Submitted:
17 August 2024
Posted:
19 August 2024
You are already at the latest version
Abstract
Keywords:
Introduction
Materials and Methods
Results
Discussion
Conclusions
Funding
Conflicts of Interest
References
- Delluc, A.; Ianotto, J.-C.; Tromeur, C.; De Moreuil, C.; Couturaud, F.; Lacut, K. , et al. Real-world incidence of cancer following a first unprovoked venous thrombosis: Results from the EPIGETBO study. Thrombosis Research. 2018, 164, 79–84. [Google Scholar] [CrossRef]
- White RH, Chew HK, Zhou H, Parikh-Patel A, Harris D, Harvey D, et al. Incidence of Venous Thromboembolism in the Year Before the Diagnosis of Cancer in 528 693 Adults. Archives of Internal Medicine. 2005, 165, 1782. [CrossRef]
- Douketis, J.D.; Gu, C.; Piccioli, A.; Ghirarduzzi, A.; Pengo, V.; Prandoni, P. The long-term risk of cancer in patients with a first episode of venous thromboembolism. J Thromb Haemost. 2009, 7, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Taccone, F.S.; Jeangette, S.M.; Blecic, S.A. First-ever stroke as initial presentation of systemic cancer. J Stroke Cerebrovasc Dis. 2008, 17, 169–174. [Google Scholar] [CrossRef]
- Selvik, H.A.; Bjerkreim, A.T.; Thomassen, L.; Waje-Andreassen, U.; Naess, H.; Kvistad, C.E. When to Screen Ischaemic Stroke Patients for Cancer. Cerebrovasc Dis. 2018, 45, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Quintas S, Rogado J, Gullón P, Pacheco-Barcia V, Dotor García-Soto J, Reig-Roselló G, et al. Predictors of unknown cancer in patients with ischemic stroke. J Neurooncol. 2018, 137, 551–557. [CrossRef]
- Robertson, L.; Yeoh, S.E.; Stansby, G.; Agarwal, R. Effect of testing for cancer on cancer- and venous thromboembolism (VTE)-related mortality and morbidity in people with unprovoked VTE. Cochrane Database Syst Rev. 2017, 8, CD010837. [Google Scholar] [CrossRef]
- Fang J, Wu J, Hong G, Zheng L, Yu L, Liu X, et al. Cancer screening in hospitalized ischemic stroke patients: a multicenter study focused on multiparametric analysis to improve management of occult cancers. EPMA J. 2024, 15, 53–66. [CrossRef]
- Pernod G, Cohen A, Mismetti P, Sanchez O, Mahé I, INNOVTE CAT Working Group. Cancer-related arterial thromboembolic events. Arch Cardiovasc Dis. 2024, 117, 101–113. [CrossRef] [PubMed]
- Martens KL, Li A, La J, May SB, Swinnerton KN, Tosi H, et al. Epidemiology of Cancer-Associated Venous Thromboembolism in Patients With Solid and Hematologic Neoplasms in the Veterans Affairs Health Care System. JAMA Netw Open. 2023, 6, e2317945. [CrossRef]
- Miroddi M, Sterrantino C, Simmonds M, Caridi L, Calapai G, Phillips RS, et al. Systematic review and meta-analysis of the risk of severe and life-threatening thromboembolism in cancer patients receiving anti-EGFR monoclonal antibodies (cetuximab or panitumumab). Intl Journal of Cancer. 2016, 139, 2370–2380. [CrossRef] [PubMed]
- Arnold D, Fuchs CS, Tabernero J, Ohtsu A, Zhu AX, Garon EB, et al. Meta-analysis of individual patient safety data from six randomized, placebo-controlled trials with the antiangiogenic VEGFR2-binding monoclonal antibody ramucirumab. Annals of Oncology. 2017, 28, 2932–2942. [CrossRef]
- Haguet, H.; Douxfils, J.; Mullier, F.; Chatelain, C.; Graux, C.; Dogné, J.-M. Risk of arterial and venous occlusive events in chronic myeloid leukemia patients treated with new generation BCR-ABL tyrosine kinase inhibitors: a systematic review and meta-analysis. Expert Opinion on Drug Safety. 2017, 16, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Grover, S.P.; Hisada, Y.M.; Kasthuri, R.S.; Reeves, B.N.; Mackman, N. Cancer Therapy–Associated Thrombosis. ATVB. 2021, 41, 1291–1305. [Google Scholar] [CrossRef]
- Herrmann J, Lenihan D, Armenian S, Barac A, Blaes A, Cardinale D, et al. Defining cardiovascular toxicities of cancer therapies: an International Cardio-Oncology Society (IC-OS) consensus statement. European Heart Journal. 2021, ehab674.
- Sørensen, H.T.; Pedersen, L.; van Es, N.; Büller, H.R.; Horváth-Puhó, E. Impact of venous thromboembolism on the mortality in patients with cancer: a population-based cohort study. Lancet Reg Health Eur. 2023, 34, 100739. [Google Scholar] [CrossRef]
- Mulder FI, Horváth-Puhó E, van Es N, Pedersen L, Büller HR, Bøtker HE, et al. Arterial Thromboembolism in Cancer Patients: A Danish Population-Based Cohort Study. JACC CardioOncol. 2021, 3, 205–218.
- Hsu, C.; Patell, R.; Zwicker, J.I. The prevalence of thrombocytopenia in patients with acute cancer-associated thrombosis. Blood Adv. 2023, 7, 4721–4727. [Google Scholar] [CrossRef]
- Wang, T.-F.; Leader, A.; Sanfilippo, K.M. Thrombosis and bleeding in hematological malignancy. Best Practice & Research Clinical Haematology. 2022, 35, 101353. [Google Scholar]
- Giustozzi M, Connors JM, Ruperez Blanco AB, Szmit S, Falvo N, Cohen AT, et al. Clinical characteristics and outcomes of incidental venous thromboembolism in cancer patients: Insights from the Caravaggio study. Journal of Thrombosis and Haemostasis. 2021, 19, 2751–2759. [CrossRef]
- Navi BB, Reiner AS, Kamel H, Iadecola C, Okin PM, Elkind MSV, et al. Risk of Arterial Thromboembolism in Patients With Cancer. Journal of the American College of Cardiology. 2017, 70, 926–938. [CrossRef]
- Giustozzi M, Agnelli G, del Toro-Cervera J, Klok FA, Rosovsky RP, Martin A-C, et al. Direct Oral Anticoagulants for the Treatment of Acute Venous Thromboembolism Associated with Cancer: A Systematic Review and Meta-Analysis. Thromb Haemost. 2020, 120, 1128–1136. [CrossRef] [PubMed]
- Falanga A, Leader A, Ambaglio C, Bagoly Z, Castaman G, Elalamy I, et al. EHA Guidelines on Management of Antithrombotic Treatments in Thrombocytopenic Patients With Cancer. HemaSphere. 2022, 6, e750. [CrossRef] [PubMed]
- Bohacekova, M.; Kaldararova, M.; Valkovicova, T.; Remkova, A.; Vesely, J.; Simkova, I. Risk factors detection in chronic thromboembolic pulmonary hypertension, a tool for risk quantification? Bratisl Lek Listy. 2016, 117, 577–582. [Google Scholar] [CrossRef]
- Montani D, Thoré P, Mignard X, Jaïs X, Boucly A, Jevnikar M, et al. Clinical Phenotype and Outcomes of Pulmonary Hypertension Associated with Myeloproliferative Neoplasms: A Population-based Study. Am J Respir Crit Care Med. 2023, 208, 600–612. [CrossRef]
- Ferrari, A.; Scandura, J.; Masciulli, A.; Krichevsky, S.; Gavazzi, A.; Barbui, T. Prevalence and risk factors for Pulmonary Hypertension associated with chronic Myeloproliferative Neoplasms. Eur J Haematol. 2021, 106, 250–259. [Google Scholar] [CrossRef]
- Humbert M, Kovacs G, Hoeper MM, Badagliacca R, Berger RMF, Brida M, et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. European Heart Journal. 2022, 43, 3618–3731. [CrossRef]
- Falanga A, Ay C, Di Nisio M, Gerotziafas G, Jara-Palomares L, Langer F, et al. Venous thromboembolism in cancer patients: ESMO Clinical Practice Guideline. Ann Oncol. 2023, 34, 452–467. [CrossRef] [PubMed]
- Key NS, Khorana AA, Kuderer NM, Bohlke K, Lee AYY, Arcelus JI, et al. Venous Thromboembolism Prophylaxis and Treatment in Patients With Cancer: ASCO Guideline Update. J Clin Oncol. 2023, 41, 3063–3071. [CrossRef]
- Sanfilippo KM, Luo S, Wang T, Fiala M, Schoen M, Wildes TM, et al. Predicting venous thromboembolism in multiple myeloma: development and validation of the IMPEDE VTE score. American J Hematol. 2019, 94, 1176–1184. [CrossRef]
- Khorana, A.A.; Kuderer, N.M.; Culakova, E.; Lyman, G.H.; Francis, C.W. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008, 111, 4902–4907. [Google Scholar] [CrossRef]
- Khorana AA, Soff GA, Kakkar AK, Vadhan-Raj S, Riess H, Wun T, et al. Rivaroxaban for Thromboprophylaxis in High-Risk Ambulatory Patients with Cancer. N Engl J Med. 2019, 380, 720–728. [CrossRef] [PubMed]
- Carrier M, Abou-Nassar K, Mallick R, Tagalakis V, Shivakumar S, Schattner A, et al. Apixaban to Prevent Venous Thromboembolism in Patients with Cancer. N Engl J Med. 2019, 380, 711–719. [CrossRef]
- Paterno G, Palmieri R, Forte V, Del Prete V, Gurnari C, Guarnera L, et al. Predictors of Early Thrombotic Events in Adult Patients with Acute Myeloid Leukemia: A Real-World Experience. Cancers. 2022, 14, 5640. [CrossRef]
- Lyman, G.H.; Eckert, L.; Wang, Y.; Wang, H.; Cohen, A. Venous Thromboembolism Risk in Patients With Cancer Receiving Chemotherapy: A Real-World Analysis. The Oncologist. 2013, 18, 1321–1329. [Google Scholar] [CrossRef]
- Libourel EJ, Klerk CPW, van Norden Y, de Maat MPM, Kruip MJ, Sonneveld P, et al. Disseminated intravascular coagulation at diagnosis is a strong predictor for thrombosis in acute myeloid leukemia. Blood. 2016, 128, 1854–1861. [CrossRef]
- Khorana, A.A. Venous thromboembolism and prognosis in cancer. Thrombosis Research. 2010, 125, 490–493. [Google Scholar] [CrossRef]
- Ku, G.H.; White, R.H.; Chew, H.K.; Harvey, D.J.; Zhou, H.; Wun, T. Venous thromboembolism in patients with acute leukemia: incidence, risk factors, and effect on survival. Blood. 2009, 113, 3911–3917. [Google Scholar] [CrossRef]
- Faiz, A.S.; Sridharan, A.; Guo, S.; Lin, Y.; Philipp, C.S. Risk factors and mortality associated with venous thromboembolism in the elderly US population with acute lymphocytic leukemia. Thrombosis Update. 2024, 14, 100155. [Google Scholar] [CrossRef]
- Johannesdottir SA, Horváth-Puhó E, Dekkers OM, Cannegieter SC, Jørgensen JOL, Ehrenstein V, et al. Use of Glucocorticoids and Risk of Venous Thromboembolism: A Nationwide Population-Based Case-Control Study. JAMA Intern Med. 2013, 173, 743. [CrossRef]
- Orsi FA, Lijfering WM, Geersing G, Rosendaal FR, Dekkers OM, Le Cessie S, et al. Glucocorticoid use and risk of first and recurrent venous thromboembolism: self-controlled case-series and cohort study. Br J Haematol. 2021, 193, 1194–1202. [CrossRef] [PubMed]
- Raskob GE, van Es N, Verhamme P, Carrier M, Di Nisio M, Garcia D, et al. Edoxaban for the Treatment of Cancer-Associated Venous Thromboembolism. New England Journal of Medicine. 2018, 378, 615–624. [CrossRef]
- Agnelli G, Becattini C, Meyer G, Muñoz A, Huisman MV, Connors JM, et al. Apixaban for the Treatment of Venous Thromboembolism Associated with Cancer. N Engl J Med. 2020, 382, 1599–1607. [CrossRef] [PubMed]
- Lyon AR, López-Fernández T, Couch LS, Asteggiano R, Aznar MC, Bergler-Klein J, et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). European Heart Journal. 2022, ehac244.
- Giustozzi M, Curcio A, Weijs B, Field TS, Sudikas S, Katholing A, et al. Variation in the Association between Antineoplastic Therapies and Venous Thromboembolism in Patients with Active Cancer. Thromb Haemost. 2020, 120, 847–856. [CrossRef]
- Herrmann, J. Vascular toxic effects of cancer therapies. Nature Reviews Cardiology. 2020, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Navi BB, Reiner AS, Kamel H, Iadecola C, Okin PM, Elkind MSV, et al. Risk of Arterial Thromboembolism in Patients With Cancer. Journal of the American College of Cardiology. 2017, 70, 926–938.
- Verso M, Agnelli G, Munoz A, Connors JM, Sanchez O, Huisman M, et al. Recurrent venous thromboembolism and major bleeding in patients with localised, locally advanced or metastatic cancer: an analysis of the Caravaggio study. Eur J Cancer. 2022, 165, 136–145. [CrossRef] [PubMed]
- Feldman S, Gupta D, Navi BB, Grace Ho K-W, Willeit P, Devlin S, et al. Tumor Genomic Profile Is Associated With Arterial Thromboembolism Risk in Patients With Solid Cancer. JACC CardioOncol. 2023, 5, 246–255. [CrossRef]
- Moik F, Chan W-SE, Wiedemann S, Hoeller C, Tuchmann F, Aretin M-B, et al. Incidence, risk factors, and outcomes of venous and arterial thromboembolism in immune checkpoint inhibitor therapy. Blood. 2021, 137, 1669–1678. [CrossRef]
- Mulder FI, Horváth-Puhó E, van Es N, van Laarhoven HWM, Pedersen L, Moik F, et al. Venous thromboembolism in cancer patients: a population-based cohort study. Blood. 2021, 137, 1959–1969. [CrossRef]
- Wang, J.; Kim, Y.D.; Kim, C.H. Incidence and Risk of Various Types of Arterial Thromboembolism in Patients With Cancer. Mayo Clinic Proceedings. 2021, 96, 592–600. [Google Scholar] [CrossRef]
- Aboumsallem, J.P.; Moslehi, J.; de Boer, R.A. Reverse Cardio-Oncology: Cancer Development in Patients With Cardiovascular Disease. J Am Heart Assoc. 2020, 9, e013754. [Google Scholar] [CrossRef] [PubMed]
- Imran S, Rao MS, Shah MH, Gaur A, Guernaoui AE, Roy S, et al. Evolving perspectives in reverse cardio-oncology: A review of current status, pathophysiological insights, and future directives. Curr Probl Cardiol. 2024, 49, 102389. [CrossRef]
- Liang, Z.; He, Y.; Hu, X. Cardio-Oncology: Mechanisms, Drug Combinations, and Reverse Cardio-Oncology. Int J Mol Sci. 2022, 23, 10617. [Google Scholar] [CrossRef]
- Sinha A, Bavishi A, Hibler EA, Yang EH, Parashar S, Okwuosa T, et al. Interconnected Clinical and Social Risk Factors in Breast Cancer and Heart Failure. Front Cardiovasc Med. 2022, 9, 847975. [CrossRef] [PubMed]
- Di Fusco SA, Cianfrocca C, Bisceglia I, Spinelli A, Alonzo A, Mocini E, et al. Potential pathophysiologic mechanisms underlying the inherent risk of cancer in patients with atherosclerotic cardiovascular disease. Int J Cardiol. 2022, 363, 190–195. [CrossRef]
- Koelwyn, G.J.; Aboumsallem, J.P.; Moore, K.J.; de Boer, R.A. Reverse cardio-oncology: Exploring the effects of cardiovascular disease on cancer pathogenesis. J Mol Cell Cardiol. 2022, 163, 1–8. [Google Scholar] [CrossRef] [PubMed]
- de Wit S, de Boer RA. From Studying Heart Disease and Cancer Simultaneously to Reverse Cardio-Oncology. Circulation. 2021, 144, 93–95. [CrossRef] [PubMed]
- Lyon AR, Dent S, Stanway S, Earl H, Brezden-Masley C, Cohen-Solal A, et al. Baseline cardiovascular risk assessment in cancer patients scheduled to receive cardiotoxic cancer therapies: a position statement and new risk assessment tools from the C ardio- O ncology S tudy G roup of the H eart F ailure A ssociation of the E uropean S ociety of C ardiology in collaboration with the I nternational C ardio- O ncology S ociety. Eur J Heart Fail. 2020, 22, 1945–1960.
| Number | Percentage | ||
|---|---|---|---|
| Sex | men | 673 | 52.46 |
| Older age | ≥ 70 years | 390 | 30.4 |
| Hematological malignancies | AML - acute myeloid leukemia | 270 | 21.04 |
| NHL - non-Hodgkin's lymphoma | 250 | 19.49 | |
| MM - multiple myeloma | 223 | 17,38 | |
| CLL - chronic lymphocytic leukemia | 101 | 7.87 | |
| ALL - acute lymphocytic leukemia | 76 | 5.92 | |
| MDS - myelodysplastic syndrome | 70 | 5.46 | |
| HL - Hodgkin lymphoma | 56 | 4.36 | |
| CML - chronic myeloid leukemia | 51 | 3.98 | |
| Chronic myeloproliferative disease | 48 | 3.74 | |
| Other rarer (together) | 138 | ||
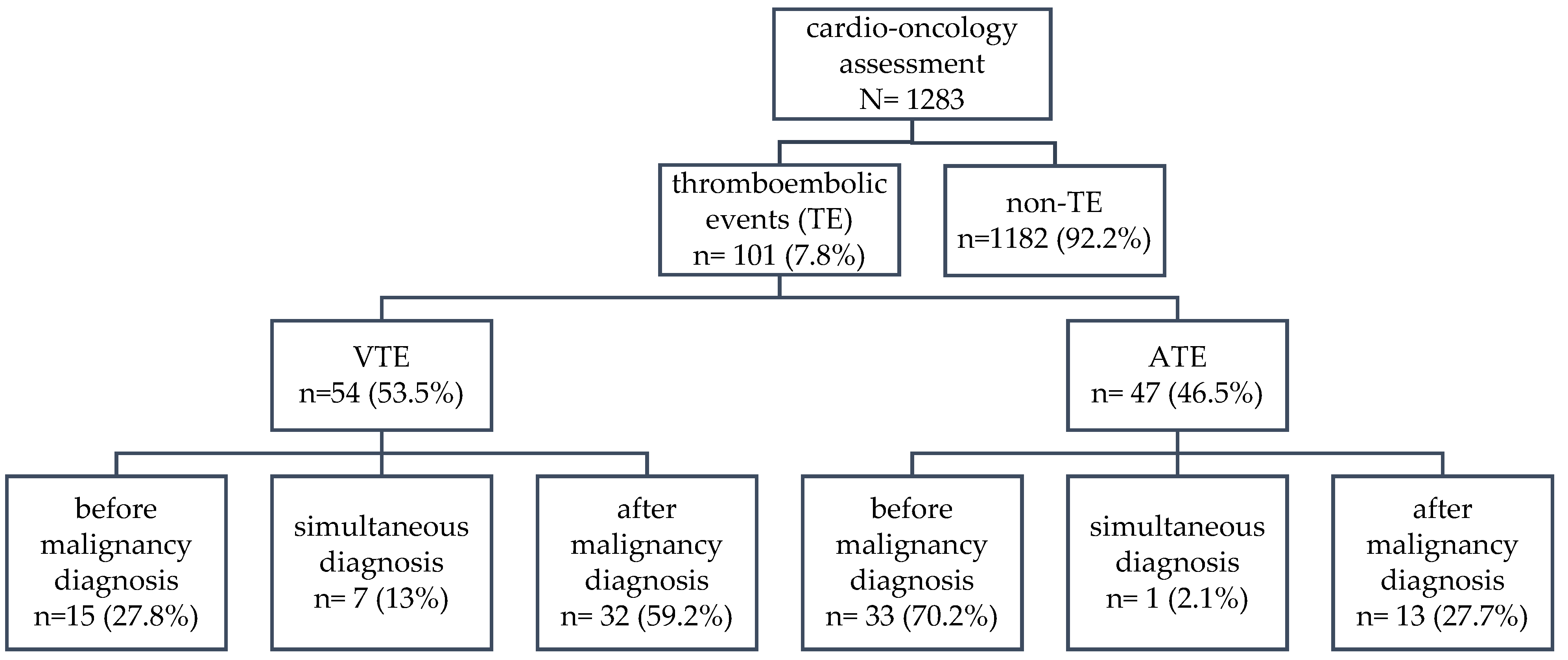
| Thromboembolism | All | 101 | 7.87 |
| VTE - venous thromboembolism | 54 | 4.21 | |
| ATE - arterial thromboembolism | 47 | 3.66 | |
| Coexisting diseases | Hypertension | 521 | 40.61 |
| Arrhythmia | 203 | 15.82 | |
| AF - atrial fibrillation | 105 | 8.18 | |
| HF - heart failure | 142 | 11.07 | |
| IHD - ischemic heart disease | 107 | 8.34 | |
| DM - diabetes melitus | 128 | 9.98 |
| VTE | ATE | |||
|---|---|---|---|---|
| frequency | p-value | frequency | p-value | |
| All | 54 of 1283 4.21% |
- | 47 of 1283 3.66% |
- |
| AML- acute myeloid leukemia | 17 of 270 6.30% |
0.055 | 8 of 270 2.96% |
0.491 |
| NHL- non-Hodgkin's lymphoma | 7 of 250 2.80% |
0.216 | 6 of 250 2.40% |
0.236 |
| MM- multiple myeloma | 10 of 223 4.48% |
0.822 | 11 of 223 4.93% |
0.267 |
| CLL- chronic lymphocytic leukemia | 3 of 101 2.97% |
0.698* | 6 of 101 5.94% |
0.204 |
| ALL- acute lymphocytic leukemia | 7 of 76 9.21% |
0.025 | 2 of 76 2.63% |
0.858* |
| MDS- myelodysplastic syndrome | 1 of 70 1.43% |
0.376* | 3 of 70 4.29% |
0.966* |
| HL- Hodgkin lymphoma | 0 of 56 0% |
0.206* | 1 of 56 1.79% |
0.688* |
| CML- chronic myeloid leukemia | 0 of 51 0% |
0.241* | 4 of 51 7.84% |
0.215* |
| Chronic myeloproliferative disease | 7 of 48 14.58% |
0.0003 | 4 of 48 8.33% |
0.173* |
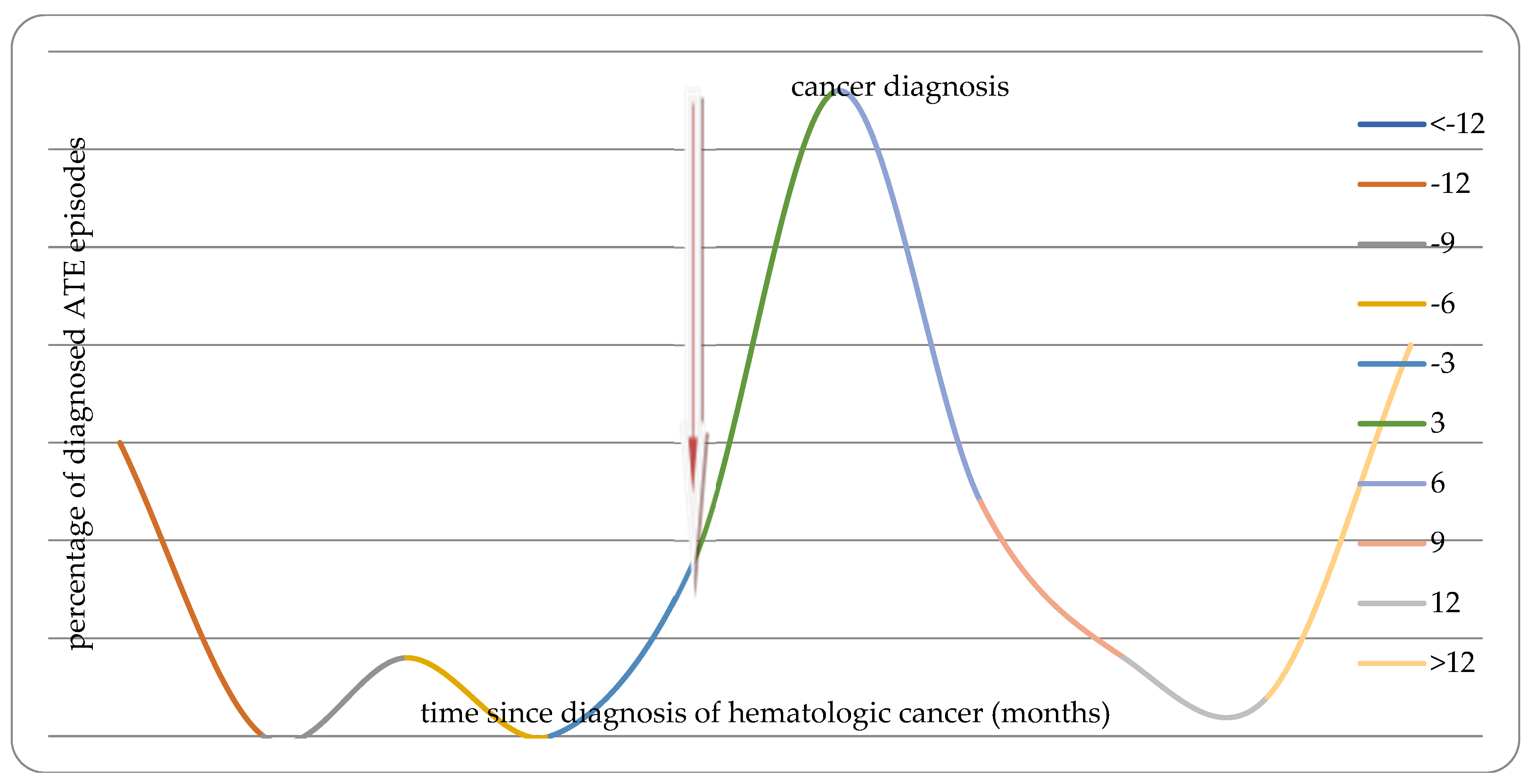
| All ATE | ATE before diagnosis of malignancy | ATE after diagnosis of malignancy | |
|---|---|---|---|
| Older age (≥70 y) | OR=3.55 (1.96-6.44) p=0.00003 |
OR=4.17 (2.03-8.57) p=0.0001 |
NS |
| Hypertension | OR=4.5 (2.31-8.76) p<0.00001 |
OR=5.67 (2.44-13.16) p=0.00005 |
NS |
| Arrhythmia | OR=2.61 (1.39-4.92) p=0.003 |
OR=3.17 (1.53-6.55) p=0.002 |
NS |
| Atrial Fibrillation | NS | NS | NS |
| Heart Failure | OR=3.28 (1.69-6.38) p=0.0004 |
OR=4.92 (2.37-10.24) p=0.00002 |
NS |
| Ischemic Heart Disease | OR=83.74 (37.63-186.32) p<0.00001 |
OR=108.94 (37.32-317.98) p<0.00001 |
OR=30.21 (9.29-98.2) p<0.00001 |
| Diabetes Mellitus | NS | NS | NS |
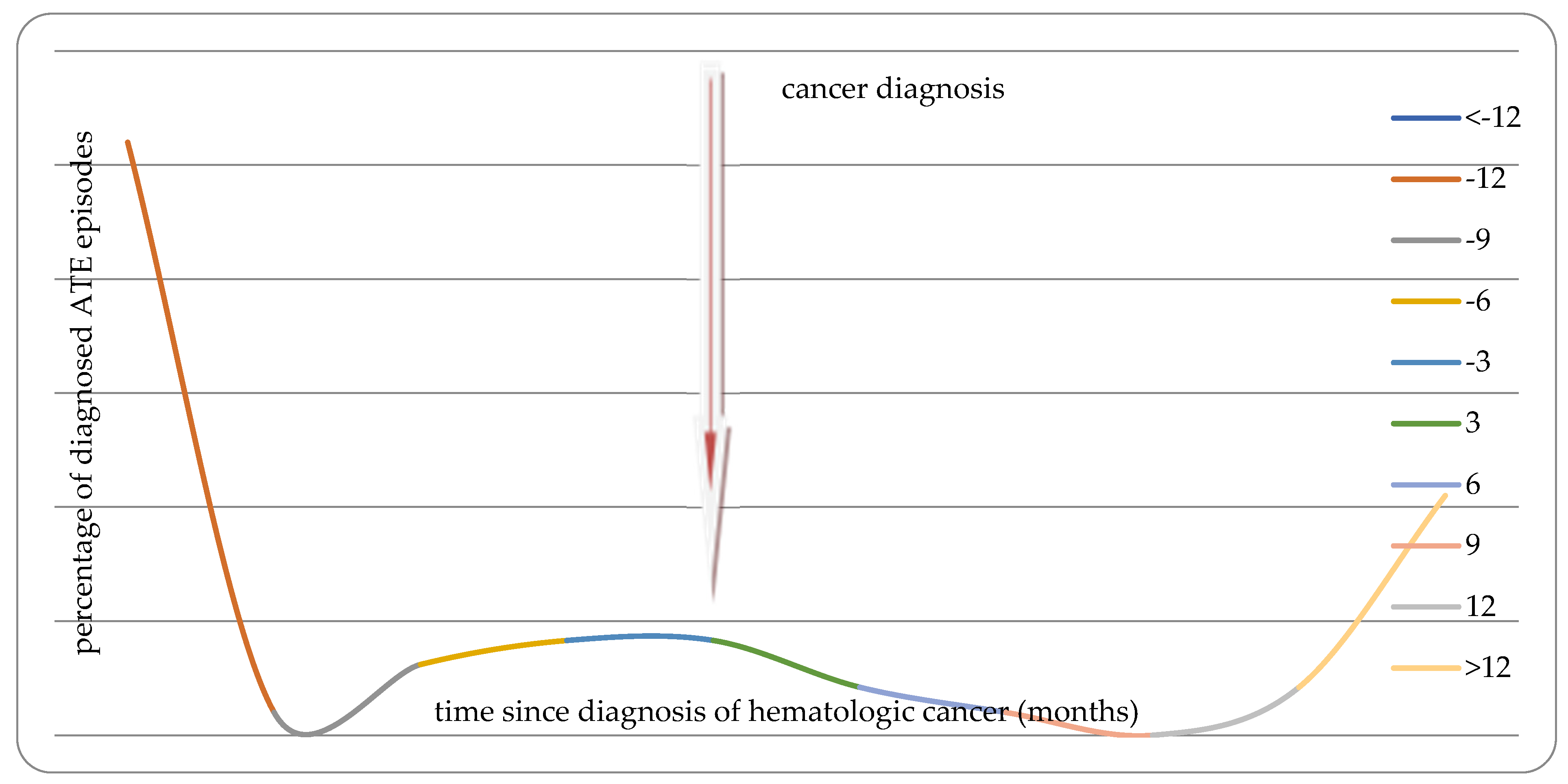
| All VTE | VTE before diagnosis of malignancy | VTE after diagnosis of malignancy | |
|---|---|---|---|
| AML- acute myeloid leukemia | NS | NS | NS |
| ALL- acute lymphocytic leukemia | OR=2.5 (1.09-5.75) p=0.03 |
NS | OR=3.05 (1.24-7.53) p=0.015 |
| Chronic myeloproliferative disease | OR=4.32 (1.84-10.13) p=0.0008 |
OR=6.79 (1.85-24.95) p=0.004 |
OR=3.12 (1.06-9.16) p=0.04 |
| Older age (≥70 y) | NS | NS | NS |
| Hypertension | NS | NS | NS |
| Arrhythmia | NS | NS | NS |
| Atrial Fibrillation | NS | NS | NS |
| Heart Failure | NS | NS | NS |
| Ischemic Heart Disease | NS | NS | NS |
| Diabetes Mellitus | OR=2.43 (1.22-4.85) p=0.01 |
NS | OR=2.42 (1.09-5.38) p=0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





