Submitted:
09 September 2024
Posted:
11 September 2024
You are already at the latest version
Abstract
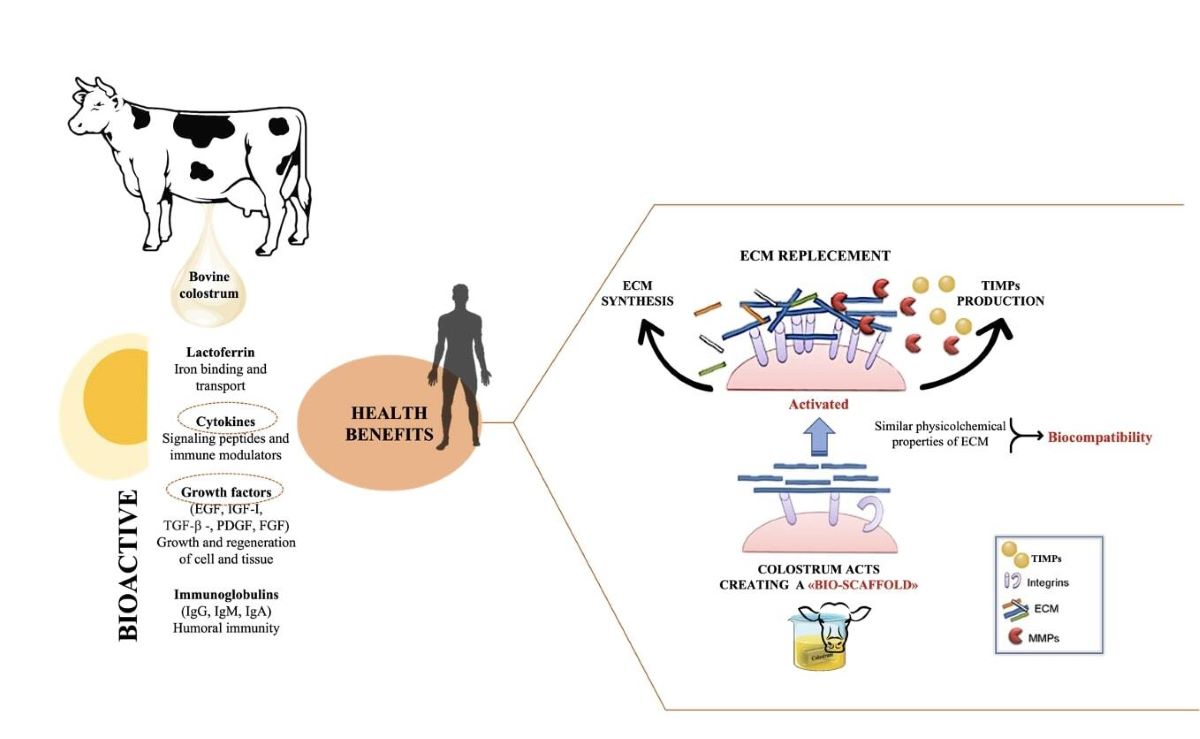
Keywords:
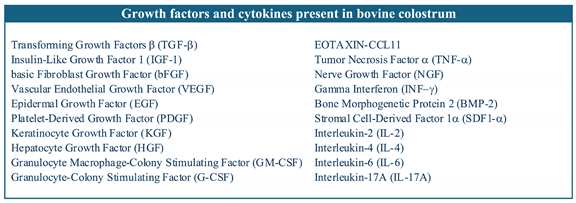
1. Introduction
2. Materials and Methods
2.1. Colostrum Derivative Mixture Preparation
2.2. Cell Lines
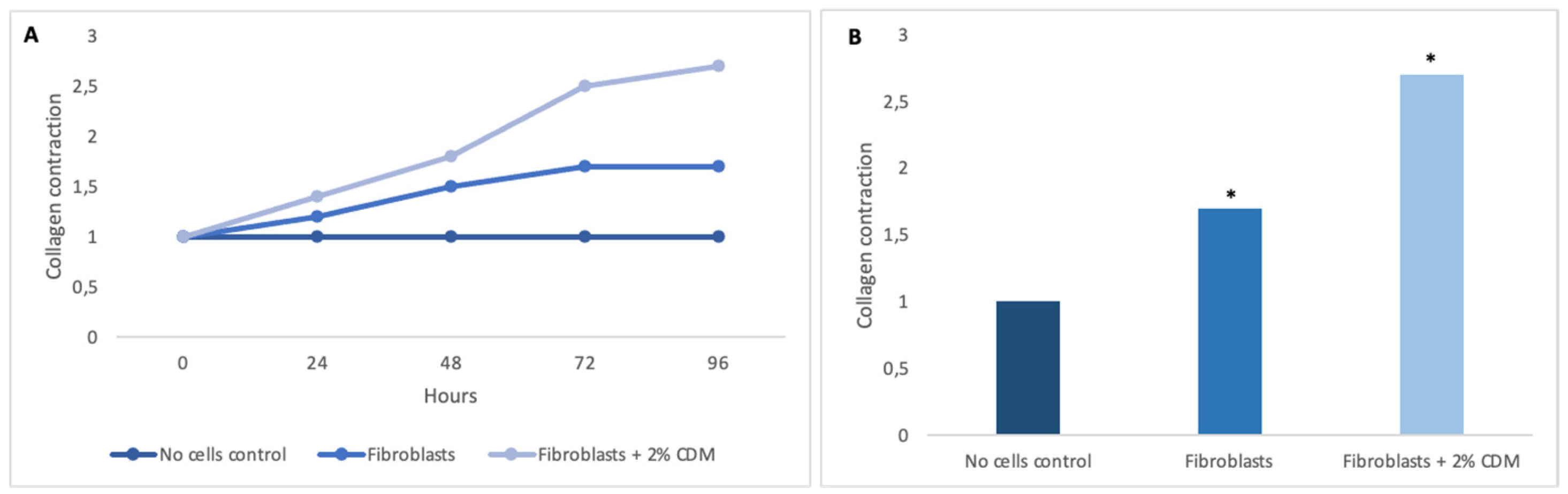
2.3. Collagen Contraction
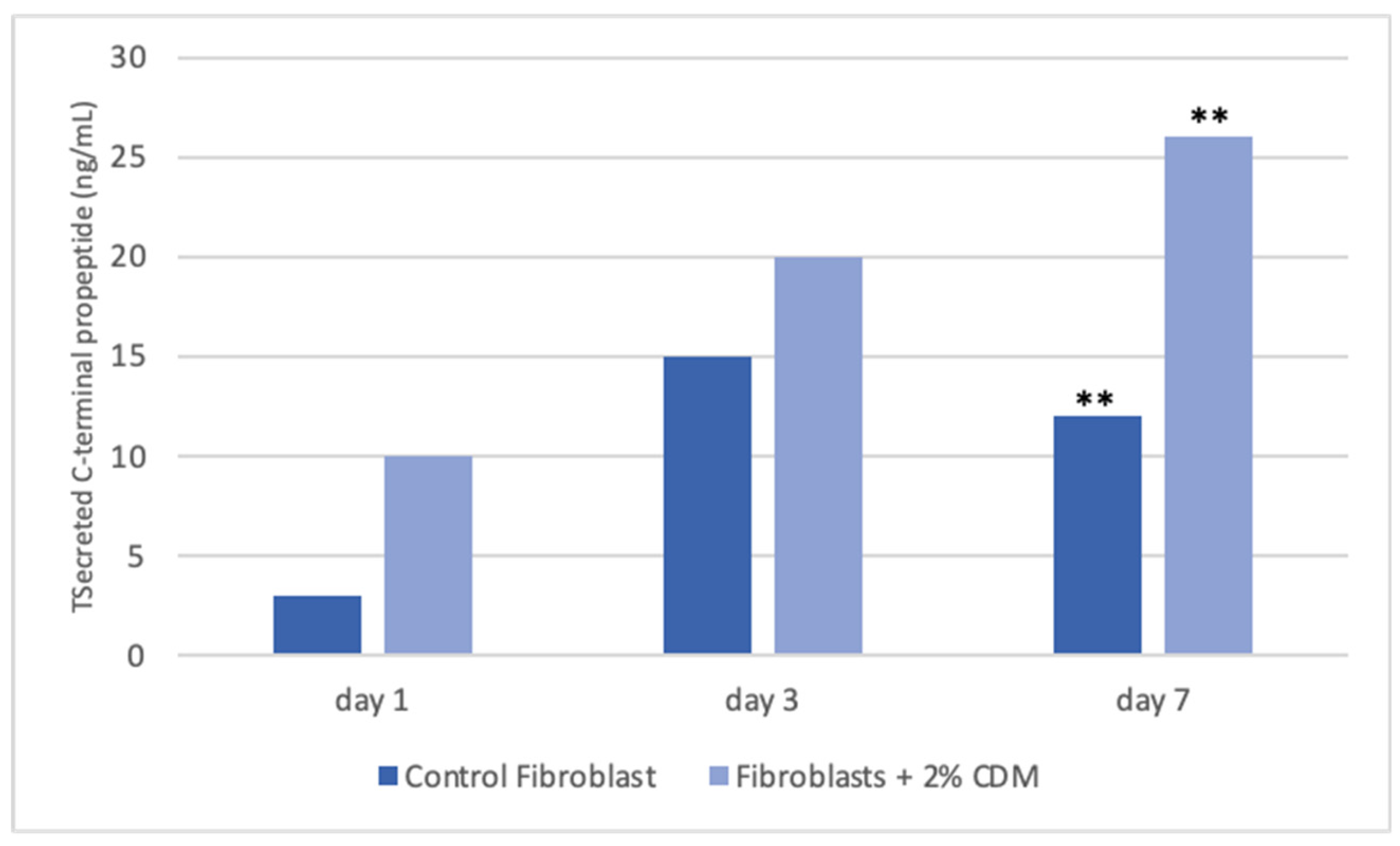
2.4. Collagen Production
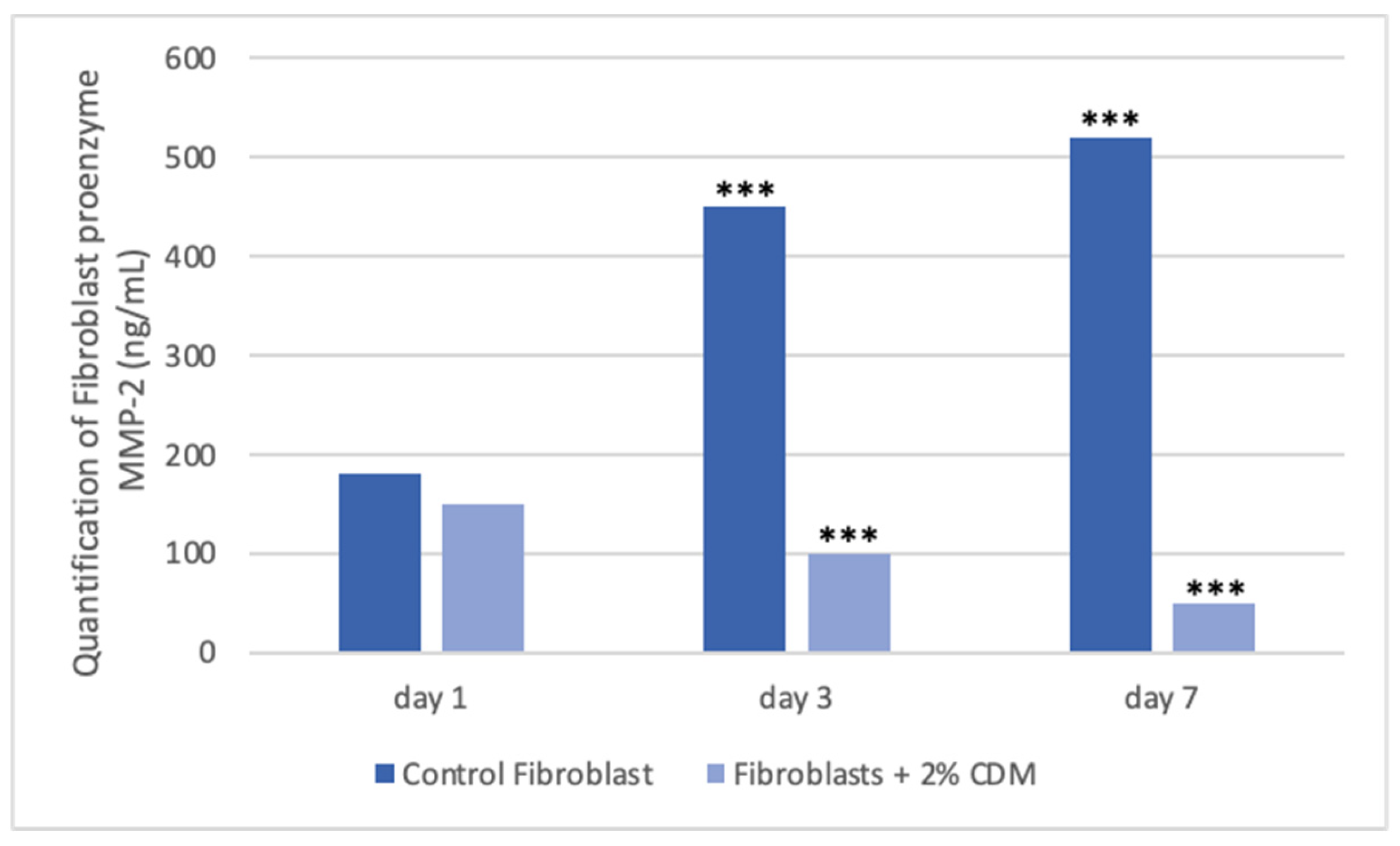
2.5. Determination of MMP-2 Concentration
2.6. Statistical Analysis
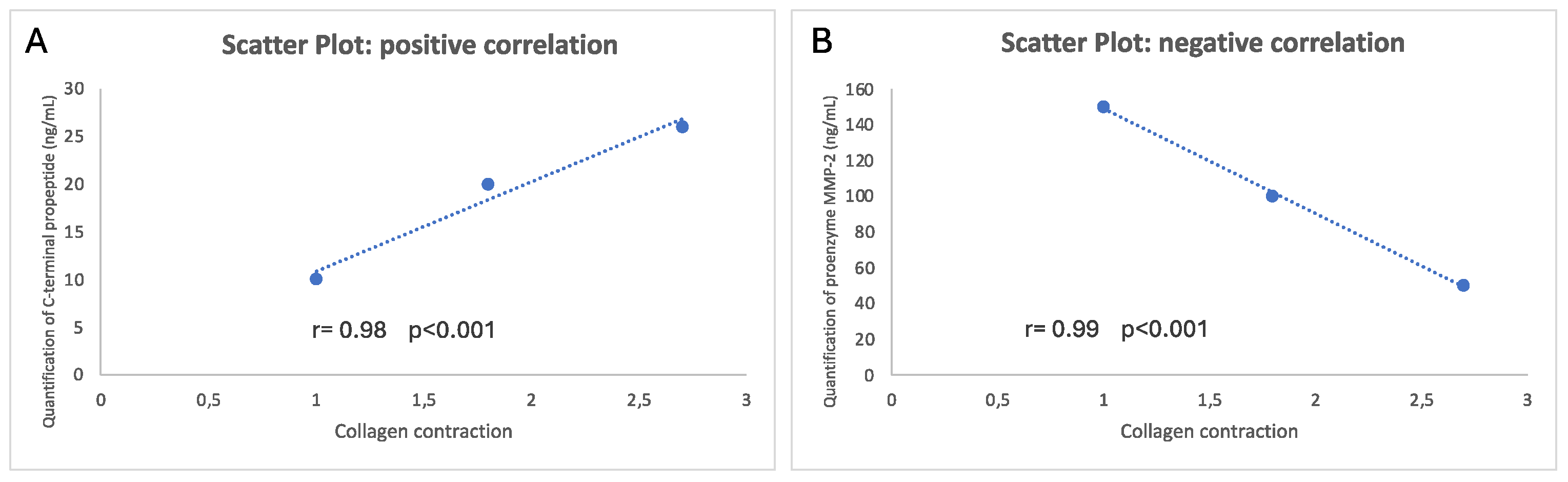
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Mierke, C.T. Bidirectional Mechanical Response Between Cells and Their Microenvironment. Front Phys 2021, 9, 749830. [Google Scholar] [CrossRef]
- Xie, N.; Xiao, C.; Shu, Q.; Cheng, B.; Wang, Z.; Xue, R.; Wen, Z.; Wang, J.; Shi, H.; Fan, D.; Liu, N.; Xu, F. Cell response to mechanical microenvironment cues via Rho signaling: From mechanobiology to mechanomedicine. Acta Biomater 2023, 159, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Jansen KA, Donato DM, Balcioglu HE, Schmidt T, Danen EH, Koenderink GH. A guide to mechanobiology: Where biology and physics meet. Biochim Biophys Acta. 2015, 1853, 3043–3052. [Google Scholar] [CrossRef]
- Boyd, D.F.; Thomas, P.G. Towards integrating extracellular matrix and immunological pathways. Cytokine 2017, 98, 79–86. [Google Scholar] [CrossRef]
- Di, X.; Gao, X.; Peng, L.; Ai, J.; Jin, X.; Qi, S.; Li, H.; Wang, K.; Luo, D. Cellular mechanotransduction in health and diseases: from molecular mechanism to therapeutic targets. Sig Transduct Target Ther 2023, 8, 282. [Google Scholar]
- Lu, P.; Takai, K.; Weaver, V.M.; Werb, Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol 2011, 3, a005058. [Google Scholar] [CrossRef] [PubMed]
- Caley, M.P.; Martins, V.L.; O’Toole, E.A. Metalloproteinases and Wound Healing. Adv Wound Care (New Rochelle) 2015, 4, 225–234. [Google Scholar] [CrossRef]
- Zhao, X.; Li, Q.; Guo, Z.; Li, Z. Constructing a cell microenvironment with biomaterial scaffolds for stem cell therapy. Stem Cell Res Ther 2021, 12, 583. [Google Scholar] [CrossRef]
- Kozaniti, F.K.; Manara, A.E.; Kostopoulos, V.; Mallis, P.; Michalopoulos, E.; Polyzos, D.; Deligianni, D.D.; Portan, D.V. Computational and Experimental Investigation of the Combined Effect of Various 3D Scaffolds and Bioreactor Stimulation on Human Cells’ Feedback. Appl Biosci 2023, 2, 249–277. [Google Scholar] [CrossRef]
- Caliari, S.R.; Harley, B.A. The effect of anisotropic collagen-GAG scaffolds and growth factor supplementation on tendon cell recruitment, alignment, and metabolic activity. Biomaterials 2011, 32, 5330–5340. [Google Scholar] [CrossRef]
- Harmansa, S.; Erlich, A.; Eloy, C.; Zurlo, G.; Lecuit, T. Growth anisotropy of the extracellular matrix shapes a developing organ. Nat Commun 2023, 14, 1220. [Google Scholar] [CrossRef] [PubMed]
- Datta, P.; Vyas, V.; Dhara, S.; Chowdhury, A.; Barui, A. Anisotropy Properties of Tissues: A Basis for Fabrication of Biomimetic Anisotropic Scaffolds for Tissue Engineering. J Bionic Eng 2019, 16, 842–868. [Google Scholar] [CrossRef]
- Reid, J.A.; Dwyer, K.D.; Schmitt, P.R.; Soepriatna, A.H.; Coulombe, K.L.; Callanan, A. Architected fibrous scaffolds for engineering anisotropic tissues. Biofabrication 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Ross, T.D.; Coon, B.G.; Yun, S.; Baeyens, N.; Tanaka, K.; Ouyang, M.; Schwartz, M.A. Integrins in mechanotransduction. Curr Opin Cell Biol 2013, 25, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Ross, M.; Atalla, H.; Karrow, N.; Mallard, B.A. The bioactivity of colostrum and milk exosomes of high, average, and low immune responder cows on human intestinal epithelial cells. J Dairy Sci 2021, 104, 2499–2510. [Google Scholar] [CrossRef]
- Yue, B. Biology of the extracellular matrix: an overview. J Glaucoma 2014, 23(8 Suppl 1), S20-3. [Google Scholar] [CrossRef]
- Diller, R.B.; Tabor, A.J. The Role of the Extracellular Matrix (ECM) in Wound Healing: A Review. Biomimetics (Basel) 2022, 7, 87. [Google Scholar] [CrossRef]
- Kuehlmann, B.; Bonham, C.A.; Zucal, I.; Prantl, L.; Gurtner, G.C. Mechanotransduction in Wound Healing and Fibrosis. J Clin Med 2020, 9, 1423. [Google Scholar] [CrossRef]
- Daniels, J.T.; Cambrey, A.D.; Occleston, N.L.; Garrett, Q.; Tarnuzzer, R.W.; Schultz, G.S.; Khaw, P.T. Matrix metalloproteinase inhibition modulates fibroblast-mediated matrix contraction and collagen production in vitro. Invest Ophthalmol Vis Sci 2003, 44, 1104–1110. [Google Scholar] [CrossRef]
- Singh, D.; Rai, V.; Agrawal, D.K. Regulation of Collagen I and Collagen III in Tissue Injury and Regeneration. Cardiol Cardiovasc Med 2023, 7, 5–16. [Google Scholar] [CrossRef]
- Sacerdote, P.; Mussano, F.; Franchi, S.; Panerai, A.E.; Bussolati, G.; Carossa, S.; Bartorelli, A.; Bussolati, B. Biological components in a standardized derivative of bovine colostrum. J Dairy Sci 2013, 96, 1745–1754. [Google Scholar] [CrossRef]
- Hu, M.; Ling, Z.; Ren, X. Extracellular matrix dynamics: tracking in biological systems and their implications. J Biol Eng 2022, 16, 13. [Google Scholar] [CrossRef] [PubMed]
- Franchi, M.; Piperigkou, Z.; Mastronikolis, N.S.; Karamanos, N. Extracellular matrix biomechanical roles and adaptation in health and disease. FEBS J 2024, 291, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Holle, A.W.; Young, J.L.; Van Vliet, K.J.; Kamm, R.D.; Discher, D.; Janmey, P.; Spatz, J.P.; Saif, T. Cell-Extracellular Matrix Mechanobiology: Forceful Tools and Emerging Needs for Basic and Translational Research. Nano Lett 2018, 18, 1–8. [Google Scholar] [CrossRef]
- Arnold, M.; Cavalcanti-Adam, E.A.; Glass, R.; Blümmel, J.; Eck, W.; Kantlehner, M.; Kessler, H.; Spatz, J.P. Activation of integrin function by nanopatterned adhesive interfaces. Chemphyschem 2004, 5, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Boudreau, A.; Bissell, M.J. Tissue architecture and function: dynamic reciprocity via extra- and intra-cellular matrices. Cancer Metastasis Rev 2009, 28, 167–176. [Google Scholar] [CrossRef]
- Olczyk, P.; Mencner, Ł.; Komosinska-Vassev, K. The role of the extracellular matrix components in cutaneous wound healing. Biomed Res Int 2014, 2014, 747584. [Google Scholar] [CrossRef]
- Potekaev, N.N.; Borzykh, O.B.; Medvedev, G.V.; Pushkin, D.V.; Petrova, M.M.; Petrov, A.V.; Dmitrenko, D.V.; Karpova, E.I.; Demina, O.M.; Shnayder, N.A. The Role of Extracellular Matrix in Skin Wound Healing. J Clin Med 2021, 10, 5947. [Google Scholar] [CrossRef]
- Tracy, L.E.; Minasian, R.A.; Caterson, E.J. Extracellular Matrix and Dermal Fibroblast Function in the Healing Wound. Adv Wound Care (New Rochelle) 2016, 5, 119–136. [Google Scholar] [CrossRef]
- Kular, J.K.; Basu, S.; Sharma, R.I. The extracellular matrix: Structure, composition, age-related differences, tools for analysis and applications for tissue engineering. J Tissue Eng 2014, 5, 2041731414557112. [Google Scholar] [CrossRef]
- Sun, B. The mechanics of fibrillar collagen extracellular matrix. Cell Rep Phys Sci 2021, 2, 100515. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.E.; Parks, W.C. Metalloproteinases and their inhibitors: regulators of wound healing. Int J Biochem Cell Biol 2008, 40, 1334–1347. [Google Scholar] [CrossRef] [PubMed]
- Lukes, A.; Mun-Bryce, S.; Lukes, M.; Rosenberg, G.A. Extracellular matrix degradation by metalloproteinases and central nervous system diseases. Mol Neurobiol 1999, 19, 267–284. [Google Scholar] [CrossRef]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; Castruita-De la Rosa, C.; Ramirez-Acuña, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int J Mol Sci 2020, 21, 9739. [Google Scholar] [CrossRef]
- Burbridge, M.F.; Cogé, F.; Galizzi, J.P.; Boutin, J.A.; West, D.C.; Tucker, G.C. The role of the matrix metalloproteinases during in vitro vessel formation. Angiogenesis 2002, 5, 215–226. [Google Scholar] [CrossRef]
- Lombard, C.; Saulnier, J.; Wallach, J. Assays of matrix metalloproteinases (MMPs) activities: a review. Biochimie 2005, 87, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Agren, M.S.; Mirastschijski, U.; Karlsmark, T.; Saarialho-Kere, U.K. Topical synthetic inhibitor of matrix metalloproteinases delays epidermal regeneration of human wounds. Exp Dermatol 2001, 10, 337–348. [Google Scholar] [CrossRef]
- Wong, V.W.; Akaishi, S.; Longaker, M.T.; Gurtner, G.C. Pushing back: wound mechanotransduction in repair and regeneration. J Invest Dermatol 2011, 131, 2186–2196. [Google Scholar] [CrossRef] [PubMed]
- DuFort, C.C.; Paszek, M.J.; Weaver, V.M. Balancing forces: architectural control of mechanotransduction. Nat Rev Mol Cell Biol 2011, 12, 308–319. [Google Scholar] [CrossRef]
- Ricca, B.L.; Venugopalan, G.; Fletcher, D.A. To pull or be pulled: parsing the multiple modes of mechanotransduction. Curr Opin Cell Biol 2013, 25, 558–564. [Google Scholar] [CrossRef]
- Saraswathibhatla, A.; Indana, D.; Chaudhuri, O. Cell-extracellular matrix mechanotransduction in 3D. Nat Rev Mol Cell Biol 2023, 24, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Wei, X.; Kang, H.; Jiang, H.; Chu, Z.; Lin, Y.; Hou, Y.; Wei, Q. Static and Dynamic: Evolving Biomaterial Mechanical Properties to Control Cellular Mechanotransduction. Adv Sci (Weinh) 2023, 10, e2204594. [Google Scholar] [CrossRef] [PubMed]
- Urciuolo, F.; Imparato, G.; Netti, P.A. In vitro strategies for mimicking dynamic cell–ECM reciprocity in 3D culture models. Front Bioeng Biotechnol 2023, 11, 1197075. [Google Scholar] [CrossRef] [PubMed]
 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





