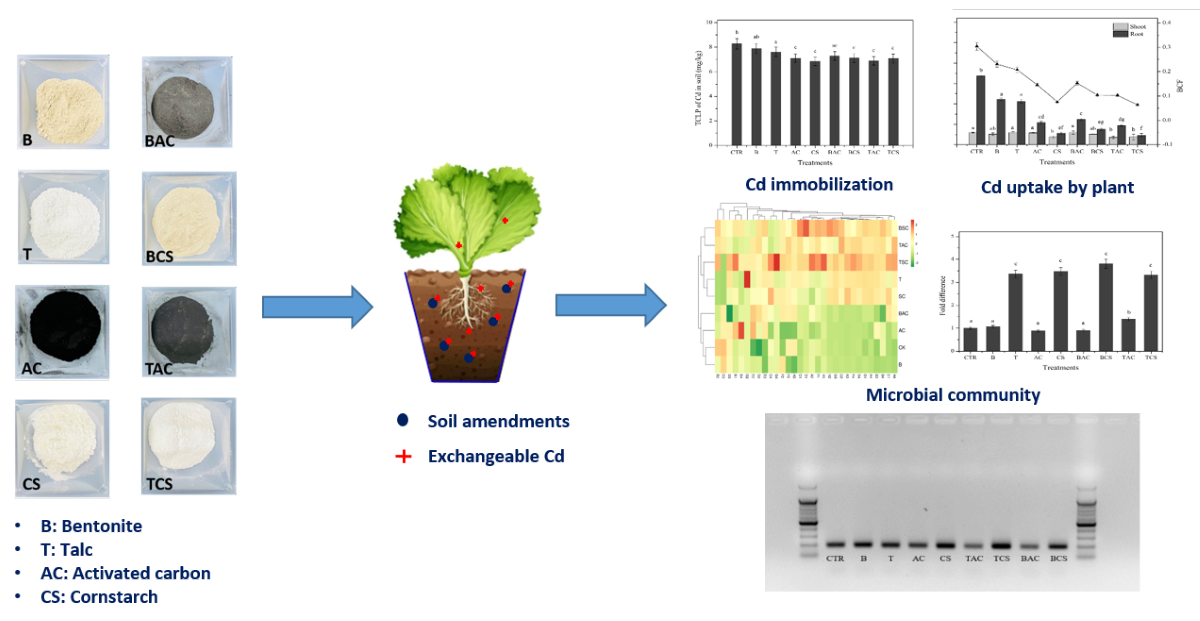
1. Introduction
The presence of cadmium (Cd) in the soil was considered a big problem due to its potential harm to human health through the food chain, even at low levels of accumulation. (Ali & Chidambaram, 2021). Cadmium is a non-essential element for plants but it can have a negative effect on plants including the inhibition of respiration, transpiration, photosynthesis, and mineral uptake if the accumulated amount exceeds the standard concentration.
Various technologies for remediation of Cd-contaminated soils have been reported, such as soil washing with a strong reagent (Rui et al., 2019), using materials with high specific surface area to absorb Cd in aqueous and soil phases (Li et al., 2022), and phytoremediation (Zhang et al., 2021). However, those remediation technologies still have many disadvantages that can limit their usage, such as the problem of chelator consumption, the washing effluent treatment methods, the growth of hyper-accumulators, and secondary pollution from Cd accumulation in biomass (Xu et al., 2019).
In recent years, immobilization technology for Cd remediation in soil has been developed rapidly due to the low cost, environmental friendliness, and community acceptance (Bolan et al., 2014). Although traditional amendments like lime, and natural clay minerals have significant effects on Cd immobilization, however, their limitations are also obvious (Nejad et al., 2018). For example, lime can increase soil pH trigger the precipitation of metal carbonates, oxides, or hydroxides, and reduce heavy metal solubility (Palansooriya et al., 2020). However, long-term application of lime can damage the soil aggregate structure, soil hardening, and the growth of crops, especially the soil microorganisms.
Recently, many studies have focused on applying commercial amendments like activated carbon, and bentonite for the immobilization of heavy metals in soil because of their cost-effectiveness, convenience, and wide application (Vrînceanu et al., 2019; Wu et al., 2016; Xie et al., 2018). Besides, talc and cornstarch were also recently applied for heavy metal ion (e.g., Cd2+) removal in water as absorbents due to the capacity of their surface of a functional cross-linking group (Kirn & Lim, 1999; Ossman et al., 2014). However, the application of talc and cornstarch in the remediation of heavy metals in soil is still limited. In addition, the research on the effect of activated carbon, bentonite, talc, and cornstarch (sole and combination) on the Cd immobilization, phytotoxicity, and soil microbial community in Cd-contaminated soil is lacking
This study investigated the effect of four amendments of activated carbon, bentonite, corn starch, and talc, and their combination on the availability of Cd immobilization in soil, Cd phytotoxicity assessed by lettuce (Lactuva sativa), and soil microorganisms. This study was also conducted to find which amendments were the best for not only reducing Cd mobility in soils and Cd uptake by plants but also supporting the soil ecology.
2. Materials and Methods
2.1. Soil Sampling and Property Characterization
Sub-surface soil samples were collected from a paddy soil surrounding the abandoned mining site in Korea (36o55’57”N and 129o06’43”S). The soils were then dried in air and sieved to the size fractions < 2 mm. To assess the effect of various amendments toward the Cd mobility in soil, plant uptake, and soil microbial diversity, the sampled soil was merged with Cd(NO3)2 solution for 1 month in the dark at constant humidity to become stable and homogeneous, and reaching a 15 mg/kg concentration of Cd in soil.
Soil pH was measured by pH meter (Orion StarTM A211 pH Benchtop Meter, Waltham, MA, USA) in the solution mixxed with the ratio of 5:1 water: soil (v:v). The total Cd concentration in soil was digested by HNO3/HCl (3:1 v/v) and analyzed by Atomic Absorption Spectrometry (AAS, AA240, Varian, Australia). Total nitrogen (TN) was determined by the Kjeldahl method (USEPA Method 351.2). Total phosphorus (TP) and potassium (TK) were analyzed using the NaOH fusion method (Sommers, 1982). Soil organic matter (OM) was measured by dry combustion techniques (ASTM D 2974).
To assess the leachability of Cd in soil, the Toxicity Characteristic Leaching Procedure (TCLP) was conducted following US-EPA, 1992 guideline.
2.2. Experimental Setting
The Cd-merged soils were weighed with 1 kg and transferred to each pot. Four amendments including bentonite (B), talc (T), activated carbon (AC), and corn starch (CS) and the composite amendments including bentonite + activated carbon (B+AC), bentonite + cornstarch (B+CS), talc + activated carbon (T+AC), and talc + cornstarch (T+CS) were prepared and added to Cd contaminated soil at a rate of 2% w/w (amendment/soil). After that they will be kept and placed in the dark to equilibrate for 8 weeks. A control soil was also prepared with the same procedure with no amendments. After finishing the equilibration, two lettuce seedlings (
Lactuca sativa) were planted for 45 days. Pots were irrigated every 2 days with the same amount (100 ml d
−1) of deionized water (DIW). After 45 days, lettuce was harvested, rinsed with DIW, dried in an oven at 40
oC for 7 days, and ground to fine samples before analysis treatment. Plant samples were digested in a solution of H
2O
2 and HCl on a hot plate at 120
oC. The resultant solutions were filtered and then measured Cd concentrations using AAS. The soil after harvesting was collected and kept in a plastic bag for later analysis. The bioconcentration factor (BCF) was calculated to analyze the transport of Cd from soil to plant as following the formula;
2.3. Semi-Quantitative of Microbial Community: DNA Isolation and Polymerase Chain Reaction (PCR)
The total DNA of the microbial community in each treatment soil was extracted from the same amount of 0.25 g. Experiments were performed by a GenElute Soil DNA Isolation kit (Sigma-Aldrich, USA) according to the manufacturer’s instructions. The same volume of elution buffer of 50 µL was used in the final step of DNA isolation, and DNA concentration was measured using the NanoDrop OneC Spectrophotometer (NanoDrop Technologies, Wilmington, USA). All samples were stored at -20 oC for long-term experiments.
Two microliters of the total DNA from each sample were used in a 20 μL PCR reaction using AccuPower® PyroHotStart TaqPCR PreMix. The PCR conditions for amplification were as follows: initial denaturation at 95 oC for 5 mins (1 cycle), 20 cycles of denaturation at 95 oC for 30 s, annealing at 58oC for 30 s, and extension step 72 oC for 1 min, and the final extension step at 72 oC for 10 mins. Amplified DNA was run on a 1,5 % agarose gel, DNA was stained with RedSafe™ Nucleic Acid Staining Solution (iNtRON Biotechnology, Korea) and was visualized by iBright CL1000 (Invitrogen, USA)
2.4. Quantitative Real-Time Polymerase Chain Reaction (Real-Time PCR)
The qPCR was used for the confirmation of increased or decreased microbial enrichment. The 16s RNA gene, which has been by far the most common housekeeping genetic marker represented in almost all bacteria was amplified with two universal primers 357F (5′- CTCCTACGGGAGGCAGCAG−3′) and 518-R (5′-ATTACCGCGGCTGCTGG−3′). qPCR was performed using a 7500 real-time PCR system (Applied Biosystems, Woolston Warrington, UK) in a 20 μL volume containing 2 μL of DNA template, 4 μL Betaine solution 5 M to decrease non-specific amplification, 3 μL of distilled water, and 10 μL of SYBR® green PCR master mix (Applied Biosystems, UK). The amplification was performed as follows: 95 °C for 15 s and 58 °C for 30 s (40 cycles). The fluorescent signal was obtained and measured during the annealing and dissociation steps, and the Ct value of each sample was normalized to the control sample to calculate the significant differentiation.
The C
t value is how many cycles the sample reached the thresholds. At the start of the run, when the amount of PCR product is low, this produces very little fluorescence. This section of cycles (between cycles 0 and 15 in the above figure) is known as the background signal. Ideally, the threshold is placed at the point in which the reaction is in the exponential phase and will therefore not be confused with the background signal. The threshold is placed at the point in which the reaction is in the exponential phase (when the amount of PCR product doubles for every PCR cycle), and the placement of this threshold line is often determined by the qPCR software. To quantify the effect of amendment treatment on the soil microbial population, our research compared the C
t value of each DNA extracted from amended soil and control soil. In detail, we calculate how much fold difference of DNA content in amended soil compared to control soil following the formula;
2.5. Biog Ecoplate Approach: Microbial Diversity
The Biolog Ecoplate method has been applied by several researchers to compare the functional diversity of microorganisms from contaminated and uncontaminated soils. In this study, the influence of commercial amendments on microbial communities in heavy metal-contaminated soils was examined. To measure how the amendments influence the substrate utilization of the microbial communities in the soil applying the Biolog Ecoplate, the soil was suspended in sterile NaCl (Feigl et al., 2017). The suspension was used to transfer to the plate and incubated in the dark at room temperature. The absorbance was determined by measuring optical density (OD) with a DIALAB EL800 Microplate Reader every 24h for 120h at 590nm wavelength.
The OD value was used for data analysis for average well color development (AWCD), and substrate average well color development (SWACD). Average AWCD was calculated using this equation (Garland & Mills, 1991; Gryta et al., 2014);
where OD
i is the value of substrate number i, and N is 31.
SWACD was determined by: SAWCD = ∑(Ci – R)/(number of the substrate) (4) where Ci is OD590 of the substrate within the substrate category (Kenarova et al., 2014).
2.6. Statistical Analysis
The experimental data were calculated from triple independently replicated experiments. The data were statistically analyzed by applying a one-way analysis of variance (ANOVA) and Tukey’s test (p < 0.05) via SPSS version 20.0. Origin software version 9.1 was applied to figure out the data.
3. Results and Discussion
3.1. The Change of Soil Properties in the Presence of Applied Amendments
The soil sample from the abandoned mine site was slightly acidic with a pH of 4.79, and low organic matter content as shown in
Table 1. The soil texture was loamy sand. The samoled soil showed low values of total N and P with 0.12 mg/kg and 55.5 mg/kg, respectively. The soil was high in Cd concentration (15 mg/kg) compared to the standard threshold (4 mg/kg) for farmland soil in Korea (KMOE, 2013).
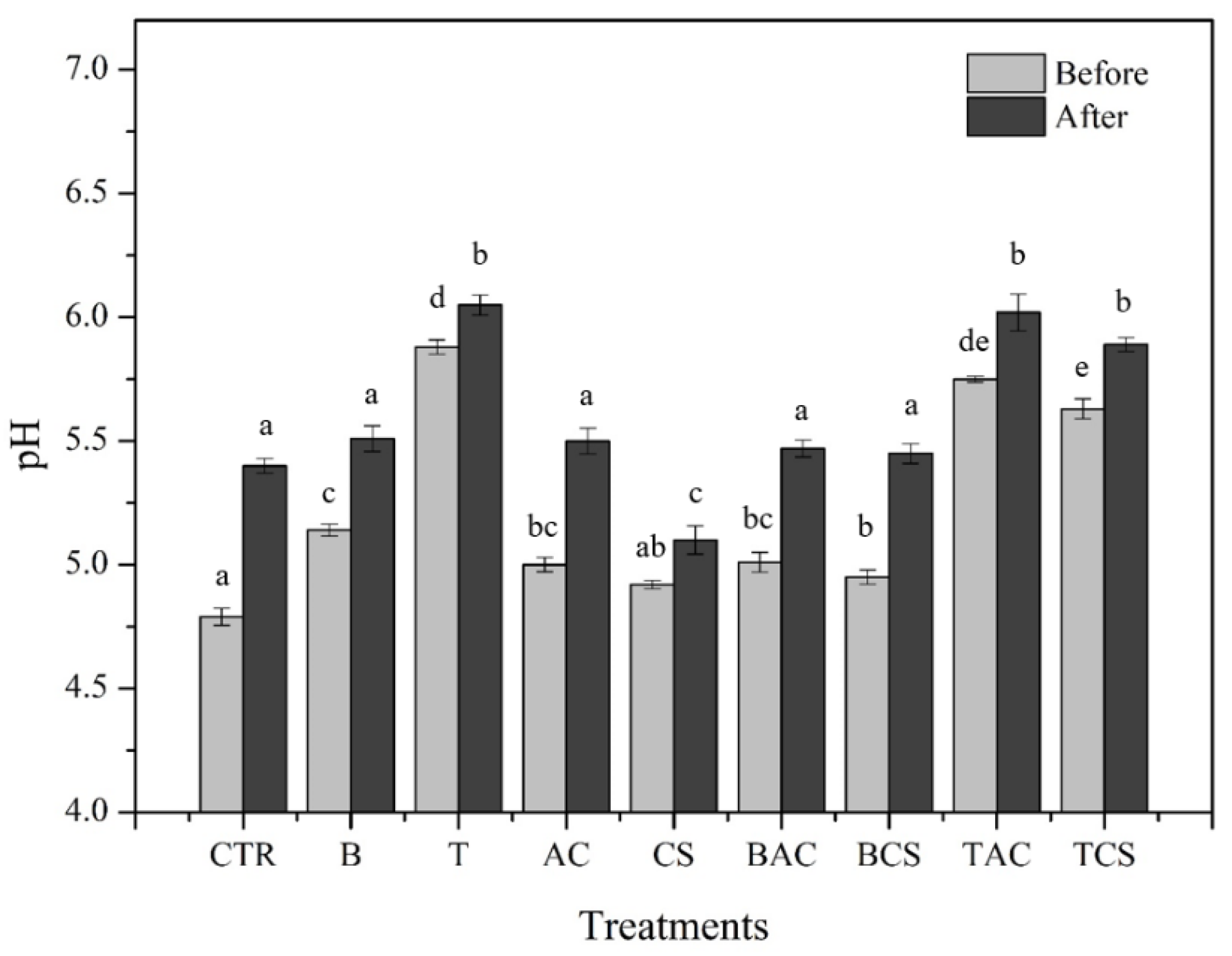
The soil pH values for each treatment and control before and after lettuce planting were shown in
Figure 1. Results indicated that the addition of amendments to the soil led to a slight increase in pH compared to the control soil, except for CS. The treatments with T and its composites (T+AC and T+CS) showed the most significant difference to the control soil, corresponding to an increase in pH of the soil to 6.05, 6.02, and 5.89, respectively, compared to the control soil (p < 0.05). Indeed, Quoc et al. (2021) confirmed that talc consisted of calcite which can release Ca
2+ and further react with HCO
3- leading to an increase in the pH of the soil.
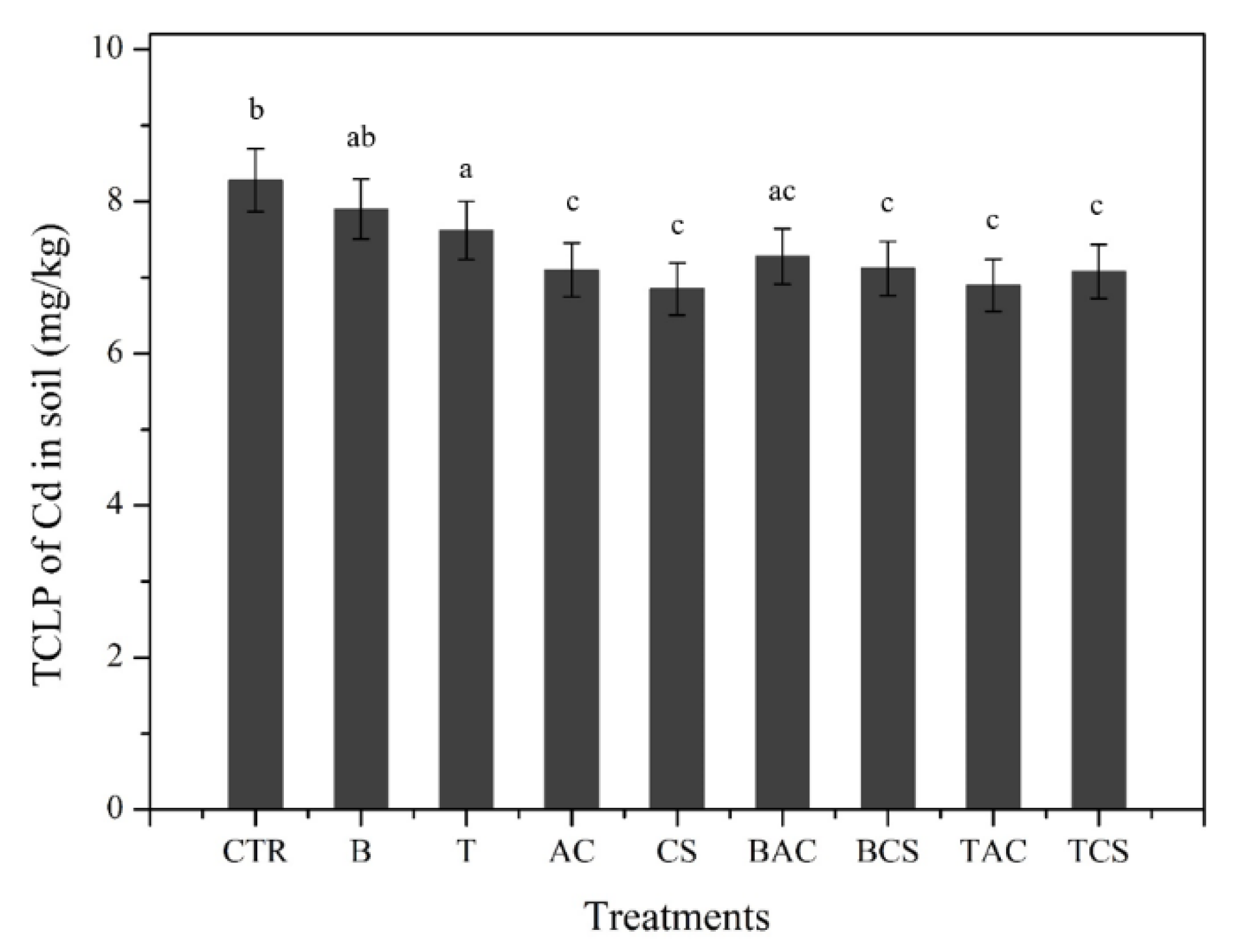
To access the mobility of heavy metals, the TCLP was applied in our study. The TCLP is conducted to test the mobility of organic and inorganic compounds in aqueous or solid phases. This test was initially developed to assess the environmental risk of solid wastes, but it has been applied to assess the remediation of contaminated soils (Lu et al., 2018). In this study, the TCLP of Cd as shown in
Figure 2 was reduced in most of the treatments except for B and T. The leachable Cd was decreased up to 10% (in CS) compared to the control soil. The order of treatment efficiency by reducing leachable Cd is as follows: CS > T+AC > T+CS > AC > B+CS > B+AC > T > B. These results concurred with other researchers, which revealed the effect of B, T, and AC on the immobilization of Cd in soil (Huang and Fuerstenau, 2001; Sun et al., 2016; Vrînceanu et al., 2019). The immobilization of Cd in the presence of B, T, and AC can be explained by the presence of some substance such as calcite which can lead to an increase in the pH of soil, and therefore Cd could be precipitated as CdCO
3 (Bandara et al., 2021). The high specific surface area of AC also improves the capacity of Cd adsorption which increases the Cd immobilization in soil (Quoc et al., 2022). For the CS, to our knowledge, it is the first time CS was studied in terms of the effectiveness of CS on Cd immobilization in soil. That may be the cause of functional groups in the surface of CS such as O-H, C-H, and C-O, and the crystalline structure of amylose-lipid complex helps to make adsorption, co-precipitation, and complexation between CS with Cd species.
3.2. Effect of Amendments Treatments on Cd Uptake by Lettuce
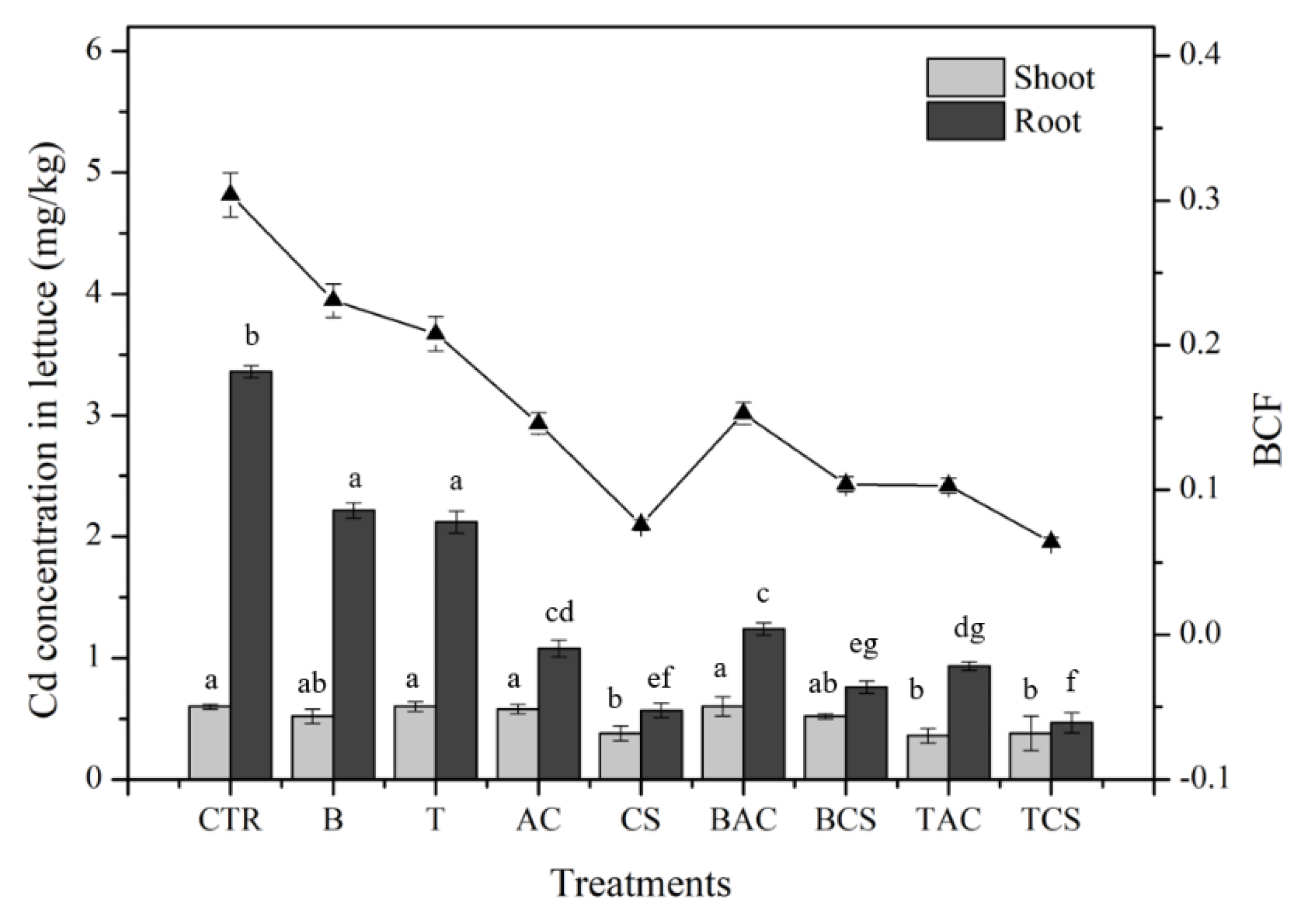
The concentration of Cd in lettuce shoots and roots was presented in
Figure 3. The results indicated that adding amendments reduced Cd uptake by the plant in roots and shoots. Among the amendments, amending soil with CS and its composites (B+CS, and T+CS) showed a significant effect on reducing Cd uptake by lettuce root and shoot. In detail, the Cd concentration in lettuce reduced from 3.36 ± 0.05 and 0.6 ± 0.02 mg/kg in root and shoot in control soil to 0.57 ± 0.06 and 0.38 ± 0.06; 0.76 ± 0.05 and 0.52 ± 0.02; 0.47 ± 0.08 and 0.38 ± 0.14 mg/kg in root and shoot in CS, B+CS, and T+CS, respectively. The Cd uptake by lettuce in soil treated with amendments is strongly related to the mobility of Cd in soil. The treatments with CS and its composites showed the highest effect on the immobilization of Cd in the soil as discussed above.
The bioaccumulation factor is also consistent with the Cd concentration uptake by lettuce. The BCF values of Cd decreased from 0.30 ± 0.01 in control soil to 0.07 ± 0.004, 0.10 ± 0.005, and 0.06 ± 0.003 in soil treated with CS, B+CS, and T+CS, respectively.
Besides, B and AC also showed the effect of reducing Cd uptake by lettuce. Those results also concurred with other studies that using B and AC could reduce the phytotoxicity of heavy metal uptake by plants (Brendova et al., 2016; Sun et al., 2015).
3.3. Effect of Amendments on Microbial Community in Cd-Contaminated Soil
Microorganisms are abundant in soil and very sensitive to respond promptly to environmental change. That is the reason why microbial toxicity tests of amendments affecting heavy metal immobilization in soil have attracted more attention from recent research (Song et al., 2017).
In our study, to assess the effect of amendments on microbial community in soil, the Biolog Ecoplate and molecular methods were applied. Biolog Ecoplate uses the lyophilized substrate which havs 31 different carbon sources that microorganisms give a characteristic response pattern when utilized (Gryta et al., 2014). That is called a metabolic fingerprint. However, it has been limited by low resolution to prove more accurate for the microbial population (Wu et al., 2017). Via the molecular technique approach like Quantitative Real-time Polymerase chain reaction, this study confirms a more accurate understanding of the effect of amendments on the microbial community.
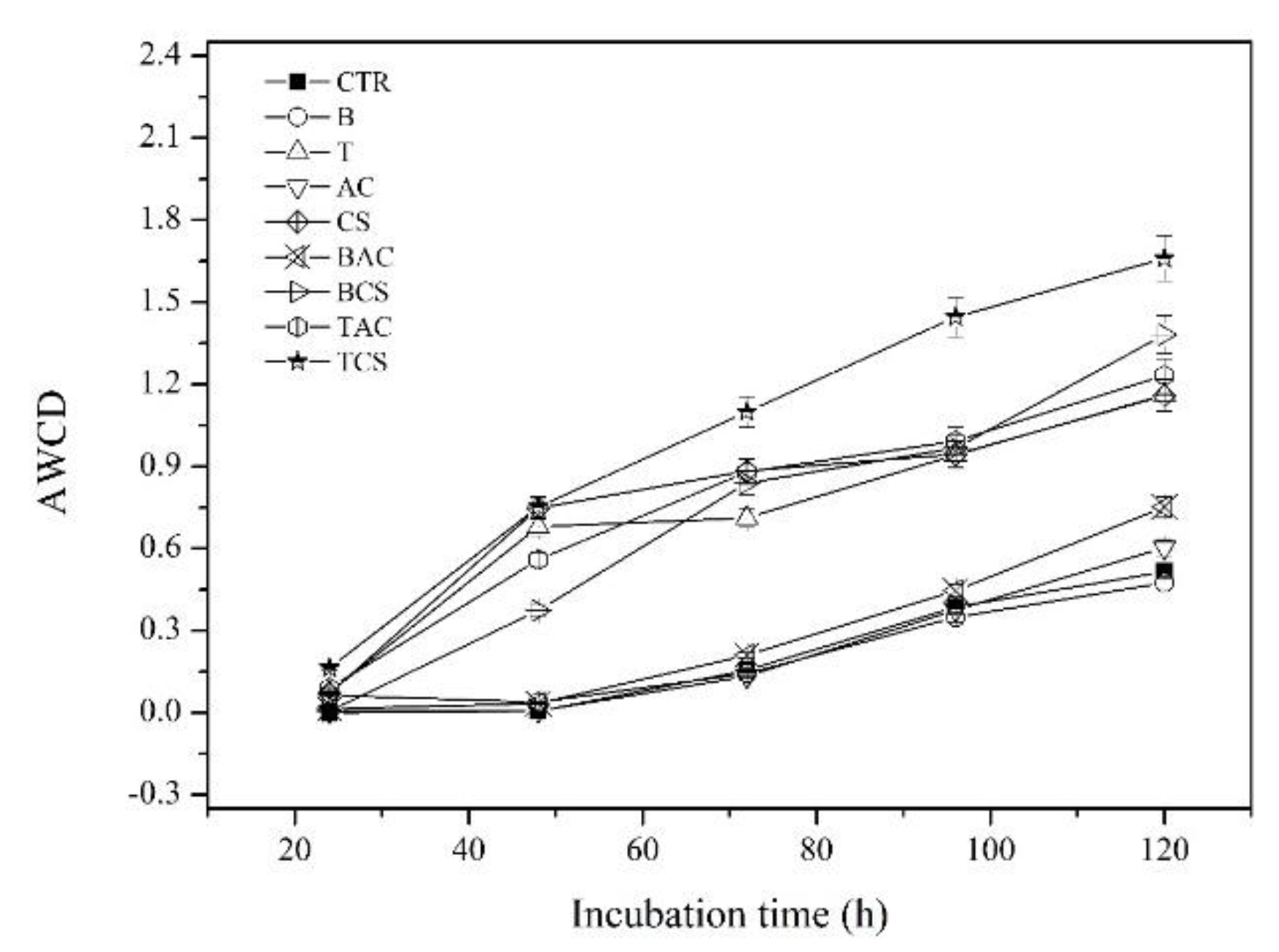
Among these treatments,
Figure 4 showed that the AWCD of treatments with B, AC, and B+AC was stabilized at 0.47, 0.60, and 0.75, respectively, and had no significant difference compared to the control where the AWCD of the control was 0.51 after 120 h incubation time. Meanwhile, the AWCD of treatments with CS, B+CS, and T+CS were significantly different compared to the control at 1.16, 1.38, and 1.66, respectively. Generally, the AWCD was slightly inhibited with the presence of B, AC, and B+AC in the soil; meanwhile, CS, B+CS, and T+CS promoted microorganism activity by improving the AWCD index.
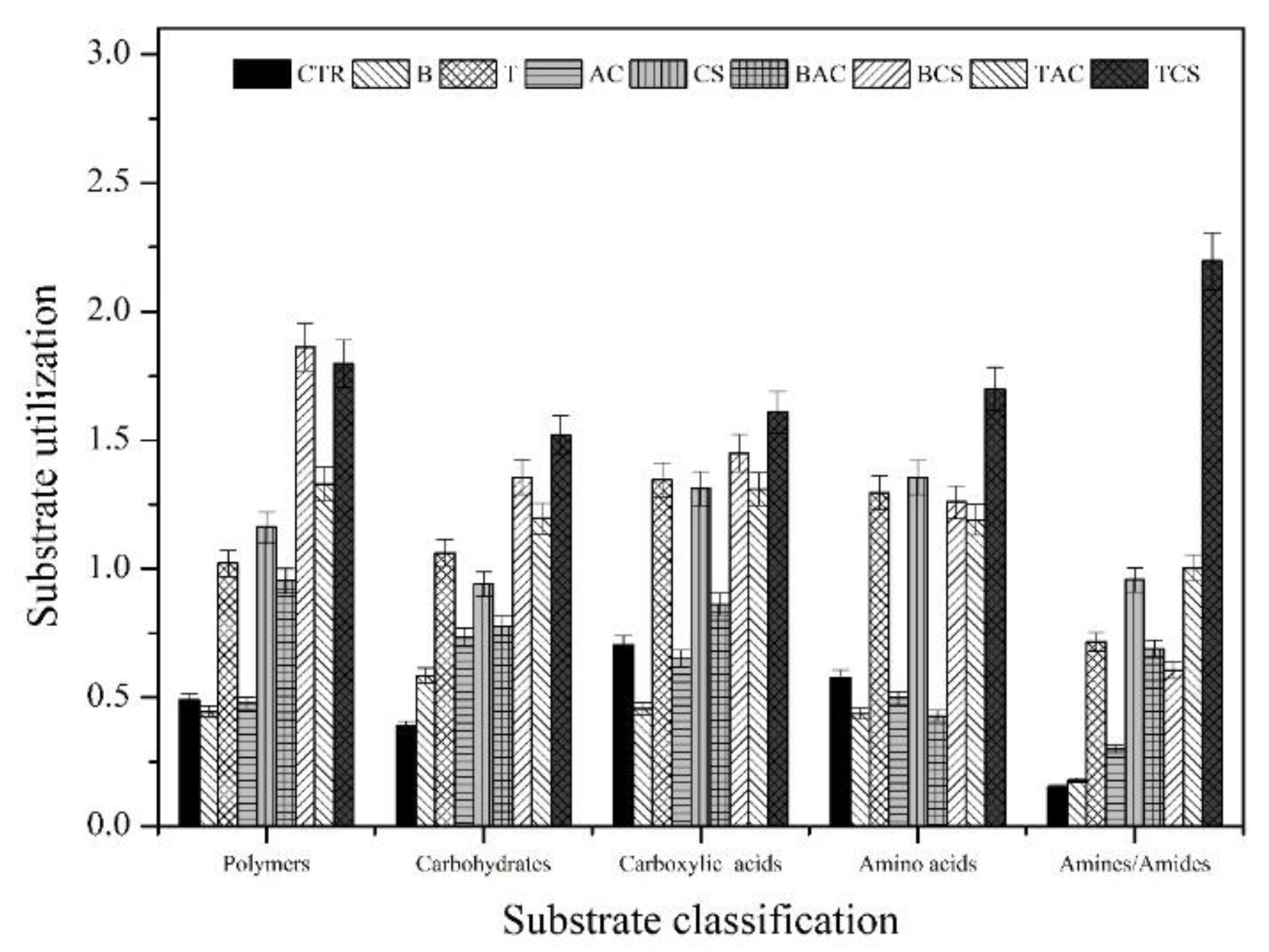
The SAWCD value is presented in
Figure 5. Comparing the distribution of SAWCD for each amendment treatment, one could see that by applying the amendments the carbohydrates and amines/amides SAWCD value increased compared to the untreated soil. In the polymers, amino acids, and carboxylic acid utilization, most of the amendment treatments showed a higher value of SAWCD than control soil, except B, AC, and B+AC.
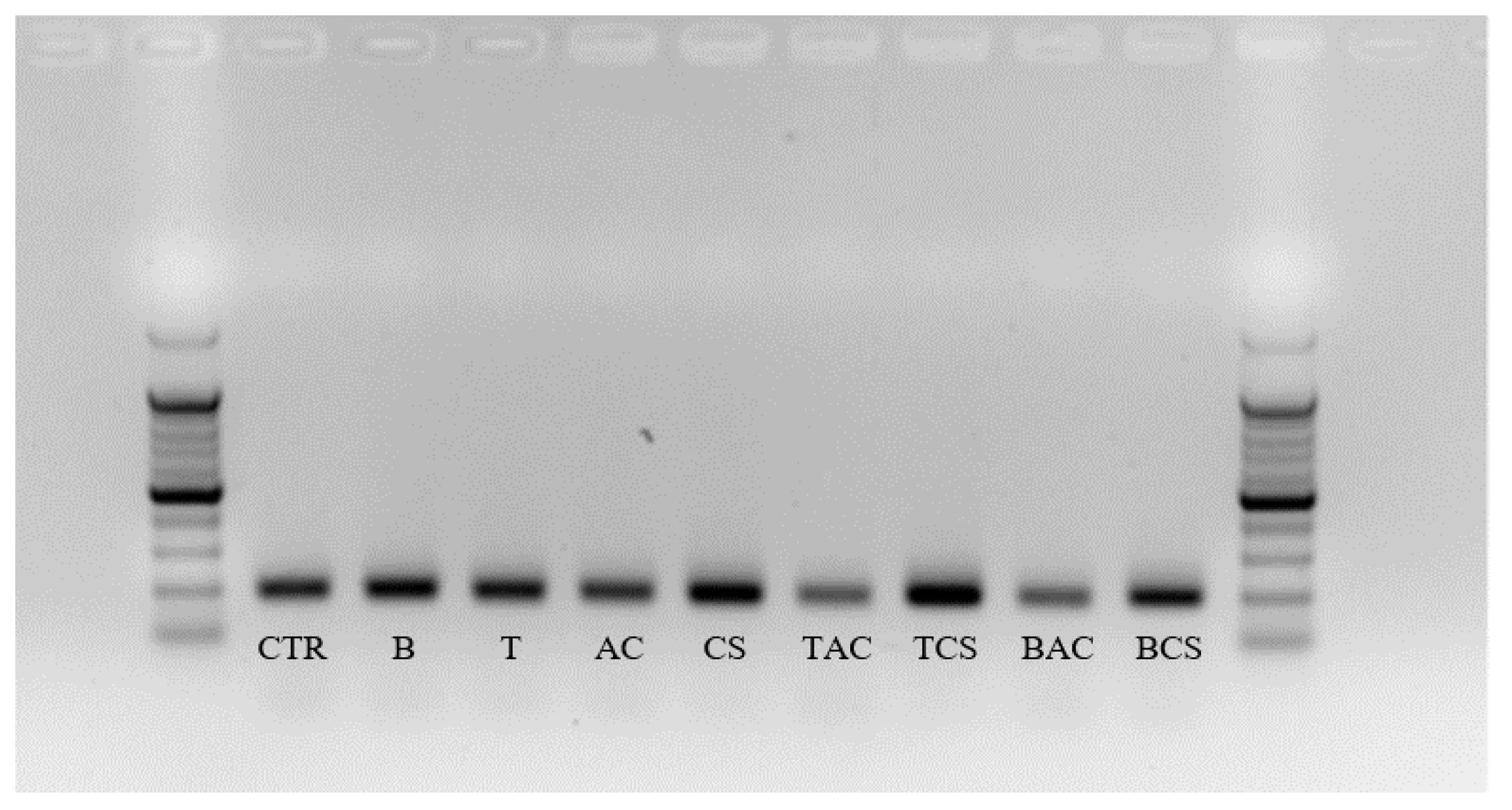
By using the molecular method approach, our results again confirmed that the effect of amendment treatment on soil microbial was similar to results from Biolog Ecoplate. In
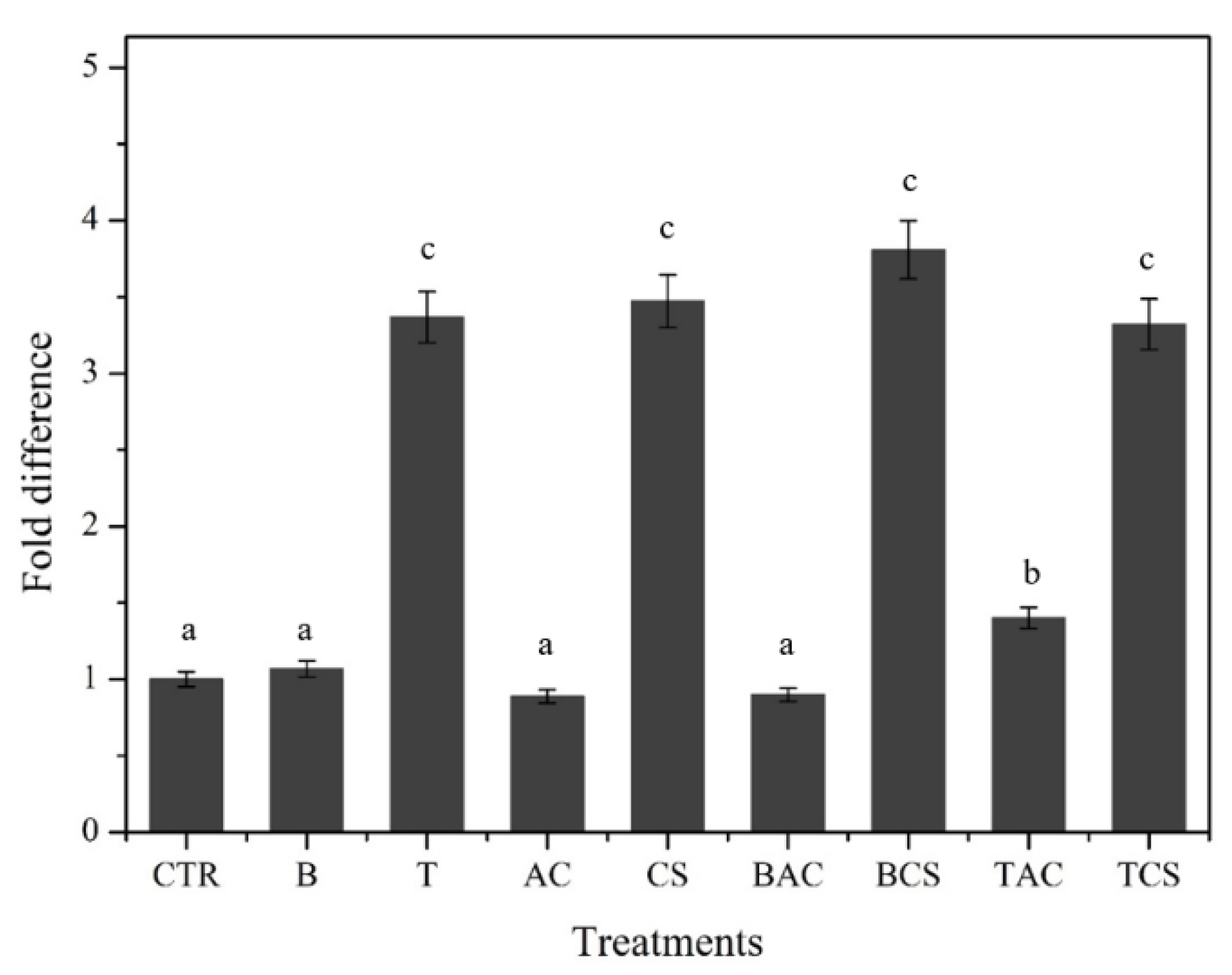
Figure 6, the semi-quantitative of the microbial community in the soil is represented in the concentration of the DNA band. The bands of T, CS, T+CS, and B+CS showed more clearly than the others. This result is also consistent with the quantitative results from real-time PCR which was shown in
Figure 7 to have a greater signal from T, CS, T+CS, T+AC, and B+CS than the control (3.36, 3.47, 3.32, 1.40, and 3.81-fold difference, respectively).
The results from Biolog Ecoplate and real-time PCR in this study concurred with other research on the effects of B and AC on microbial communities. For example, Sun et al., (2013) showed that bentonite had a negative effect on a soil microbial community (Sun et al., 2013). Que et al., also indicated that the microorganism was destroyed when AC was amended to the sediment (Que et al., 2019). Furthermore, there were numerous research works confirmed the relationship between changing soil pH and soil microorganisms (Deng et al., 2015; Hmid et al., 2015). Most microorganisms had an intracellular pH around neutral, which is why increasing or decreasing pH is sufficient to impose stress and likely affects the microbial community (Wang et al., 2019). In this study, B, AC, and B+AC applied to soil slightly changed pH but not significantly different than the control while T, T+AC, and T+CS increased the soil pH nearly neutrally. That is the reason why the microbial community in soil treated with T, CS, and T+CS was more abundant than B, AC, and B+AC. Besides, a previous study reported that an increase in the concentration of Cd-nitrate in soil caused abiotic stress, which was reflected by a continuous decrease in soil microbial biomass (Muhammad et al., 2005). This study confirmed that by applying amendments such as CS, B+CS, and T+CS, the Cd mobility in soil was reduced which means it can make a favorable environment for microbial community development.
4. Conclusions
A pot trial proceeded to assess the effectiveness of these sole amendments including B, T, AC, CS, and their combination (B+AC, B+CS, T+AC, and T+CS) on the immobilization of exchangeable Cd merged-soil by using a set of variables including pH, TCLP, Cd uptake by the plant, and microbial community. To assess the effect of these amendments on the soil microbial community, both Biolog Ecoplate and molecular methods were utilized to quantify and qualify the microbial population in the soil after the application of the amendments. Results showed that most of the applied amendments changed the soil properties like increasing the pH and reducing the Cd exchangeable in soil, which led to the reduction of the Cd uptake by lettuce, especially for CS, B+CS, and T+CS. Biolog Ecoplate data revealed that using the amendments such as T, CS, B+CS, T+AC, and T+CS, significantly increased the microbial population and diversity in soil. By applying the molecular method (quantitative real-time PCR), the microbial population in soil treated by these amendments increased a maximum of 3.81-fold difference in B+CS compared to the control soil. This study provided more options to consider when immobilizing Cd-contaminated soil through combining commercial amendments to achieve more efficiency with low Cd availability in soil, low Cd uptake and translocation in the plants, and promoting soil microbial community.
Plant material collection and use permission
No permission is required for plant material as it was purchased from a certified dealer in the local area.
Complies with international, national, and/or institutional guidelines
Experimental research and field studies on plants (either cultivated or wild), comply with relevant institutional, national, and international guidelines and legislation.
Author Contributions
Tuan Nguyen Quoc: Experiment set-up, Investigation, Writing - original draft. Myung Chae Jung: Writing - review and editing, Supervision.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Acknowledgments
This research was supported by Korea Basic Science Institute (National Research Facilities and Equipment Center) grant funded by the Ministry of Education (2022R1A6C101A774).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Ali, A. & Chidambaram, S. (2021). Assessment of trace inorganic contaminates in water and sediment to address its impact on common fish varieties along Kuwait Bay. Environmental Geochemistry and Health, 43(2), 855-883. [CrossRef]
- Bandara, T., Chathurika, J. B. A. J., Franks, A., Xu, J., & Tang, C. (2021). Interactive effects of biochar type and pH on the bioavailability of As and Cd and microbial activities in co-contaminated soils. Environmental Technology & Innovation, 23, 101767. [CrossRef]
- Bolan, N., Kunhikrishnan, A., Thangarajan, R., Kumpiene, J., Park, J., Makino, T., Kirkham, M. B., & Scheckel, K. (2014). Remediation of heavy metal(loid)s contaminated soils - To mobilize or to immobilize? Journal of Hazardous Materials, 266, 141-166. [CrossRef]
- Brendova, K., Zemanova, V., Pavlikova, D., & Tlustos, P. (2016). Utilization of biochar and activated carbon to reduce Cd, Pb and Zn phytoavailability and phytotoxicity for plants. J Environ Manage, 181, 637-645. [CrossRef]
- Deng, L., Zeng, G., Fan, C., Lu, L., Chen, X., Chen, M., Wu, H., He, X., & He, Y. (2015). Response of rhizosphere microbial community structure and diversity to heavy metal co-pollution in arable soil. Applied Microbiology and Biotechnology, 99(19), 8259-8269. [CrossRef]
- Feigl, V., Ujaczki, E., Vaszita, E., & Molnar, M. (2017). Influence of red mud on soil microbial communities: Application and comprehensive evaluation of the Biolog EcoPlate approach as a tool in soil microbiological studies. Sci Total Environ, 595, 903-911. [CrossRef]
- Garland, J. L. & Mills, A. L. (1991). Classification and characterization of heterotrophic microbial communities on the basis of patterns of community-level sole-carbon-source utilization. Applied and environmental microbiology, 57(8), 2351-2359. [CrossRef]
- Gryta, A., Frac, M., & Oszust, K. (2014). The application of the Biolog EcoPlate approach in ecotoxicological evaluation of dairy sewage sludge. Appl Biochem Biotechnol, 174(4), 1434-1443. [CrossRef]
- Hmid, A., Al Chami, Z., Sillen, W., De Vocht, A., & Vangronsveld, J. (2015). Olive mill waste biochar: a promising soil amendment for metal immobilization in contaminated soils. Environ Sci Pollut Res Int, 22(2), 1444-1456. [CrossRef]
- Huang, P. & Fuerstenau, D. W. (2001). The effect of the adsorption of lead and cadmium ions on the interfacial behavior of quartz and talc. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 177, 147 - 156.
- Kenarova, A., Radeva, G., Traykov, I., & Boteva, S. (2014). Community level physiological profiles of bacterial communities inhabiting uranium mining impacted sites. Ecotoxicology and Environmental Safety, 100, 226-232. [CrossRef]
- Kirn, B. S. & Lim, S. T. (1999). Removal of heavy metal ions from water by cross-linked carboxymethyl corn starch. Carbohydrate Polymers, 39(3), 217-223. [CrossRef]
- KMOE. (2013). Detailed survey for soil and water contamination in abandoned metal mines in Korea KMOE, Sejong-city, South Korea. (in Korean).
- Li, B., Li, M., Zhang, P., Pan, Y., Huang, Z., & Xiao, H. (2022). Remediation of Cd (II) ions in aqueous and soil phases using novel porous cellulose/chitosan composite spheres loaded with zero-valent iron nanoparticles. Reactive and Functional Polymers, 173, 105210. [CrossRef]
- Lu, H. P., Li, Z. A., Gascó, G., Méndez, A., Shen, Y., & Paz-Ferreiro, J. (2018). Use of magnetic biochars for the immobilization of heavy metals in a multi-contaminated soil. Science of The Total Environment, 622-623, 892-899. [CrossRef]
- Muhammad, A., Xu, J., Li, Z., Wang, H., & Yao, H. (2005). Effects of lead and cadmium nitrate on biomass and substrate utilization pattern of soil microbial communities. Chemosphere, 60(4), 508-514. [CrossRef]
- Nejad, Z. D., Jung, M. C., & Kim, K.-H. (2018). Remediation of soils contaminated with heavy metals with an emphasis on immobilization technology. Environmental Geochemistry and Health, 40(3), 927-953. [CrossRef]
- Ossman, M. E., Mansour, M. S., Fattah, M. A., Taha, N., Kiros, Y. (2014). Peanut shells and talc powder for removal of hexavalent chromium from aqueous solutions. Bulgarian Chemical Communications, 46(3), 629 - 639.
- Palansooriya, K. N., Shaheen, S. M., Chen, S. S., Tsang, D. C. W., Hashimoto, Y., Hou, D., Bolan, N. S., Rinklebe, J., & Ok, Y. S. (2020). Soil amendments for immobilization of potentially toxic elements in contaminated soils: A critical review. Environment International, 134, 105046. [CrossRef]
- Que, W., Zhou, Y.-h., Liu, Y.-g., Wen, J., Tan, X.-f., Liu, S.-j., & Jiang, L.-h. (2019). Appraising the effect of in-situ remediation of heavy metal contaminated sediment by biochar and activated carbon on Cu immobilization and microbial community. Ecological Engineering, 127, 519-526. [CrossRef]
- Quoc, N. T., Kim, J. W., Nejad, Z. D., Le Thanh, T., & Jung, M. C. (2022). Influence of commercial amendments on Cu and Zn mobility, phytoavailability, and microbial activities on two contaminated soils. Journal of Environmental Chemical Engineering, 10(1), 107098. [CrossRef]
- Quoc, N. T., Nejad, Z. D., & Jung, M. C. (2021). Effect of Commercial Amendments on Immobilization of Arsenic, Copper, and Zinc in Contaminated Soil: Comprehensive Assessing to Plant Uptake Combined with a Microbial Community Approach. Minerals, 11(10). [CrossRef]
- Rui, D., Wu, Z., Ji, M., Liu, J., Wang, S., & Ito, Y. (2019). Remediation of Cd- and Pb- contaminated clay soils through combined freeze-thaw and soil washing. Journal of Hazardous Materials, 369, 87-95. [CrossRef]
- Sommers, O. A. (1982) Phosphorus. In: Page, A.L., Ed., Methods of Soil Analysis Part 2 Chemical and Microbiological Properties (pp. 403-430): American Society of Agronomy, Soil Science Society of America, Madison.
- Song, B., Zeng, G., Gong, J., Liang, J., Xu, P., Liu, Z., Zhang, Y., Zhang, C., Cheng, M., Liu, Y., Ye, S., Yi, H., & Ren, X. (2017). Evaluation methods for assessing effectiveness of in situ remediation of soil and sediment contaminated with organic pollutants and heavy metals. Environ Int, 105, 43-55. [CrossRef]
- Sun, Y., Li, Y., Xu, Y., Liang, X., & Wang, L. (2015). In situ stabilization remediation of cadmium (Cd) and lead (Pb) co-contaminated paddy soil using bentonite. Applied Clay Science, 105-106, 200-206. [CrossRef]
- Sun, Y., Sun, G., Xu, Y., Liu, W., Liang, X., & Wang, L. (2016). Evaluation of the effectiveness of sepiolite, bentonite, and phosphate amendments on the stabilization remediation of cadmium-contaminated soils. J Environ Manage, 166, 204-210. [CrossRef]
- Sun, Y., Sun, G., Xu, Y., Wang, L., Liang, X., & Lin, D. (2013). Assessment of sepiolite for immobilization of cadmium-contaminated soils. Geoderma, 193-194, 149-155. [CrossRef]
- Vrînceanu, N. O., Motelică, D. M., Dumitru, M., Calciu, I., Tănase, V., & Preda, M. (2019). Assessment of using bentonite, dolomite, natural zeolite and manure for the immobilization of heavy metals in a contaminated soil: The Copșa Mică case study (Romania). Catena, 176, 336-342. [CrossRef]
- Wang, M., Chen, S., Han, Y., Chen, L., & Wang, D. (2019). Responses of soil aggregates and bacterial communities to soil-Pb immobilization induced by biofertilizer. Chemosphere, 220, 828-836. [CrossRef]
- Wu, B., Cheng, G., Jiao, K., Shi, W., Wang, C., & Xu, H. (2016). Mycoextraction by Clitocybe maxima combined with metal immobilization by biochar and activated carbon in an aged soil. Sci Total Environ, 562, 732-739. [CrossRef]
- Wu, W., Wu, J., Liu, X., Chen, X., Wu, Y., & Yu, S. (2017). Inorganic phosphorus fertilizer ameliorates maize growth by reducing metal uptake, improving soil enzyme activity and microbial community structure. Ecotoxicol Environ Saf, 143, 322-329. [CrossRef]
- Xie, Y., Xiao, K., Sun, Y., Gao, Y., Yang, H., & Xu, H. (2018). Effects of amendments on heavy metal immobilization and uptake by Rhizoma chuanxiong on copper and cadmium contaminated soil. R Soc Open Sci, 5(8), 181138. [CrossRef]
- Xu, J., Yu, Y., Chen, K., & Huang, Y. (2019). Intersex regulates female external genital and imaginal disc development in the silkworm. Insect Biochemistry and Molecular Biology, 108, 1-8. [CrossRef]
- Zhang, J., Cao, X., Yao, Z., Lin, Q., Yan, B., Cui, X., He, Z., Yang, X., Wang, C.-H., & Chen, G. (2021). Phytoremediation of Cd-contaminated farmland soil via various Sedum alfredii-oilseed rape cropping systems: Efficiency comparison and cost-benefit analysis. Journal of Hazardous Materials, 419, 126489. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).