1. Introduction
Aroma is one of the crucial indicators of fruit flavor quality, and fruits with enticing aromas often enhance consumers' purchasing desire [
1]. Different types of fruits usually have distinctive aroma characteristics, and products with unique flavor qualities are often more likely to prevail in business competition. Mulberry (
Fructus Mori) is a renowned fruit originating from Asia and is widely cultivated in Asia, Europe, America, and Africa. Mulberries are juicy, sweet-tasting, and highly nutritious. They can be eaten fresh or processed into mulberry wine, mulberry beverages, or other mulberry products, making them highly favored in the market. Recently, we discovered a new variety of mulberry known as 'Baichang' that emits a unique, creamy aroma when ripe, which is uncommon among other mulberry varieties. As a distinctly different variety, this mulberry holds immense value for breeding and potential commercial value.
The aroma of fruit is primarily determined by Volatile Organic Compounds (VOCs), which include esters, alcohols, aldehydes, ketones, terpenes, and heterocyclic compounds [
2,
3]. Although they constitute only a small portion of the fruit's weight, they play a decisive role in the fruit's fragrance and also have a certain impact on the fruit's taste. Different VOCs give different flavor perceptions, so the contribution of VOCs to aroma components depends not only on concentration but also closely relates to their sensory threshold [
4]. For a single VOC, its concentration and sensory threshold together determine its contribution to the aroma composition, usually measured by the odor activity value (OAV) [
5].
Existing research shows that esters are an important component of VOCs and play a decisive role in the sensory flavor of many fruits. For example, the main aroma source of European pear (Pyrus spp) is derived from decadienoate and hexyl ester [
6]; in peach (Prunus persica), among the more than 100 identified VOCs, esters have a decisive impact on its aroma [
7,
8]. Volatile esters typically accumulate more during fruit ripening, and provide unique fruit flavors for a variety of fruits such as strawberries (Fragaria × ananassa), bananas (Musa acuminata), and apples (Malus domestica) [
9,
10]. In plants, esters are synthesized through the fatty acid pathway, involving multiple structural genes, including
pyruvate decarboxylase (
PDC), aldehyde dehydrogenase (
ALDH),
acyl carrier protein-acyltransferase (
ACP),
fatty acid desaturase (
FAD),
acyl-CoA oxidase (
ACX),
cytochrome P450 (
CYP),
fatty acidhydr oxylase (
FAH),
lipoxygenase (
LOX),
epoxyde hydrolase (
EHL),
hydroperoxide lyase (
HPL),
alcohol dehydrogenase (
ADH) and
acetyltransferase (
AAT) [
11,
12,
13,
14]。
In this study, we employed GC-MS technology to analyze the VOCs and gene expression status of 'Baichang' mulberries and 'Hongchang' mulberries, a variety without a unique aroma. We identified key VOCs that contribute to the distinct scent of 'Baichang' mulberries. Key structural genes related to aroma formation were identified through RNA-seq. This research will provide new insights into the mechanism of aroma formation in mulberries.
2. Materials and Methods
2.1. Plant Material
The experimental materials used in this study are the fruits of 'Baichang' and 'Hongchang' mulberry trees cultivated in the Germplasm Resources Nursery of the Institute of Environment and Plant Protection, Chinese Academy of Tropical Agricultural Sciences (Danzhou, Hainan, China). Nine healthy mature trees were selected, with every three trees constituting one biological replicate, and fully ripe fruit samples were collected. Immediately after sampling, the fruits were frozen in liquid nitrogen and subsequently stored in a -80°C refrigerator for later use.
2.2. Detection and Analysis of VOCs
A homogenized sample of 500 mg was added to the headspace vial, followed by the addition of saturated NaCl solution, and 10μL of internal standard solution (50 μg/mL), and then subjected to automatic headspace solid-phase microextraction (HS-SPME) for later use. Extraction conditions: constant temperature at 60 °C, shaking for 5 minutes, 120 µm DVB/CWR/PDMS extraction head placed in the sample headspace vial, extraction for 15 minutes, desorption for 5 minutes at 250 °C, followed by GC-MS separation and identification. Prior to sampling, the extraction head was aged for 5 minutes in the Fiber Conditioning Station at a temperature of 250°C. Chromatographic conditions were set as follows: DB-5MS capillary column, high-purity helium as carrier gas, constant flow rate set at 1.2 mL/min, injection port temperature set at 250 °C, non-split injection, solvent delay of 3.5 minutes. The temperature program setting was set to maintain at 40 °C for 3.5 minutes, then rise at 10 °C/min to 100 °C, then rise at 7 °C/min to 180 °C, finally rise at 25 °C/min to 280 °C, and hold for 5 minutes. Mass spectrometry conditions were set as follows: electron impact source (EI), ion source set at 230°C, quadrupole temperature set at 150°C, mass spectrometry interface temperature at 280 °C, electron energy 70 eV, scanning mode was selected ion detection mode (SIM), qualitative and quantitative ion precise scanning (GB 23200.8-2016).
The raw data obtained from mass spectrometry analysis is processed using MassHunter software and qualitative and quantitative analysis is performed based on the self-built database of Metetware company. Principal Component Analysis (PCA) is used to verify the differences and reliability of the metabolites in the sample. R programming scripts are used to draw cluster heat maps, and the built-in cor function of R software is used to calculate the Pearson correlation coefficient. Differential metabolites are screened based on standards of VIP > 1, fold change≥2, and fold change≤0.5, and the sensory flavor characteristics of VOCs are annotated using LRI& odour database, Flavornet and human odor space, and Flavor Ingredient Library databases. The rOAV values of VOCs are calculated using the R package [
15].
2.3. RNA Extraction and Sequencing
The methods for RNA extraction, library construction, and sequencing are consistent with those of Qian et al. [
16], and sequencing is performed using the Illumina HiSeq platform of MetWare (Wuhan, China). Clean Reads are aligned with the reference genome using HISAT2 to obtain gene location information and unique sequence feature information of the sequencing samples [
17,
18]. StringTie is used to assemble reads into transcripts, and gene expression is measured by the number of fragments per kilobase of transcript per million mapped reads (FPKM) [
19]. The DESeq R package (1.10.1) is used to analyze differential expression between two groups, with |log
2 fold change| > 1 and p-value< 0.05 considered as differentially expressed genes (DEGs). The raw data of RNA-seq has been submitted to NCBI with the following ID numbers: PRJNA1084918 and PRJNA1142089.
2.4. qRT-PCR Analysis
The protocol for cDNA synthesis and real-time quantitative PCR (Q-PCR) is described by Shi et al. [
20]. Primers for q-PCR of genes are designed using Primer 3.0 (
https://primer3.ut.ee/) [
21], with the mulberry actin gene used as an internal reference [
22]. The qPCR primers used in this study can be found in
Supplementary Table S1.
2.5. HeatMap and Statistical Analysis
The present study mainly used TBtools software [
23] and the Metware Cloud platform (
https://cloud.metware.cn) for heatmap plotting and data analysis. Independent sample T-tests were performed using SPSS 27.0. A p-value of < 0.05 and < 0.01 were considered statistically significant and are denoted by "*" and "**" respectively.
3. Results
3.1. Metabolome Analysis
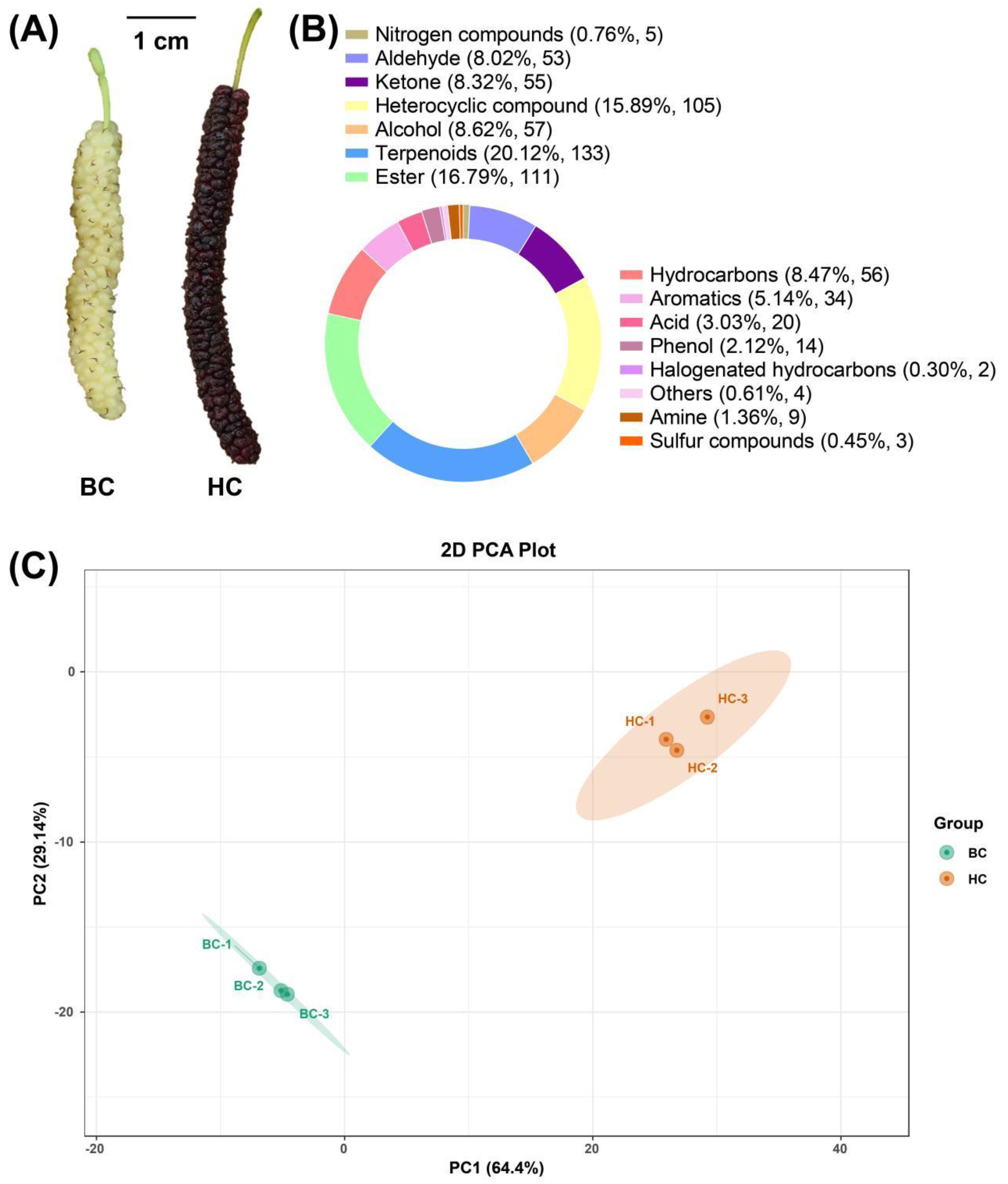
The mature fruits of 'Baichang' mulberry and 'Hongchang' mulberry are shown in
Figure 1A. The 'Baichang' mulberry emits a unique buttery aroma when ripe, which the 'Hongchang' mulberry lacks at ripening. A total of 661 volatile metabolites were identified in the mature fruits of both mulberry varieties, classified into 15 types. Terpenoids were the most abundant, accounting for 20.12% of all identified volatile metabolites, followed by Esters and Heterocyclic compounds, which made up 16.79% and 15.89% respectively. Other components detected include Nitrogen compounds, Aldehydes, Ketones, Alcohols, Hydrocarbons, Aromatics, Acids, Phenols, Halogenated hydrocarbons, Amines, and Sulfur compounds (
Figure 1B). Interestingly, 47 volatile metabolites were only detected in 'Baichang' mulberries, suggesting a richer aroma composition in this variety. PCA analysis showed that the three repetitions within the same mulberry variety were close to each other, indicating good reproducibility of the samples; there was a distinct separation trend between the two groups, indicating significant differences in VOCs between the two varieties. The first principal component accounted for 64.4% of the variance, while the second principal component accounted for 29.14% of the variance (
Figure 1C).
3.2. Differential VOCs Analysis
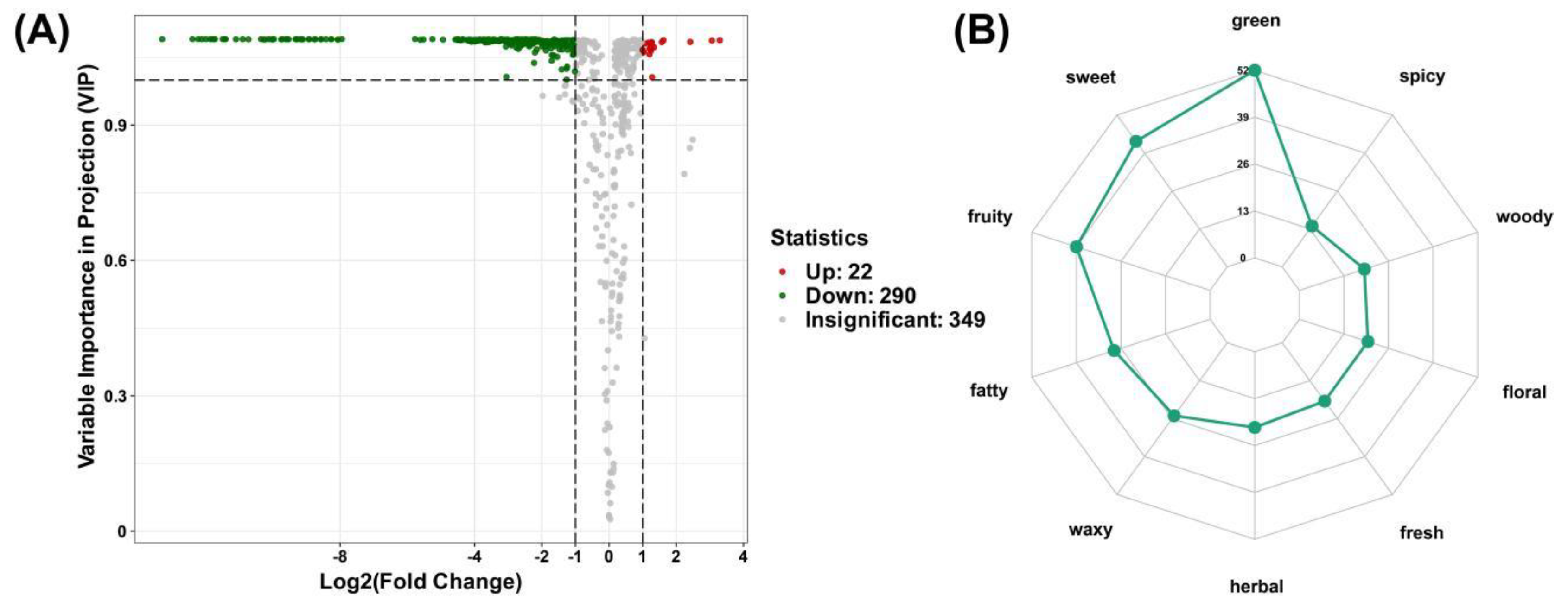
A total of 312 differential metabolites were identified between the two mulberry varieties. Compared with 'Baichang' fruits, most differential metabolites in 'Hongchang' fruits were downregulated (290 types), with only 22 types of metabolites upregulated. This might be the cause for the distinctive aroma components in ‘Baichang’ mulberries. According to sensory experience, ‘Baichang’ mulberries have a fragrant smell, while 'Hongchang' mulberries are not fragrant, so the key metabolites affecting the aroma of ‘Baichang’ mulberries are likely to be these downregulated differential metabolites (
Figure 2A). By annotating the sensory flavor characteristics of the differential metabolites in the two types of mulberries, it was found that green, spicy, woody, floral, fresh, herbal, waxy, fatty, fruity, and sweet were the top ten differential metabolite sensory flavors, with the highest number of differential metabolites found in green, sweet, fruity, and fatty flavors (
Figure 2B,
Supplementary Table S2).
3.3. Screening of Key Differential Metabolites of Aroma Components
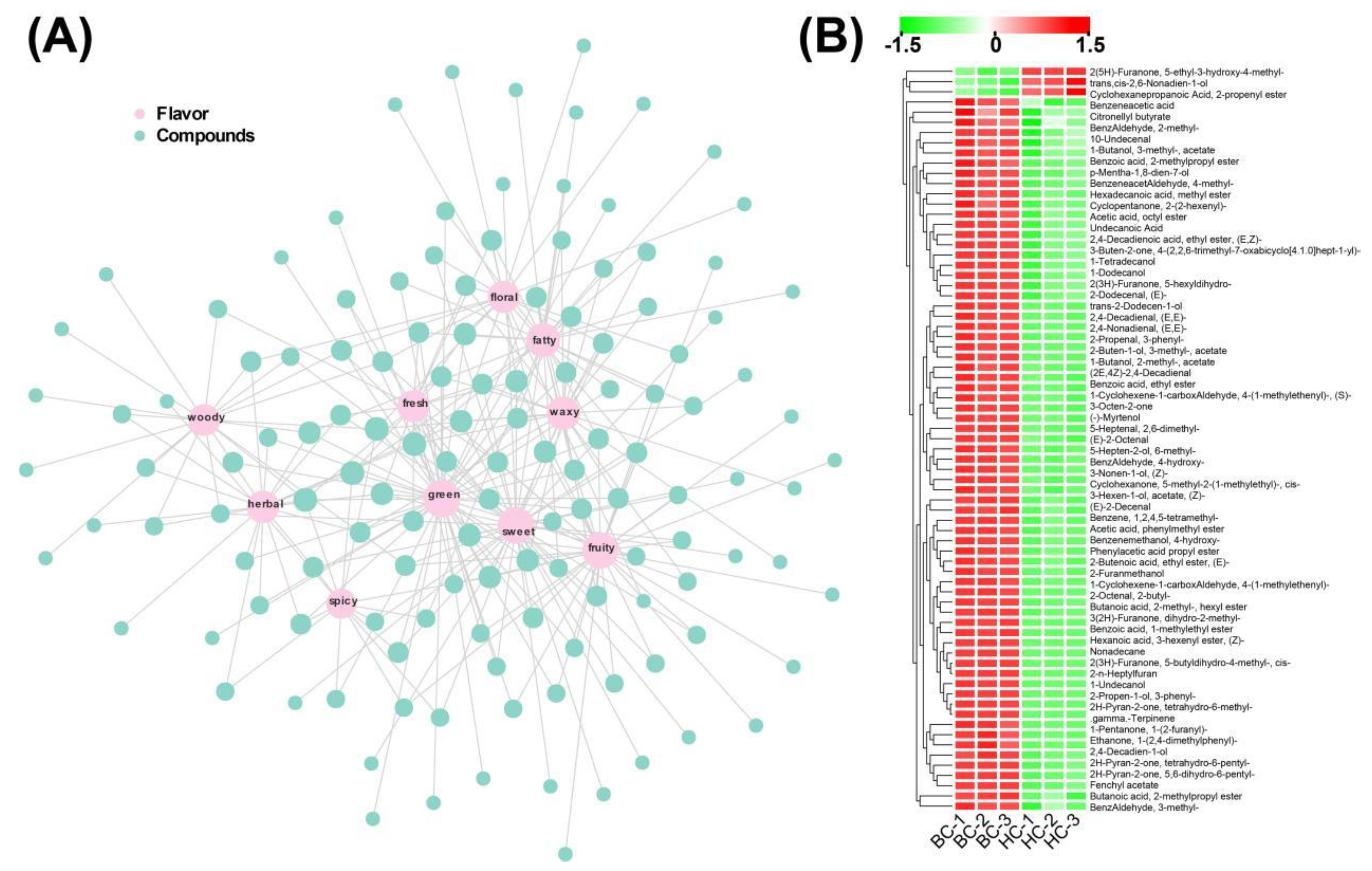
We constructed a sensory flavor network diagram to identify the key differential metabolites for each sensory flavor (
Figure 3A,
Supplementary Table S3). The sensory flavors of sweet, waxy, coconut, and oily are consistent with the olfactory experience of "Baichang" mulberries, and based on these four sensory flavors, we screened out 73 associated differential metabolites. The odor of 'Baichang' mulberries is rich in milky aroma, therefore, the relative content of key differential metabolites related to aroma should be relatively higher in 'Baichang' mulberry fruits. The contents of 2(5H)-Furanone, 5-ethyl-3-hydroxy-4-methyl-, trans,cis-2,6-Nonadien-1-ol, and 1-Nonanol are higher in 'Hongchang' fruits, indicating that they are not the key substances forming the aroma of 'Baichang'. The relative contents of the remaining 70 differential metabolites are in line with the trend, suggesting that they could be the candidate key metabolites causing the differential aroma of the two mulberry fruit species (
Figure 3B).
3.4. Relative Odor Activity Value (rOAV) Analysis
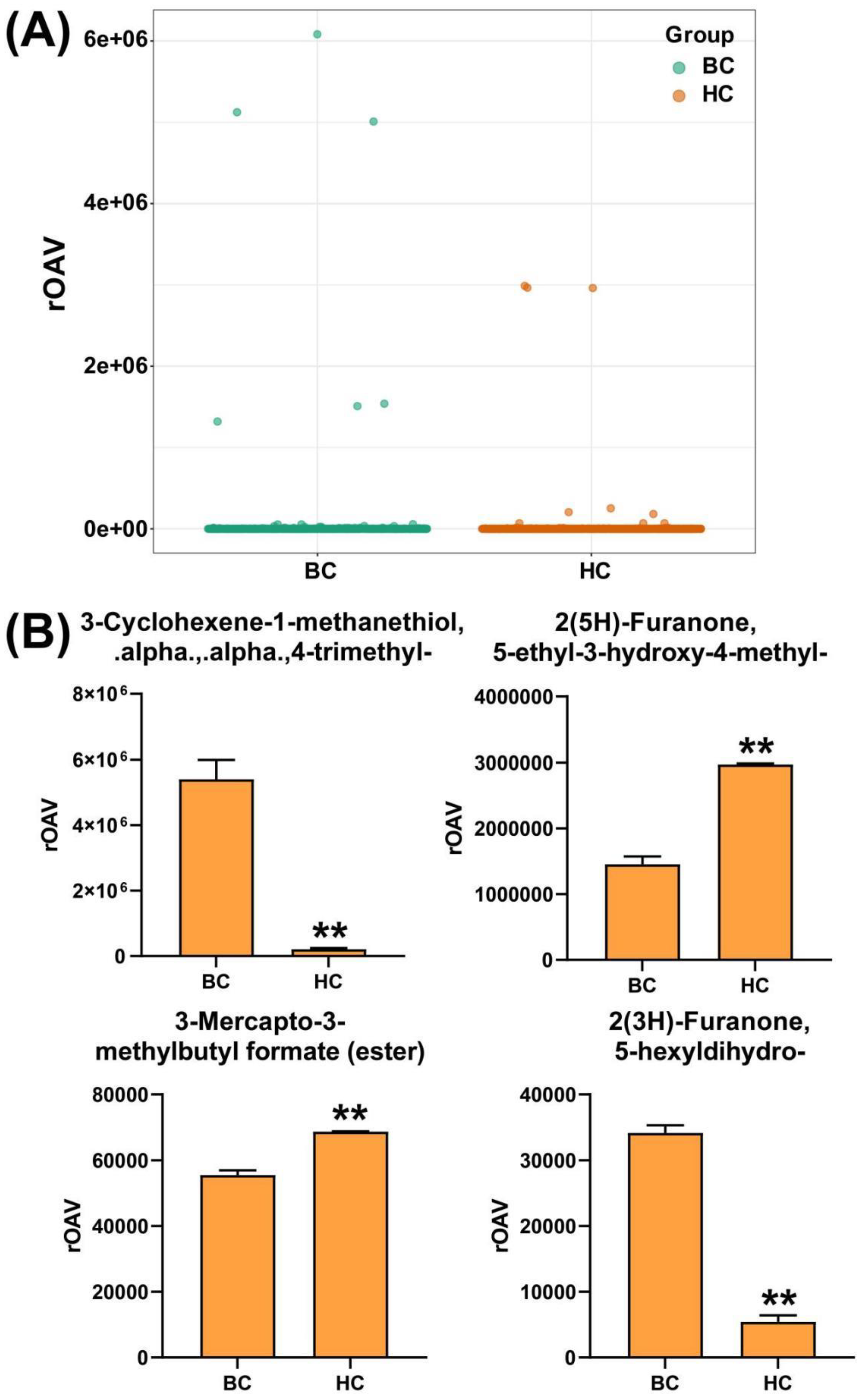
The relative odor activity value (rOAV) can elucidate the contribution of each aroma compound to the overall aroma characteristics of the fruit. Combined with the content of differential metabolites, it can further help identify the key substances causing the aroma differences between 'Baichang' and 'Hongchang' mulberries. The rOAV analysis results show that the four substances that have the greatest impact on the aroma of 'Baichang' mulberries are 3-Cyclohexene-1-methanethiol, .alpha.,.alpha.,4-trimethyl-, 2(5H)-Furanone, 5-ethyl-3-hydroxy-4-methyl-, 3-Mercapto-3-methylbutyl formate (ester), and 2(3H)-Furanone, 5-hexyldihydro- (
Figure 4A,
Supplementary Table S4). The rOAV values of 2(5H)-Furanone, 5-ethyl-3-hydroxy-4-methyl-, and 3-Mercapto-3-methylbutyl formate (ester) are high in 'Hongchang' mulberries but low in 'Baichang' mulberries. This is inconsistent with the sensory experience that 'Baichang' mulberries have a strong aroma while 'Hongchang' mulberries have no distinct aroma. Therefore, we infer that these two substances are not the key substances causing the aroma differences between the two types of mulberries. The odor annotation of 3-Cyclohexene-1-methanethiol, .alpha.,.alpha.,4-trimethyl- is sulfury, aromatic, grapefruit, naphthyl, resinous, woody, which is far different from the actual creamy aroma sensory experience of 'Baichang' mulberries (
Figure 4B). Therefore, after comprehensive analysis, it is speculated that 2(3H)-Furanone, 5-hexyldihydro- is the main substance responsible for the creamy aroma of 'Baichang' mulberries and is also the key metabolic product that forms the aroma difference between the two types of mulberries.
3.5. RNA-seq and Differential Gene Analysis
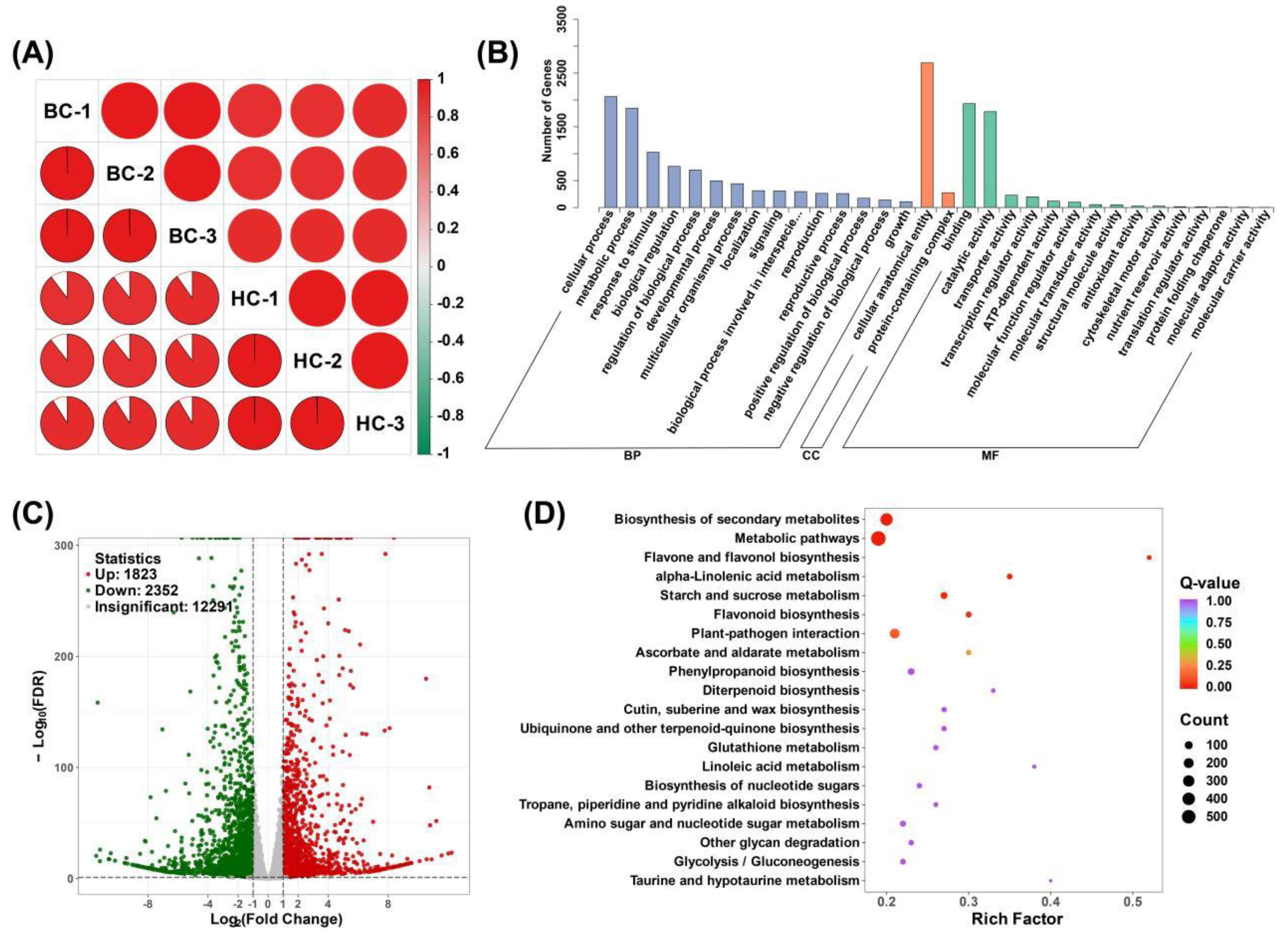
Through RNA-seq, 42,731,000 to 51,231,176 raw reads were obtained from six mulberry fruit samples, and after filtering, 41,862,788 to 49,701,590 clean reads and 6.28 to 7.46 G of clean base were obtained. The error rate for all samples is 0.02%, the Q20 content is between 98.06% and 98.14%, the Q30 content is between 94.42% and 94.76%, and the GC content is between 46.3% and 47.04% (
Supplementary Table S4). Correlation analysis showed good repeatability of the samples (
Figure 5A). Differential analysis showed that in the BC vs HC comparison group, 1823 genes were up-regulated and 2352 genes were down-regulated (
Figure 5C).
GO analysis classified the differential genes into three categories: Biological process (BP), Cellular component (CC), and Molecular function (MF). Among the three comparison groups, the category with the most annotated genes in Biological process (BP) was the cellular process (with 2063 genes), in Cellular component (CC) it was cellular anatomical entity (with 2689 genes), and in Molecular function (MF) it was binding (with 1932 genes) (
Figure 5B). KEGG analysis enriched the differential genes into multiple metabolic pathways, including Biosynthesis of secondary metabolites, Metabolic pathways, Flavone and flavonol biosynthesis, alpha-Linolenic acid metabolism, Starch and sucrose metabolism, etc., demonstrating the significant differences in gene expression between the two varieties (
Figure 5D).
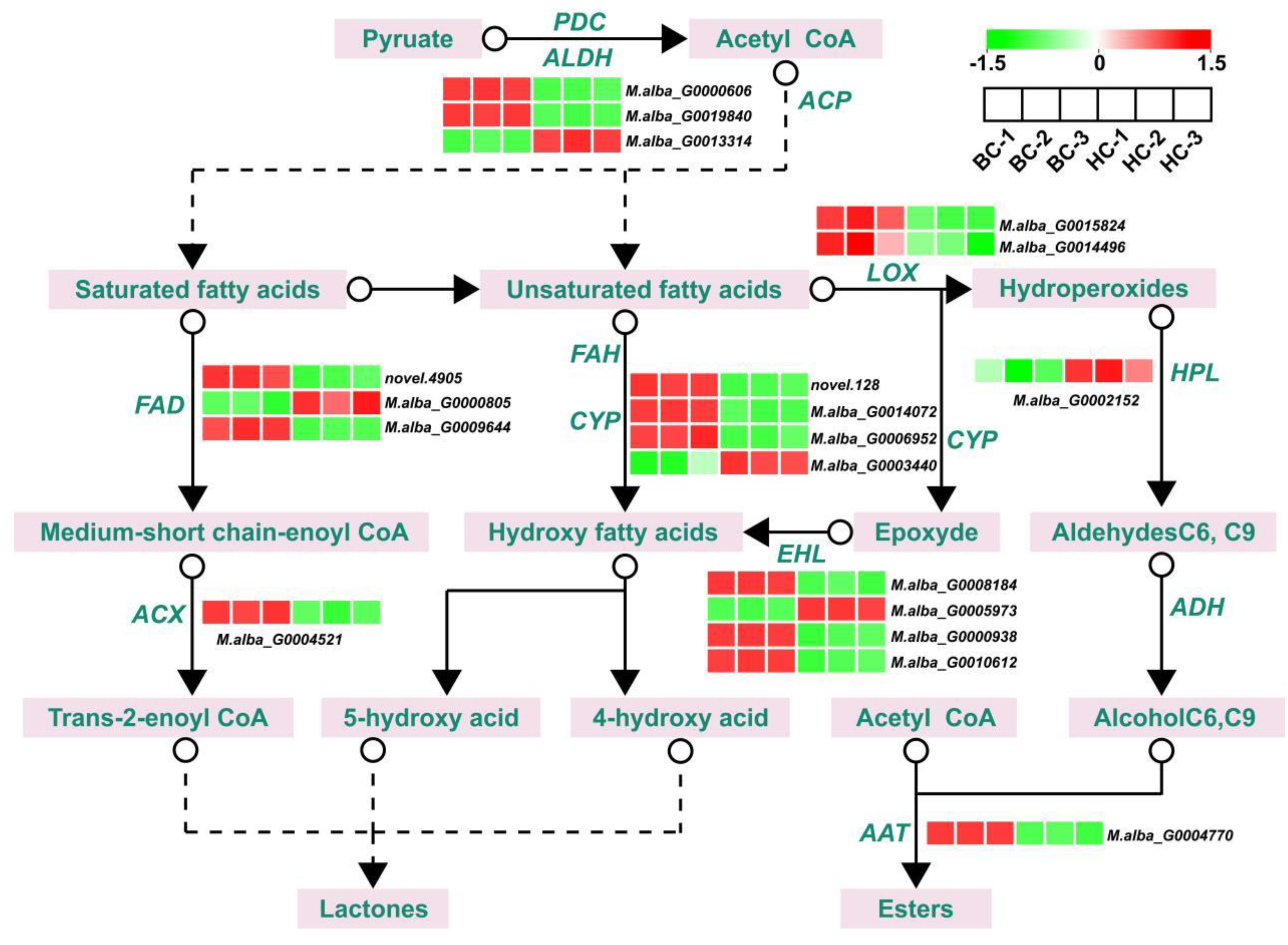
3.6. Expression Patterns of Genes Related to Ester Synthesis Pathway
We screened structural genes related to ester synthesis from the differential genes. These structural genes include 1
AAT (
M.alba_G0004770), 1
ACX (
M.alba_G0004521), 3
ALDHs (
M.alba_G0000606,
M.alba_G0019840, and
M.alba_G0013314), 4
CYPs (
novel.128,
M.alba_G0014072,
M.alba_G0006952, and
M.alba_G0003440), 4
EHLs (
M.alba_G0008184,
M.alba_G0005973,
M.alba_G0000938, and
M.alba_G0010612), 3
FADs (
novel.4905,
M.alba_G0000805, and
M.alba_G0009644), 1
HPL (
M.alba_G0002152), and 2
LOXs (
M.alba_G0015824 and
M.alba_G0014496). Among these structural genes, the majority are highly expressed in the 'Baichang' variety, with a few highly expressed in the 'Hongchang' variety (
Figure 6,
Supplementary Table S5). For instance, compared to the 'Hongchang' fruits, the expression of
M.alba_G0000606 (
ALDH) in 'Baichang' fruits was upregulated by 14.50 times,
M.alba_G0014072 (
CYP) was upregulated by 11.14 times,
M.alba_G0004521 (
ACX) was upregulated by 7.06 times, and
M.alba_G0004770 (
AAT) was upregulated by 2.71 times, among others (
Figure 6,
Supplementary Table S5). These genes could potentially be key factors in the formation of the unique aromatic components of 'Baichang' mulberries.
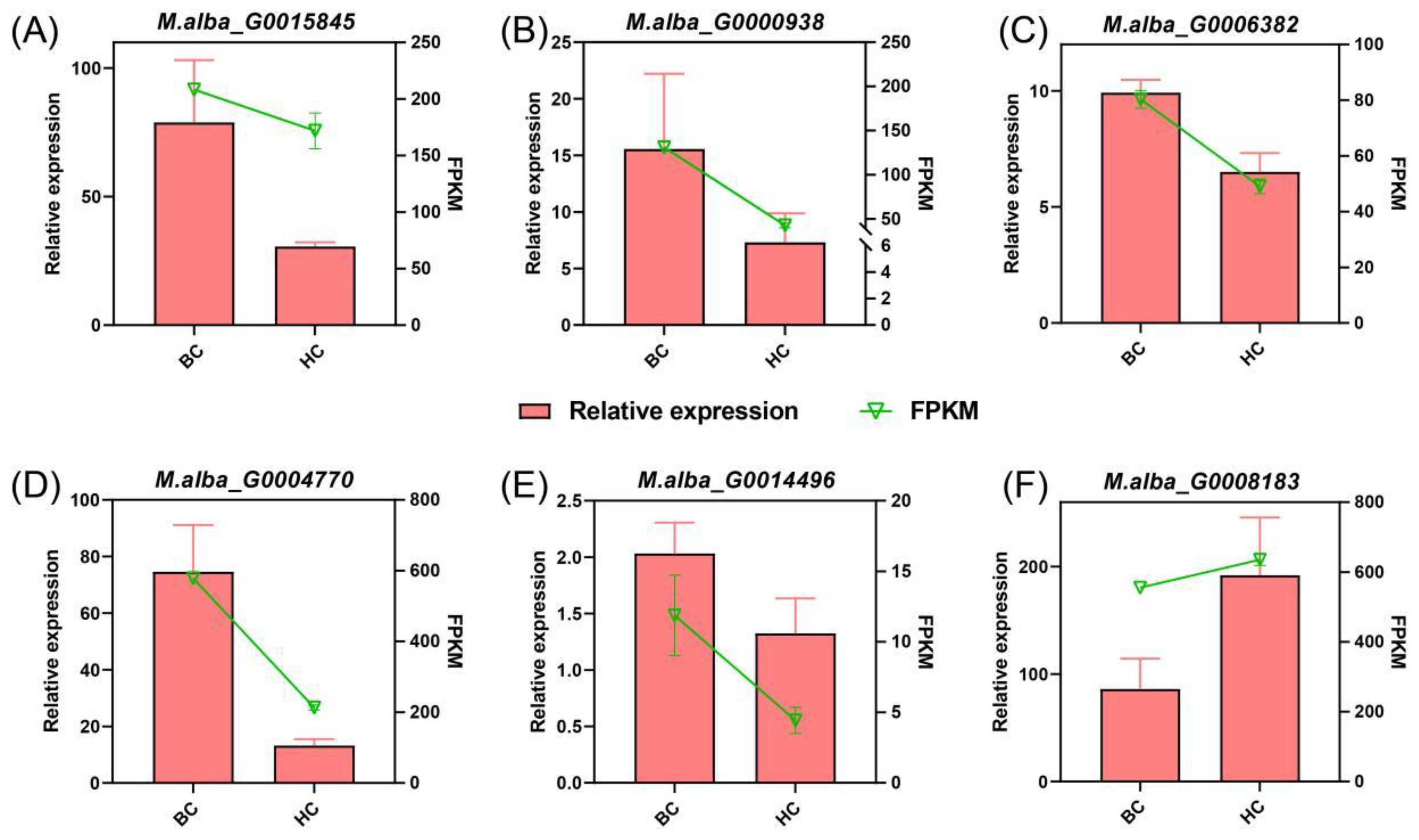
3.7. Validation of Genes Expression by qPCR
To evaluate the accuracy of RNA-seq data, we randomly selected 6 genes from the transcriptome for qPCR analysis. Among them, the qPCR results of the majority of genes such as M.alba_G0000938, M.alba_G0006382, M.alba_G0004770, and M.alba_G0014496 were highly consistent with the RNA-seq results; the trends of M.alba_G0015845 and M.alba_Go008183 were slightly different but the relative sizes were also consistent. This indicates that the qPCR data highly matches the RNA-seq data (
Figure 7).
4. Discussion
Aroma is one of the important criteria for measuring fruit quality, influencing the price and sales of the fruit. New fruit varieties that emit a captivating aroma often gain consumer preference [
24,
25]. The aroma not only has a profound impact on fresh fruit but also on subsequent processed products, such as wine, canned goods, freeze-dried fruit, and jam, making it extremely significant in their production [
26,
27]. Certain aromatic substances, such as lactones, can also be used in the production of perfumes, food flavorings, or pharmaceuticals [
28]. The aroma of fruits is usually determined by the types of Volatile Organic Compounds (VOCs), their varying concentrations, and the odor threshold value of each component [
29]. Among the VOCs in fruits, terpenes, esters, and fatty acid derivatives are the most common [
30]. Research by Zhu et al. on the aromatic components of three cultivated varieties of mulberry (Morus nigra, Morus Macroura, and Morus Alba) shows that Hexanal, (E)-2-Hexenal, Benzaldehyde, Methyl benzoate, Ethyl benzoate, Ethyl acetate, Ethyl butanoate, Ethyl hexanoate, methional, 3-mercaptohexyl acetate, etc., are the primary substances contributing to the aroma of mulberries [
31]. However, to date, no studies have reported the presence of a distinct butter-like aroma in any mulberry varieties. This study used 'Baichang' and 'Hongchang' mulberries as samples, and detected a total of 661 types of volatile organic compounds (VOCs). These included a large number of esters, terpenes, aldehydes, and heterocyclic compounds, which is similar to previous research findings. However, 2(3H)-Furanone, 5-hexyldihydro- was found to have a higher relative content only in 'Baichang' mulberries and has not been reported in other varieties. This demonstrates its uniqueness in terms of aroma components and indicates its significant potential market value [
32,
33].
2(3H)-Furanone, 5-hexyldihydro- (also known as γ-decalactone) is described as having a 'fresh, oily, waxy, peach, coconut, buttery, sweet' aroma. It is the most volatile substance contributing to the scent of peaches, making them distinct from other fruits [
34,
35]. However, from a sensory perspective, the aroma emitted by 'Baichang' mulberries is strong and unique, differing from that of peaches. This could be due to variations in the composition and content of other VOCs. Wang and colleagues studied the aroma components of 8 strawberry varieties and found that 2(3H)-Furanone, 5-hexyldihydro- plays a decisive role in the formation of the unique flavor of 'Jingtaoxiang' strawberries [
36]. In addition to being an important component of fruit aroma, 2(3H)-Furanone, 5-hexyldihydro- is also one of the most widely used lactones in the fragrance and food industry [
37].
The synthesis pathway of esters in plants has not yet been fully elucidated, but fatty acids (FAs) are considered to be the main precursors for ester synthesis. The aroma of bananas, strawberries, and cantaloupes (Cucumis melo) is mainly due to fatty acid derivatives [
9,
38]. Some key structural genes of the fatty acid pathway have been reported, including
fatty acid desaturase (
FAD),
alcohol dehydrogenase (
ADH),
lipoxygenase (
LOX), and
acetyltransferase (
AAT) [
39]. Research on passion fruit (Passiflora edulis Sims) has shown that
ACX13/14/15/20,
ADH13/26/33,
ALDH1/4/21,
HPL4/6,
FAD13/50/52/53/55,
PeLOX5/18,
PeFAE6,
PeFAH2,
PeCYP7/13,
PeEHL13/15,
PeACP-AP1/5/6/7, and
PeAAT3 may be involved in the regulation of ester synthesis [
14].
AdFAD1,
AdALDH2, and
AdAAT17 are involved in the regulation of ester synthesis during the ripening process of kiwi (Actinidia deliciosa) [
40]. 1-MCP significantly regulates the release of esters and VOCs in pear (Pyrus ussuriensis Maxim.), as well as the expression of
AATs and
LOX [
41].
AAT catalyzes the final step of ester biosynthesis, which typically increases synchronously with the maturation level and ester content of the fruit [
42]. The
AATs of various fruits such as apricot (Prunus armeniaca), papaya (Carica papaya), kiwi, grape (Vitis vinifera), and strawberry have been cloned and functionally verified [
10,
43,
44,
45,
46]. Peng and colleagues have used an oil yeast expression system to confirm that
PpAAT1 can catalyze the synthesis of 2(3H)-Furanone, 5-hexyldihydro- [
47]. In this study, most structural genes of the fatty acid pathway are highly expressed in 'Baichang' mulberry fruit, which also includes an
AAT (
M.alba_G0004770). The high expression of these genes might result in 'Baichang' mulberry having a more complex aroma composition and unique flavor.
In terms of regulation of volatile esters, most focus on transcriptional regulation of key structural genes in the fatty acid pathway by transcription factors. Zhang et al. demonstrated through a dual-luciferase assay that AdNAC5 and AdDof4 respectively activated and suppressed
AdFAD1 promoter activity, thereby regulating the synthesis of kiwifruit esters [
40]. NAC transcription factors can activate
AAT expression, catalyzing the formation of volatile esters in various fruits; such as PpNAC1 in peaches activating
PpAAT1 expression, and MdNAC5 in apples activating
MdAAT1 expression. Cao et al.'s research indicated that this regulation is subject to epigenetic regulation, closely related to the removal of the inhibitory marker H3K27me3 during the maturation process of fruit trees [
42]. The regulatory mechanism for the synthesis of 2(3H)-Furanone, 5-hexyldihydro- in mulberries remains to be further explored.
5. Conclusions
This study utilized HS-SPME extraction and GC-MS technology to detect the volatile metabolites in 'Baichang' and 'Hongchang' mulberries, and key genes affecting the aroma formation of 'Baichang' mulberries were screened through RNA-seq. A total of 661 volatile metabolites were detected in the metabolome, of which 47 were unique to 'Baichang' mulberries. A total of 312 differential metabolites were screened. By combining their relative content, sensory annotations, and rOAV analysis, 2(3H)-Furanone, 5-hexyldihydro- was ultimately determined to be the key VOC contributing to the unique aroma of 'Baichang' mulberries. By RNA-seq, 19 key structural genes related to ester synthesis were identified, including AAT (M.alba_G0004770), ACX (M.alba_G0004521), ALDHs (M.alba_G0000606, M.alba_G0019840, and M.alba_G0013314), CYPs (novel.128, M.alba_G0014072, M.alba_G0006952, and M.alba_G0003440), EHLs (M.alba_G0008184, M.alba_G0005973, M.alba_G0000938, and M.alba_G0010612), FADs (novel.4905, M.alba_G0000805, and M.alba_G0009644), HPL (M.alba_G0002152), and LOXs (M.alba_G0015824 and M.alba_G0014496). The high expression of these structural genes in 'Baichang' mulberry fruit may be the main factor contributing to its unique creamy aroma. This research provides insights into the aroma differences among different types of mulberries and offers new ideas for the subsequent improvement of mulberry varieties.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Supplementary Table S1 Sequences of the Q-PCR primers used in this study; Supplementary Table S2 VOCs statistics of differences affecting different odor senses; Supplementary Table S3 rOAV analysis of VOCs; Supplementary Table S4 Statistics on the quality and output of the RNA-Seq; Supplementary Table S5 FPKM values of key structural genes in ester synthesis pathway.
Author Contributions
Conceptualization, W.Y. and J.H.; methodology, C.Y.; software, C.Y.; validation, W.H.; formal analysis, W.Y.; investigation, C.Y.; resources, H.W.; data curation, C.Y.; writing—original draft preparation, C.Y.; writing—review and editing, H.W. and S.W.; visualization, C.Y.; supervision, J.H.; project administration, C.Y.; funding acquisition, S.W. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the earmarked funds for the China Agriculture Research System-Sericulture (CARS-18), and Central Public-interest Scientific Institution Basal Research Fund for Chinese Academy of Tropical Agricultural Sciences (No.1630042024023)
Data Availability Statement
The datasets presented in this study can be found in online repositories. The transcriptome raw data were submitted to NCBI with the following ID number: PRJNA1084918 and PRJNA1142089.
Acknowledgments
In this section, you can acknowledge any support given which is not covered by the author contribution or funding sections. This may include administrative and technical support, or donations in kind (e.g., materials used for experiments).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhu, J.; Chen, F.; Wang, L.; Niu, Y.; Chen, H.; Wang, H.; Xiao, Z. Characterization of the Key Aroma Volatile Compounds in Cranberry (Vaccinium macrocarpon Ait.) Using Gas Chromatography-Olfactometry (GC-O) and Odor Activity Value (OAV). J Agric Food Chem 2016, 64, 4990–4999. [Google Scholar] [CrossRef]
- Goff, S.A.; Klee, H.J. Plant volatile compounds: sensory cues for health and nutritional value? Science 2006, 311, 815–819. [Google Scholar] [CrossRef]
- Pichersky, E.; Gershenzon, J. The formation and function of plant volatiles: perfumes for pollinator attraction and defense. Current Opinion in Plant Biology 2002, 5, 237–243. [Google Scholar] [CrossRef]
- Klee, H.J. Improving the flavor of fresh fruits: genomics, biochemistry, and biotechnology. The New phytologist 2010, 187, 44–56. [Google Scholar] [CrossRef]
- He, Y.; Qin, H.; Wen, J.; Cao, W.; Yan, Y.; Sun, Y.; Yuan, P.; Sun, B.; Fan, S.; Lu, W.; et al. Characterization of Key Compounds of Organic Acids and Aroma Volatiles in Fruits of Different Actinidia argute Resources Based on High-Performance Liquid Chromatography (HPLC) and Headspace Gas Chromatography–Ion Mobility Spectrometry (HS-GC-IMS). Foods 2023, 12. [Google Scholar] [CrossRef]
- Rapparini, F.; Predieri, S. Pear Fruit Volatiles. In Horticultural Reviews; 2002; pp. 237-324.
- Jia, H.; Hirano, K.; Okamoto, G.J.J.o.t.J.S.f.H.S. Effects of Fertilizer Levels on Tree Growth and Fruit Quality of 'Hakuho' Peaches (Prunus persica). 2008, 68, 487-493.
- Eduardo, I.; Chietera, G.; Bassi, D.; Rossini, L.; Vecchietti, A. Identification of key odor volatile compounds in the essential oil of nine peach accessions. J Sci Food Agric 2010, 90, 1146–1154. [Google Scholar] [CrossRef] [PubMed]
- Souleyre, E.J.F.; Chagné, D.; Chen, X.; Tomes, S.; Turner, R.M.; Wang, M.Y.; Maddumage, R.; Hunt, M.B.; Winz, R.A.; Wiedow, C.; et al. The 1 locus is critical for the biosynthesis of esters contributing to ‘ripe apple’ flavour in ‘Royal Gala’ and ‘Granny Smith’ apples. The Plant Journal 2014, 78, 903–915. [Google Scholar] [CrossRef] [PubMed]
- Beekwilder, J.; Alvarez-Huerta, M.; Neef, E.; Verstappen, F.W.; Bouwmeester, H.J.; Aharoni, A. Functional characterization of enzymes forming volatile esters from strawberry and banana. Plant Physiol 2004, 135, 1865–1878. [Google Scholar] [CrossRef] [PubMed]
- Echeverrı́a, G.; Graell, J.; López, M.L.; Lara, I. Volatile production, quality and aroma-related enzyme activities during maturation of ‘Fuji’ apples. Postharvest Biology and Technology 2004, 31, 217–227. [Google Scholar] [CrossRef]
- Li, X.; Tieman, D.; Liu, Z.; Chen, K.; Klee, H.J. Identification of a lipase gene with a role in tomato fruit short-chain fatty acid-derived flavor volatiles by genome-wide association. The Plant journal: for cell and molecular biology 2020, 104, 631–644. [Google Scholar] [CrossRef]
- Song, J.; Bangerth, F. Fatty acids as precursors for aroma volatile biosynthesis in pre-climacteric and climacteric apple fruit. Postharvest Biology and Technology 2003, 30, 113–121. [Google Scholar] [CrossRef]
- Xia, Z.; Huang, D.; Zhang, S.; Wang, W.; Ma, F.; Wu, B.; Xu, Y.; Xu, B.; Chen, D.; Zou, M.; et al. Chromosome-scale genome assembly provides insights into the evolution and flavor synthesis of passion fruit (Passiflora edulis Sims). Hortic Res 2021, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Wang, C.; Chen, Y.; Zheng, M.; Zhang, Y.; Deng, Q.; Zhou, Q. Aroma characteristics of flaxseed milk via GC–MS-O and odor activity value calculation: Imparts and selection of different flaxseed varieties. Food Chemistry 2024, 432, 137095. [Google Scholar] [CrossRef] [PubMed]
- Qian, M.; Wu, H.; Yang, C.; Zhu, W.; Shi, B.; Zheng, B.; Wang, S.; Zhou, K.; Gao, A. RNA-Seq reveals the key pathways and genes involved in the light-regulated flavonoids biosynthesis in mango (Mangifera indica L.) peel. Front Plant Sci 2022, 13, 1119384. [Google Scholar] [CrossRef]
- Jiao, F.; Luo, R.; Dai, X.; Liu, H.; Yu, G.; Han, S.; Lu, X.; Su, C.; Chen, Q.; Song, Q.; et al. Chromosome-Level Reference Genome and Population Genomic Analysis Provide Insights into the Evolution and Improvement of Domesticated Mulberry (Morus alba). Molecular Plant 2020, 13, 1001–1012. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nature biotechnology 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Pertea, M.; Kim, D.; Pertea, G.M.; Leek, J.T.; Salzberg, S.L. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat Protoc 2016, 11, 1650–1667. [Google Scholar] [CrossRef]
- Shi, B.; Wu, H.; Zheng, B.; Qian, M.; Gao, A.; Zhou, K. Analysis of Light-Independent Anthocyanin Accumulation in Mango (Mangifera indica L. ). 2021, 7, 423. [Google Scholar] [CrossRef]
- Kõressaar, T.; Lepamets, M.; Kaplinski, L.; Raime, K.; Andreson, R.; Remm, M. Primer3_masker: integrating masking of template sequence with primer design software. Bioinformatics (Oxford, England) 2018, 34, 1937–1938. [Google Scholar] [CrossRef]
- Dai, F.; Zhao, X.; Tang, C.; Wang, Z.; Kuang, Z.; Li, Z.; Huang, J.; Luo, G. Identification and validation of reference genes for qRT-PCR analysis in mulberry (Morus alba L.). PLoS One 2018, 13, e0194129. [Google Scholar] [CrossRef]
- Gong, P.; Shen, Q.; Zhang, M.; Qiao, R.; Jiang, J.; Su, L.; Zhao, S.; Fu, S.; Ma, Y.; Ge, L.; et al. Plant and animal positive-sense single-stranded RNA viruses encode small proteins important for viral infection in their negative-sense strand. Molecular Plant 2023, 16, 1794–1810. [Google Scholar] [CrossRef] [PubMed]
- Nuzzi, M.; Lo Scalzo, R.; Testoni, A.; Rizzolo, A. Evaluation of Fruit Aroma Quality: Comparison Between Gas Chromatography–Olfactometry (GC–O) and Odour Activity Value (OAV) Aroma Patterns of Strawberries. Food Analytical Methods 2008, 1, 270–282. [Google Scholar] [CrossRef]
- Lu, H.; Zhao, H.; Zhong, T.; Chen, D.; Wu, Y.; Xie, Z. Molecular Regulatory Mechanisms Affecting Fruit Aroma. Foods 2024, 13, 1870. [Google Scholar] [CrossRef] [PubMed]
- Siegmund, B. 7 - Biogenesis of aroma compounds: flavour formation in fruits and vegetables. In Flavour Development, Analysis and Perception in Food and Beverages, Parker, J.K., Elmore, J.S., Methven, L., Eds.; Woodhead Publishing: 2015; pp. 127-149.
- El Hadi, M.A.; Zhang, F.J.; Wu, F.F.; Zhou, C.H.; Tao, J. Advances in fruit aroma volatile research. Molecules (Basel, Switzerland) 2013, 18, 8200–8229. [Google Scholar] [CrossRef] [PubMed]
- Sartori, S.K.; Diaz, M.A.N.; Diaz-Muñoz, G. Lactones: Classification, synthesis, biological activities, and industrial applications. Tetrahedron 2021, 84, 132001. [Google Scholar] [CrossRef]
- Niu, Y.; Zhu, Q.; Xiao, Z. Characterization of perceptual interactions among ester aroma compounds found in Chinese Moutai Baijiu by gas chromatography-olfactometry, odor Intensity, olfactory threshold and odor activity value. Food research international (Ottawa, Ont.) 2020, 131, 108986. [Google Scholar] [CrossRef]
- Liu, J.; Yin, X.; Kou, C.; Thimmappa, R.; Hua, X.; Xue, Z. Classification, biosynthesis, and biological functions of triterpene esters in plants. Plant Communications 2024, 5, 100845. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, L.; Xiao, Z.; Niu, Y. Characterization of the key aroma compounds in mulberry fruits by application of gas chromatography–olfactometry (GC-O), odor activity value (OAV), gas chromatography-mass spectrometry (GC–MS) and flame photometric detection (FPD). Food Chemistry 2018, 245, 775–785. [Google Scholar] [CrossRef]
- Elmacı, Y.; Altuğ, T. Flavour evaluation of three black mulberry (Morus nigra) cultivars using GC/MS, chemical and sensory data. Journal of the Science of Food and Agriculture 2002, 82, 632–635. [Google Scholar] [CrossRef]
- Feng, Y.; Liu, M.; Ouyang, Y.; Zhao, X.; Ju, Y.; Fang, Y. Comparative study of aromatic compounds in fruit wines from raspberry, strawberry, and mulberry in central Shaanxi area. Food & nutrition research 2015, 59, 29290. [Google Scholar] [CrossRef]
- Peng, B.; Yu, M.; Zhang, B.; Xu, J.; Ma, R. Differences in PpAAT1 Activity in High- and Low-Aroma Peach Varieties Affect γ-Decalactone Production. Plant Physiol 2020, 182, 2065–2080. [Google Scholar] [CrossRef]
- Zhang, L.; Li, H.; Gao, L.; Qi, Y.; Fu, W.; Li, X.; Zhou, X.; Gao, Q.; Gao, Z.; Jia, H. Acyl-CoA oxidase 1 is involved in γ-decalactone release from peach (Prunus persica) fruit. Plant Cell Reports 2017, 36, 829–842. [Google Scholar] [CrossRef] [PubMed]
- Wang Juan; Sun Rui; Wang Guixia; Chang Linlin; Sun Jian; Zhong Chuanfei; Dong Jing; Zhang Yuntao; Detlef. ULRICH. Comparative Analysis of Characteristic Aromatic Components of Fruits in Eight Strawberry Varieties/Cultivars. Journal of Fruit Science (Chinese). 2018, 35, 967–976. [CrossRef]
- Waché, Y.; Aguedo, M.; Nicaud, J.M.; Belin, J.M. Catabolism of hydroxyacids and biotechnological production of lactones by Yarrowia lipolytica. Applied microbiology and biotechnology 2003, 61, 393–404. [Google Scholar] [CrossRef] [PubMed]
- El-Sharkawy, I.; Manríquez, D.; Flores, F.B.; Regad, F.; Bouzayen, M.; Latché, A.; Pech, J.C. Functional characterization of a melon alcohol acyl-transferase gene family involved in the biosynthesis of ester volatiles. Identification of the crucial role of a threonine residue for enzyme activity*. Plant molecular biology 2005, 59, 345–362. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Sun, Y.; Li, M.; Zhu, T.; Tu, K. Postharvest hot air and UV-C treatments enhance aroma-related volatiles by simulating the lipoxygenase pathway in peaches during cold storage. Food Chem 2019, 292, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Zhang, Q.; Li, J.; Gong, H.; Fan, X.; Yang, Y.; Liu, X.; Yin, X. Transcriptome co-expression network analysis identifies key genes and regulators of ripening kiwifruit ester biosynthesis. BMC Plant Biol 2020, 20, 103. [Google Scholar] [CrossRef]
- Li, G.; Jia, H.; Li, J.; Li, H.; Teng, Y. Effects of 1-MCP on volatile production and transcription of ester biosynthesis related genes under cold storage in ‘Ruanerli’ pear fruit (Pyrus ussuriensis Maxim.). Postharvest Biology and Technology 2016, 111, 168–174. [Google Scholar] [CrossRef]
- Cao, X.; Wei, C.; Duan, W.; Gao, Y.; Kuang, J.; Liu, M.; Chen, K.; Klee, H.; Zhang, B. Transcriptional and epigenetic analysis reveals that NAC transcription factors regulate fruit flavor ester biosynthesis. The Plant journal: for cell and molecular biology 2021, 106, 785–800. [Google Scholar] [CrossRef]
- Wang, J.; De Luca, V. The biosynthesis and regulation of biosynthesis of Concord grape fruit esters, including 'foxy' methylanthranilate. The Plant journal: for cell and molecular biology 2005, 44, 606–619. [Google Scholar] [CrossRef]
- Günther, C.S.; Chervin, C.; Marsh, K.B.; Newcomb, R.D.; Souleyre, E.J. Characterisation of two alcohol acyltransferases from kiwifruit (Actinidia spp.) reveals distinct substrate preferences. Phytochemistry 2011, 72, 700–710. [Google Scholar] [CrossRef] [PubMed]
- Balbontín, C.; Gaete-Eastman, C.; Fuentes, L.; Figueroa, C.R.; Herrera, R.; Manriquez, D.; Latché, A.; Pech, J.C.; Moya-León, M.A. VpAAT1, a gene encoding an alcohol acyltransferase, is involved in ester biosynthesis during ripening of mountain papaya fruit. J Agric Food Chem 2010, 58, 5114–5121. [Google Scholar] [CrossRef] [PubMed]
- González-Agüero, M.; Troncoso, S.; Gudenschwager, O.; Campos-Vargas, R.; Moya-León, M.A.; Defilippi, B.G. Differential expression levels of aroma-related genes during ripening of apricot (Prunus armeniaca L.). Plant physiology and biochemistry 2009, 47, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Xu, J.; Cai, Z.; Zhang, B.; Yu, M.; Ma, R. Different Roles of the Five Alcohol Acyltransferases in Peach Fruit Aroma Development. Journal of the American Society for Horticultural Science 2020, 145, 374–381. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).