1. Introduction
Biomaterials are used in medical applications to replace or assist in the healing process of soft or hard tissue, either internally or externally within the human body. These materials are designed to be compatible with living tissues, interact with biological systems, and promote biocompatibility, cellular viability, and because of their low rejection rates [
1]. Over the years, numerous studies and investigations have been conducted to evaluate and support the properties and applications of Ti6Al4V as a biomaterial [
2,
3,
4,
5,
6,
7], as it is recognized for its excellent mechanical strength and biocompatibility. However, challenges remain regarding its ability to promote cellular adhesion and integration with biological tissues [
8,
9,
10], as well as long-term health issues caused by vanadium ion release [
11,
12].
Coatings have historically been used to protect and enhance the functionality of materials. With the evolution of technology, new coating techniques have been developed to improve material characteristics, one of which is magnetron-assisted sputtering. This technique is used to deposit thin films of elements or compounds onto substrates of various materials using a magnetic field and plasma discharges [
13]. Surface coatings for medical use can enhance biocompatibility by creating a protective layer that reduces immune response and prevents the release of toxic substances. Additionally, they improve resistance to corrosion, wear, lubrication capacity, and antimicrobial properties [
14].
Carbon-based coatings are an attractive option for enhancing biocompatibility in medical applications due to their low chemical reactivity, high stability, and unique mechanical properties. Various techniques have been used to evaluate the biocompatibility of carbon coatings, including cellular adhesion, cellular viability, and biomineralization [
15], as well as the corrosion behavior using blood donor tests [
16]. While carbon-based coatings offer significant advances in enhancing biocompatibility, another element of interest in biomedical applications is rhenium (Re), which exhibits a wide range of oxidation states and possesses outstanding chemical and mechanical properties, such as high wear resistance and a high valence electron density. It is also used as an alloying element with other metals like tungsten and molybdenum to enhance properties such as hardness, tensile strength, and corrosion resistance [
17]. Despite its potential, analyses on the use of rhenium-carbon composite materials as coatings on common metals used in the biomedical industry have not been conducted.
The aim of this study is to assess the performance of rhenium-carbon coatings on Ti6Al4V substrates, deposited via magnetron sputtering with a molybdenum anchoring layer. Despite the confirmed formation of rhenium carbides and oxides, poor adhesion and significant delamination were observed in SEM images. Corrosion resistance tests indicated an adverse effect on the Ti6Al4V substrate, while biocompatibility assessments showed a reduction in cell viability, particularly with coatings of higher surface roughness. This study underscores the need for improved surface preparation and coating processes to optimize both adhesion and biocompatibility.
In the pages that follow, the production and characterization of rhenium and carbon-based coatings on Ti6Al4V substrates are discussed and the biocompatible properties of the coatings are analyzed with the aim of prevent direct exposure of vanadium to the body and examining its behavior in a biological environment.
2. Materials and Methods
2.1. Coatings’ Deposition
Ti6Al4V alloy pieces produced by additive manufacture via electron beam fusion (EBM) process carried out in vacuum and with the construction chamber maintained at an elevated temperature throughout manufacturing, were used as substrates to coat. Dimensions of the substrates were diameter of 17.7 mm and a thickness of 2.8 mm. The surface of the substrates was prepared using alumina in a cloth to reach a mirror-like polish and 2000 grit SiC paper. The average surface roughness was measured using Mahr brand roughness tester (MarSirf PS1 series) and the values were Ra = 0.035μm and Ra = 0.102 μm respectively (see
Table 1). Then, cleaning was carried out using isopropanol for 10 minutes using a 130 W, 40 kHz Branson 2510 ultrasonic cleaner before the coating process.
Re-C based thin films with a Mo anchor interlayer were deposited using a YKY Three-target magnetron sputtering coating system. For this purpose, 2-inches diameter targets of high purity (99.999%) of Molybdenum (Mo), Rhenium (Re) and Graphite (C) were used. In the deposition process, a base pressure of 1.7×10
-3 Pa and a working pressure of 2.2×10
-2 Pa were maintained. Argon (Ar) was used as carrier gas with a flow rate of 4 sccm, all the tests were carried out at 350°C.
Table 2 presents coating process deposition time and DC power applied for each target.
2.2. Coatings’ Characerization
A Gamry 750 potentiostat/galvanostat was used to carry out the potentiodynamic polarization tests. Hank's solution consisting of a combination of ions, such as sodium chloride (NaCl), dibasic potassium phosphate (K
2HPO
4), potassium chloride (KCl), calcium chloride (CaCl
2) and sodium bicarbonate (NaHCO
3) as electrolyte, which in turn simulates the extracellular fluid conditions inside the human body, was used. In the assay, an Ag/AgCl reference electrode was used, a platinum electrode was used as a counter electrode, and the Ti6Al4V specimen was used as the working electrode. The test was performed with a scan rate of 0.3 mV/s and a voltage range between -150 mV and 550 mV in relation to the open circuit potential (Ecorr) was established. The exposed area of the samples was 0,196 cm
2. To ensure stabilization of the open circuit potential, the solution was allowed to stabilize by maintaining fluid contact with the sample for 1 hour according to the specifications of the ASTM G5 [
18].
Faraday's law was used to calculate the corrosion rate according to equation 1.
where,
CR is corrosion rate in mm/yr,
icorr current corrosion density in μA/cm
2, K
1 = 3.27× 10
-3 in mm g/μA cm yr and density
in g/cm
3.
For surface morphology evaluation, scanning electron microscopy (ZEIZZ EVO MA10 series) using 20.00 kV and at magnifications of 500X and 200X was used. A semiquantitative EDS analysis (OXFORD probe) was also performed using this technique to obtain an elemental chemical overview of the sample.
To determine whether the coatings have potential biocompatible properties, cells of the PK15 line derived from pig kidney were incubated in minimum essential medium (MEM - Gibco CAT 61100061) supplemented with 10% fetal bovine serum (FBS – Gibco CAT 11573397) and 1% antibiotic/antimycotic (Gibco CAT 15240062). The cell culture was carried out in a controlled atmosphere at 5% CO2 and at 37° and approximately 1.500.000 cells were seeded in a 6-well plate. After 24 hours of growth, the cells were exposed to the surface of the coated samples. Cell viability was assessed at 24, 48 and 72 hours post exposure using the 3-(4,5-dimethyl-2-thiazoyl)-2,5-diphenyltetrazolium bromide (MTT) reduction assay. The absorbance was read using a Biotek ELX800 microplate reader. The wavelength used was 570 nm.
In accordance with ISO 10993-5:2009, the reduction in viability relative to the control group was calculated using Equation 2.
Where: OD570e is the mean value of the optical density measured from the samples at 100% of the test sample and OD570b is the mean value of the optical density measured from the blanks at 100% of the test sample. The lower the viability percentage the higher the cytotoxic potential of the specimen tested. A cytotoxic potential exists if the viability is reduced to 70% of the blank.
3. Results
3.1. Surface Characterization
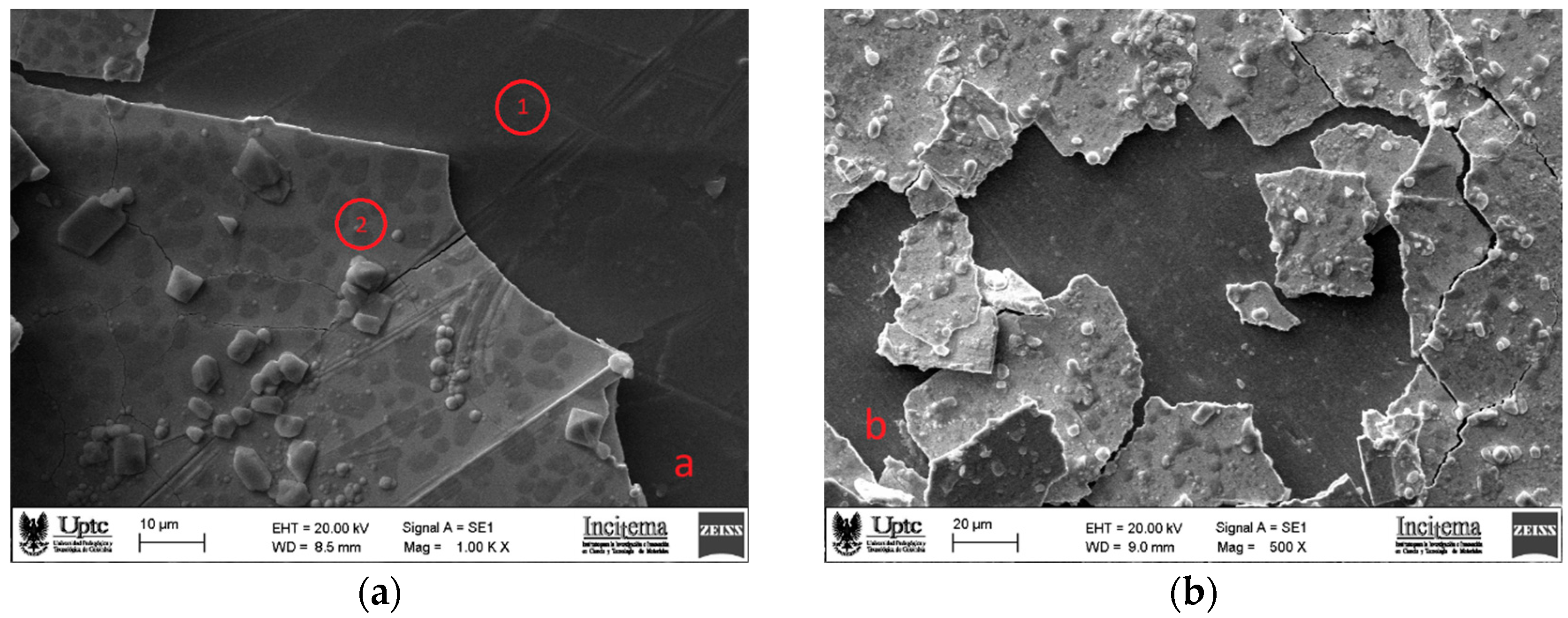
Surface morphology of as-deposited ReC coatings is shown in
Figure 1 for the coatings on samples substrates prepared with mirror-like polish (a) and 2000 grit SiC paper (b). It is clearly observed that delamination occurs in both cases. It is well known that during deposition process, all morphological features of the substrate surface that are formed while its mechanical preparation are transferred onto the coating surface and even, they are magnified mainly because of the shadowing effect that this morphology causes on the coating process. As can be seen on
Figure 1 b (2000 grit SiC paper), the rougher the surface the greater the surface defects on the coating. But not also this is the cause of surface coating defects, it is also known that nodular defects occur in all PVD coatings and are promoted by a seed for example small particle or substrate protrusions which raises the nucleation and formation of inverted cone-like shapes forming dome protrusions out of the coating surface [
18].
Figure 1 presents two points named as 1 and 2 and red circled. These zones represent surface places where EDS analysis was made. Zone 1 was located specifically in the area where the coating peeled off and reveals the presence of titanium, aluminum and vanadium, with weight percentages presented in
Table 3 which are in accordance with the weight of these elements in Ti6Al4V compound of substrate material. Similarly,
Table 4 provides weight percentage values from EDS analysis of zone 2 in
Figure 1, the surface of the coating; these values suggest the ReC compound formation due to its agreement with the weight of the elements in that compound. This distribution of elements suggests that rhenium carbide is homogeneously deposited on the surface, also oxygen presence suggest the formation of rhenium oxides in smaller amounts, due to contact with the environment as soon as it leaves the coating equipment. Molybdenum presence also confirms the formation of the interlayer used to try to enhance the adhesion problem.
3.2. X-ray Diffrraction Analysis
Figure 2 shows the XRD pattern of the as-deposited ReC based coating with Mo interlayer on Ti6Al4V substrate that was previously prepared with 2000 grit SiC paper; it is worth to mention that the XRD patterns of the samples with different surface preparation showed similar peaks pattern. As can be seen, black stars represent sharp peaks associated with substrate material according to titanium reference pattern number ICSD 98-007-6144. Sharp peaks associated with rhenium oxide are marked with blue circles in the figure according to ICSD 98-000-1395 and the presence of this compound is attributed to the reaction of rhenium with oxygen adsorbed on the coating surface after the deposition process. From the figure, it can be seen red triangles attached to peaks showing that crystalline rhenium carbide compound was formed according to ICSD 98-007-7350 reference pattern; however, significant broadening of this XRD peaks is evident this can be attributed to several factors, including small crystallite size or poor crystallinity of the material. By using Scherrer equation, crystallite size of 5,0524 nm was calculated suggesting that crystallites are small, and the rhenium carbide coatings are predominantly nanocrystalline with increased surface area and the presence of more grain boundaries.
EDS analysis confirmed the presence of rhenium, carbon, and molybdenum in the coatings. Additionally, the formation of rhenium carbides and rhenium oxides was detected by XRD analysis, which also identified the presence of elemental rhenium. Despite the successful deposition of the intended elements, the coatings did not adhere well to the substrates, resulting in partial detachment and exposure of the underlying material.
3.3. Corrosion Resistance
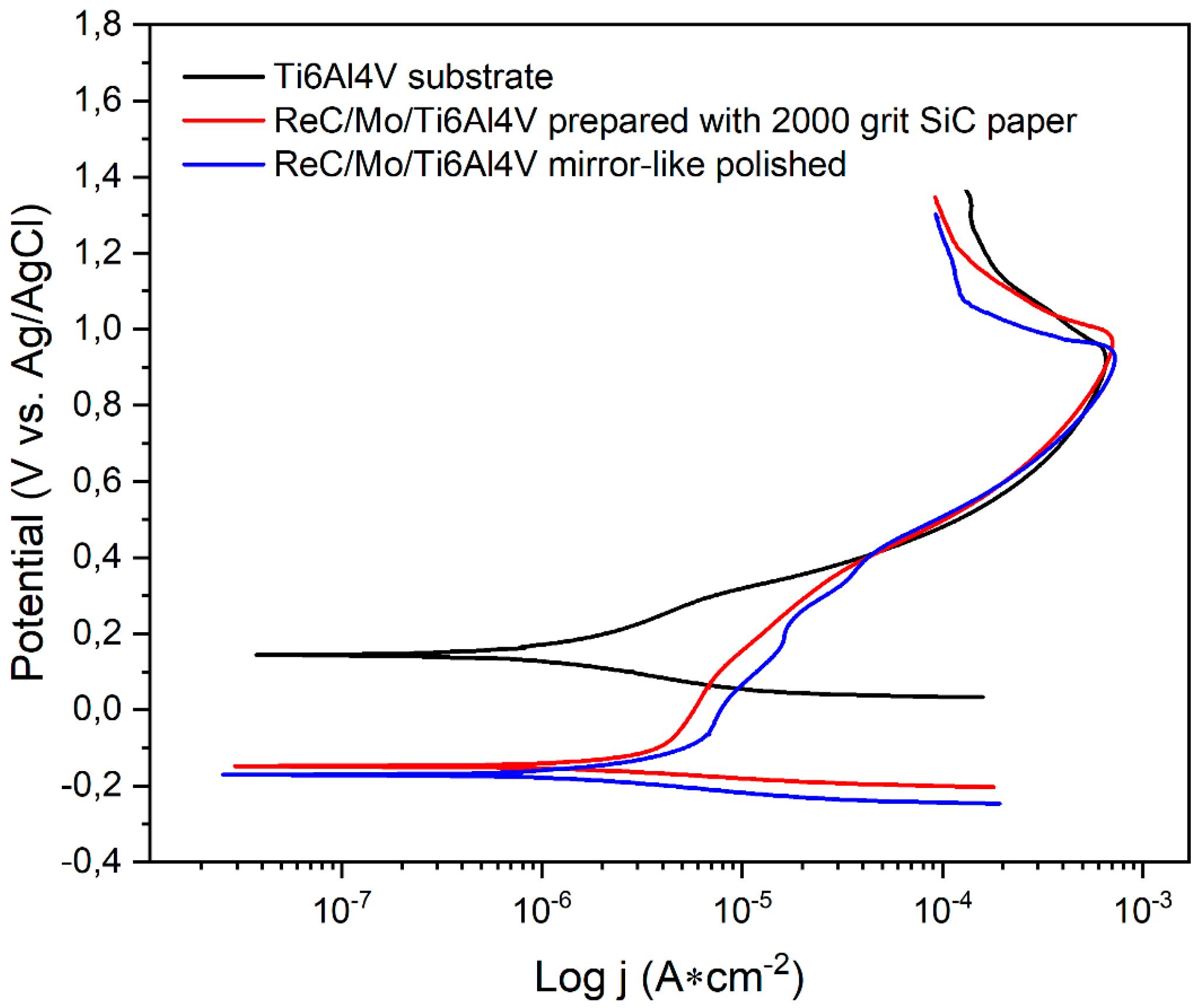
Figure 3 compares potentiodynamic polarization curves experimentally obtained for naked substrate, ReC based coated substrate and mirror-like polished surface, and ReC based coated C2 substrate. The differences between the naked substrate and the two other conditions are highlighted in
Table 5. It can be seen that the substrate without coating presents lower current corrosion density compared to the coated samples, suggesting that coatings do not offer protection against corrosion process. This may be explained due to the low adherence showed by the delamination phenomenon showed in
Figure 1, which can be attributed to the substrate surface preparation. The sample prepared with 2000 grit SiC paper, produced rougher surface (
Table 1) and, in turn, higher defects acting as corrosion promoters not only on the substrate itself, but also on the coating surface. This leads to the electrolyte penetration and posterior delamination. Potentiodynamic polarization tests revealed that the corrosion resistance of the Ti6Al4V substrates was adversely affected by the Re-C coatings, as the coated samples exhibited increased corrosion current densities and more negative corrosion potentials compared to the uncoated substrates. The results indicated that the Re-C coatings did not provide adequate protection against corrosion in Hank's solution, potentially due to the observed delamination and poor adhesion.
From
Figure 3, it is clear that the differences related to the corrosion potential (
Ecorr) are significant. The uncoated substrate exhibited a positive
Ecorr value, indicating noble behavior and low chemical reactivity. In contrast, the coated samples showed negative
Ecorr values and higher corrosion rates compared to the uncoated substrate. Although both coatings displayed passivation areas with higher slopes, suggesting protection against corrosion by a passive layer, this layer degraded after around 400 mV, and the behavior matched that of the substrate due to delamination and surface defects. These findings highlight several challenges associated with the use of Re-C coatings on Ti6Al4V substrates. The poor adhesion and significant delamination observed in the SEM images suggest that the current surface preparation and coating deposition processes are inadequate for achieving durable coatings. The negative impact of the coatings on corrosion resistance further underscores the need for improved adhesion and uniform coverage to protect the underlying substrate.
3.4. Biocompatibility
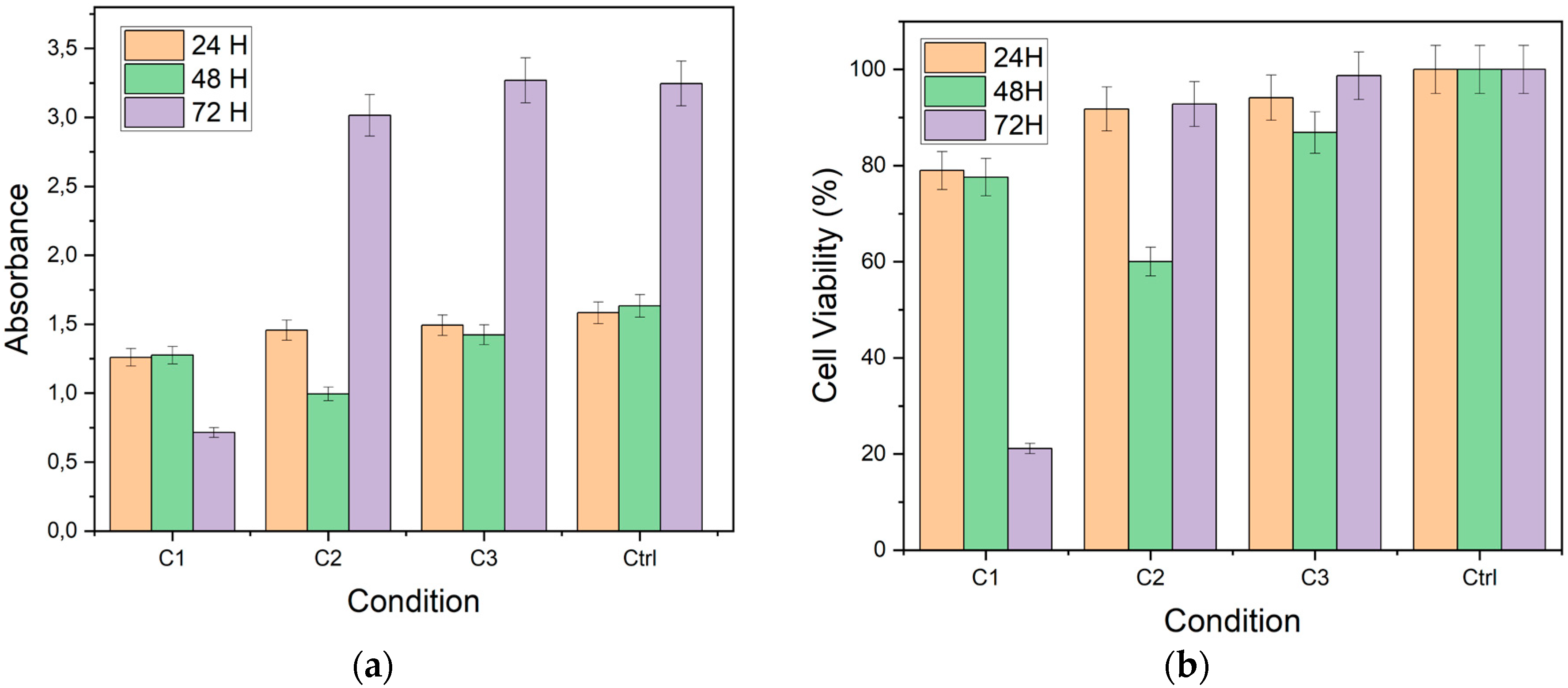
Figure 4 presents cell viability (a) and absorbance (b) values obtained by the MTT assay on the coated pieces of the study. Absorbance of cells exposed to C1 and C2 decreases at all exposure times compared to cells exposed to C3 (uncoated Ti6Al4V substrate) and Ctrl (control group with PK15 cells). Cell viability was calculated by considering the absorbance of the control group compared to that obtained for each condition. It is observed that cell viability remains above 90% for cells exposed to the uncoated substrates (C3) at the times evaluated. However, cell viability decreased in cells exposed to the other conditions (C1 and C2).
The biocompatibility assessments revealed that the coatings hindered cell viability and metabolic activity, particularly on rougher surfaces. These results emphasize the importance of surface roughness in influencing cellular responses and suggest that smoother coatings may be more favorable for biocompatibility [
19,
20,
21]. However, the observed cytotoxicity indicates that further optimization of the coating process is necessary to enhance biocompatibility and ensure safe application in biomedical contexts.
The MTT assay results showed that cell viability decreased with increasing surface roughness of the coatings. Coatings deposited on the C1 substrates (higher roughness) exhibited lower cell viability compared to those on the C2 substrates (lower roughness). This trend suggests that surface roughness plays a critical role in cellular responses, with rougher surfaces potentially hindering cell adhesion and proliferation.
The cytotoxic potential of the coatings was evaluated by comparing the cell viability to the control group. The results indicated that the coatings reduced cell viability to less than 70% of the control, classifying them as cytotoxic according to ISO 10993-5:2009 standards. The observed cytotoxicity may be attributed to the poor adhesion and delamination of the coatings, which could expose the cells to toxic elements or compounds from the coating-substrate interface.
The results obtained in this study demonstrate that rhenium and carbon coatings significantly impact the cell viability and metabolic activity of PK15 cells, as evaluated by the MTT assay. The surface roughness of the coatings emerged as a crucial factor influencing cellular response. Firstly, conditions with higher roughness (C1) showed a notable decrease in cell viability and absorbance over time. At 24 hours, cell viability in this condition was high, close to 90%. However, a significant decline was observed at 72 hours, with viability dropping below 20%. This progressive decrease suggests that rough surfaces may induce additional mechanical stress or release toxic particles, negatively affecting the cells. Moreover, absorbance reflected this trend, with a considerable reduction at 72 hours, indicating decreased cellular metabolic activity.
In contrast, condition C2 showed relatively higher viability and absorbance compared to C1. Cell viability remained around 60% at 72 hours, suggesting that a smoother surface may mitigate adverse effects on the cells. Absorbance was also consistently higher in this condition, indicating lower cytotoxicity and better compatibility with PK15 cells. These findings highlight the importance of surface roughness in the biocompatibility of rhenium and carbon coatings.
Condition C3 (uncoated substrate) and the control group of cells (Ctrl) provided essential comparative environments. In both conditions, cell viability and absorbance remained high and stable across all evaluated times. Cell viability in C3 was above 90%, and absorbance remained high, similar to the observations in the control. These results indicate that the absence of coating does not negatively affect cell viability, reinforcing the notion that surface roughness and treatment are determinant factors in cellular response.
In terms of implications, these findings are crucial for the development of biomedical coatings, especially in applications where cell-material interaction is critical. The evidence suggests that coatings with lower roughness may offer better biocompatibility, reducing cytotoxicity and improving cell viability. This study provides a solid foundation for optimizing rhenium and carbon coatings, guiding future efforts towards developing smoother and more biocompatible surfaces.
Future research should focus on understanding the specific mechanisms of cytotoxicity associated with the roughness of the coatings. Additional studies evaluating the release of ions or particles from rough coatings can provide deeper insights into the factors affecting cell viability. Moreover, expanding the evaluation to different cell types and biological contexts will help validate these findings and develop more versatile and safer coatings for various clinical applications.
4. Conclusions
The coatings produced through magnetron sputtering with a molybdenum anchoring layer exhibited very low adhesion, as evidenced by significant delamination observed in the SEM analysis. However, EDS and XRD results confirmed the formation of rhenium carbides, elemental rhenium, and rhenium oxides, the latter likely formed due to exposure to the environment upon exiting the coating equipment. The XRD analysis reveals that the synthesized rhenium carbide is nanocrystalline, with crystallite sizes ranging from 5.05 nm to 6.19 nm. The broadening of XRD peaks corresponds to these small crystallite sizes and suggests the presence of lattice strain and defects.
Corrosion resistance tests indicate that the coatings decrease the corrosion resistance of the Ti6Al4V substrate. This is attributed to the detachment of the coating in the fluid, which increases the dissolution of elements that can accelerate the corrosion rate. The potentiodynamic polarization results showed higher corrosion current densities and more negative corrosion potentials for the coated samples compared to the uncoated substrate, suggesting that the coatings did not provide effective corrosion protection.
Biocompatibility tests using PK15 cells demonstrated that the coatings significantly impacted cell viability and metabolic activity. The surface roughness of the coatings was a critical factor influencing the cellular response. Coatings with higher roughness showed a significant decrease in cell viability over time, while smoother coatings exhibited relatively better biocompatibility but still lower than the uncoated substrate.
These findings underscore the importance of optimizing surface preparation and coating processes to improve the adhesion and biocompatibility of rhenium-carbon coatings on Ti6Al4V substrates. Future research should aim to enhance coating adhesion and further investigate the mechanisms of cytotoxicity associated with surface roughness. Additionally, expanding biocompatibility evaluations to encompass various cell types and biological environments will contribute to the development of safer and more effective biomedical coatings.
Author Contributions
Conceptualization, G. Orozco, W. Aperador and Y. Pineda.; methodology, G. Orozco.; validation, G. Orozco., Y. Pineda, A. Corredor. and W. Aperador.; formal analysis, G. Orozco, S. Mosquera, J. Monroy and A. Corredor; investigation, G. Orozco, S. Mosquera and J. Monroy; resources, W. Aperador and Y. Pineda.; data curation, G. Orozco.; writing—original draft preparation, G. Orozco and S. Mosquera; writing—review and editing, G. Orozco, W. Aperador, A. Corredor and Y. Pineda; visualization, G. Orozco.; supervision, Y. Pineda.; project administration, Y. Pineda.; funding acquisition, W. Aperador and Y. Pineda. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by MINCIENCIAS, under the convocatoria fortalecimiento de vocaciones y formación en CTeI para la reactivación económica en el arco de la postpandemia 2020 and under the contract number 80740-575-2021. The APC was funded by Universidad Pedagógica y Tecnológica de Colombia and Universidad ECCI”.
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Basu, B.; Katti, D.S.; Kumar, A. Advanced Biomaterials: fundamentals, processing and applications; John Wiley & Sons, Publisher location, country, 2010; pp. 768.
- Caballero Sarmiento, J.; Correa Muñoz, E.; Estupiñan Duran, H. Análisis de la biocompatibilidad de Ti6Al4V y acero inoxidable 316LVM basado en efectos de pH, aplicando criterios de la norma ASTMF2129. Ingeniare Revista Chilena de Ingeniería 2017, 25(1), 95-105. [CrossRef]
- Bordi, K.; Jouzeau, J.Y.; Mainard, D.; Payan, E.; Netter, P.; Rie, K.T.; Stucky, T.; Hage-Ali, M. Cytocompatibility of Ti-6Al-4V and Ti-5Al-2.5 Fe alloys according to three surface treatments, using human fibroblasts and osteoblasts. Biomaterials 1996, 17(9), 929-940. [CrossRef]
- Guden, M.; Celik, E.; Akar, E.; Cetiner, S. Compression testing of a sintered Ti6Al4V powder compact for biomedical applications. Materials Characterization 2005, 54(4-5), 399-408. [CrossRef]
- Niinomi, M. Mechanical biocompatibilities of titanium alloys for biomedical applications. Journal of the mechanical behavior of biomedical materials 2008, 1(1), 30-42. [CrossRef]
- Zhou, Y.L.; Niinomi, M.; Akahori, T.; Fukui, H.; Toda, H. Corrosion resistance and biocompatibility of Ti-Ta alloys for biomedical applications. Materials Science & Engineering A 2005, 398, 28-36. [CrossRef]
- Fasasi, A.Y.; Mwenifumbo, S.; Rahbar, N.; Chen, J.; Li, M.; Beye, A.C.; Arnold, C.B.; Soboyejo, W.O. Nano-second UV laser processed micro-grooves on Ti6Al4V for biomedical applications. Materials Science and Engineering C 2009, 29, 5-13. [CrossRef]
- Disegi, J.A. Titanium alloys for fracture fixation implants. Injury 2000, 31(4), D14-D17. [CrossRef]
- Gaona Latorre, M. Recubrimientos biocompatibles obtenidos por proyección térmica y estudio in vitro de la función osteoblástica. PhD thesis, Universitat de Barcelona, Barcelona – España, June 2007. http://hdl.handle.net/2445/36451.
- Fernández, J.; Gilemany, J.M.; Gaona, M. La proyección térmica en la obtención de recubrimientos biocompatibles ventajas de la proyección térmica por alta velocidad (HVOF) sobre la proyección térmica por plasma atmosférico (APS). Biomecánica 2005, 13(1), 16-39. [CrossRef]
- Costa, B.C.; Tokuhara, C.K.; Rocha, L.A.; Oliveira, R.C.; Lisboa-Filho, P.N.; Pessoa, J.C. Vanadium ionic species forom degradation of Ti-6Al-4V metallic implants: In vitro cytotoxicity and speciation evaluation. Mater. Sci. Eng. C 2019, 96, 730-739. [CrossRef]
- Catalani, S.; Stea, S.; Beraudi, A.; Gilberti, M.E.; Bordini, B.; Toni, A.; Apostoli, P. Vanadium reléase in whole blood, serum and urine of patients implanted with a titanium alloy hip prosthesis. Clinical Toxicology 2013, 51(7), 550-556. [CrossRef]
- Frías, A. Y. A.; Rodil-Posada, S. E.; Depablos-Rivera, O.; Navarro, C. H.; Gómez, K. A. C.; Bustos, E. G. Caracterización de recubrimientos de TiN mediante pulverización catódica con magnetrón (characterization of TiN coatings by magnetron sputtering). Pistas Educativas 2020, 42(136), 206-214.
- Garzón, A.; Aguirre, N.; Olaya J. Estado del arte en biocompatibilidad de recubrimientos (biocompatibility of coatings: survey paper). Visión Electrónica 2013, 7(1), 160-177.
- Valencia, C.R. Recubrimientos biomiméticos y bioactivos. Procesamiento y esudio de su biocompatibilidad in vitro. PhD thesis, Universidade de Vigo, España, 2016.
- Fernandez, P.C. Producción de material híbrido de óxidos de renio, cobre y nanotubos de carbono para la electrólisis del agua. Undergraduate thesis, Universidad de Chile, Chile, 2017.
- ASTM G5-14. Standard reference test method for making potentiostatic and potentiodynamic anodic polarization measurements. 2014, ASTM International, West Conshohocken, PA.
- Panjan, P.; Drnovsek, A.; Gselman, P.; Cekada, M.; Panjan, M. Review of growth defects in thin films prepared by PVD techniques Coatings 2020, 10(5), 447. [CrossRef]
- Gittens, R. A.; McLachlan, T.; Olivares-Navarrete, R.; Cai, Y.; Berner, S.; Tannenbaum, R.; Boyan, B. D. The effects of combined micron-/submicron-scale surface roughness and nanoscale features on cell proliferation and differentiation. Biomaterials 2011, 32(13), 3395-3403. [CrossRef]
- Majhy, B.; Priyadarshini, P.; Sen, A. K. Effect of surface energy and roughness on cell adhesion and growth–facile surface modification for enhanced cell culture. RSC Advances 2021, 11(25), 15467-15476. [CrossRef]
- Schwartz, Z.; Martin, J. Y.; Dean, D. D.; Simpson, J.; Cochran, D. L.; Boyan, B. D. Effect of titanium surface roughness on chondrocyte proliferation, matrix production, and differentiation depends on the state of cell maturation. Journal of Biomedical Materials Research: An Official Journal of The Society for Biomaterials and The Japanese Society for Biomaterials 1996, 30(2), 145-155. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).