1. Introduction
Collagen helps to maintain skin’s firmness, elasticity, and hydration. As we age, the body’s natural collagen production decreases, leading to the formation of wrinkles, sagging skin, and the loss of volume. Collagen treatments, such as collagen supplements or injections, aim to replenish lost collagen and improve skin elasticity, hydration, and overall appearance [
1].
The global tissue-engineered collagen biomaterial market will be valued at over 6.63 billion US dollars by 2025 [
2]. The market for oral collagen supplements has already surpassed 1 billion US dollars in 2022 and is projected to grow at more than 6.5% annually from 2023 to 2032. Collagen supplements are available as powder, liquid, pills, and gummies and are sourced from bovine, porcine, marine, and other sources [
3,
4]. However, despite their widespread use for cosmetic purposes to improve skin functions, there is insufficient clinical evidence to support these claims [
5]. Although some clinical trials appear promising, it is still unclear if the skin improvements are due to oral collagen supplements.
On the other hand, the market for injectable skin boosters is already valued at 1.08 billion US dollars in 2023 and is expected to grow at a rate of 9.0% annually from 2024 to 2030 [
6]. Skin boosters are commonly injected into the epidermis [
7,
8,
9] and multiple studies/trials have demonstrated revitalization, hydration, and rejuvenation of the skin [
7,
8,
9,
10,
11,
12]. For injectable skin boosters to be considered effective, they must be safe, natural, have low downtime, be rapidly effective, and consistently delivered via minimally invasive methods [
13]. Overall, direct injection of skin boosters appears to be more beneficial/effective for skin improvements compared to oral collagen supplements.
Previously commercialized injectable collagens were aseptically processed by filtration in the clean bench or cleanroom to avoid collagen degradation due to sterilization limitations [
14]. Autoimmunity of injectable collagen has been reported in animal models and clinical studies [
15,
16,
17]. The most critical issues of injectable collagen processes are not only the efficacy, safety, and side effects but also the ease of use by the consumer. It is therefore essential to establish advanced processing technologies for injectable collagen products to avoid protein denaturation and undesirable modifications after the processing [
18,
19].
Despite the safety concerns, injectable collagen can mediate physiological effects during tissue repair [
20]. Collagen products play an important role in maintaining tissue health and in the treatment of many conditions such as skin wound healing, gastroesophageal reflux, and conditions affecting the elderly such as skin regeneration, sarcopenia (muscle wasting), and bone loss [
21]. Numerous clinical trials show collagen-based therapies are effective [
21]. Degraded proteins of the extracellular matrix generate fragments called matrikines that not only aid the pathology of skin diseases and aging but also provide potential mechanisms of repair [
22]. The manufacturing process for animal-derived collagen typically involves several steps such as source selection, extraction, cleaning and washing, acid or enzymatic treatment, purification, concentration and drying, and sterilization [
23]. However, there are some deficiencies and challenges associated with these processes, which can affect collagen quality, increase the risk of contamination, and lead to degradation [
24]. To mitigate all safety concerns of injectable collagen, a consistent and verified manufacturing process is necessary to confirm a minimal immune response, consistent integrity after sterilization, viral inactivation, and a controlled degradation profile.
To address these requirements, we conducted a study on pre-filtered type I collagen purified with multiple filtration steps, post-sterilized by low-temperature steamed sterilization. We tested sterility and degradation in vitro, and biocompatibility in vivo after intramuscular implantation test in rats. The goal of the study is to confirm the biological safety of type I collagen after these improved purification and sterilization steps.
2. Results and Discussion
2.1. Sterility Test
We used a microbiology sterility test to confirm the sterility of the collagen gel by inoculating the test samples on Tryptic Soy Agar (TSA) which can detect aerobic microbes and fungus at 22.5±2.5°C, and Fluid Thioglycollate Medium (FTM) which can detect anaerobic microbes at 32.5±2.5°C. After culturing for 7 and 14 days, the cultured media were visually inspected for microbe or fungus colony formation. The microbiology sterility test results showed that none of the TSA and FTM media cultured with test samples or negative controls indicated the presence of any microbes after the culture (
Table 1).
2.2. SDS-PAGE Gel Electrophoresis
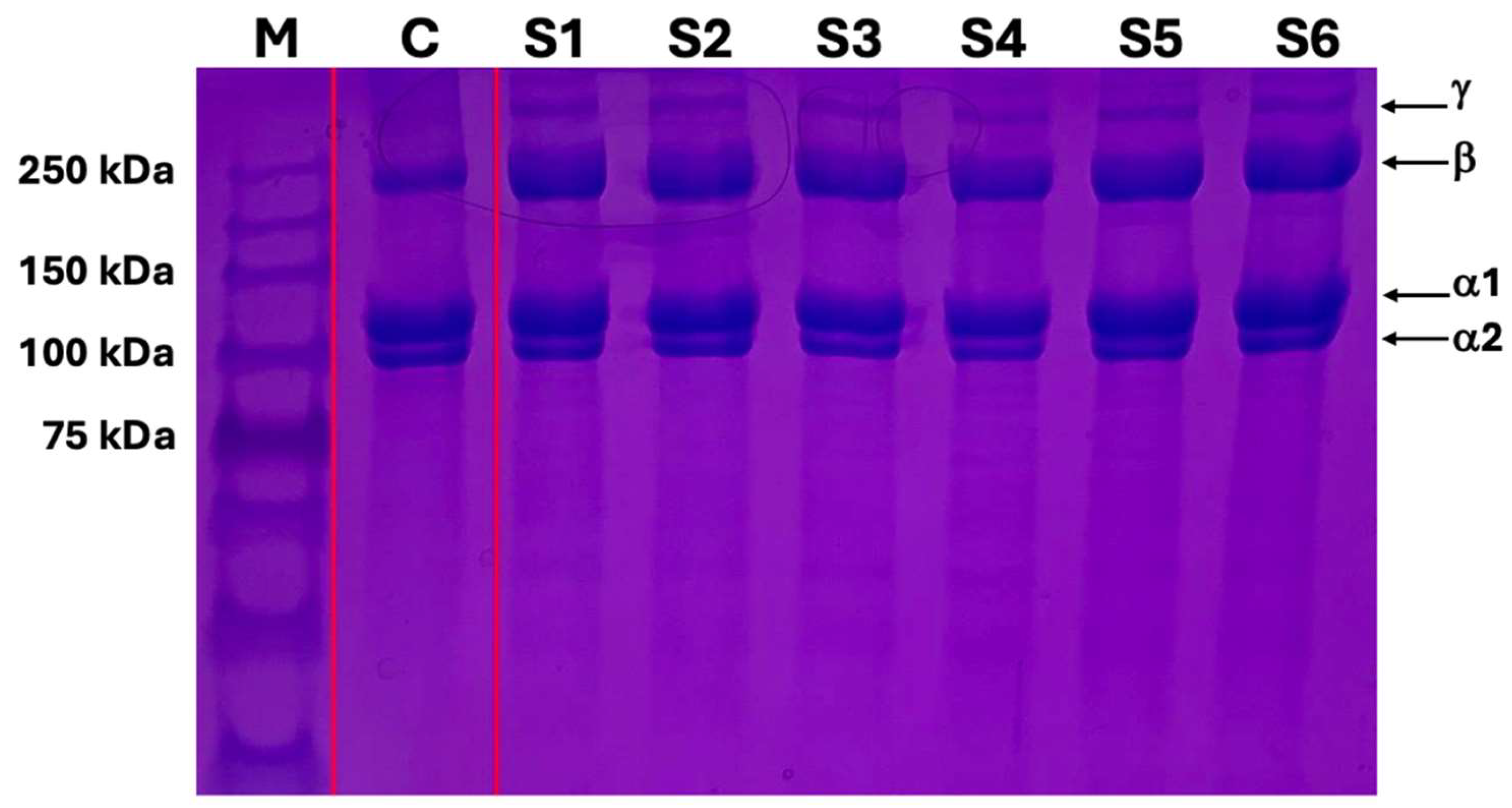
SDS-PAGE gel electrophoresis showed that the bands of terminally sterilized collagen gel were the same as the control material, type I collagen gel. Moreover, there were no smeared bands and smaller-sized protein fragments on the gel suggesting that the terminally filtered and sterilized collagen gel did not induce collagen degradation nor polypeptide chain modification (
Figure 1).
2.3. Microscopic Evaluations
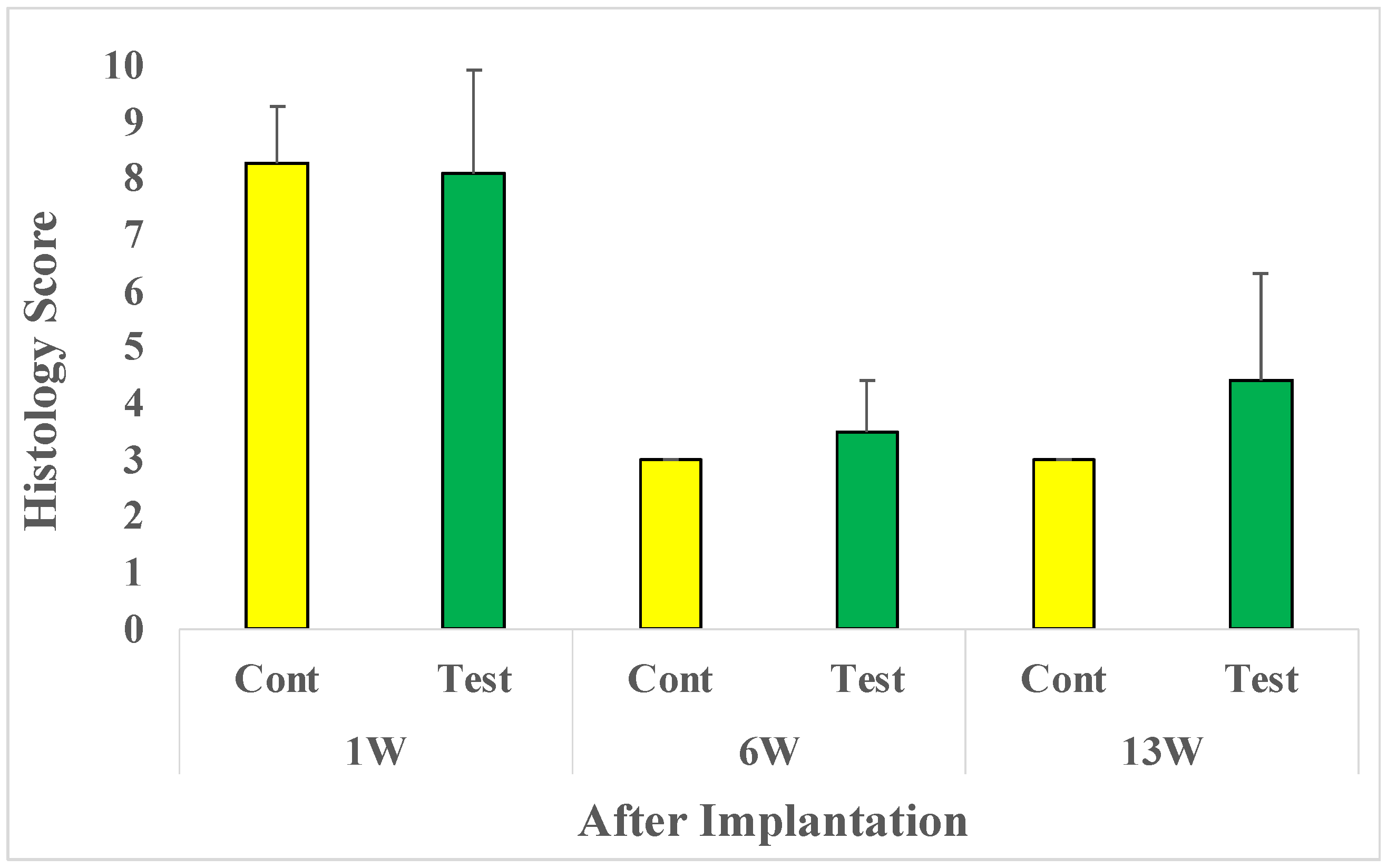
After 1-week implantation, the average microscopic evaluation score was 8.3 in the control group and 8.1 in the test group. After 6-week implantation, the average microscopic evaluation score was reduced to 3.0 in the control group and 3.5 in the test group. After the 13-week implantation, the average score was 3.0 in the control group and 4.4 in the test group (
Figure 2).
One week after implantation, there was no significant difference between groups. The histopathological evaluation index for the test sample was evaluated as 0.0 (Test average – Control average) compared with the control group. After 6-weeks, no significant difference between groups was observed. The histopathological evaluation index for the test sample was evaluated as 0.5 compared with the control group. And after 13 weeks no significant difference between groups was noted. The histopathological evaluation index for the test sample was evaluated as 1.4 compared with the control group. Therefore, for all time points tested after implantation minimal or no reaction for bio-reactivity rating was seen (
Table 2).
A skin booster is an injectable treatment used to restore and improve the overall quality and health of the skin [
7]. However, it can cause side effects such as swelling, redness, bruising, and pain at the injected site. A major factor leading to side effects is the reaction to ingredients, making it important to validate the process used for hydrogel production before clinical use [
25].
Improved sterilization of sensitive biomaterials is technically limited however, it is very important to explore [
26]. To ensure the safe use of a collagen type I gel as a skin booster, it must undergo verified processing technique(s) and standard testing for sterility, adverse reaction, and stability. In this paper, we are proposing a new processing technique, which involves a double filtration process using filters with different cut-off sizes, and low-temperature steam sterilization. The verification of the process technique is very critical because degraded or modified collagen by post-processing steps may have potential safety risks such as autoimmunity, allergic reactions, inflammation, and impaired functionality on the skin [
27].
Other studies have reported that post-processing and sterilization can degrade collagen [
28,
29]. To assess the integrity of the collagen gel after processing and sterilization in vitro, we performed SDS-PAGE gel electrophoresis to analyze protein bands. Protein gel electrophoresis results showed that the type I collagen gel maintained the basic structure of monomeric alpha-1, alpha-2, beta, and gamma chains compared to other commercially available type I collagen gel. The results of SDS-PAGE gel electrophoresis confirmed the maintenance of collagen integrity after our processing and sterilization methods.
To evaluate the local effects of the finally processed collagen gel in vivo, the collagen gel was implanted in the intramuscular tissue of rats and evaluated at 1 week, 6 weeks, and 13 weeks after implantation. On histologic evaluation, there was no significant increase in polymorphonuclear leukocyte infiltration, lymphocyte infiltration, macrophage infiltration, neovascularization, and fibrosis at the test implantation sites relative to control. The histopathological evaluation index was 0.0 after 1 week, 0.5 after 6 weeks, and 1.4 after 13 weeks of implantation.
The in vivo evaluations also supported that the implanted collagen gel in the intramuscular tissue of rats did not show any critical tissue reactions and confirmed the biological safety. However, other collagen-related studies have shown tissue inflammation and immune reactions using irradiated, chemically processed, or high-temperature sterilized methods typically employed for other collagens [
30,
31]. Based on the collagen integrity, sterility, and animal study for biological safety evaluation, the study’s findings support that the implanted collagen gel by double filtration and low-temperature sterilization processing methods demonstrates favorable outcomes, and the methods are effective and safe for potential clinical applications.
Demonstration of relative biological effectiveness requires further investigation with an animal model. Future studies such as full-thickness wound healing and assessment of sub-chronic systemic toxicity will evaluate the efficacy of treating soft tissue defects in appropriate animal models.
3. Conclusions
This study aimed to examine the protein integrity and the biological compatibility of pre-filtered and low-temperature sterilized collagen gels both in vitro and in vivo experiments. Based on these results, the collagen polypeptide structure was maintained without any protein denaturation and modification in vitro, and the bioactivity rating of the implanted test sample was considered non-irritant in the in vivo rat model. Therefore, the processed collagen type I gel by double filtration and low-temperature steam sterilization is a promising processing platform as a safe skin booster for clinical use.
4. Materials and Methods
4.1. Preparation of Test Samples
Collagen (D-med, Seoul, Korea) was extracted from porcine skin as described earlier [
32]. The extracted collagen gel was filtered sequentially using a primary filter (Polysep II Cartridge, MilliporeSigma, MA) with a 300kDa cut-off size and a secondary filter with a 100kDa cut-off size (Milligard Cartridge, MilliporeSigma, MA). The final filtered collagen gel (3% w/v, 150kDa) was then sterilized at 40°C and 0.7 atm for 40 minutes in a steam sterilization chamber (HS-1000R, Hanshinmed, Seoul, Korea). The collagen gel for the study was prepared in a 1 mL syringe. High-density polyethylene rod (diameter: 1mm, length: 5mm) was sterilized at 121°C for 15 minutes and used as a control sample. To confirm the sterility of the test sample, Tryptic Soy Agar (TSA, Synergyinno, SeongNam, Korea) and Fluid Thioglycollate Medium (FTM, Synergyinno, SeongNam, Korea) were used for the microbiology sterility test according to the USP 29<71> test method [
33]. As a positive control for the sterility test, Staphylococcus aureus (ATCC 6538, ThermoFisher Scientific, Seoul, Korea), Pseudomonas aeruginosa (ATCC 9027, ThermoFisher Scientific, Seoul, Korea), Bacillus subtilis (ATCC 6633, ThermoFisher Scientific, Seoul, Korea), Candida albicans (ATCC 10231, ThermoFisher Scientific, Seoul, Korea), and Aspergillus niger (ATCC 16404, ThermoFisher Scientific, Seoul, Korea) were used during the culture.
4.2. SDS-PAGE of Processed and Sterilized Collagen Gel
The test collagen samples (n=6) were diluted to 1mg/ml with DI water. The diluted collagen was mixed with 5x loading buffer (#426311, BioLegend®, Seoul, Korea) and boiled at 95°C for 5 minutes. The samples were separated by 5%-7% resolving gel and stained with 0.05% Coomassie brilliant blue (B0149-25G, Sigma-Aldrich, St. Louis, MO) for an hour and destained with a destaining solution for 20 minutes and DI water for 30 minutes. The protein ladder (HQM14500, BioD, Seoul, Korea) was used to evaluate the molecular weights of the type I collagen. After the destaining, the gel was scanned by a gel scanner (Bio-6000, BIOIMAGER, Seoul, Korea). The molecular weight of stained gel bands was identified by comparing them with the protein ladder.
4.3. Rat Muscle Implantation Model
This animal study was approved by the institutional animal care of use committee of the Korea Testing and Research Institute (IAC202846). A total of 18 rats (Sprague Dawley specific-pathogen-free rats, 18 males, 9 weeks old, Orient Bio Co., Ltd., Korea) were used for muscle implantation under GLP (Good Laboratory Practice) and ISO10993-6:2016 standard [
34]. For each implantation group of 1, 6, and 13 weeks, 6 rats were used for each time point. Each rat had 2 test implantation and 2 control implantation sites so a total of 12 test implantation and 12 control implantation sites per time point.
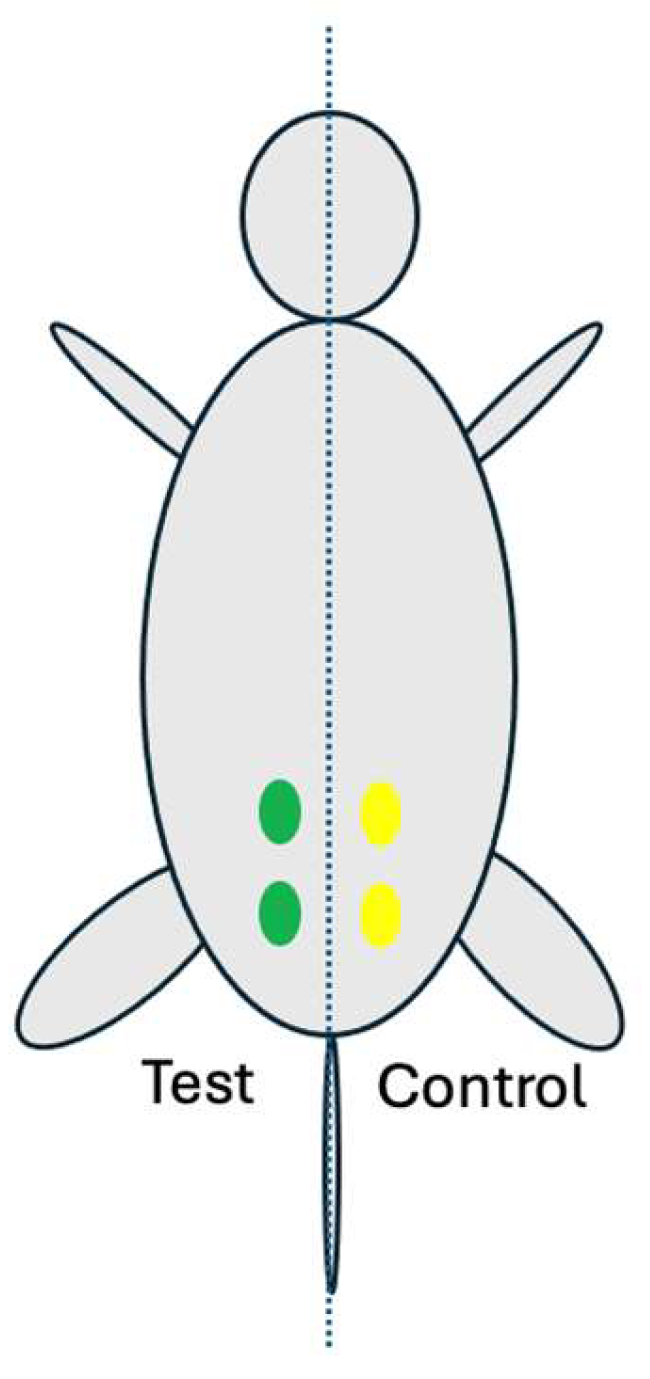
Rompun (xylazine, Bayer, Korea) and Ketamine (ketamine, Yuhan, Korea) were injected intraperitoneally for anesthesia. After inducing anesthesia, the fur over the gluteal muscle area was removed using a clipper and the implantation area was disinfected with betadine. The skin was incised to expose the gluteal muscle. For each animal, two test samples (0.2mL each) were implanted in the left gluteal muscle, and two control samples (0.2mL each) were implanted in the right gluteal muscle (
Figure 3).
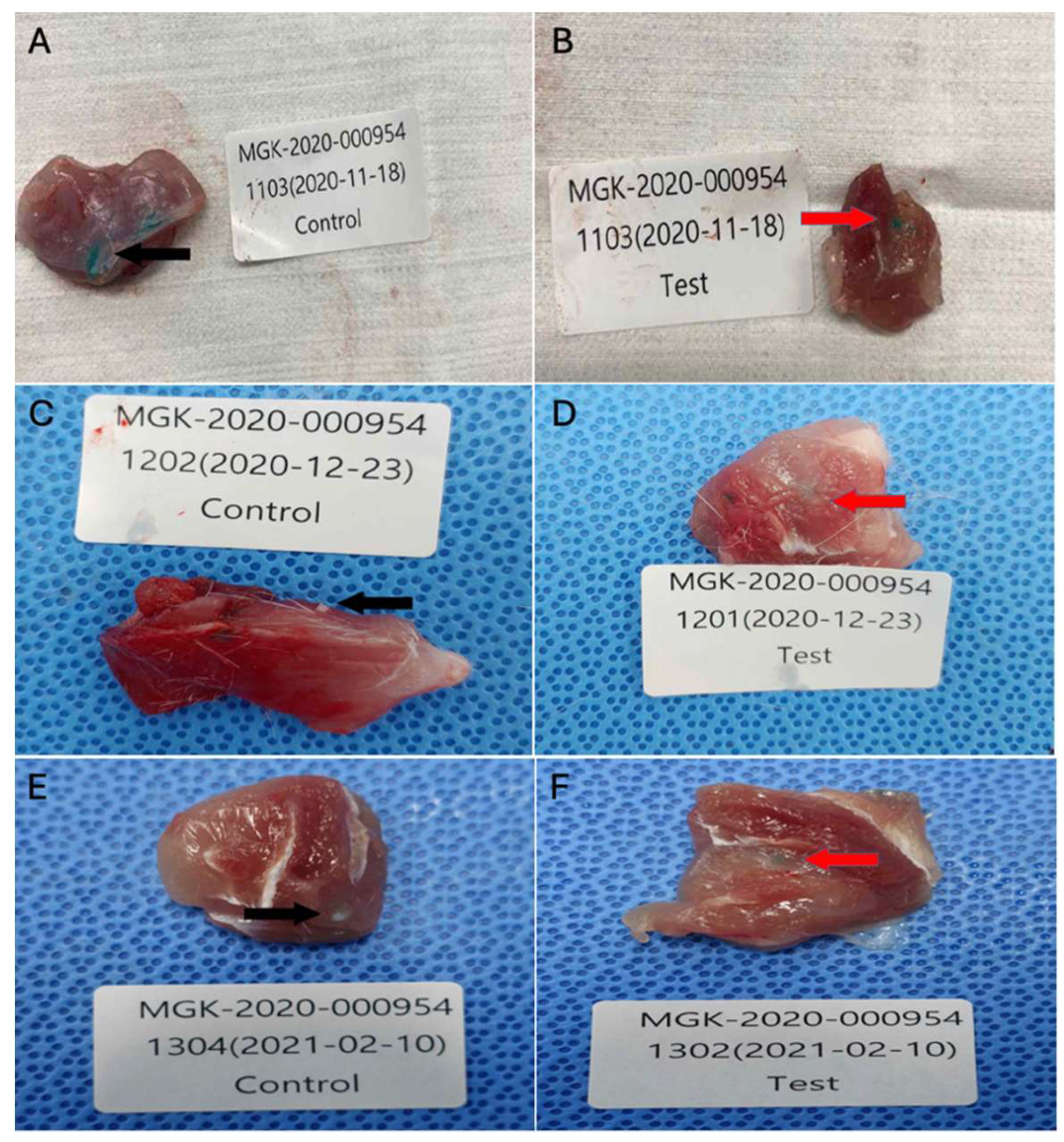
After recovery from the anesthesia, the animals were placed in a cage. All animals were observed daily for nonspecific clinical signs such as anorexia, lethargy, emaciation, ruffled fur, and acute death. At the appropriate time points, the animals were euthanized by carbon dioxide. The macroscopic lesion was observed after the excision of the implantation site with the adjacent tissue (
Figure 4).
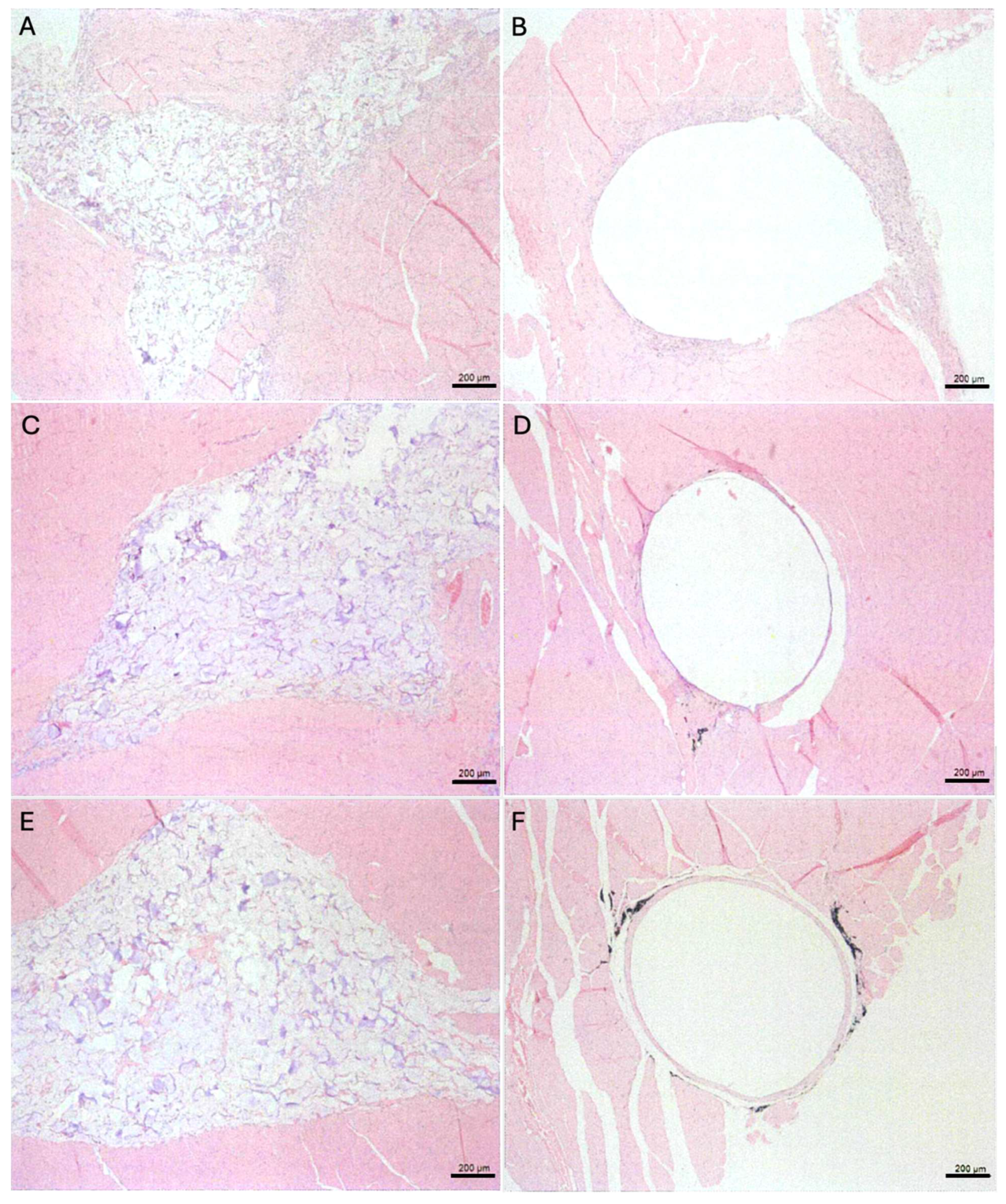
After full fixation in 10% neutral buffered formalin for up to 3 days, the tissue specimens were embedded in paraffin for Hematoxylin and Eosin stain (H&E), and histopathological examination (
Figure 5). Tissue specimens were trimmed into 10-um pieces by using a rotary microtome (RM2125, Leica Biosystems, Korea) and then cut into 4-um sections. The histology sections were immersed in water, stained with H&E, and mounted. The stained slides were examined under an inverted microscope at a magnification of 50×.
4.4. Microscopic Evaluation
The histological evaluation of implantation sites was evaluated using a previously described method [
32] outlined in
Table 3 and
Table 4. The mean scores by double-blind evaluation were averaged from 10-12 histology samples per group at each time point. The total score of biological response was divided into the number of implantation sites.
After calculating the difference in mean score between the test sample and the control sample, the bio-reactivity rating was evaluated (
Table 4).
Author Contributions
T.K. performed the research and improved the paper. T.K., J.L., and H.R. designed the research study. H.R. and S.G. analyzed the data. J.L., S.G., and D.D. wrote the paper.
Funding
This research received no external funding.
Institutional Review Board Statement
This animal study was approved by the institutional animal care of use committee of the Korea Testing and Research Institute.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors thank all the investigators and technical staff who have participated in this pre-clinical study.
Conflicts of Interest
The authors have no conflict of interest to declare.
References
- Al-Atif, H. Collagen Supplements for Aging and Wrinkles: A Paradigm Shift in the Fields of Dermatology and Cosmetics. Dermatol Pract Concept 2022, 12, e2022018. [Google Scholar] [CrossRef] [PubMed]
- Avila Rodríguez, M.I.; Barroso, L.G.R.; Sánchez, M.L. Collagen: A review on its sources and potential cosmetic applications. J Cosmet Dermatol 2018, 17, 20–26. [Google Scholar] [CrossRef]
- Liu, D.; et al. Collagen and gelatin. Annu Rev Food Sci Technol 2015, 6, 527–557. [Google Scholar] [CrossRef]
- Meyer, M. Processing of collagen based biomaterials and the resulting materials properties. Biomed Eng Online 2019, 18, 24. [Google Scholar] [CrossRef]
- Perez-Sanchez, A.C.; et al. Skin, Hair, and Nail Supplements: Marketing and Labeling Concerns. Cureus 2020, 12, e12062. [Google Scholar] [CrossRef]
-
Skin Boosters Market Size, Share & Trends Analysis Report By Type (Mesotherapy, Micro-needle), By Gender (Female, Male), By End-use (Dermatology Clinics, Medspa), By Region, And Segment Forecasts, 2024 - 2030. Grand View Research, 108.
- Ghatge, A.S.; Ghatge, S.B. The Effectiveness of Injectable Hyaluronic Acid in the Improvement of the Facial Skin Quality: A Systematic Review. Clin Cosmet Investig Dermatol 2023, 16, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; et al. Clinical efficacy and safety of targeted injection of PRP with skin booster in the treatment of aging face. Biotechnol Genet Eng Rev 2023, 1–17. [Google Scholar] [CrossRef]
- Lee, J.H.; et al. The efficacy of intradermal hyaluronic acid filler as a skin quality booster: A prospective, single-center, single-arm pilot study. J Cosmet Dermatol 2024, 23, 409–416. [Google Scholar] [CrossRef]
- Arora, G.; et al. Biorevitalization of the skin with skin boosters: Concepts, variables, and limitations. J Cosmet Dermatol 2021, 20, 2458–2462. [Google Scholar] [CrossRef]
- Yi, K.H.; et al. Skin boosters: Definitions and varied classifications. Skin Res Technol 2024, 30, e13627. [Google Scholar] [CrossRef] [PubMed]
- Ayatollahi, A.; Firooz, A.; Samadi, A. Evaluation of safety and efficacy of booster injections of hyaluronic acid in improving the facial skin quality. J Cosmet Dermatol 2020, 19, 2267–2272. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, L.; et al. An Update on the Clinical Efficacy and Safety of Collagen Injectables for Aesthetic and Regenerative Medicine Applications. Polymers (Basel) 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Matuska, A.M.; McFetridge, P.S. The effect of terminal sterilization on structural and biophysical properties of a decellularized collagen-based scaffold; implications for stem cell adhesion. J Biomed Mater Res B Appl Biomater 2015, 103, 397–406. [Google Scholar] [CrossRef]
- Elson, M.L. Injectable collagen and autoimmune disease. J Dermatol Surg Oncol 1993, 19, 165–168. [Google Scholar] [CrossRef]
- Stuart, J.M.; Townes, A.S.; Kang, A.H. Collagen autoimmune arthritis. Annu Rev Immunol 1984, 2, 199–218. [Google Scholar] [CrossRef]
- Fujiyoshi, T.; et al. Molecular basis of type II collagen autoimmune disease: observations of arthritis, auricular chondritis and tympanitis in mice. ORL J Otorhinolaryngol Relat Spec 1997, 59, 215–229. [Google Scholar] [CrossRef]
- Pearlman, R.L.; McClung, S.J.W.; Schlesinger, T. Introduction to soft tissue fillers. Dermatological Reviews 2023, 4, 79–81. [Google Scholar] [CrossRef]
- Açil, Y.; et al. Proof of direct radiogenic destruction of collagen in vitro. Strahlenther Onkol 2007, 183, 374–379. [Google Scholar] [CrossRef]
- Keefe, J.; et al. Clinical use of injectable bovine collagen: a decade of experience. Clin Mater 1992, 9, 155–162. [Google Scholar] [CrossRef]
- Wang, H. A Review of the Effects of Collagen Treatment in Clinical Studies. Polymers (Basel) 2021, 13. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. The extracellular matrix in myocardial injury, repair, and remodeling. J Clin Invest 2017, 127, 1600–1612. [Google Scholar] [CrossRef] [PubMed]
- Matinong, A.M.E.; et al. Collagen Extraction from Animal Skin. Biology (Basel) 2022, 11. [Google Scholar] [CrossRef]
- Xu, S.; et al. Improving the Sustainability of Processing By-Products: Extraction and Recent Biological Activities of Collagen Peptides. Foods 2023, 12. [Google Scholar] [CrossRef] [PubMed]
- Haneke, E. Managing Complications of Fillers: Rare and Not-So-Rare. J Cutan Aesthet Surg 2015, 8, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Galante, R.; et al. Sterilization of hydrogels for biomedical applications: A review. Journal of Biomedical Materials Research Part B: Applied Biomaterials 2018, 106, 2472–2492. [Google Scholar] [CrossRef] [PubMed]
- Amirrah, I.N.; et al. A Comprehensive Review on Collagen Type I Development of Biomaterials for Tissue Engineering: From Biosynthesis to Bioscaffold. Biomedicines 2022, 10. [Google Scholar] [CrossRef]
- Delgado, L.M.; Pandit, A.; Zeugolis, D.I. Influence of sterilisation methods on collagen-based devices stability and properties. Expert Review of Medical Devices 2014, 11, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Šupová, M.; et al. The electron beam irradiation of collagen in the dry and gel states: The effect of the dose and water content from the primary to the quaternary levels. International Journal of Biological Macromolecules 2023, 253, 126898. [Google Scholar] [CrossRef]
- Martínez-Puig, D.; et al. Collagen Supplementation for Joint Health: The Link between Composition and Scientific Knowledge. Nutrients 2023, 15. [Google Scholar] [CrossRef]
- Rýglová, Š.; Braun, M.; Suchý, T. Collagen and Its Modifications—Crucial Aspects with Concern to Its Processing and Analysis. Macromolecular Materials and Engineering 2017, 302, 1600460. [Google Scholar] [CrossRef]
- Lee, K.I.; et al. Subcutaneous toxicity of a dual ionically cross-linked atelocollagen and sodium hyaluronate gel: Rat in vivo study for biological safety evaluation of the injectable hydrogel. Toxicol Rep 2021, 8, 1651–1656. [Google Scholar] [CrossRef] [PubMed]
- Parveen, S.; et al. Evaluation of growth based rapid microbiological methods for sterility testing of vaccines and other biological products. Vaccine 2011, 29, 8012–8023. [Google Scholar] [CrossRef] [PubMed]
- Tungtasana, H.; et al. Tissue response and biodegradation of composite scaffolds prepared from Thai silk fibroin, gelatin and hydroxyapatite. J Mater Sci Mater Med 2010, 21, 3151–3162. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
SDS-PAGE gel electrophoresis of collagen type I gel: M; protein marker, C; control, commercial type I collagen gel, S1-S6; filtered and sterilized collagen type I gel.
Figure 1.
SDS-PAGE gel electrophoresis of collagen type I gel: M; protein marker, C; control, commercial type I collagen gel, S1-S6; filtered and sterilized collagen type I gel.
Figure 2.
Microscopic evaluation: 1, 6, and 13 weeks after implantation: Cont; Control sample implanted rat group (n=10-12), Test; Test sample implanted rat group (n=10-12).
Figure 2.
Microscopic evaluation: 1, 6, and 13 weeks after implantation: Cont; Control sample implanted rat group (n=10-12), Test; Test sample implanted rat group (n=10-12).
Figure 3.
Implantation locations (four) on the gluteal muscle of the rat: The left two green marks are implantation sites for two test samples and the right two yellow marks are implantation sites for two control samples.
Figure 3.
Implantation locations (four) on the gluteal muscle of the rat: The left two green marks are implantation sites for two test samples and the right two yellow marks are implantation sites for two control samples.
Figure 4.
Collected tissue specimens from implantation sites after implantation periods of 1, 6, or 13 weeks: (A) Control sample implanted tissue after 1 week (n=11), (B) Test sample implanted tissue after 1 week (n=12), (C) Control sample implanted tissue after 6 weeks (n=10), (D) Test sample implanted tissue after 6 weeks (n=12), (E) Control sample implanted tissue after 13 weeks (n=11), (F) Test sample implanted tissue after 13 weeks (n=10).
Figure 4.
Collected tissue specimens from implantation sites after implantation periods of 1, 6, or 13 weeks: (A) Control sample implanted tissue after 1 week (n=11), (B) Test sample implanted tissue after 1 week (n=12), (C) Control sample implanted tissue after 6 weeks (n=10), (D) Test sample implanted tissue after 6 weeks (n=12), (E) Control sample implanted tissue after 13 weeks (n=11), (F) Test sample implanted tissue after 13 weeks (n=10).
Figure 5.
H&E staining of muscle sections at the implantation site: (A) Test sample implanted tissue after 1 week, (B) Control sample implanted tissue after 1 week, (C) Test sample implanted tissue after 6 weeks, (D) Control sample implanted tissue after 6 weeks, (E) Test sample implanted tissue after 13 weeks, (F) Control sample implanted tissue after 13 weeks.
Figure 5.
H&E staining of muscle sections at the implantation site: (A) Test sample implanted tissue after 1 week, (B) Control sample implanted tissue after 1 week, (C) Test sample implanted tissue after 6 weeks, (D) Control sample implanted tissue after 6 weeks, (E) Test sample implanted tissue after 13 weeks, (F) Control sample implanted tissue after 13 weeks.
Table 1.
Sterility test of the collagen gel at D-0, D+7, and D+14.
Table 1.
Sterility test of the collagen gel at D-0, D+7, and D+14.
| Media |
Test Samples |
D-0 |
D+7 |
D+14 |
| TSA |
#01 |
No growth |
No growth |
No growth |
| #02 |
No growth |
No growth |
No growth |
| #03 |
No growth |
No growth |
No growth |
| Negative control |
No growth |
No growth |
No growth |
| Positive control |
No growth |
Growth |
Growth |
| FTM |
Test Samples |
D-0 |
D+7 |
D+14 |
| #01 |
No growth |
No growth |
No growth |
| #02 |
No growth |
No growth |
No growth |
| #03 |
No growth |
No growth |
No growth |
| Negative control |
No growth |
No growth |
No growth |
| Positive control |
No growth |
Growth |
Growth |
Table 2.
Bioactivity rating after 1-week, 6-week, and 13-week implantation.
Table 2.
Bioactivity rating after 1-week, 6-week, and 13-week implantation.
| Implantation Period |
Mean Score (Control) |
Mean Score (Test) |
Mean Score Difference
(Test - Control) |
Bioreactivity Rating |
| 1 week |
8.3 |
8.1 |
8.1 – 8.3 = -0.2
(Considered as 0.0) |
Minimal or No Reaction |
| 6 weeks |
3.0 |
3.5 |
3.5 - 3.0 = 0.5 |
Minimal or No Reaction |
| 13 weeks |
3.0 |
4.4 |
4.4 - 3.0 = 1.4 |
Minimal or No Reaction |
Table 3.
Histological evaluation system.
Table 3.
Histological evaluation system.
| Category |
Score |
| 0 |
1 |
2 |
3 |
4 |
| Leukocyte |
0 |
Rare, 1-5/phf* |
5-10/phf |
Heavy infiltrate |
Packed |
| Lymphocytes |
0 |
Rare, 1-5/phf |
5-10/phf |
Heavy infiltrate |
Packed |
| Macrophages |
0 |
Rare, 1-5/phf |
5-10/phf |
Heavy infiltrate |
Packed |
| Neo-vascularization |
0 |
Minimal capillary proliferation, focal, 1-3 buds |
Groups of 4-7 capillaries with supporting fibroblastic structures |
Broad band of capillaries with supporting structures |
Extensive band of capillaries with supporting fibroblastic structures |
| Fibrosis |
0 |
Narrow band |
Moderately thick band |
Thick band |
Extensive band |
| *phf: per high powered (50x) field |
Table 4.
Bio-reactivity rating.
Table 4.
Bio-reactivity rating.
| Bio-reactivity rating |
Mean score (Animal score) |
| Minimal or no reaction |
0.0 2.9 |
| Slight reaction |
3.0 - 8.9 |
| Moderate reaction |
9.0 – 15.0 |
| Severe reaction |
> 15 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).