1. Introduction
The field of pediatric urology is changing as a result of artificial intelligence (AI) applications, which are providing novel answers to persistent problems with patient diagnosis and therapy. This study examines the role of AI in pediatric urology, offering a thorough examination of implications, current uses, and future potential to improve standards of care.
1.1. Background on Pediatric Urology
Pediatric urology focuses on the diagnosis and management of urinary tract problems in children and adolescents, which can include a broad range of conditions such as urologic cancers, voiding dysfunction, urinary tract infections, and complex congenital defects. A multidisciplinary strategy that combines pediatricians, urologists, nephrologists, radiologists, and other allied healthcare specialists is typically necessary for the treatment of pediatric urological problems [
1]. Depending on the exact illness and severity, several treatment plans may be used, with possible interventions ranging from medication to surgery.
Diagnosis and management of juvenile urological disorders have regularly been transformed by technological progress, including minimally invasive surgery and sophisticated imaging techniques. However, this discipline still confronts a number of major challenges due to the intricacy and diversity of certain conditions, the requirement for pediatric patient-specific techniques, and the need for long-term follow-up to monitor complications and ensure optimal results. Complexity is further compounded by the variation in diseases and how they appear across pediatric patients, since ailments can present in different ways depending on age, genetics, and comorbidities. Furthermore, due to the dynamic nature of childhood development, treatment plans must be continuously modified to account for changes in growth and development [
2]. In recent years, use of AI in pediatric urology has shown promise for improving patient diagnosis, treatment planning, surgical results, and postoperative care. The purpose of this study is to present a thorough analysis of recent progress in AI applications for pediatric urology, emphasizing the many advantages, current difficulties, and future possibilities in this quickly developing area.
1.2. Importance of AI in Pediatric Urology
AI has potential to reshape the field of pediatric urology by delivering better clinical judgment, increasing diagnostic precision, and improving patient outcomes. The special challenges of treating young patients make the use of AI in pediatric urology all the more important. Juveniles frequently exhibit intricate and varied urological ailments that require customized methods for precise identification and efficient therapy as shown in
Figure 1.
The ability of AI to quickly and accurately assess enormous volumes of clinical and imaging data is one of the technology's main advantages. By sorting through complex patterns and nuanced details in patient information, machine learning (ML) algorithms can aid doctors with early detection, risk assessment, and individualized therapy planning. In pediatric populations, where prompt intervention can have a substantial influence on quality of life and long-term outcomes, this capacity is extremely valuable [
3] (
Table 1).
By synthesizing information from many sources, AI-powered decision support systems provide healthcare providers with crucial evidence-based advice. These technologies can assist pediatric urologists in navigating intricate treatment algorithms, optimizing surgical planning, and reducing intervention-related risks to ultimately improve results for pediatric urology patients [
4]. In particular, healthcare professionals can now employ AI tools to provide more individualized, accurate, and efficient care.
With enhanced tools for image interpretation, segmentation, and visualization, AI has potential to completely transform medical imaging in pediatric urology. Deep learning (DL) algorithms can evaluate radiological images with unprecedented accuracy, making it easier to identify tiny anomalies and direct focused interventions. Furthermore, AI-driven image analysis methods make it possible to assess clinical course of disease, response to medication, and impact of treatment, providing clinicians with important new data on patient care [
1].
Table 1.
Role of AI in enhancing pediatric urology.
Table 1.
Role of AI in enhancing pediatric urology.
| Aspect |
Importance of AI in Pediatric Urology |
| Diagnosis |
AI can aid the accurate and early diagnosis of pediatric urological conditions through analysis of medical images and patient data [5]. |
| Treatment Planning |
AI algorithms can assist in developing personalized treatment plans based on patient-specific factors, improving outcomes, and reducing risks [6]. |
| Surgical Assistance |
AI-enabled surgical tools can enhance precision and safety during pediatric urological procedures, reducing complications and recovery times [7]. |
| Predictive Analytics |
AI can help predict progression of certain urological conditions in pediatric patients, allowing for proactive intervention and management [3]. |
| Research and Innovation |
AI facilitates the analysis of large datasets to identify trends, patterns, and novel insights, driving advances in pediatric urology [8]. |
| Education and Training |
AI-powered simulations and virtual reality environments offer valuable educational resources for training pediatric urologists and residents [8]. |
| Patient Monitoring |
AI-driven monitoring systems can continuously track pediatric urology patients, providing real-time alerts for any concerning developments [6]. |
Beyond diagnosis and therapy, AI is also transforming pediatric urology research and innovation. Enormous datasets can now be evaluated using ML techniques to discern new information on prognostic variables, therapy responses, and disease causes. AI-powered virtual reality simulations are also revolutionizing surgical education by giving pediatric urology trainees hands-on experiences in a safe setting. AI will therefore make pediatric urology more precise, efficient, and patient-centered in the future by accelerating scientific innovation and discovery [
3].
1.3. Objectives of this Review
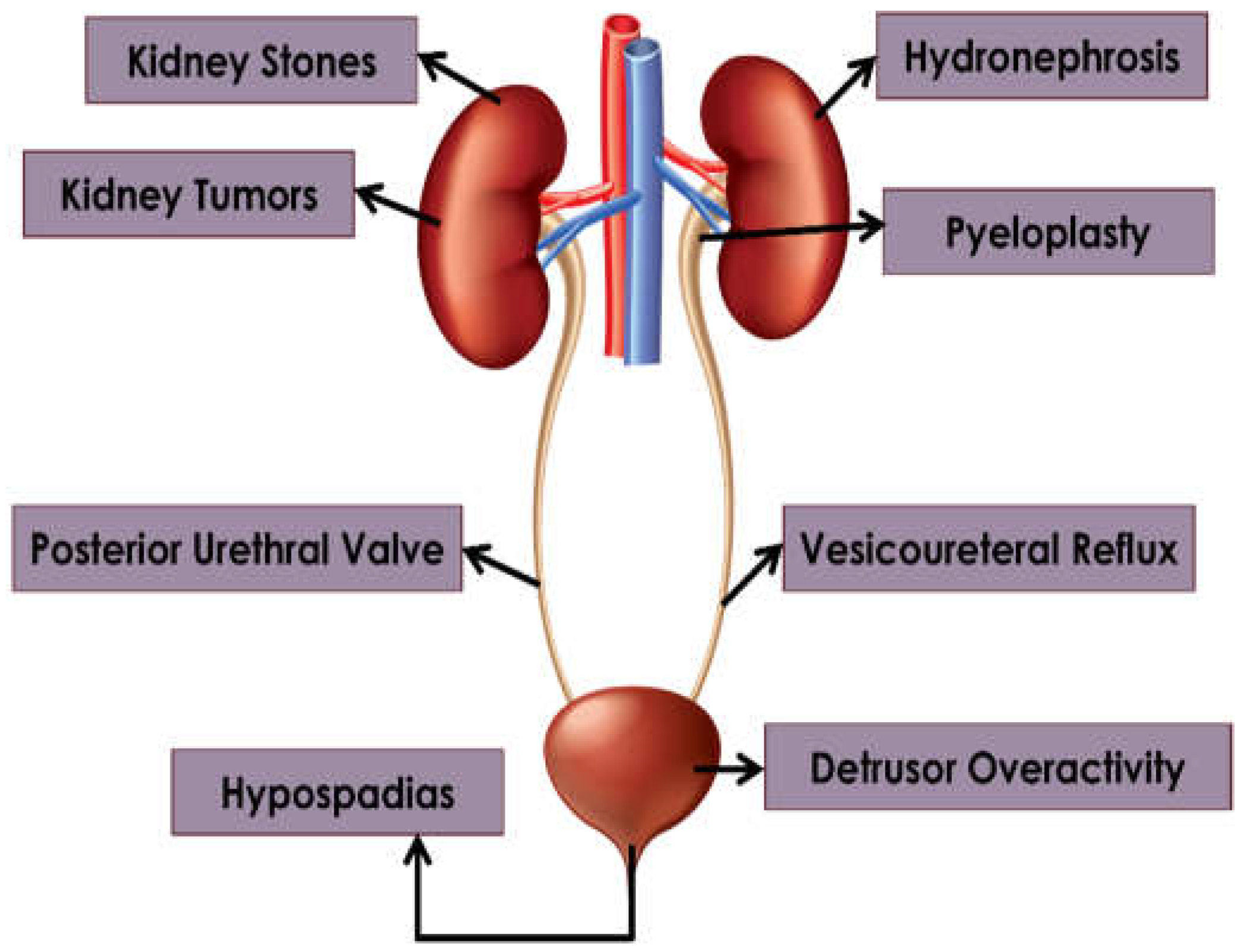
This review aims to shed light on the potential of AI to drive revolutionary advances in key pediatric urological disorders and surgeries (
Figure 2), while navigating the ethical, regulatory, and practical challenges of adopting new technologies:
Examining the technical landscape: Our initial goal is to present a broad overview of the technical environment where pediatric urology and artificial intelligence converge. We aim to summarize current research and recent breakthroughs to provide readers with a thorough grasp of AI tools and approaches that are changing clinical practice in this specialized sector.
Clinical implications: Going beyond the technical details, we aim to clarify the real-world effects of adopting AI in pediatric urology, both promise and pitfalls. We explore how AI-driven innovations are revolutionizing diagnostic routes, treatment paradigms, and patient outcomes through a critical examination of current research and case studies.
Regulatory and ethical boundaries: Mindful of bioethics and legal issues that accompany technological advances in healthcare, we endeavor to traverse these boundaries, identify ethical concerns, highlight privacy issues, and outline legal frameworks that will influence incorporation of AI into pediatric urology.
Knowledge gaps and future directions: Identifying knowledge gaps and potential topics for additional research into AI applications for pediatric urology is a major goal of this review. Through a critical assessment of the current literature, we aim to identify key areas for future AI-driven research initiatives and innovations.
Encouraging medical professionals and scholars: Our main goal is to provide healthcare professionals, researchers, and other stakeholders with the information and understanding necessary to fully realize AI's transformational potential in the field of pediatric urology. Our aim is to present a range of evidence-based discoveries and viewpoints that stimulate meaningful debate, cooperation, and innovation in this dynamic and quickly changing sector.
Figure 2.
Pediatric urological disorders discussed in this review.
Figure 2.
Pediatric urological disorders discussed in this review.
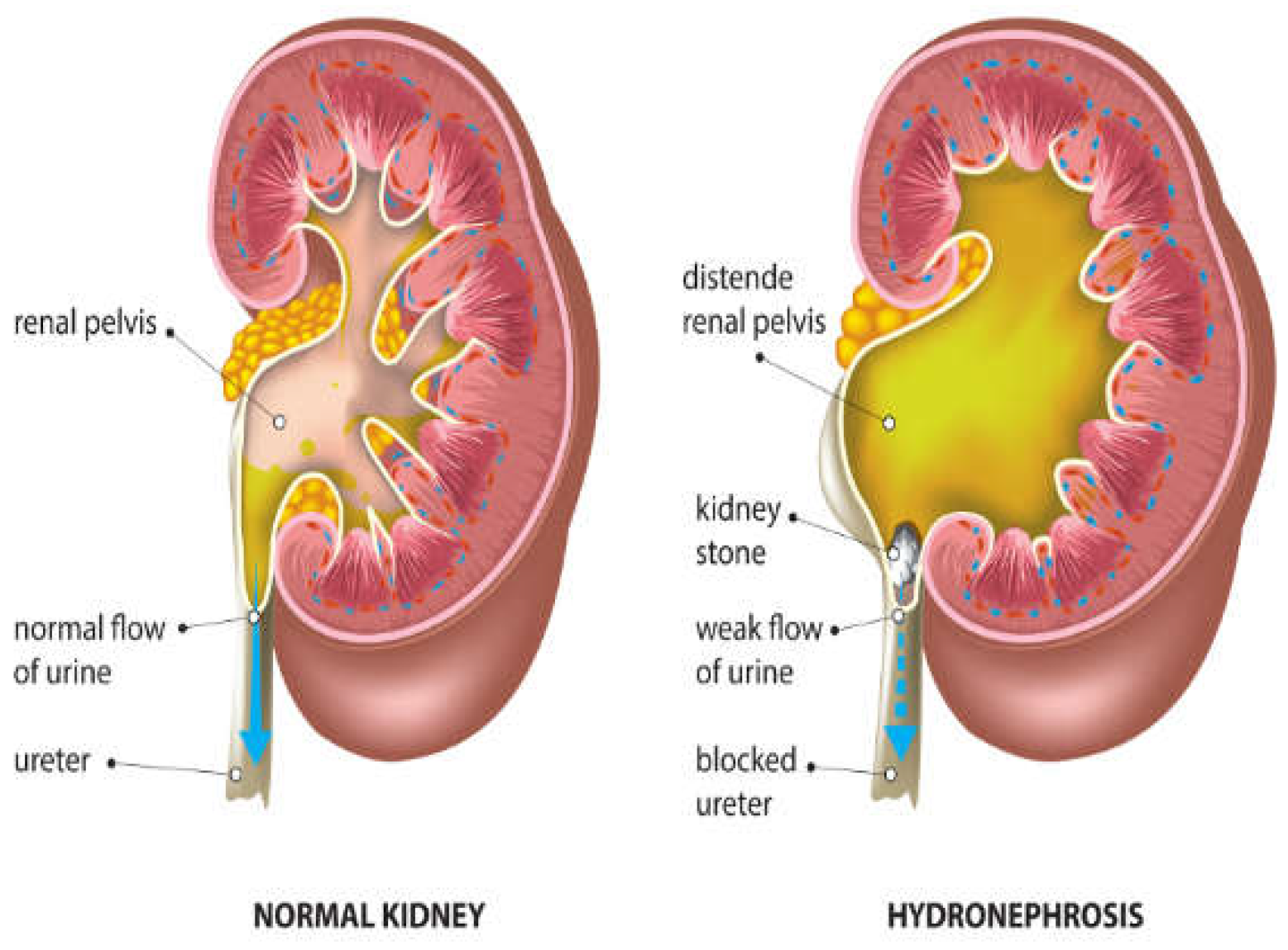
2. Hydronephrosis
Hydronephrosis is a medical condition that involves enlargement and distension of one or both kidneys due to accumulation of urine (
Figure 3).
Table 2 shows the challenges of managing hydronephrosis in pediatric urology, including the need for precise diagnosis and customized therapeutic approaches. Historically, evaluation of hydronephrosis has relied on subjective grading systems and intrusive diagnostic procedures, resulting in inconsistency in medical practice and possible hazards for patients. However, AI tools now offer promising alternatives to boost the precision of diagnoses, simplify clinical processes, and improve patient results. According to the Society for Fetal Urology (SFU) grading system, ultrasound scans can be used to determine hydronephrosis severity from Grade 1-4 (
Figure 4).
2.1. Diagnostic Challenges in Pediatric Hydronephrosis
Traditional methods of diagnosing pediatric hydronephrosis are limited by the subjective nature of ultrasound imaging and variability of interpretation [
10]. Semi-quantitative ultrasonic analysis can result in observer-dependent outcomes, with a lack of explicit instructions for subsequent monitoring and management. In addition, establishing precise criteria for intervention based on grading methods such as the SFU [
11] and the Urinary Tract Dilatation (UTD) [
12] classification systems remain challenging. This ambiguity increases the need for invasive treatments, radiation exposure, and frequent testing, which places additional strain on young patients, their families, and healthcare systems [
13].
Figure 4.
Kidney ultrasound scans indicating different levels of hydronephrosis: a) Grade-1, b) Grade-2, c) Grade-3, and d) Grade-4. Based on SFU grading system. [
14].
Figure 4.
Kidney ultrasound scans indicating different levels of hydronephrosis: a) Grade-1, b) Grade-2, c) Grade-3, and d) Grade-4. Based on SFU grading system. [
14].
The constraints of conventional approaches also apply to imaging techniques such as abdominal CT, which is highly efficient but also raises issues regarding radiation exposure and cost. While ultrasound is easily accessible, this method is also hindered by inter-observer variability, which compromises reliability [
15]. The lack of clear guidelines and understanding of hydronephrosis natural history further complicates decision-making, thus impacting patient counseling and management [
16].
DL models may help address these difficulties by offering more impartial and replicable diagnostic tools [
17]. These models can reduce the subjective, inconsistent, and variable nature of existing diagnostics, although further work will be required to maximize accuracy of these approaches, particularly for grading of severe cases [
18].
Table 2.
Diagnostic challenges in pediatric hydronephrosis.
Table 2.
Diagnostic challenges in pediatric hydronephrosis.
| Diagnostic Challenge |
Description |
Mitigation Strategies |
| Subjective nature of ultrasound imaging [19] |
Traditional methods for diagnosing pediatric hydronephrosis rely heavily on ultrasound imaging, which is subjective and prone to variability. |
Implementing DL models can offer more impartial and replicable diagnostic tools, reducing the subjective and variable nature of ultrasound interpretation. |
| Lack of clear guidelines for intervention [20] |
Establishing precise criteria for intervention based on grading systems presents challenges. |
Continued research is needed to develop clearer guidelines for intervention, considering factors such as patient age, severity of hydronephrosis, and potential risks. |
| Issues with imaging techniques [15] |
Although imaging techniques like abdominal CT are efficient, they pose risks of radiation exposure and higher cost. |
Exploring alternative imaging modalities with lower radiation exposure, such as magnetic resonance urography (MRU), can mitigate the risks associated with radiation exposure while maintaining diagnostic efficiency. |
2.2. AL Solutions for Hydronephrosis Management
Quantitative imaging and ML methods can now be used to assess renal sonograms with the goal of predicting need for diuretic nuclear renography [
10]. By analyzing key morphological features, this type of approach was successfully used to determine ultrasound-based safety criteria that correspond to washout times, potentially leading to a 62% reduction in use of invasive diagnostic procedures. In addition, researchers have created DL algorithms that can accurately identify obstructive hydronephrosis using only ultrasound scans, which could speed-up this process and significantly improve clinical care [
13].
AI has demonstrated potential in the management and grading of hydronephrosis, providing more consistent and accurate evaluations. Based on renal pelvis dilation—a critical component in the diagnosis of urinary tract obstructions—hydronephrosis is graded [
21]. The ability of AI to differentiate between hydronephrosis resulting from Posterior Urethral Valves (PUV) and other etiologies is demonstrated by research conducted by Ostrowski et al. (2023) [
22]. Their research shows how AI might improve diagnosis accuracy, resulting in better patient outcomes through prompt and targeted therapies.
Advanced DL models such as U-Net, Res-UNet, and UNet++ have also been used to identify hydronephrosis from ultrasound images, aiming to improve efficiency and uniformity in clinical environments [
15]. The combination of these models, along with pre-processing and post-processing techniques, exhibited a remarkable level of accuracy in identifying moderate/severe hydronephrosis. ML algorithms have also been used to distinguish between high and low-grade hydronephrosis to facilitate standardized automatic grading [
20]. These algorithms have created predictive models using a combination of radiomic texture analysis, support-vector machines, and DL approaches. In addition, DL algorithms have also been used to segment ultrasound pictures and calculate the hydronephrotic area as a ratio of the renal parenchyma, which serves as a useful biomarker of disease severity. Overall, AI technologies offer higher diagnostic accuracy, clinical efficiency, and improved care of hydronephrosis management.
2.3. Impact Treatment Planning and Long-Term Monitoring
AI approaches have significant implications for treatment planning and long-term monitoring of pediatric hydronephrosis. Cerrolaza et al. (2016) [
10] showcased the ability of AI algorithms to accurately forecast the need for diuretic renography. This approach provides a non-invasive means of evaluating the extent of hydronephrotic renal units. Clinicians can optimize treatment planning and reduce unnecessary invasive procedures by determining thresholds of clinically meaningful washout times. This will allow efficient selection of patients who would benefit from diuretic renography, thereby decreasing radiation exposure and associated healthcare costs. This technique is particularly relevant to the continuing debate over whether postnatal imaging is necessary in newborns with prenatally diagnosed hydronephrosis.
Erdman et al. (2020) [
13] revolutionized treatment planning in pediatric urology by introducing DL algorithms that can predict obstructive hydronephrosis from ultrasound pictures alone. The exceptional accuracy attained by these models allows medical professionals to identify patients at high risk who require urgent surgical intervention, thereby minimizing the need for unnecessary invasive testing and follow-up. Implementation of standardized management protocols across all urological departments also ensures uniform and precise identification of hydronephrosis, particularly in remote regions lacking specialized pediatric facilities.
In addition, Lien et al. (2023) [
15] highlighted the importance of identifying hydronephrosis promptly and correctly, particularly in emergency and acute care environments. AI algorithms can precisely identify moderate/severe hydronephrosis to speed up medical treatment and potentially decrease the length of emergency room visits. Implementation of automated hydronephrosis grading from ultrasound scans also improves diagnostic methods and standardizes evaluation, facilitating customization of treatment plans for individual patients.
Sloan et al. (2023) [
16] introduced AI systems that can forecast clinical outcomes by analyzing ultrasound images collected over an extended period. This technique offers insight into the evolution of hydronephrosis, success of different treatments, and ongoing patient management. In addition, the HARP ratio can be calculated using DL techniques, as explained by S. H. Song et al. (2022) [
18] This AI technique streamlines the calculation process and reduces inconsistencies among observers, enabling precise and consistent monitoring of hydronephrosis progression to ensure reliable long-term care.
Overall, the incorporation of AI methods into the management of pediatric hydronephrosis has great promise to improve treatment planning and long-term monitoring tactics. AI-driven solutions in pediatric urology provide more accurate, effective, and tailored approaches to patient treatment by predicting the need for diagnostic procedures, automating severity grading, and tracking disease development.
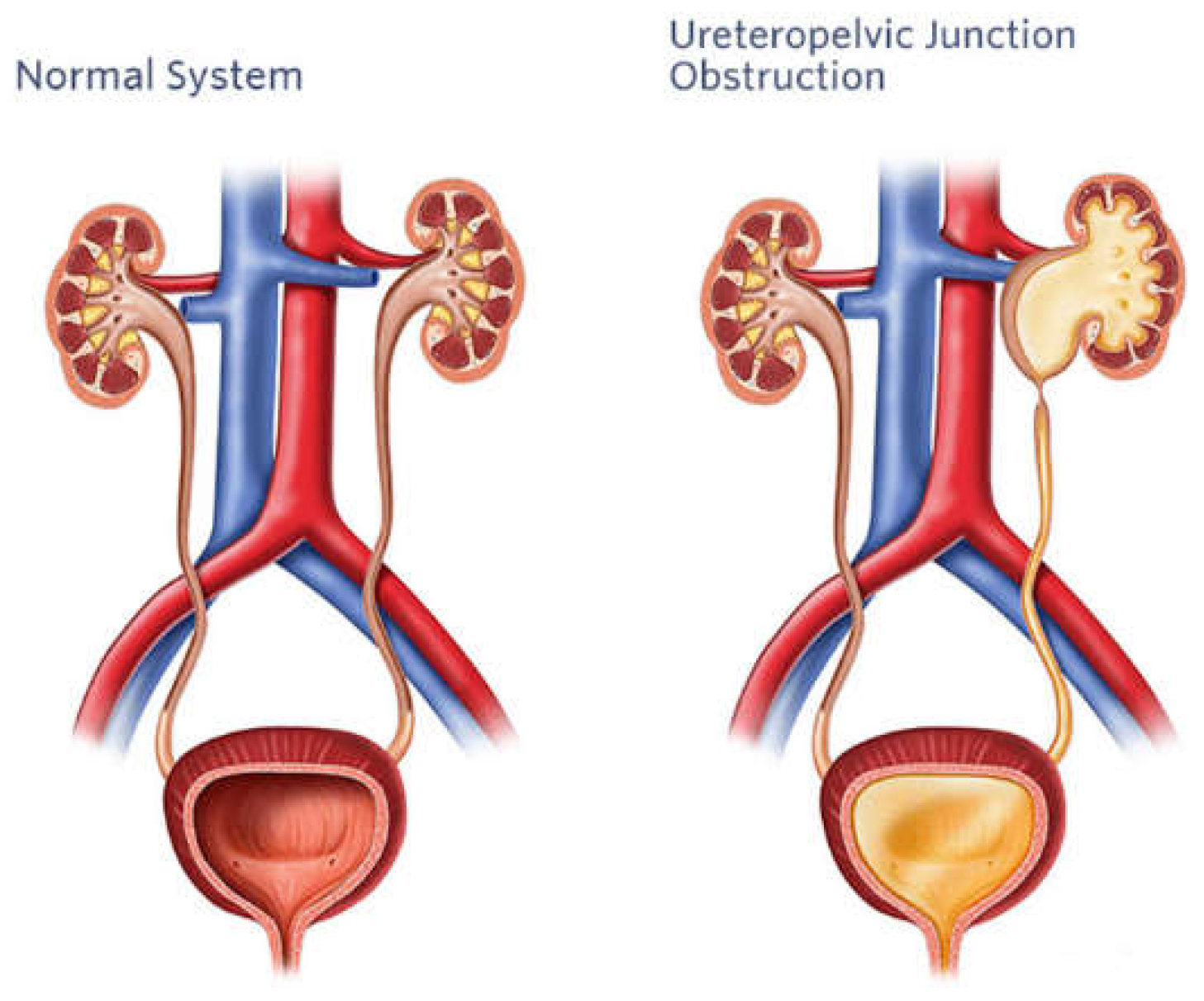
3. Pyeloplasty
A cornerstone of surgical care for pediatric urological disorders is pyeloplasty, a surgical operation designed to correct blockage of the ureteropelvic junction (UPJ) as shown in
Figure 5. Despite historically high rates of success, pediatric pyeloplasty poses specific difficulties because of the tiny stature and fragile anatomy of young patients. The complex surgical challenges of pediatric pyeloplasty, development of AI-assisted operating methods, and potential future applications are outlined in this section (Kelley et al., 2016) [
23].
3.1. Surgical Challenges in Pediatric Pyeloplasty
Although pediatric pyeloplasty is a fundamental surgical procedure for treating blockage of the UPJ, the fragile anatomy of pediatric patients requires extreme accuracy and attention to detail. A primary obstacle is the restricted area available for surgery and the requirement for careful tissue manipulation to maximize results while reducing harm [
25]. The diverse appearance of UPJ blockage also complicates surgical planning and management. Individualized surgical techniques may be required for pediatric patients due to a variety of anatomical variables, including crossing vessels, aberrant anatomy, and related congenital defects. To successfully address these factors, one must have a solid grasp of pediatric anatomy and pathology in addition to flexibility of surgical approach [
24,
25,
26].
Management of postoperative complications in pediatric pyeloplasty remains complex [
27]. Urine leakage, blockage recurrence, and renal parenchymal loss are example complications that need to be identified and treated promptly to minimize side effects and maximize long-term renal function (
Table 4). In order to reduce morbidity and maintain renal health, surgical treatments must also be carefully considered and strategically planned in the light of possible effects on future renal growth and development [
28]. Structural and physiological variation in pediatric patients creates additional surgical hurdles for pyeloplasty. During these procedures, a number of major issues have been identified by [
29,
30], along with possible mitigation techniques;
Small Anatomy: The smaller anatomical components of pediatric patients make precision surgical operations more difficult. Surgeons use pediatric surgical procedures that are adapted to the reduced scale of pediatric anatomy in conjunction with tiny tools to solve this challenge.
Reduced Vision: Pediatric patients typically confer reduced vision during surgery due to the small size of the operative region. Surgeons use magnification techniques such as surgical loupes or endoscopic magnification equipment to increase visibility. This allows clearer visualization of fine anatomical features within the operative site.
Tissue Handling: Delicate tissues from pediatric patients must be handled with extreme caution to avoid harm and ensure positive results. To reduce tissue stress and improve outcomes, surgeons prioritize precise hemostasis, use thin surgical equipment intended for pediatric operations, and manipulate tissue gently.
Ensuring safety and efficacy of pediatric pyeloplasty requires attention to detail and specific strategies to address surgical obstacles. Nonetheless, pediatric pyeloplasty remains a very popular and successful surgical procedure for treating UPJ blockage in young children.
3.2. AI-Assisted Surgical Techniques
AI is transforming pediatric urology by providing new methods and instruments to improve the accuracy, security, and effectiveness of major surgical procedures. Preoperative planning and simulation are one of the main ways in which AI has been deployed in pediatric pyeloplasty. AI algorithms can analyze preoperative images including CT, MRI, and ultrasound to produce three-dimensional reconstructions of the urinary system and determine optimal surgical technique. AI-driven preoperative planning technologies also allow surgeons to customize treatment plans based on the distinct anatomy of each pediatric patient by giving them access to comprehensive anatomical data in virtual simulations [
31] .
Intraoperative surgical navigation systems improve accuracy and precision by combining AI algorithms with real-time imaging data to give surgeons enhanced visibility and guide the surgical process. AI-assisted or robotic navigation systems make precise dissection, tissue manipulation, and suture insertion easier by superimposing anatomical landmarks, vascular maps, and trajectory planning onto the surgical field. This reduces error and improves surgical results [
31]. AI systems can identify minute variations in surgical dynamics and notify surgeons of possible consequences or departures from best practices by evaluating intraoperative data, such as motion of surgical instruments, tissue properties, and physiological factors. Proactive advising improves situational awareness and makes it possible to take prompt action to reduce risks and improve patient outcomes [
32].
AI-driven surgical education and training systems are also revolutionizing the way pediatric urology trainees learn and hone their surgical skills outside of the operating room. AI-powered virtual reality simulations give students rich, interactive settings in which to refine their procedural abilities, practice advanced surgical methods, and model intricate surgical situations. AI-assisted surgical training platforms also help pediatric urology trainees gain proficiency and confidence in executing pyeloplasty operations by providing a secure and regulated learning environment [
33].
3.3. Outcomes and Future Directions
Offering excellent long-term results and high success rates, pediatric pyeloplasty is a mainstay in the surgical treatment of UPJ blockage. Improvements in perioperative care, follow-up procedures, and surgical methods have led to outstanding results in juvenile pyeloplasty, including remission of symptoms, preservation of renal function, and avoidance of sequelae [
23].
Studies that track patients over an extended period of time have shown how durable surgical results are after juvenile pyeloplasty; most patients report long-lasting symptom alleviation and maintenance of renal function. Furthermore, advances in less invasive surgical methods, such laparoscopic and robotic-assisted procedures, have improved outcomes further by decreasing surgical morbidity, improving cosmesis, and hastening recovery [
29].
AI has the potential to significantly impact the future of pediatric pyeloplasty by providing personalized treatment recommendations catered to the specific requirements of each patient, real-time intraoperative guidance, and predictive modeling for risk stratification [
30]. By using cutting-edge imaging modalities, predictive analytics, and ML algorithms, AI-driven surgical platforms and decision support systems can increase surgical accuracy, improve outcomes, and minimize complications. AI-powered virtual reality training programs will also encourage professional growth in the field of pediatric pyeloplasty that will positively impact on treated patients [
30]. At the same, a number of ethical, regulatory, and practical challenges must be carefully addressed to ensure appropriate deployment of AI in pediatric surgical settings [
29].
4. Pyeloplasty, Kidney Tumors and Stones
Pediatric kidney tumors and stone care call for a multidisciplinary approach to ensure accurate diagnosis and efficient treatment. In this section we explore the diagnostic nuances and treatment issues related to kidney cancers and stones, then explore the revolutionary effects of AI applications on patient management and outcomes.
4.1. Outcomes and Future Directions, Diagnostic Challenges and Treatment Options
Given the intricacy of kidney tumors and stones, as well as the distinct physiological features of juvenile anatomy, diagnosing and treating these illnesses in young patients presents considerable obstacles:
Diagnostic Difficulties: Kidney tumors and stones in children may exhibit nonspecific symptoms such hematuria, stomach discomfort, or urinary tract infections, which can be mistaken for other common pediatric ailments. Therefore, a combination of clinical evaluation, imaging exams, and laboratory investigations is crucial for accurate diagnosis. However, it can be difficult to differentiate between different forms of stones or distinguish between benign and malignant tumors, requiring a thorough diagnostic approach [
34].
Imaging modalities: A number of imaging modalities, such as intravenous pyelography (IVP), CT, MRI, and ultrasound, are essential for the diagnosis of juvenile kidney cancers and stones. Ultrasound is non-invasive and emits no ionizing radiation, so this method is used as a first-line imaging modality to examine renal morphology and identify structural problems (
Figure 6). CT and MRI are more sensitive and specific for assessing stone load and identifying renal masses, but also entail radiation exposure and require patient sedation [
35].
Treatment Options: Tumor type, size, location, and patient age are among the characteristics that influence how juvenile kidney tumors and stones are managed. Options for treatment vary from minimally invasive techniques and surgical intervention to cautious maintenance and attentive waiting. The primary therapy for kidney cancers that are localized in nature is surgical excision; nephron-sparing techniques are recommended whenever possible in order to maintain renal function. Alternatively, depending on the size and composition of stones, medicinal therapy, dietary changes, and minimally invasive techniques like ureteroscopy or shock wave lithotripsy (SWL) may be employed [
36].
Clinicians should take into account the individual requirements and preferences of each patient when navigating the diagnostic hurdles and treatment options for kidney tumors and stones in children. Pediatric patients with kidney tumors and stones can receive better care and achieve superior results when practitioners use a multidisciplinary team and combine cutting-edge imaging methods with evidence-based therapy algorithms.
4.2. AI Applications in the Management of Kidney Tumors and Stones
AI technologies are transforming the way young patients with kidney tumors and stones are managed, in particular by guiding diagnosis and treatment planning to raise standards of care:
4.2.1. Pediatric Kidney Stones
Improved Diagnostics: AI-driven image analysis tools are improving the identification and measurement of juvenile kidney stones, enabling more precise diagnosis and therapy. Clinicians can estimate stone load and composition by applying ML techniques, making it possible to customize treatment and patient management [
35].
Personalized Therapy Planning: AI systems create individualized therapy suggestions based on the analysis of patient-specific information, including imaging data and medical history. This improves outcomes and lowers the likelihood of recurrence [
34].
Predictive Modeling and Risk Stratification: Physicians can identify juvenile kidney stone patients who are more likely to experience problems or a recurrence by using AI-powered predictive modeling. ML algorithms can forecast future stone occurrences and direct treatment and preventative actions by evaluating a variety of clinical factors and imaging data to achieve better long-term results [
36].
Ethics and Regulations: Careful consideration of ethical and regulatory problems is required when integrating AI into the treatment of juvenile kidney stones, which should employ defined protocols and encourage openness and accountability as standard. For AI to be used ethically and responsibly, cooperation between researchers, regulatory agencies, and healthcare practitioners will be crucial [
34].
4.2.2. Pediatric Kidney Tumors
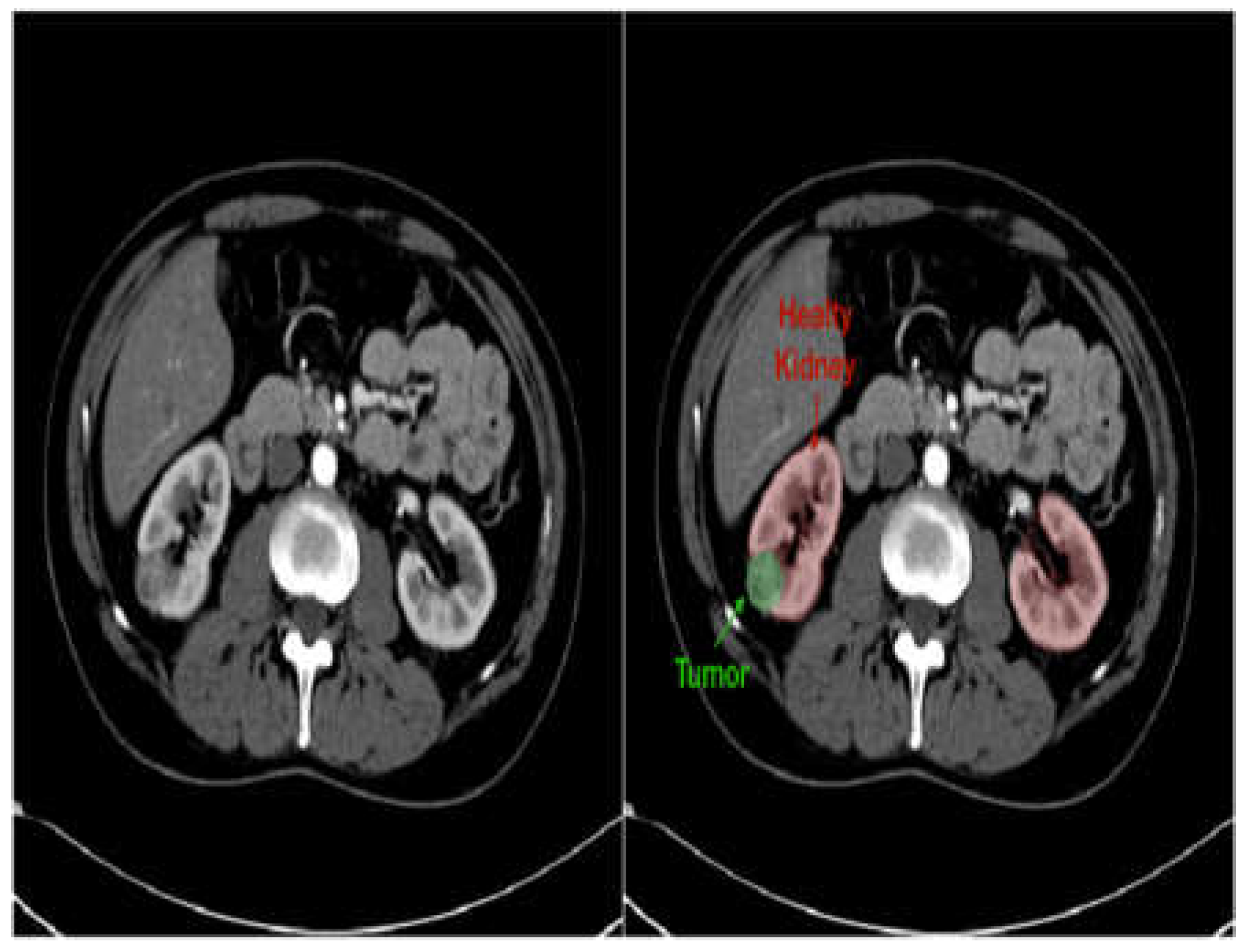
Improved Diagnostics: AI-driven image analysis tools can enhance the identification and description of pediatric kidney cancers and tumors. Radiologists can spot minor abnormalities, characterize renal masses, and estimate tumor burden more accurately by using ML algorithms that have been trained on large datasets of pediatric renal images (
Figure 7). AI algorithms provide early identification of kidney cancers and tumors by methods including pattern recognition, masking, segmentation, and quantitative analysis, allowing for confident diagnosis and timely treatment [
19].
Personalized Treatment Planning: AI systems enable personalized therapy recommendations and prognostic evaluation of patient-specific data, including clinical history, imaging results, and laboratory values. For young patients with kidney cancers, this customized strategy enables medical practitioners to maximize therapeutic efficacy, reduce treatment-related morbidity, and enhance long-term outcomes [
38].
Predictive Modeling and Risk Stratification: AI predictive modeling enables doctors to identify children with kidney tumors who are more likely to experience treatment failure or disease progression. ML algorithms identify prognostic characteristics and biomarkers through analysis of varied datasets. This improves treatment outcomes and survival rates for children with kidney cancers by empowering doctors to use tailored risk mitigation strategies and early treatment [
39].
Ethical and Regulatory Considerations: Incorporating AI into the treatment of juvenile kidney cancers and tumors requires careful negotiation to overcome legal, ethical, and practical challenges. Collaboration between developers, healthcare providers, and other stakeholders will be essential to ensure ethical and effective use of AI in clinical practice [
40].
Figure 7.
Kidney CT image in grayscale (left) and with overlaid mask (right) to highlight suspected stones or tumors [
41].
Figure 7.
Kidney CT image in grayscale (left) and with overlaid mask (right) to highlight suspected stones or tumors [
41].
4.3. Outcomes and Future Directions Impact on Treatment Strategies and Outcomes
Treatment plans and patient outcomes will be significantly impacted by use of AI in the management of kidney tumors and stones in young patients (
Table 3).
5. Vesicoureteral Reflux
VUR is a common pediatric urological condition characterized by retrograde flow of urine from the bladder into the ureters and kidneys. Failing to treat this condition can result in renal scarring and chronic kidney damage, highlighting the need for precise diagnosis and efficient management. Previously, diagnosis of VUR depended on invasive techniques such as VCUG and radionuclide cystography, which causes discomfort and poses significant risks for pediatric patients.
Figure 8 illustrates the VUR grading system, which classifies this disorder into five groups based on the extent of urine backflow and dilation of urinary tract structures.
Advances in medical imaging and AI offer new possibilities for enhancing diagnosis and treatment of VUR. Microwave radiometry provides a non-intrusive method for identifying VUR by monitoring temperature changes in the kidney as a result of urine reflux [
42].
ML methods have also been used to improve the grading and diagnosis of VUR based on automated analysis of features collected from imaging studies [
43,
44]. This section aims to explore the diverse array of methods currently used for diagnosis and management of VUR in pediatric patients. Grade I: urine backflow into ureters but not kidney. Grade II: urine reaches the kidney but no renal pelvis dilation. Grade III: mild to moderate ureter and renal pelvis dilation. Grade IV: dilation of ureter, renal pelvis, and calyces. Grade V: severe dilation of ureters, renal pelvis, and calyces [
45].
5.1. Diagnosis and Management of VUR
Conventional diagnosis and treatment of VUR are limited by their invasive nature. VCUG is the current gold standard method, but requires catheterization and exposes patients to radiation. While commonly used, VCUG exhibits significant variability between observers due to reliance on visual cues to grade severity, which can result in uneven grading and inconsistent decision-making [
43].
To overcome the challenges of VCUG, researchers have investigated alternate non-invasive methods. Voiding urosonography (VUS) with contrast agents has demonstrated enhanced sensitivity in identifying VUR compared with earlier contrast-based methods. Despite an excellent safety record, there are still concerns over the invasiveness of applying contrast agents. It is therefore crucial to continue searching for non-invasive techniques that offer high sensitivity and specificity for diagnosis of VUR [
42].
At present, diagnosis is heavily dependent on VCUG and the grading system established by the International Reflux Committee, which confers subjectivity and variability of image interpretation. Inconsistency of VUR grading impacts diagnostic accuracy, particularly when differentiating between many classes, underscoring the need for a more objective categorization method [
44]. Further progress in imaging technologies is also needed to aid detection of small lesions and structural disorders [
46]. New approaches such as contrast-enhanced voiding ultrasonography (ceVUS) may offer alternatives to conventional methods [
47]. CeVUS is radiation-free and dependable for diagnosing VUR, surpassing VCUG in terms of sensitivity and specificity. This method effectively addresses concerns over radiation exposure and invasiveness. Nevertheless, it is imperative to tackle issues such as reliance on operators and inaccurate negative results by conducting additional research [
48]. Ultimately, while traditional diagnostic approaches remain useful, continuous progress in non-invasive techniques and the creation of objective categorization systems are warranted to enhance the precision and uniformity of VUR diagnosis and treatment.
5.2. AI-Based Imaging Techniques
AI-based imaging tools represent a major breakthrough in diagnosis and treatment of VUR. One proposed method entails the use of ML models to automate assessment of VUR severity from VCUG data [
43]. This strategy trains models using annotated images that have been assessed by multiple experts. Using ML techniques, this approach presents a possible transition towards more dependable and uniform diagnostic procedures in the management of VUR.
Quantitative Vesicoureteral Reflux (qVUR) is a novel method that uses supervised ML to assess severity of VUR by analyzing VCUG image features [
44]. The use of a random forest classifier results in a high level of accuracy and clarity when differentiating between low and high-grade VUR. The creation of a web program named qVUR enables automated grading, providing a more impartial and consistent method for assessing VUR.
CeVUS uses contrast-specific harmonic imaging modes and AI algorithms to improve ultrasound imaging, offering a dependable and radiation-free alternative to conventional radiological methods for diagnosing VUR [
47]. This method shows potential to effectively visualize VUR while reducing the dangers associated with exposure to ionizing radiation. In addition, the Vesicoureteral Reflux Index (VURx) predictive tool can be used to assess rates of resolution and improvement of VUR in children <2 years old [
49]. VURx was created using multivariate survival analysis and random forest modeling to accurately predict primary reflux improvement and resolution, which can help in developing tailored treatment plans.
Table 4 summarizes a variety of imaging techniques that employ AI to guide diagnosis and treatment of VUR.
Table 4.
AI-based approaches to diagnosis and treatment of vesicoureteral reflux.
Table 4.
AI-based approaches to diagnosis and treatment of vesicoureteral reflux.
| Approach |
Description |
Advantages |
| Machine Learning Models for VUR [43] |
Utilizes ML to automate VUR severity assessment from VCUG images, aiming to reduce subjectivity and improve reliability of evaluation. |
Decreases influence of subjectivity, offers uniform diagnostic procedures. |
| Quantitative Vesicoureteral Reflux (qVUR) [44] |
Employs supervised ML to analyze VCUG images for VUR severity, achieving high accuracy and clarity in grading. |
Provides a web program for automated grading, ensures impartial and consistent assessment. |
| Contrast-enhanced Voiding Ultrasonography (ceVUS) [47] |
Uses second-generation ultrasound contrast agents and AI algorithms to diagnose VUR, offering a radiation-free alternative to traditional methods. |
Enhances ultrasound imaging, reduces risks associated with ionizing radiation. |
| Vesicoureteral Reflux Index (VURx) [49] |
Predictive tool developed for assessing improvement and resolution rates of VUR in children under two years, aiding in tailored treatment plans. |
Accurately predicts reflux improvement and resolution, guides clinical decision-making. |
5.3. Prediction Models and Long-Term Follow-Up
Multiple studies have developed predictive models that can inform both short and long-term management of VUR. Kabir et al. (2024) [
34] focused on creating ML models to accurately diagnose VUR using VCUG images, although they also included long-term treatment outcomes and management strategies. Khondker, Kwong, Rickard, et al. (2022) [
44] also proposed a ML method to forecast and assess VUR using VCUG data. Their supervised method offers exceptional accuracy, sensitivity, and specificity for differentiating between low and high-grade VUR. The authors also proposed that these models could be used to monitor VUR patients over an extended period of time, offering an automated and uniform approach that does not rely on subjective human interpretation.
In the same way, Kirsch et al. (2014) [
49] proposed the VURx prognostic tool to predict improvement of primary reflux in children. This model provides useful insights into long-term treatment results and aids in decision-making and patient care. This is achieved by identifying characteristics related to reflux resolution, such as VUR grade and the existence of ureteral abnormalities. While many researchers concentrate exclusively on enhancing diagnostic accuracy, others have attempted to create models that predict long-term treatment outcomes, ultimately helping to individualize patient care. Overall, the use of predictive models in VUR management represents significant progress in treatment of affected patients.
6. Detrusor Overactivity
Urge, frequency, and incontinence are symptoms of a common urological disorder known as detrusor overactivity (DO), which is characterized by involuntary contraction of bladder muscle. This section explores the complex nature of DO, typical clinical symptoms, and current diagnostic procedures, as well as key areas for deployment for AI tools (
Table 5).
6.1. Current Diagnosis and Management Challenges
6.1.1. Current Diagnosis Challenges in Pediatric Detrusor Overactivity (DO):
Traditional diagnostics for DO in juvenile patients are highly variable, including urodynamic studies (UDS) which rely on operator skill and are thus subjective [
50]. Diversity of opinion makes it difficult to identify DO confidently, particularly in young patients. Furthermore, conventional diagnostic criteria may not sufficiently encompass the subtleties of DO, especially in populations with specific disorders such as spina bifida. The absence of standardized interpretation methods impedes precise diagnosis and may lead to suboptimal treatment choices [
51].
6.1.2. Challenges in Managing Pediatric Detrusor Overactivity:
Addressing DO in juvenile patients poses numerous difficulties, mainly stemming from the lack of clear diagnostic criteria and appropriate therapies. Although conservative treatment choices are commonly favored, a significant proportion of patients do not receive any treatment whatsoever. Furthermore, current epidemiological data on DO are limited and ambiguous, thus impeding our comprehension of incidence and consequences. In addition, while new developments like transcutaneous electrotherapy display potential, there remains a clear need for more effective treatment methods. The historical disregard for DO has resulted in a renewed focus, although obstacles remain in the effort to decrease problems related to inadequate bladder emptying [
56].
6.1.3. Progress and Prospects for the Future:
Advances in ML algorithms and diagnostic imaging techniques provide new options for identifying and controlling DO in juvenile patients. ML algorithms have demonstrated clear efficacy in detecting DO patterns in UDS, which can enable consistent interpretation and enhance diagnostic precision [
56]. Advanced imaging techniques like Contrast-Enhanced Ultrasound (CEUS) also provide radiation-free and extremely sensitive diagnostic techniques. These developments emphasize the need to continue formulating more precise diagnostic criteria and efficient treatments for DO, ultimately enhancing outcomes for pediatric patients experiencing lower urinary tract symptoms [
57].
6.2. AI Solutions for Detrusor Overactivity
ML algorithms provide creative methods to standardize interpretation of urodynamic data and enable automated identification of DO events. Moreover, progress in diagnostic imaging methods such as ceVUS offer radiation-free options for precise diagnosis.
6.2.1. AI Solutions for the Diagnosis of Detrusor Overactivity in Pediatric Patients:
DO diagnosis in pediatric patients has historically been difficult due to the inconsistent interpretation of urodynamic tests (UDT). Hobbs et al. (2022) [
50] tackled this problem by creating a ML system called the Pediatric Detrusor Overactivity Identification system (PDOIA), which was designed exclusively for pediatric patients with spina bifida. PDOIA displayed exceptional performance in detecting DO, with an overall accuracy of 85% using both time and frequency-based methods. Incorporating ML algorithms like PDOIA can standardize UDS interpretation and enable shared decision making to enhance efficiency and accuracy, thereby reducing overall healthcare expenditure.
6.2.2. Artificial Intelligence Solutions for Automated Detection of Detrusor Overactivity:
H. S. Wang et al. (2021) and J. Wang et al. (2023) [
51,
53] used the Urodynamic Detrusor Overactivity Recognition Algorithm (UDORA) to detect DO events in UDS data. UDORA achieved an overall accuracy of 87%, with 80% sensitivity and 92% specificity 92%. These algorithms have the capacity to acquire knowledge from diverse patterns of DO and represent substantial progress in automating detection [
7,
51]
6.2.3. AI Solutions for the Management of Detrusor Underactivity (DUA):
Addressing detrusor underactivity (DUA) is challenging due to the scarcity of diagnostic standards and viable therapies [
57]. While there are conservative treatment options available, a significant proportion of patients may not receive treatment for an extended period, emphasizing the necessity for novel interventions. ML methods, like the Detrusor Underactivity Management Algorithm (DUMA), show potential in enhancing therapy choice and enhancing results for patients with DUA and underactive bladder (UAB) [
57]. DUMA demonstrated a comprehensive accuracy of 82% in guiding treatment options by considering individual patient characteristics and treatment responses.
6.2.4. Progress in Diagnostic Imaging Techniques:
Advances in diagnostic imaging techniques such as ceVUS have significantly improved the diagnosis of urinary tract problems in children. Roic et al. (2022) [
56] provided evidence for safety and efficacy of VUD-ceVUS in identifying intrarenal reflux (IRR) and VUR without the use of ionizing radiation. The combination of this novel strategy and ML algorithms such as the VUD-ceVUS Detection Algorithm (VUDA) provides numerous benefits compared to traditional methods, including enhanced detection rates and prolonged duration of observations. VUDA demonstrated sensitivity of 90% and specificity of 95% in detecting VUR and IRR, while also reducing radiation exposure in juvenile populations [
56].
6.3. Case Studies and Clinical Applications
Diagnosing and treating DO in children, especially those with spina bifida, presents considerable difficulties due to inconsistent interpretation of conventional tests. ML algorithms have now emerged as a possible solution for detecting DO in UDS (Hobbs et al., 2022), but significant obstacles persist, including the need for more algorithm refinement and testing of applicability across diverse clinical environments.
6.3.1. Case Studies and Clinical Applications in Pediatric Patients:
A previous case study created a ML algorithm to detect DO in UDS from individuals with spina bifida. This analysis examined 805 UDS testing files obtained from 546 pediatric patients with a median age of 8.7 years [
50]. The algorithm demonstrated impressive performance metrics, with the time-based model that included all three pressure channels (intravesical, abdominal, and detrusor) achieving a maximum area under the curve (AUC) of 91.9%. This proof-of-concept study showcased the capacity of ML algorithms to standardize interpretation of UDS, promote collaborative decision-making, and enhance care for patients with spina bifida.
6.3.2. Machine Learning Models and Performance:
The time-domain models developed by Hobbs et al. (2022) [
50] examined UDS traces and retrieved parameters such as mean, minimum, maximum, median, and standard deviation of pressure signals. By implementing data windowing, the models' performance was enhanced. This involved dividing each file into segments of 60 seconds, with overlapping intervals of 20 seconds. The time-based model, utilizing all three pressure channels, demonstrated a maximum AUC of 91.9%, sensitivity of 84.2%, and specificity of 86.4%.
The frequency-domain models used the Fast Fourier Transform to convert pressure data into the frequency domain [
50]. Dimensionality reduction approaches were then employed to decrease the quantity of features and enhance performance of the model which achieved an AUC of 90.5%, sensitivity of 68.3%, and specificity of 92.9%. A separate study also devised a ML model to automatically detect DO in UDS [
53]. This alternative method employed manifold learning and dynamic time warping algorithms to attain an overall accuracy of 81.35%, with sensitivity of 76.92%, and a specificity of 81.41% when identifying DO in the testing set.
6.3.3. Challenges and Future Directions:
Hobbs et al. (2022) [
50] demonstrated encouraging findings but also acknowledged areas that require improvement. The bulk of false-positive occurrences were attributed to patient movement difficulties, underscoring the need for algorithm refinement and optimization. In addition, the study was carried out at a solitary center, so it will now be important to test applicability to other centers. The authors further stressed the need to follow defined protocols and interpretation guidelines in conjunction with ML algorithms in order to establish clear benefit and maximize influence on clinical outcomes [
50].
To summarize, the use of ML algorithms in interpreting UDS for the detection of DO has yielded encouraging outcomes, especially in individuals with spina bifida. However, additional study is required to tackle obstacles, fine-tune algorithms, and assess their effectiveness in real-world clinical environments across various facilities.
7. Posterior Urethral Valves
This section explores the clinical characteristics, AI diagnostics, and use of cystoscopy in PUV, a congenital defect that is characterized by obstructive tissue folds within the male urethra that manifest as a range of urinary tract symptoms in newborns and infants.
7.1. Clinical Features and Diagnosis
Antenatal Diagnosis: Deshpande (2018) found that approximately 50% of PUV instances can be diagnosed prenatally using ultrasound. The diagnostic criteria include bilateral hydronephrosis, an enlarged bladder (megacystis), or a keyhole sign (a dilated posterior urethra narrowing at the point of valve obstruction). Unfortunately, the accuracy of prenatal ultrasound is quite low, ranging from 40% to 80%. Fishberg et al. (2018) and Kwong et al. (2022) [
58,
59] have also reported that PUV can be indicated by prenatal ultrasound findings including bilateral hydroureteronephrosis (dilation and swelling of the ureters), a distended and thickened bladder, oligohydramnios (low levels of amniotic fluid), presence of keyhole sign, and increased renal echogenicity (higher than normal reflection of sound waves in the kidneys).
Postnatal Presentation: Neonates with PUVs may present diverse symptoms such as difficulty breathing, bluish discoloration of the skin, weak or irregular urine flow, lack of energy, difficulty with feeding, and widespread swelling [
58,
59]. Physical examination may uncover an enlarged abdomen caused by urine ascites, a swollen bladder, or hydronephrosis. Pellegrino et al. (2023) observed that some neonates may exhibit respiratory distress or sepsis, while others show no symptoms. This study also noted that in newborns and older children, symptoms such as urinary tract infections, reduced urine flow, inability to control urination, and bedwetting may also indicate the presence of PUV.
Bladder and Renal Function: According to Deshpande (2018) [
60], blood creatinine levels are crucial for predicting outcomes after birth. An elevated minimum level of blood creatinine in the first year of life (>0.8-1.0 mg/dL) is linked to increased likelihood of long-term kidney complications. The velocity of decline in serum creatinine following valve ablation, along with the existence of prolonged renal tubular acidosis, also serve as indicators for renal outcomes.
Taskinen et al. (2012) [
61] found that PUV frequently results in atypical bladder function characterized by reduced compliance or excessive activity throughout infancy. The bladder wall is characterized by a thickened structure with trabeculae and the presence of pseudodiverticulae. The posterior urethra is dilated, and the bladder neck is conspicuous. Urodynamic studies indicate that upon initial examination, 74-88% of patients exhibit an overactive or poorly compliant bladder, characterized by elevated voiding pressures. Nevertheless, bladder hypercontractility and compliance typically show signs of improvement following valve ablation.
As children age, their bladder becomes excessively stretched and difficult to empty, resulting in higher amounts of leftover urine due to variables such as excessive urination, less sensation, and an enlarged bladder neck. A significant number of children experience delayed toilet training and urine incontinence, with approximately 49-55% establishing continence by the age of 5, as reported by Taskinen et al. (2012) [
61].
Methods of Diagnosis: VCUG is widely recognized as the most reliable method of validating a PUV diagnosis. According to Fishberg et al. (2018) and Kwong et al. (2022), VCUG enables the observation of valvular obstruction, a bladder that is thickened and has trabeculations, diverticuli, and vesicoureteral reflux.
Weaver et al. (2023) [
62] emphasized the application of DL to extract characteristics from postnatal kidney ultrasounds that forecast progression to chronic kidney disease (CKD) in children with PUV. At present, assessment of CKD risk relies on the lowest level of creatinine during the initial year of life. Levels below 0.8 mg/dL indicate negligible risk, whereas levels above 1.2 mg/dL indicate increased likelihood of kidney failure.
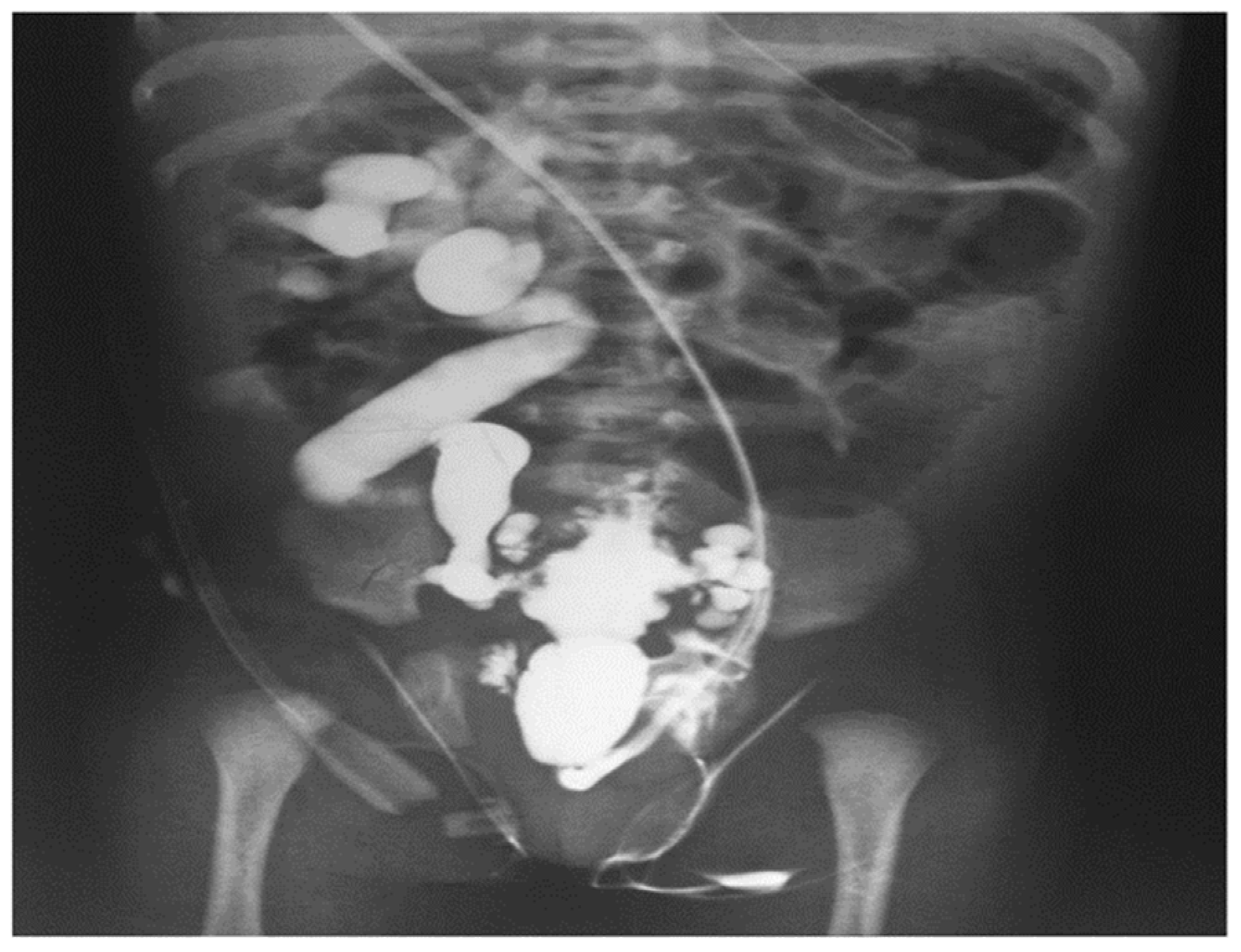
Figure 9 shows that upon examination, a micturating cystourethrogram can confirm the presence of type 1 PUV, in addition to matching abnormalities in the bladder (trabeculation) and upper urinary system.
To summarize, the clinical characteristics and diagnosis of PUV encompass a blend of antenatal ultrasound observations, postnatal clinical symptoms, assessment of kidney function / bladder dynamics, and diagnostic techniques such as voiding cystourethrography. Prompt identification and diagnosis are essential for timely action and prevention of sequelae.
Figure 9.
Micturating cystourethrogram revealing the presence of type 1 PUV together with corresponding alterations in the bladder (trabeculation) and upper urinary system (vesicoureteral reflux) [
60].
Figure 9.
Micturating cystourethrogram revealing the presence of type 1 PUV together with corresponding alterations in the bladder (trabeculation) and upper urinary system (vesicoureteral reflux) [
60].
7.2. AI Applications in Prenatal Diagnosis and Risk Stratification
Prenatal detection of PUV is essential for timely intervention and improving outcomes for affected fetuses. DL techniques such as CNNs have been created to automatically detect PUV using prenatal ultrasound pictures. These models have achieved reported accuracies of up to 92%, surpassing traditional methods [
63]. In addition, AI algorithms have been used to accurately segment and analyze the volume of fetal anatomy related to PUV, such as measures of the bladder and kidneys. This allows for exact monitoring of anatomical changes over time [
64].
In addition, researchers have investigated the use of AI predictive models to categorize prenatal PUV observations to determine the likelihood of postnatal problems. These models have demonstrated encouraging outcomes, achieving accuracies of up to 86% in forecasting the probability of postnatal renal impairment, thereby guiding tailored counseling and management decisions.
Deep neural network models have also shown capacity to accurately diagnose PUV patients with 89% accuracy from maternal serum analyte levels in the first trimester [
65]. AI applications can therefore facilitate early detection, precise risk assessment, and personalized intervention planning. Ultimately, this can lead to better outcomes for fetuses affected by PUV as well as their families (
Table 6).
7.3. Role of Cystoscopy in the Diagnosis and Management of PUV
Cystourethroscopy is used as a supportive and confirmative imaging modality to further investigate children with an episode of febrile urinary tract infection in which VCUG findings are inconclusive. Valves are likely to be missed on VCUG when no urethral dilatation is present. Hakan discusses a case report of a 26 day-old boy who presented with febrile urosepsis in which VCUG revealed left-sided grade V vesicoureteral reflux without a posterior valve. In contrast, cystourethroscopy showed an anterior urethral valve, a rare congenital anomaly, that was missed by VCUG.
Use of AI technology for detection and diagnosis of pathological cystoscopy findings in the pediatric patient is sparse in comparison to the adult population. A review by Ikeda & Nosato (2024) [
66] of AI applications in cystoscopy and transurethral resection of bladder tumors showed that performance surpassed urologists in diagnosing lesions as benign or malignant. AI results were also compared to physicians in the detection of interstitial cystitis, bladder intraepithelial carcinoma, and BCG-induced cystitis.
Diagnostic Role: Cystoscopy is the definitive diagnostic modality for PUV, as highlighted by Pellegrino et al. (2023) [
67]. This method enables direct observation of the membrane of the posterior urethral valve, thereby establishing diagnosis following first suspicion based on prenatal ultrasound or postnatal clinical presentation. Moreover, these studies assert that cystoscopy is essential for assessing bladder dysfunction resulting from PUV. It can evaluate for any remaining valve, stricture caused by medical intervention, or enlargement of the bladder neck, all of which can lead to ongoing dilation of the upper urinary tract and decline in function.
Therapeutic Role: According to Deshpande (2018) [
60], post-natal treatment of choice for PUV is endoscopic ablation of the valve using diathermy, cold knife, or holmium-YAG laser, performed under cystoscopic guidance. Cystoscopy is the main technique used for endoscopic valve ablation, which is the ideal initial surgical treatment for PUVs in newborns, as stated by Fishberg et al. (2018), Kwong et al. (2022), and Taskinen et al. (2012) [
58,
59,
61]. They observed that in certain situations, bladder neck incision can be performed cystoscopically in conjunction with valve ablation to enhance emptying. In addition, Fishberg et al. (2018) and Kwong et al. (2022) [
58,
59] examined the application of cystoscopy for the endoscopic administration of botulinum toxin into the bladder. This procedure aims to enhance bladder compliance and reduce detrusor overactivity in patients experiencing ongoing bladder dysfunction following valve ablation.
Follow-up and Management: Following valve ablation, it is advised to repeat VCUG or cystoscopy within 1 to 3 months to verify full removal of the valves, as indicated by Fishberg et al. (2018) [
58]. According to Taskinen et al. (2012) [
61], after surgery it is advisable to do cystoscopy or voiding cystourethrogram to eliminate the possibility of partial ablation, scar tissue, or remaining obstruction.
Cystoscopy is essential to assess bladder dysfunction which is a significant consequence of PUV [
60,
61]. This evaluation is particularly important in the long-term monitoring of PUV patients, allowing observation of enduring symptoms, suspected constrictions, and other consequences. In addition, as stated by (Pellegrino et al., 2023) [
67], cystoscopy can be used in conjunction with urodynamic tests (videourodynamics) to evaluate bladder function and detect distinct urodynamic patterns related to the "valve bladder syndrome", aiding in the proper treatment of bladder dysfunction.
7.4. Importance, Impact, and Application of Cystoscopy in Pediatric Urology:
Cystoscopy is considered to be an essential diagnostic and therapeutic tool in the field of pediatric urology. This method allows direct visualization of the bladder, urethra, and associated structures to assist in diagnosis of congenital anomalies, urethral strictures, bladder tumors, vesicoureteral reflux, and other urological conditions in pediatric patients. From a therapeutic standpoint, cystoscopy plays a crucial role in allowing urologists to carry out minimally invasive procedures including biopsies, stone removal, urethral dilation, and endoscopic treatment of conditions such as posterior urethral valves [
68]. Cystoscopy is also an essential tool for researchers to use in the postoperative monitoring of pediatric patients, allowing assessment of treatment efficacy, detection of complications, and identification of residual abnormalities. The information obtained by cystoscopy guides treatment planning in pediatric urology by providing detailed insight into anatomical and functional aspects of the lower urinary tract, enabling tailored and individualized treatment strategies [
66].
Previous studies have shown that cystoscopy plays a crucial role in improving the quality of care for pediatric urology patients. By enabling accurate diagnosis, targeted interventions, and comprehensive management of urological conditions, cystoscopy ultimately leads to better patient outcomes and overall satisfaction [
69]. Cystoscopy also enables researchers to investigate urological conditions, evaluate treatment efficacy, and explore advances in endoscopic techniques [
70]. It is important to note that pediatric endourology also plays a vital role in the education of healthcare professionals and the training of future urologists. Based on these findings, it can be concluded that cystoscopy plays a crucial role in pediatric urology by offering numerous advantages in terms of diagnosing, treating, monitoring, and conducting research.
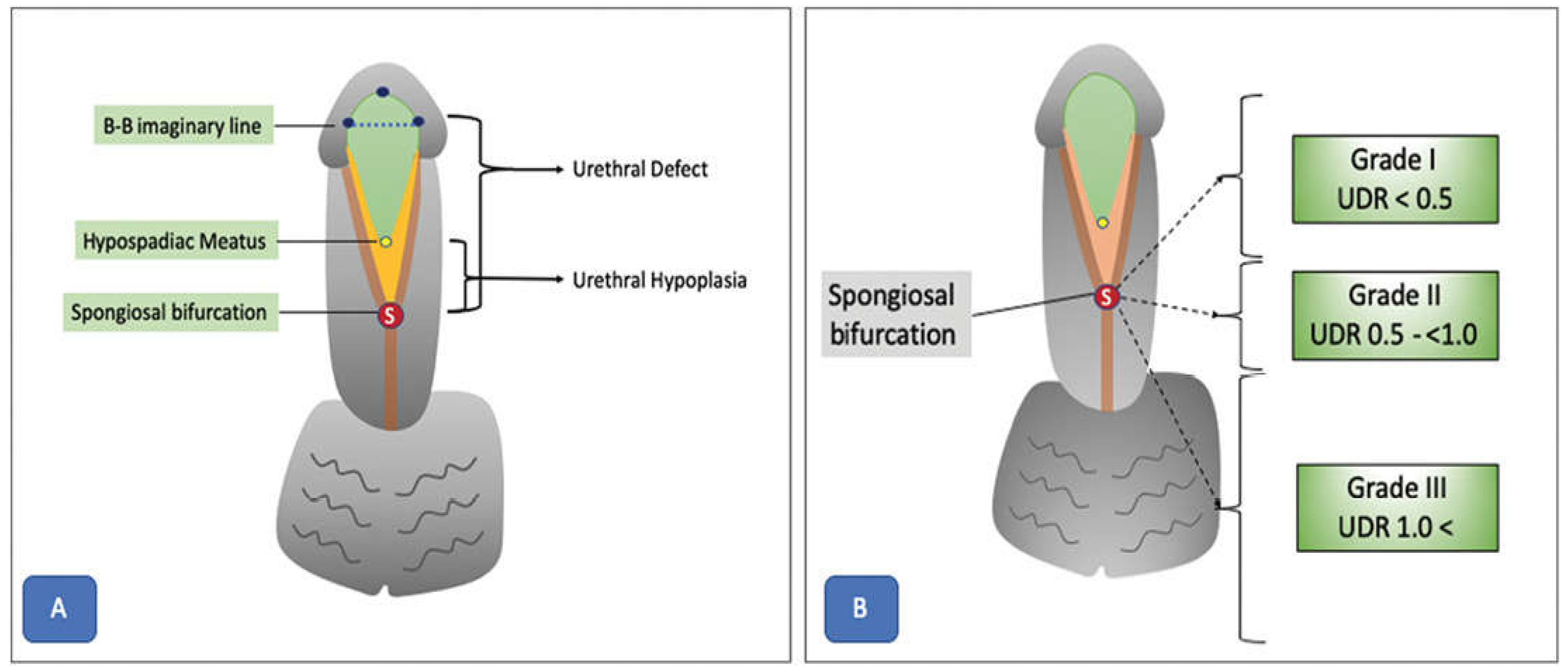
8. Hypospadias
Hypospadias is a congenital disorder of the urethra in males. Due to a wide range of presentations, variety of surgical approaches, and diverse possible outcomes, this condition is highly challenging for pediatric urologists to manage. Urethral defect ratio (UDR) and an imaginary line between the glanular knobs (B-B) are key features used to classify urethral defects (
Figure 10). The latest developments in AI and ML have created new opportunities for enhancing the diagnosis, surgery planning, and postoperative care of individuals with hypospadias. Scientists have investigated the use of DL algorithms to automatically classify and assess hypospadias severity using medical images. Prediction models have also been used to detect risk factors and improve surgical outcomes. In addition, advances in AI-assisted surgical planning systems can offer tailored suggestions for procedures and graft selection for individual patients. The ongoing development of AI technology presents an opportunity to improve patient care, optimize clinical workflows, and deepen our understanding of the intricate nature of hypospadias phenotypes and treatments.
8.1. Surgical Considerations and Complications
To minimize potential problems, surgical interventions require careful preparation and consideration. Each individual stage of the procedure, from pre-operative evaluation to post operative care, demands an accurate and meticulous approach.
Objective Assessment of Hypospadias Severity: Objective evaluation methods such as the Plate Objective Scoring Tool (POST) represent major advances in the field of hypospadias surgery [
71]. POST provides a systematic way to assess urethral plate quality, which helps surgeons select appropriate repair methods and anticipate postoperative results. However, even standardized methods like POST remain subjective, notably regarding the interpretation of anatomical landmarks from 2D pictures [
72]. The need for accurate and reproducible methods to standardize evaluation and reduce variability between surgeons is therefore clear. Objective evaluation methods like POST are an important step towards this aim, but additional refinement and validation are required before they can be used widely in clinical practice.
Surgical considerations in hypospadias-associated penile curvature (HAPC): Treatment of hypospadias-associated penile curvature (HAPC) is complex, as explained by Fernandez et al. (2021) [
73]. A critical aspect is the initial assessment and quantification of HAPC, where variable evaluation techniques highlight the need for standardized, reliable, and repeatable instruments. The lack of agreement on surgical correction thresholds also affects decision-making. Surgical method selection can range from dorsal plication to ventral lengthening operations, as described by Abbas et al. (2023) [
71]. Complications linked with HAPC correction, such as graft contracture and recurrent curvature, demand careful planning and judicious use of surgical procedures. Overall, treating HAPC requires a multimodal strategy that includes several standardized evaluation tools, evidence-based protocols, and careful surgical method selection.
Impact of Anatomical Variables on Surgical Outcomes: Anatomical characteristics are critical determinants of surgical results and potential problems in hypospadias correction, as highlighted by Fruntelata & Stoica (2021) [
74]. A previous study by Fernandez et al. (2021)[
73] reported that glans size, quality of the urethral plate, and degree of penile curvature all exert a major impact on surgical difficulty and likelihood of attaining good esthetic and functional outcomes. These studies present information on the many kinds of hypospadias operations performed and related consequences, emphasizing the need to conduct preoperative screening for concomitant abnormalities and tailor surgical techniques accordingly. Despite acknowledgement that these anatomical features are key predictors of surgical results, there is still substantial subjectivity in their evaluation, making it difficult to standardize categorization criteria and compare outcomes between surgical facilities and individual procedures. Addressing these issues necessitates continued work to provide uniform screening tools, improve categorization criteria, and establish evidence.
8.2. AI Applications in Hypospadias Surgery
AI-based UP Quality Assessment: Research by Abbas et al. (2023) [
71] explored the potential of AI and DL algorithms to streamline and optimize assessment of urethral plate (UP) quality from 2D images. The authors proposed a framework that combines glans localization, landmark detection, and POST (Plate Objective Scoring Tool) calculation using DL models. The localization step detects the glans area within an image using a YOLOv5 network, which achieved highly accurate results (mean average precision 99.5%, overall sensitivity 99.1%) [
71]. Isolating the region of interest from image background improves feature extraction and landmark detection in subsequent stages.
Landmark Detection and Benefits: For landmark detection, the authors employed a deep convolutional neural network (CNN) architecture called HRNetV2, which maintains high-resolution representations throughout the network [
71]. This approach results in spatially precise landmark predictions, which are essential for accurate calculation of POST score. The proposed model achieved a normalized mean error of 0.07152 in predicting the coordinates of all five POST landmarks. The authors highlight the potential benefits of this AI-based framework in increasing inter-rater consistency and standardizing UP quality scoring. Less experienced surgeons could benefit from real-time intraoperative application of the algorithm to aid decision-making and potentially improve postoperative outcomes.
AI for Hypospadias Parameter Recognition: Wahyudi et al. (2022) [
75] proposed the development of an artificial neural network (ANN) for automated recognition of various hypospadias parameters from digital images. The key parameters include hypospadias status, meatal location and shape, urethral plate quality, glans diameter, and glans shape. This type of model needs to be trained on a database of labeled images from hypospadias cases and normal penile anatomy controls. To achieve this, parents or guardians capture standardized photographs using a mobile app, then upload these for analysis by the ANN model. Performance can then be validated against evaluations by pediatric urologists, with measures including accuracy, precision, and interrater reliability. Several other AI-based approaches have also been proposed to help standardize hypospadias assessment, reduce inter-observer variability, and facilitate diagnosis, especially in regions with limited access to specialized healthcare personnel (
Table 7).
8.3. Impact on Surgical Outcomes
The study of Abbas et al. (2023) [
71] emphasized the crucial role of objective and reproducible UP assessment in predicting surgical outcomes and aiding selection of surgical techniques. Historically, assessment of anatomical factors such as UP quality has been highly subjective, which poses difficulties when comparing outcomes between medical centers and surgeons, even when standardized techniques are used. The introduction of POST scoring as proposed by Abbas et al. (2023) [
71], offers a quantitative assessment of upper limb quality based on a set of well-defined anatomical landmarks. While POST is both standardized and objective, manual measurement of landmarks from two-dimensional (2D) images can still introduce a degree of variability and subjectivity that limits accuracy.
The authors therefore proposed using AI to automate the process of landmark detection and objectively calculate POST score for increased consistency (Abbas et al., 2023) [
71]. The potential benefits of this approach include more reliable comparisons of surgical outcomes across centers and surgeons by eliminating the need for manual measurements. Moreover, the researchers posit that surgical outcomes in the context of hypospadias AI-assisted POST scoring can also serve as an instrument for assessing UP quality and predicting repair [
72]. Precise evaluation of UP quality prior to operating allows surgeons to enhance their decision-making process by selecting the most appropriate techniques, leading to improved outcomes and decreased complication rates.
Use of AI and DL technologies in the context of hypospadias surgery shows great potential in establishing standardized assessments, enhancing the decision-making process, and ultimately improving quality of care. By employing a systematic approach to assess anatomical factors and anticipate surgical outcomes, it may be possible to develop personalized treatment strategies, enhance surgical methods, and decrease variability of outcomes. Moreover, the systematic and reproducible evaluation of hypospadias cases has potential to enhance collaborative research efforts among multiple centers, thereby facilitating larger-scale investigations and fostering a more comprehensive understanding of the condition's origins, therapeutic options, and long-term consequences.
9. Other Emerging AI Applications in Pediatric Urology
This section examines three innovative AI technologies that are transforming the practice of pediatric urology: virtual reality simulation for surgical training, personalized treatment planning, and telemedicine for remote consultations. We aim to clarify the possible influence of these new technologies on pediatric urology and draw attention to the opportunities and obstacles they present for healthcare stakeholders, researchers, and practitioners.
9.1. Customized Treatment Planning
Treatment planning in pediatric urology frequently requires a customized approach to suit each patient's unique anatomical and physiological features. For juvenile urological diseases, AI-enabled decision support systems can integrate a wide range of patient-specific factors, including the demographics, medical history, imaging results, and biomarker data, to provide personalized treatment recommendations that optimize outcomes and mitigate risk [
78]. AI decision support systems can also assist physicians use time and resources more effectively by automating repetitive processes such as data analysis [
6]. This will eventually lead to significant advances in patient care and operational efficiency (
Table 8). To fully realize these benefits, issues including data quality, model interpretability, and ethical concerns around algorithmic decision-making must also be properly addressed.
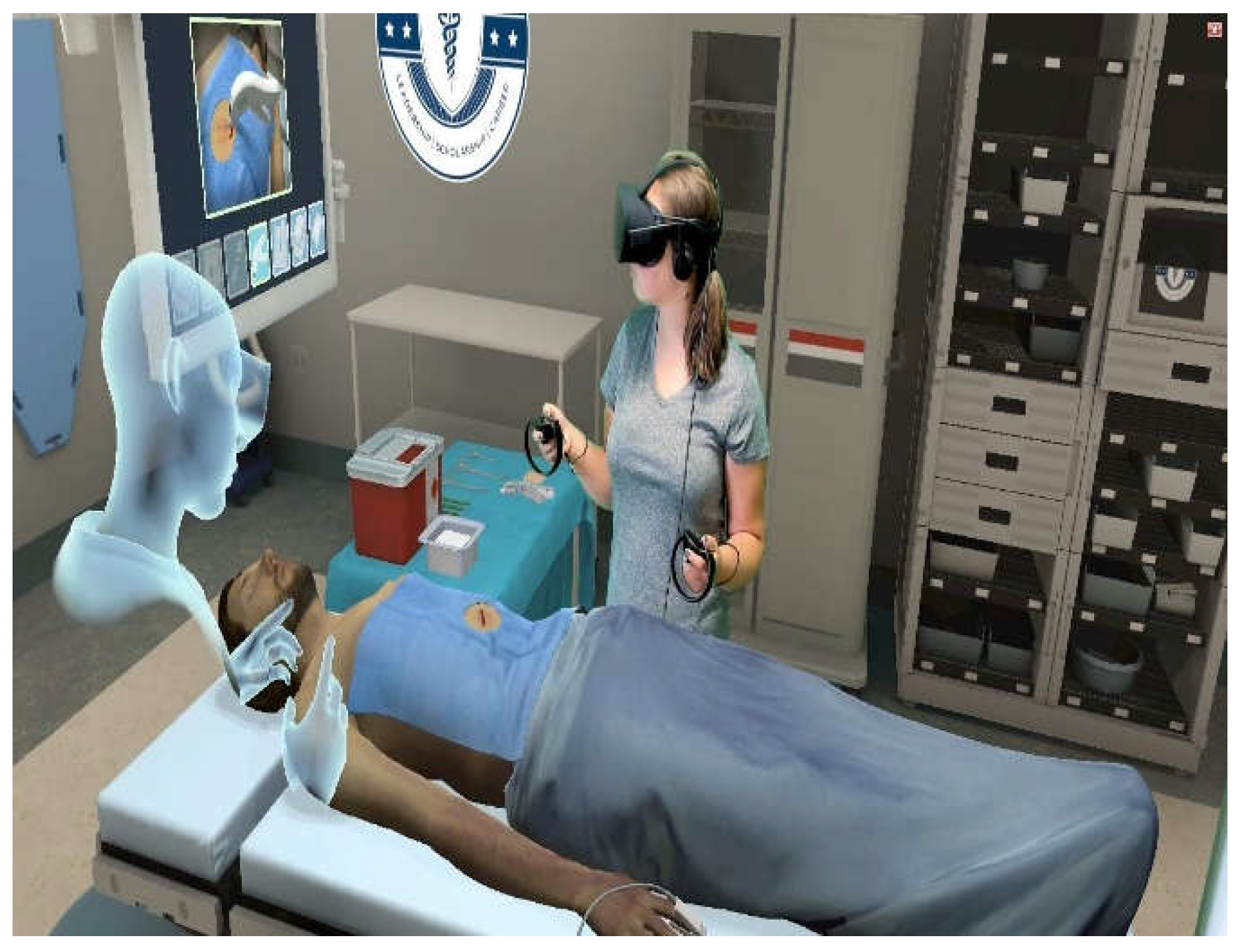
9.2. Virtual Reality Simulation for Real-time Surgical Training and Guidance
Virtual reality (VR) simulation represents a ground-breaking technology for improving surgical teaching and training. VR technology provides trainees with hands-on, realistic experiences that more closely resemble the complexities and problems of pediatric urology treatments. A major benefit of VR simulation is the capacity to give students a secure setting in which to practice surgery methods without endangering patients [
3].
Before entering the operating room, trainees can improve their abilities, perfect methods, and become familiar with difficult surgical procedures using realistic anatomical models and dynamic simulations. VR also facilitates customized learning experiences that cater to the unique requirements and proficiency levels of each student (
Figure 11). Advanced training modules allow trainees to focus on areas that need development and advance at their own speed. These modules can be tailored to address specific parts of pediatric urological surgery, such as robotic-assisted or laparoscopic operations [
4].
VR simulation also makes it easier for multidisciplinary teams to collaborate and train together. Surgeons, nurses, anesthesiologists, and other surgical team members can rehearse and coordinate their duties in a virtual surgical setting. In practical clinical settings, this cooperative method improves communication, cooperation, and situational awareness, which in-turn improves surgical results and patient safety. VR simulation provides a dynamic and adaptable platform for improving training and competency, from fundamental procedural skills to sophisticated surgical methods [
3].
Developments in Image Recognition: Advanced ML techniques allow real-time analysis of medical imaging data to precisely detect lesions, highlight anatomical features, and identify possible surgery site concerns. These AI-driven tools provide surgeons with timely and precise insights to support surgical planning and decision-making by quickly and effectively integrating large volumes of imaging data [
6].
Guidance for Instrument Placement and Surgical Maneuvers: Real-time surgical guidance systems use AI algorithms to provide real-time feedback on the depth, trajectory, and placement of instruments [
7]. These technologies aid in traversing complicated anatomy and producing the best possible surgical results by superimposing virtual representations of anatomical features onto the surgeon's field of view.
Prospective Advantages and Future Courses: AI tools can supplement surgical experience, potentially improving precision, decreasing complications, and expediting operating timeframes. As AI technology develops further and thorough clinical validation studies are conducted, these systems will likely be expanded and refined even further, transforming the field of pediatric urology in the process [
5].
9.3. Integration of Decision Support Mechanisms
Real-time surgical guidance systems provide surgeons with evidence-based suggestions and practical insights by analyzing a wide range of patient-specific data intraoperatively. Image enhancement methods also allow more accurate diagnosis and surgical planning by interpreting medical pictures more precisely. Incorporating these approaches with new imaging techniques will eventually improve the efficacy of decision support systems in pediatric urology [
4].
Pediatric Image Enhancement Techniques: Improving picture quality is crucial for precise diagnosis and efficient surgical planning. To meet this demand, a variety of image enhancement techniques have been created to improve clarity, raise contrast, and reduce noise (
Table 9).
Ground truth building techniques for segmentation: High-quality image segmentation is standard for certifying and training AI systems. Ground truth construction by combining multiple inputs to provide a consensus segmentation is essential to obtain precise and reliable decision support systems. A summary of current segmentation ground truth methods in medical imaging is shown in (
Table 10).
10. Conclusion
10.1. Summary of Key Findings
AI technologies can assist with treatment planning and long-term monitoring of pediatric disorders, thereby reducing the need for invasive procedures and reducing associated costs. Predictive modeling can also aid prognosis and risk stratification in patients affected by kidney tumors and stones. Personalized risk mitigation strategies and early interventions are made possible by algorithms that analyze diverse datasets to identify predictive biomarkers and indicators of therapeutic response. The field of pediatric urology is likely to be significantly reshaped by the impact of these AI technologies, leading to better treatment planning, high precision surgery, and a more individualized approach to care [
10,
13]
Image-enhancing tools have produced substantial advances in pediatric urology, specifically in the domains of patient diagnosis, treatment planning, and surgical results. These tools employ sophisticated imaging technology and AI algorithms to improve the visualization of anatomical components and offer clinicians significant insight into different urological diseases:
Improved Diagnostic Accuracy: Image-enhancing techniques such as ceVUS and quantitative analysis software have greatly advanced diagnostic precision in the field of pediatric urology. These techniques offer crisper and more detailed images that enhance doctors' ability to identify tiny abnormalities that may have been overlooked by traditional imaging methods. These advanced technologies enable the early identification and treatment of medical conditions, leading to improved results for patients [
47].
Personalized Treatment Planning: AI-driven preoperative planning technologies analyze imaging data (CT, MRI, ultrasound), to create three-dimensional reconstructions of the urinary system. This allows pediatric urologists to tailor treatment approaches to the distinct anatomy and pathology of individual patients, thereby maximizing results and reducing problems [
44].
Enhanced Surgical Guidance: AI-assisted surgical navigation systems improve accuracy and precision by offering real-time guidance during critical procedures. These devices employ AI algorithms to apply anatomical landmarks and trajectory planning onto the surgical field, enabling accurate dissection, tissue manipulation, and suture placement. These instruments enhance outcomes and patient safety by minimizing surgical errors.
Efficient Productivity: Image-enhancing technologies equipped with automated functions and user-friendly interfaces can optimize productivity. These tools facilitate rapid processing of complex data, enabling doctors to prioritize patient care over manual duties, thereby improving satisfaction through increased efficiency [
90].
Enhanced Education and Training: AI-powered virtual reality simulations provide a secure and regulated learning environment that greatly improves the education and training of pediatric urology trainees. These tools offer interactive environments to engage in practice operations, enhance techniques, and simulate intricate surgical scenarios, leading to increased proficiency and confidence of trainees.
10.2. Ethical and Regulatory Considerations
It will be crucial to address the ethical and legal issues raised by advances in AI and incorporation of these technologies into pediatric urology clinical practice. Patient privacy and data security are key concerns. AI algorithms use large volumes of patient data, including genetic information, imaging results, and medical records, which raises questions about data security, permissions, and possible confidentiality violations. In the age of AI-driven healthcare, finding appropriate balance between respect for patient privacy and use of data to further research and innovation remains challenging [
7].
Use of AI in clinical decision-making also raises concerns about bias, accountability, and transparency. AI systems may unintentionally reinforce pre-existing biases in the provision of healthcare, resulting in divergent treatment results and access to care. To reduce the possibility of bias and advance equitable healthcare delivery, strict validation, monitoring, and auditing procedures must be used to guarantee fairness, accountability, and transparency [
6].
AI is being developed much faster than the necessary regulatory frameworks that will control applications in healthcare. Regulatory bodies must modify current laws to account for the special features of AI technologies, such as their dynamic nature, opaque algorithms, and ever-evolving capabilities. It is imperative to establish clear rules, standards, and oversight mechanisms to guarantee patient safety, quality of treatment, and ethical practice [
7]. The ethical implications of AI extend beyond the domain of the clinical treatment to include wider social issues including algorithmic responsibility, economic impact, and labor displacement. For pediatric urologists to responsibly traverse the complicated ethical environment of AI, interdisciplinary partnerships, professional guidelines, and ethical frameworks will be essential to promote responsible innovation that puts patients and societal welfare first [
4].
10.3. Recommendations for Future Research and Clinical Practice
Further research on incorporating AI into multimodal treatment approaches for pediatric urological disorders is necessary. In particular, researchers will need to explore how AI-driven decision support systems can improve treatment planning by evaluating patient-specific variables to identify the best therapeutic options [
100]. Enhancing the interpretability and transparency of AI algorithms in pediatric urology should also be a priority, thereby building confidence and promoting cooperation between AI developers and healthcare practitioners [
101].
Big data analytics and ML techniques also offer the possibility to identify latent patterns, biomarkers, and predictive variables that lead to new understanding of pediatric urological disorders [
102]. Standardization and validation will be essential to guarantee robustness, dependability, and generalizability of AI models across populations and healthcare settings. Application of AI tools in pediatric urology has social, legal, and ethical ramifications that need to be considered in parallel. These include concerns about patient permissions, data privacy, and fair access to AI-driven medical technology [
100], which will undoubtedly lead to major improvements in clinical decision-making, patient care, and innovation in pediatric urology (
Table 11).
Author Contributions
Conceptualization, MEHC and TOA.; methodology, ATC and AS; validation, MEHC.; formal analysis, ATC and AS; investigation, ATC, AS, MN and DA; resources, TOA; data curation, ATC and MN; writing—original draft preparation, ATC, AS and DA; writing—review and editing, ATC, MN, LE and MEHC; visualization, LE; supervision, MEHC and TOA.; project administration, MEHC and TOA. (Adiba Tabassum Chowdhury ATC, Abdus Salam AS, Mansura Naznine MN, Da’ad Abdalla DA, Lauren Erdman LE, Muhammad E.H. Chowdhury MEHC, Tariq O Abbas TOA)
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article
Acknowledgments
The open access publication of this article is supported by Qatar National Library.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Tsai, M.-C., Lu, H. H.-S., Chang, Y.-C., Huang, Y.-C., & Fu, L.-S. (2022). Automatic Screening of Pediatric Renal Ultrasound Abnormalities: Deep Learning and Transfer Learning Approach. JMIR Medical Informatics, 10(11), e40878. [CrossRef]
- Bar-Sever, Z., Shammas, A., Gheisari, F., & Vali, R. (2022). Pediatric Nephro-Urology: Overview and Updates in Diuretic Renal Scans and Renal Cortical Scintigraphy. Seminars in Nuclear Medicine, 52(4), 419–431. [CrossRef]
- Checcucci, E., De Cillis, S., Granato, S., Chang, P., Afyouni, A. S., Okhunov, Z., & Uro-technology and SoMe Working Group of the Young Academic Urologists Working Party of the European Association of Urology. (2020). Applications of neural networks in urology: A systematic review. Current Opinion in Urology, 30(6), 788–807. [CrossRef]
- Khondker, A., Kwong, J., Malik, S., Erdman, L., Keefe, D., Fernandez, N., Tasian, G., Wang, H.-H., Estrada, C., Nelson, C., Lorenzo, A., & Rickard, M. (2022). The state of artificial intelligence in pediatric urology. Frontiers in Urology, 2. [CrossRef]
- Hameed, B. M. Z., S Dhavileswarapu, A. V. L., Raza, S. Z., Karimi, H., Khanuja, H. S., Shetty, D. K., Ibrahim, S., Shah, M. J., Naik, N., Paul, R., Rai, B. P., & Somani, B. K. (2021). Artificial Intelligence and Its Impact on Urological Diseases and Management: A Comprehensive Review of the Literature. Journal of Clinical Medicine, 10(9), 1864. [CrossRef]
- Matsushita, F. Y., Krebs, V. L. J., & de Carvalho, W. B. (2022). Artificial intelligence and ML in pediatrics and neonatology healthcare. Revista Da Associação Médica Brasileira, 68(6), 745–750. [CrossRef]
- Wang, H.-H. S., Vasdev, R., & Nelson, C. P. (2024). Artificial Intelligence in Pediatric Urology. The Urologic Clinics of North America, 51(1), 91–103. [CrossRef]
- Matta, R., & Schaeffer, A. (2021). The top 100 cited articles in pediatric urology: A bibliometric analysis. Journal of Pediatric Urology, 17(5), 709.e1-709.e12. [CrossRef]
- Maini, Dr. A. (2020). Hydronephrosis. SAI Nephrology. https://sainephrology.com/hydronephrosis/.
- Cerrolaza, J. J., Peters, C. A., Martin, A. D., Myers, E., Safdar, N., & Linguraru, M. G. (2016). Quantitative Ultrasound for Measuring Obstructive Severity in Children with Hydronephrosis. The Journal of Urology, 195(4 Pt 1), 1093–1099. [CrossRef]
- Cho, H. Y., Jung, I., Kim, Y. H., & Kwon, J.-Y. (2019). Reliability of society of fetal urology and Onen grading system in fetal hydronephrosis. Obstetrics & Gynecology Science, 62(2), 87–92. [CrossRef]
- Hwang, J., Kim, P. H., Yoon, H. M., Song, S. H., Jung, A. Y., Lee, J. S., & Cho, Y. A. (2023). Application of the postnatal urinary tract dilation classification system to predict the need for surgical intervention among neonates and young infants. Ultrasonography, 42(1), 136–146. [CrossRef]
- Erdman, L., Skreta, M., Rickard, M., McLean, C., Mezlini, A., Keefe, D. T., Blais, A.-S., Brudno, M., Lorenzo, A., & Goldenberg, A. (2020). Predicting Obstructive Hydronephrosis Based on Ultrasound Alone. In A. L. Martel, P. Abolmaesumi, D. Stoyanov, D. Mateus, M. A. Zuluaga, S. K. Zhou, D. Racoceanu, & L. Joskowicz (Eds.), Medical Image Computing and Computer Assisted Intervention – MICCAI 2020 (Vol. 12263, pp. 493–503). Springer International Publishing. [CrossRef]
- Alshoabi, S. A., Alhamodi, D. S., Alhammadi, M. A., & Alshamrani, A. F. (2021). Etiology of Hydronephrosis in adults and children: Ultrasonographic Assessment in 233 patients. Pakistan Journal of Medical Sciences, 37(5). [CrossRef]
- Lien, W.-C., Chang, Y.-C., Chou, H.-H., Lin, L.-C., Liu, Y.-P., Liu, L., Chan, Y.-T., & Kuan, F.-S. (2023). Detecting Hydronephrosis Through Ultrasound Images Using State-of-the-Art Deep Learning Models. Ultrasound in Medicine & Biology, 49(3), 723–733. [CrossRef]
- Sloan, M., Li, H., Lescay, H. A., Judge, C., Lan, L., Hajiyev, P., Giger, M. L., & Gundeti, M. S. (2023). Pilot study of ML in the task of distinguishing high and low-grade pediatric hydronephrosis on ultrasound. Investigative and Clinical Urology, 64(6), 588. [CrossRef]
- Kazlauskas, V., Bilius, V., Jakutis, V., Komiagiene, R., Burnyte, B., & Verkauskas, G. (2022). Urine Biomarkers Combined With Ultrasound for the Diagnosis of Obstruction in Pediatric Hydronephrosis. Frontiers in Pediatrics, 9, 762417. [CrossRef]
- Song, S. H., Han, J. H., Kim, K. S., Cho, Y. A., Youn, H. J., Kim, Y. I., & Kweon, J. (2022). Deep-learning segmentation of ultrasound images for automated calculation of the hydronephrosis area to renal parenchyma ratio. Investigative and Clinical Urology, 63(4), 455. [CrossRef]
- Roshanitabrizi, P., Zember, J., Sprague, B. M., Hoefer, S., Sanchez-Jacob, R., Jago, J., Bulas, D., Pohl, H. G., & Linguraru, M. G. (2021). Standardized Analysis of Kidney Ultrasound Images for the Prediction of Pediatric Hydronephrosis Severity. Machine Learning in Medical Imaging: 12th International Workshop, MLMI 2021, Held in Conjunction with MICCAI 2021, Strasbourg, France, September 27, 2021, Proceedings, 366–375. [CrossRef]
- Smail, L. C., Dhindsa, K., Braga, L. H., Becker, S., & Sonnadara, R. R. (2020). Using Deep Learning Algorithms to Grade Hydronephrosis Severity: Toward a Clinical Adjunct. Frontiers in Pediatrics, 8, 1. [CrossRef]
- Onen, A. (2020). Grading of Hydronephrosis: An Ongoing Challenge. Frontiers in Pediatrics, 8, 458. [CrossRef]
- Ostrowski, D. A., Logan, J. R., Antony, M., Broms, R., Weiss, D. A., Batavia, J. V., Long, C. J., Smith, A. L., Zderic, S. A., Edwins, R. C., Pominville, R. J., Hannick, J. H., Woo, L. L., Fan, Y., Tasian, G. E., & Weaver, J. K. (2023). Automated Society of Fetal Urology (SFU) grading of hydronephrosis on ultrasound imaging using a convolutional neural network. Journal of Pediatric Urology, 19(5), 566.e1-566.e8. [CrossRef]
- Kelley, J. C., White, J. T., Goetz, J. T., Romero, E., Leslie, J. A., & Prieto, J. C. (2016). Sonographic Renal Parenchymal Measurements for the Evaluation and Management of Ureteropelvic Junction Obstruction in Children. Frontiers in Pediatrics, 4. [CrossRef]
- Ariyanagam, Dr. M. (2024, March 8). A Guide To Laparoscopic Pyeloplasty And How It Works. Urology Specialist. https://urologyspecialist.com.au/enhancing-kidney-health-comprehensive-guide-laparoscopic-pyeloplasty-robotic-surgery/.
- Vauth, F., Zöhrer, P., Girtner, F., Rösch, W. H., & Hofmann, A. (2023). Open Pyeloplasty in Infants under 1 Year—Proven or Meaningless? Children, 10(2), 257. [CrossRef]
- Abbas, T., Elifranji, M., Al-Salihi, M., Ahmad, J., Vallasciani, S., Elkadhi, A., Özcan, C., Burgu, B., Akinci, A., Alnaimi, A., & Salle, J. L. P. (2022). Functional recoverability post-pyeloplasty in children with ureteropelvic junction obstruction and poorly functioning kidneys: Systematic review. Journal of Pediatric Urology, 18(5), 616–628. [CrossRef]
- Abbas, T. O., Ali, M., & Moog, R. (2020). “Double-Lumen Valve-Controlled Intra-Operative Pyeloplasty Stent (VIPs)”: A New Technology for Post-Pyeloplasty Stenting—Proof of Concept Study in a Preclinical Large Animal Model. Research and Reports in Urology, 12, 61–74. [CrossRef]
- Helmy, T., Harraz, A., Sharaf, D., Demerdash, Y., Hafez, A., Gad, H., & Dawaba, M. (2014). Can Renal Ultrasonography Predict Early Success after Pyeloplasty in Children? A Prospective Study. Urologia Internationalis, 93. [CrossRef]
- Esposito, C., Cerulo, M., Lepore, B., Coppola, V., D’Auria, D., Esposito, G., Carulli, R., Conte, F. D., & Escolino, M. (2023). Robotic-assisted pyeloplasty in children: A systematic review of the literature. Journal of Robotic Surgery, 17(4), 1239. [CrossRef]
- Masieri, L., Sforza, S., Grosso, A. A., Valastro, F., Tellini, R., Cini, C., Landi, L., Taverna, M., Elia, A., Mantovani, A., Minervini, A., & Carini, M. (2020). Robot-assisted laparoscopic pyeloplasty in children: A systematic review. Minerva Urologica E Nefrologica = The Italian Journal of Urology and Nephrology, 72(6), 673–690. [CrossRef]
- Cheung, C. L., Looi, T., Lendvay, T. S., Drake, J. M., & Farhat, W. A. (2014). Use of 3-dimensional printing technology and silicone modeling in surgical simulation: Development and face validation in pediatric laparoscopic pyeloplasty. Journal of Surgical Education, 71(5), 762–767. [CrossRef]
- Muralidharan, V., Burgart, A., Daneshjou, R., & Rose, S. (2023). Recommendations for the use of pediatric data in artificial intelligence and ML ACCEPT-AI. Npj Digital Medicine, 6(1), 1–6. [CrossRef]
- Avery, D. I., Herbst, K. W., Lendvay, T. S., Noh, P. H., Dangle, P., Gundeti, M. S., Steele, M. C., Corbett, S. T., Peters, C. A., & Kim, C. (2015). Robot-assisted laparoscopic pyeloplasty: Multi-institutional experience in infants. Journal of Pediatric Urology, 11(3), 139.e1-5. [CrossRef]
- Ahmed, F., Abbas, S., Athar, A., Shahzad, T., Khan, W. A., Alharbi, M., Khan, M. A., & Ahmed, A. (2024). Identification of kidney stones in KUB X-ray images using VGG16 empowered with explainable artificial intelligence. Scientific Reports, 14(1), 6173. [CrossRef]
- Babajide, R., Lembrikova, K., Ziemba, J., Ding, J., Li, Y., Fermin, A. S., Fan, Y., & Tasian, G. E. (2022). Automated Machine Learning Segmentation and Measurement of Urinary Stones on CT Scan. Urology, 169, 41–46. [CrossRef]
- Islam, M. N., Hasan, M., Hossain, M. K., Alam, M. G. R., Uddin, M. Z., & Soylu, A. (2022). Vision transformer and explainable transfer learning models for auto detection of kidney cyst, stone and tumor from CT-radiography. Scientific Reports, 12(1), 11440. [CrossRef]
- Li, D., Xiao, C., Liu, Y., Chen, Z., Hassan, H., Su, L., Liu, J., Li, H., Xie, W., Zhong, W., & Huang, B. (2022). Deep Segmentation Networks for Segmenting Kidneys and Detecting Kidney Stones in Unenhanced Abdominal CT Images. Diagnostics, 12(8), Article 8. [CrossRef]
- Lee, H., Nguyen, H., Hwang, S., Lee, H. J., Hong, S. K., & Byun, S.-S. (2018). Personalized 3D kidney model produced by rapid prototyping method and its usefulness in clinical applications. International Braz j Urol : Official Journal of the Brazilian Society of Urology, 44. [CrossRef]
- Raina, R., Nada, A., Shah, R., Aly, H., Kadatane, S., Abitbol, C., Aggarwal, M., Koyner, J., Neyra, J., & Sethi, S. K. (2023). Artificial intelligence in early detection and prediction of pediatric/neonatal acute kidney injury: Current status and future directions. Pediatric Nephrology (Berlin, Germany). [CrossRef]
- Filler, G., Gipson, D., Iyamuremye, D., & Ferris, M. (2022). Artificial Intelligence in Pediatric Nephrology—A Call for Action. Advances in Kidney Disease and Health, 30. [CrossRef]
- Santini, PhD, G. S., PhD. (2018). AI in Medical Imaging: The Kidney Tumor Segmentation Challenge. https://blog.keosys.com/ai-in-medical-imaging-the-kidney-tumor-segmentation-challenge.
- Jacobsen, S., Klemetsen, Ø., & Birkelund, Y. (2012). Vesicoureteral reflux in young children: A study of radiometric thermometry as detection modality using an ex vivo porcine model. Physics in Medicine and Biology, 57(17), 5557–5573. [CrossRef]
- Kabir, S., Pippi Salle, J. L., Chowdhury, M. E. H., & Abbas, T. O. (2024). Quantification of vesicoureteral reflux using ML. Journal of Pediatric Urology, 20(2), 257–264. [CrossRef]
- Khondker, A., Kwong, J. C. C., Rickard, M., Skreta, M., Keefe, D. T., Lorenzo, A. J., & Erdman, L. (2022). A ML-based approach for quantitative grading of vesicoureteral reflux from voiding cystourethrograms: Methods and proof of concept. Journal of Pediatric Urology, 18(1), 78.e1-78.e7. [CrossRef]
- Vesicoureteral Reflux (VUR). (2014, April 29). The Children’s Hospital of Philadelphia. https://www.chop.edu/conditions-diseases/vesicoureteral-reflux-vur.
- Koçyiğit, A., Yüksel, S., Bayram, R., Yılmaz, İ., & Karabulut, N. (2014). Efficacy of magnetic resonance urography in detecting renal scars in children with vesicoureteral reflux. Pediatric Nephrology, 29(7), 1215–1220. [CrossRef]
- Kuzmanovska, D., Risteski, A., Kambovska, M., Trpcevski, T., Sahpazova, E., & Petrovski, M. (2017). Voiding Urosonography with Second-Generation Ultrasound Contrast Agent for Diagnosis of Vesicoureteric Reflux: First Local Pilot Study. Open Access Macedonian Journal of Medical Sciences, 5(2), 215–221. [CrossRef]
- Yousefifard, M., Toloui, A., Rafiei Alavi, S. N., Madani Neishaboori, A., Ahmadzadeh, K., Ghelichkhani, P., Safari, S., Abbasi, A., Ataei, N., & Hosseini, M. (2022). Contrast-enhanced voiding urosonography, a possible candidate for the diagnosis of vesicoureteral reflux in children and adolescents; a systematic review and meta-analysis. Journal of Pediatric Urology, 18(1), 61–74. [CrossRef]
- Kirsch, A. J., Arlen, A. M., Leong, T., Merriman, L. S., Herrel, L. A., Scherz, H. C., Smith, E. A., & Srinivasan, A. K. (2014). Vesicoureteral reflux index (VURx): A novel tool to predict primary reflux improvement and resolution in children less than 2 years of age. Journal of Pediatric Urology, 10(6), 1249–1254. [CrossRef]
- Hobbs, K. T., Choe, N., Aksenov, L. I., Reyes, L., Aquino, W., Routh, J. C., & Hokanson, J. A. (2022). Machine Learning for Urodynamic Detection of Detrusor Overactivity. Urology, 159, 247–254. [CrossRef]
- Wang, J., Ren, L., Liu, X., Liu, J., & Ling, Q. (2023). Underactive Bladder and Detrusor Underactivity: New Advances and Prospectives. International Journal of Molecular Sciences, 24(21), 15517. [CrossRef]
- Zhou, Q., Chen, Z., Wu, B., Lin, D., Hu, Y., Zhang, X., & Liu, J. (2023). A Pilot Study: Detrusor Overactivity Diagnosis Method Based on Deep Learning. Urology, 179, 188–195. [CrossRef]
- Wang, H. S., Cahill, D., Panagides, J., Nelson, C. P., Wu, H., & Estrada, C. (2021). Pattern recognition algorithm to identify detrusor overactivity on urodynamics. Neurourology and Urodynamics, 40(1), 428–434. [CrossRef]
- Niederhauser, T., Gafner, E. S., Cantieni, T., Grämiger, M., Haeberlin, A., Obrist, D., Burkhard, F., & Clavica, F. (2018). Detection and quantification of overactive bladder activity in patients: Can we make it better and automatic? Neurourology and Urodynamics, 37(2), 823–831. [CrossRef]
- Abbaraju, V., Lewis, K., & Majerus, S. J. A. (2022). Machine Learning for Automated Bladder Event Classification from Single-Channel Vesical Pressure Recordings. 2022 IEEE Signal Processing in Medicine and Biology Symposium (SPMB), 1–6. [CrossRef]
- Roic, A. C., Milošević, D., Turudić, D., & Roic, G. (2022). An innovative diagnostic procedure in children: Videourodynamics with contrast-enhanced voiding urosonography. Journal of Ultrasound, 26(2), 583–587. [CrossRef]
- Osman, N. I., Esperto, F., & Chapple, C. R. (2018). Detrusor Underactivity and the Underactive Bladder: A Systematic Review of Preclinical and Clinical Studies. European Urology, 74(5), 633–643. [CrossRef]
- Fishberg, S. E., Landau, E. H., Duvdevani, M., Gofrit, O. N., Friedman, S. E., & Hidas, G. (2018). Posterior Urethral Valves: Prenatal, Neonatal, and Long-Term Management. NeoReviews, 19(12), e753–e761. [CrossRef]
- Kwong, J. Cc., Khondker, A., Kim, J. K., Chua, M., Keefe, D. T., Dos Santos, J., Skreta, M., Erdman, L., D’Souza, N., Selman, A. F., Weaver, J., Weiss, D. A., Long, C., Tasian, G., Teoh, C. W., Rickard, M., & Lorenzo, A. J. (2022). Posterior Urethral Valves Outcomes Prediction (PUVOP): A ML tool to predict clinically relevant outcomes in boys with posterior urethral valves. Pediatric Nephrology, 37(5), 1067–1074. [CrossRef]
- Deshpande, A. V. (2018). Current strategies to predict and manage sequelae of posterior urethral valves in children. Pediatric Nephrology, 33(10), 1651–1661. [CrossRef]
- Taskinen, S., Heikkilä, J., & Rintala, R. (2012). Effects of posterior urethral valves on long-term bladder and sexual function. Nature Reviews Urology, 9(12), 699–706. [CrossRef]
- Weaver, J. K., Milford, K., Rickard, M., Logan, J., Erdman, L., Viteri, B., D’Souza, N., Cucchiara, A., Skreta, M., Keefe, D., Shah, S., Selman, A., Fischer, K., Weiss, D. A., Long, C. J., Lorenzo, A., Fan, Y., & Tasian, G. E. (2023). DL imaging features derived from kidney ultrasounds predict chronic kidney disease progression in children with posterior urethral valves. Pediatric Nephrology, 38(3), 839–846. [CrossRef]
- Cohen, H. L., Zinn, H. L., Patel, A., Zinn, D. L., & Haller, J. O. (1998). Prenatal sonographic diagnosis of posterior urethral valves: Identification of valves and thickening of the posterior urethral wall. Journal of Clinical Ultrasound, 26(7), 366–370. [CrossRef]
- Buffin-Meyer, B., Klein, J., Van Der Zanden, L. F. M., Levtchenko, E., Moulos, P., Lounis, N., Conte-Auriol, F., Hindryckx, A., Wühl, E., Persico, N., Oepkes, D., Schreuder, M. F., Tkaczyk, M., Ariceta, G., Fossum, M., Parvex, P., Feitz, W., Olsen, H., Montini, G., … Chehade, H. (2020). The ANTENATAL multicentre study to predict postnatal renal outcome in fetuses with posterior urethral valves: Objectives and design. Clinical Kidney Journal, 13(3), 371–379. [CrossRef]
- Krzemień, G., Szmigielska, A., Wawer, Z., & Roszkowska-Blaim, M. (2013). [Significance of early diagnosis of posterior urethral valves in fetus for further development—Own experience]. Medycyna Wieku Rozwojowego, 17(4), 301–305.
- Ikeda, A., & Nosato, H. (2024). Overview of current applications and trends in artificial intelligence for cystoscopy and transurethral resection of bladder tumours. Current Opinion in Urology, 34(1), 27–31. [CrossRef]
- Pellegrino, C., Capitanucci, M. L., Forlini, V., Zaccara, A., Lena, F., Sollini, M. L., Castelli, E., & Mosiello, G. (2023). Posterior urethral valves: Role of prenatal diagnosis and long-term management of bladder function; a single center point of view and review of literature. Frontiers in Pediatrics, 10, 1057092. [CrossRef]
- Abdelghany, M., Elkousy, M. M., Emran, A., Zamel, S., & Nabil, A. E. W. (2022). The importance of second look cystoscopy in children with PUV and its impact on future bladder, kidney functions and fertility. International Journal of Health Sciences, 3189–3195. [CrossRef]
- Hakan, N., Karakus, S. C., Aydin, M., Suzen, A., & Cengiz, N. (2019). An Anterior Urethral Valve without Urethral Dilatation Diagnosed by Cystoscopy in a Neonate. Pediatric Oncall, 16(4). [CrossRef]
- Topuz, B., Ebiloğlu, T., Kaya, E., Çoğuplugil, A. E., Gürdal, M., Bedir, S., & Yalçın, S. (2019). Comparison of Ultrasonography and Cystoscopy in the Evaluation of Hematuria. Journal of Urological Surgery, 6(1), 27–31. [CrossRef]
- Abbas, T. O., AbdelMoniem, M., Khalil, I. A., Abrar Hossain, M. S., & Chowdhury, M. E. H. (2023). DL based automated quantification of urethral plate characteristics using the plate objective scoring tool (POST). Journal of Pediatric Urology, 19(4), 373.e1-373.e9. [CrossRef]
- Abbas, T. O., Sennert, M., Tiryaki, S., Fernandez, N., Fawzy, M., & Hadidi, A. (2024). Hypospadias-associated penile curvature assessment and management: A global survey of current practice. Journal of Pediatric Urology, S1477-5131(24)00086-X. [CrossRef]
- Fernandez, N., Lorenzo, A. J., Rickard, M., Chua, M., Pippi-Salle, J. L., Perez, J., Braga, L. H., & Matava, C. (2021). Digital Pattern Recognition for the Identification and Classification of Hypospadias Using Artificial Intelligence vs Experienced Pediatric Urologist. Urology, 147, 264–269. [CrossRef]
- Fruntelata, R., & Stoica, G. A. (2021). Retrospective Study Over the Hypospadias Surgery in a Single Tertiary Center. Current Health Sciences Journal, 2, 177–183. [CrossRef]
- Wahyudi, I., Utomo, C. P., Djauzi, S., Fathurahman, M., Situmorang, G. R., Rodjani, A., Yonathan, K., & Santoso, B. (2022). Digital Pattern Recognition for the Identification of Various Hypospadias Parameters via an Artificial Neural Network: Protocol for the Development and Validation of a System and Mobile App. JMIR Research Protocols, 11(11), e42853. [CrossRef]
- He Z, Yang B, Tang Y, Wang X. Development and verification of machine learning model based on anogenital distance, penoscrotal distance, and 2D:4D finger ratio before puberty to predict hypospadias classification. Front Pediatr. 2024 Apr 30;12:1297642. doi: 10.3389/fped.2024.1297642. PMID: 38745832; PMCID: PMC11091291.
- Baray, S. B., Abdelmoniem, M., Mahmud, S., Kabir, S., Faisal, Md. A. A., Chowdhury, M. E. H., & Abbas, T. O. (2023). Automated measurement of penile curvature using DL-based novel quantification method. Frontiers in Pediatrics, 11, 1149318. [CrossRef]
- Diamond, D. A., Chan, I. H. Y., Holland, A. J. A., Kurtz, M. P., Nelson, C., Estrada, C. R., Bauer, S., & Tam, P. K. H. (2017). Advances in paediatric urology. The Lancet, 390(10099), 1061–1071. [CrossRef]
- Matta, R., & Schaeffer, A. (2021). The top 100 cited articles in pediatric urology: A bibliometric analysis. Journal of Pediatric Urology, 17(5), 709.e1-709.e12. [CrossRef]
- Envision a Surgery. (2020). Virtually with Immersive Healthcare Simulation | Arch Virtual VR Training and Simulation for Education and Enterprise. https://archvirtual.com/project/envision-a-surgery-virtually-with-immersive-healthcare-simulation/.
- Bhan, B., & Patel, S. (2017). Efficient Medical Image Enhancement using CLAHE Enhancement and Wavelet Fusion. International Journal of Computer Applications, 167, 1–5. [CrossRef]
- Lidong, H., Wei, Z., Jun, W., & Zebin, S. (2015). Combination of contrast limited adaptive histogram equalisation and discrete wavelet transform for image enhancement. IET Image Processing, 9(10), 908–915. [CrossRef]
- Song, Y., Li, C., Xiao, S., Xiao, H., & Guo, B. (2022). Unsharp masking image enhancement the parallel algorithm based on cross-platform. Scientific Reports, 12(1), 20175. [CrossRef]
- Ali, H. M. (2018). MRI Medical Image Denoising by Fundamental Filters. In High-Resolution Neuroimaging—Basic Physical Principles and Clinical Applications. IntechOpen. [CrossRef]
- Kachouie, N. (2010). Anisotropic Diffusion for Medical Image Enhancement. Kachouie International Journal Of Image Processing, 4.
- Radhika, R., & Mahajan, R. (2022). Medical Image Enhancement: A Review. In M. Saraswat, S. Roy, C. Chowdhury, & A. H. Gandomi (Eds.), Proceedings of International Conference on Data Science and Applications (pp. 105–118). Springer. [CrossRef]
- Dabass, Jyoti & Vig, Rekha. (2018). Biomedical Image Enhancement Using Different Techniques - A Comparative Study. 10.1007/978-981-10-8527-7_22.
- Delisle, P.-L., Anctil-Robitaille, B., Desrosiers, C., & Lombaert, H. (2021). Realistic image normalization for multi-Domain segmentation. Medical Image Analysis, 74, 102191. [CrossRef]
- Guo, M., Li, Y., Su, Y., Lambert, T., Nogare, D. D., Moyle, M. W., Duncan, L. H., Ikegami, R., Santella, A., Rey-Suarez, I., Green, D., Beiriger, A., Chen, J., Vishwasrao, H., Ganesan, S., Prince, V., Waters, J. C., Annunziata, C. M., Hafner, M., … Shroff, H. (2020). Rapid image deconvolution and multiview fusion for optical microscopy. Nature.
- Yamashita, K., & Markov, K. (2020). Medical Image Enhancement Using Super Resolution Methods. Computational Science – ICCS 2020, 12141, 496–508. [CrossRef]
- Nazir, N., Sarwar, A., & Saini, B. S. (2024). Recent developments in denoising medical images using DL: An overview of models, techniques, and challenges. Micron, 180, 103615. [CrossRef]
- Athanasiou, G., Arcos, J. L., & Cerquides, J. (2023). Enhancing Medical Image Segmentation: Ground Truth Optimization through Evaluating Uncertainty in Expert Annotations. Mathematics, 11(17), Article 17. [CrossRef]
- Liu, X., Montillo, A., Tan, E. T., & Schenck, J. F. (2013). iSTAPLE: Improved label fusion for segmentation by combining STAPLE with image intensity (S. Ourselin & D. R. Haynor, Eds.; p. 86692O). [CrossRef]
- Akhondi-Asl, A., & Warfield, S. K. (2013). Simultaneous Truth and Performance Level Estimation Through Fusion of Probabilistic Segmentations. IEEE Transactions on Medical Imaging, 32(10), 1840–1852. [CrossRef]
- Landman, B. A., Bogovic, J. A., & Prince, J. L. (2010). Simultaneous Truth and Performance Level Estimation with Incomplete, Over-complete, and Ancillary Data. Proceedings of SPIE--the International Society for Optical Engineering, 7623, 76231N.
- Deng, Q., Zhang, R., Li, S., Hong, J., Zhang, Y., Chu, W., & Shi, L. (2022). Voting-Based Contour-Aware Framework for Medical Image Segmentation. Applied Sciences, 13, 84. [CrossRef]
- Zhou, T., Ruan, S., & Canu, S. (2020). A review: DL for medical image segmentation using multi-modality fusion.
- Andrews, S., Hamarneh, G., & Saad, A. (2010). Fast Random Walker with Priors Using Precomputation for Interactive Medical Image Segmentation. In Medical image computing and computer-assisted intervention: MICCAI ... International Conference on Medical Image Computing and Computer-Assisted Intervention (Vol. 13, p. 16). [CrossRef]
- Keskinoğlu, Ahmet & Özgür, Su. (2020). The Use of Artificial Neural Networks for Differential Diagnosis between Vesicoureteral Reflux and Urinary Tract Infection in Children. The Journal of Pediatric Research. 7. 10.4274/jpr.galenos.2019.24650.
- Blum, E. S., Porras, A. R., Biggs, E., Tabrizi, P. R., Sussman, R. D., Sprague, B. M., Shalaby-Rana, E., Majd, M., Pohl, H. G., & Linguraru, M. G. (2018). Early Detection of Ureteropelvic Junction Obstruction Using Signal Analysis and Machine Learning: A Dynamic Solution to a Dynamic Problem. The Journal of Urology, 199(3), 847–852. [CrossRef]
- Liang, X., Du, M., & Chen, Z. (2023). Artificial intelligence-aided ultrasound in renal diseases: A systematic review. Quantitative Imaging in Medicine and Surgery, 13(6), 3988–4001. [CrossRef]
- Logvinenko, T., Chow, J. S., & Nelson, C. P. (2015). Predictive value of specific ultrasound findings when used as a screening test for abnormalities on VCUG. Journal of Pediatric Urology, 11(4), 176.e1-176.e7. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).