Submitted:
15 August 2024
Posted:
16 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
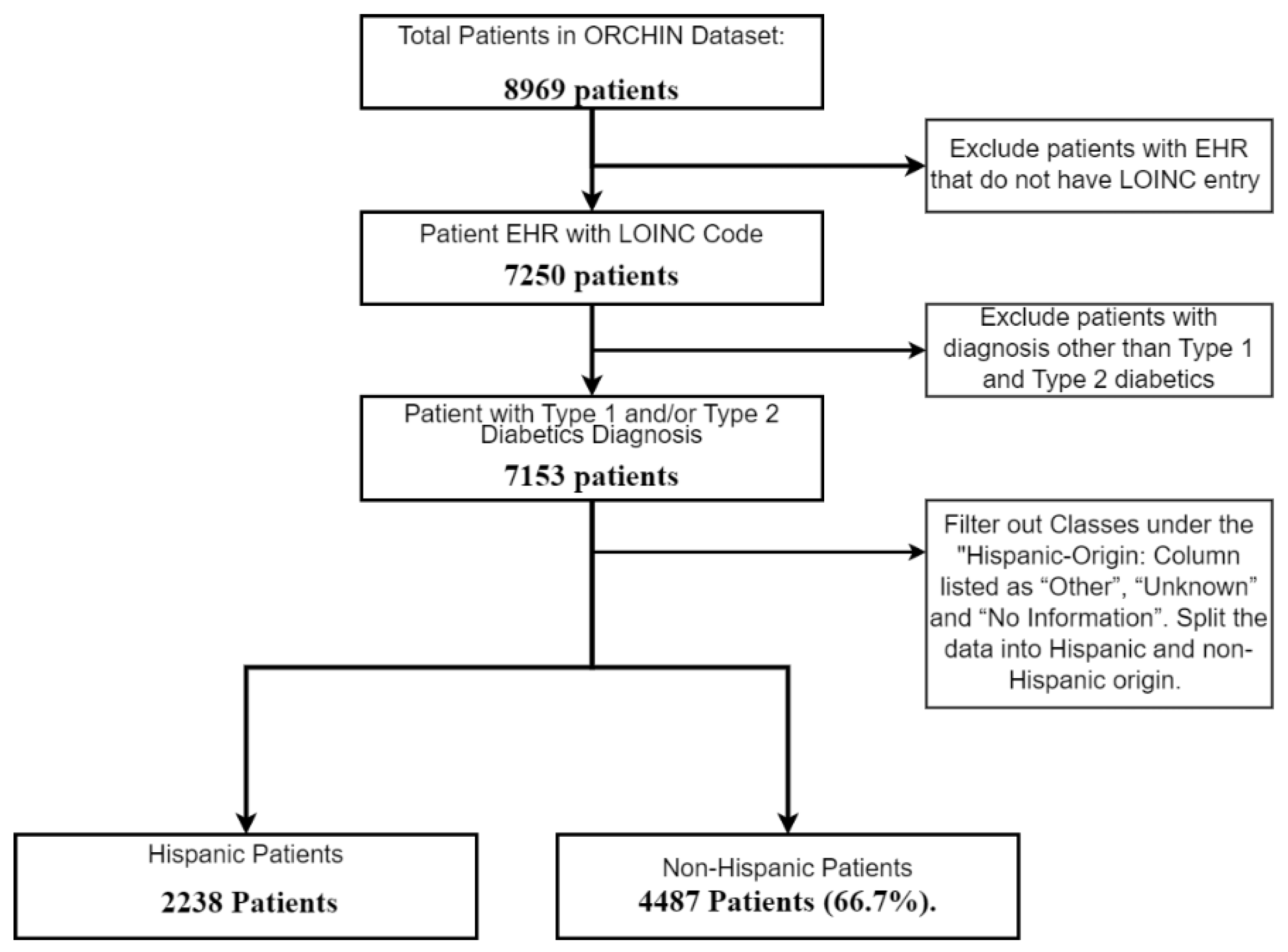
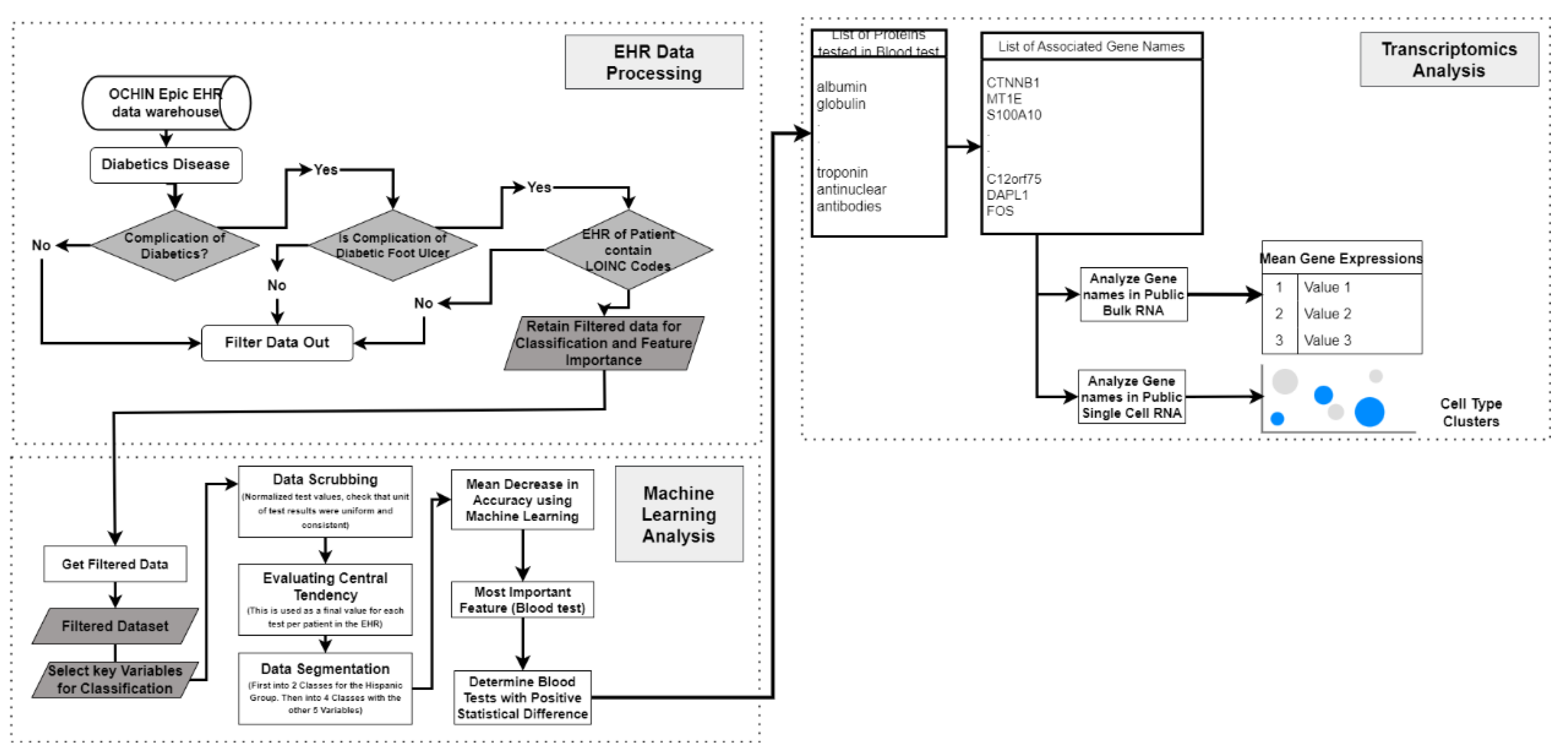
2.1. Analysis Using Electronic Health Record
2.1.1. Analysis Using Electronic Health Record
- (1)
- Data Preprocessing and Interpretation from EHR
- (2)
- Data Preprocessing and Interpretation from EHR
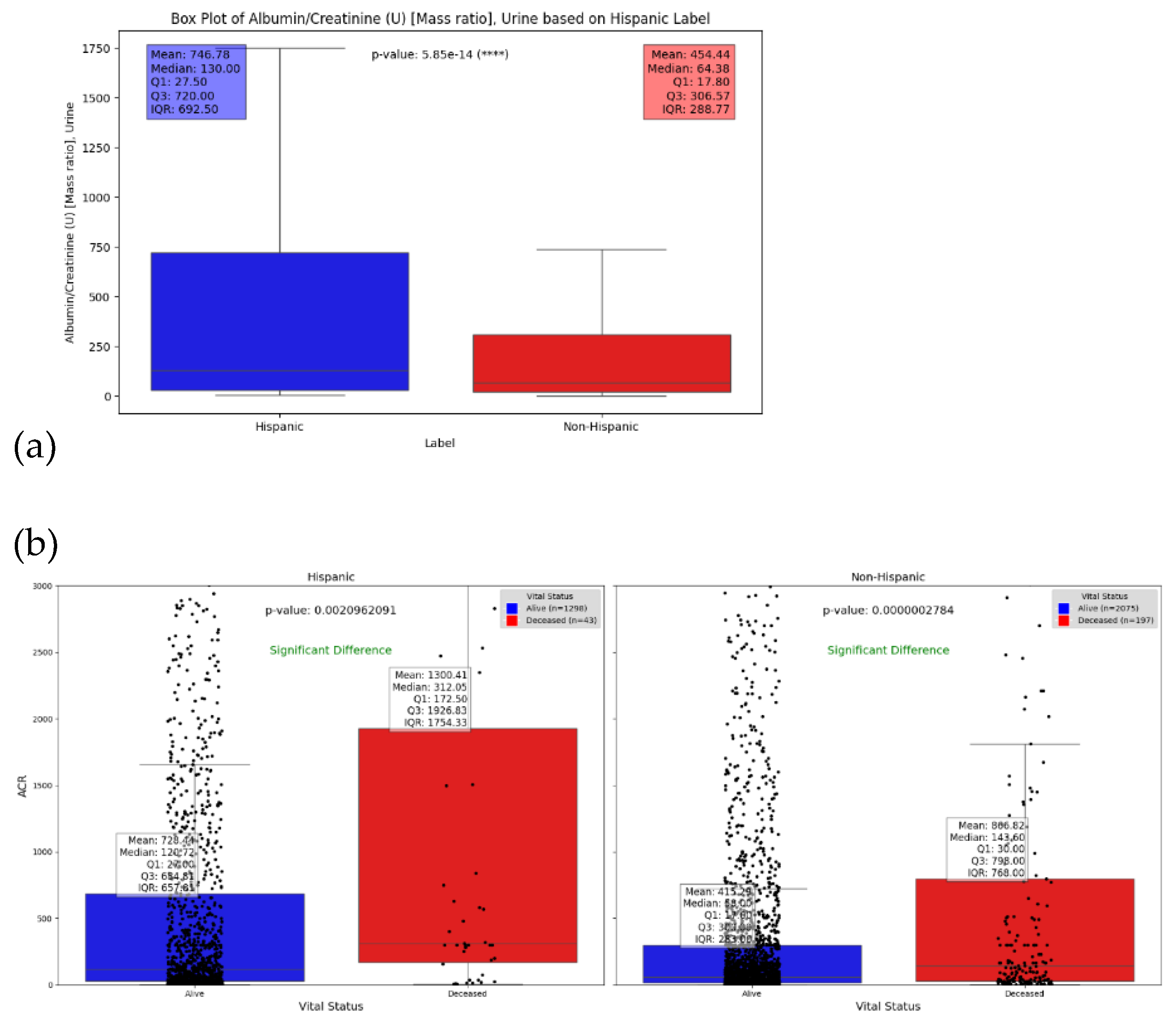
- (3)
- Albumin-Creatinine Ratio Test as Basis for Assessing DFU Severity in Hispanics
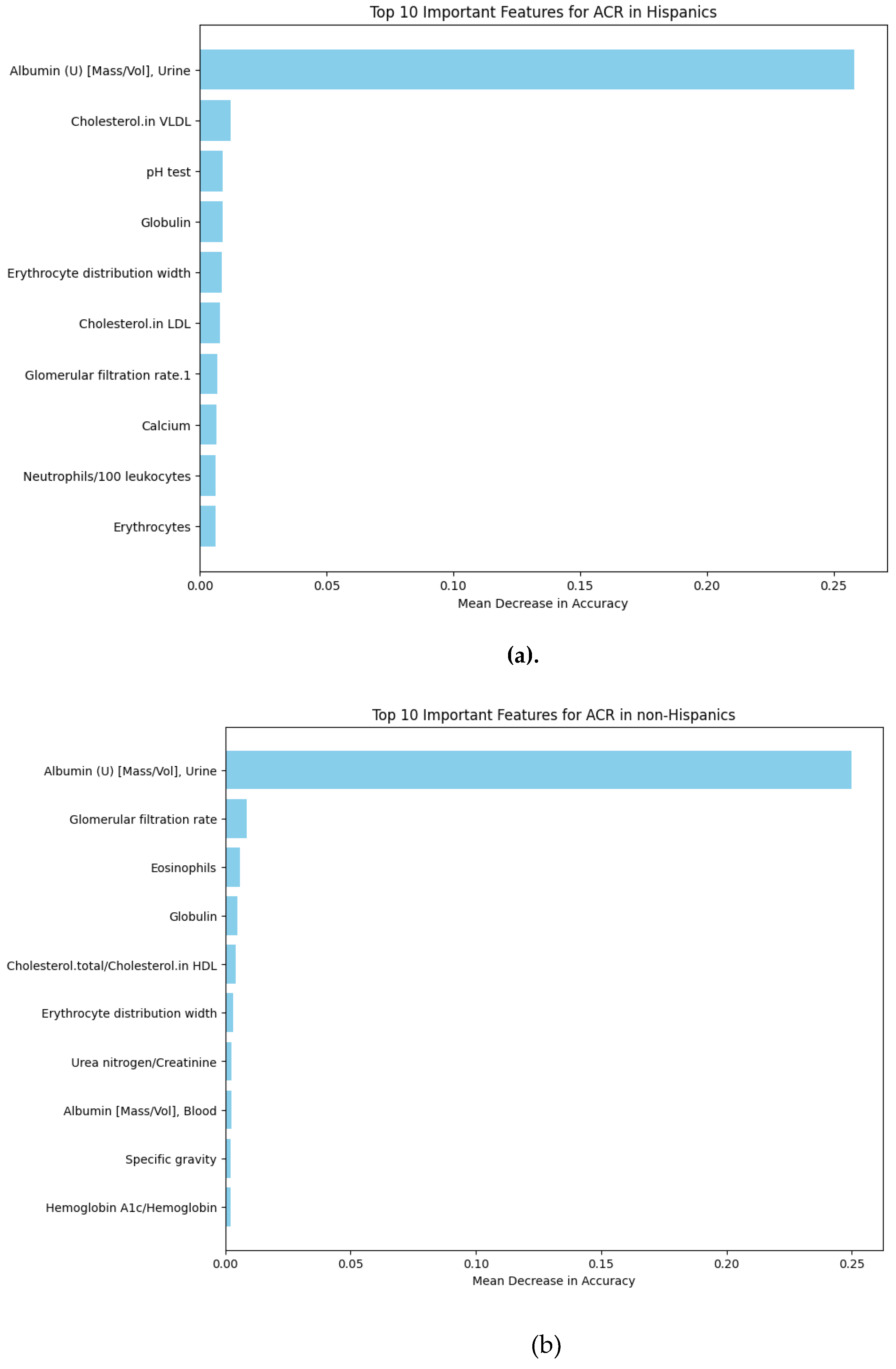
1.1.2. Analysis Using Bulk RNA Dataset
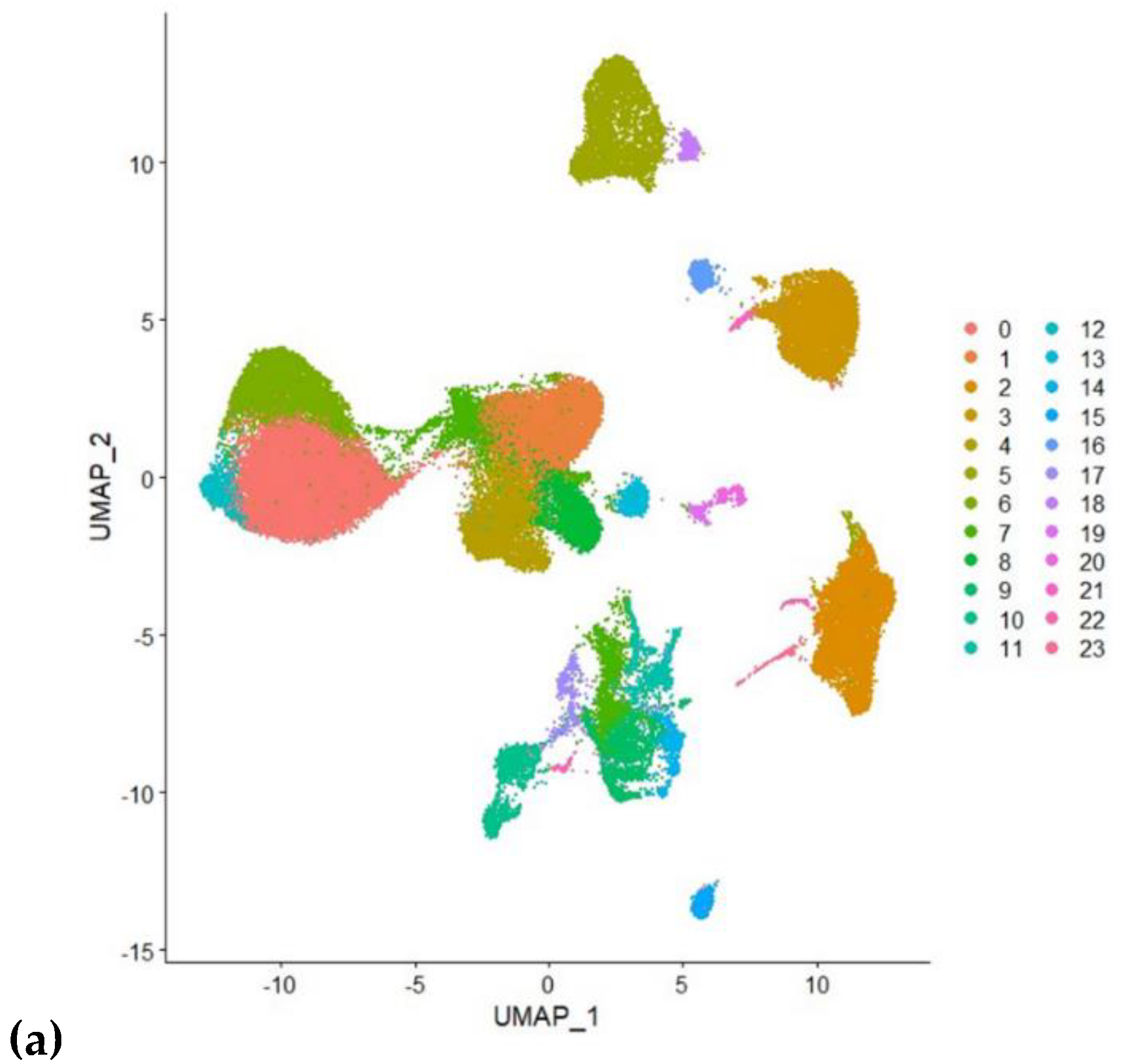
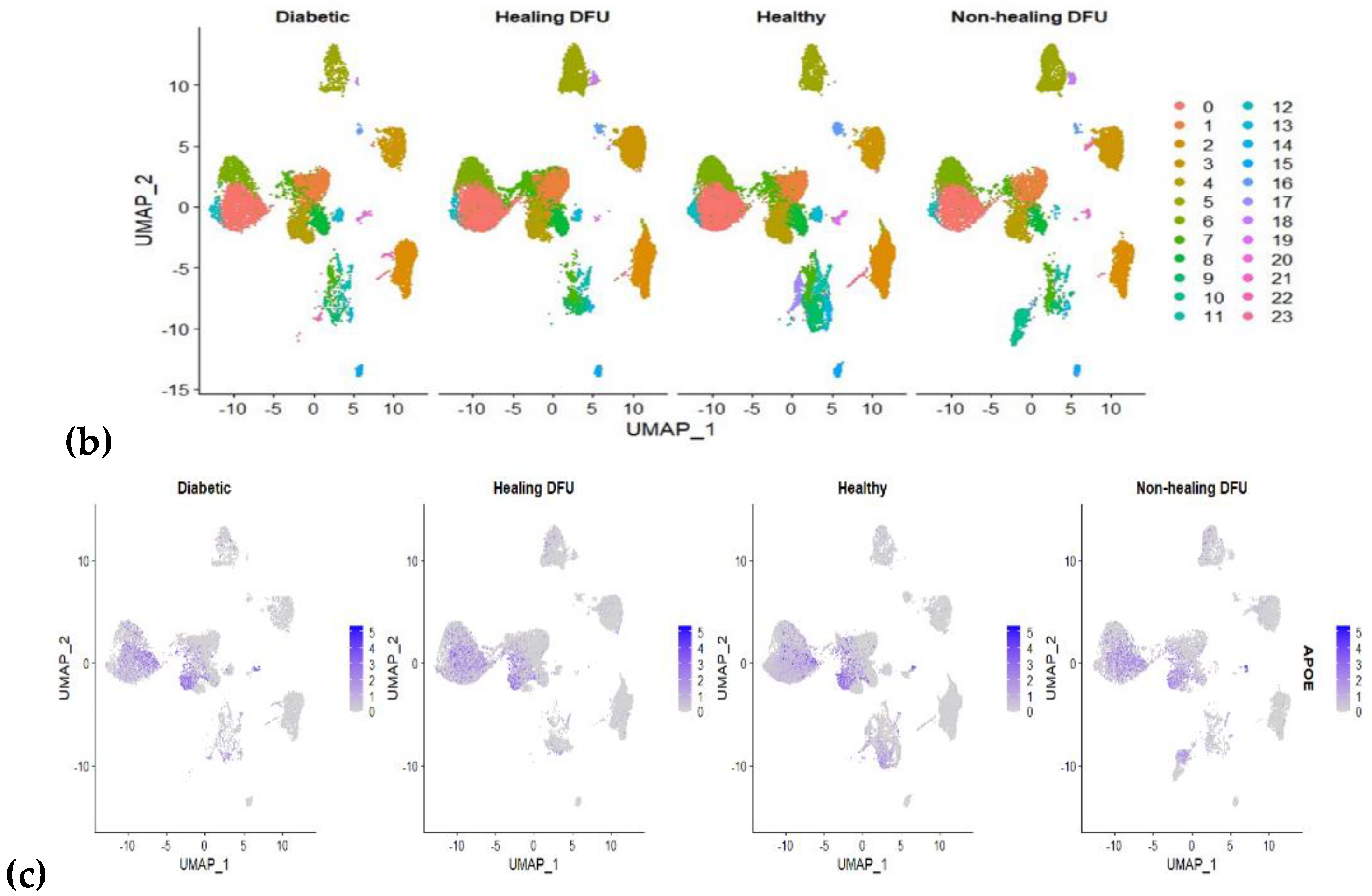
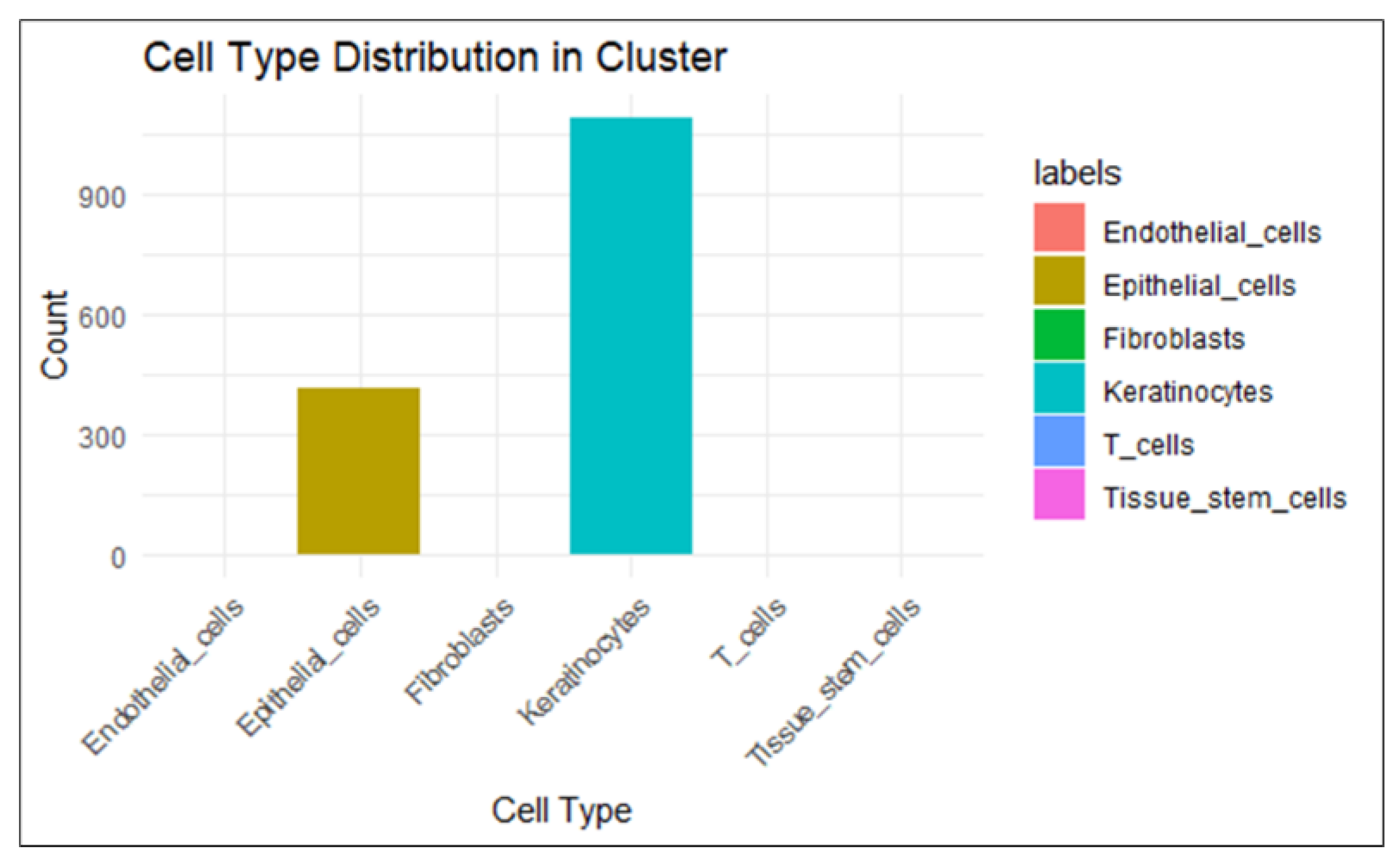
2.1.3. Analysis Using Single Cell RNA Sequenced Dataset
3. Discussion
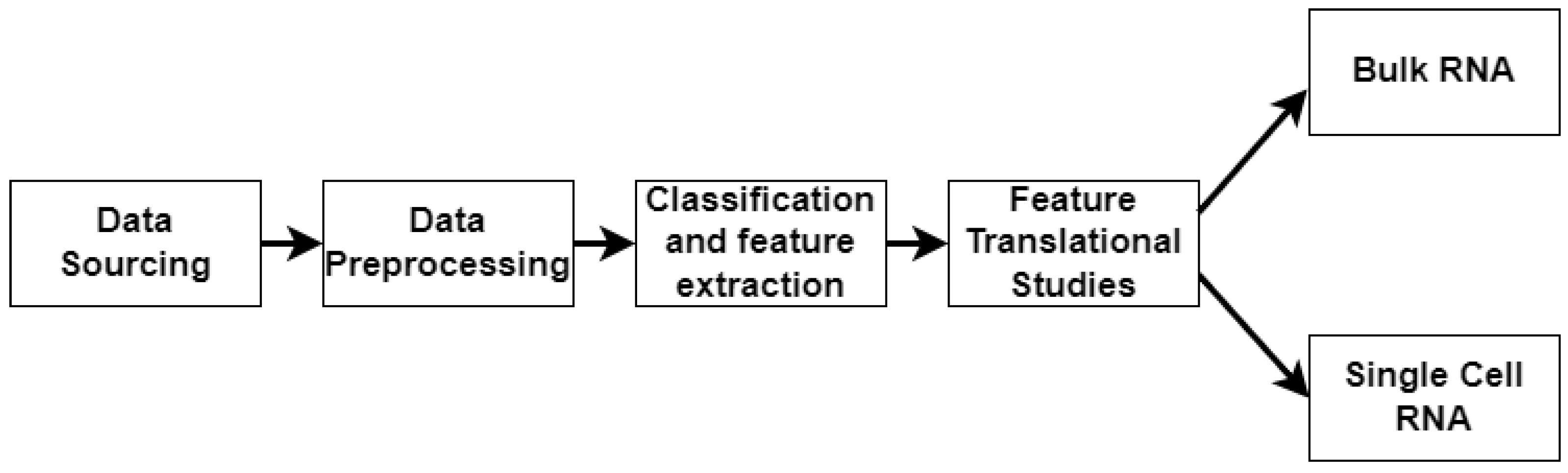
4. Materials and Methods
4.2. Data Preprocessing
4.3. Machine Learning
4.4. Transcriptomics
References
- Lavery, L.A., et al. , Diabetic foot syndrome: evaluating the prevalence and incidence of foot pathology in Mexican Americans and non-Hispanic whites from a diabetes disease management cohort. Diabetes Care, 2003, 26, 1435–8. [Google Scholar] [CrossRef] [PubMed]
- Clayton, E.O. , et al., Racial and Ethnic Disparities in the Management of Diabetic Feet. Curr Rev Musculoskelet Med, 2023, 16, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Oliver, T.I. and M. Mutluoglu, StatPearls. Diabetic Foot Ulcer. 2023, Treasure Island (FL): StatPearls Publishing.
- Wagner, F.W., Jr. , The dysvascular foot: a system for diagnosis and treatment. Foot Ankle, 1981, 2, 64–122. [Google Scholar] [CrossRef] [PubMed]
- Arruda-Olson, A.M. , et al., Leveraging the Electronic Health Record to Create an Automated Real-Time Prognostic Tool for Peripheral Arterial Disease. Journal of the American Heart Association, 2018, 7, e009680. [Google Scholar] [CrossRef] [PubMed]
- Wang, R. , et al., Derivation and validation of essential predictors and risk index for early detection of diabetic retinopathy using electronic health records. Journal of Clinical Medicine, 2021, 10, 1473. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y. , et al., Single-cell RNA sequencing integrated with bulk RNA sequencing analysis identifies a tumor immune microenvironment-related lncRNA signature in lung adenocarcinoma. BMC Biology, 2024, 22, 69. [Google Scholar] [CrossRef] [PubMed]
- Wang, X. , et al., Integration of scRNA-seq and bulk RNA-seq constructs a stemness-related signature for predicting prognosis and immunotherapy responses in hepatocellular carcinoma. Journal of Cancer Research and Clinical Oncology, 2023, 149, 13823–13839. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, J.-B. and A. Sultan, Narrative Review of the Relationship Between CKD and Diabetic Foot Ulcer. Kidney International Reports, 2022, 7, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Chen, R. Deng, and L. Zou, Analysis of Bulk Transcriptome Sequencing Data and in vitro Experiments Reveal SIN3A as a Potential Target for Diabetic Foot Ulcer. Diabetes Metab Syndr Obes, 2023, 16, 4119–4132. [Google Scholar] [CrossRef] [PubMed]
- He, X. , et al., Association of remnant cholesterol with decreased kidney function or albuminuria: a population-based study in the U.S. Lipids in Health and Disease, 2024, 23, 2. [Google Scholar] [CrossRef] [PubMed]
- Theocharidis, G. , et al., Single cell transcriptomic landscape of diabetic foot ulcers. Nature Communications, 2022, 13, 181. [Google Scholar] [CrossRef]
- Aran, D. , et al., Reference-based analysis of lung single-cell sequencing reveals a transitional profibrotic macrophage. Nature Immunology, 2019, 20, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Infusino, I. and M. Panteghini, Serum albumin: Accuracy and clinical use. Clinica Chimica Acta 2013, 419, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Shi, L. , et al., A potent weighted risk model for evaluating the occurrence and severity of diabetic foot ulcers. Diabetol Metab Syndr, 2021, 13, 92. [Google Scholar] [CrossRef] [PubMed]
- Jain, A., R. Jain, and S. Jain, Determination of Blood Creatinine, in Basic Techniques in Biochemistry, Microbiology and Molecular Biology: Principles and Techniques. 2020, Springer US: New York, NY. 201-203.
- Wang, Y.-X. , et al., Elevated triglycerides rather than other lipid parameters are associated with increased urinary albumin to creatinine ratio in the general population of China: a report from the REACTION study. Cardiovascular Diabetology, 2019, 18, 57. [Google Scholar] [CrossRef] [PubMed]
- Xue, J. , et al., Triglycerides to high-density lipoprotein cholesterol ratio is superior to triglycerides and other lipid ratios as an indicator of increased urinary albumin-to-creatinine ratio in the general population of China: a cross-fsectional study. Lipids in Health and Disease, 2021, 20, 13. [Google Scholar] [CrossRef] [PubMed]
- Mehmet, E. , et al., The relationship of the apolipoprotein E gene polymorphism in Turkish Type 2 Diabetic Patients with and without diabetic foot ulcers. Diabetes & Metabolic Syndrome: Clinical Research & Reviews, 2016, 10 (Supplement 1), S30–S33. [Google Scholar]
- OCHIN. [cited 2024 2/1/2024]; Available from: https://ochin.org/research/.
- Chamine, I. , et al., Acute and Chronic Diabetes-Related Complications Among Patients With Diabetes Receiving Care in Community Health Centers. Diabetes Care, 2022, 45, e141–e143. [Google Scholar] [CrossRef] [PubMed]
- Gemelas, J. , et al., Changes in diabetes prescription patterns following Affordable Care Act Medicaid expansion. BMJ Open Diabetes Research and Care, 2021, 9 (Suppl 1), e002135. [Google Scholar] [CrossRef] [PubMed]
- Li, J. , et al., Why is there variation in test ordering practices for patients presenting to the emergency department with undifferentiated chest pain? A qualitative study. Emergency Medicine Journal, 2021, 38, 820. [Google Scholar] [CrossRef]
- Breiman, L. , Random Forests. Machine Learning, 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Forrey, A.W. , et al., Logical observation identifier names and codes (LOINC) database: a public use set of codes and names for electronic reporting of clinical laboratory test results. Clin Chem, 1996, 42, 81–90. [Google Scholar] [CrossRef] [PubMed]
| Feature |
Normal Values |
Mean (Hispanic) | Mean (non-Hispanic) |
Median (Hispanic) | Median (non-Hispanic) | P-value (Mann Whitney U test) |
|---|---|---|---|---|---|---|
| Alkaline phosphatase | 44-147 IU/L | 107.74 | 99.64 | 96.5 | 90.5 | 1.26e-14 |
| Albumin/Creatinine (U) [Mass ratio], Urine | 0-30 mg/g | 746.78 | 454.4 | 130 | 64.375 | 5.85e-14 |
| Urea nitrogen/Creatinine | 10-20 | 20.76 | 18.88 | 20 | 18 | 2.7e-18 |
| Coagulation tissue factor-induced | 0.8-1.1 | 2 | 2.99 | 1.2 | 2.65 | 0.000986 |
| Albumin/Globulin, [ratio] Blood | 1-2 | 1.34 | 1.38 | 1.35 | 1.375 | 0.00022 |
| Monocytes | 0.02-0.08 | 0.56 | 0.62 | 0.52 | 0.5845 | 2e-19 |
| Glomerular filtration rate | 90- 120 | 86.71 | 81.18 | 91 | 82.5 | 9.9e-13 |
| Erythrocyte mean corpuscular hemoglobin concentration | 32-36 | 32.99 | 32.79 | 33.15 | 32.9 | 2.57e-9 |
| Erythrocytes | 4.2-6.1 | 4.364 | 4.48 | 4.375 | 4.5 | 1.16e-11 |
| Platelet mean volume | 8-12 | 10.48 | 10.32 | 10.4 | 10.3 | 0.000266 |
| Lymphocytes/100 leukocytes | 20%-40% | 26.80 | 26.37 | 26.6 | 25.875 | 0.00306 |
| Neutrophils | 2500-7000 | 3294.91 | 2339.42 | 3893.5 | 52 | 0.000549 |
| Associated Gene Names | Control | Healer | Non-Healer | Kruskal-Wallis Test - Statistic | P-value by Mann Whitney U Test (P>= 0.01) | |
|---|---|---|---|---|---|---|
| Albumin | ALB | 0.205592661 | 0.008977797 | 0.029324667 | 8.92857 | 0.0115 |
| Creatinine | CKM | 0.499565985 | 0.173955302 | 0.207658008 | 3.56275 | 0.168406596 |
| SLC22A12 | 0.006241889 | 0 | 0.077868848 | 3.1312025 | 0.208962 | |
| SLC22A2 | 0.010733984 | 0.004172606 | 0.006355688 | |||
| GATM | 55.79607655 | 8.327619645 | 9.674862405 | 9.7249536 | 0.0077313 | |
| CKB | 93.50450349 | 52.65157717 | 54.05675365 | 3.030303 | 0.2197748 | |
| CKMT1A | 46.5820514 | 50.68992477 | 43.91039501 | 0.8126159 | 0.6661049 | |
| CKMT1B | 36.42784045 | 37.23741686 | 36.16401885 | 0.3178726 | 0.853050 | |
| CKMT2 | 4.13219376 | 0.822784598 | 0.728522893 | 11.32522 | 0.0034734 | |
| Cholesterol | LDLR | 70.72075147 | 92.85914778 | 118.6011276 | 3.725572 | 0.155239 |
| PCSK9 | 5.25025018 | 2.867216518 | 3.207883812 | 5.454545 | 0.0653974 | |
| APOE | 117.7178759 | 27.56887668 | 15.82637224 | 13.18058 | 0.0013736 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





