1. Introduction
Since the late 1930s, perfluoroalkyl substances (PFAS) have been used in a variety of consumer and commercial goods, including textiles, surfactants, and food packaging materials (Sant et al., 2019). These compounds are comprised of short or long carbon chains, where short-chain PFAS are considered less harmful than long-chain PFAS [
1]. According to the Danish Environmental Protection Agency, as long-chain PFAS persist in the environment due to their chemical makeup, their production usage has been gradually phased out since the early 2000s [
2]. Perfluorooctanesulfonamide (PFOSA,
C8H2F17NO2S) is a common precursor of
perfluorooctane sulfonic acid (PFOS), a long-chain PFAS [
3]. Most studies to date focus on the presence and toxicity of other commonly used PFAS, such as PFOS or perfluorooctanoic acid (PFOA) [4-6]. Currently, toxicity data regarding the precursor PFOSA are lacking.
There are a few studies that report on the environmental presence of PFOSA. Konwick et al. (2008) found that PFOSA ranged from 162-283 ng/L in the Conasauga River, Georgia, United States [
7]. Additionally, other studies report that PFOSA can range from 0.09-20,000 µg/kg in surface-soil, 0.07-2500 µg/kg in subsurface-soil, 15 µg/L in surface water, and 12 µg/L in groundwater across various testing sites worldwide [
8,
9]. In regards to PFOSA within fish tissues, Fair et al. (2019) measured different PFAS within edible fish species from South Carolina, United States [
10]. In whole fish, the average relative percent of PFOSA in mullet, spot, croaker, red drum, and seatrout was 2.04, 3.81, 3.64, 3.12, and 4.66%, respectively, whereas, in fillets, the same species had an average relative percent of 1.44, 2.03, 0.70, 2.18, 4.65, and 1.79%, respectively. PFOSA was also found to range between 0.105-16.4 ng/mL in serum across various fish species, including common carp
(Cyprinus carpio), crucian carp
(Carassius auratus), tilapia
(Oreochromis niloticus), and leather catfish
(Clarias lazera) [
11]. However, the mechanism of uptake, metabolism, and toxicity of PFOSA are relatively unknown for aquatic species. One study reports that the half-life of PFOSA in rainbow trout (
Oncorhynchus mykiss) is 6.0 ± 0.4 days following a 30-day dietary exposure to 10 µg/g wet weight PFOSA and a 30-day depurination period [
12]. Thus, PFOSA is measurable in fish tissues and may pose a health risk to wildlife and humans.
According to studies, adverse morphological and physiological effects in aquatic organisms are potential consequences associated with the environmental presence of long-chain PFAS [
13,
14]. For example, studies show that PFOSA exerts cardiotoxicity in zebrafish (
Danio rerio). Exposure to 0.1-100 µg/L PFOSA has been reported to reduce cardiac output, heart rate, stroke volume and reduce cardiac vasoconstriction-related genes[
15]. PFOSA has also been reported to significantly increase sinus venosus and bulbus arteriosus distance at 10 and 100 µg/L [
15,
16]. Other studies report that exposure to PFOSA can induce hepatic and renal toxicity in zebrafish [
17,
18]; however, limited studies investigate the neurotoxic potential of PFOSA in developing fish. Consequently, the objectives of this study were to evaluate the neurotoxicity potential of PFOSA. To do this, we measured developmental endpoints, reactive oxygen species (ROS), locomotor behavior, and genes related to oxidative damage response, apoptosis, and neurotoxicity as indicators of central nervous system damage. We hypothesized that neurotoxicity endpoints would reflect dose response increased in PFOSA exposure, suggesting adverse effects on the nervous system.
2. Materials and Methods
2.1. Chemical Preparation
Perfluorooctanesulfonamide (PFOSA, (1,1,2,2,3,3,4,4,5,5,6,6,7,7,8,8,8-heptadecafluorooctane-1-sulfonamide Perfluorooctane sulfonamide) (CAS Number:
754-91-6, purity > 95%) was purchased from Fisher Scientific (Cat# AC459640010). Stock solutions of PFOSA were prepared in dimethyl sulfoxide (DMSO, Dimethyl Sulfoxide, CAS 67-68-5, Sigma Aldrich) and added to embryo rearing media (ERM) containing the zebrafish embryos. Recipes for ERM can be found in [
19]. Exposure solutions were prepared daily to yield final nominal concentrations of 0.1, 1, 10, and 100 µg/L PFOSA with a final concentration < 0.1% v/v DMSO in experimental treatments.
2.2. Maintenance and Egg Production of Zebrafish
Adult zebrafish (AB x Tübingen,
Danio rerio) were raised in a flow-through Pentair system in the Cancer-Genetics Research Center at the University of Florida as outlined previously [
20,
21]. The University of Florida maintains a breeding colony for research and outbreeds extraneous fish to maintain high genetic diversity. Rearing and staging of zebrafish embryos followed that described by [
22]. Adult fish were fed Zeigler Zebrafish Diet ad libitum. Zebrafish at 6 months of age were selected at random from a breeding stock and placed into a shallow water breeding tank the night before embryo collection (2 males and 2 females). A divider was used to separate the males and females overnight and it was removed at 8:00 am when the facility lights turned on. Typically, three or four breeding tanks are prepared the night before to maximize eggs, and adult fish are bred once every 1 to 2 weeks. Embryos were rinsed with saline phosphate buffer (PBS) and ERM several times once they were collected and they were sorted under a light microscope in the late morning (around 4 hours post-fertilization, hpf). All experiments were conducted at pH = 7.2 ± 0.5, a conductivity value of 600 ± 100 μS/cm, light/dark cycle of 14:10 h, dissolved oxygen concentration ~80% air saturation, and temperature of 27 ± 1 °C. Institutional Animal Care and Use Committee of University of Florida approved all experiments (UFIACUC202300000140).
2.3. PFOSA Exposure Regime
Fertilized and normally developing eggs were selected at ∼6 h post-fertilization (hpf) using a dissecting microscope. Zebrafish eggs were assigned in random fashion into experimental groups [ERM, 0.1% DMSO, or one dose of 0.1, 1, 10, 100 µg/L PFOSA]. Four independent experiments were conducted using embryos that were generated from separate breeders of fish. For each experiment, there were 5 to 6 replicate glass beakers for each experimental group containing 20-30 embryos and 10 mL of embryo rearing media (ERM). Following addition of chemicals to the water, the glass beakers were placed into an incubator that was maintained at 27 ± 1 °C. Each day, data related to mortality, deformities, hatch, as well as images using an EVOS™ FL Auto Imaging System (ThermoFisher Scientific, USA) were collected. Deformity assessments included the presence of spinal lordosis and edema (yolk sack/pericardial) were noted over the duration of the exposure. Exposure solutions were prepared fresh daily from a stock stored at -20°C in glass amber vials and water was renewed every day with a 90% water change.
2.4. Reactive Oxygen Species
Six hpf embryos were obtained immediately after fertilization and treated as per above for 7 days in the ERM with designated concentrations of PFOSA. Embryos were rinsed three times in ERM and assessed under a light microscope. Fertilized embryos were evenly distributed using sterile micropipettes into sterile 25 mL glass beakers containing the assigned concentration of ERM, 0.1% DMSO, 0.1, 1, 10 µg/L PFOSA (n=5 beakers of 10 fish each /treatment) in a 10 mL volume. Embryos were maintained in the controlled environment of an incubator at 27 ± 1.0°C. Media changes were conducted every 24 hours with new sterile ERM or renewed PFOSA in ERM. Following a 7-day exposure, larvae were quickly transferred from beakers into 1.7 mL microcentrifuge tubes, homogenized in 200 µL of ice-cold PBS, and centrifuged at 12,000 g for 20 min at 4° C. Following centrifugation, 20 µL of the supernatant was transferred to a black fluorescence 96-well plate and incubated for 5 minutes at room temperature. After incubation, 8.3 µL of 1 mg/mL 2′,7′-Dichlorofluorescin Diacetate (Calbiochem, Millipore Sigma, CAS 4091–99, or H2-DCFDA) dissolved in DMSO and 200 µL of PBS were added to each well. Then, the plate was incubated in the dark for 30 minutes at 37 ± 1.0 °C. Following this, excitation at 485 nm and emission at 520 nm were recorded using a Synergy™ H4 Hybrid Multi-Mode Microplate Reader. Total protein using a BCA assay (Thermo Scientific) was measured to express ROS as normalized signal intensity/(µg/mL) protein.
2.5. Visual Motor Response Test
Experiments were performed to test the dark photokinesis response in larvae. Fish were exposed continuously for 7 days with 90% daily water changes with PFOSA as described above and assessed for locomotor activity behavior at a temperature of 27 ± 1 °C and photoperiod pf 14:10 h. In each trial, zebrafish embryos at 6 hpf were randomly assigned to an experimental group of either ERM, 0.1% DMSO, or 0.1, 1, 10, or 100 µg/L PFOSA (5 beakers per treatment). Each group contained 20 zebrafish embryos and 10 mL of ERM. In mid-afternoon on the 7th day, 2 normally developed larva were selected from each replicate beaker and placed in a random fashion into a clear 96-well plate (N=10 fish per treatment/per experiment). Each well contained 200 μL of ERM. The 96-well plate was placed into DanioVision™ Observation Chamber (Noldus Information Technology, Leesburg, VA) with an infrared analog camera (25 frames/ second) to track the activities of zebrafish larvae. The assay proceeded as per our previous methods [
23]. Data were analyzed independently for each trial, and total distance moved was used as an indicator of overall locomotor activity. Data were also analyzed by normalizing each of the three independent runs using a relative value = 1 for the solvent control group, and then relative data was combined into a single graph representing 30 larvae per treatment.
2.6. Real-Time PCR
Zebrafish larvae at 6 hpf were exposed to either ERM, 0.1% DMSO, 0.1, 1, or 10 µg/L PFOSA. Each beaker contained 10-15 embryos and exposure conditions were maintained as that above. Following the 7-day exposure period, larvae were pooled within a beaker, subjected to liquid nitrogen, and placed at −80 °C for RNA extraction. Extraction of RNA from larvae pools was performed using 500 mL TRIzol® Reagent (Life Technologies, Carlsbad, CA, USA) as per manufacturer’s protocol. Samples were DNase treated with DNA TURBO (Ambion). DNase treated samples were assessed for quality using the 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). The mean RIN value for RNA was >7 and concentrations ranged approximately 50-200 ng/µl sample. The cDNA synthesis was performed using ~500 ng of column purified RNA using iScript (BioRad) following the manufacturer’s protocol in a final sample volume of 15 µL. Once prepared, samples were placed into a T100™ Thermal Cycler (BioRad, USA). The cDNA was generated using the following steps: 25 °C for 5 min, 42 °C for 30 min, 85 °C for 5 min, and 4 °C for 5 min. Prior to real-time PCR, cDNA stocks were diluted 1:25 in RNAse-DNase free water. The no reverse transcriptase (NRT) controls were prepared in the same way as above without enzyme using 3 randomly selected RNA samples.
Real-time PCR was performed using the CFX Connect™ Real-Time PCR Detection System (BioRad) with SsoFast™ EvaGreen® Supermix (BioRad, Hercules, CA, USA), 200-300 nM of each forward and reverse primer, and 3.33 µL of diluted cDNA. The two-step thermal cycling parameters were as follows: initial 1-cycle Taq polymerase activation at 95 °C for 30 s, followed by 95 °C for 5 s, and 60 °C primer annealing temperature for 5 s. After 40 cycles, a dissociation curve was generated, starting at 65.0 and ending at 95.0°C, with increments of 0.5 °C every 5 s. Primers used in this study were obtained from published literature [24-33](Supplemental
Table S1). Two housekeeping genes (ribosomal subunit 18,
rps18, and beta actin,
b-actin) were used to normalize expression levels of all target genes. Target genes included acetylcholinesterase (
ache), BCL2 Associated X Apoptosis Regulator (
bax), BCL2 Apoptosis Regulator (
bcl2), catalase (
cat), caspase 3 (
caspase3), ELAV like RNA binding protein 3 (
elavl3), growth associated protein 43 (
gap43), glial fibrillary acidic protein (
gfap), heat shock protein 70 (
hsp70), mesencephalic astrocyte derived neurotrophic factor (
manf), myelin basic protein (
mbp), nestin (
nestin), tumor protein 53 (
p53), sonic hedgehog signaling molecule (
shha), superoxide dismutase 1 (
sod1) (Cu/Zn SOD), superoxide dismutase 2 (s
od2) (Mn SOD), synapsin IIa (
syn2a), and
tubulin. Normalized expression was obtained for each target gene using CFX Manager™ software (v3.1) (baseline subtracted) and the Cq method was employed. The qPCR analysis included 3 NRT samples and 1 NTC sample. Negative controls indicated that RNA column purification and DNase treatment sufficiently removed gDNA. Sample sizes ranged from 4 to 6 for gene expression analysis. All primers used in the qPCR analysis amplified one product, indicated by a single melt curve.
2.7. Statistical Analysis
All data were compared to the solvent control (DMSO group). A Log-rank test (Mantel-Cox) was employed to evaluate survival data. Data for hatch time was evaluated using a One-Way ANOVA at each time point. Levels of ROS and relative mRNA levels were first log(10) transformed following a Shapiro-Wilk test for normality. Group mean differences were then tested using a One-Way ANOVA (Dunnett’s multiple comparisons test). A simple linear regression was also conducted on the gene expression data to determine whether expression varied with concentration. Because there was no difference in expression between the ERM and DMSO group, these two experimental groups were combined for the regression as a “control” or baseline group. For the VMR, distance moved for larval fish in each treatment across the three independent experiments were binned into a single graph, but each individual run is shown in Supplemental Figures. The distance moved in the DMSO group was normalized to a value of 1, and all treatments were compared relative to this group. A Kruskal-Wallis test followed by a Dunn’s multiple comparisons test was used to evaluate differences in locomotor activity, which was analyzed as discrete temporal units (light and dark sections to corresponding control group). Data are presented as mean ± S.D. Significance of difference was determined using a threshold of P < 0.05. Statistics, and graphing, was performed using GraphPad V9.3 (La Jolla, CA, USA).
4. Discussion
PFOSA is a synthetic compound used to produce non-stick, waterproof, and stain-repellent coatings [
34]. PFOSA is a precursor to PFOS, a PFAS that is regulated by the United States Environmental Protection Agency due to its known toxicity towards various organisms. It is important to recognize that not all PFOSA breaks down into PFOS. For example, Galatius et al. (2013) studied three top predators in the Danish North Sea and found evidence that the ability to metabolize PFOSA to PFOS was variable [
35]. There remains a lack of focus on PFOSA despite its prevalence alongside PFOS. To address this knowledge gap, we investigated the effects of waterborne PFOSA exposure on developmental and behavior endpoints in zebrafish. Additionally, we measured transcripts associated with oxidative stress and neurotoxicity to account for potential mechanisms underlying locomotor activity alterations.
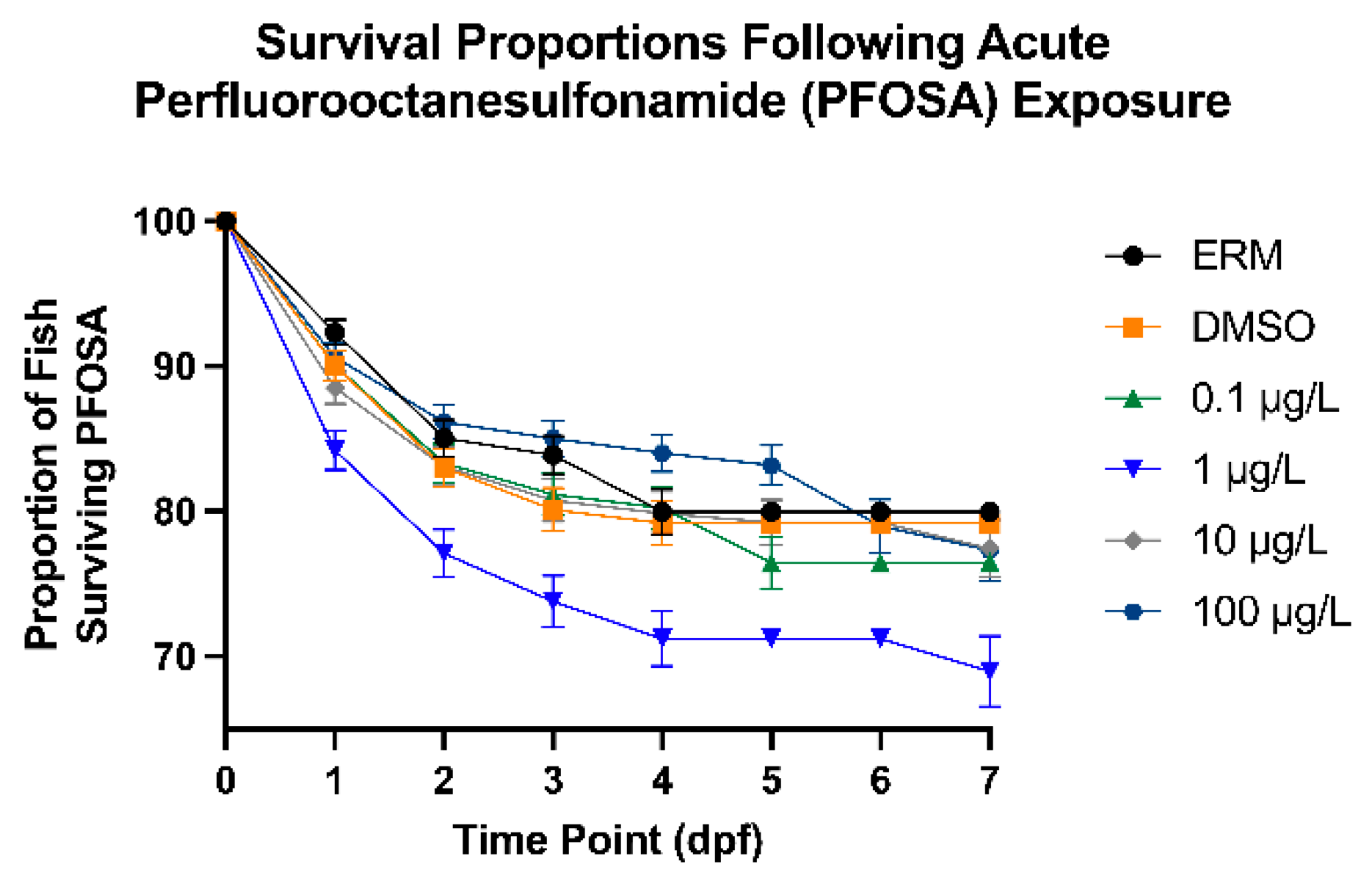
We observed that PFOSA affected the survival of fish treated with 1 µg/L (10% decline at this concentration relative to the DMSO control). Dasgupta et al. (2020) exposed zebrafish embryos to 0.78-50 µM (389-24,900 µg/L) PFOSA for up to 72 hpf and 100% mortality was observed in all treated embryos by 72 hpf [
17]. Regarding abnormalities, we observed only a few deformities (less than 2-3 percent) across all treatment groups; several studies examining toxicity effects of PFOSA report significant deformities, which are likely due to much higher treatment concentrations. It was also reported that all treated embryos exhibiting concentration- and duration-dependent abnormalities, as well as developmental delays, at 24 hpf. For instance, embryos exposed to PFOSA starting at 0.75 hpf exhibited stronger concentration-dependent delays in epiboly compared to embryos exposed at 4 or 5 hpf. Truong et al. (2022) exposed dechorionated zebrafish embryos to 0.015-100 µM (7.4-49,900 µg/L) of various PFAS, including PFOSA from 6 to 120 hpf [
36]. PFAS were ranked on potency based on morphological effects (i.e., pericardial and yolk sac edema, brain and eye malformation) in which PFOSA was ranked second highest. Various studies also report cardiac abnormalities, including heart elongation and reduced cardiac output, heart rate, and stroke volume, in zebrafish exposed from 0.01-100 µg/L [
15,
16]. In our case with survival, 1 µg/L may have been too low a concentration to sufficiently activate defense mechanisms to PFOSA exposure, leading to lower overall survival in developing fish while higher concentrations of PFOSA may elicit a stronger defense response to mitigate toxicity, leading to higher survival. Such dose-dependent responses have been observed for other chemical exposures in zebrafish [
37,
38]. This hypothesis is supported by the increase in anti-oxidant defense enzymes with higher concentrations of PFOSA. Nevertheless, survival remained relatively high for fish up to the 100 µg/L exposure, suggesting that PFOSA, spanning environmental concentrations, is not overtly toxic up to 100 µg/L.
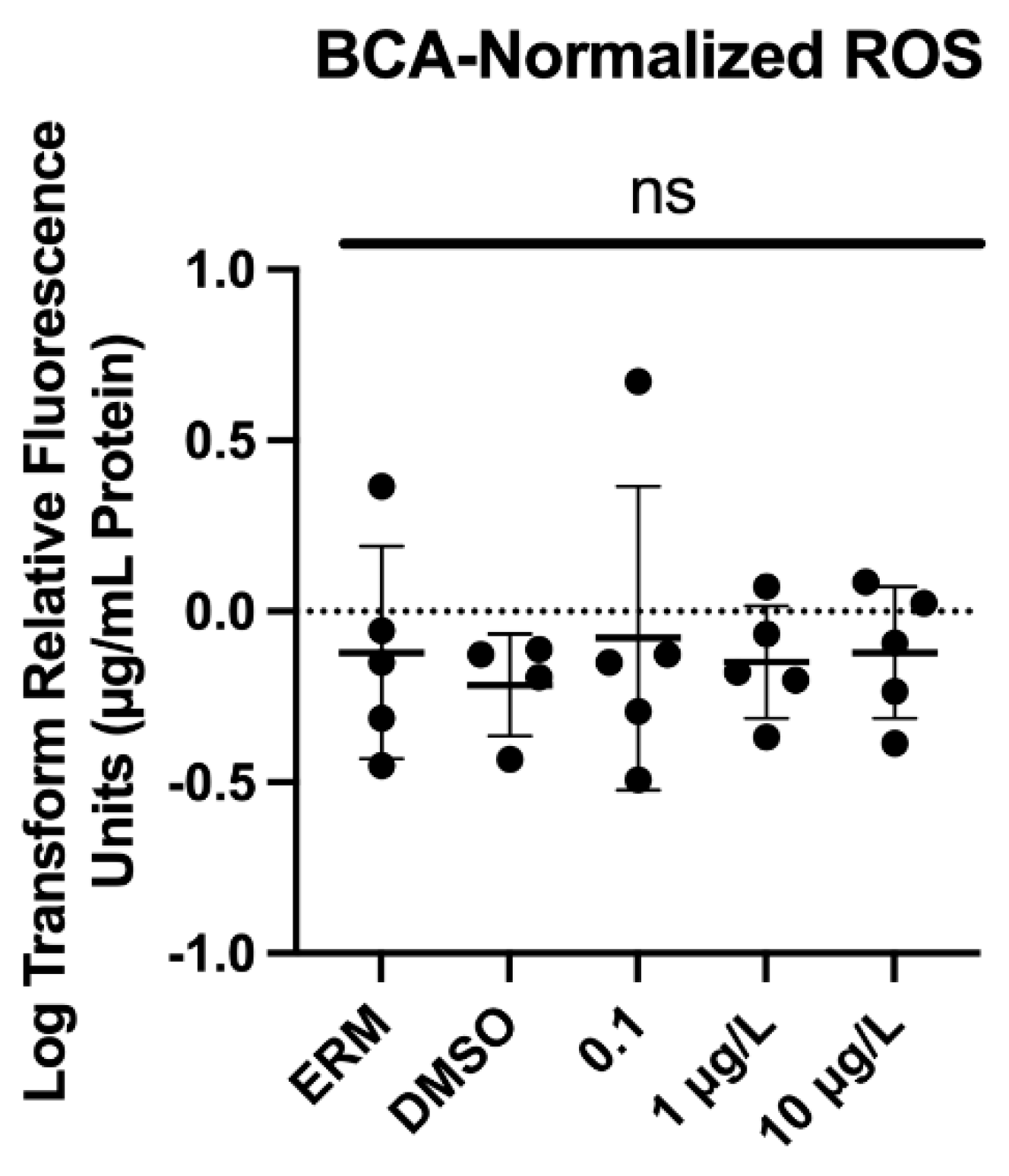
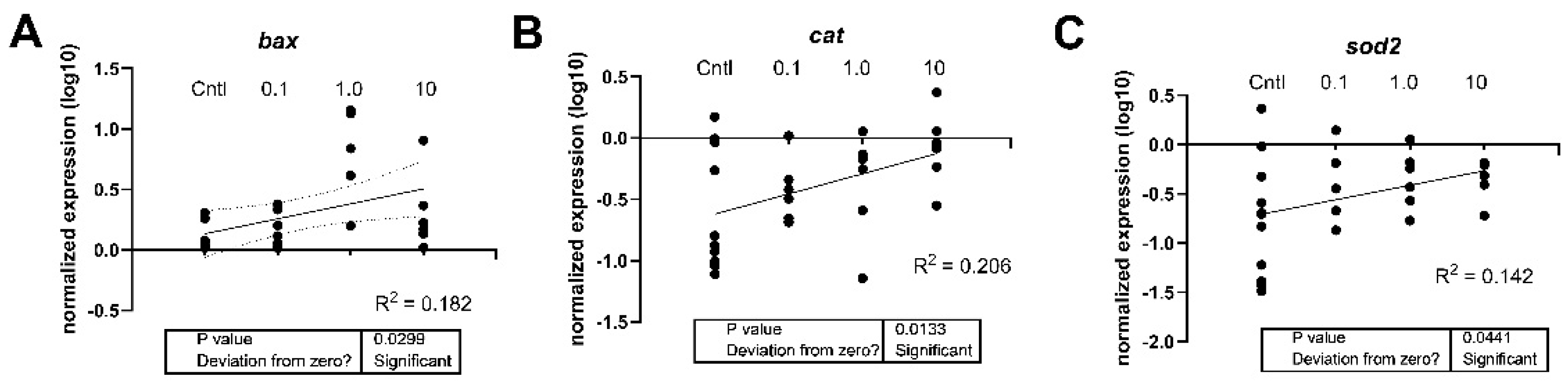
The amount of ROS is often indicative of the amount of oxidative stress in cells, and an excess of ROS can contribute to damage at the molecular level. Limited studies in literature examine the impact PFOSA has on organisms. In our study, we did not observe any increase in ROS in zebrafish treated with PFOSA; however, there was a concentration dependent increase in both
cat and
sod2 expression. Mitochondrial dysfunction has been thought to contribute to the progression of neurodegenerative disorders and the presence of ROS is one clear indicator of dysfunction as antioxidant systems are implemented to counteract oxidative stress. This supports our oxidative stress-related gene responses (PFOSA-induced increase in
cat and
sod2 mRNA levels) which could have mitigated any change in ROS levels in the larval zebrafish. Similar results have also been observed in rodent models where oxidative stress-related genes, like
cat, were significantly increased to counteract damage by PFOA-induced lipid peroxidation in mouse brain and liver tissues [
39]. ROS can also trigger apoptosis to mediate inflammation. Though PFOSA was found to only significantly upregulate two apoptosis-related genes (
bax and
cycs) in our study, another study reports increased apoptotic cells in the brain and upregulated
bcl-2,
caspase3, and
p53 zebrafish exposed to PFOS (Mahapatra et al., 2023). Bax, is a pro-apoptotic factor in the Bcl-2 family, signaling mitochondria and cell death while cytochrome c is an intrinsic apoptotic signal activating downstream caspase enzymes. Other studies investigating PFOSA report mixed results for antioxidant gene expression and proteins. Olufsen and Arukwe (2015) exposed Atlantic salmon (
Salmo salar) hepatocytes to 25 or 50 µM (12,400-24,900 µg/L) PFOSA for 24 or 48 hours and analyzed catalase (
cat), glutathione peroxidase (
gpx), glucocorticoid receptor (
gr), and glutathione S-transferase (
gst) mRNA levels, which were not significantly impacted [
40]. Another study also exposed Atlantic salmon hepatocytes to 2, 20, or 50 µM (998, 9,900, or 24,900 µg/L) PFOSA for 12 or 24 hours [
3]. No significant changes to
gpx mRNA levels were found, but
cat mRNA levels were significantly increased by 20 and 50 µM PFOSA following 24 hours of exposure, suggesting that antioxidant defense mechanisms were activated. Differences among studies may occur due to the type of model used to investigate PFOSA toxicity (e.g. cells versus larvae). Taken together, there is evidence that PFOSA initiates an antioxidant defense and any elevation in ROS may lead to higher levels of apoptosis in larval zebrafish.
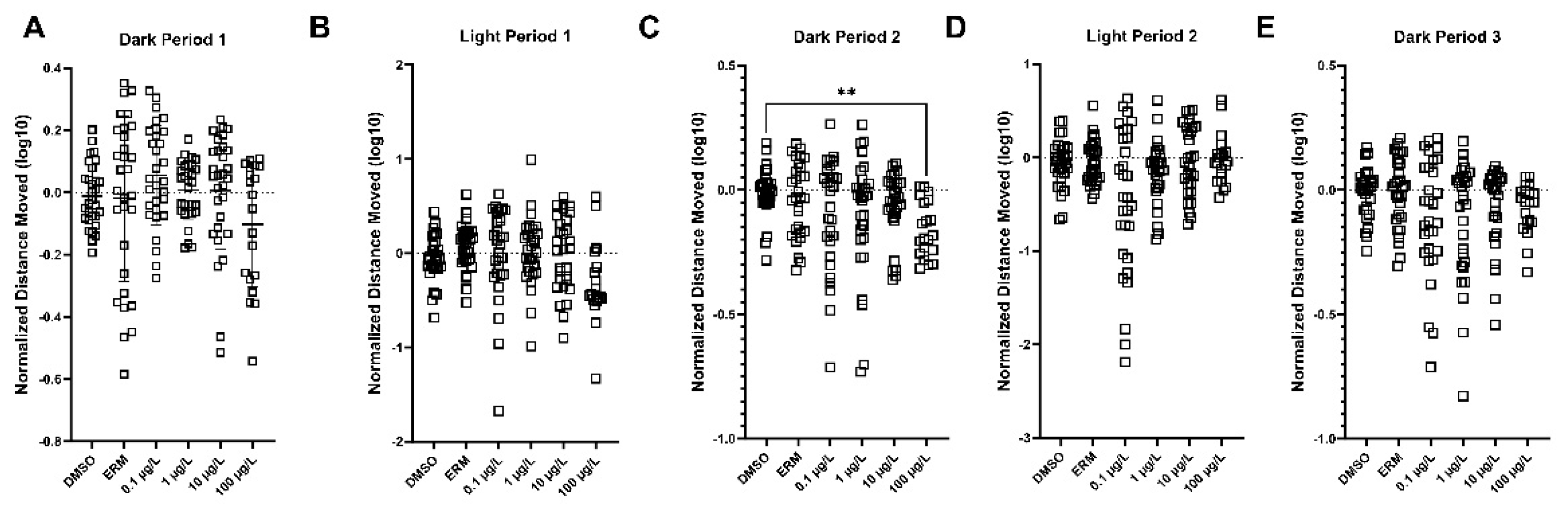
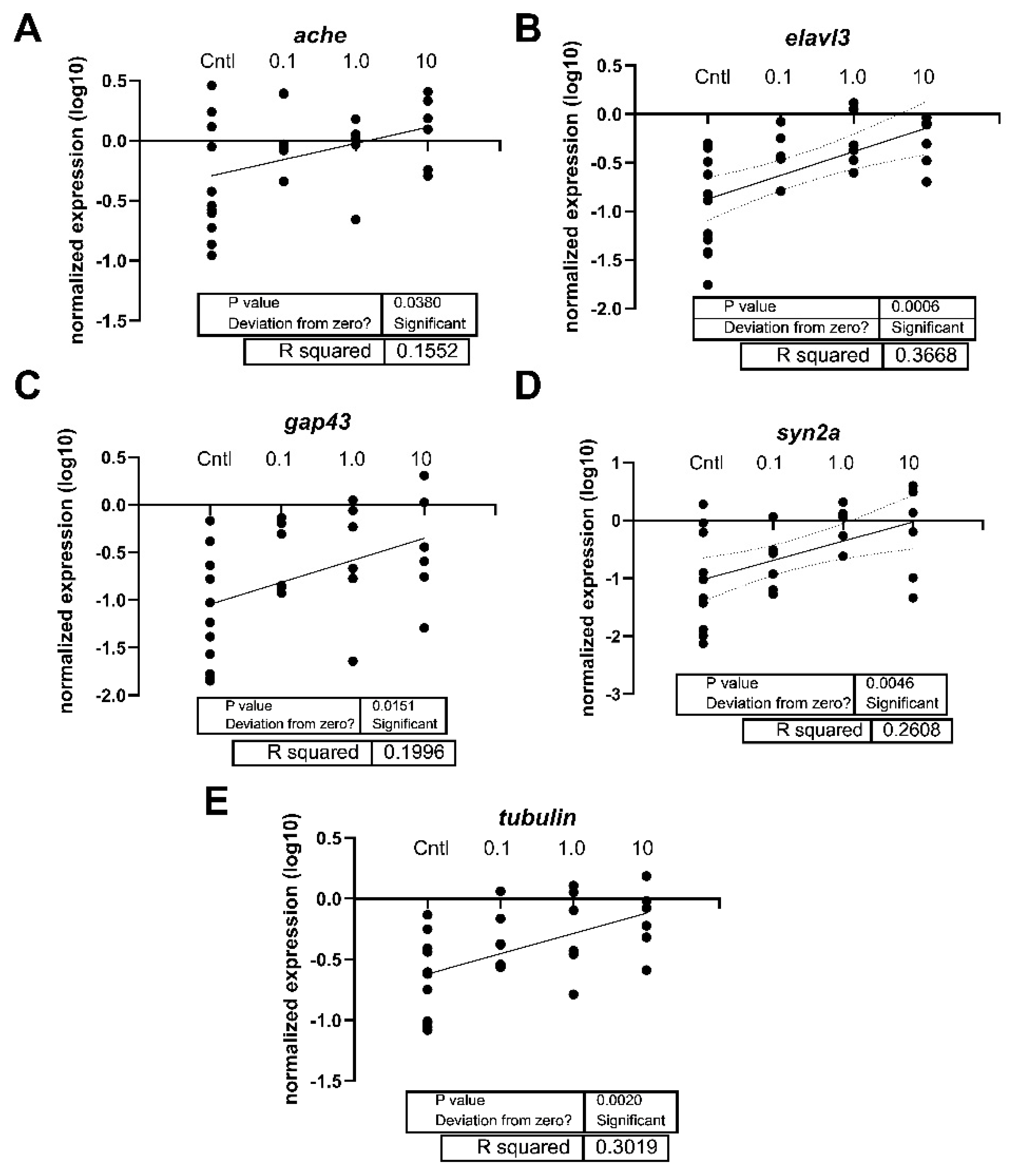
We hypothesized that PFOSA would induce neurotoxicity in the form of behavioral changes and altered expression of genes related to neurotoxicity. Indeed, we observed hypoactivity at 100 µg/L which corresponded to the highest expression levels of several neurotoxicity biomarkers. These responses indicate some form of neurological impairment [
41]. Chemical neurotoxins cause damage to, or death of, cells in the nervous system, disrupting neuronal pathways linked to neurodegenerative illnesses and other neurodevelopmental issues (i.e., Parkinson’s disease and schizophrenia). For instance, zebrafish exposed to PFOS had transcriptome changes linked to disturbance of the neuromuscular system [
42] and zebrafish exposed to perfluorononanoic acid (PFNA) showed evidence of altered neuroinflammatory pathways [
43]. To our knowledge, this is the first study to examine neurotoxicity mechanism in zebrafish exposed to PFOSA. We observed hypoactivity effects on locomotor activity in larval zebrafish, suggesting neurotoxicity or motor deficits with PFOSA exposure. Fish treated with 100 µg/L PFOSA showed reduced activity in Dark Period 2 of the combined VMR. Truong et al. (2022) exposed dechorionated zebrafish embryos to 0.015-100 µM PFOSA from 6 to 120 hpf and found that PFOSA induced both a refractory and an excitatory phase of hyperactivity [
36]. Consistent with our observation, after exposing zebrafish embryos to 1 or 10 µg/L PFOSA for 120 hours, Liu et al. (2022) observed reductions in total distance moved, average swimming velocity, and maximum acceleration in fish treated with 1 µg/L PFOSA [
16]. Our results also revealed that PFOSA alters the expression of neurotoxicity-related genes, as notable effects were observed in
elavl3, and positive associations were detected between PFOSA concentration and expression levels (e.g.
ache, elavl3, gap43, syn2a, and tubb3).
Elavl3 is expressed in different nervous system structures and is known to regulate neurogenesis [
44]. Additionally,
ache is involved in cholinergic functioning and
syn2a is involved in dopamine and serotonin release. PFAS exposure has previously been shown to alter these transcripts; PFOS, the metabolic product of PFOSA, was reported to decrease
ache expression [
45] and perfluorododecanoate (PFDoA) decreased mRNA levels of
elavl3,
gap43, and
syn2a [
46]. Here we report an elevation in the expression of
elavl3 and many other neurotoxic-related transcripts with PFOSA exposure, and this may reflect a compensatory response to impaired neurogenesis and neurotransmitter release. Conversely, different PFAS may elicit unique responses in the CNS in relation to gene expression patterns. Regardless, there is evidence from the molecular response that PFOSA alters genes related to neuronal integrity and structure, suggesting the potential for neurotoxicity in developing larval fish. Thus, early developmental exposures to PFOSA may have long lasting detrimental effects on the adult brain and this should be further investigated.
Author Contributions
Conceptualization, C.J.M.; Investigation, A.S., C.D.E., C.E., D.S., E.I., E.M.A.V., I.K., L.A., M.K., M.R., and N.D.; writing—original draft preparation, E.I.; writing—review and editing, E.I. and C.J.M.; supervision, C.J.M; funding acquisition, C.J.M.,
Figure 1.
Total percent of surviving zebrafish embryos/larvae exposed to ERM, 0.1% DMSO, 0.1, 1, 10, or 100 µg/L PFOSA over 7 days. .
Figure 1.
Total percent of surviving zebrafish embryos/larvae exposed to ERM, 0.1% DMSO, 0.1, 1, 10, or 100 µg/L PFOSA over 7 days. .
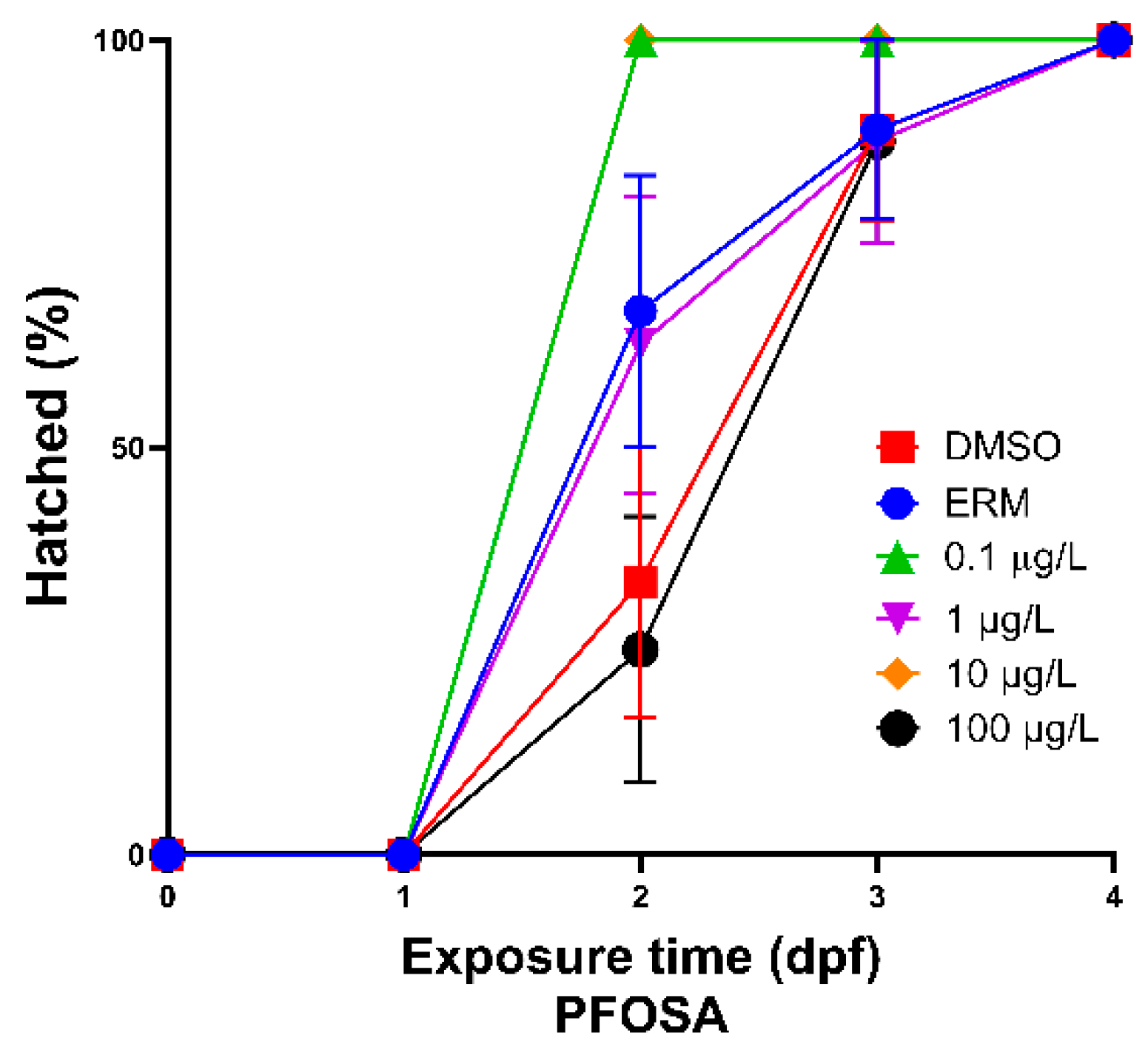
Figure 2.
Total percent of hatched zebrafish embryos/larvae exposed to ERM, 0.1% DMSO, 0.1, 1, 10, or 100 µg/L PFOSA over 4 days.
Figure 2.
Total percent of hatched zebrafish embryos/larvae exposed to ERM, 0.1% DMSO, 0.1, 1, 10, or 100 µg/L PFOSA over 4 days.
Figure 3.
Normalized reactive oxygen species (to µg/mL media/protein) present in zebrafish at 7 dpf. Each circle represents a biological replicate, the horizontal line indicates the mean (± S.D.), and ns = not significant (One-Way ANOVA, Dunnett’s multiple comparisons test, n=5/treatment).
Figure 3.
Normalized reactive oxygen species (to µg/mL media/protein) present in zebrafish at 7 dpf. Each circle represents a biological replicate, the horizontal line indicates the mean (± S.D.), and ns = not significant (One-Way ANOVA, Dunnett’s multiple comparisons test, n=5/treatment).
Figure 4.
The distance moved in each of the light and dark zones (10-minute bins) of 7-day zebrafish larvae exposed to 0.1% DMSO, ERM, 0.1, 1, 10, or 100 µg/L PFOSA. Graphs are the combined output from three independent VMR runs. Mean values are depicted by the columns in each dark-light phase (mean ± S.D.) (Kruskal-Wallis test followed by a multiple comparisons test, n=8-12 fish/treatment/run). Asterisk indicates **P<0.01. .
Figure 4.
The distance moved in each of the light and dark zones (10-minute bins) of 7-day zebrafish larvae exposed to 0.1% DMSO, ERM, 0.1, 1, 10, or 100 µg/L PFOSA. Graphs are the combined output from three independent VMR runs. Mean values are depicted by the columns in each dark-light phase (mean ± S.D.) (Kruskal-Wallis test followed by a multiple comparisons test, n=8-12 fish/treatment/run). Asterisk indicates **P<0.01. .
Figure 5.
Linear regression of the expression levels of (A) bax, (B) cat, and (C) sod2 in 7-day old larval zebrafish exposed to either 0.1% DMSO, ERM, 0.1, 1, or 10 µg/L PFOSA. The DMSO and ERM group were combined to yield the control (Cntl) group. Each circle is a biological replicate (n=4 to 6).
Figure 5.
Linear regression of the expression levels of (A) bax, (B) cat, and (C) sod2 in 7-day old larval zebrafish exposed to either 0.1% DMSO, ERM, 0.1, 1, or 10 µg/L PFOSA. The DMSO and ERM group were combined to yield the control (Cntl) group. Each circle is a biological replicate (n=4 to 6).
Figure 6.
The expression levels of (A) bax and (B) elavl3 in 7-day old larval zebrafish exposed to 0.1% DMSO, ERM, 0.1, 1, or 10 µg/L PFOSA. Each circle is a biological replicate, and the horizontal line indicates the mean (± S.D.) (One-Way ANOVA, Dunnett’s multiple comparisons test, n=4 to 6). Asterisk indicates *P<0.05 or **P<0.01. .
Figure 6.
The expression levels of (A) bax and (B) elavl3 in 7-day old larval zebrafish exposed to 0.1% DMSO, ERM, 0.1, 1, or 10 µg/L PFOSA. Each circle is a biological replicate, and the horizontal line indicates the mean (± S.D.) (One-Way ANOVA, Dunnett’s multiple comparisons test, n=4 to 6). Asterisk indicates *P<0.05 or **P<0.01. .
Figure 7.
Linear regression of the expression levels of (A) ache, (B) elavl3, (C) gap43, (D) syn2a, and (E) tubulin (tubb3) in 7-day old larval zebrafish exposed to either 0.1% DMSO, ERM, 0.1, 1, or 10 µg/L PFOSA. The DMSO and ERM group were combined to yield the control (Cntl) group. Each circle is a biological replicate (n=4 to 6).
Figure 7.
Linear regression of the expression levels of (A) ache, (B) elavl3, (C) gap43, (D) syn2a, and (E) tubulin (tubb3) in 7-day old larval zebrafish exposed to either 0.1% DMSO, ERM, 0.1, 1, or 10 µg/L PFOSA. The DMSO and ERM group were combined to yield the control (Cntl) group. Each circle is a biological replicate (n=4 to 6).