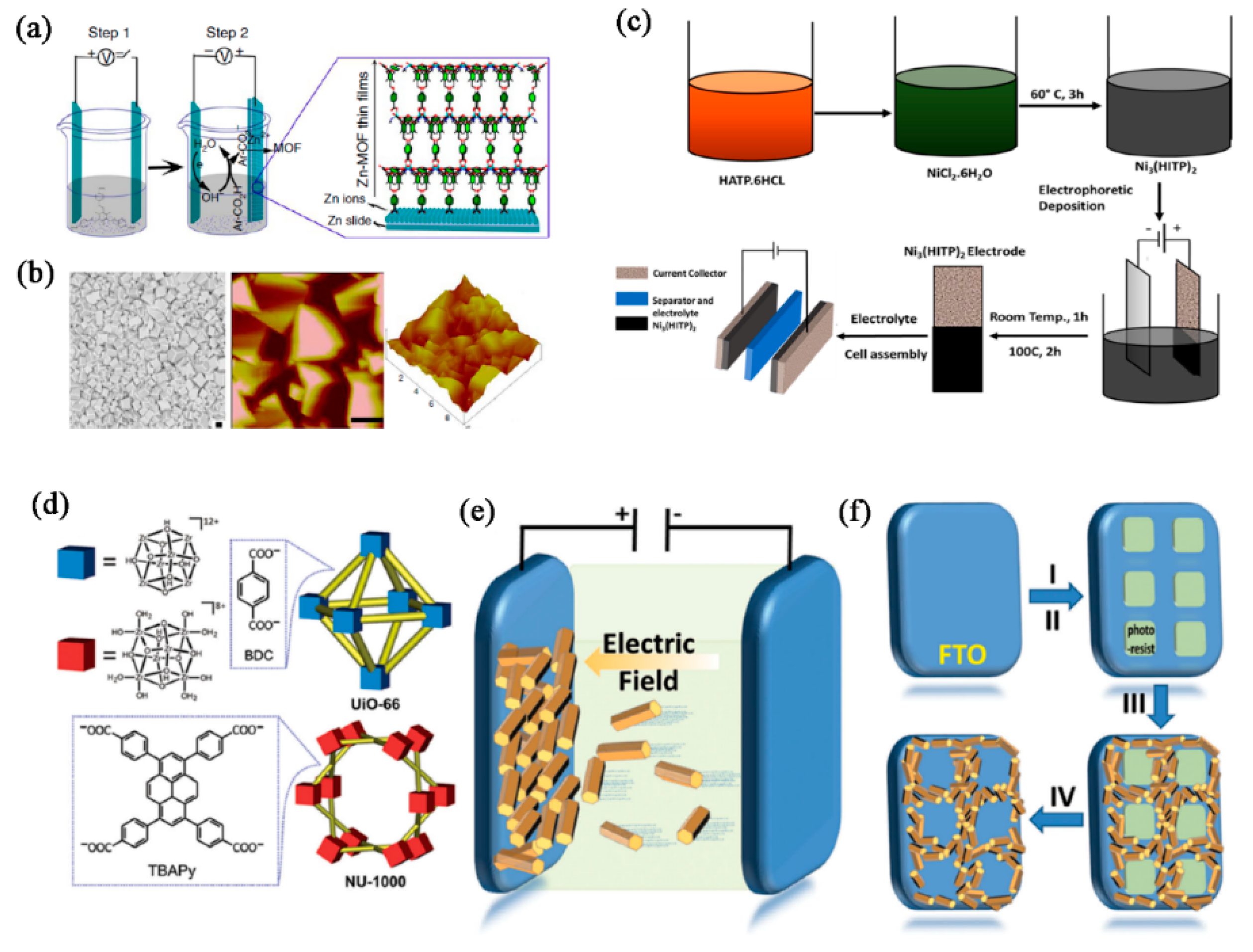
Electrophoretic deposition (EPD) is a versatile method for fabricating MOFs films on various substrates. It involves the migration of charged MOFs in a suspension under the influence of an electric field, leading to their deposition on an electrode. EPD is a promising technique for creating MOFs films, particularly for applications requiring high surface area, controlled porosity, and specific chemical functionalities. For example, Cao et al. [
139] introduce a straightforward method for depositing interpenetrated, crystalline MOFs films onto conductive metal-plate anodes through an in situ electrochemical assembly process. The thickness and uniformity of the MOFs film are well controlled by the temperature, reaction time and voltage (
Figure 7a,
b).
EPD can produce uniform and dense films, which are essential for various applications. This technique is particularly advantageous for creating uniform and well-adhered films of MOFs, which are useful in applications such as sensors, catalysis, and separation membranes. as illustrated in
Figure 7c, the creation of an ultra-high cycling stability supercapacitor involves the use of electrophoretic deposition (EPD), employing the 2D MOFs Ni₃(HITP)₂ as the active material for the electrodes [
140]. The electrophoretic deposition produced highly dense films, resulting in a Ni₃(HITP)₂ supercapacitor achieving an exceptionally high areal specific capacitance of 15.69 mF cm⁻². This is the highest capacitance value recorded for MOFs to date.
The first step involves preparing a stable colloidal suspension of the desired MOFs particles (
Figure 7d-
e) [
141]. The MOFs particles can be synthesized beforehand and dispersed in a suitable solvent. Stabilizers or surfactants may be added to prevent aggregation of MOFs particles and to ensure a stable suspension. Two electrodes are used, typically made of conductive materials such as metal or conductive glass (
Figure 7f). The substrate on which the MOFs film is to be deposited serves as the working electrode, while the counter electrode completes the circuit. An electric field is applied between the electrodes. The charged MOFs particles migrate towards the electrode with the opposite charge, where they accumulate and form a film. However, Precise control over film thickness can be challenging and requires careful optimization of deposition parameters. Maintaining a stable suspension of MOFs particles is crucial for consistent film quality. The charge of the MOFs particles affects their mobility in the electric field and must be controlled for effective deposition.
Figure 7.
(a) Diagram illustrating the electrochemical formation of MOFs films. (b) SEM image alongside a representative height AFM image. Scale bar, 1 mm. Reproduced with permission [
139]. (c) Diagram illustrating the fabrication process and electrophoretic deposition of Ni₃(HITP)₂ [
140]. (d) Components and illustration of the crystal structures of of UiO-66 and NU-1000 include Zr-containing nodes. (e) A diagram demonstrating the principle of MOF film growth via electrophoretic deposition. (f) Illustration of the MOF EPD film patterning procedure. Reproduced with permission [
141].
Figure 7.
(a) Diagram illustrating the electrochemical formation of MOFs films. (b) SEM image alongside a representative height AFM image. Scale bar, 1 mm. Reproduced with permission [
139]. (c) Diagram illustrating the fabrication process and electrophoretic deposition of Ni₃(HITP)₂ [
140]. (d) Components and illustration of the crystal structures of of UiO-66 and NU-1000 include Zr-containing nodes. (e) A diagram demonstrating the principle of MOF film growth via electrophoretic deposition. (f) Illustration of the MOF EPD film patterning procedure. Reproduced with permission [
141].
4.5. Polymer-Assisted Strategy for Creating MOFs Films
The polymer such as Polyvinyl alcohol (PVA), poly(methyl methacrylate) (PMMA), or polydopamine (PDA) serves as a stabilizer or binder and helps in controlling the MOFs films growth [
142,
143,
144]. Dissolve the polymer in a solvent that can effectively dissolve it without affecting the MOF precursors. Adjust the polymer concentration to achieve the desired viscosity and film properties. Apply the polymer solution onto the substrate using techniques such as spin-coating, dip-coating, or spray-coating. Introduce the MOF precursor solution onto the polymer-coated substrate. This can be done by sequential application or co-dissolution, depending on the method used. This polymer-assisted strategy can help achieve uniform and high-quality MOF films with tailored properties, making it useful for various applications in materials science and engineering. Wang et al. [
145] proposed three strategies we for MOFs film fabrication (Fig. (a)). For example, a novel approach has been developed to reduce the bulk electrical resistance of MOFs by incorporating polyaniline (PANI) chains into the MOFs structure. As shown in
Figure 8b, this is achieved through the electrochemical deposition of PANI onto the MOF crystals. In this method, cobalt-based MOF crystals, specifically ZIF-67, were first synthesized onto a carbon cloth (CC) substrate [
65]. Subsequently, PANI was electrochemically deposited onto the ZIF-67, resulting in the creation of a flexible, conductive porous electrode, designated as PANI-ZIF-67-CC. This strategy effectively maintains the integrity of the underlying MOF structure while enhancing electrical conductivity. Electrochemical evaluations reveal that the PANI-ZIF-67-CC electrode exhibits an exceptional areal capacitance of 2146 mF cm
−2 at a scan rate of 10 mV s
−1, demonstrating its high performance and potential for advanced applications.
Figure 8.
(a) Three strategies for the fabrication of MOFs films. Reproduced with permission [
145]. (b) Schematic Representation of ZIF-67 onto carbon cloth and further electrically deposited PANI to give a flexible conductive porous electrode [
65]. (c) Schematic illustration of the two-step fabrication process and SEM images of MOFs interwoven by electrochemically-ceposited PANI [
146].
Figure 8.
(a) Three strategies for the fabrication of MOFs films. Reproduced with permission [
145]. (b) Schematic Representation of ZIF-67 onto carbon cloth and further electrically deposited PANI to give a flexible conductive porous electrode [
65]. (c) Schematic illustration of the two-step fabrication process and SEM images of MOFs interwoven by electrochemically-ceposited PANI [
146].
5. Application of MOFs-Based Flexible Electrochemical Sensors
MOFs-based electrochemical sensors have promising applications across several domains due to their unique properties and capabilities. MOFs-based electrochemical sensors represent a versatile and promising technology with significant potential to address critical challenges in environmental monitoring, healthcare diagnostics, food safety, and industrial processes. Continued advancements in MOF materials and sensor design are essential to realize their full impact across these diverse application areas.
5.1. Environmental Monitoring
MOFs can be used to remove contaminants from water, including heavy metals, organic pollutants, and radionuclides [
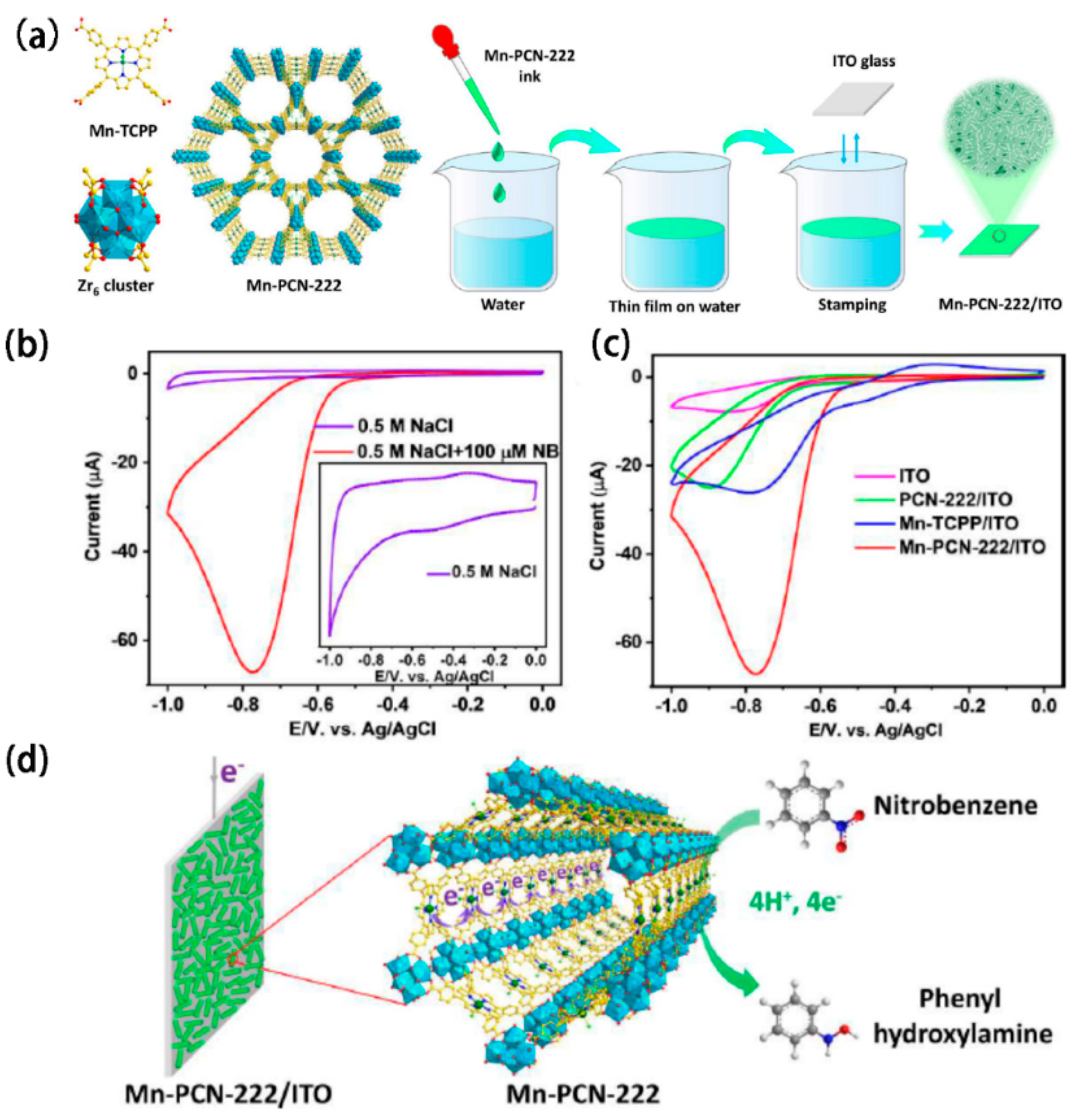
147]. Their large surface area and functional groups enable them to adsorb and trap a wide range of pollutants. In a study by Roland A. Fischer group [
148], the porphyritic MOFs Mn-PCN-222 is deposited onto a conductive ITO surface. The high surface area of Mn-PCN-222/ITO as a working electrode supports elevated current densities, facilitating highly sensitive analyses toward nitrobenzene (NB). The metalloporphyrin center in Mn-PCN-222 enables analyte-specific redox catalysis, allowing for the simultaneous detection of multiple analytes in binary and ternary systems. This capability makes MOFs film effective for detecting a wide range of trace pollutants under real-world conditions with high sensitivity. MOF films can be easily integrated with flexible electronic substrates, such as conductive polymers or graphene-based materials, allowing for the development of flexible, wearable sensors for detecting pollutants and hazardous substances in air and water. In summary, MOF films combine flexibility with high sensitivity and tunable properties, making them a promising material for advanced electrochemical sensors in environmental monitoring applications.
Figure 9.
(a) Diagram illustrating the film of Mn-PCN-222 is deposited on a conductive indium tin oxide (ITO) surface. (b) Cyclic voltammetry (CV) profiles of the Mn-PCN-222/ITO electrode in a 0.5 M NaCl solution, both with and without 100 μM NB. (c) CV curves obtained from different electrodes focusing on the reduction of 100 μM NB. (d) Schematic representation of the possible mechanism for NB reduction on the Mn-PCN-222/ITO electrode. Reproduced with permission [
148].
Figure 9.
(a) Diagram illustrating the film of Mn-PCN-222 is deposited on a conductive indium tin oxide (ITO) surface. (b) Cyclic voltammetry (CV) profiles of the Mn-PCN-222/ITO electrode in a 0.5 M NaCl solution, both with and without 100 μM NB. (c) CV curves obtained from different electrodes focusing on the reduction of 100 μM NB. (d) Schematic representation of the possible mechanism for NB reduction on the Mn-PCN-222/ITO electrode. Reproduced with permission [
148].
5.2. Health Diagnostics
In this regard, flexible sensors provide real-time monitoring of various physiological parameters such as heart rate [
149,
150], blood pressure [
151,
152], and biomarkers [
153,
154], offering accurate and reliable data support for healthcare and fitness applications. These data aid precise health assessments by medical professionals and empower fitness enthusiasts to better understand their physical condition for scientifically informed exercise routines. Electrochemical biosensors monitor biomolecular reactions at electrode surfaces, enabling real-time detection of biomarkers, drugs, and disease indicators crucial for early diagnosis and health management [
155,
156,
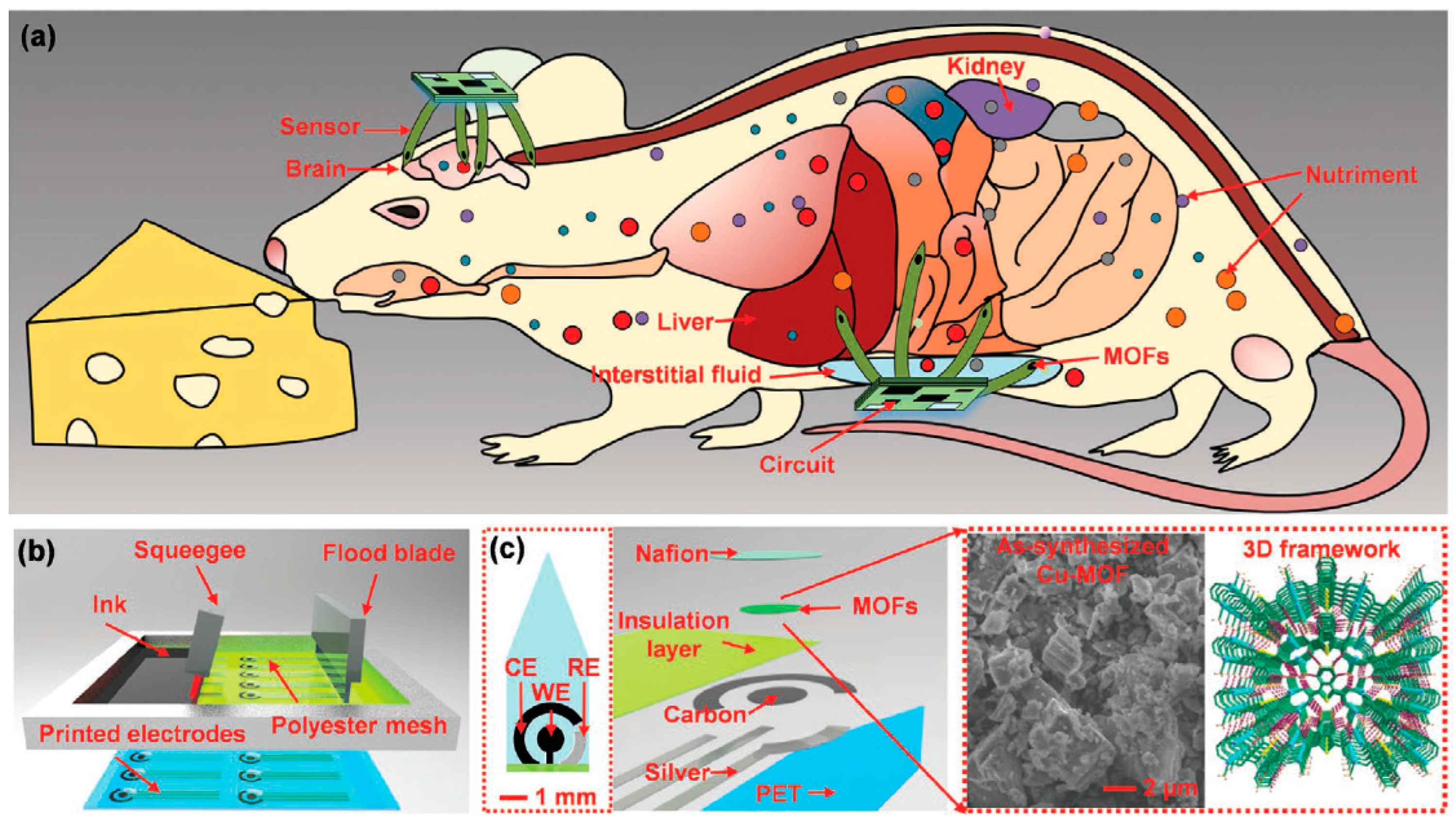
157]. However, integrating MOFs with flexible electronic devices for wearable sensing poses challenges due to most MOFs have inherently low electrical conductivity, which limits their effectiveness in electronic applications. Conductive MOFs represent a burgeoning class of multifunctional materials characterized by abundant catalytic active sites, highly porous structures, and intrinsic conductivity. These attributes make them highly desirable for electrochemical sensing applications. Ling et al. [
158] demonstrated multichannel implantable electrochemical sensors that were surface-modified with conductive copper-MOFs and cobalt-MOFs. Experiments with live cells and animals indicate that the MOF-modified sensors are biologically safe for cells and capable of detecting l-Trp in both blood and interstitial fluid. This research represents the first effort to integrate MOFs film with flexible sensors to achieve highly specific and sensitive implantable electrochemical detection.
Figure 10.
Schematics of MOF-based flexible electrochemical sensors for implant detection. Reproduced with permission [
158].
Figure 10.
Schematics of MOF-based flexible electrochemical sensors for implant detection. Reproduced with permission [
158].
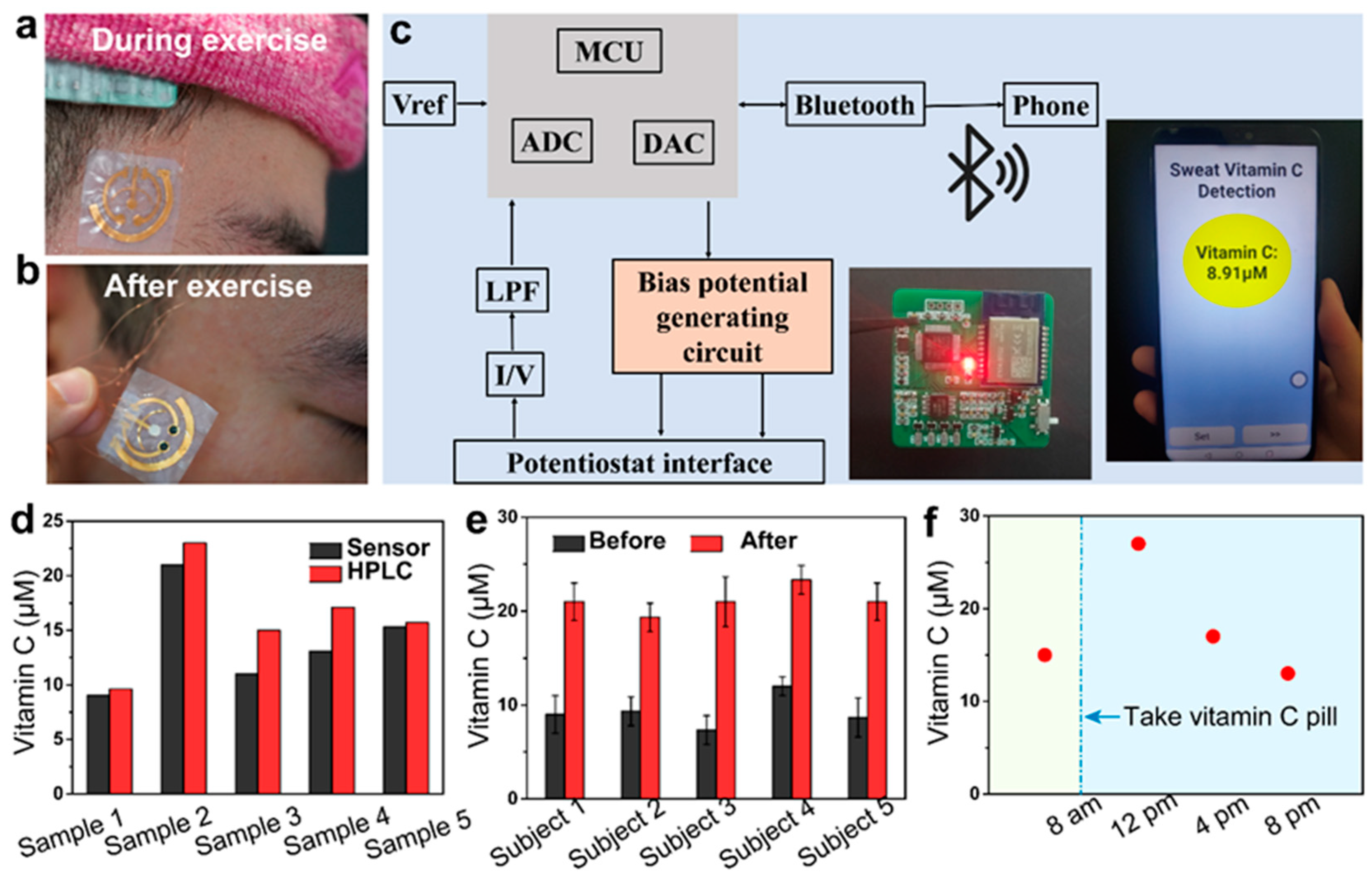
Conductive MOFs offer a lot of potential for advancing wearable sensors, especially with ongoing research and development aimed at overcoming these challenges. Yang et al. [
159] demonstrated the development of bacterial nanocellulose (BNC)-based wearable sensors that utilize conductive MOFs as the sole electrode material for sweat sensing. The MOFs-based layered film electrodes, characterized by inherent conductivity, permanent porosity, and tunable catalytic properties, enable selective and simultaneous monitoring of nutritional and metabolic biomarkers. Additionally, BNC serves as a highly skin-adherent sensor platform, providing excellent permeability to the underlying skin, which facilitates natural sweating and evaporative cooling—key factors for wearable comfort and prolonged use of epidermal electronics. The successful demonstration of a wireless system capable of continuously monitoring dynamic trends in sweat vitamin C following supplement intake highlights the potential monitoring biomarkers in bodily fluids for early disease detection.
Figure 11.
Demonstration of conductive MOFs-based wearable sweat sensor for wireless ascorbic acid analysis. Reproduced with permission [
159].
Figure 11.
Demonstration of conductive MOFs-based wearable sweat sensor for wireless ascorbic acid analysis. Reproduced with permission [
159].
5.3. Food Safety
In the context of food safety, MOFs-based flexible electrochemical sensors have gained significant attention for their potential to efficiently detect harmful substances and contaminants [
160]. These sensors can play a crucial role in ensuring the safety and quality of food products by identifying contaminants such as heavy metal ions, pesticide residues, adulterants, and indicators of spoilage. MOFs can be engineered to selectively bind heavy metal ions like lead, cadmium, and mercury, enabling the detection of trace amounts of these harmful metals in food products. Additionally, certain MOFs can interact with organophosphates and other pesticide residues, facilitating their detection in agricultural produce. For detecting adulterants, such as melamine in milk or Sudan dyes in spices, MOF-based sensors can identify specific chemical signatures associated with these substances. Moreover, the freshness of perishable goods can be monitored by detecting volatile organic compounds (VOCs) released during spoilage. MOFs can adsorb these VOCs, leading to measurable changes in the sensor’s electrical properties [
161,
162]. However, for these sensors to be widely adopted in the food industry, they must meet regulatory standards for food safety testing. While they show promise in laboratory settings, scaling up the production of MOF-based sensors for commercial applications poses challenges. Ensuring the long-term stability and durability of MOFs in different environments is also crucial, as these factors can impact sensor performance over time.
In summary, MOF-based flexible electrochemical sensors hold significant promise for enhancing food safety by providing rapid and accurate detection of contaminants and pathogens. Ongoing advances in materials science and engineering are likely to improve their performance and expand their range of applications, further supporting food safety efforts.
Figure 12.
Schematic representation of MOFs-based sensors for food safety. Reproduced with permission [
160].
Figure 12.
Schematic representation of MOFs-based sensors for food safety. Reproduced with permission [
160].
6. Challenges and Future Directions
6.1. Challenges
Preparing flexible electrochemical sensors based on MOFs films is a challenging field involving multiple aspects. Firstly, a core challenge lies in the preparation and control of high-quality MOFs films. Despite various methods such as atomic layer deposition, electrochemical methods, and cross-linking-induced assembly strategies being available, each method has its limitations, particularly in precisely controlling the morphology, orientation, and thickness of the MOFs films. Secondly, the stability of MOFs films presents another significant issue, encompassing chemical stability in electrochemical environments and mechanical stability against bending and stretching. Ensuring the effective integration of MOFs films with flexible substrates like polymers or carbon-based materials requires consideration of compatibility, adhesion, and optimization of process parameters.
Additionally, practical challenges in real-world applications include sensor reproducibility, long-term stability, and cost-effectiveness. MOFs are sensitive to environmental factors such as humidity, temperature, and pH, which can impact the stability and performance of sensors. Maintaining the structural integrity of MOFs films under mechanical stress (bending, stretching) is crucial for flexible sensors. Ensuring durability without compromising performance poses a significant challenge. Achieving consistent quality and reproducibility in MOFs film synthesis is difficult but crucial for reliable sensor performance.
Researchers are actively exploring new preparation methods and processes to address these challenges, optimize sensors structure and performance, and seek practical solutions for applications. These efforts include developing protective coatings to enhance stability, utilizing advanced manufacturing technologies for precise MOFs films production, and developing wireless communication systems for real-time data transmission and remote monitoring. Addressing these challenges and exploring future directions is critical for advancing the field of flexible electrochemical sensors integrated with MOFs films. Continued research and development in these areas will lead to more robust, reliable, and versatile sensors capable of meeting diverse application needs from healthcare diagnostics to environmental monitoring. Developing cost-effective, scalable methods for producing high-quality MOFs films suitable for commercial applications remains challenging. Many MOFs have poor conductivity, limiting their application in electrochemical sensors. Improving the conductivity of MOFs films without sacrificing their intrinsic properties is a challenge. Effectively integrating MOF films with flexible substrates and electronic components into signal processing and data transmission remains a technical barrier. Achieving high selectivity for specific analytes while minimizing interference from complex matrices is challenging. For many applications such as medical diagnostics and environmental monitoring, improving the sensitivity of MOFs-based sensors to detect analytes at extremely low concentrations is necessary. Ensuring that MOFs films maintain their performance over long periods, especially under harsh conditions, is crucial for practical applications.
6.2. Future Directions
Electrochemical sensors, renowned for their exceptional accuracy and reliability, are essential tools in environmental and healthcare applications. MOFs, with their high porosity, low density, and large surface area, offer outstanding performance in areas such as gas adsorption and ion exchange. Integrating MOFs thin films with flexible electrochemical sensors opens new possibilities for enhanced detection accuracy and sensitivity, presenting broad prospects for future development. With rapid advancements in nanotechnology and continuous improvements in measurement techniques, flexible electrochemical sensors integrated with MOFs thin films show immense potential for applications in sectors such as biomedicine, smart homes, and intelligent transportation. To enhance the stability of MOFs materials without compromising their performance, developing protective coatings or surface treatments is crucial. Additionally, combining MOFs thin films with other flexible substrate materials, such as polymers or carbon-based materials, not only improves mechanical strength and flexibility but also offers greater customization possibilities for sensors.
Advanced manufacturing technologies such as 3D printing and micromachining enable the production of complex and precise MOFs thin films on flexible substrates. The optimization of in situ growth techniques allows for better control over film morphology, orientation, and thickness, thereby enhancing reproducibility and scalability. Additionally, surface functionalization of MOFs films by introducing specific functional groups significantly improves selectivity toward target analytes. The integration of conductive materials like graphene or conductive polymers further enhances the electrical and overall performance of sensors. Looking ahead, the integration of MOFs-based sensors with flexible and stretchable electronic devices is set to usher in a new era of seamless incorporation into wearable technologies. Concurrently, the development of wireless communication systems will facilitate real-time data transmission and remote monitoring, revolutionizing fields such as environmental monitoring and traffic management. Designing MOFs thin films capable of detecting multiple analytes simultaneously will enhance sensor versatility. Furthermore, research into self-healing materials will help extend the lifespan of flexible sensors, contributing to more durable and reliable devices.
In personalized health monitoring, customized MOFs-based sensors can detect specific biomarkers related to individual health conditions, facilitating early disease diagnosis and treatment. Furthermore, networks of MOFs-based sensors can be deployed for large-scale environmental monitoring, providing real-time data on pollution levels and climate conditions. Currently, electrochemical sensors are extensively used in environmental protection, food safety, pharmaceuticals, and the chemical industry. The incorporation of MOFs thin films will further expand their application scope, especially in scenarios that demand high sensitivity and selectivity. As artificial intelligence and automation technologies continue to advance, flexible electrochemical sensors integrated with MOFs thin films are expected to become smarter and more automated. They will be capable of automatically identifying and quantifying multiple chemical substances, as well as providing real-time monitoring and data transmission. In conclusion, these sensors will play critical roles across various fields, significantly contributing to human well-being and economic development. We anticipate ongoing progress and innovation in this technology, heralding new advancements in precise detection and monitoring.
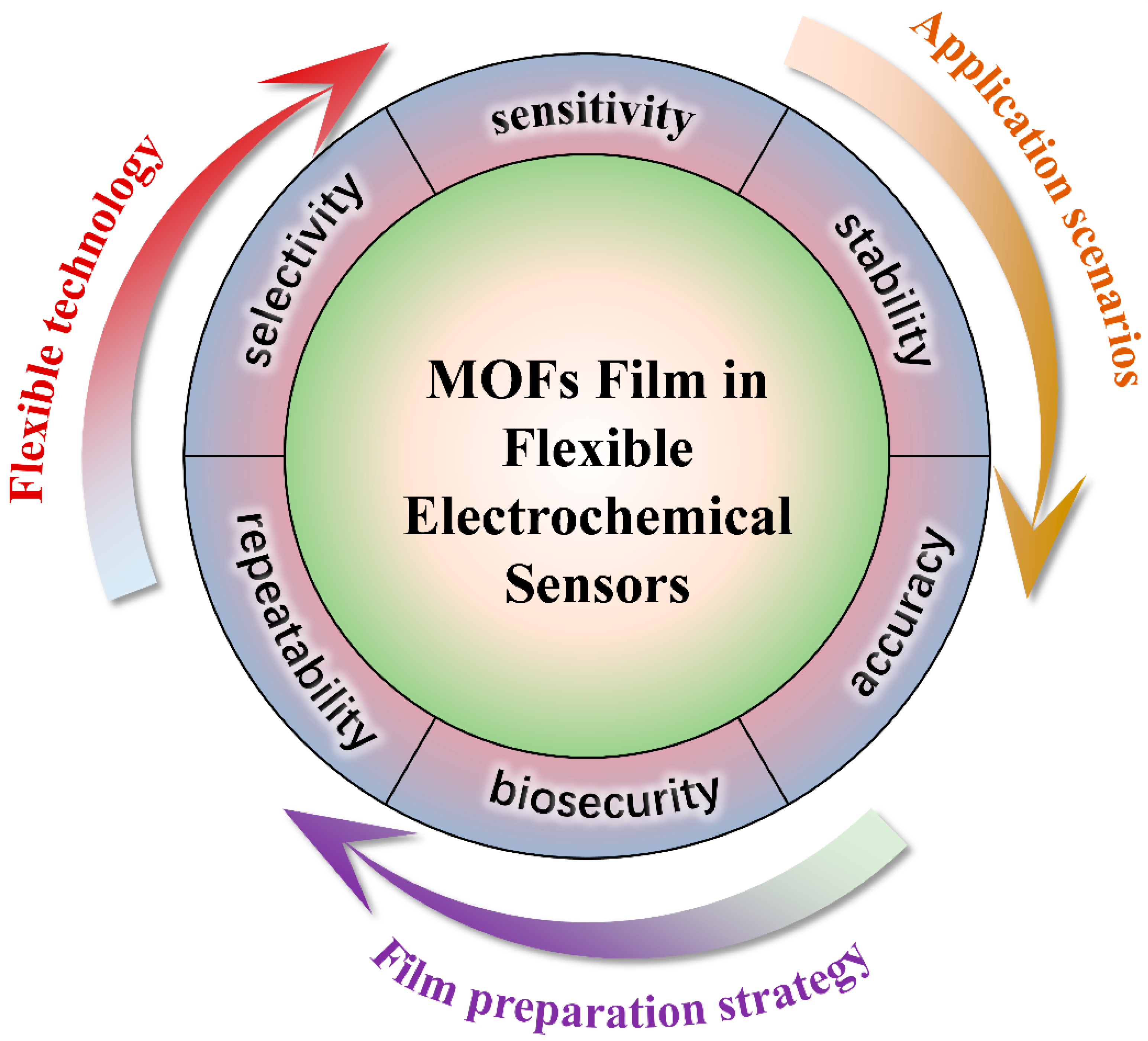
Figure 13.
An overview of MOF films for flexible electrochemical sensing.
Figure 13.
An overview of MOF films for flexible electrochemical sensing.
7. Conclusions
MOFs offer significant potential in the field of flexible electrochemical sensors due to their unique properties, such as high surface area, adjustable porosity, and chemical versatility. These attributes can greatly enhance sensor performance in terms of sensitivity, selectivity, and durability, making MOFs ideal for a wide range of applications, including environmental monitoring and biomedical diagnostics. In this review, we summarize the research progress on flexible electrochemical sensors based on MOFs and discuss the challenges and key technical issues in this area. Although considerable efforts have been made to produce high-quality MOFs films and integrate them with flexible electronics technology for use in flexible sensors, several challenges remain. While various methods, such as atomic layer deposition and electrochemical techniques, can be employed to fabricate MOFs films, these approaches have their own advantages and limitations, making it difficult to precisely control film morphology, orientation, and thickness simultaneously. Flexible electronic devices must endure mechanical deformations such as bending and stretching during use, necessitating MOF films with excellent mechanical stability. Ensuring compatibility between MOFs films and common flexible substrates, such as polymers and carbon-based materials, is crucial to prevent delamination or detachment during operation. Additionally, when MOFs serve as the active layer in sensors, they must provide both high sensitivity and selectivity to accurately detect target substances. For practical applications, MOF-based flexible electronic devices need to demonstrate good repeatability and long-term stability to ensure accurate and reliable measurements. Unlike well-established materials like silicon and conventional semiconductors, the fundamental charge transport and photonic properties of MOFs are not yet fully understood. Future research must address current challenges, such as improving the stability, repeatability, and scalability of MOF thin film deposition. Moreover, exploring new methods for integrating MOFs with advanced sensor technologies could expand the boundaries of sensor design and functionality. By unlocking the full potential of MOFs in sensor applications, researchers can develop innovative solutions that meet emerging societal and technological needs.
Declaration of competing interest
The authors declare that they have no conflict of interests.
Acknowledgements
The authors are grateful for the financial support of Shanghai Pujiang program (No. 22PJD026) and Suzhou PPM Institute of Functional Materials Co., Ltd. (No.2023008); the China Postdoctoral Science Foundation (grant number: 2022M722034); Natural Science Foundation of Fujian Province (2023J05087)
References
- S. S. Timilsina, P. Jolly, N. Durr, M. Yafia,D. E. Ingber, Enabling Multiplexed Electrochemical Detection of Biomarkers with High Sensitivity in Complex Biological Samples, Acc. Chem. Res. 54 (2021) 3529-3539. [CrossRef]
- L. Xiang, X. Zeng, F. Xia, W. Jin, Y. Liu,Y. Hu, Recent Advances in Flexible and Stretchable Sensing Systems: From the Perspective of System Integration, ACS Nano 14 (2020) 6449-6469. [CrossRef]
- G. Fiori, F. Bonaccorso, G. Iannaccone, T. Palacios, D. Neumaier, A. Seabaugh, S. K. Banerjee,L. Colombo, Electronics based on two-dimensional materials, Nat. Nanotechnol. 9 (2014) 768-779. [CrossRef]
- G. Eda, G. Fanchini,M. Chhowalla, Large-area ultrathin films of reduced graphene oxide as a transparent and flexible electronic material, Nat. Nanotechnol. 3 (2008) 270-274. [CrossRef]
- H. Park, S. Kim, J. Lee, I. Lee, S. Bontapalle, Y. Na,K. Sim, Organic flexible electronics with closed-loop recycling for sustainable wearable technology, Nat. Electron. 7 (2023) 39-50. [CrossRef]
- S. Mishra, S. Mohanty,A. Ramadoss, Functionality of Flexible Pressure Sensors in Cardiovascular Health Monitoring: A Review, ACS Sens. 7 (2022) 2495-2520. [CrossRef]
- W. Wu,H. Haick, Materials and Wearable Devices for Autonomous Monitoring of Physiological Markers, Adv. Mater. 30 (2018). [CrossRef]
- T. Dong, N. M. Matos Pires, Z. Yang,Z. Jiang, Advances in Electrochemical Biosensors Based on Nanomaterials for Protein Biomarker Detection in Saliva, Adv. Sci. (Weinheim, Ger.) 10 (2023) e2205429. [CrossRef]
- J. Lee, M. C. Kim, I. Soltis, S. H. Lee,W. H. Yeo, Advances in Electrochemical Sensors for Detecting Analytes in Biofluids, Adv. Sens. Res. 2 (2023). [CrossRef]
- X. Liu, X. Li, X. Gao, L. Ge, X. Sun,F. Li, A Universal Paper-Based Electrochemical Sensor for Zero-Background Assay of Diverse Biomarkers, ACS Appl. Mater. Interfaces 11 (2019) 15381-15388. [CrossRef]
- P. Bollella, G. Fusco, C. Tortolini, G. Sanzo, G. Favero, L. Gorton,R. Antiochia, Beyond graphene: Electrochemical sensors and biosensors for biomarkers detection, Biosens. Bioelectron. 89 (2017) 152-166. [CrossRef]
- I. Grabowska, N. Sharma, A. Vasilescu, M. Iancu, G. Badea, R. Boukherroub, S. Ogale,S. Szunerits, Electrochemical Aptamer-Based Biosensors for the Detection of Cardiac Biomarkers, ACS Omega 3 (2018) 12010-12018. [CrossRef]
- A.J. Bandodkar, R. Nuñez-Flores, W. Jia,J. Wang, All-Printed Stretchable Electrochemical Devices, Adv. Mater. 27 (2015) 3060-3065. [CrossRef]
- W. Tang, L. Yin, J. R. Sempionatto, J. M. Moon, H. Teymourian,J. Wang, Touch-Based Stressless Cortisol Sensing, Adv. Mater. 2021; 33. [CrossRef]
- Y. Yang,W. Gao, Wearable and flexible electronics for continuous molecular monitoring, Chem. Soc. Rev. 48 (2019) 1465-1491. [CrossRef]
- B. H. Moghadam, M. Hasanzadeh,A. Simchi, Self-Powered Wearable Piezoelectric Sensors Based on Polymer Nanofiber–Metal–Organic Framework Nanoparticle Composites for Arterial Pulse Monitoring, ACS Appl. Nano Mater. 3 (2020) 8742-8752. [CrossRef]
- Y. Shu, T. Su, Q. Lu, Z. Shang, Q. Xu,X. Hu, Highly Stretchable Wearable Electrochemical Sensor Based on Ni-Co MOF Nanosheet-Decorated Ag/rGO/PU Fiber for Continuous Sweat Glucose Detection, Anal. Chem. 93 (2021) 16222-16230. [CrossRef]
- G. Aragay, J. Pons,A. Merkoçi, Recent Trends in Macro-, Micro-, and Nanomaterial-Based Tools and Strategies for Heavy-Metal Detection, Chem. Rev. 111 (2011) 3433-3458. [CrossRef]
- X. Fang, B. Zong,S. Mao, Metal–Organic Framework-Based Sensors for Environmental Contaminant Sensing, Nano-Micro Lett. 10 (2018) 64. [CrossRef]
- S. Tajik, H. Beitollahi, F. Garkani Nejad, I. Sheikhshoaie, A. S. Nugraha, H. W. Jang, Y. Yamauchi,M. Shokouhimehr, Performance of metal–organic frameworks in the electrochemical sensing of environmental pollutants, J. Mater. Chem. A 9 (2021) 8195-8220. [CrossRef]
- H. Lee, T. K. Choi, Y. B. Lee, H. R. Cho, R. Ghaffari, L. Wang, H. J. Choi, T. D. Chung, N. Lu, T. Hyeon, S. H. Choi,D.-H. Kim, A graphene-based electrochemical device with thermoresponsive microneedles for diabetes monitoring and therapy, Nat. Nanotechnol. 11 (2016) 566-572. [CrossRef]
- M. Segev-Bar,H. Haick, Flexible Sensors Based on Nanoparticles, ACS Nano 7 (2013) 8366-8378. [CrossRef]
- M. Mayer,A. J. Baeumner, A Megatrend Challenging Analytical Chemistry: Biosensor and Chemosensor Concepts Ready for the Internet of Things, Chem. Rev. 119 (2019) 7996-8027. [CrossRef]
- D. Chen, H. Feng,J. Li, Graphene Oxide: Preparation, Functionalization, and Electrochemical Applications, Chem. Rev. 112 (2012) 6027-6053. [CrossRef]
- H. Y. Y. Nyein, W. Gao, Z. Shahpar, S. Emaminejad, S. Challa, K. Chen, H. M. Fahad, L.-C. Tai, H. Ota, R. W. Davis,A. Javey, A Wearable Electrochemical Platform for Noninvasive Simultaneous Monitoring of Ca2+ and pH, ACS Nano 10 (2016) 7216-7224. [CrossRef]
- V. Mani, B. V. Chikkaveeraiah, V. Patel, J. S. Gutkind,J. F. Rusling, Ultrasensitive Immunosensor for Cancer Biomarker Proteins Using Gold Nanoparticle Film Electrodes and Multienzyme-Particle Amplification, ACS Nano 3 (2009) 585-594. [CrossRef]
- J. Zhang, S. Song, L. Zhang, L. Wang, H. Wu, D. Pan,C. Fan, Sequence-Specific Detection of Femtomolar DNA via a Chronocoulometric DNA Sensor (CDS): Effects of Nanoparticle-Mediated Amplification and Nanoscale Control of DNA Assembly at Electrodes, J. Am. Chem. Soc. 128 (2006) 8575-8580. [CrossRef]
- D. J. Lipomi, M. Vosgueritchian, B. C. K. Tee, S. L. Hellstrom, J. A. Lee, C. H. Fox,Z. Bao, Skin-like pressure and strain sensors based on transparent elastic films of carbon nanotubes, Nat. Nanotechnol. 6 (2011) 788-792. [CrossRef]
- P. Gayen,B. P. Chaplin, Selective Electrochemical Detection of Ciprofloxacin with a Porous Nafion/Multiwalled Carbon Nanotube Composite Film Electrode, ACS Appl. Mater. Interfaces 8 (2016) 1615-1626. [CrossRef]
- A.Umar, M. M. Rahman, S. H. Kim,Y.-B. Hahn, Zinc oxide nanonail based chemical sensor for hydrazine detection, Chem. Commun. (2008) 166-168. [CrossRef]
- T. Zhang, J. Zhu, M. Xie, K. Meng, G. Yao, T. Pan, M. Gao, H. Cheng,Y. Lin, Highly Sensitive Wearable Sensor Based on (001)-Orientated TiO2 for Real-Time Electrochemical Detection of Dopamine, Tyrosine, and Paracetamol, Small 20 (2024) 2312238. [CrossRef]
- C.-S. Liu, J. Li,H. Pang, Metal-organic framework-based materials as an emerging platform for advanced electrochemical sensing, Coord. Chem. Rev. 410 (2020) 213222. [CrossRef]
- W. Pan, Y. Wang, G. Ouyang, M. Ren,M. Cao, High-Sensitivity and Low-Cost Wearable Flexible Pressure Sensor Based on MOFs, ACS Appl. Electron. Mater. 5 (2023) 3851-3858. [CrossRef]
- L. Li, Y. Shi, L. Pan, Y. Shi,G. Yu, Rational design and applications of conducting polymer hydrogels as electrochemical biosensors, J. Mater. Chem. B 3 (2015) 2920-2930. [CrossRef]
- F. S. Omar, N. Duraisamy, K. Ramesh,S. Ramesh, Conducting polymer and its composite materials based electrochemical sensor for Nicotinamide Adenine Dinucleotide (NADH), Biosens. Bioelectron. 79 (2016) 763-775. [CrossRef]
- M. Liu, M. Peng, B. Dong, Y. Teng, L. Feng,Q. Xu, Explicating the Role of Metal Centers in Porphyrin-Based MOFs of PCN-222(M) for Electrochemical Reduction of CO2, Chin. J. Struct. Chem. 41 (2022) 2207046-2207052. [CrossRef]
- H. Sun, Z. Li, Y. Gu,C. Guo, A Review on the Progress of Metal-Organic Frameworks in Electrochemiluminescence Sensors, Chin. J. Struct. Chem. 41 (2022) 2211018-2211030. [CrossRef]
- P. G. Boyd, A. Chidambaram, E. García-Díez, C. P. Ireland, T. D. Daff, R. Bounds, A. Gładysiak, P. Schouwink, S. M. Moosavi, M. M. Maroto-Valer, J. A. Reimer, J. a. R. Navarro, T. K. Woo, S. Garcia, K. C. Stylianou,B. Smit, Data-driven design of metal–organic frameworks for wet flue gas CO2 capture, Nature 576 (2019) 253-256. [CrossRef]
- H. Furukawa, K. E. Cordova, M. O’keeffe,O. M. Yaghi, The Chemistry and Applications of Metal-Organic Frameworks, Science 341 (2013) 1230444. [CrossRef]
- N. L. Rosi, J. Eckert, M. Eddaoudi, D. T. Vodak, J. Kim, M. O’keeffe,O. M. Yaghi, Hydrogen Storage in Microporous Metal-Organic Frameworks, Science 300 (2003) 1127-1129. [CrossRef]
- M. Eddaoudi, J. Kim, N. Rosi, D. Vodak, J. Wachter, M. O’keeffe,O. M. Yaghi, Systematic Design of Pore Size and Functionality in Isoreticular MOFs and Their Application in Methane Storage, Science 295 (2002) 469-472. [CrossRef]
- B. Y. Xia, Y. Yan, N. Li, H. B. Wu, X. W. Lou,X. Wang, A metal–organic framework-derived bifunctional oxygen electrocatalyst, Nat. Energy 1 (2016) 15006. [CrossRef]
- X. F. Lu, B. Y. Xia, S.-Q. Zang,X. W. Lou, Metal–Organic Frameworks Based Electrocatalysts for the Oxygen Reduction Reaction, Angew. Chem., Int. Ed. 59 (2020) 4634-4650. [CrossRef]
- L. E. Kreno, K. Leong, O. K. Farha, M. Allendorf, R. P. Van Duyne,J. T. Hupp, Metal–Organic Framework Materials as Chemical Sensors, Chem. Rev. 112 (2012) 1105-1125. [CrossRef]
- S. Wu, H. Min, W. Shi,P. Cheng, Multicenter Metal–Organic Framework-Based Ratiometric Fluorescent Sensors, Adv. Mater. 32 (2020) 1805871. [CrossRef]
- Y. Men, Z. Qin, Z. Yang, P. Zhang, M. Li, Q. Wang, D. Zeng, X. Yin,H. Ji, Antibacterial Defective-ZIF-8/PPY/BC-Based Flexible Electronics as Stress-Strain and NO2 Gas Sensors, Adv. Funct. Mater. 34 (2024) 2316633. [CrossRef]
- X. Chen, Y. Lu, J. Dong, L. Ma, Z. Yi, Y. Wang, L. Wang, S. Wang, Y. Zhao, J. Huang,Y. Liu, Ultrafast In Situ Synthesis of Large-Area Conductive Metal–Organic Frameworks on Substrates for Flexible Chemiresistive Sensing, ACS Appl. Mater. Interfaces 12 (2020) 57235-57244. [CrossRef]
- W. Xu, Z. Lu, X. Sun, L. Jiang,X. Duan, Superwetting Electrodes for Gas-Involving Electrocatalysis, Acc. Chem. Res. 51 (2018) 1590-1598. [CrossRef]
- D. Antuña-Jiménez, M. B. González-García, D. Hernández-Santos,P. Fanjul-Bolado, Screen-Printed Electrodes Modified with Metal Nanoparticles for Small Molecule Sensing, Biosensors 10 (2020) 9. [CrossRef]
- A.Kaliyaraj Selva Kumar, Y. Zhang, D. Li,R. G. Compton, A mini-review: How reliable is the drop casting technique?, Electrochem. Commun. 121 (2020) 106867. [CrossRef]
- M. G. Campbell, D. Sheberla, S. F. Liu, T. M. Swager,M. Dincă, Cu3(hexaiminotriphenylene)2: An Electrically Conductive 2D Metal–Organic Framework for Chemiresistive Sensing, Angew. Chem., Int. Ed. 54 (2015) 4349-4352. [CrossRef]
- Z. Qiu, T. Yang, R. Gao, G. Jie,W. Hou, An electrochemical ratiometric sensor based on 2D MOF nanosheet/Au/polyxanthurenic acid composite for detection of dopamine, J. Electroanal. Chem. 835 (2019) 123-129. [CrossRef]
- C.-S. Liu, Z.-H. Zhang, M. Chen, H. Zhao, F.-H. Duan, D.-M. Chen, M.-H. Wang, S. Zhang,M. Du, Pore modulation of zirconium–organic frameworks for high-efficiency detection of trace proteins, Chem. Commun. 53 (2017) 3941-3944. [CrossRef]
- R. Zhong, Q. Tang, S. Wang, H. Zhang, F. Zhang, M. Xiao, T. Man, X. Qu, L. Li, W. Zhang,H. Pei, Self-Assembly of Enzyme-Like Nanofibrous G-Molecular Hydrogel for Printed Flexible Electrochemical Sensors, Adv. Mater. 30 (2018) 1706887. [CrossRef]
- M. Santhiago, M. Strauss, M. P. Pereira, A. S. Chagas,C. C. B. Bufon, Direct Drawing Method of Graphite onto Paper for High-Performance Flexible Electrochemical Sensors, ACS Appl. Mater. Interfaces 9 (2017) 11959-11966. [CrossRef]
- N. Thakur, A. Chaturvedi, D. Mandal,T. C. Nagaiah, Ultrasensitive and highly selective detection of dopamine by a NiFeP based flexible electrochemical sensor, Chem. Commun. 56 (2020) 8448-8451. [CrossRef]
- J. Zhong, Y. Ma, Y. Song, Q. Zhong, Y. Chu, I. Karakurt, D. B. Bogy,L. Lin, A Flexible Piezoelectret Actuator/Sensor Patch for Mechanical Human–Machine Interfaces, ACS Nano 13 (2019) 7107-7116. [CrossRef]
- Y. J. Park, B. K. Sharma, S. M. Shinde, M.-S. Kim, B. Jang, J.-H. Kim,J.-H. Ahn, All MoS2-Based Large Area, Skin-Attachable Active-Matrix Tactile Sensor, ACS Nano 13 (2019) 3023-3030. [CrossRef]
- H.-K. Chang, F. N. Ishikawa, R. Zhang, R. Datar, R. J. Cote, M. E. Thompson,C. Zhou, Rapid, Label-Free, Electrical Whole Blood Bioassay Based on Nanobiosensor Systems, ACS Nano 5 (2011) 9883-9891. [CrossRef]
- N. Noriega, M. Shekhirev, C. E. Shuck, J. Salvage, A. Vahidmohammadi, M. K. Dymond, J. Lacey, S. Sandeman, Y. Gogotsi,B. A. Patel, Pristine Ti3C2Tx MXene Enables Flexible and Transparent Electrochemical Sensors, ACS Appl. Mater. Interfaces 16 (2024) 6569-6578. [CrossRef]
- D. E. Oh, C.-S. Lee, T. W. Kim, S. Jeon,T. H. Kim, A Flexible and Transparent PtNP/SWCNT/PET Electrochemical Sensor for Nonenzymatic Detection of Hydrogen Peroxide Released from Living Cells with Real-Time Monitoring Capability, Biosensors 13 (2023) 704. [CrossRef]
- Y. Liu, J. Canoura, O. Alkhamis,Y. Xiao, Immobilization Strategies for Enhancing Sensitivity of Electrochemical Aptamer-Based Sensors, ACS Appl. Mater. Interfaces 13 (2021) 9491-9499. [CrossRef]
- L. Wang, L. Wang, Y. Zhang, J. Pan, S. Li, X. Sun, B. Zhang,H. Peng, Weaving Sensing Fibers into Electrochemical Fabric for Real-Time Health Monitoring, Adv. Funct. Mater. 28 (2018) 1804456. [CrossRef]
- K. Zou, Q. Li, D. Li, Y. Jiao, L. Wang, L. Li, J. Wang, Y. Li, R. Gao, F. Li, E. He, T. Ye, W. Tang, J. Song, J. Lu, X. Li, H. Zhang, X. Cao,Y. Zhang, A Highly Selective Implantable Electrochemical Fiber Sensor for Real-Time Monitoring of Blood Homovanillic Acid, ACS Nano 18 (2024) 7485-7495. [CrossRef]
- J.-Q. Xu, Y.-L. Liu, Q. Wang, H.-H. Duo, X.-W. Zhang, Y.-T. Li,W.-H. Huang, Photocatalytically Renewable Micro-electrochemical Sensor for Real-Time Monitoring of Cells, Angew. Chem., Int. Ed. 54 (2015) 14402-14406. [CrossRef]
- Y.-L. Liu, Z.-H. Jin, Y.-H. Liu, X.-B. Hu, Y. Qin, J.-Q. Xu, C.-F. Fan,W.-H. Huang, Stretchable Electrochemical Sensor for Real-Time Monitoring of Cells and Tissues, Angew. Chem., Int. Ed. 55 (2016) 4537-4541. [CrossRef]
- K. Zhou, V. Kammarchedu, D. Butler, P. Soltan Khamsi,A. Ebrahimi, Electrochemical Sensors Based on MoSx-Functionalized Laser-Induced Graphene for Real-Time Monitoring of Phenazines Produced by Pseudomonas aeruginosa, Adv. Healthcare Mater. 11 (2022) 2200773. [CrossRef]
- Y. Zhao, K.-Q. Jin, J.-D. Li, K.-K. Sheng, W.-H. Huang,Y.-L. Liu, Flexible and Stretchable Electrochemical Sensors for Biological Monitoring, Adv. Mater. n/a (2023) 2305917. [CrossRef]
- Y.-L. Liu, Y. Qin, Z.-H. Jin, X.-B. Hu, M.-M. Chen, R. Liu, C. Amatore,W.-H. Huang, A Stretchable Electrochemical Sensor for Inducing and Monitoring Cell Mechanotransduction in Real Time, Angew. Chem., Int. Ed. 56 (2017) 9454-9458. [CrossRef]
- H. Teymourian, A. Barfidokht,J. Wang, Electrochemical glucose sensors in diabetes management: an updated review (2010–2020), Chem. Soc. Rev. 49 (2020) 7671-7709. [CrossRef]
- J. Kim, I. Jeerapan, B. Ciui, M. C. Hartel, A. Martin,J. Wang, Edible Electrochemistry: Food Materials Based Electrochemical Sensors, Adv. Healthcare Mater. 6 (2017) 1700770. [CrossRef]
- S. P. Nichols, A. Koh, W. L. Storm, J. H. Shin,M. H. Schoenfisch, Biocompatible Materials for Continuous Glucose Monitoring Devices, Chem. Rev. 113 (2013) 2528-2549. [CrossRef]
- M. S. Mannoor, H. Tao, J. D. Clayton, A. Sengupta, D. L. Kaplan, R. R. Naik, N. Verma, F. G. Omenetto,M. C. Mcalpine, Graphene-based wireless bacteria detection on tooth enamel, Nat. Commun. 3 (2012) 763. [CrossRef]
- R. Wu, L. Li, L. Pan, K. Yan, Y. Shi, L. Jiang,J.-J. Zhu, Long-term cell culture and electrically in situ monitoring of living cells based on a polyaniline hydrogel sensor, J. Mater. Chem. B 9 (2021) 9514-9523. [CrossRef]
- V. B. Juska, A. Walcarius,M. E. Pemble, Cu Nanodendrite Foams on Integrated Band Array Electrodes for the Nonenzymatic Detection of Glucose, ACS Appl. Nano Mater. 2 (2019) 5878-5889. [CrossRef]
- S. G. R. Avuthu, J. T. Wabeke, B. B. Narakathu, D. Maddipatla, J. S. Arachchilage, S. O. Obare,M. Z. Atashbar, A Screen Printed Phenanthroline-Based Flexible Electrochemical Sensor for Selective Detection of Toxic Heavy Metal Ions, IEEE Sens. J. 16 (2016) 8678-8684. [CrossRef]
- S. Nasraoui, A. Al-Hamry, P. R. Teixeira, S. Ameur, L. G. Paterno, M. Ben Ali,O. Kanoun, Electrochemical sensor for nitrite detection in water samples using flexible laser-induced graphene electrodes functionalized by CNT decorated by Au nanoparticles, J. Electroanal. Chem. 880 (2021) 114893. [CrossRef]
- W.-Y. Wu, X. Zhong, W. Wang, Q. Miao,J.-J. Zhu, Flexible PDMS-based three-electrode sensor, Electrochem. Commun. 12 (2010) 1600-1604. [CrossRef]
- Z.-H. Zeng, N. Wu, J.-J. Wei, Y.-F. Yang, T.-T. Wu, B. Li, S. B. Hauser, W.-D. Yang, J.-R. Liu,S.-Y. Zhao, Porous and Ultra-Flexible Crosslinked MXene/Polyimide Composites for Multifunctional Electromagnetic Interference Shielding, Nano-Micro Lett. 14 (2022) 59. [CrossRef]
- J. Zhang, D. Wang,Y. Li, Ratiometric Electrochemical Sensors Associated with Self-Cleaning Electrodes for Simultaneous Detection of Adrenaline, Serotonin, and Tryptophan, ACS Appl. Mater. Interfaces 11 (2019) 13557-13563. [CrossRef]
- Y. Yao,C. Zhang, A Novel One-Step Fabricated, Droplet-Based Electrochemical Sensor for Facile Biochemical Assays, Sensors 16 (2016) 1231. [CrossRef]
- Y. Liu, X. Dong,P. Chen, Biological and chemical sensors based on graphene materials, Chem. Soc. Rev. 41 (2012) 2283-2307. [CrossRef]
- A.Heller,B. Feldman, Electrochemical Glucose Sensors and Their Applications in Diabetes Management, Chem. Rev. 108 (2008) 2482-2505. [CrossRef]
- H. Tang, F. Yan, P. Lin, J. Xu,H. L. W. Chan, Highly Sensitive Glucose Biosensors Based on Organic Electrochemical Transistors Using Platinum Gate Electrodes Modified with Enzyme and Nanomaterials, Adv. Funct. Mater. 21 (2011) 2264-2272. [CrossRef]
- P. Li, M. Zhang, X. Liu, Z. Su,G. Wei, Electrostatic Assembly of Platinum Nanoparticles along Electrospun Polymeric Nanofibers for High Performance Electrochemical Sensors, Nanomaterials 7 (2017) 236. [CrossRef]
- A.Ramanavičius, A. Ramanavičienė,A. Malinauskas, Electrochemical sensors based on conducting polymer—polypyrrole, Electrochim. Acta 51 (2006) 6025-6037. [CrossRef]
- D. M. Fernandes, M. Costa, C. Pereira, B. Bachiller-Baeza, I. Rodríguez-Ramos, A. Guerrero-Ruiz,C. Freire, Novel electrochemical sensor based on N-doped carbon nanotubes and Fe3O4 nanoparticles: Simultaneous voltammetric determination of ascorbic acid, dopamine and uric acid, J. Colloid Interface Sci. 432 (2014) 207-213. [CrossRef]
- R. Devi, S. Yadav, R. Nehra, S. Yadav,C. S. Pundir, Electrochemical biosensor based on gold coated iron nanoparticles/chitosan composite bound xanthine oxidase for detection of xanthine in fish meat, J. Food Eng. 115 (2013) 207-214. [CrossRef]
- P. Guo, R. D. Schaller, L. E. Ocola, B. T. Diroll, J. B. Ketterson,R. P. H. Chang, Large optical nonlinearity of ITO nanorods for sub-picosecond all-optical modulation of the full-visible spectrum, Nat. Commun. 7 (2016) 12892. [CrossRef]
- A.Menzel, K. Subannajui, F. Güder, D. Moser, O. Paul,M. Zacharias, Multifunctional ZnO-Nanowire-Based Sensor, Adv. Funct. Mater. 21 (2011) 4342-4348. [CrossRef]
- W.-W. Zhan, Q. Kuang, J.-Z. Zhou, X.-J. Kong, Z.-X. Xie,L.-S. Zheng, Semiconductor@Metal–Organic Framework Core–Shell Heterostructures: A Case of ZnO@ZIF-8 Nanorods with Selective Photoelectrochemical Response, J. Am. Chem. Soc. 135 (2013) 1926-1933. [CrossRef]
- A.Chen,S. Chatterjee, Nanomaterials based electrochemical sensors for biomedical applications, Chem. Soc. Rev. 42 (2013) 5425-5438. [CrossRef]
- M. Labib, E. H. Sargent,S. O. Kelley, Electrochemical Methods for the Analysis of Clinically Relevant Biomolecules, Chem. Rev. 116 (2016) 9001-9090. [CrossRef]
- X. Gao, W. Ma, J. Mao, C.-T. He, W. Ji, Z. Chen, W. Chen, W. Wu, P. Yu,L. Mao, A single-atom Cu–N2 catalyst eliminates oxygen interference for electrochemical sensing of hydrogen peroxide in a living animal brain, Chem. Sci. 12 (2021) 15045-15053. [CrossRef]
- K. Abnous, N. M. Danesh, M. Ramezani, M. Alibolandi,S. M. Taghdisi, A novel electrochemical sensor for bisphenol A detection based on nontarget-induced extension of aptamer length and formation of a physical barrier, Biosens. Bioelectron. 119 (2018) 204-208. [CrossRef]
- S. G. Kim, J. Song, B. Ryplida, H. J. Jo, G.-J. Jeong, I. Y. Kang, J. M. Patel, E.-J. Jin, Y. C. Jang,S. Y. Park, Touchable Electrochemical Hydrogel Sensor for Detection of Reactive Oxygen Species-induced Cellular Senescence in Articular Chondrocytes, Adv. Funct. Mater. 33 (2023) 2213887. [CrossRef]
- H. Shafique, J. De Vries, J. Strauss, A. Khorrami Jahromi, R. Siavash Moakhar,S. Mahshid, Advances in the Translation of Electrochemical Hydrogel-Based Sensors, Adv. Healthcare Mater. 12 (2023) 2201501. [CrossRef]
- X. Tian, Z. Tan, Z. Zhang, T. Zhan,X. Liu, An Electrochemical Sensor Based on an Ionic Liquid Covalently Functionalized Graphene Oxide for Simultaneous Determination of Copper (II) and Antimony (III), ChemistrySelect 3 (2018) 8252-8258. [CrossRef]
- P. Kuberský, J. Altšmíd, A. Hamáček, S. Nešpůrek,O. Zmeškal, An Electrochemical NO2 Sensor Based on Ionic Liquid: Influence of the Morphology of the Polymer Electrolyte on Sensor Sensitivity, Sensors 15 (2015) 28421-28434. [CrossRef]
- L. Killedar, D. Ilager, N. P. Shetti, T. M. Aminabhavi,K. Raghava Reddy, Synthesis of ruthenium doped titanium dioxide nanoparticles for the electrochemical detection of diclofenac sodium, J. Mol. Liq. 340 (2021) 116891. [CrossRef]
- S. A. Ansari, A. Ahmed, F. K. Ferdousi, M. A. Salam, A. A. Shaikh, H. R. Barai, N. S. Lopa,M. M. Rahman, Conducting poly(aniline blue)-gold nanoparticles composite modified fluorine-doped tin oxide electrode for sensitive and non-enzymatic electrochemical detection of glucose, J. Electroanal. Chem. 850 (2019) 113394. [CrossRef]
- P. K. Sekhar, J. Kysar, E. L. Brosha,C. R. Kreller, Development and testing of an electrochemical methane sensor, Sens. Actuators, B 228 (2016) 162-167. [CrossRef]
- M. A. Ehsan, M. M. Hasan, T. Islam, M. D. Hossain, M. A. Aziz,A. J. S. Ahammad, Fabrication of Nanostructured Pd Thin Films Using Aerosol-Assisted Chemical Vapor Deposition for the Nonenzymatic Electrochemical Detection of H2O2, ACS Appl. Electron. Mater. 1 (2019) 417-429. [CrossRef]
- X. Dong, X. Wang, L. Wang, H. Song, H. Zhang, W. Huang,P. Chen, 3D Graphene Foam as a Monolithic and Macroporous Carbon Electrode for Electrochemical Sensing, ACS Appl. Mater. Interfaces 4 (2012) 3129-3133. [CrossRef]
- J. A. Hondred, L. R. Stromberg, C. L. Mosher,J. C. Claussen, High-Resolution Graphene Films for Electrochemical Sensing via Inkjet Maskless Lithography, ACS Nano 11 (2017) 9836-9845. [CrossRef]
- Y. Qin, A. U. Alam, M. M. R. Howlader, N.-X. Hu,M. J. Deen, Inkjet Printing of a Highly Loaded Palladium Ink for Integrated, Low-Cost pH Sensors, Adv. Funct. Mater. 26 (2016) 4923-4933. [CrossRef]
- S. Park, H. Kang, C. M. Kang, Y. Kim,E. J. Kim, NiO electrochemical sensor fabricated via electrodeposition and spin-coating, Electron. Lett. 59 (2023) e12761. [CrossRef]
- R. Zheng, Z.-H. Fu, W.-H. Deng, Y. Wen, A.-Q. Wu, X.-L. Ye,G. Xu, The Growth Mechanism of a Conductive MOF Thin Film in Spray-based Layer-by-layer Liquid Phase Epitaxy, Angew. Chem., Int. Ed. 61 (2022) e202212797. [CrossRef]
- R. Hu, X. Zhang, K.-N. Chi, T. Yang,Y.-H. Yang, Bifunctional MOFs-Based Ratiometric Electrochemical Sensor for Multiplex Heavy Metal Ions, ACS Appl. Mater. Interfaces 12 (2020) 30770-30778. [CrossRef]
- X. Peng, X. Wu, F. Yang, Y. Tian, M. Zhang,H. Yuan, Gas sensors based on metal-organic frameworks: Challenges and opportunities, Chin. J. Struct. Chem. 43 (2024) 100251. [CrossRef]
- R. J. Kuppler, D. J. Timmons, Q.-R. Fang, J.-R. Li, T. A. Makal, M. D. Young, D. Yuan, D. Zhao, W. Zhuang,H.-C. Zhou, Potential applications of metal-organic frameworks, Coord. Chem. Rev. 253 (2009) 3042-3066. [CrossRef]
- N. S. Lopa, M. M. Rahman, F. Ahmed, S. Chandra Sutradhar, T. Ryu,W. Kim, A base-stable metal-organic framework for sensitive and non-enzymatic electrochemical detection of hydrogen peroxide, Electrochim. Acta 274 (2018) 49-56. [CrossRef]
- L. Gao, C. Jiao, H. Chai, Y. Ren, G. Zhang, H. Yu,L. Tang, A highly sensitive multifunctional Eu-MOF sensor with pentacarboxylate for fluorescence detecting acetone, Cu2+ and Cr2O72−, and electrochemical detection of TNP, J. Solid State Chem. 284 (2020) 121199. [CrossRef]
- Z. Xu, Q. Wang, H. Zhangsun, S. Zhao, Y. Zhao,L. Wang, Carbon cloth-supported nanorod-like conductive Ni/Co bimetal MOF: A stable and high-performance enzyme-free electrochemical sensor for determination of glucose in serum and beverage, Food Chem. 349 (2021) 129202. [CrossRef]
- N. Stock,S. Biswas, Synthesis of Metal-Organic Frameworks (MOFs): Routes to Various MOF Topologies, Morphologies, and Composites, Chem. Rev. 112 (2012) 933-969. [CrossRef]
- K. J. Lee, J. H. Lee, S. Jeoung,H. R. Moon, Transformation of Metal–Organic Frameworks/Coordination Polymers into Functional Nanostructured Materials: Experimental Approaches Based on Mechanistic Insights, Acc. Chem. Res. 50 (2017) 2684-2692. [CrossRef]
- J. H. Cavka, S. Jakobsen, U. Olsbye, N. Guillou, C. Lamberti, S. Bordiga,K. P. Lillerud, A New Zirconium Inorganic Building Brick Forming Metal Organic Frameworks with Exceptional Stability, J. Am. Chem. Soc. 130 (2008) 13850-13851. [CrossRef]
- B. Chen, S. Xiang,G. Qian, Metal−Organic Frameworks with Functional Pores for Recognition of Small Molecules, Acc. Chem. Res. 43 (2010) 1115-1124. [CrossRef]
- Y. Du, X. Li, X. Lv,Q. Jia, Highly Sensitive and Selective Sensing of Free Bilirubin Using Metal–Organic Frameworks-Based Energy Transfer Process, ACS Appl. Mater. Interfaces 9 (2017) 30925-30932. [CrossRef]
- P. Nugent, Y. Belmabkhout, S. D. Burd, A. J. Cairns, R. Luebke, K. Forrest, T. Pham, S. Ma, B. Space, L. Wojtas, M. Eddaoudi,M. J. Zaworotko, Porous materials with optimal adsorption thermodynamics and kinetics for CO2 separation, Nature 495 (2013) 80-84. [CrossRef]
- S.-Q. Yang, R. Krishna, H. Chen, L. Li, L. Zhou, Y.-F. An, F.-Y. Zhang, Q. Zhang, Y.-H. Zhang, W. Li, T.-L. Hu,X.-H. Bu, Immobilization of the Polar Group into an Ultramicroporous Metal–Organic Framework Enabling Benchmark Inverse Selective CO2/C2H2 Separation with Record C2H2 Production, J. Am. Chem. Soc. 145 (2023) 13901-13911. [CrossRef]
- S. M. Pirot, K. M. Omer, A. H. Alshatteri, G. K. Ali,O. B. A. Shatery, Dual-template molecularly surface imprinted polymer on fluorescent metal-organic frameworks functionalized with carbon dots for ascorbic acid and uric acid detection, Spectrochim. Acta, Part A 291 (2023) 122340. [CrossRef]
- W.-H. Chen, G.-F. Luo, M. Vázquez-González, R. Cazelles, Y. S. Sohn, R. Nechushtai, Y. Mandel,I. Willner, Glucose-Responsive Metal–Organic-Framework Nanoparticles Act as “Smart” Sense-and-Treat Carriers, ACS Nano 12 (2018) 7538-7545. [CrossRef]
- C. Lei, J. Gao, W. Ren, Y. Xie, S. Y. H. Abdalkarim, S. Wang, Q. Ni,J. Yao, Fabrication of metal-organic frameworks@cellulose aerogels composite materials for removal of heavy metal ions in water, Carbohydr. Polym. 205 (2019) 35-41. [CrossRef]
- F. Moghzi, J. Soleimannejad, E. C. Sañudo,J. Janczak, Dopamine Sensing Based on Ultrathin Fluorescent Metal–Organic Nanosheets, ACS Appl. Mater. Interfaces 12 (2020) 44499-44507. [CrossRef]
- R. Liu, L. Zhao, S. Yu, X. Liang, Z. Li,G. Li, Enhancing Proton Conductivity of a 3D Metal–Organic Framework by Attaching Guest NH3 Molecules, Inorg Chem. 57 (2018) 11560-11568. [CrossRef]
- Z. Han, K. Wang, H. Min, J. Xu, W. Shi,P. Cheng, Bifunctionalized Metal–Organic Frameworks for Pore-Size-Dependent Enantioselective Sensing, Angew. Chem., Int. Ed. 61 (2022) e202204066. [CrossRef]
- C.-Z. Wang, J. Chen, Q.-H. Li, G.-E. Wang, X.-L. Ye, J. Lv,G. Xu, Pore Size Modulation in Flexible Metal-Organic Framework Enabling High Performance Gas Sensing, Angew. Chem., Int. Ed. 62 (2023) e202302996. [CrossRef]
- M. C. So, S. Jin, H.-J. Son, G. P. Wiederrecht, O. K. Farha,J. T. Hupp, Layer-by-Layer Fabrication of Oriented Porous Thin Films Based on Porphyrin-Containing Metal–Organic Frameworks, J. Am. Chem. Soc. 135 (2013) 15698-15701. [CrossRef]
- M.-S. Yao, X.-J. Lv, Z.-H. Fu, W.-H. Li, W.-H. Deng, G.-D. Wu,G. Xu, Layer-by-Layer Assembled Conductive Metal–Organic Framework Nanofilms for Room-Temperature Chemiresistive Sensing, Angew. Chem., Int. Ed. 56 (2017) 16510-16514. [CrossRef]
- A.Demessence, C. Boissière, D. Grosso, P. Horcajada, C. Serre, G. Férey, G. J. a. A. Soler-Illia,C. Sanchez, Adsorption properties in high optical quality nanoZIF-8 thin films with tunable thickness, J. Mater. Chem. 20 (2010) 7676-7681. [CrossRef]
- O.Shekhah, Layer-by-Layer Method for the Synthesis and Growth of Surface Mounted Metal-Organic Frameworks (SURMOFs), Materials 3 (2010) 1302-1315. [CrossRef]
- Y.-B. Tian, N. Vankova, P. Weidler, A. Kuc, T. Heine, C. Wöll, Z.-G. Gu,J. Zhang, Oriented Growth of In-Oxo Chain Based Metal-Porphyrin Framework Thin Film for High-Sensitive Photodetector, Adv. Sci. (Weinheim, Ger.) 8 (2021) 2100548. [CrossRef]
- S. Sachdeva, M. R. Venkatesh, B. E. Mansouri, J. Wei, A. Bossche, F. Kapteijn, G. Q. Zhang, J. Gascon, L. C. P. M. De Smet,E. J. R. Sudhölter, Sensitive and Reversible Detection of Methanol and Water Vapor by In Situ Electrochemically Grown CuBTC MOFs on Interdigitated Electrodes, Small 13 (2017) 1604150. [CrossRef]
- V. Bon, E. Brunner, A. Pöppl,S. Kaskel, Unraveling Structure and Dynamics in Porous Frameworks via Advanced In Situ Characterization Techniques, Adv. Funct. Mater. 30 (2020) 1907847. [CrossRef]
- S. H. Kim, J. S. Yeon, R. Kim, K. M. Choi,H. S. Park, A functional separator coated with sulfonated metal–organic framework/Nafion hybrids for Li–S batteries, J. Mater. Chem. A 6 (2018) 24971-24978. [CrossRef]
- C.-H. Shen, Y.-N. Chang, Y.-L. Chen,C.-W. Kung, Sulfonate-Grafted Metal–Organic Framework─A Porous Alternative to Nafion for Electrochemical Sensors, ACS Mater. Lett. 5 (2023) 1938-1943. [CrossRef]
- H. Ji, S. Hwang, K. Kim, C. Kim,N. C. Jeong, Direct in Situ Conversion of Metals into Metal–Organic Frameworks: A Strategy for the Rapid Growth of MOF Films on Metal Substrates, ACS Appl. Mater. Interfaces 8 (2016) 32414-32420. [CrossRef]
- W.-J. Li, J. Liu, Z.-H. Sun, T.-F. Liu, J. Lü, S.-Y. Gao, C. He, R. Cao,J.-H. Luo, Integration of metal-organic frameworks into an electrochemical dielectric thin film for electronic applications, Nat. Commun. 7 (2016) 11830. [CrossRef]
- D. K. Nguyen, I. M. Schepisi,F. Z. Amir, Extraordinary cycling stability of Ni3(HITP)2 supercapacitors fabricated by electrophoretic deposition: Cycling at 100,000 cycles, Chem. Eng. J. 378 (2019) 122150. [CrossRef]
- I.Hod, W. Bury, D. M. Karlin, P. Deria, C.-W. Kung, M. J. Katz, M. So, B. Klahr, D. Jin, Y.-W. Chung, T. W. Odom, O. K. Farha,J. T. Hupp, Directed Growth of Electroactive Metal-Organic Framework Thin Films Using Electrophoretic Deposition, Adv. Mater. 26 (2014) 6295-6300. [CrossRef]
- H. Molavi, A. Shojaei,S. A. Mousavi, Improving mixed-matrix membrane performance via PMMA grafting from functionalized NH2–UiO-66, J. Mater. Chem. A 6 (2018) 2775-2791. [CrossRef]
- P. Jiamjirangkul, T. Inprasit, V. Intasanta,A. Pangon, Metal organic framework-integrated chitosan/poly(vinyl alcohol) (PVA) nanofibrous membrane hybrids from green process for selective CO2 capture and filtration, Chem. Eng. Sci. 221 (2020) 115650. [CrossRef]
- D. T. Sun, L. Peng, W. S. Reeder, S. M. Moosavi, D. Tiana, D. K. Britt, E. Oveisi,W. L. Queen, Rapid, Selective Heavy Metal Removal from Water by a Metal–Organic Framework/Polydopamine Composite, ACS Cent. Sci. 4 (2018) 349-356. [CrossRef]
- X. Ma, Y. Chai, P. Li,B. Wang, Metal–Organic Framework Films and Their Potential Applications in Environmental Pollution Control, Acc. Chem. Res. 52 (2019) 1461-1470. [CrossRef]
- L. Wang, X. Feng, L. Ren, Q. Piao, J. Zhong, Y. Wang, H. Li, Y. Chen,B. Wang, Flexible Solid-State Supercapacitor Based on a Metal–Organic Framework Interwoven by Electrochemically-Deposited PANI, J. Am. Chem. Soc. 137 (2015) 4920-4923. [CrossRef]
- M. M. Langari, M. M. Antxustegi,J. Labidi, Nanocellulose-based sensing platforms for heavy metal ions detection: A comprehensive review, Chemosphere 302 (2022) 134823. [CrossRef]
- Z. Zhou, S. Mukherjee, S. Hou, W. Li, M. Elsner,R. A. Fischer, Porphyrinic MOF Film for Multifaceted Electrochemical Sensing, Angew. Chem., Int. Ed. 60 (2021) 20551-20557. [CrossRef]
- S. Chen, J. Qi, S. Fan, Z. Qiao, J. C. Yeo,C. T. Lim, Flexible Wearable Sensors for Cardiovascular Health Monitoring, Adv. Healthcare Mater. 10 (2021) e2100116. [CrossRef]
- J. Lee, D. Kim, H.-Y. Ryoo,B.-S. Shin, Sustainable Wearables: Wearable Technology for Enhancing the Quality of Human Life, Sustainability 8 (2016) 466. [CrossRef]
- N. Luo, W. Dai, C. Li, Z. Zhou, L. Lu, C. C. Y. Poon, S. C. Chen, Y. Zhang,N. Zhao, Flexible Piezoresistive Sensor Patch Enabling Ultralow Power Cuffless Blood Pressure Measurement, Adv. Funct. Mater. 26 (2015) 1178-1187. [CrossRef]
- K. Meng, J. Chen, X. Li, Y. Wu, W. Fan, Z. Zhou, Q. He, X. Wang, X. Fan, Y. Zhang, J. Yang,Z. L. Wang, Flexible Weaving Constructed Self-Powered Pressure Sensor Enabling Continuous Diagnosis of Cardiovascular Disease and Measurement of Cuffless Blood Pressure, Adv. Funct. Mater. 29 (2018). [CrossRef]
- Z. Pu, X. Zhang, H. Yu, J. Tu, H. Chen, Y. Liu, X. Su, R. Wang, L. Zhang,D. Li, A thermal activated and differential self-calibrated flexible epidermal biomicrofluidic device for wearable accurate blood glucose monitoring, Sci. Adv. 7 (2021). [CrossRef]
- X. Jin, G. Li, T. Xu, L. Su, D. Yan,X. Zhang, Fully integrated flexible biosensor for wearable continuous glucose monitoring, Biosens. Bioelectron. 196 (2022) 113760. [CrossRef]
- P.-H. Ling, X.-N. Zang, C.-H. Qian,F. Gao, A metal–organic framework with multienzyme activity as a biosensing platform for real-time electrochemical detection of nitric oxide and hydrogen peroxide, Analyst 146 (2021) 2609-2616. [CrossRef]
- H. Yang, J. Dong, Q. Li, L. Wen, N. Qi, X. Wang, F. Xu, D. Huo,C. Hou, Au and Pt Nanoparticles Grown on Flexible Carbon Fiber Cloth Supports Decorated with Cerium Metal Organic Frameworks for the Real-Time Detection of H2O2 in Live Cancer Tissue, ACS Appl. Nano Mater. 5 (2022) 18328-18336. [CrossRef]
- Y. Xia, T. Su, Z. Mi, Z. Feng, Y. Hong, X. Hu,Y. Shu, Wearable electrochemical sensor based on bimetallic MOF coated CNT/PDMS film electrode via a dual-stamping method for real-time sweat glucose analysis, Anal. Chim. Acta 1278 (2023) 341754. [CrossRef]
- W. Ling, G. Liew, Y. Li, Y. Hao, H. Pan, H. Wang, B. Ning, H. Xu,X. Huang, Materials and Techniques for Implantable Nutrient Sensing Using Flexible Sensors Integrated with Metal–Organic Frameworks, Adv. Mater. 30 (2018) 1800917. [CrossRef]
- X. Yang, J. Yi, T. Wang, Y. Feng, J. Wang, J. Yu, F. Zhang, Z. Jiang, Z. Lv, H. Li, T. Huang, D. Si, X. Wang, R. Cao,X. Chen, Wet-Adhesive On-Skin Sensors Based on Metal–Organic Frameworks for Wireless Monitoring of Metabolites in Sweat, Adv. Mater. 34 (2022) 2201768. [CrossRef]
- A.Hitabatuma, P. Wang, X. Su,M. Ma, Metal-Organic Frameworks-Based Sensors for Food Safety, Foods 11 (2022) 382. [CrossRef]
- B.Siu, A. R. Chowdhury, Z. Yan, S. M. Humphrey,T. Hutter, Selective adsorption of volatile organic compounds in metal-organic frameworks (MOFs), Coord. Chem. Rev. 485 (2023) 215119. [CrossRef]
- L.-H. Xie, X.-M. Liu, T. He,J.-R. Li, Metal-Organic Frameworks for the Capture of Trace Aromatic Volatile Organic Compounds, Chem 4 (2018) 1911-1927. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).