Submitted:
16 August 2024
Posted:
19 August 2024
You are already at the latest version
Abstract
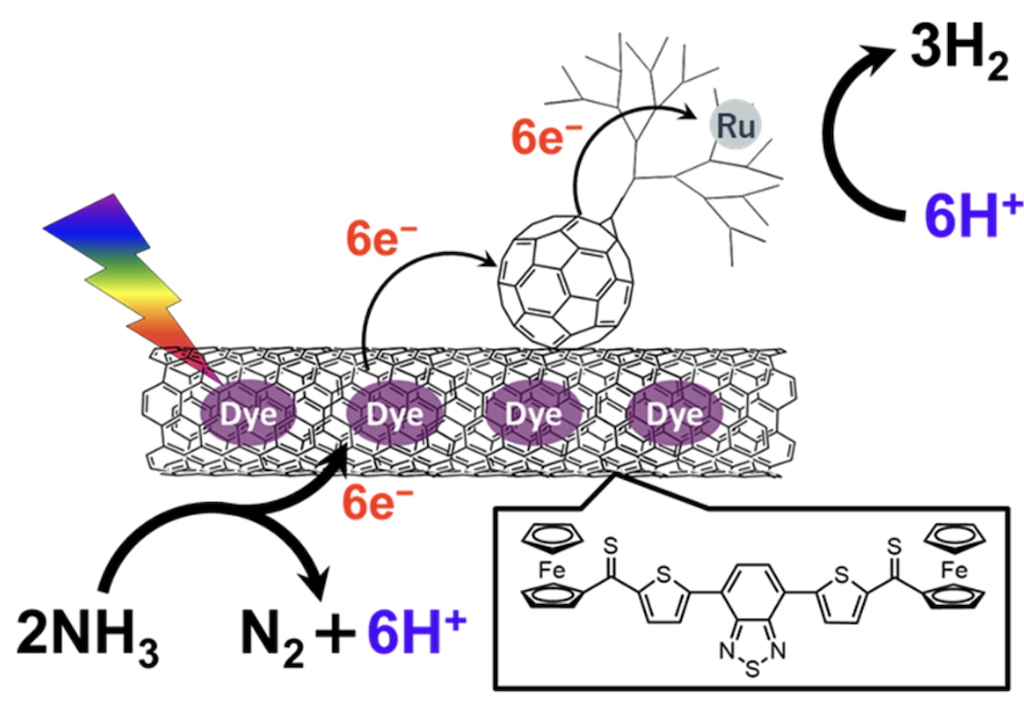
Keywords:
1. Introduction
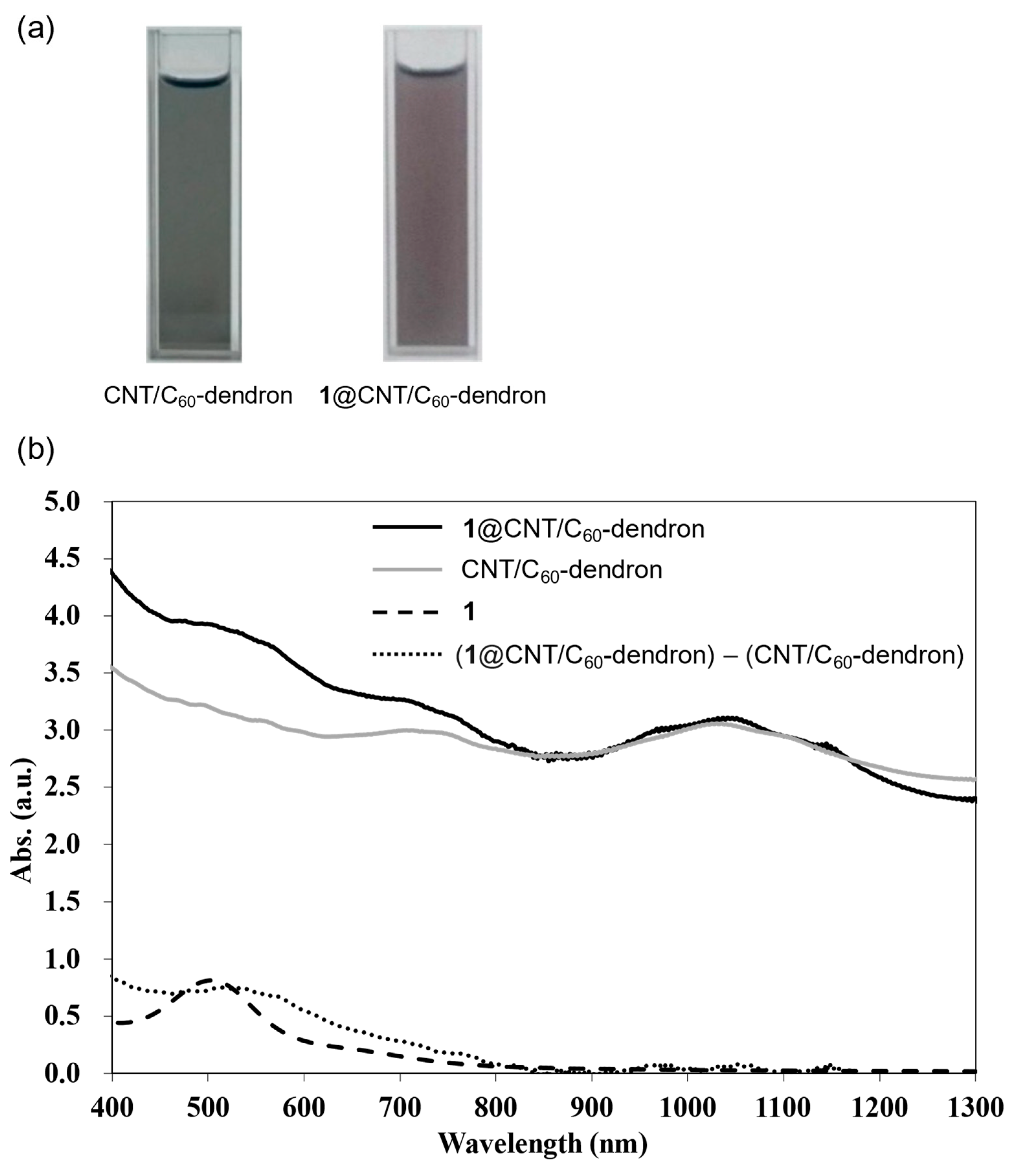
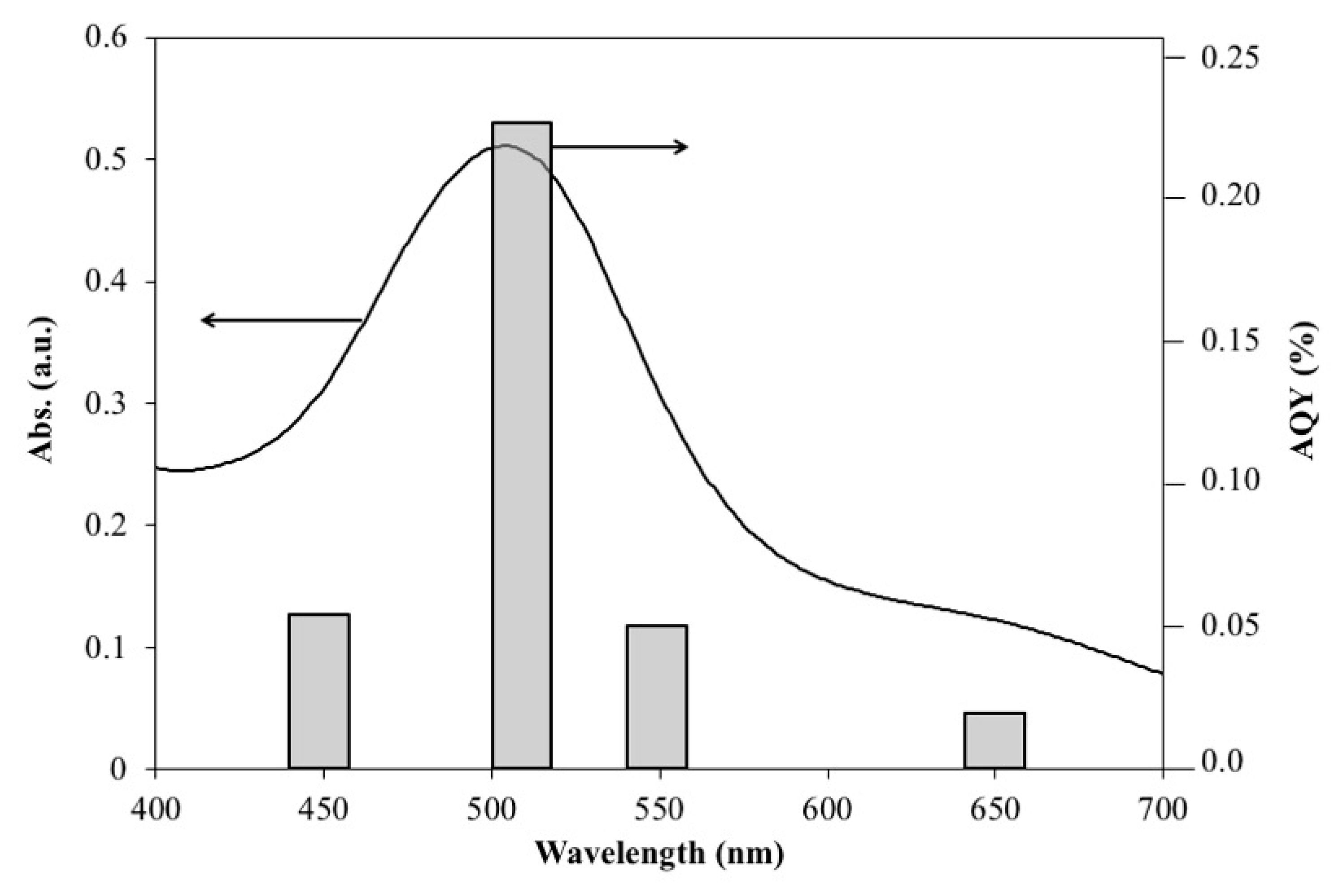
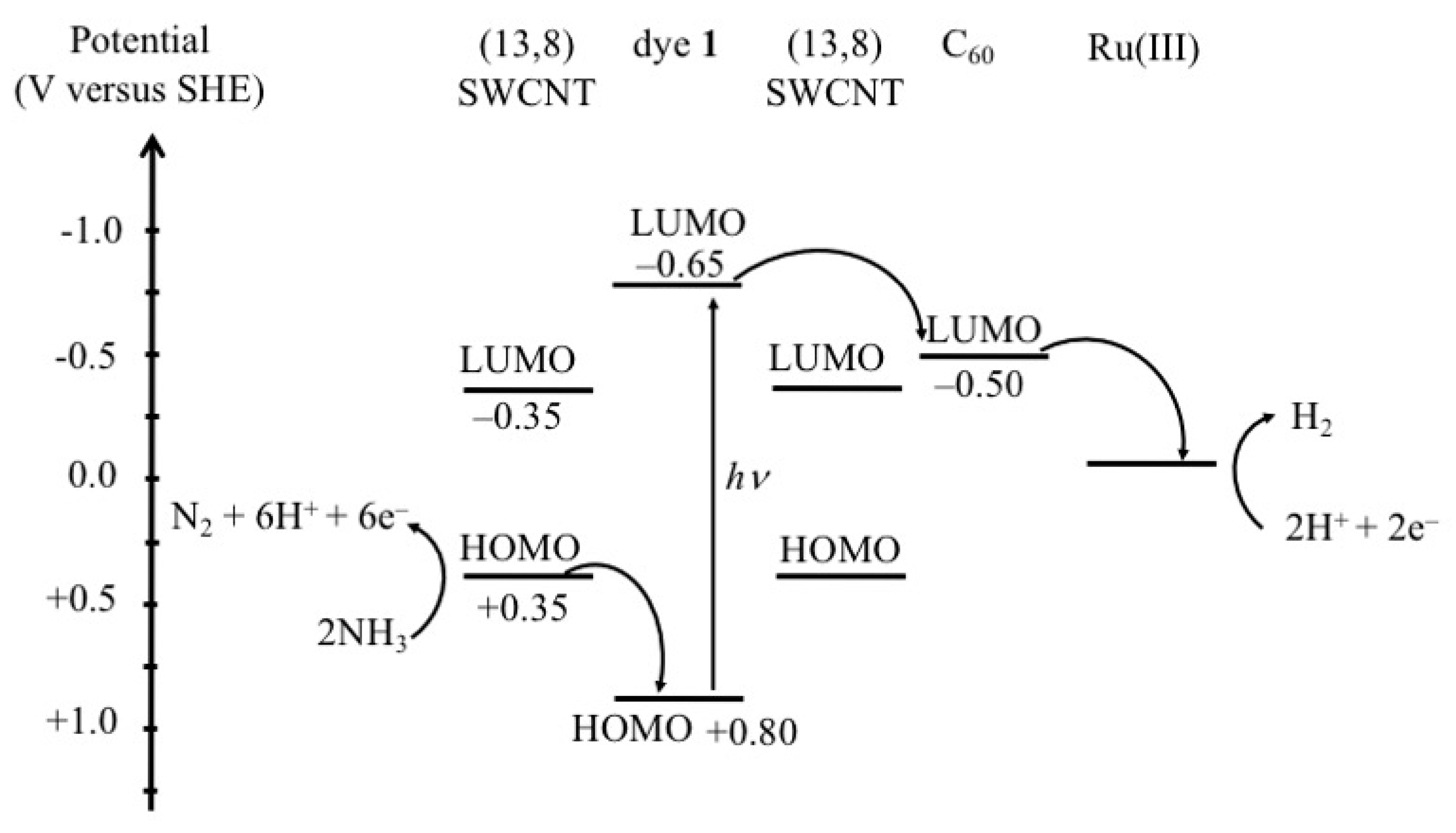
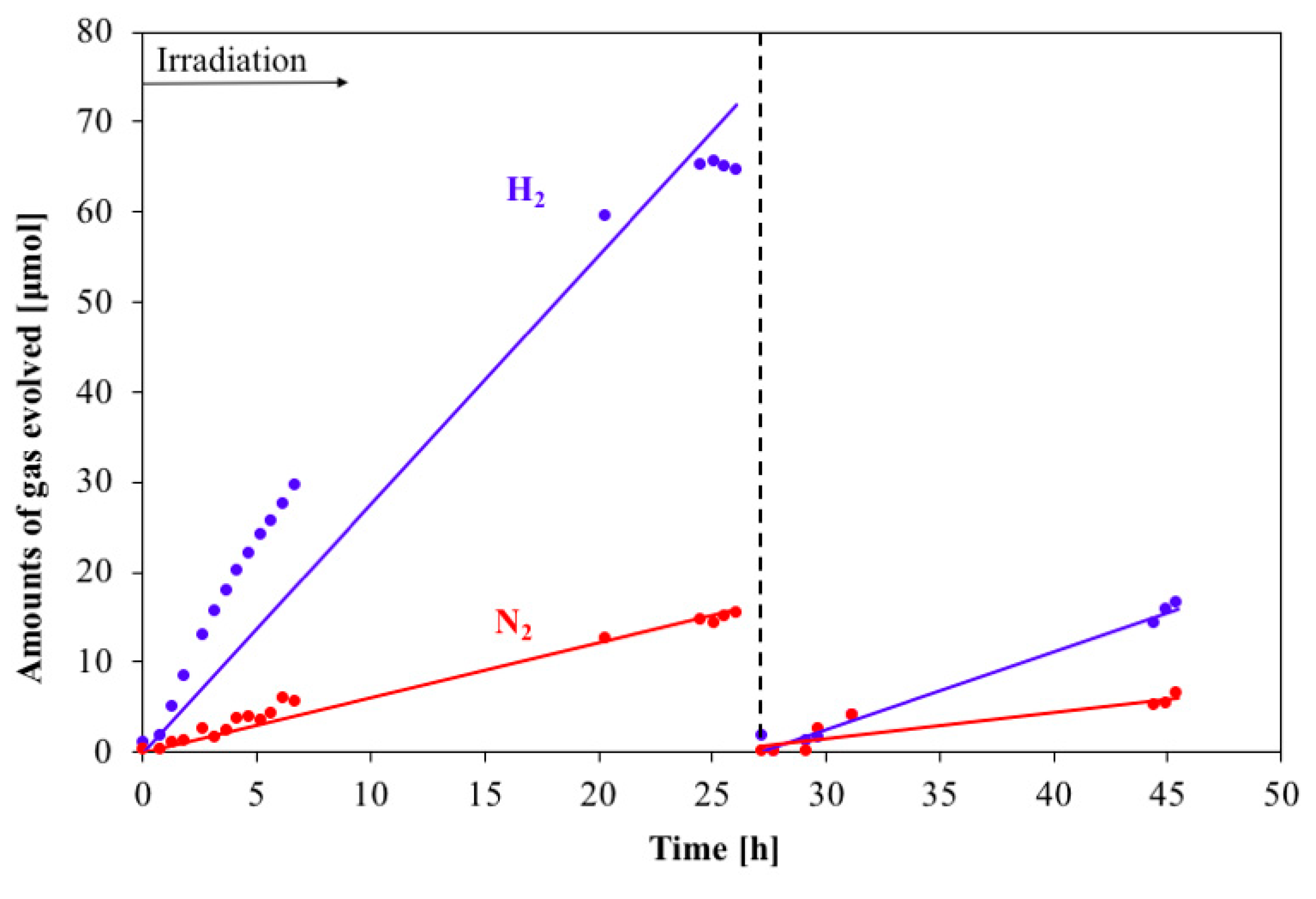
2. Result and Discussions
3. Materials and Methods
3.1. Materials
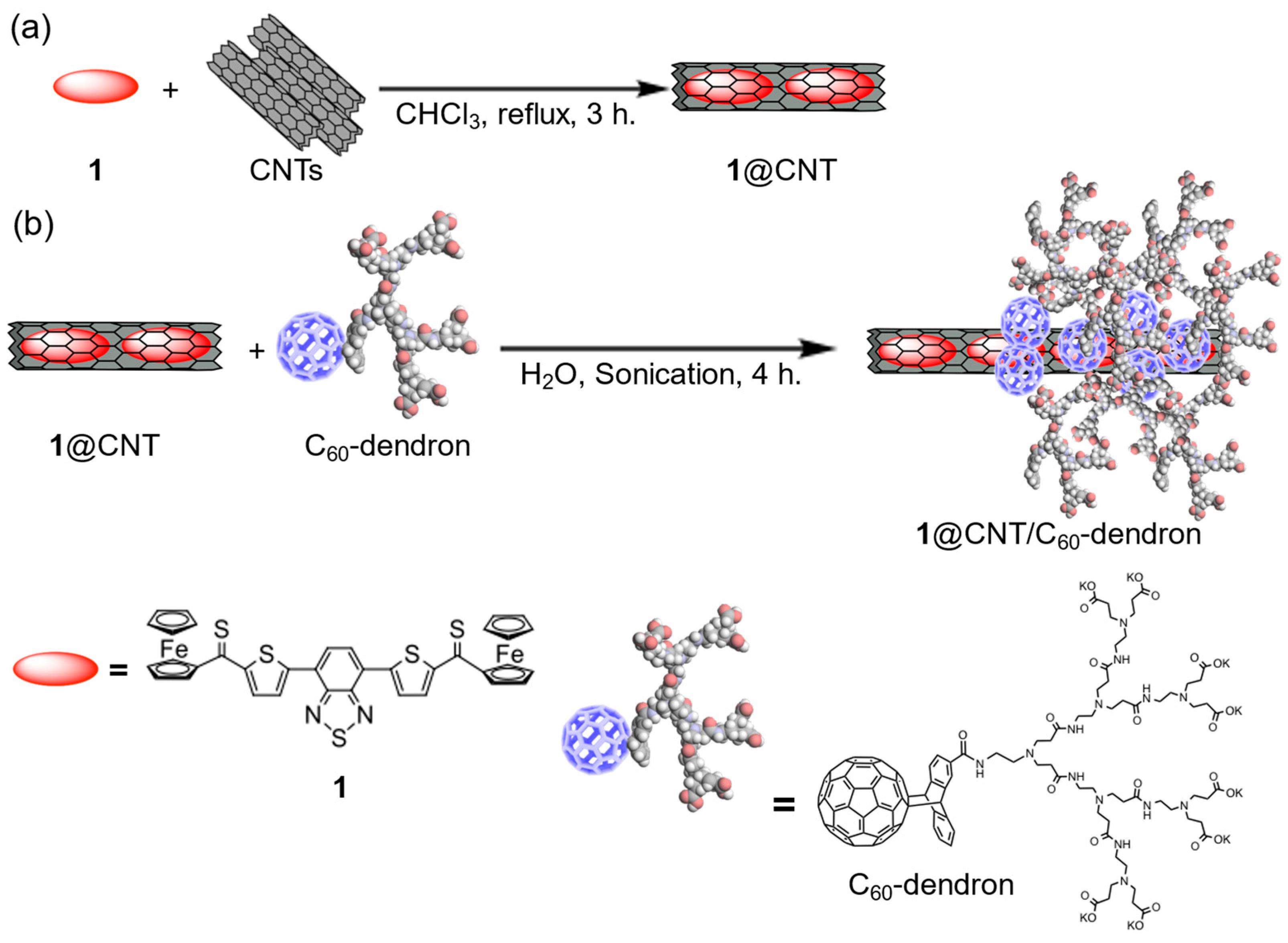
3.2. Preparation of a Stock Solution of 1@CNT/C60-Dendron
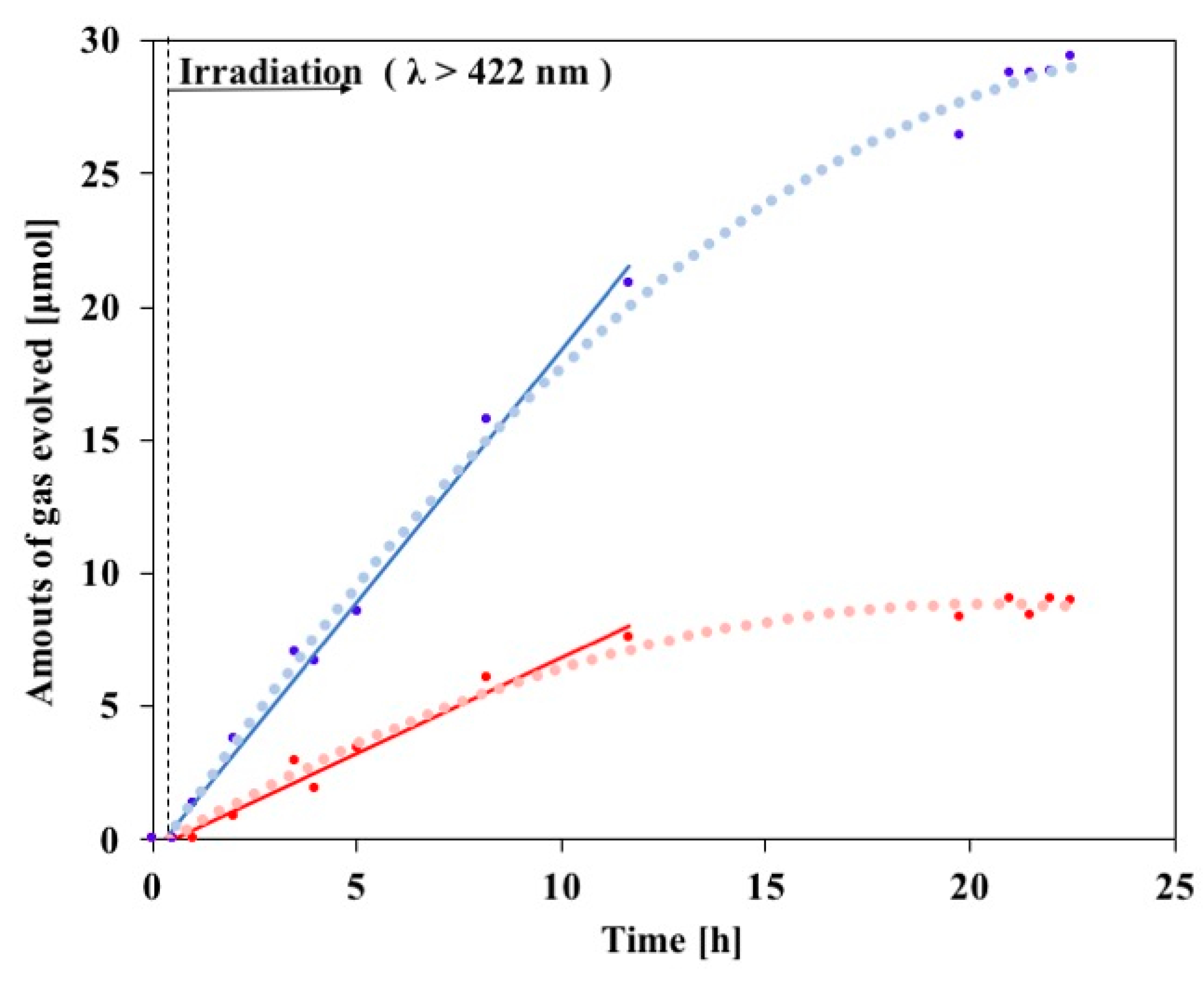
3.3. Photocatalytic Decomposition of Ammonia Using 1@CNT/C60-Dendron
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guo, J.; Chen, P. Catalyst: NH3 as an energy carrier. Chem 2017, 3, 709–714. [CrossRef]
- Zhang, S.; He, Z.; Li, X.; Zhang, J.; Zang, Q; Wang, S. Building heterogeneous nanostructures for photocatalytic ammonia decomposition. Nanoscale Adv. 2020, 2, 3610–3623. [CrossRef]
- Iwase, A.; Ii, K.; Kudo, A. Decomposition of an aqueous ammonia solution as a photon energy conversion reaction using a Ru-loaded ZnS photocatalyst. Chem. Commun. 2018, 54, 6117–6119. [CrossRef]
- Obata, K.; Kishishita, K.; Okemoto, A.; Taniya, K.; Ichihashi, Y.; Nishiyama, S. Photocatalytic decomposition of NH3 over TiO2 catalysts doped with Fe. Appl. Catal., B. 2014, 160–161, 200-203. [CrossRef]
- Zhang, H.; Gu, Q.-Q.; Zhou, Y.-W.; Liu, S.-Q.; Liu, W.-X.; Luo, L.; Meng, Z.-D. Direct Z-scheme photocatalytic removal of ammonia via the narrow band gap MoS2/N-doped graphene hybrid catalyst upon near-infrared irradiation. Appl. Surf. Sci. 2020, 504 144065. [CrossRef]
- Tajima, T.; Sakata, W.; Wada, T.; Tsutui, A.; Nishimoto, S.; Miyake, M.; Takaguchi, Y. Photosensitized Hydrogen Evolution from water using a single-walled carbon nanotube/fullerodendron/SiO2 coaxial nanohybrid. Adv. Mater. 2011, 23, 5850–5754. [CrossRef]
- Sasada, Y.; Tajima, T.; Wada, T.; Uchida, T.; Nishi, M.; Ohkubo, T.; Takaguchi, Y. Photosensitized hydrogen evolution from water using single-walled carbon nanotube/fullerodendron/Pt(II) coaxial nanohybrids. New J. Chem. 2013, 37, 4214–4219. [CrossRef]
- Kurniawan, K.; Tajima, T.; Kubo, Y.; Miyake, H.; Kurashige, W.; Negishi, Y.; Takaguchi, Y. Incorporating a TiOx shell in single-walled carbon nanotube/fullerodendron coaxial nanowires: increasing the photocatalytic evolution of H2 from water under irradiation with visible light. RSC Adv. 2017, 7, 31767–31770. [CrossRef]
- Izawa, T.; Kalousek, V.; Miyamoto, D.; Murakami, N.; Miyake, H.; Tajima, T.; Kurashige, W.; Negishi, Y.; Ikeue, K.; Ohkubo, T.; Takaguchi, Y. Carbon-nanotube-based photocatalysts for water splitting in cooperation with BiVO4 and [Co(bpy)3]3+/2+. Chem. Lett. 2019, 48, 410–413. [CrossRef]
- Ishimoto, K.; Tajima, T.; Miyake, H.; Yamagami, M.; Kurashige, W.; Negishi, Y.; Takaguchi, Y. Photo-induced H2 evolution from water via the dissociation of excitones in water-dispersible single-walled carbon nanotube sensitizers. Chem. Commun. 2018, 54, 393–396. [CrossRef]
- Yamagami, M.; Tajima, T.; Zhang, Z.; Kano, J.; Yashima, K.; Matsubayashi, T., Nguyen, H. K.; Nishiyama, N.; Hayashi, T.; Takaguchi, Y. Hot electron extraction in SWCNT/TiO2 for photocatalytic H2 evolution from water. Nanomaterials 2022, 12, 3826. [CrossRef]
- Murakami, N.; Miyake, H.; Tajima, T.; Nishikawa, K.; Hirayama, R.; Takaguchi, Y. Enhanced photosensitized hydrogen production by encapsulation of ferrocenyl dyes into single-walled carbon nanotubes. J. Am. Chem. Soc. 2018, 140, 3821–3824. [CrossRef]
- Takaguchi, Y.; Miyake, H.; Izawa, T.; Miyamoto, D.; Sagawa, K.; Tajima, T. Molecular design of benzothiadiazole-based dyes for working with carbon nanotube photocatalysts. Phosphorus Sulfur Silicon Relat. Elem. 2019, 194, 707–711. [CrossRef]
- Tajima, T.; Yamagami, M.; Sagawa, R.; Miyake, H.; Takaguchi, Y. Dye-sensitized H2 evolution from water facilitated by photoinduced electron transfer between molecules on the inside and the outside of a carbon nanotube. J. Appl. Phys. 2021, 129, 014303. [CrossRef]
- Watanabe, H.; Ekuni, K.; Okuda, Y.; Nakayama, R.; Kawano, K.; Iwanaga, T.; Yamaguchi, A.; Kiyomura, T.; Miyake, H.; Yamagami, M.; Tajima, T.; Kitai, T.; Hayashi, T.; Nishiyama, N.; Kusano, Y.; Kurata, H.; Takaguchi, Y.; Orita, A. Composite formation of anthrylene- and ferrocenoyl-substituted phenyleneethynylenes with single-wall carbon nanotubes (SWCNTs). Bull. Chem. Soc. Jpn. 2023, 96, 57–64. [CrossRef]
- Miyake, H.; Tajima, T.; Takaguchi, Y. Synthesis and light-absorption characteristics of thiophene derivatives bearing ferrocenylthiocarbonyl groups. Chem. Lett. 2017, 46, 48–50. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




