Submitted:
15 August 2024
Posted:
20 August 2024
You are already at the latest version
Abstract
Keywords:
Introduction
Methods
Search Strategy
Inclusion and Exclusion Criteria
Data Extraction
Quality Assessment
Statistical Analysis
Effect Size Aggregation
Moderator Analyses
Results
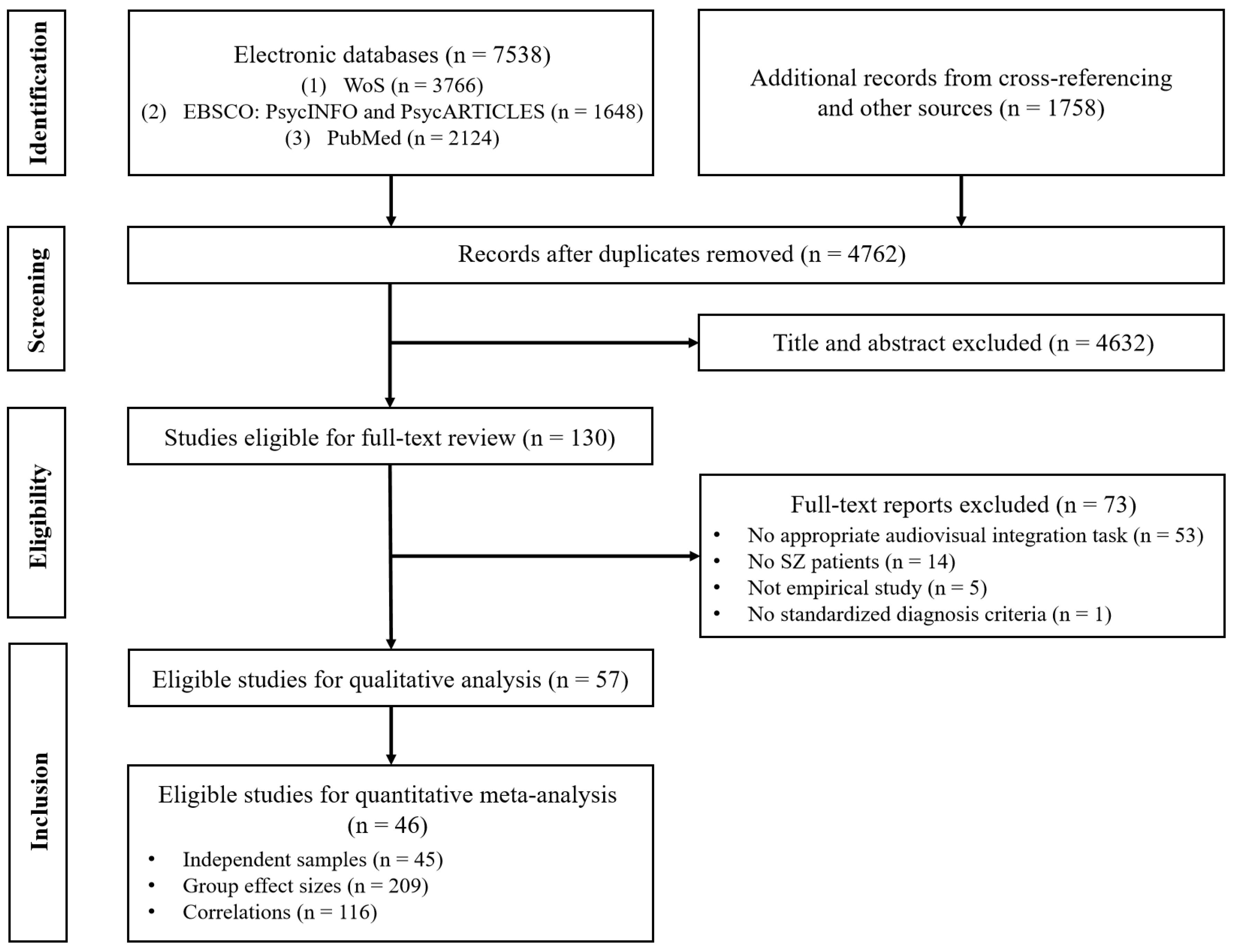
Literature Search
Primary Outcomes
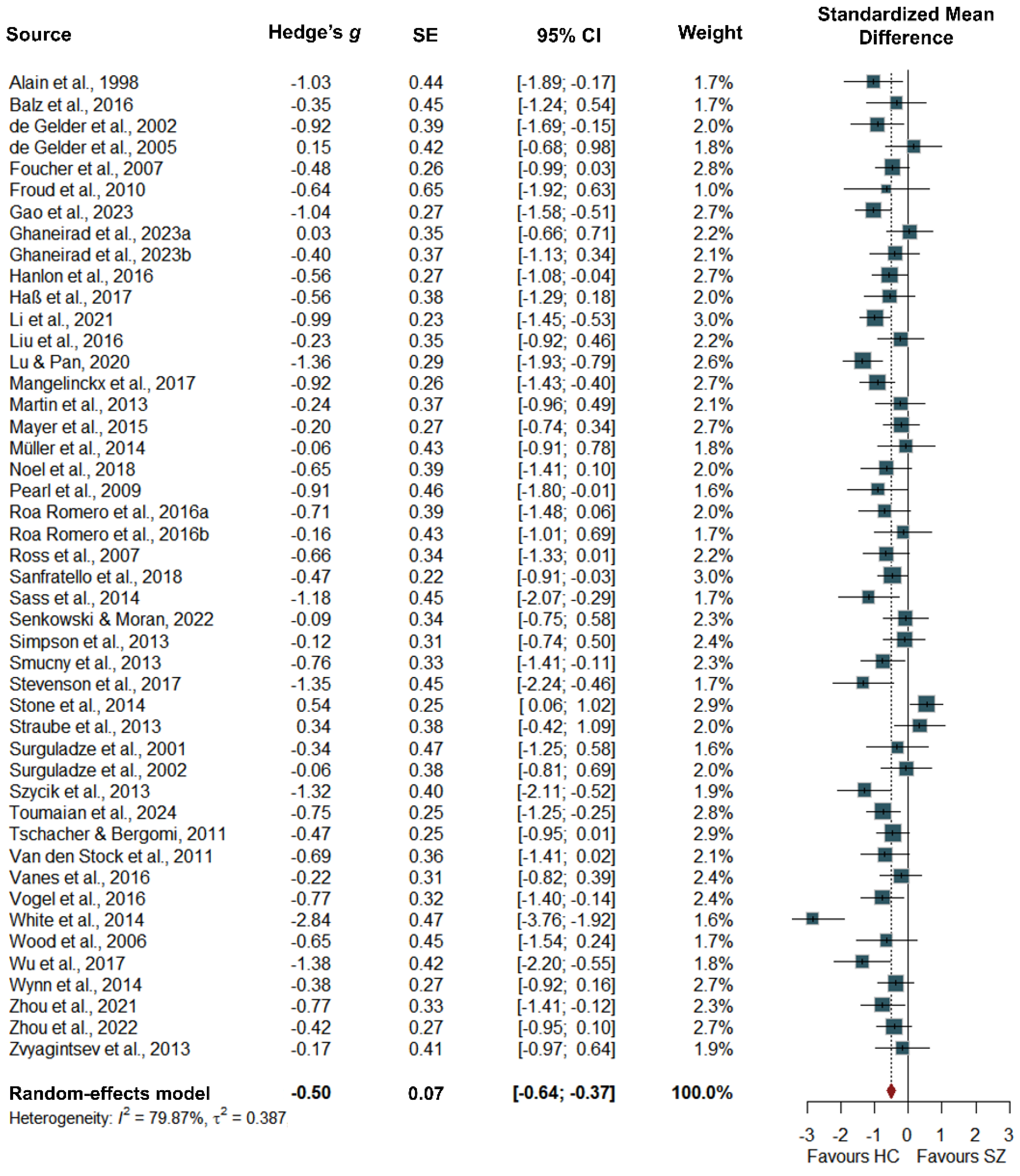
Group Differences
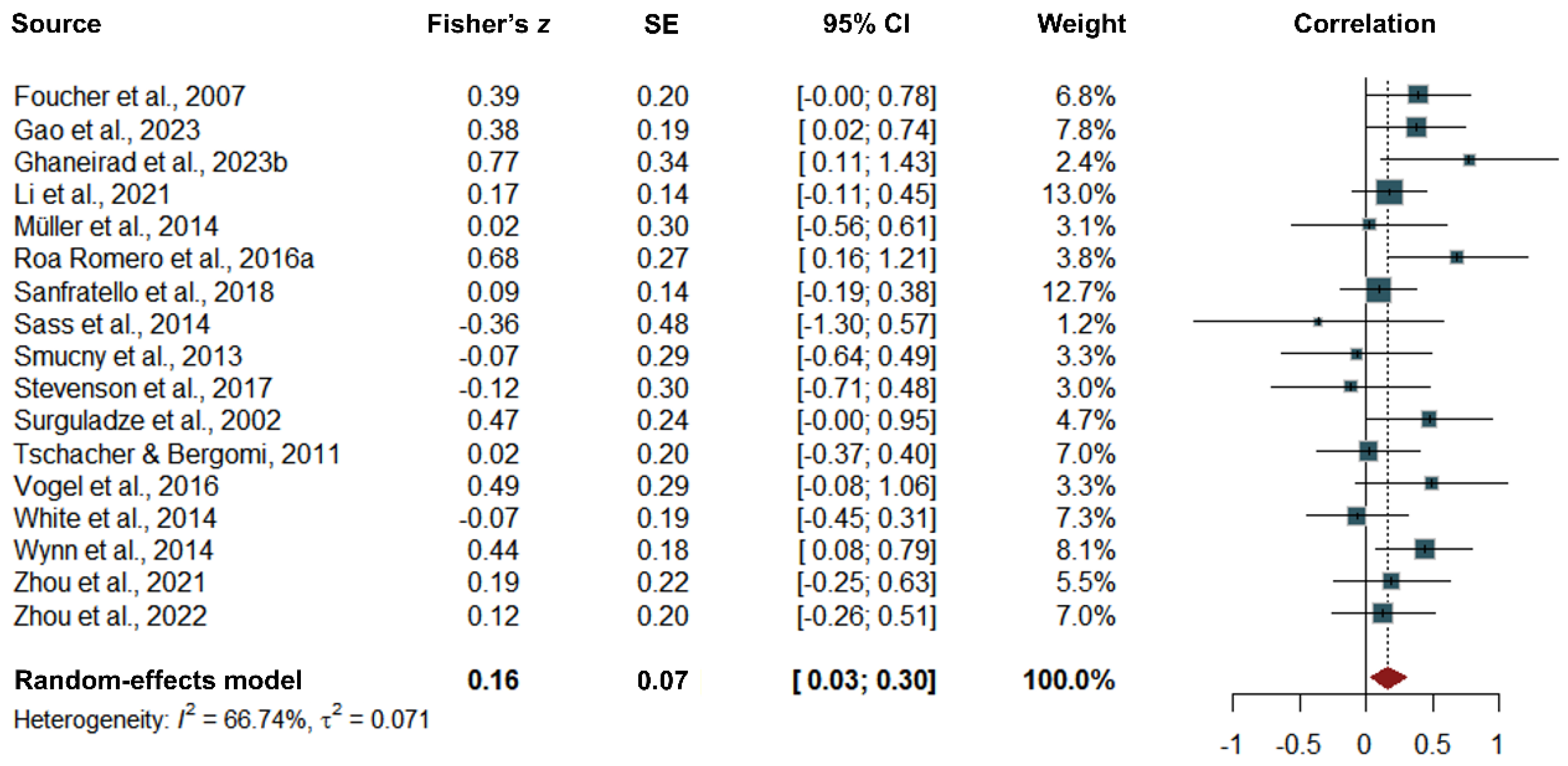
Correlations
Moderator Analyses
Quality Assessment, Publication Bias and Sensitivity Analyses
Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Haß K, Sinke C, Reese T, et al. Enlarged temporal integration window in schizophrenia indicated by the double-flash illusion. Cogn Neuropsychiatry. [CrossRef]
- Noel JP, Stevenson RA, Wallace MT. Atypical audiovisual temporal function in autism and schizophrenia: Similar phenotype, different cause. Eur J Neurosci. 1230. [CrossRef]
- Vanes LD, White TP, Wigton RL, Joyce D, Collier T, Shergill SS. Reduced susceptibility to the sound-induced flash fusion illusion in schizophrenia. Psychiatry Res. [CrossRef]
- Wallace MT, Stevenson RA. The construct of the multisensory temporal binding window and its dysregulation in developmental disabilities. Neuropsychologia. [CrossRef]
- Mayer AR, Hanlon FM, Teshiba TM, et al. An fMRI study of multimodal selective attention in schizophrenia. Br J Psychiatry. [CrossRef]
- Wood SM, Potts GF, Hall JF, Ulanday JB, Netsiri C. Event-related potentials to auditory and visual selective attention in schizophrenia. Int J Psychophysiol. [CrossRef]
- Gao T, Wang X, Cen H, et al. Cross-modal associative memory impairment in schizophrenia. Neuropsychologia, 1087. [CrossRef]
- Ghaneirad E, Saenger E, Szycik GR, et al. Deficient audiovisual speech perception in schizophrenia: an ERP study. Brain Sci. [CrossRef]
- Ross LA, Saint-Amour D, Leavitt VM, Molholm S, Javitt DC, Foxe JJ. Impaired multisensory processing in schizophrenia: Deficits in the visual enhancement of speech comprehension under noisy environmental conditions. Schizophr Res. [CrossRef]
- Mangelinckx C, Belge JB, Maurage P, Constant E. Impaired facial and vocal emotion decoding in schizophrenia is underpinned by basic perceptivo-motor deficits. Cogn Neuropsychiatry. [CrossRef]
- Van den Stock J, de Jong SJ, Hodiamont PP, de Gelder B. Perceiving emotions from bodily expressions and multisensory integration of emotion cues in schizophrenia. Soc Neurosci. [CrossRef]
- Zvyagintsev M, Parisi C, Chechko N, Nikolaev AR, Mathiak K. Attention and multisensory integration of emotions in schizophrenia. Front Hum Neurosci. [CrossRef]
- Liu T, Pinheiro AP, Zhao Z, Nestor PG, McCarley RW, Niznikiewicz M. Simultaneous face and voice processing in schizophrenia. Behav Brain Res. [CrossRef]
- Senkowski D, Moran JK. Early evoked brain activity underlies auditory and audiovisual speech recognition deficits in schizophrenia. Neuroimage Clin, 1029. [CrossRef]
- Gao C, Green JJ, Yang X, Oh S, Kim J, Shinkareva SV. Audiovisual integration in the human brain: a coordinate-based meta-analysis. Cereb Cortex, 5574. [CrossRef]
- Ding H, Zhang Y. Speech prosody in mental disorders. Annu Rev Linguist. [CrossRef]
- Adámek P, Langová V, Horáček J. Early-stage visual perception impairment in schizophrenia, bottom-up and back again. Schizophrenia. [CrossRef]
- Javitt DC, Sweet RA. Auditory dysfunction in schizophrenia: integrating clinical and basic features. Nat Rev Neurosci. [CrossRef]
- Li Z, Huang J, Hung KSY, et al. Cerebellar hypoactivation is associated with impaired sensory integration in schizophrenia. J Abnorm Psychol. [CrossRef]
- Pearl D, Yodashkin-Porat D, Katz N, et al. Differences in audiovisual integration, as measured by McGurk phenomenon, among adult and adolescent patients with schizophrenia and age-matched healthy control groups. Compr Psychiatry. [CrossRef]
- Stevenson RA, Park S, Cochran C, et al. The associations between multisensory temporal processing and symptoms of schizophrenia. Schizophr Res. [CrossRef]
- Surguladze SA, Calvert GA, Brammer MJ, et al. Audio-visual speech perception in schizophrenia: An fMRI study. Psychiatry Res Neuroimaging. [CrossRef]
- Feldman JI, Dunham K, Cassidy M, Wallace MT, Liu Y, Woynaroski TG. Audiovisual multisensory integration in individuals with autism spectrum disorder: a systematic review and meta-analysis. Neurosci Biobehav Rev. [CrossRef]
- Gröhn C, Norgren E, Eriksson L. A systematic review of the neural correlates of multisensory integration in schizophrenia. Schizophr Res Cogn. [CrossRef]
- Tseng HH, Bossong MG, Modinos G, et al. A systematic review of multisensory cognitive-affective integration in schizophrenia. Neurosci Biobehav Rev. [CrossRef]
- Bremner AJ, Lewkowicz DJ, Spence C. The multisensory approach to development, 1: AJ, Lewkowicz DJ, Spence C, eds. Multisensory Development. Oxford, UK: Oxford University Press; 2012, 2012.
- Hershenson, M. Reaction time as a measure of intersensory facilitation. J Exp Psychol. [CrossRef]
- Miller, J. Divided attention: Evidence for coactivation with redundant signals. Cogn Psychol. [CrossRef]
- de Boer-Schellekens L, Stekelenburg JJ, Maes JP, Van Gool AR, Vroomen J. Sound improves diminished visual temporal sensitivity in schizophrenia. Acta Psychol. [CrossRef]
- Martin B, Giersch A, Huron C, van Wassenhove V. Temporal event structure and timing in schizophrenia: preserved binding in a longer “now”. Neuropsychologia. [CrossRef]
- Szycik GR, Ye Z, Mohammadi B, et al. Maladaptive connectivity of Broca’s area in schizophrenia during audiovisual speech perception: An fMRI study. Neuroscience. [CrossRef]
- Gao C, Weber CE, Shinkareva SV. The brain basis of audiovisual affective processing: evidence from a coordinate-based activation likelihood estimation meta-analysis. Cortex. [CrossRef]
- Balz J, Roa Romero Y, Keil J, et al. Beta/Gamma oscillations and event-related potentials indicate aberrant multisensory processing in schizophrenia. Front Psychol, 1896. [CrossRef]
- Roa Romero Y, Keil J, Balz J, Niedeggen M, Gallinat J, Senkowski D. Alpha-band oscillations reflect altered multisensory processing of the McGurk illusion in schizophrenia. Front Hum Neurosci. [CrossRef]
- Stone DB, Urrea LJ, Aine CJ, Bustillo JR, Clark VP, Stephen JM. Unisensory processing and multisensory integration in schizophrenia: a high-density electrical mapping study. Neuropsychologia, 3178. [CrossRef]
- Thaler NS, Strauss GP, Sutton GP, et al. Emotion perception abnormalities across sensory modalities in bipolar disorder with psychotic features and schizophrenia. Schizophr Res. [CrossRef]
- Zvyagintsev M, Parisi C, Mathiak K. Temporal processing deficit leads to impaired multisensory binding in schizophrenia. Cogn Neuropsychiatry. [CrossRef]
- Foucher JR, Lacambre M, Pham BT, Giersch A, Elliott MA. Low time resolution in schizophrenia: lengthened windows of simultaneity for visual, auditory and bimodal stimuli. Schizophr Res. [CrossRef]
- Müller VI, Kellermann TS, Seligman SC, Turetsky BI, Eickhoff SB. Modulation of affective face processing deficits in schizophrenia by congruent emotional sounds. Soc Cogn Affect Neurosci. [CrossRef]
- Vogel BD, Bruck C, Jacob H, Eberle M, Wildgruber D. Effects of cue modality and emotional category on recognition of nonverbal emotional signals in schizophrenia. BMC Psychiatry. [CrossRef]
- Zhou HY, Cui XL, Yang BR, et al. Audiovisual temporal processing in children and adolescents with schizophrenia and children and adolescents with autism: evidence from simultaneity-judgment tasks and eye-tracking data. Clin Psychol Sci. [CrossRef]
- Lema YY, Gamo NJ, Yang K, Ishizuka K. Trait and state biomarkers for psychiatric disorders: Importance of infrastructure to bridge the gap between basic and clinical research and industry. Psychiat Clin Neurosci. [CrossRef]
- Garrido-Vásquez P, Jessen S, Kotz SA. Perception of emotion in psychiatric disorders: on the possible role of task, dynamics, and multimodality. Soc Neurosci. [CrossRef]
- Lin Y, Ding H, Zhang Y. Multisensory integration of emotion in schizophrenic patients. Multisens Res. [CrossRef]
- Thoenes S, Oberfeld D. Meta-analysis of time perception and temporal processing in schizophrenia: differential effects on precision and accuracy. Clin Psychol Rev. [CrossRef]
- Zhou HY, Cai XL, Weigl M, Bang P, Cheung EF, Chan RC. Multisensory temporal binding window in autism spectrum disorders and schizophrenia spectrum disorders: a systematic review and meta-analysis. Neurosci Biobehav Rev. [CrossRef]
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med, 1000. [CrossRef]
- Hornix BE, Havekes R, Kas MJH. Multisensory cortical processing and dysfunction across the neuropsychiatric spectrum. Neurosci Biobehav Rev. [CrossRef]
- Aslam S, Emmanuel P. Formulating a researchable question: a critical step for facilitating good clinical research. Indian J Sex Transm Dis Aids. [CrossRef]
- Moran JK, Keil J, Masurovsky A, Gutwinski S, Montag C, Senkowski D. Multisensory processing can compensate for top-down attention deficits in schizophrenia. Cereb Cortex, 5536. [CrossRef]
- Nikitova N, Keane BP, Demmin D, Silverstein SM, Uhlhaas PJ. The audio-visual abnormalities questionnaire (AVAQ): development and validation of a new instrument for assessing anomalies in sensory perception in schizophrenia spectrum disorders. Schizophr Res. [CrossRef]
- Curtis MT, Ren X, Coffman BA, Salisbury DF. Attentional M100 gain modulation localizes to auditory sensory cortex and is deficient in first-episode psychosis. Hum Brain Mapp. [CrossRef]
- Stekelenburg JJ, Maes JP, Van Gool AR, Sitskoorn M, Vroomen J. Deficient multisensory integration in schizophrenia: an event-related potential study. Schizophr Res. [CrossRef]
- Hirano Y, Nakamura I, Tamura S. Abnormal connectivity and activation during audiovisual speech perception in schizophrenia. Eur J Neurosci, 1918. [CrossRef]
- Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Commun Health. [CrossRef]
- Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses, /: Health Research Institute. Available from: http.
- Viechtbauer W, Cheung MW. Outlier and influence diagnostics for meta-analysis. Res Synth Methods. [CrossRef]
- Pustejovsky, JE. clubSandwich: cluster-robust (sandwich) variance estimators with small-sample corrections. 2022. Available from: URL: https://CRAN.R-project.
- Pustejovsky JE, Tipton E. Meta-analysis with robust variance estimation: expanding the range of working models. Prev Sci. [CrossRef]
- Hedges LV, Tipton E, Johnson MC. Robust variance estimation in meta-regression with dependent effect size estimates. Res Synth Methods. [CrossRef]
- Tipton, E. Small sample adjustments for robust variance estimation with meta-regression. Psychol Methods. [CrossRef]
- Radua J, Mataix-Cols D, Phillips ML, et al. A new meta-analytic method for neuroimaging studies that combines reported peak coordinates and statistical parametric maps. Eur Psychiatry. [CrossRef]
- Albajes-Eizagirre A, Solanes A, Vieta E, Radua J. Voxel-based meta-analysis via permutation of subject images (PSI): theory and implementation for SDM. Neuroimage. [CrossRef]
- Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. J Clin Epidemiol. [CrossRef]
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ, 7109. [CrossRef]
- Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. [CrossRef]
- Rosenthal, R. Meta-analytic procedures for social research. Thousand Oaks, CA: SAGE; 1991.
- Sass K, Heim S, Sachs O, et al. Neural correlates of semantic associations in patients with schizophrenia. Eur Arch Psychiatry Clin Neurosci. [CrossRef]
- Smucny J, Rojas DC, Eichman LC, Tregellas JR. Neural effects of auditory distraction on visual attention in schizophrenia. PLoS One, 6060. [CrossRef]
- Straube B, Green A, Sass K, Kirner-Veselinovic A, Kircher T. Neural integration of speech and gesture in schizophrenia: evidence for differential processing of metaphoric gestures. Hum Brain Mapp, 1696. [CrossRef]
- Stone DB, Coffman BA, Bustillo JR, Aine CJ, Stephen JM. Multisensory stimuli elicit altered oscillatory brain responses at gamma frequencies in patients with schizophrenia. Front Hum Neurosci. [CrossRef]
- Surguladze S, Rossell S, Rabe-Hesketh S, David AS. Cross-modal semantic priming in schizophrenia. J Int Neuropsychol Soc. [CrossRef]
- Sanfratello L, Aine C, Stephen J. Neuroimaging investigations of dorsal stream processing and effects of stimulus synchrony in schizophrenia. Psychiatry Res Neuroimaging. [CrossRef]
- Wynn JK, Jahshan C, Green MF. Multisensory integration in schizophrenia: a behavioral and event-related potential study. Cogn Neuropsychiatry. [CrossRef]
- de Jong JJ, Hodiamont PP, de Gelder B. Modality-specific attention and multisensory integration of emotions in schizophrenia: reduced regulatory effects. Schizophr Res. [CrossRef]
- de Gelder B, Vroomen J, de Jong SJ, Masthoff ED, Trompenaars FJ, Hodiamont P. Multisensory integration of emotional faces and voices in schizophrenics. Schizophr Res. [CrossRef]
- Zhou HY, Lai IYS, Hung KSY, et al. Audiovisual temporal processing in adult patients with first-episode schizophrenia and high-functioning autism. Schizophrenia. [CrossRef]
- Jones SA, Noppeney U. Multisensory integration and causal inference in typical and atypical populations, 5: Y, Zaidel A, eds. Advances of Multisensory Integration in the Brain. Adv Exp Med Biol. Singapore: Springer; 2024;1437, 2024. [CrossRef]
- Roa Romero Y, Keil J, Balz J, Gallinat J, Senkowski D. Reduced frontal theta oscillations indicate altered crossmodal prediction error processing in schizophrenia. J Neurophysiol, 1396. [CrossRef]
- Brown C, Cromwell RL, Filion D, Dunn W, Tollefson N. Sensory processing in schizophrenia: missing and avoiding information. Schizophr Res. [CrossRef]
- van den Boogert F, Klein K, Spaan P, et al. Sensory processing difficulties in psychiatric disorders: a meta-analysis. J Psychiatr Res. [CrossRef]
- Zhou HY, Yang HX, Cui XL, et al. Self-reported sensory responsiveness patterns in typically-developing and early-onset schizophrenia adolescents: its relationship with schizotypal and autistic traits. J Psychiatr Res. [CrossRef]
- Dondé C, Mondino M, Brunelin J, Haesebaert F. Sensory-targeted cognitive training for schizophrenia. Expert Rev Neurother. [CrossRef]
- Bechi M, Bosia M, Spangaro M, et al. Visual and audio emotion processing training for outpatients with schizophrenia: an integrated multisensory approach. Neuropsychol Rehabil, 1131. [CrossRef]
- Scoriels L, Genaro LT, Keffer S, et al. Changes in emotion processing and social cognition with auditory versus visual neuroscience-informed cognitive training in individuals with schizophrenia. Schizophr Res. [CrossRef]
- Jeong JW, Kim HT, Lee SH, Lee H. Effects of an audiovisual emotion perception training for schizophrenia: a preliminary study. Front Psychiatry, 5220. [CrossRef]
- Zerr M, Freihorst C, Schütz H, et al. Brief sensory training narrows the temporal binding window and enhances long-term multimodal speech perception. Front Psychol, 2489. [CrossRef]
- Powers AR III, Hillock-Dunn A, Wallace MT. Generalization of multisensory perceptual learning. Sci Rep, 2337. [CrossRef]
- De Niear MA, Gupta PB, Baum SH, Wallace MT. Perceptual training enhances temporal acuity for multisensory speech. Neurobiol Learn Mem. [CrossRef]
- Stein BE, Stanford TR. Multisensory integration: current issues from the perspective of the single neuron. Nat Rev Neurosci. [CrossRef]
| ESa | SZ | HC | Experiment Methods | |||||||||||
| Cluster/Study | g | r | n | % male | Mage | n | % male | Mage | Task(s) | Stimuli | ||||
| Alain et al. (1998) | 5 | 0 | 15 | 100.00% | 46.00 | 15 | 53.33% | 46.00 | TI | flash-beep | ||||
| Balz et al. (2016) | 3 | 0 | 15 | 80.00% | 33.87 | 15 | 80.00% | 36.13 | SIFI | flash-beep | ||||
| de Gelder et al. (2002) | 1 | 0 | 18 | 83.33% | 36.40 | 12 | 91.67% | 41.20 | McGurk | face-syllable | ||||
| de Gelder et al. (2005) | 2 | 0 | 13 | 84.62% | 37.00 | 13 | N/A | N/A | EI | face-sentence/prosody | ||||
| Foucher et al. (2007) | 2 | 1 | 29 | 70.00% | 33.00 | 33 | 66.67% | 32.00 | SJ | flash-beep | ||||
| Froud et al. (2010) | 5 | 0 | 6 | 50.00% | 44.90 | 7 | 42.86% | 43.20 | LD | word-word | ||||
| Gao et al. (2023) | 1 | 1 | 32 | 59.38% | 28.30 | 29 | 51.72% | 28.00 | LTM | picture-sound | ||||
| Ghaneirad et al. (2023a) | 8 | 0 | 21 | 61.90% | 37.86 | 20 | 33.33% | 31.90 | LTM | picture-sound | ||||
| Ghaneirad et al. (2023b) | 8 | 5 | 20 | 60.00% | 41.90 | 21 | 57.14% | 38.20 | SN: WI | face-word | ||||
| Hanlon et al. (2016) | 8 | 0 | 33 | 81.82% | 38.09 | 34 | 82.35% | 37.50 | TI | flash-beep | ||||
| Haß et al. (2017) | 2 | 0 | 15 | 46.67% | 41.60 | 15 | 46.67% | 39.50 | SIFI | flash-beep | ||||
| Li et al. (2021) | 5 | 2 | 52 | 51.90% | 26.33 | 52 | 44.20% | 27.85 | TI | flash-beep | ||||
| Liu et al. (2016) | 10 | 0 | 18 | 94.12% | 46.90 | 19 | 94.44% | 44.60 | TI | face-burst | ||||
| Lu & Pan (2020) | 1 | 0 | 33 | 51.50% | 31.79 | 26 | 46.15% | 32.38 | MJ | face-syllable | ||||
| Mangelinckx et al. (2017) | 2 | 0 | 32 | 53.13% | 47.31 | 32 | N/A | 46.41 | EI | face-word/prosody | ||||
| Martin et al. (2013) | 7 | 0 | 26 | 65.38% | 37.00 | 26 | 65.38% | 38.60 | McGurk, SJ | face-syllable | ||||
| Mayer et al. (2015) | 6 | 0 | 33 | 87.88% | 36.00 | 33 | 87.88% | 34.55 | TI | number-number | ||||
| Müller et al. (2014) | 4 | 18 | 15 | 73.33% | 35.10 | 15 | 80.00% | 40.80 | EI | face-burst | ||||
| Pearl et al. (2009) | 3 | 0 | 30 | 76.67% | 26.40 | 20 | 60.00% | 25.90 | McGurk | face-syllable | ||||
| Roa Romero et al. (2016a) | 6 | 1 | 17 | 70.59% | 35.24 | 17 | 76.47% | 36.00 | SI | face-syllable | ||||
| Roa Romero et al. (2016b) | 6 | 0 | 14 | 71.43% | 35.57 | 14 | 71.43% | 36.79 | McGurk | face-syllable | ||||
| Ross et al. (2007) | 1 | 0 | 18 | 94.44% | 39.00 | 18 | 61.11% | 35.00 | SN: WI | face-word | ||||
| Sanfratello et al. (2018) | 4 | 32 | 53 | 83.02% | 39.50 | 56 | 69.64% | 36.30 | DJ | soccer-beep | ||||
| Sass et al. (2014) | 8 | 3 | 14 | 57.14% | 36.40 | 14 | 85.71% | 31.20 | LD | word-word | ||||
| Senkowski & Moran (2022) | 5 | 0 | 24 | 62.50% | 39.12 | 21 | 57.14% | 37.76 | SN: SI | face-syllable | ||||
| Simpson et al. (2013) | 3 | 0 | 31 | 51.61% | 40.61 | 30 | 50.00% | 40.87 | EI | face-sentence/prosody | ||||
| Smucny et al. (2013) | 10 | 3 | 21 | 66.67% | 46.90 | 23 | 56.52% | 39.40 | TI | number-noise | ||||
| Stone et al. (2014) | 3 | 0 | 46 | 84.78% | 39.20 | 57 | 70.18% | 39.40 | DJ | soccer-beep | ||||
| Straube et al. (2013) | 21 | 0 | 16 | 62.50% | 38.00 | 16 | 100.00% | 27.90 | TI | face/gesture-sentence | ||||
| Surguladze et al. (2001) | 1 | 0 | 14 | 71.43% | 35.90 | 7 | 71.43% | 35.70 | WI | face-word | ||||
| Surguladze et al. (2002) | 3 | 1 | 20 | 55.00% | 34.00 | 24 | 46.15% | 30.30 | LD | word-word | ||||
| Szycik et al. (2013) | 2 | 0 | 15 | 46.67% | 38.20 | 15 | 46.67% | 36.50 | TI | face-word | ||||
| Toumaian et al. (2024) | 1 | 0 | 35 | 74.29% | 28.29 | 32 | 43.75% | 26.62 | TI | flash-beep | ||||
| Tschacher & Bergomi (2011) | 2 | 6 | 34 | 79.41% | 27.88 | 34 | 76.47% | 27.94 | CJ | flash-beep | ||||
| Van den Stock et al. (2011) | 2 | 0 | 16 | 93.80% | 36.80 | 16 | 56.30% | 38.00 | EI | body action-human burst | ||||
| Vanes et al. (2016) | 4 | 0 | 40 | 80.00% | 37.00 | 22 | 86.00% | 36.40 | SIFI | flash-beep | ||||
| Vogel et al. (2016) | 1 | 3 | 21 | 61.90% | 36.52 | 21 | 61.90% | 36.29 | EI | face-word/prosody | ||||
| Wallace Cluster | ||||||||||||||
| Noel et al. (2018) | 2 | 0 | 14 | 50.00% | 42.30 | 15 | 37.50% | 41.90 | SJ | face-syllable | ||||
| Stevenson et al. (2017) | 4 | 6 | 16 | 50.00% | 42.30 | 16 | 37.50% | 41.90 | SJ | flash-beep; face-syllable | ||||
| White et al. (2014) | 1 | 2 | 30 | 90.00% | 39.03 | 24 | 87.50% | 36.67 | McGurk | face-syllable | ||||
| Wood et al. (2006) | 6 | 0 | 13 | N/A | 48.43 | 13 | N/A | 49.71 | TI | flash-beep | ||||
| Wu et al. (2017) | 5 | 0 | 22 | 63.55% | 29.00 | 16 | 43.75% | 27.44 | MJ | face-phrase | ||||
| Wynn et al. (2014) | 9 | 2 | 33 | 69.70% | 48.40 | 30 | 70.00% | 48.00 | TI | letter-beep | ||||
| Zhou et al. (2021) | 7 | 22 | 23 | 32.26% | 14.84 | 28 | 36.67% | 14.20 | SJ, AD | flash-beep; face-syllable; face-sentence | ||||
| Zhou et al. (2022) | 4 | 8 | 34 | 62.79% | 25.93 | 48 | 62.50% | 25.02 | SJ | flash-beep; face-syllable | ||||
| Zvyagintsev et al. (2013) | 5 | 0 | 15 | 65.00% | 42.90 | 18 | 65.00% | 42.50 | TI, EI | flash-beep; face-pseudowords | ||||
| Outcome | k | g/z (95% CI) | PI | p | τ2 | I2, % |
| Group difference | ||||||
| Overall | 209 | -0.50 (-0.64 to -0.37) | -1.73 to 0.72 | <.001 | 0.387 | 79.87 |
| MSI components | ||||||
| MM | 37 | -0.71 (-0.99 to -0.42) | -1.86 to 0.45 | < .001 | 0.324 | 73.31 |
| AVP | 141 | -0.53 (-0.71 to -0.35) | -1.73 to 0.67 | < .001 | 0.369 | 79.68 |
| MU | 31 | -0.23 (-0.56 to 0.11) | -1.23 to 0.78 | .225 | 0.234 | 71.14 |
| Measures | ||||||
| Behavioral | 108 | -0.52 (-0.66 to -0.37) | -1.65 to 0.62 | < .001 | 0.330 | 77.16 |
| Neural | 101 | -0.51 (-0.74 to -0.27) | -1.87 to 0.86 | < .001 | 0.469 | 82.78 |
| Correlation | ||||||
| Overall | 116 | 0.16 (0.03 to 0.30) | -0.38 to 0.70 | .030 | 0.071 | 66.74 |
| Measures | ||||||
| Behavioral | 56 | 0.10 (-0.10 to 0.29) | -0.57 to 0.76 | .348 | 0.107 | 70.42 |
| Neural | 60 | 0.25 (0.04 to 0.46) | -0.25 to 0.75 | .077 | 0.053 | 63.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





