Submitted:
15 August 2024
Posted:
19 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
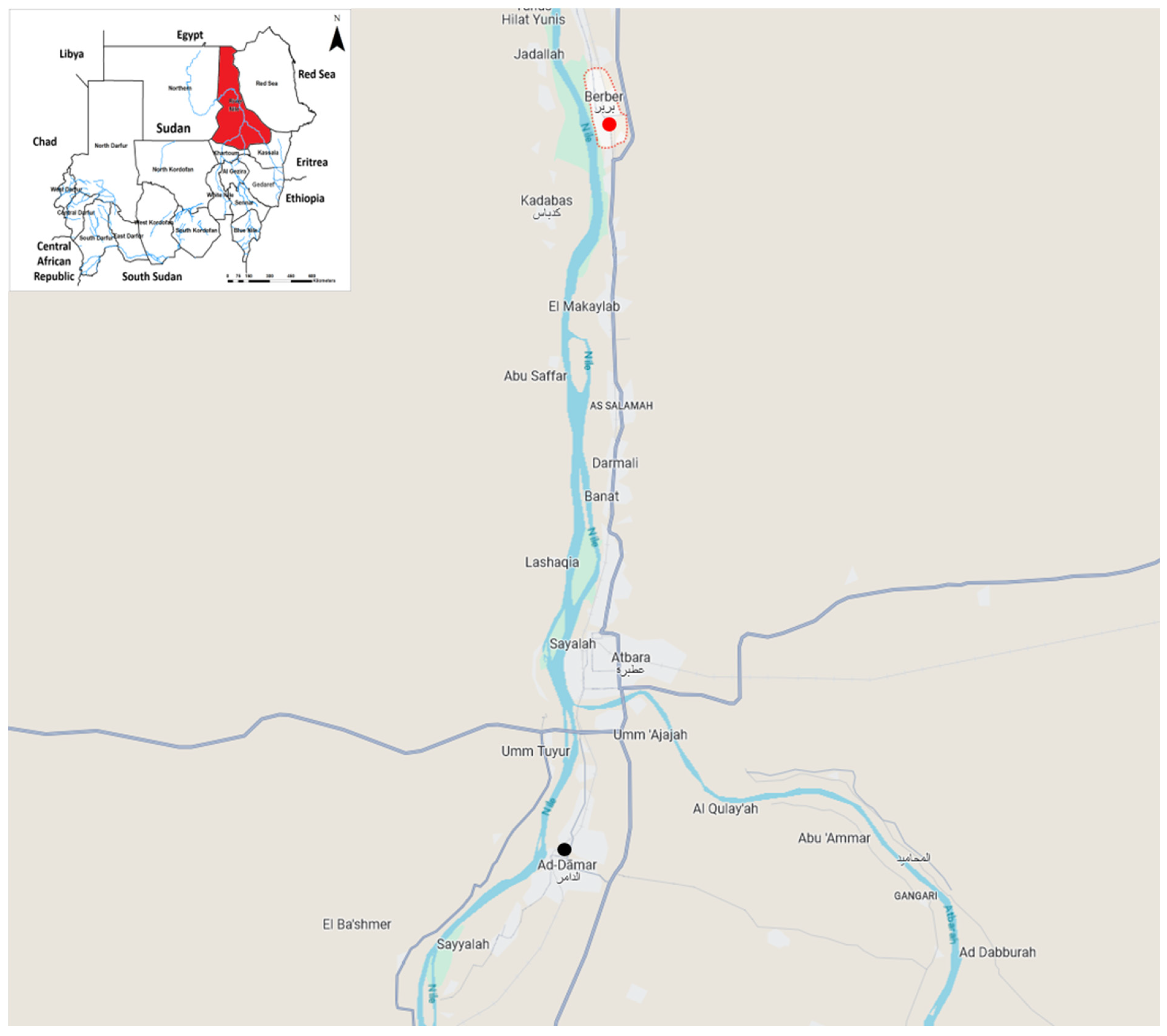
3. Results
3.1. Literature analysis:
3.2. The outbreak:
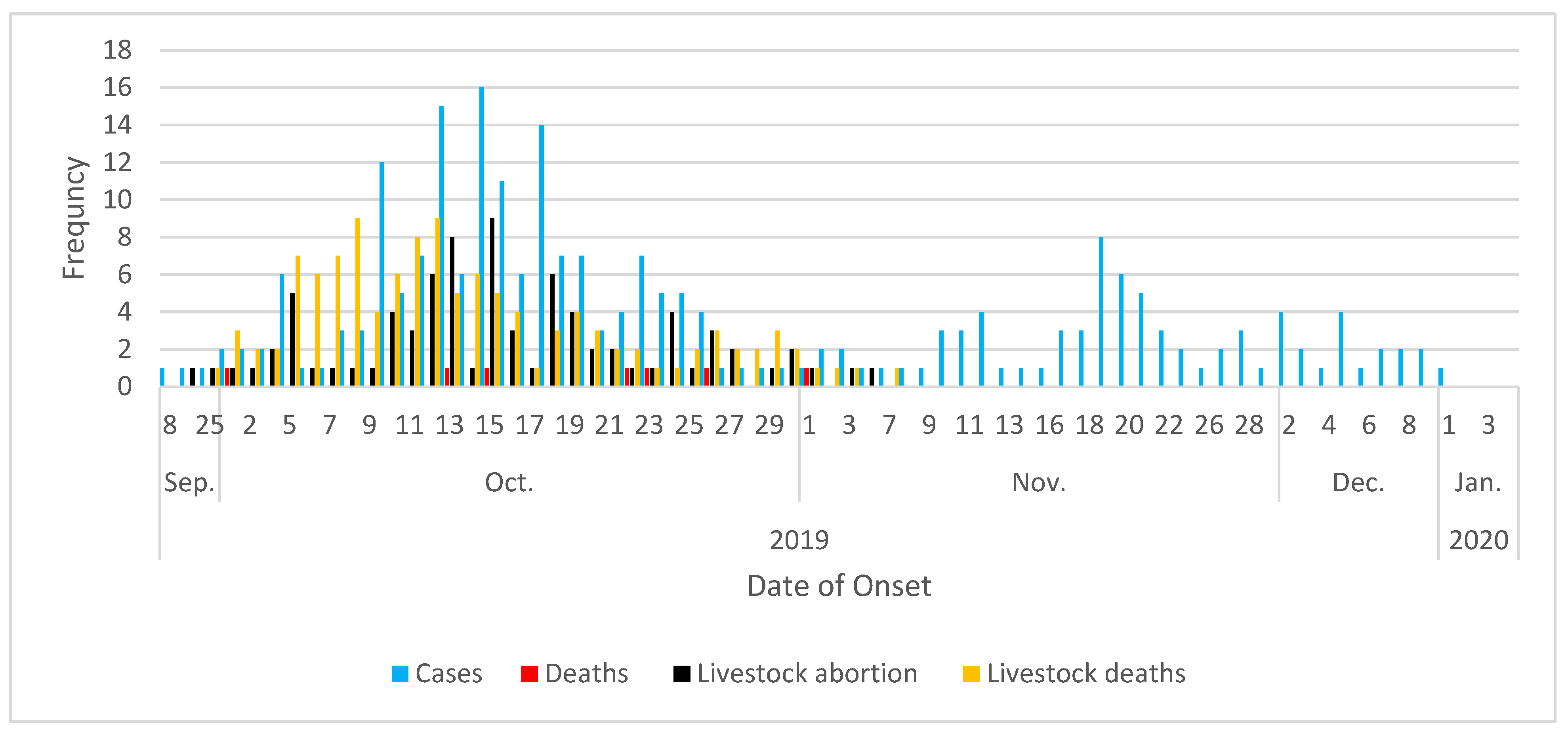
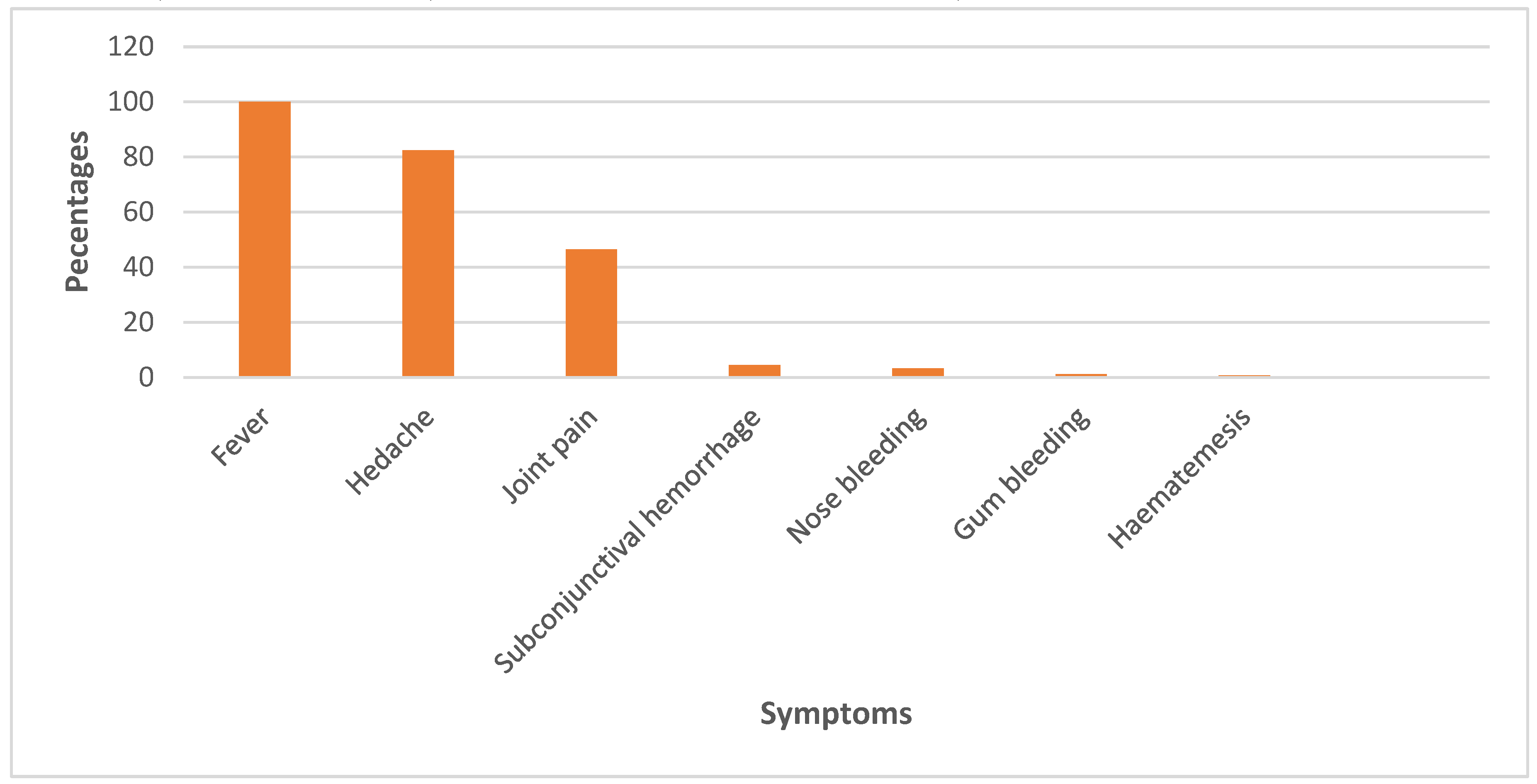
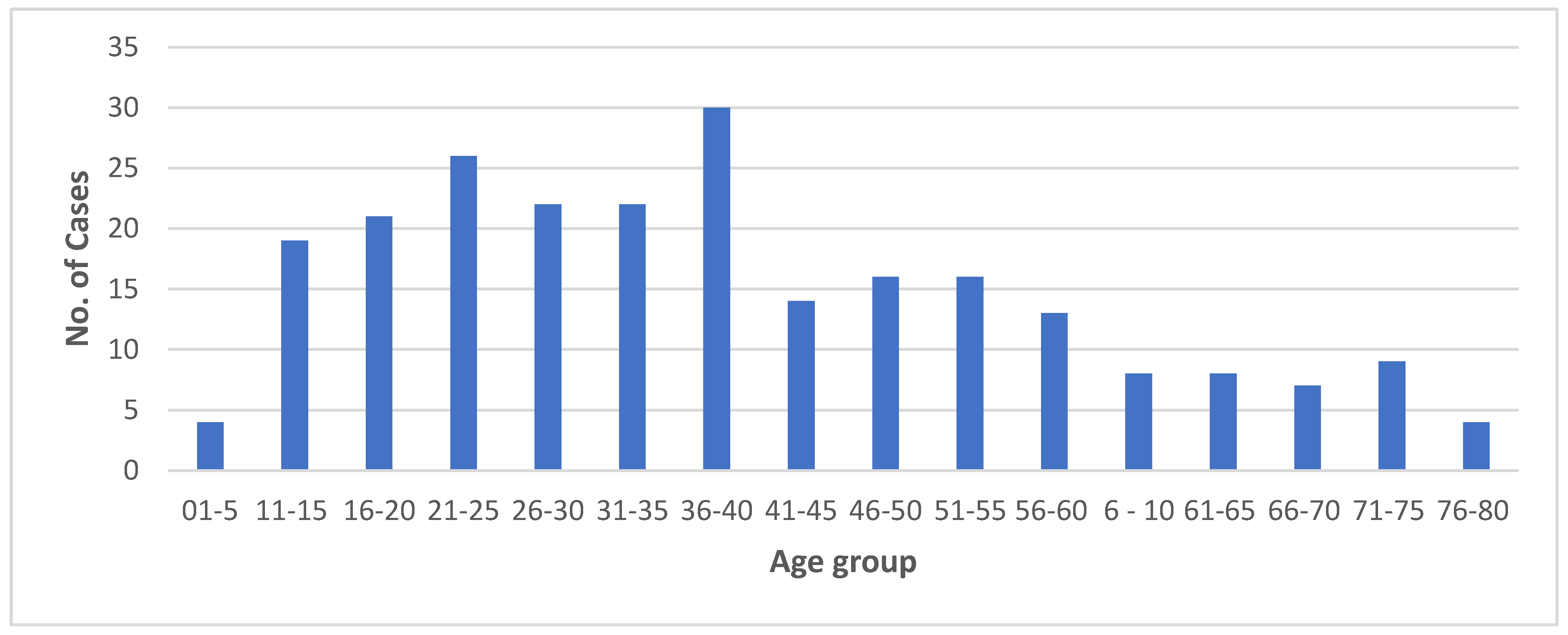
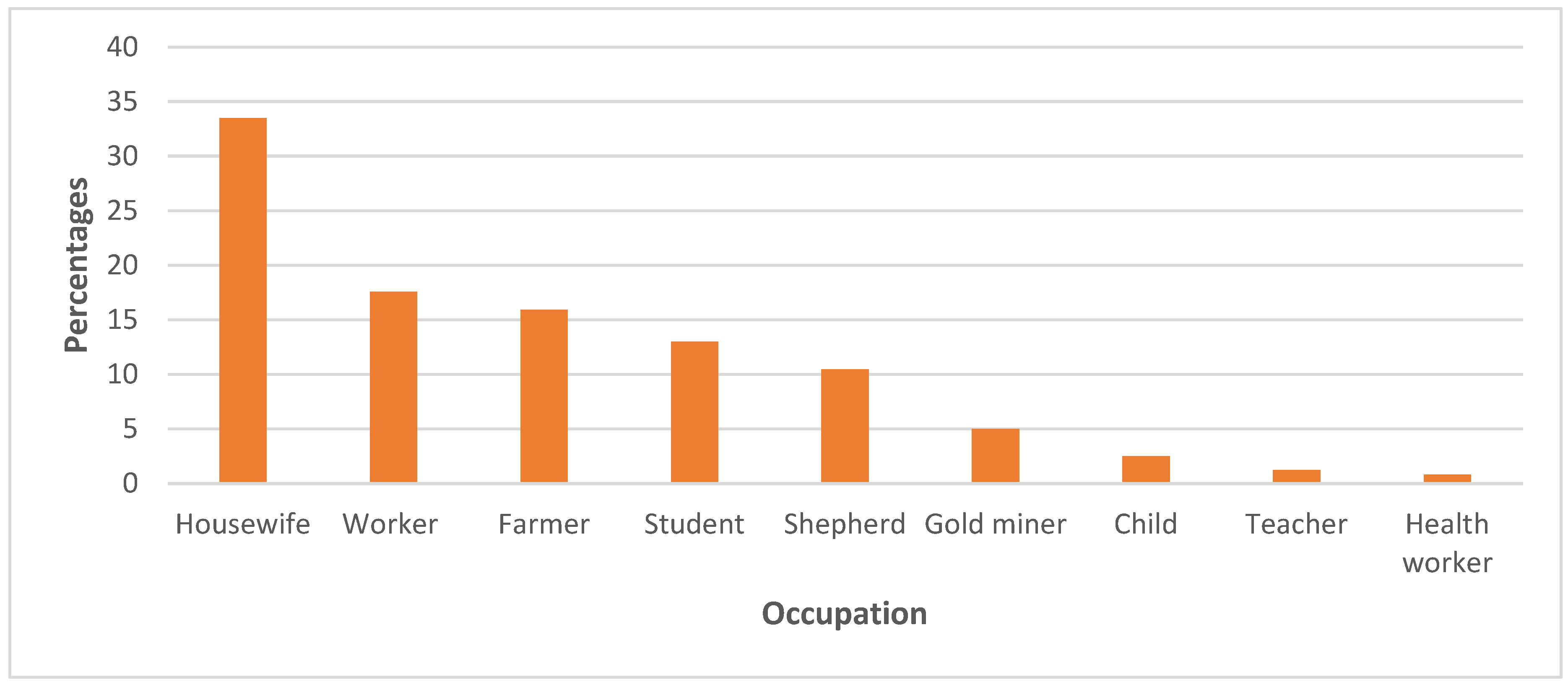
3.3. The One Health response:
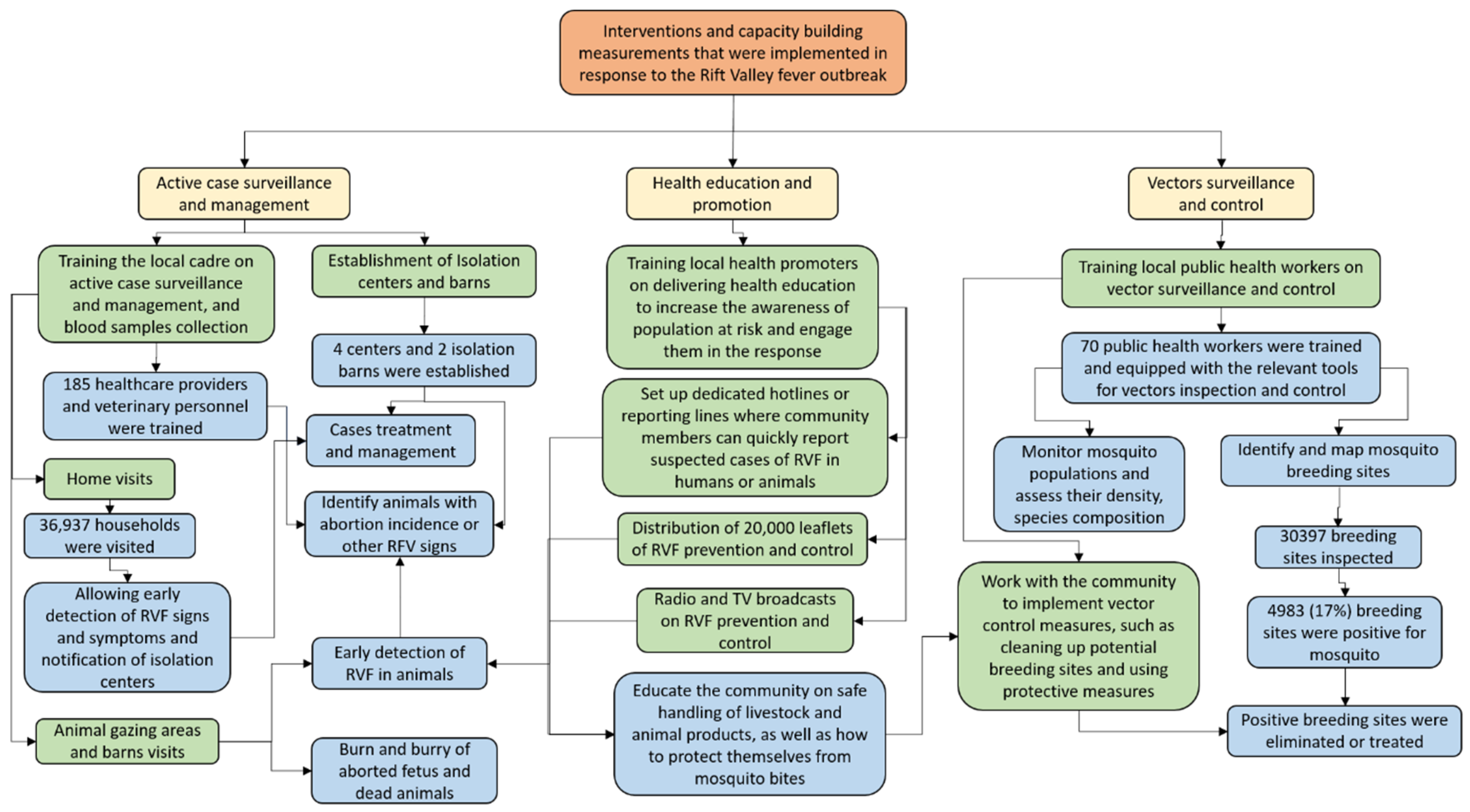
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weaver, S.C.; Barrett, A.D.T. Transmission Cycles, Host Range, Evolution and Emergence of Arboviral Disease. Nat Rev Microbiol 2004, 2, 789–801. [Google Scholar] [CrossRef] [PubMed]
- Sick, F.; Beer, M.; Kampen, H.; Wernike, K. Culicoides Biting Midges—Underestimated Vectors for Arboviruses of Public Health and Veterinary Importance. Viruses 2019, 11, 376. [Google Scholar] [CrossRef]
- Cleton, N.; Koopmans, M.; Reimerink, J.; Godeke, G.-J.; Reusken, C. Come Fly with Me: Review of Clinically Important Arboviruses for Global Travelers. Journal of Clinical Virology 2012, 55, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.; Campo, J.M.D.; Constenla, D. A Review of the Economic Evidence of Aedes-Borne Arboviruses and Aedes-Borne Arboviral Disease Prevention and Control Strategies. Expert Review of Vaccines 2020, 19, 143–162. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Ali, Y.; Salim, B.; Dietrich, I.; Zinsstag, J. Epidemics of Crimean-Congo Hemorrhagic Fever (CCHF) in Sudan between 2010 and 2020. Microorganisms 2022, 10, 928. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Dietrich, I.; LaBeaud, A.D.; Lindsay, S.W.; Musa, A.; Weaver, S.C. Risks and Challenges of Arboviral Diseases in Sudan: The Urgent Need for Actions. Viruses 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Ali, Y.; Elduma, A.; Eldigail, M.H.; Mhmoud, R.A.; Mohamed, N.S.; Ksiazek, T.G.; Dietrich, I.; Weaver, S.C. Unique Outbreak of Rift Valley Fever in Sudan, 2019 - Volume 26, Number 12—December 2020 - Emerging Infectious Diseases Journal - CDC. Emerging Infectious Diseases 2020, 26, 3030–3033. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Mahmoud, I.; Eldigail, M.; Elhassan, R.M.; Weaver, S.C. The Emergence of Rift Valley Fever in Gedaref State Urges the Need for a Cross-Border One Health Strategy and Enforcement of the International Health Regulations. Pathogens 2021, 10, 885. [Google Scholar] [CrossRef] [PubMed]
- Remera, E.; Rwagasore, E.; Muvunyi, C.M.; Ahmed, A. Emergence of the First Molecularly Confirmed Outbreak of Rift Valley Fever among Humans in Rwanda, Calls for Institutionalizing the One Health Strategy. IJID One Health 2024, 4. [Google Scholar] [CrossRef]
- Nsengimana, I.; Juma, J.; Roesel, K.; Gasana, M.N.; Ndayisenga, F.; Muvunyi, C.M.; Hakizimana, E.; Hakizimana, J.N.; Eastwood, G.; Chengula, A.A.; et al. Genomic Epidemiology of Rift Valley Fever Virus Involved in the 2018 and 2022 Outbreaks in Livestock in Rwanda. Viruses 2024, 16, 1148. [Google Scholar] [CrossRef] [PubMed]
- Weaver, S.C. Prediction and Prevention of Urban Arbovirus Epidemics: A Challenge for the Global Virology Community. Antiviral Res 2018, 156, 80–84. [Google Scholar] [CrossRef]
- Pigott, D.M.; Deshpande, A.; Letourneau, I.; Morozoff, C.; Reiner, R.C.; Kraemer, M.U.G.; Brent, S.E.; Bogoch, I.I.; Khan, K.; Biehl, M.H.; et al. Local, National, and Regional Viral Haemorrhagic Fever Pandemic Potential in Africa: A Multistage Analysis. The Lancet 2017, 390, 2662–2672. [Google Scholar] [CrossRef]
- Kraemer, M.U.G.; Reiner, R.C.; Brady, O.J.; Messina, J.P.; Gilbert, M.; Pigott, D.M.; Yi, D.; Johnson, K.; Earl, L.; Marczak, L.B.; et al. Past and Future Spread of the Arbovirus Vectors Aedes Aegypti and Aedes Albopictus. Nat Microbiol 2019, 4, 854–863. [Google Scholar] [CrossRef] [PubMed]
- Weaver, S.C.; Reisen, W.K. Present and Future Arboviral Threats. Antiviral Research 2010, 85, 328–345. [Google Scholar] [CrossRef]
- Coffey, L.L.; Forrester, N.; Tsetsarkin, K.; Vasilakis, N.; Weaver, S.C. Factors Shaping the Adaptive Landscape for Arboviruses: Implications for the Emergence of Disease. Future Microbiology 2013, 8, 155–176. [Google Scholar] [CrossRef]
- Esser, H.J.; Mögling, R.; Cleton, N.B.; van der Jeugd, H.; Sprong, H.; Stroo, A.; Koopmans, M.P.G.; de Boer, W.F.; Reusken, C.B.E.M. Risk Factors Associated with Sustained Circulation of Six Zoonotic Arboviruses: A Systematic Review for Selection of Surveillance Sites in Non-Endemic Areas. Parasites Vectors 2019, 12, 265. [Google Scholar] [CrossRef] [PubMed]
- Whiteman, A.; Loaiza, J.R.; Yee, D.A.; Poh, K.C.; Watkins, A.S.; Lucas, K.J.; Rapp, T.J.; Kline, L.; Ahmed, A.; Chen, S.; et al. Do Socioeconomic Factors Drive Aedes Mosquito Vectors and Their Arboviral Diseases? A Systematic Review of Dengue, Chikungunya, Yellow Fever, and Zika Virus. One Health 2020, 100188. [Google Scholar] [CrossRef]
- Huang, Y.-J.S.; Higgs, S.; Vanlandingham, D.L. Emergence and Re-Emergence of Mosquito-Borne Arboviruses. Current Opinion in Virology 2019, 34, 104–109. [Google Scholar] [CrossRef]
- Elaagip, A.; Alsedig, K.; Altahir, O.; Ageep, T.; Ahmed, A.; Siam, H.A.; Samy, A.M.; Mohamed, W.; Khalid, F.; Gumaa, S.; et al. Seroprevalence and Associated Risk Factors of Dengue Fever in Kassala State, Eastern Sudan. PLoS Negl Trop Dis 2020, 14. [Google Scholar] [CrossRef]
- Elduma, A.H.; LaBeaud, A.D.; Plante, J.A.; Plante, K.S.; Ahmed, A. High Seroprevalence of Dengue Virus Infection in Sudan: Systematic Review and Meta-Analysis. Tropical Medicine and Infectious Disease 2020, 5, 120. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Mohamed, N.S.; Siddig, E.E.; Algaily, T.; Sulaiman, S.; Ali, Y. The Impacts of Climate Change on Displaced Populations: A Call for Actions. The Journal of Climate Change and Health 2021, 100057. [Google Scholar] [CrossRef]
- Ahmed, A.; Ali, Y.; Mohamed, N.S. Arboviral Diseases: The Emergence of a Major yet Ignored Public Health Threat in Africa. The Lancet Planetary Health 2020, 4, e555. [Google Scholar] [CrossRef]
- Ahmed, A.; Abubakr, M.; Sami, H.; Mahdi, I.; Mohamed, N.S.; Zinsstag, J. The First Molecular Detection of Aedes Albopictus in Sudan Associates with Increased Outbreaks of Chikungunya and Dengue. International Journal of Molecular Sciences 2022, 23, 11802. [Google Scholar] [CrossRef]
- Eisa, M. Preliminary Survey of Domestic Animals of the Sudan for Precipitating Antibodies to Rift Valley Fever Virus. J Hyg (Lond) 1984, 93, 629–637. [Google Scholar] [CrossRef]
- Abdallah, M.M.M.; Adam, I.A.; Abdalla, T.M.; Abdelaziz, S.A.; Ahmed, M.E.; Aradaib, I.E. A Survey of Rift Valley Fever and Associated Risk Factors among the One-Humped Camel (Camelus Dromedaries) in Sudan. Irish Veterinary Journal 2016, 69, 6. [Google Scholar] [CrossRef]
- Seufi, A.M.; Galal, F.H. Role of Culex and Anopheles Mosquito Species as Potential Vectors of Rift Valley Fever Virus in Sudan Outbreak, 2007. BMC Infectious Diseases 2010, 10, 65. [Google Scholar] [CrossRef]
- Aradaib, I.E.; Erickson, B.R.; Elageb, R.M.; Khristova, M.L.; Carroll, S.A.; Elkhidir, I.M.; Karsany, M.E.; Karrar, A.E.; Elbashir, M.I.; Nichol, S.T. Rift Valley Fever, Sudan, 2007 and 2010. Emerg Infect Dis 2013, 19, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Mansour, M.E.A.; Ahmed, A.; Kamal, S.; Elhassan, T.M.A.; Abdelgadir, A.E. Seroprevalence and Geographical Distribution of Rift Valley Fever in Livestock in Sudan. Journal of Applied Veterinary Science And Technology 2024, 5, 78–82. [Google Scholar] [CrossRef]
- de Glanville, W.A.; Allan, K.J.; Nyarobi, J.M.; Thomas, K.M.; Lankester, F.; Kibona, T.J.; Claxton, J.R.; Brennan, B.; Carter, R.W.; Crump, J.A.; et al. An Outbreak of Rift Valley Fever among Peri-Urban Dairy Cattle in Northern Tanzania. Trans R Soc Trop Med Hyg 2022, 116, 1082–1090. [Google Scholar] [CrossRef] [PubMed]
- Hassan, O.A.; Ahlm, C.; Sang, R.; Evander, M. The 2007 Rift Valley Fever Outbreak in Sudan. PLOS Neglected Tropical Diseases 2011, 5, e1229. [Google Scholar] [CrossRef]
- Lagare, A.; Fall, G.; Ibrahim, A.; Ousmane, S.; Sadio, B.; Abdoulaye, M.; Alhassane, A.; Mahaman, A.E.; Issaka, B.; Sidikou, F.; et al. First Occurrence of Rift Valley Fever Outbreak in Niger, 2016. Veterinary Medicine and Science 2019, 5, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Munyua, P.; Murithi, R.M.; Wainwright, S.; Githinji, J.; Hightower, A.; Mutonga, D.; Macharia, J.; Ithondeka, P.M.; Musaa, J.; Breiman, R.F.; et al. Rift Valley Fever Outbreak in Livestock in Kenya, 2006–2007. The American Journal of Tropical Medicine and Hygiene 2010, 83, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Youssouf, H.; Subiros, M.; Dennetiere, G.; Collet, L.; Dommergues, L.; Pauvert, A.; Rabarison, P.; Vauloup-Fellous, C.; Le Godais, G.; Jaffar-Bandjee, M.-C.; et al. Rift Valley Fever Outbreak, Mayotte, France, 2018–2019. Emerg Infect Dis 2020, 26, 769–772. [Google Scholar] [CrossRef] [PubMed]
- Zinsstag, J.; Hediger, K.; Osman, Y.M.; Abukhattab, S.; Crump, L.; Kaiser-Grolimund, A.; Mauti, S.; Ahmed, A.; Hattendorf, J.; Bonfoh, B.; et al. The Promotion and Development of One Health at Swiss TPH and Its Greater Potential. Diseases 2022, 10, 65. [Google Scholar] [CrossRef] [PubMed]
- Zinsstag, J.; Utzinger, J.; Probst-Hensch, N.; Shan, L.; Zhou, X.-N. Towards Integrated Surveillance-Response Systems for the Prevention of Future Pandemics. Infectious diseases of poverty 2020, 9, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hassan, O.A.; Ahlm, C.; Evander, M. A Need for One Health Approach – Lessons Learned from Outbreaks of Rift Valley Fever in Saudi Arabia and Sudan. Infection Ecology & Epidemiology 2014, 4, 20710. [Google Scholar] [CrossRef]
- Ahmed, A.; Elduma, A.; Magboul, B.; Higazi, T.; Ali, Y. The First Outbreak of Dengue Fever in Greater Darfur, Western Sudan. Tropical Medicine and Infectious Disease 2019, 4, 43. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, N.S.; Ali, Y.; Abdalrahman, S.; Ahmed, A.; Siddig, E.E. The Use of Cholera Oral Vaccine for Containment of the 2019 Disease Outbreak in Sudan. Trans R Soc Trop Med Hyg 2022, 116, 763–766. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Eldigail, M.; Elduma, A.; Breima, T.; Dietrich, I.; Ali, Y.; Weaver, S.C. First Report of Epidemic Dengue Fever and Malaria Co-Infections among Internally Displaced Persons in Humanitarian Camps of North Darfur, Sudan. International Journal of Infectious Diseases 2021, 108, 513–516. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Ali, Y.; Elmagboul, B.; Mohamed, O.; Elduma, A.; Bashab, H.; Mahamoud, A.; Khogali, H.; Elaagip, A.; Higazi, T. Dengue Fever in the Darfur Area, Western Sudan. Emerging Infect. Dis. 2019, 25, 2126. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Ali, Y.; Siddig, E.E.; Hamed, J.; Mohamed, N.S.; Khairy, A.; Zinsstag, J. Hepatitis E Virus Outbreak among Tigray War Refugees from Ethiopia, Sudan. Emerg Infect Dis 2022, 28, 1722–1724. [Google Scholar] [CrossRef]
- Mohamed, N.S.; Ali, Y.; Muneer, M.S.; Siddig, E.E.; Sibley, C.H.; Ahmed, A. Malaria Epidemic in Humanitarian Crisis Settings the Case of South Kordofan State, Sudan. The Journal of Infection in Developing Countries 2021, 15, 168–171. [Google Scholar] [CrossRef]
- El-Sadig, S.M.; Mohamed, N.S.; Ahmed, E.S.; Alayeib, M.A.; Tahir, L.H.; Edris, A.M.M.; Ali, Y.; Siddig, E.E.; Ahmed, A. Obstacles Faced by Healthcare Providers during COVID-19 Pandemic in Sudan. J Infect Dev Ctries 2021, 15, 1615–1617. [Google Scholar] [CrossRef] [PubMed]
- El-Sadig, S.M.; El-Amin, S.O.; El-Amin, R.O.; Siddig, E.E.; Ahmed, A. Humanitarian Crisis in Sudan: The Collapsed Health System Threats the Public and Global Health. QJM: An International Journal of Medicine 2023, 116, 810. [Google Scholar] [CrossRef]
- Ahmed, A.; Mohamed, N.S.; EL-Sadig, S.M.; Fahal, L.A.; Abelrahim, Z.B.; Ahmed, E.S.; Siddig, E.E. COVID-19 in Sudan. The Journal of Infection in Developing Countries 2021, 15, 204–208. [Google Scholar] [CrossRef]
- Grossi-Soyster, E.N.; Lee, J.; King, C.H.; LaBeaud, A.D. The Influence of Raw Milk Exposures on Rift Valley Fever Virus Transmission. PLOS Neglected Tropical Diseases 2019, 13, e0007258. [Google Scholar] [CrossRef] [PubMed]
- CDC: Centers for Disease Control and Prevention About Rift Valley Fever (RVF). Available online: https://www.cdc.gov/rift-valley-fever/about/index.html (accessed on 14 August 2024).
- The World Health Organization (WHO) Rift Valley Fever. Available online: https://www.who.int/news-room/fact-sheets/detail/rift-valley-fever (accessed on 27 January 2024).
- McIntosh, B.M. Rift Valley Fever: 1. Vector Studies in the Field. Journal of the South African Veterinary Association 1972, 43, 391–395. [Google Scholar] [CrossRef]
- Abdelgadir, D.M.; Bashab, H.M.M.; Elhadi Mohamed, R.A.; Abuelmaali, S.A. Risk Factor Analysis for Outbreak of Rift Valley Fever in Khartoum State of Sudan. Journal of Entomological Science 2010, 45, 239–251. [Google Scholar] [CrossRef]
- Abdo-Salem, S.; Tran, A.; Grosbois, V.; Gerbier, G.; Al-Qadasi, M.; Saeed, K.; Etter, E.; Thiry, E.; Roger, F.; Chevalier, V. Can Environmental and Socioeconomic Factors Explain the Recent Emergence of Rift Valley Fever in Yemen, 2000-2001? Vector Borne Zoonotic Dis 2011, 11, 773–779. [Google Scholar] [CrossRef]
- Linthicum, K.J.; Britch, S.C.; Anyamba, A. Rift Valley Fever: An Emerging Mosquito-Borne Disease. Annual Review of Entomology 2016, 61, 395–415. [Google Scholar] [CrossRef]
- Mohamed, R.; Abdelgadir, D.M.; Bashab, H.M. Transovarian Transmission of Rift Valley Fever Virus by Two Species of Mosquitoes in Khartoum State (Sudan): Aedes Vexans (Meigen) and Culex Quinquefasciatus (Say). Sudanese J Public Health 2013, 8, 164Á170. [Google Scholar]
- Outammassine, A.; Zouhair, S.; Loqman, S. Global Potential Distribution of Three Underappreciated Arboviruses Vectors (Aedes Japonicus, Aedes Vexans and Aedes Vittatus) under Current and Future Climate Conditions. Transboundary and Emerging Diseases 2022, 69, e1160–e1171. [Google Scholar] [CrossRef] [PubMed]
- Sanborn, M.A.; Klein, T.A.; Kim, H.-C.; Fung, C.K.; Figueroa, K.L.; Yang, Y.; Asafo-adjei, E.A.; Jarman, R.G.; Hang, J. Metagenomic Analysis Reveals Three Novel and Prevalent Mosquito Viruses from a Single Pool of Aedes Vexans Nipponii Collected in the Republic of Korea. Viruses 2019, 11, 222. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO) Rift Valley Fever: Vigilance Needed in the Coming Months. EMPRES WATCH. Rome 2012, 27.
- Birungi, D.; Aceng, F.L.; Bulage, L.; Nkonwa, I.H.; Mirembe, B.B.; Biribawa, C.; Okethwangu, D.; Opio, N.D.; Monje, F.; Muwanguzi, D.; et al. Sporadic Rift Valley Fever Outbreaks in Humans and Animals in Uganda, October 2017-January 2018. J Environ Public Health 2021, 2021, 8881191. [Google Scholar] [CrossRef]
- Nyakarahuka, L.; Whitmer, S.; Klena, J.; Balinandi, S.; Talundzic, E.; Tumusiime, A.; Kyondo, J.; Mulei, S.; Patel, K.; Baluku, J.; et al. Detection of Sporadic Outbreaks of Rift Valley Fever in Uganda through the National Viral Hemorrhagic Fever Surveillance System, 2017–2020. Am J Trop Med Hyg 2023, 108, 995–1002. [Google Scholar] [CrossRef]
- Nguku, P.M.; Sharif, S.K.; Mutonga, D.; Amwayi, S.; Omolo, J.; Mohammed, O.; Farnon, E.C.; Gould, L.H.; Lederman, E.; Rao, C.; et al. An Investigation of a Major Outbreak of Rift Valley Fever in Kenya: 2006–2007. Am J Trop Med Hyg 2010, 83, 05–13. [Google Scholar] [CrossRef]
- Sindato, C.; Karimuribo, E.D.; Pfeiffer, D.U.; Mboera, L.E.G.; Kivaria, F.; Dautu, G.; Bernard, B.; Paweska, J.T. Spatial and Temporal Pattern of Rift Valley Fever Outbreaks in Tanzania; 1930 to 2007. PLOS ONE 2014, 9, e88897. [Google Scholar] [CrossRef]
- Ali, Y.; Siddig, E.E.; Mohamed, N.; Ahmed, A. Rift Valley Fever and Malaria Co-infection: A Case Report. Clin Case Rep 2023, 11, e7926. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M. c.; Haberberger, R. l.; Salib, A. w.; Soliman, B. a.; El-Tigani, A.; Khalid, I. o.; Watts, D. m. Evaluation of Arthropod-Borne Viruses and Other Infectious Disease Pathogens as the Causes of Febrile Illnesses in the Khartoum Province of Sudan. J. Med. Virol. 1996, 48, 141–146. [Google Scholar] [CrossRef]
- Watts, D.M.; el-Tigani, A.; Botros, B.A.; Salib, A.W.; Olson, J.G.; McCarthy, M.; Ksiazek, T.G. Arthropod-Borne Viral Infections Associated with a Fever Outbreak in the Northern Province of Sudan. J Trop Med Hyg 1994, 97, 228–230. [Google Scholar] [PubMed]
- Seruyange, E.; Ljungberg, K.; Muvunyi, C.M.; Gahutu, J.B.; Katare, S.; Nyamusore, J.; Gwon, Y.-D.; Evander, M.; Norder, H.; Liljeström, P.; et al. Seroreactivity to Chikungunya and West Nile Viruses in Rwandan Blood Donors. Vector-Borne and Zoonotic Diseases 2019, 19, 731–740. [Google Scholar] [CrossRef]
- Seruyange, E.; Gahutu, J.-B.; Muvunyi, C.M.; Katare, S.; Ndahindwa, V.; Sibomana, H.; Nyamusore, J.; Rutagarama, F.; Hannoun, C.; Norder, H.; et al. Seroprevalence of Zika Virus and Rubella Virus IgG among Blood Donors in Rwanda and in Sweden. Journal of Medical Virology 2018, 90, 1290–1296. [Google Scholar] [CrossRef]
- Cordes, K.M.; Cookson, S.T.; Boyd, A.T.; Hardy, C.; Malik, M.R.; Mala, P.; El Tahir, K.; Everard, M.; Jasiem, M.; Husain, F. Real-Time Surveillance in Emergencies Using the Early Warning Alert and Response Network. Emerg Infect Dis 2017, 23, S131–S137. [Google Scholar] [CrossRef] [PubMed]
- Bordier, M.; Delavenne, C.; Nguyen, D.T.T.; Goutard, F.L.; Hendrikx, P. One Health Surveillance: A Matrix to Evaluate Multisectoral Collaboration. Frontiers in veterinary science 2019, 6, 109. [Google Scholar] [CrossRef]
- Osman, Y.; Ali, S.M.; Schelling, E.; Tschopp, R.; Hattendorf, J.; Muhumed, A.; Zinsstag, J. Integrated Community Based Human and Animal Syndromic Surveillance in Adadle District of the Somali Region of Ethiopia. One Health 2021, 13, 100334. [Google Scholar] [CrossRef]
- Siddig, E.E.; Ahmed, A. When Parasites Stray from the Path: A Curious Case of Ectopic Cutaneous Schistosoma Haematobium. QJM: An International Journal of Medicine 2023, 116, 794–795. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Hemaida, M.A.; Hagelnur, A.A.; Eltigani, H.F.; Siddig, E.E. Sudden Emergence and Spread of Cutaneous Larva Migrans in Sudan: A Case Series Calls for Urgent Actions. IDCases 2023, 32, e01789. [Google Scholar] [CrossRef]
- Ahmed, A.; Abubakr, M.; Ali, Y.; Siddig, E.E.; Mohamed, N.S. Vector Control Strategy for Anopheles Stephensi in Africa. The Lancet Microbe 2022, 3, e403. [Google Scholar] [CrossRef]
- Ahmed, A.; Irish, S.R.; Zohdy, S.; Yoshimizu, M.; Tadesse, F.G. Strategies for Conducting Anopheles Stephensi Surveys in Non-Endemic Areas. Acta Tropica 2022, 236, 106671. [Google Scholar] [CrossRef]
- Hemming-Schroeder, E.; Ahmed, A. Anopheles Stephensi in Africa: Vector Control Opportunities for Cobreeding An. Stephensi and Aedes Arbovirus Vectors. Trends in Parasitology 2023, 39, 86–90. [Google Scholar] [CrossRef]
- AU PSC report, T.I. for S.S. Climate Change and Violence in Africa: No Time to Lose. Available online: https://issafrica.org/iss-today/climate-change-and-violence-in-africa-no-time-to-lose (accessed on 18 May 2021).
- Carlson, C.J.; Albery, G.F.; Merow, C.; Trisos, C.H.; Zipfel, C.M.; Eskew, E.A.; Olival, K.J.; Ross, N.; Bansal, S. Climate Change Increases Cross-Species Viral Transmission Risk. Nature 2022, 607, 555–562. [Google Scholar] [CrossRef]
- Gayer, M.; Legros, D.; Formenty, P.; Connolly, M.A. Conflict and Emerging Infectious Diseases. Emerg Infect Dis 2007, 13, 1625–1631. [Google Scholar] [CrossRef]
- Abubakr, M.; Sami, H.; Mahdi, I.; Altahir, O.; Abdelbagi, H.; Mohamed, N.S.; Ahmed, A. The Phylodynamic and Spread of the Invasive Asian Malaria Vectors, Anopheles Stephensi, in Sudan. Biology 2022, 11, 409. [Google Scholar] [CrossRef]
- Ahmed, A.; Khogali, R.; Elnour, M.-A.B.; Nakao, R.; Salim, B. Emergence of the Invasive Malaria Vector Anopheles Stephensi in Khartoum State, Central Sudan. Parasites & Vectors 2021, 14, 511. [Google Scholar] [CrossRef]
- Baudin, M.; Jumaa, A.M.; Jomma, H.J.E.; Karsany, M.S.; Bucht, G.; Näslund, J.; Ahlm, C.; Evander, M.; Mohamed, N. Association of Rift Valley Fever Virus Infection with Miscarriage in Sudanese Women: A Cross-Sectional Study. The Lancet Global Health 2016, 4, e864–e871. [Google Scholar] [CrossRef] [PubMed]
- Adam, I.; Karsany, M.S. Case Report: Rift Valley Fever with Vertical Transmission in a Pregnant Sudanese Woman. J. Med. Virol. 2008, 80, 929. [Google Scholar] [CrossRef] [PubMed]
- Adam, A.A.; Karsany, M.S.; Adam, I. Manifestations of Severe Rift Valley Fever in Sudan. International Journal of Infectious Diseases 2010, 14, e179–e180. [Google Scholar] [CrossRef]
- Bashir, R.S.E.; Hassan, O.A. A One Health Perspective to Identify Environmental Factors That Affect Rift Valley Fever Transmission in Gezira State, Central Sudan. Tropical Medicine and Health 2019, 47, 54. [Google Scholar] [CrossRef]
- Hassan, O.A.; Affognon, H.; Rocklöv, J.; Mburu, P.; Sang, R.; Ahlm, C.; Evander, M. The One Health Approach to Identify Knowledge, Attitudes and Practices That Affect Community Involvement in the Control of Rift Valley Fever Outbreaks. PLoS Negl Trop Dis 2017, 11. [Google Scholar] [CrossRef] [PubMed]
- Valente, A.; Jiolle, D.; Ravel, S.; Porciani, A.; Vial, L.; Michaud, V.; Kwiatek, O.; Pedarrieu, A.; Misse, D.; Ferraris, P.; et al. Flying Syringes for Emerging Enzootic Virus Screening: Proof of Concept for the Development of Noninvasive Xenosurveillance Tools Based on Tsetse Flies. Transboundary and Emerging Diseases 2023, 2023, 9145289. [Google Scholar] [CrossRef]
- Grubaugh, N.D.; Sharma, S.; Krajacich, B.J.; Iii, L.S.F.; Bolay, F.K.; Ii, J.W.D.; Johnson, W.E.; Ebel, G.D.; Foy, B.D.; Brackney, D.E. Xenosurveillance: A Novel Mosquito-Based Approach for Examining the Human-Pathogen Landscape. PLOS Neglected Tropical Diseases 2015, 9, e0003628. [Google Scholar] [CrossRef] [PubMed]
- Fauver, J.R.; Gendernalik, A.; Weger-Lucarelli, J.; Grubaugh, N.D.; Brackney, D.E.; Foy, B.D.; Ebel, G.D. The Use of Xenosurveillance to Detect Human Bacteria, Parasites, and Viruses in Mosquito Bloodmeals. Am J Trop Med Hyg 2017, 97, 324–329. [Google Scholar] [CrossRef]
- Cameron, M.M.; Ramesh, A. The Use of Molecular Xenomonitoring for Surveillance of Mosquito-Borne Diseases. Philosophical Transactions of the Royal Society B: Biological Sciences 2021, 376, 20190816. [Google Scholar] [CrossRef] [PubMed]
- The World Health Organization (WHO) Defining Collaborative Surveillance: A Core Concept for Strengthening the Global Architecture for Health Emergency Preparedness, Response, and Resilience (HEPR); World Health Organization, 2023; ISBN 92-4-007406-6.
- CEPI, T.C. for E.P.I. Priority Diseases. Available online: https://cepi.net/research_dev/priority-diseases/ (accessed on 19 January 2023).
- The World Health Organization (WHO) Ensuring Health Security in The African Region through Emergency Preparedness and Response Flagship Programmes in Rwanda; 2022.
- WHO Emergencies Preparedness, Response: Chikungunya in Sudan. Available online: http://www.who.int/csr/don/15-october-2018-chikungunya-sudan/en/ (accessed on 24 November 2018).
- Krajacich, B.J.; Samaké, D.; Dao, A.; Diallo, M.; Sanogo, Z.L.; Yaro, A.S.; Zeguime, A.; Poudiougo, J.; Cissé, K.; Traoré, M.; et al. Tracking SARS-CoV-2 Seropositivity in Rural Communities Using Blood-Fed Mosquitoes: A Proof-of-Concept Study. Front. Epidemiol. 2023, 3. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, A.; Nitsche, A.; Kohl, C. Viral Metagenomics on Blood-Feeding Arthropods as a Tool for Human Disease Surveillance. International Journal of Molecular Sciences 2016, 17, 1743. [Google Scholar] [CrossRef]
- Bird, B.H.; Nichol, S.T. Breaking the Chain: Rift Valley Fever Virus Control via Livestock Vaccination. Current Opinion in Virology 2012, 2, 315–323. [Google Scholar] [CrossRef]
- Jenkin, D.; Wright, D.; Folegatti, P.M.; Platt, A.; Poulton, I.; Lawrie, A.; Tran, N.; Boyd, A.; Turner, C.; Gitonga, J.N.; et al. Safety and Immunogenicity of a ChAdOx1 Vaccine against Rift Valley Fever in UK Adults: An Open-Label, Non-Randomised, First-in-Human Phase 1 Clinical Trial. The Lancet Infectious Diseases 2023, 23, 956–964. [Google Scholar] [CrossRef]
- WHO, T.W.H.O. Prioritizing Diseases for Research and Development in Emergency Contexts. Available online: https://www.who.int/activities/prioritizing-diseases-for-research-and-development-in-emergency-contexts (accessed on 1 July 2021).
- WHO, T.W.H.O. WHO | List of Blueprint Priority Diseases. Available online: http://www.who.int/blueprint/priority-diseases/en/ (accessed on 19 May 2019).
- GAVI, T.G.A. for V. and I. The next Pandemic: Rift Valley Fever? Available online: https://www.gavi.org/vaccineswork/next-pandemic/rift-valley-fever (accessed on 30 May 2021).
- Ali, Y.; Ahmed, A.; Siddig, E.E.; Mohamed, N.S. The Role of Integrated Programs in the Prevention of COVID-19 in a Humanitarian Setting. Transactions of The Royal Society of Tropical Medicine and Hygiene 2021. [Google Scholar] [CrossRef]
- Ali, Y.; Siddig, E.E.; Osman, M.; Mohamed, N.S.; Musa, A.; Ahmed, A. Preparedness, Prevention, Investigation, and Response to the Emergence of Mpox in Khartoum, Sudan in 2022. 2024.
- Lucey, D.R.; Gostin, L.O. The Emerging Zika Pandemic: Enhancing Preparedness. JAMA 2016, 315, 865–866. [Google Scholar] [CrossRef]
- Khairy, A.; Mahgoob, E.; Nimir, M.; Ahmed, M.; Jubara, M.; Eltayeb, D.; Ahmed, A. Acceptability of COVID-19 Vaccination among Healthcare Workers in Sudan: A Cross Sectional Survey. 2021.
- Ahmed, A.; Ali, Y.; Mohamed, N.S.; Zinsstag, J.; Siddig, E.E.; Khairy, A. Hepatitis E Virus Outbreak among Tigray War Refugees from Ethiopia, Sudan (Response). Emerg Infect Dis 2023, 29, 460–461. [Google Scholar] [CrossRef] [PubMed]
| Date | Event or action taken |
| December 2018 | Violence escalated throughout the country and increased the dynamic of human and animal populations between RVF-endemic and disease-free areas. |
| January 2019 | Public services totally or partially paralyzed |
| August - October 2019 | Unexpected heavy rains and flash flooding occurred in River Nile |
| September 11th 2019 | Indexed case was reported from Berber locality, River Nile state |
| September 2019 | Alert from the state Ministry of Health |
| September 2019 | Investigation team was deployed and logistic and technical support was provided |
| September 2019 | RVFV infections in human and animal were confirmed |
| October 2019 | RVF epidemic and epizootic were officially declared |
| October 2019 | Ministry of Health, Ministry of Livestock, and WHO joint One Health response and containment mission was launched |
| January 2020 | Successful containment of the outbreak. |
| Outcomes | Routine intervention | Integrated One Health intervention | Added value of One Health strategy |
| Location | Eldamar locality | Berber locality | Health system strengthened |
| Period | May-July 2019 | September 2019-January 2020 | Less cases and deaths |
| Human cases | 1,129 | 246 | 78% less human cases |
| Human fatalities | 19 | 7 | 63% less human deaths |
| Death/abortion among animals | At least 1,104 deaths and/or abortions | 201 deaths and/or abortions | 82% less loss in livestock |
| Associated vector composition | Not identified | Anopheles arabiensis, An. stephensi, Aedes aegypti, Ae. vexans, Culex pipiens, Cx. Quinquefasciatus, and Cx. theileri | Implementation of vector species targeted interventions. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





