1. Introduction
The neurotransmitter dopamine (DA) is well known for its role within the central nervous system (CNS) where it modulates movement, cognition, and reward [
1]. Over the past decades, the focus has expanded to elucidate DA’s effects also on the peripheral immune system, especially how it modulates diverse immune cell functions in humans. The initial evidence for DA as an immune regulator emerged from the discovery of dopamine receptors (DR) and other proteins involved in the dopaminergic pathway in immune cells [
2,
3,
4], as well as in their ability to produce, store and metabolize DA [
5,
6,
7].
DA can bind two types of DRs, D
1-like (DRD
1 and DRD
5) and D
2-like receptors (DRD
2, DRD
3, and DRD
4). The D
1-like family typically activates downstream signaling pathways in the brain including an increase in cAMP levels, whereas binding of D
2-like receptors inhibits this signaling [
8,
9]. Besides cAMP-involved signaling, there are also other DR pathways in the brain including for example β-arrestin 2 and PI3K-Akt [
10,
11]. It is suggested, that the dopaminergic pathways in peripheral immune cells may differ from the ones in the brain and are not fully discovered yet [
1,
12].
The effects of DA on peripheral immune cells are diverse and very context-dependent, ranging from suppression to activation. For example, while DA activates resting effector T cells, it inhibits them once they are activated [
13]. Of note, most studies have focused on T cells, describing DA’s effects on differentiation [
14], migration [
15,
16], cytokine secretion [
17] and proliferation [
18], leaving the effects on other peripheral blood mononuclear cell (PBMC) subsets like B cells or monocytes less explored. The rare studies about B cells report increased activation marker expression [
3] but contrary effects on proliferation due to DR stimulation [
3,
19,
20]. As a modulator of monocytes, DA increased their migration and adhesion secretion [
21,
22], while it decreased LPS-induced proliferation [
23]. Human monocyte-derived macrophages showed a DA-induced increase in IL6 and MCP1 with and without LPS stimulation [
24], whereas in murine macrophages and monocytes, a suppression of LPS-induced IL12p40 production and TNF release was observed [
25,
26]. Interestingly, interactions between different immune cell subsets appear to influence DA-induced immune responses [
27,
28,
29]. For example, the interplay of T
FH cells and B cells enhances germinal center output [
28], while dopamine released by monocyte-derived dendritic cells can influence T cell differentiation [
29].
Concluding, the complexity of DA’s effects is highlighted by partly controversial findings, influenced by factors such as addressed immune cell type, secondary effects on other immune cells, DA concentration, and inflammatory state [
13] that vary throughout different studies and thus make comparisons difficult. Detailed investigations into DA-induced changes in activation status including activation marker expression as well as cytokine secretion in a culture of mixed PBMCs, also taking secondary effects into account, remain limited.
One reason for the diverse and partly contradictory findings could be the influence of sex, which is rarely considered in studies examining DA’s function on peripheral immune cells. In the brain, it is already suggested that the neurotransmitter can act in a sex-specific manner [
30]. Evidence that sex hormones can influence the dopaminergic pathway is seen in a change of DRD
1 density in the brain throughout the female cycle in rats [
31]. Furthermore, male rats showed higher DRD
1 density in the striatum than females [
32]. Also in the periphery, healthy men exhibited increased DRD
1 expression on B cells compared to women [
3]. Contrary to this, chronic treatment with estradiol increased and ovariectomy decreased striatal DRD
1 in rats [
32]. Interestingly, Lee et al. have reported a half palindromic estrogen responsive element on the promotor of DRD
1 [
33] giving a potential epigenetic reason for a connection between DR pathway and sex hormones. Nevertheless, the impact of sex on the dopaminergic pathway has been little studied yet, especially in the periphery.
Beyond sex, inflammatory conditions also significantly impact DA’s mode of action. Dysregulated DA signaling is associated with various diseases, ranging from CNS-related pathologies with an involvement of the peripheral immune system, like multiple sclerosis and Parkinsons disease, to autoimmune diseases, like systemic lupus erythematosus and rheumatoid arthritis (RA) or viral infections like with Human Immunodeficiency Virus (HIV). In Parkinson’s disease, a deficit in DA is characteristic [
1,
34,
35], which is caused by degeneration of dopaminergic neurons due to chronic inflammation in the brain, partly a result of the recruitment of peripheral immune cells [
7,
35,
36]. A strong association with dopaminergic dysfunction is also shown for HIV, including fluctuations in DA level in the brain throughout the disease progression [
37] as well as a DA-induced increase of virus entry into macrophages [
38]. In RA or appropriate animal models, DR expression on diverse immune cells is correlated with disease activity [
3,
39,
40,
41] and DR stimulation affects cytokine secretion, leading to Th17/Treg imbalance and reduced ROS production [
41,
42]. In a previous study, we investigated the influence of sex and pathological conditions by stimulating the D
1-like pathway in PBMCs from female and male healthy donors and RA patients and found a sex-specific increase in IL8 and CCL3 secretion after D
1-like stimulation only for RA women compared to healthy women but not for men [
3].
These findings underline that DA signaling and its dysfunction can impact many pathological processes with the potential to appear differently in women and men. Thus, it is of high clinical relevance to understand DA’s effects in more depth with a special focus on sex-specific differences.
Our in vitro study addresses the impact of dopaminergic stimulation on PBMCs under physiological as well as inflammatory conditions, with a detailed focus on cytokine secretion and expression of activation markers on monocytes, but also B cells and T cells from women and men. We found a DA-induced anti-inflammatory phenotype of monocytes under physiological conditions only for women, while DR stimulation supported proinflammatory effects when applied together with CpG, in both sexes but stronger pronounced in women. Under physiological conditions, these effects on monocytes were B cell-driven, whereas inflammation increased the activation of monocytes, thus probably enabling the response to DR agonists without B cell help.
2. Materials and Methods
Healthy subjects and blood donation: 26 healthy women and 21 healthy men participated in this study and gave signed consent. Exclusion criteria were acute or previous hepatitis B or HIV infection, cancer diagnosis and diseases affecting the lung, liver, lymphatic-, cardiovascular-, or nervous system. The recruited female subjects completed a questionnaire regarding their use of hormonal contraception. For blood sampling, lithium heparin collection tubes were used, and the collected blood was processed within 1 h after sampling. The age range of all included participants is 21 – 72 years (average age is 39.2 years for women and 40.2 years for men). Used samples of women and men were age matched for each experiment.
Isolation of PBMCs and stimulation of cells: After blood sampling, peripheral blood mononuclear cells (PBMCs) were separated from other components of the blood via density gradient centrifugation with Pancoll™ (PAN, #P04-60500). For storage, cells were frozen in fetal bovine serum (FBS, Gibco, #10270-106) supplemented with 10% dimethyl sulfoxide (Merck, #472301-1L-M). After thawing, cells were washed with medium (RPMI 1640 medium (PAN, #P04-16515) with 10% FBS, 1% Penicillin-Streptomycin (Gibco, #15140-122), 1% sodium pyruvate (Gibco, #11360-070), 1% MEM non-essential aminoacids (Gibco, #11140-035), 0.1% HEPES (Cytiva, #SH30237.01), 1.918 µl β-mercaptoethanol (Carl Roth, #4227.3)). For stimulation, 200,000 cells per condition were seeded in 100 µl of medium into 96-well round bottom plates, and placed at 37°C, 5% CO2 for two hours, before 100 µl of respective stimulants were added.
Reagents used for stimulation of cells: For D
1-like stimulation, A68930 hydrochloride (Tocris, #1534) 10
-7, 10
-8 or 10
-9 M was added to the cells as indicated in the respective Figure Legends. For D
2-like stimulation DR agonist Ropinirole (Tocris, #3680) was used at concentrations of 10
-7, 10
-8 or 10
-9 M. An acute inflammatory stimulation was induced by the simultaneous addition of 0.195 µM CpG ODN2006 (Invivogen, #tlrl-2006) binding TLR9 as previously shown [
39]. For stimulation of sex hormone receptors, 10
-8, 10
-9 or 10
-10 M β-Estradiol (E2, Tocris, #2824/100) or 10
-7, 10
-8 or 10
-9 M 5α-Androstan-17β-ol-3-on (DHT, Sigma Aldrich, #10300-1G-F) was used.
Depletion of CD19+ B cells and CD14+ monocytes: To deplete CD19+ B cells or CD14+ monocytes, PBMCs were incubated with FBS, purified anti-human CD19 antibody (Biolegend, #302202) or purified anti-human CD14 antibody (Biolegend, #399202) and Mouse IgM (BD Pharmingen™, #555583) in depletion buffer (DPBS (Gibco, #14190-094), containing 2 mM EDTA (Carl Roth, #8043.2) and 2% FBS, sterile filtered) for 20 min at 4 °C. Dynabeads™ Pan Mouse IgG (Invitrogen, #11041) were magnetically separated for 1 min and washed with depletion buffer. After incubation, PBMCs were washed, beads were added to the cell suspension, and mixed under rotation for 15 min at RT. The tube containing the cell suspension was placed into the Dynal MPC®-15 magnet (Dynal Biotech, #120.29) for 3 min. The cells that were not attached to the beads were collected. The beads were resuspended in depletion buffer and separated a second time. The obtained depleted PBMC fractions were placed again into the magnet for separation, followed by counting of the cells with CASY counter. The efficacy of depletion was analyzed by staining for CD19 or CD14 via flow cytometry. Samples were used only if the remaining proportion of the depleted subtype was less than 1%. A detailed version of this protocol can be found in supplementary material.
Quantification of secreted cytokines: ELISA and multiplex assay: To quantify secreted IL8 and MCP1 in the supernatant of complete PBMCs and Monocyte- or B cell-depleted PBMCs, ELISA was performed: ELISA MAX™ Standard Set Human IL-8 (Biolegend, #431501) and ELISA MAX™ Standard Set Human MCP-1/CCL2 (Biolegend, #438807). The assays were performed as described in the guideline of the kits, except for the downscaling of all volumes by a factor of two. For quantification of IL1β and IL18 in the supernatant of complete PBMCs and monocyte-depleted PBMCs, LEGENDplex™ Human Inflammation Panel 1 (Biolegend, #740809) was used. The measurement was performed with undiluted samples and the protocol was followed as described in the kit guidelines but with each volume reduced by a factor of three. The samples were measured using BD LSRFortessa™ flow cytometer.
Quantification of sex hormones: Within 1 h after blood donation, blood of healthy donors was centrifuged, and plasma was frozen at -80°C for later analysis of basal levels of sex hormones using Testosterone Parameter Assay Kit (R&D, #KGE010) and 17β-Estradiol high sensitivity ELISA kit (Enzo Life Sciences, #ADI-900-174). Plasma was diluted 1:6 for measurement of estradiol and 1:10 for measurement of testosterone.
Flow cytometry staining of dopamine receptors, sex hormone receptors and activation markers including Annexin V: After stimulation, supernatant of PBMCs or depleted fractions was stored at -80°C for following cytokine analysis. Cultured or freshly thawed cells were washed with PBS. To stain for dead cells, they were incubated with Zombie UV™ Fixable Viability Dye (1:1000, Biolegend, #423108) in PBS for 20 min at 4°C in the dark and washed afterwards. PBS containing 2% Albumin Fraction V (Carl Roth, #0163.4) was added to each condition to block unspecific binding sites for 20 min at 4°C in the dark. Staining of extracellular markers in staining buffer (PBS supplemented with 2% FBS) followed for 20 min at 4°C. After washing, cells, that were previously stained with activation marker panel, were incubated with FITC Annexin V (1:1600, Biolegend, #640906) in Annexin V Binding Buffer (Biolegend, #422201) for 15 min at RT in the dark. Cells, that were previously stained with dopamine receptor panel or sex hormone receptor panel, were incubated with staining buffer containing 2% paraformaldehyde (Aldrich, #16005) for 10 min at RT in the dark and washed again. For permeabilization, FACS™ Permeabilizing Solution 2 (diluted 1:10 with dH2O, BD, #340973) was added and incubated for 10 min at RT in the dark. Cells were washed, unspecific binding sites were blocked, and intracellular antigens were stained. After washing, cells were measured with the Cytek® Aurora (5 Lasers) flow cytometer. A detailed version of this protocol including antibody panels can be found in supplementary material.
Intracellular cytokine measurement via flow cytometry: After thawing and stimulation of PBMCs, Brefeldin A (1:1000, Sigma Aldrich, #B7651-5MG) was added to the cell culture medium directly with applied stimulation reagents or 18 h after initial stimulation. Staining of dead cells, blocking of unspecific binding sites and staining of extracellular markers was conducted as described in 2.7. Afterwards, PBMCs were fixed with 2% paraformaldehyde, permeabilized with FACS™ Permeabilizing Solution 2, and unspecific binding sites were blocked with 2% Albumin Fraction V as described in 2.7. Intracellular cytokines were stained for 20 min at RT in the dark. After washing, samples were measured at the Cytek® Aurora (5 Lasers) flow cytometer. A detailed version of this protocol including antibody panels can be found in supplementary material.
Software and statistical analysis: FlowJo (Version 10.3) was used to analyze flow cytometry data, and Prism 9 (GraphPad Software, v 9.2.0) for statistical analysis. For illustration, Prism 9 (GraphPad Software, v 9.2.0) and
BioRender.com (2020) were utilized. Each figure legend mentions the statistical analyses that were used for every experiment. Single measurements were performed in flow cytometry experiments, duplicates in ELISA and multiplex assay. Each graph represents the mean with standard error.
3. Results
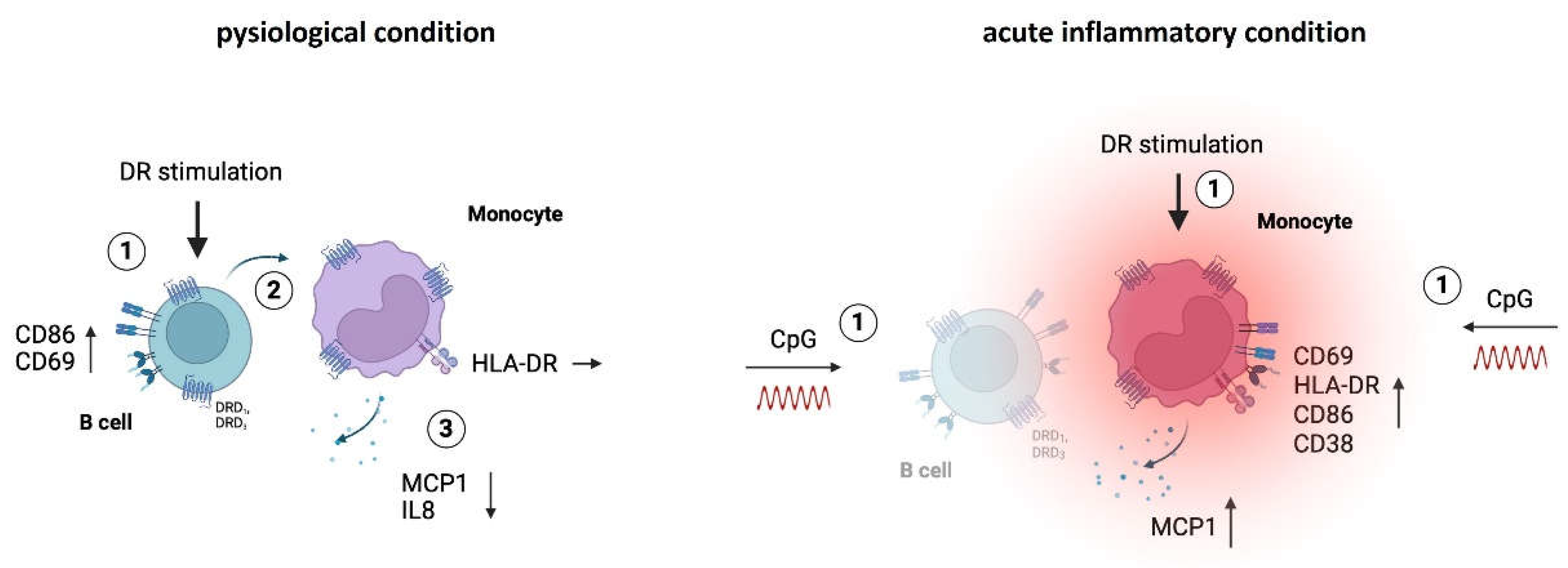
3.1. Cytokine Secretion of Monocytes Is Reduced by DR Stimulation Only in Women
Cytokine secretion is an important immune modulating property since it leads to the regulation of inflammatory responses and the coordination of cellular communication within the immune system [
43,
44]. Consequently, we first focused on whether stimulation with either D
1-like agonist A68930 or D
2-like agonist Ropinirole can influence the cytokine secretion of PBMCs from healthy women and men under physiological conditions
in vitro. Our results indicated that PBMCs from both women and men did not secrete high levels of cytokines either without stimulation or after stimulation with dopaminergic substances for 24 h (Legendplex data including 13 cytokines). The only cytokines detected at measurable levels under physiological conditions were IL8 and MCP1 (
Figure 1A and B), whereas all other cytokines were too low for reliable detection (data not shown). Notably, women exhibited a tendency towards higher secretion of these cytokines compared to men without any stimulation (
Figure 1A and B). Upon D
1- and D
2-like stimulation with varying concentrations of DR agonists, there was a significant decrease in IL8 and MCP1 secretion by PBMCs from women, while this was not observed in men, who instead showed a tendency towards upregulation of these cytokines (
Figure 1A and B,
Supplementary Figure A and B).
Previously, we already showed that DRD
1, DRD
2, DRD
3 and DRD
4, but not DRD
5 are expressed on all PBMC subsets, namely B cells, T cells, NK cells, and monocytes [
3]. Thus, we assumed that all of these cell types could respond to dopaminergic stimulation and be responsible for the observed changes in cytokine secretion. To identify the concrete subset as a source for these changes among them, we performed flow cytometry to stain for intracellular IL8 and MCP1 and found out that the monocytes exclusively produced IL8 and MCP1 in cell culture without additional stimulus (
Figure 1C and E). Furthermore, DR stimulation did not induce IL8 or MCP1 production in B cells, T cells or NK cells (
Supplementary Figure S1D and E), reinforcing the role of monocytes as the primary producers of these cytokines. This finding presents DA as a possible influencing factor for monocytes’ proper functioning.
Additionally, we investigated whether DR stimulation affected the activation status of monocytes, as indicated by the percentage of CD69
+ monocytes and the expression of activation markers HLA-DR, CD86, and CD38. While we did not observe a significant impact of dopaminergic stimulation on CD69-expressing monocytes (
Figure 1G) or on the expression of CD86 and CD38 (
Supplementary Figure S1F and G) in both sexes, D
2-like stimulation resulted in an upregulation of HLA-DR on monocytes from men but not on monocytes from women (
Figure 1H).
These findings underscore the impact of sex on the effects of dopaminergic stimulation especially on cytokine secretion of monocytes, further confirming a sex-specific interplay between DA and immune functions.
3.2. B Cells from Male Subjects Have a Higher Expression Level of DRD1 and DRD3 than B Cells from Women
Based on the above-shown results regarding sex-specific effects of DR agonists on cytokine secretion, we hypothesized that these differences could be attributed to sex-specific differences of DR expression on PBMCs. To test this hypothesis, we analyzed the expression patterns of DRs via flow cytometry. All DRs except DRD
5 were found on all PBMC subsets (data not shown). Consistent with a previous study [
3], we did not observe significant differences in the expression of DRs on monocytes between women and men (
Figure 2A). However, higher expression levels of DRD
1 and DRD
3, but not DRD
2 and DRD
4 were found on B cells from men compared to women (
Figure 2B).
Other studies have already reported that the expression of DRs seems to correlate with the level of sex hormones [
31,
32,
33]. To determine whether the observed sex-specific differences in DR expression on B cells were influenced by sex hormones, we stimulated PBMCs with 17β-estradiol (E2) and dihydrotestosterone (DHT) and looked for a change in DRD
1 and DRD
3 expression on B cells afterwards. We found an upregulation of DRD
1 on male but not female B cells (
Figure 2C and G), however, not for DRD
3 (
Figure 2E and I). This upregulation observed only in men is not related to a higher amount of sex hormone receptors on male B cells, since our flow cytometry analysis did not reveal differences in the expression levels of estrogen receptors ERα, ERβ, and GPR30, as well as androgen receptor AR between both sexes (
Supplementary Figure S2A-D).
Next, we measured 17β-estradiol and testosterone in the plasma to test for a possible correlation with DR expression. Surprisingly, we found comparable levels of estradiol in plasma from both women and men in our cohort (
Supplementary Figure S2E). This finding can be partly explained by the lower estradiol levels in women taking hormonal contraceptives (26% of total cohort) compared to those without (
Supplementary Figure S2F). As expected, women in our cohort exhibited significantly lower testosterone levels compared to men (
Supplementary Figure S2G). The direct effect, that was observed after sex hormone stimulation on DRD
1 expression, could not be confirmed in correlation analyses between E2 or testosterone levels with DR expression on B cells (
Figure 2D, F, H and J), underscoring that the level of sex hormones in plasma cannot be used as a predictor for DR expression on immune cells.
In summary, our findings suggest that the sex-specific differences in DR expression on B cells are only partly influenced by sex hormones.
3.3. DR Stimulation Led to a Slight but Women-Specific Increase in B Cell Activation
Since we observed differently expressed DRD
1 and DRD
3 on B cells from women and men, we next investigated whether dopaminergic stimulation influences activation markers on B cells. Interestingly, D
2-like stimulation significantly increased CD86 on B cells from women but not men, and the same tendency was observed for D
1-like stimulation (
Figure 3A). A similar trend was observed for CD69 (
Supplementary Figure S3A), suggesting a higher activated phenotype for B cells from women upon dopaminergic stimulation. In contrast, HLA-DR was slightly upregulated in both sexes, indicating a not-sex-specific effect (
Supplementary Figure S3B).
To test whether the increased activation of B cells was due to indirect effects mediated by monocytes, we depleted monocytes from the cell culture (
Figure 3B) and examined the effects of dopaminergic stimulation. D
2-like stimulation still caused an upregulation in CD86 expression exclusively in women (
Figure 3C) as observed for B cells in mixed PBMCs, thus excluding a possible secondary effect mediated through monocytes.
Considering that DR expression in B cells has been reported to correlate with different stages of B cell development [
3], we analyzed whether the observed differences in CD86 regulation could be attributed to specific B cell subsets. Thus, we stained for IgD
+CD27
- naïve B cells, IgD
+CD27
+ marginal zone-like B cells and IgD
-CD27
+ switched-memory B cells (
Figure 3D). All three B cell subsets showed an increase in CD86 expression exclusively in women following dopaminergic stimulation (
Figure 3E).
These findings provide strong evidence for sex-specific effects of dopaminergic stimulation on B cells.
3.4. B Cells Contributed to the Sex-Specific Cytokine Decrease of Female Monocytes after DR Stimulation
Next, we investigated whether the presence of B cells influences the downregulated cytokine secretion by monocytes observed exclusively for women. To explore this, we depleted B cells from the culture of mixed PBMCs (
Figure 4A) and measured the levels of IL8 and MCP1 in the supernatant from B cell-depleted fraction from women and men. The downregulation of IL8 and MCP1 secretion from female monocytes observed in mixed PBMC cultures after stimulation with dopaminergic agents (
Figure 1A and B) was not seen after B cell depletion (
Figure 4B and D). Similarly, the trend of upregulated IL8 and MCP1 production by male monocytes was lost in the absence of B cells (
Figure 4C and E), suggesting a possible B cell-dependent mechanism which is shown in
Figure 4F.
In summary, DR stimulation leads to increased activation of female B cells, which can subsequently suppress monocytes resulting in a reduction of their activation and cytokine secretion. Conversely, this activation in B cells in response to DR stimulation is not observed for men, and consequently, there is no downregulation in activation and cytokine secretion of monocytes (
Figure 4F).
3.5. Acute Stimulation Induced Switch to Proinflammatory Effects of DR Agonists Regarding Cytokine Secretion and Activation Marker Expression in Women
In a previous study, we demonstrated sex-specific responses to DR stimulation in patients with rheumatoid arthritis (RA), a chronic inflammatory disease [
3]. Additionally, changes in dopaminergic effects due to coactivation of the MAPK signaling pathway is reported [
45]. To elucidate whether sex-specific differences can also be observed under acute inflammatory conditions, we introduced CpG, that also leads to activated MAPK signaling [
46]. We demonstrated that the stimulation with CpG activated both B cells and monocytes without indirect B cell influence (
Supplementary Figure S5A and B).
Our findings showed that, in the presence of CpG, IL8 was still mainly produced by monocytes. However, CpG stimulation did not increase the number of IL8-expressing monocytes compared to the unstimulated sample (
Supplementary Figure S4A). Furthermore, depletion of monocytes from PBMCs resulted in a strongly reduced overall level of IL8 in the supernatant of CpG-stimulated cells (
Supplementary Figure S4B). However, elevated IL8 levels in the supernatant (
Supplementary Figure S4C), as well as the CpG-induced increase in IL8-expressing B cells (
Supplementary Figure S4A), indicated that IL8 is a cytokine produced not exclusively by monocytes. We observed a comparable increase in IL8 by CpG stimulation in both sexes, with no effect by dopaminergic stimulation, unlike under physiological conditions (
Supplementary Figure S4F).
We focused on the secretion of MCP1 since only monocytes showed a detectable level of MCP1-expressing cells, which were upregulated by CpG stimulation after 24 h (
Figure 5A). Furthermore, the depletion of monocytes resulted in nearly undetectable levels of MCP1 (
Figure 5B), underscoring that monocytes are the sole source of MCP1. Interestingly, CpG stimulation increased MCP1 expression in both sexes; however, combined with dopaminergic agents, an even more pronounced increase was observed only for women but not men (
Figure 5C). Thus, dopaminergic stimulation under inflammatory condition completely switched the anti-inflammatory phenotype to a proinflammatory effect regarding MCP1 secretion in women, whereas men exhibited only a slight tendency for upregulated MCP1 secretion under both physiological and inflammatory conditions (
Supplementary Figure S5C).
In addition to MCP1, IL1β and IL18 also appeared to be monocyte-specific since the depletion of monocytes led to an almost complete loss of these cytokines in the supernatant (
Supplementary Figure S4E and G). The release of both cytokines was induced by CpG, confirming its activating effects also on monocytes, and was further increased by DR stimulation in women (
Supplementary Figure S4 F and H), supporting the proinflammatory phenotype of female monocytes due to DR stimulants under these inflammatory conditions. This finding was further strengthened by an increase in CD69
+ monocytes and the expression of activation markers HLA-DR, CD86 and CD38 on female monocytes induced by DR stimulation, which now showed a similar upregulation as observed in men (
Figure 5D-G). Notably, the switch in the effects from physiological to inflammatory conditions was pronounced for the number of CD69-expressing monocytes, but with similar trends observed for both women and men (
Supplementary Figure S5D). These trends were also evident for CD86 and CD38, resulting in a stronger upregulation after DR stimulation in the presence of CpG than without additional stimulus (
Supplementary Figure S5F and G). However, for HLA-DR, a similar upregulation had already been observed for women without an inflammatory stimulus (
Supplementary Figure S5E).
In summary, our results indicate that the effects of dopaminergic stimulation are shifted to a proinflammatory response regarding cytokine secretion and monocyte activation by an acute inflammatory stimulus specifically in women.
Interestingly, DR stimulation also led to an increase in HLA-DR and CD86 on T cells under physiological as well as inflammatory conditions (
Supplementary Figure S6) suggesting an increased activation for this PBMC subtype. However, these effects on T cells were independent of sex and the presence of an acute inflammatory stimulus, presenting the observed responses of B cells and monocytes to be cell specific.
3.6. Monocytes Exhibited a B Cell-Independent Proinflammatory Phenotype after DR Stimulation under Inflammatory Conditions
To investigate, whether the influences of dopaminergic agonists on monocyte activation and cytokine production are still driven by B cells we depleted the B cells from the mixed PBMCs and analyzed MCP1 and activation marker expression after dopaminergic stimulation. Interestingly, we saw a slightly reduced MCP1 expression but still the trend for an upregulation overall (
Figure 6A and B). The upregulation of activation markers in women and men seemed to be B cell independent (
Figure 6E-J). Thus, we hypothesized that the B cells do only slightly support the upregulation in MCP1 by monocytes under inflammatory conditions, but that DR stimulation also has a direct effect in CpG-activated monocytes leading to an even more pronounced proinflammatory phenotype.
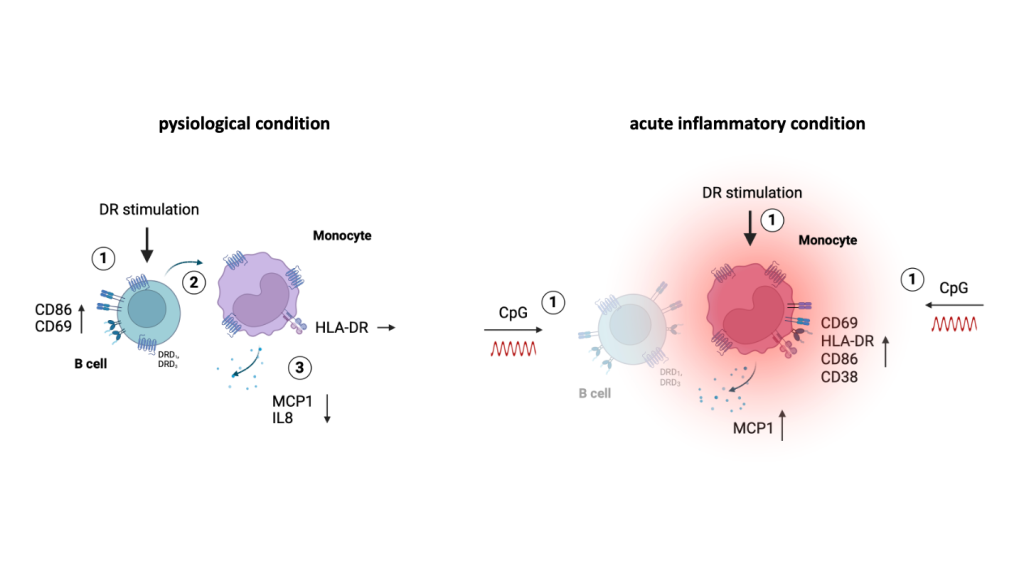
Our findings about the effects of DR stimulation on PBMCs from women under physiological and acute inflammatory conditions are summarized in
Figure 7. After DR stimulation, we observed a downregulation in activation and cytokine secretion in monocytes indirectly induced by activated B cells in the culture of mixed PBMCs from women. When dopaminergic receptors were stimulated under acute inflammatory conditions the anti-inflammatory phenotype of monocytes switched to proinflammatory effects, shown by increased activation marker expression and cytokine secretion. These effects occur without B cell help, potentially due to the CpG-induced change in the activation status of monocytes themselves (
Figure 7). These findings highlight, how important sex and the activation status of the DA-addressed immune cells is for the effects DA can have.
4. Discussion
Dopamine (DA) is a crucial bioactive compound in the human body, essential not only for physiological maintenance of various systems but also as a significant player in numerous diseases, including those involving the immune system [
1,
3,
7,
34,
35,
36,
40,
41]. Within the medical field, sex-specific differences have attracted increasing attention during the recent years. Given the limited understanding of DA’s effects on peripheral immune cells, it is of high interest to further investigate this link, especially considering sex as an important influencing factor. This study presents DA as a complex regulatory and activating immunomodulator whose effects depend on many factors namely sex, indirect influences between several cell types as well as activation status of the immune cells, which are discussed in the following sections.
First of all, DA-induced effects are not necessarily DR-specific but can also occur via binding of other receptors, such as adrenoceptors [
25,
47]. To prevent potential mixed effects resulting from the involvement of other pathways, we used the DR agonists A68930 and Ropinirole that specifically bind either D
1-like or D
2-like DRs. Besides high specificity, these agonists also offer improved stability in cell culture compared to natural DA. However, it is to be mentioned that synthetic DR-specific compounds do not fully replicate the effects that would be observed with DA
in vivo. Consequently, further research is needed to apply these findings to the whole human body, considering the complex interactions and broader context present
in vivo.
In the CNS, D
1-like receptor binding is described to stimulate cAMP-involved pathways, while D
2-like receptor signaling inhibits these pathways [
8,
9]. However, studies on dopaminergic pathway in immune cells report diverse and sometimes contradictory results. In this context, murine T cells showed decreased cAMP levels when DRD
3 was stimulated [
14], whereas human T cells displayed an increase in cAMP after DRD
2/DRD
3 stimulation [
50]. The findings of Zhao et al. support the dichotomy described for the brain also in human NK cells, which show an increase in cAMP level and NK cell cytotoxicity by D
1-like stimulation, while the opposite was observed for D
2-like stimulation [
51]. Notably, in our and other [
19] studies, no opposite effects after D
1- and D
2-like stimulation in the modulation of immune cell functions were found, suggesting that the typical classification into the two types of receptors regarding their effects may not apply to all immune cells.
Thus, the exact intracellular pathways activated by DR binding in B cells and monocytes in our study remain to be elucidated. Moreover, the observed sex-specific differences obtained under physiological conditions may be due to differently addressed pathways, other genetic background between women and men or different epigenetic regulations.
In animals, previous studies have highlighted that DA’s influence in the CNS can vary significantly based on sex [
30,
31,
32,
33], which was also reported for peripheral PBMCs from RA patients suffering from chronic inflammation [
3]. In our study, we focused on those sex-specific differences in the periphery and found increased DRD
1 and DRD
3 expression on B cells from men compared to women (
Figure 2), supporting findings by Wieber et al. [
3]. To elucidate the underlying causes of these sex-specific differences, we investigated the role of sex hormones and found an increase in DRD
1 on B cells after E2 and DHT treatment only for men (
Figure 2). Monocytes and other PBMC subsets did not show this upregulation (data not shown), suggesting that the mechanism is not conserved across all PBMCs but specific for B cells. Previous studies have described the presence of the estrogen responsive element on the promoter of DRD
1 [
33], indicating a potential direct genomic interaction.
Interestingly, despite lower expression levels of DRD1 and DRD3, the responses of female monocytes to dopaminergic stimulation were stronger pronounced than the effects observed for men. One possible explanation is that the amount of DRs does not necessarily correlate with the functional effects following receptor binding. It is also conceivable that a reduced number of DRs may promote alternative receptor pairing, thereby triggering different signaling outcomes.
In this study, we observed differences between women and men not only in DR expression but also in functional assays, especially in the cytokine secretion of monocytes. While women showed a downregulation under physiological conditions, male monocytes tended to secrete more IL8 and MCP1 after physiological DR stimulation (
Figure 1). It is of particular interest that DR stimulation strongly affects cytokine secretion since cytokines are important for modulating inflammatory responses and coordinating immune cell communication [
43,
44]. Some studies have already focused on the effect of DA on cytokine secretion, but without considering differences between sexes. For human monocyte-derived macrophages, it has been reported that DA increases the secretion of IL6 and MCP1 with and without LPS stimulation [
24], while it dampened the increase in cytokine production by PBMCs under LPS-induced inflammatory conditions in another study [
23]. Additionally, Hasko et al. reported an inhibition in LPS-induced IL12 p40 production of macrophages by DA, which was, however, mediated via β-adrenoceptors and not DRs [
25]. Overall, the mentioned studies show very diverse contexts, and their findings are only partially comparable to those in our study since we investigated the effect of DA on monocytes within the mixture of all PBMCs.
We found that the effects on cytokine secretion were secondarily influenced by B cells under physiological conditions, since they were not observed, when B cells were depleted from the PBMC culture (
Figure 4). These results could be explained by the observation that B cells from women showed an upregulation in CD86, increasing their activation and antigen-presenting capabilities [
39,
52], which was not observed for B cells from men (
Figure 3). This suggests a change in the interaction with monocytes, leading to a suppression of monocytes and thus reduced cytokine secretion. The precise mechanism of this interaction in our study still needs to be investigated. In this context, it is described that B cells and monocytes can interact either via direct cell-cell-contact [
53] or via soluble factors like B cell-derived cytokines such as IL10 or lymphotoxin, modulating the functions of monocytes [54,55]. In summary, coculture with PBMCs was essential to obtain these results since they were influenced by secondary effects due to other addressed cell types. This underlines the importance of considering secondary effects induced by other peripheral immune cells.
Besides the influencing factors of sex and secondary impacts by surrounding immune cells, a key player in modulating DA’s effects is the activation status of the cells. In line with this, it has been reported that the effects of DR stimulation vary when other receptors or signaling pathways are coactivated [
45]. In our study, the reduced cytokine secretion of monocytes from women that we observed without involvement of any other signaling pathway switched to an upregulation when the TLR9 pathway was coactivated via CpG stimulation (
Supplementary Figure S5). The MAPK pathway, reported to alter the effects of DA [
45], is also activated by TLR9 signaling [
46], supporting the hypothesis that coactivated pathways can influence the outcomes of DR signaling. Whether this switch is indeed due to MAPK involvement in monocytes after CpG stimulation requires further investigation. Unfortunately, studying the direct effect of DR stimulation on isolated monocytes is challenging, since monocytes died under comparable cell culture conditions within a few hours after thawing and isolation (data not shown) probably due to high stress and missing signals from other PBMCs.
Our results show how DR stimulation affects peripheral immune cells in a sex-specific manner and thus importantly contributes to the deepened understanding of DA’s effects. Still, several questions remain to be addressed in further studies. Firstly, the effects of dopaminergic stimulation on B cells should be examined in further research, to elucidate their interaction with monocytes and ability to influence their cytokine secretion. Furthermore, it is challenging to address whether a possible indirect effect of B cells on monocytes persists under inflammatory conditions but is covered by the strong direct effects of DR stimulation on activated monocytes. Exploring whether this effect is stimulus-specific or whether similar effects can be obtained by stimulation with other activating substances like LPS could further deepen our knowledge about the working mechanism of DA. Moreover, other PBMC subtypes might be influenced by or have an influence on the effects we observed, though addressing this complexity is challenging. We observed increased activation of T cells, shown by increased HLA-DR and CD86 expression, independent of an applied stimulus (
Supplementary Figure S6), likely due to direct DR stimulation on T cells as described in several studies. These effects on T cells were also independent of sex and show that the differences between women and men observed for monocytes and B cells are cell specific.
5. Conclusions
In summary, our study demonstrates that dopaminergic stimulation induces sex-specific effects on PBMCs from women and men. Under physiological conditions, we observed B cell-driven anti-inflammatory effects on monocytes, shown in a reduced cytokine secretion and activation marker expression exclusively in women. These sex-specific differences are accompanied by increased DR expression on B cells from men, which could further be increased by treatment with sex hormones, supporting their influencing factors in the interplay of the dopaminergic pathway and immune cell function. Interestingly, our findings showed that coactivation of the TLR9 pathway via CpG, mimicking an acute inflammatory stimulus, led to a switch from anti-inflammatory to proinflammatory responses in women after DR stimulation. These findings underscore sex, secondary effects induced by surrounding immune cells, and activation status as influencing factors in dopaminergic effects on PBMCs. Our findings enhance the understanding of DA’s mode of action under both healthy and inflammatory conditions providing valuable insights for addressing DA-related pathologies in targeted therapeutic strategies and point out the importance for sex-specific treatments. However, further research is needed to elucidate its effects in vivo.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org, Figure S1: Sex-specific effects of DR stimulation on immunological responses of monocytes under physiological conditions. Figure S2: Women and men exhibit similar estrogen levels and sex hormone receptor expression on B cells, while testosterone is higher in men. Figure S3: Effects of DR stimulation on B cells from women and men. Figure S4: Effect of DR stimulation on secretion of other cytokines of mixed PBMCs. Figure S5: Comparison of Effects after DR stimulation with and without an inflammatory stimulus. Figure S6: Increase in HLA-DR on T cells after DR stimulation is independent of sex and presence of an acute inflammatory stimulus.
Author Contributions
Conceptualization, L.F. and S.C.; methodology, L.F. and S.C..; formal analysis, L.F.; investigation, L.F.; data curation, L.F.; writing—original draft preparation, L.F.; writing—review and editing, L.F. and S.C.; visualization, L.F.; supervision, S.C.; project administration, S.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was intramurally funded by IfADo-Leibniz Research Centre for Working Environment and Human Factors.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki. Ethical review and approval for this study were waived by the Ethics Committee of IfADo as only blood samples from healthy donors after informed consent were used (decision #213 from 31.01.2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
On reasonable request, the corresponding author will provide access to the data used and/or analyzed in this study.
Acknowledgments
The authors want to thank Claudia Brockhaus and Dr. Sina Trebing for their support in blood sampling, as well as all blood donors who participated in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Channer, B.; Matt, S.M.; Nickoloff-Bybel, E.A.; Pappa, V.; Agarwal, Y.; Wickman, J.; Gaskill, P.J. Dopamine, Immunity, and Disease. Pharmacol. Rev. 2023, 75, 62–158. [Google Scholar] [CrossRef] [PubMed]
- McKenna, F.; McLaughlin, P.J.; Lewis, B.J.; Sibbring, G.C.; Cummerson, J.A.; Bowen-Jones, D.; Moots, R.J. Dopamine receptor expression on human T- and B-lymphocytes, monocytes, neutrophils, eosinophils and NK cells: A flow cytometric study. J. Neuroimmunol. 2002, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Wieber, K.; Fleige, L.; Tsiami, S.; Reinders, J.; Braun, J.; Baraliakos, X.; Capellino, S. Dopamine receptor 1 expressing B cells exert a proinflammatory role in female patients with rheumatoid arthritis. Sci. Rep. 2022, 12, 5985. [Google Scholar] [CrossRef]
- Arreola, R.; Alvarez-Herrera, S.; Pérez-Sánchez, G.; Becerril-Villanueva, E.; Cruz-Fuentes, C.; Flores-Gutierrez, E.O.; Garcés-Alvarez, M.E.; La Cruz-Aguilera, D.L. de; Medina-Rivero, E.; Hurtado-Alvarado, G.; et al. Immunomodulatory Effects Mediated by Dopamine. J. Immunol. Res. 2016, 2016, 3160486. [Google Scholar] [CrossRef] [PubMed]
- Bergquist, J.; Tarkowski, A.; Ekman, R.; Ewing, A. Discovery of endogenous catecholamines in lymphocytes and evidence for catecholamine regulation of lymphocyte function via an autocrine loop. Proc. Natl. Acad. Sci. 1994, 12912–12916. [Google Scholar] [CrossRef] [PubMed]
- Musso, N.R.; Brenci, S.; Setti, M.; Indiveri, F.; Lotti, G. Catecholamine Content and in vitro Catecholamine Synthesis in peripheral human lymphocytes. J Clin Endocrinol Metab 1996, 3553–3557. [Google Scholar] [CrossRef]
- Thomas Broome, S.; Louangaphay, K.; Keay, K.A.; Leggio, G.M.; Musumeci, G.; Castorina, A. Dopamine: An immune transmitter. Neural Regen. Res. 2020, 15, 2173–2185. [Google Scholar] [CrossRef] [PubMed]
- Kebabian, J.W.; Calne, D.B. Multiple receptors for dopamine. Nature 1979, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Missale, C.; Nash, S.; Robinson, S.W.; Jaber, M.; Caron, M.G. Dopamine Receptors: From Structure to Function. Physiological Reviews 1998, 189–225. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.O.; Battagello, D.S.; Cardoso, A.R.; Hauser, D.N.; Bittencourt, J.C.; Correa, R.G. Dopamine: Functions, Signaling, and Association with Neurological Diseases. Cell. Mol. Neurobiol. 2019, 39, 31–59. [Google Scholar] [CrossRef]
- Beaulieu, J.-M.; Del'guidice, T.; Sotnikova, T.D.; Lemasson, M.; Gainetdinov, R.R. Beyond cAMP: The Regulation of Akt and GSK3 by Dopamine Receptors. Front. Mol. Neurosci. 2011, 4, 38. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, J.-M.; Espinoza, S.; Gainetdinov, R.R. Dopamine receptors - IUPHAR Review 13. Br. J. Pharmacol. 2015, 172, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Levite, M. Dopamine and T cells: Dopamine receptors and potent effects on T cells, dopamine production in T cells, and abnormalities in the dopaminergic system in T cells in autoimmune, neurological and psychiatric diseases. Acta Physiol. (Oxf) 2016, 216, 42–89. [Google Scholar] [CrossRef] [PubMed]
- Franz, D.; Contreras, F.; González, H.; Prado, C.; Elgueta, D.; Figueroa, C.; Pacheco, R. Dopamine receptors D3 and D5 regulate CD4(+)T-cell activation and differentiation by modulating ERK activation and cAMP production. J. Neuroimmunol. 2015, 284, 18–29. [Google Scholar] [CrossRef]
- Watanabe, Y.; Nakayama, T.; Nagakubo, D.; Hieshima, K.; Jin, Z.; Katou, F.; Hashimoto, K.; Yoshie, O. Dopamine selectively induces migration and homing of naive CD8+ T cells via dopamine receptor D3. J. Immunol. 2006, 176, 848–856. [Google Scholar] [CrossRef]
- Saussez, S.; Laumbacher, B.; Chantrain, G.; Rodriguez, A.; Gu, S.; Wank, R.; Levite, M. Towards neuroimmunotherapy for cancer: The neurotransmitters glutamate, dopamine and GnRH-II augment substantially the ability of T cells of few head and neck cancer patients to perform spontaneous migration, chemotactic migration and migration towards the autologous tumor, and also elevate markedly the expression of CD3zeta and CD3epsilon TCR-associated chains. J. Neural Transm. (Vienna) 2014, 121, 1007–1027. [Google Scholar] [CrossRef] [PubMed]
- Torres, K.C.L.; Antonelli, L.R.V.; Souza, A.L.S.; Teixeira, M.M.; Dutra, W.O.; Gollob, K.J. Norepinephrine, dopamine and dexamethasone modulate discrete leukocyte subpopulations and cytokine profiles from human PBMC. J. Neuroimmunol. 2005, 166, 144–157. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, C.; Das, S.; Chakroborty, D.; Chowdhury, U.R.; Basu, B.; Dasgupta, P.S.; Basu, S. Cutting Edge: Stimulation of dopamine D4 receptors induce T cell quiescence by up-regulating Kruppel-like factor-2 expression through inhibition of ERK1/ERK2 phosphorylation. J. Immunol. 2006, 177, 7525–7529. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.W.; Lin, Y.S.; Cheng, J.T. Effect of dopamine on immune cell proliferation in mice. Life Science 1997, 361–371. [Google Scholar] [CrossRef]
- Meredith, E.J.; Holder, M.J.; Rosén, A.; Lee, A.D.; Dyer, M.; Barnes, N.M.; Gordon, J. Dopamine targets cycling B cells indeoendent of receptors/transporter for oxidative attack: Implications for non Hidgkins lymphoma. PNAS 2006, 13485–13490. [Google Scholar] [CrossRef]
- Calderon, T.M.; Williams, D.W.; Lopez, L.; Eugenin, E.A.; Cheney, L.; Gaskill, P.J.; Veenstra, M.; Anastos, K.; Morgello, S.; Berman, J.W. Dopamine Increases CD14+CD16+ Monocyte Transmigration across the Blood Brain Barrier: Implications for Substance Abuse and HIV Neuropathogenesis. J. Neuroimmune Pharmacol. 2017, 12, 353–370. [Google Scholar] [CrossRef] [PubMed]
- Coley, J.S.; Calderon, T.M.; Gaskill, P.J.; Eugenin, E.A.; Berman, J.W. Dopamine increases CD14+CD16+ monocyte migration and adhesion in the context of substance abuse and HIV neuropathogenesis. PLoS ONE 2015, 10, e0117450. [Google Scholar] [CrossRef] [PubMed]
- Bergquist, J.; Ohlsson, B.; Tarkowski, A. Nuclear factor-kappa B is involved in the catecholaminergic suppression of immunocompetent cells. Ann. N. Y. Acad. Sci. 2000, 917, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Gaskill, P.J.; Carvallo, L.; Eugenin, E.A.; Berman, J.W. Characterization and function of the human macrophage dopaminergic system: Implications for CNS disease and drug abuse. J. Neuroinflammation 2012, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Haskó, G.; Szabó, C.; Németh, Z.H.; Deitch, E.A. Dopamine suppresses IL-12 p40 production by lipopolysaccharide-stimulated macrophages via a b-adrenoceptor-mediated mechanism. J. Neuroimmunol. 2002, 34–39. [Google Scholar] [CrossRef]
- Haskó, G.; Szabó, C.; Merkel, K.; Bencsics, A.; Zingarelli, B.; Kvetan, V.; Vizi, E.S. Modulation of lipopolysaccharide-induced tumor necrosis factor-alpha and nitric oxide production by dopamine receptor agonists and antagonists in mice. Immunology Letters 1996, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Ugalde, V.; Contreras, F.; Prado, C.; Chovar, O.; Espinoza, A.; Pacheco, R. Dopaminergic signalling limits suppressive activity and gut homing of regulatory T cells upon intestinal inflammation. Mucosal Immunol. 2021, 14, 652–666. [Google Scholar] [CrossRef] [PubMed]
- Papa, I.; Saliba, D.; Ponzoni, M.; Bustamante, S.; Canete, P.F.; Gonzalez-Figueroa, P.; McNamara, H.A.; Valvo, S.; Grimbaldeston, M.; Sweet, R.A.; et al. TFH-derived dopamine accelerates productive synapses in germinal centres. Nature 2017, 547, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Nakano, K.; Higashi, T.; Takagi, R.; Hashimoto, K.; Tanaka, Y.; Matsushita, S. Dopamine released by dendritic cells polarizes Th2 differentiation. Int. Immunol. 2009, 21, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Soutschek, A.; Burke, C.J.; Raja Beharelle, A.; Schreiber, R.; Weber, S.C.; Karipidis, I.I.; Velden, J. ten; Weber, B.; Haker, H.; Kalenscher, T.; et al. The dopaminergic reward system underpins gender differences in social preferences. Nat. Hum. Behav. 2017, 1, 819–827. [Google Scholar] [CrossRef]
- Lévesque, D.; Gagnon, S.; Di Paolo, T. Striatal D1 dopamine receptor density fluctuates during the rat estrous cycle. Neuroscience Letters 1989, 345–350. [Google Scholar] [CrossRef]
- Lévesque, D.; Di Paolo, T. Chronic estradiol treatment increases ovariectomized rat striatal D-1 dopamine receptors. Life Science 1989, 1813–1820. [Google Scholar] [CrossRef]
- Lee, S.H.; Mouradian, M.M. Up-regulation of D1A dopamine receptor gene transcription by estrogen. Molecular and Cellular Endocrinology 1999, 151–157. [Google Scholar] [CrossRef]
- Ehringer, H.; Hornykiewicz, O. Distribution of noradrenaline and dopamine (3-hydroxytyramine) in the human brain and their behavior in diseases of the extrapyramidal system. Parkinsonism and Related Disorders 1998, 4, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.D.; Yi, L.X.; Wang, D.Q.; Lim, T.M.; Tan, E.K. Role of dopamine in the pathophysiology of Parkinson's disease. Transl. Neurodegener. 2023, 12, 44. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.-K.; Chao, Y.-X.; West, A.; Chan, L.-L.; Poewe, W.; Jankovic, J. Parkinson disease and the immune system - associations, mechanisms and therapeutics. Nat. Rev. Neurol. 2020, 16, 303–318. [Google Scholar] [CrossRef] [PubMed]
- Matt, S.M.; Gaskill, P.J. Dopaminergic impact of cART and anti-depressants on HIV neuropathogenesis in older adults. Brain Res. 2019, 1723, 146398. [Google Scholar] [CrossRef]
- Nickoloff-Bybel, E.A.; Mackie, P.; Runner, K.; Matt, S.M.; Khoshbouei, H.; Gaskill, P.J. Dopamine increases HIV entry into macrophages by increasing calcium release via an alternative signaling pathway. Brain Behav. Immun. 2019, 82, 239–252. [Google Scholar] [CrossRef]
- Fleige, L.; Fillatreau, S.; Claus, M.; Capellino, S. Additional use of α-IgM antibodies potentiates CpG ODN2006-induced B cell activation by targeting mainly naïve and marginal zone-like B cells. Cell. Immunol. 2024, 403–404, 104846. [Google Scholar] [CrossRef]
- Feng, Y.; Lu, Y. Immunomodulatory Effects of Dopamine in Inflammatory Diseases. Front. Immunol. 2021, 12, 663102. [Google Scholar] [CrossRef]
- Xue, L.; Li, X.; Chen, Q.; He, J.; Dong, Y.; Wang, J.; Shen, S.; Jia, R.; Zang, Q.J.; Zhang, T.; et al. Associations between D3R expression in synovial mast cells and disease activity and oxidant status in patients with rheumatoid arthritis. Clin. Rheumatol. 2018, 37, 2621–2632. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.-H.; Liu, Y.-Q.; Deng, Q.-W.; Peng, Y.-P.; Qiu, Y.-H. Dopamine D2 Receptor Is Involved in Alleviation of Type II Collagen-Induced Arthritis in Mice. Biomed Res. Int. 2015, 2015, 496759. [Google Scholar] [CrossRef] [PubMed]
- Altan-Bonnet, G.; Mukherjee, R. Cytokine-mediated communication: A quantitative appraisal of immune complexity. Nat. Rev. Immunol. 2019, 19, 205–217. [Google Scholar] [CrossRef]
- Leonard, W.J.; Lin, J.-X. Strategies to therapeutically modulate cytokine action. Nat. Rev. Drug Discov. 2023, 22, 827–854. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, J.-M.; Gainetdinov, R.R. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol. Rev. 2011, 63, 182–217. [Google Scholar] [CrossRef]
- Dongye, Z.; Li, J.; Wu, Y. Toll-like receptor 9 agonists and combination therapies: Strategies to modulate the tumour immune microenvironment for systemic anti-tumour immunity. Br. J. Cancer 2022, 127, 1584–1594. [Google Scholar] [CrossRef] [PubMed]
- Parrado, A.C.; Salaverry, L.S.; Mangone, F.M.; Apicella, C.E.; Gentile, T.; Canellada, A.; Rey-Roldán, E.B. Differential Response of Dopamine Mediated by β-Adrenergic Receptors in Human Keratinocytes and Macrophages: Potential Implication in Wound Healing. Neuroimmunomodulation 2017, 24, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Ilani, T.; Strous, R.D.; Fuchs, S. Dopaminergic regulation of immune cells via D3 dopamine receptor: A pathway mediated by activated T cells. FASEB J. 2004, 18, 1600–1602. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Huang, Y.; Liu, Z.; Cao, B.-B.; Peng, Y.-P.; Qiu, Y.-H. Dopamine receptors modulate cytotoxicity of natural killer cells via cAMP-PKA-CREB signaling pathway. PLoS ONE 2013, 8, e65860. [Google Scholar] [CrossRef] [PubMed]
- Lim, T.S.; Goh, J.K.H.; Mortellaro, A.; Lim, C.T.; Hämmerling, G.J.; Ricciardi-Castagnoli, P. CD80 and CD86 differentially regulate mechanical interactions of T-cells with antigen-presenting dendritic cells and B-cells. PLoS ONE 2012, 7, e45185. [Google Scholar] [CrossRef] [PubMed]
- Mueller, C.G.; Boix, C.; Kwan, W.-H.; Daussy, C.; Fournier, E.; Fridman, W.H.; Molina, T.J. Critical role of monocytes to support normal B cell and diffuse large B cell lymphoma survival and proliferation. J. Leukoc. Biol. 2007, 82, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Mauri, C.; Bosma, A. Immune regulatory function of B cells. Annu. Rev. Immunol. 2012, 30, 221–241. [Google Scholar] [CrossRef] [PubMed]
- Fillatreau, S. B cells and their cytokine activities implications in human diseases. Clin. Immunol. 2018, 186, 26–31. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
DR stimulation reduced physiological cytokine secretion and activation marker expression of monocytes from women compared to men. A, B) Secreted IL8 (A) and MCP1 (B) in supernatant from PBMCs from women and men after 24 h in cell culture without stimulation (left) and secreted IL8 (A) and MCP1 (B) after in vitro stimulation of PBMCs with A68930 (10-7 M) and Ropinirole (10-6 M) for 24 h normalized to unstimulated control (right) measured via ELISA; n=11-13 each group. C, E) Percentage of IL8+ (C) and MCP1+ (E) B cells, monocytes, T cells and NK cells after 24 h in cell culture without stimulation measured via flow cytometry; n=5 each subtype. D, F) Secreted IL8 (D) and MCP1 (F) in supernatant from mixed PBMCs and Monocyte-depleted PBMCs after 24 h in cell culture without stimulation measured via ELISA; n=11-13 each condition. G, H) Percentage of CD69+ monocytes (G) and expression of HLA-DR on monocytes (H) from women and men after 24 h in cell culture of mixed PBMCs without stimulation (left) and after stimulation with A68930 (10-7 M) and Ropinirole (10-6 M) for 24 h normalized to unstimulated control (right) measured via flow cytometry; n=13-14 each group. Mann-Whitney test was used for testing statistical significance between unpaired data of women and men. For comparison of paired data including unstimulated vs. stimulated samples as well as mixed PBMCs vs. monocytes depleted, Wilcoxon test was used.; *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001.
Figure 1.
DR stimulation reduced physiological cytokine secretion and activation marker expression of monocytes from women compared to men. A, B) Secreted IL8 (A) and MCP1 (B) in supernatant from PBMCs from women and men after 24 h in cell culture without stimulation (left) and secreted IL8 (A) and MCP1 (B) after in vitro stimulation of PBMCs with A68930 (10-7 M) and Ropinirole (10-6 M) for 24 h normalized to unstimulated control (right) measured via ELISA; n=11-13 each group. C, E) Percentage of IL8+ (C) and MCP1+ (E) B cells, monocytes, T cells and NK cells after 24 h in cell culture without stimulation measured via flow cytometry; n=5 each subtype. D, F) Secreted IL8 (D) and MCP1 (F) in supernatant from mixed PBMCs and Monocyte-depleted PBMCs after 24 h in cell culture without stimulation measured via ELISA; n=11-13 each condition. G, H) Percentage of CD69+ monocytes (G) and expression of HLA-DR on monocytes (H) from women and men after 24 h in cell culture of mixed PBMCs without stimulation (left) and after stimulation with A68930 (10-7 M) and Ropinirole (10-6 M) for 24 h normalized to unstimulated control (right) measured via flow cytometry; n=13-14 each group. Mann-Whitney test was used for testing statistical significance between unpaired data of women and men. For comparison of paired data including unstimulated vs. stimulated samples as well as mixed PBMCs vs. monocytes depleted, Wilcoxon test was used.; *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001.
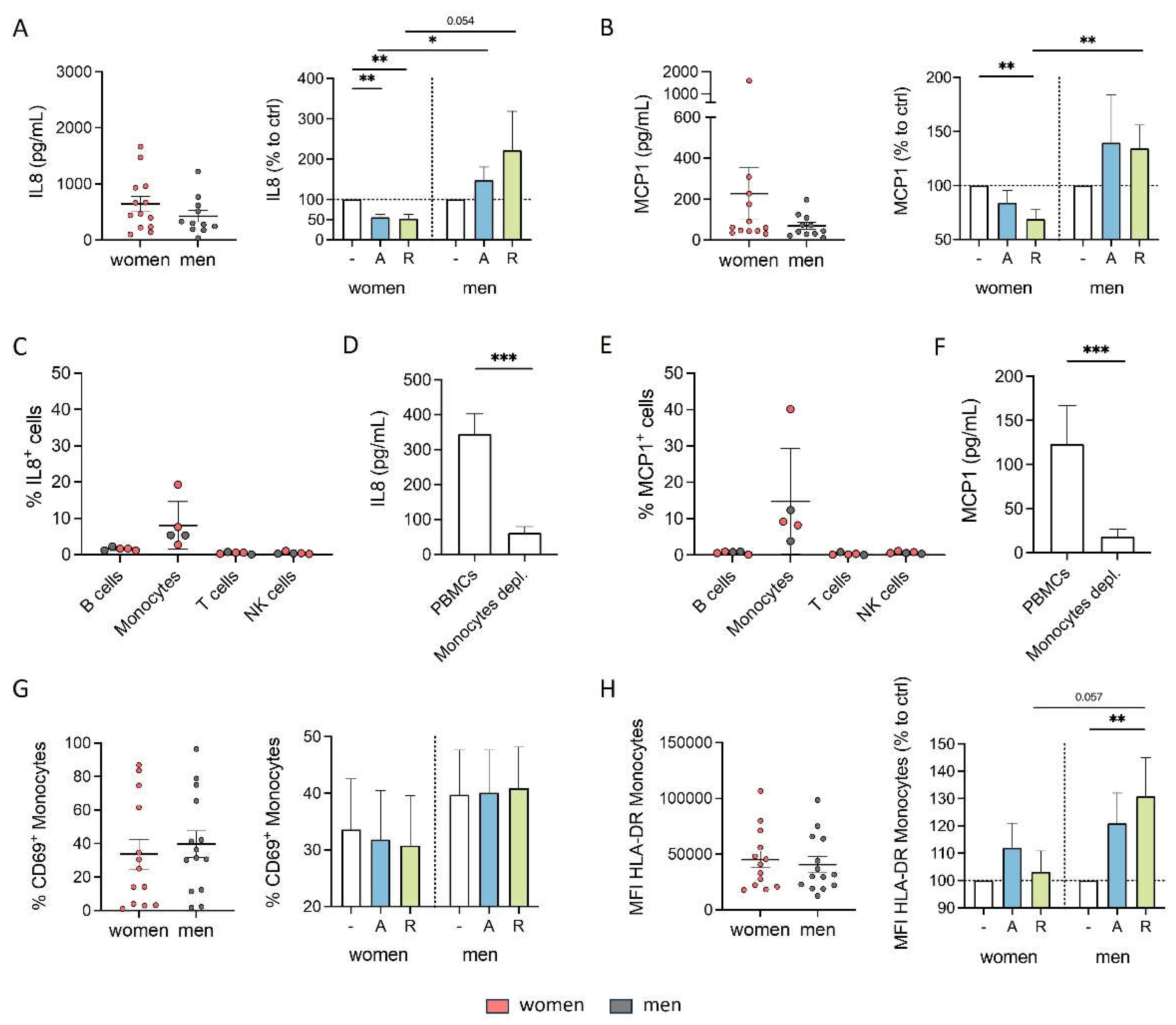
Figure 2.
Higher DRD1 and DRD3 expression on B cells from men was partly regulated by estradiol and testosterone. A, B) Basal expression of DRD1, DRD2, DRD3 and DRD4 on monocytes (A) and B cells (B) from women and men measured via flow cytometry; n=17-19 each group. C, E) Expression of DRD1 (C) and DRD3 (E) on B cells from women and men after 24 h stimulation with E2 (10-8, 10-9, 10-10 M) in mixed PBMC culture. Samples were measured via flow cytometry and normalized to DMSO control; n=8 each condition. D, F) Correlation of expression of DRD1 (D) and DRD3 (F) on B cells from women (pink) and men (grey) with basal estrogen level in plasma; n=17-18 each group. G, I) Expression of DRD1 (G) and DRD3 (I) on B cells from women and men after 24 h in cell culture of mixed PBMCs after stimulation with DHT (10-7, 10-8, 10-9 M) for 24 h normalized to DMSO control measured via flow cytometry; n=8 each condition. H, J) Correlation of expression of DRD1 (H) and DRD3 (J) on B cells from women (pink) and men (grey) with basal testosterone level in plasma; n=17-18 each group. Unpaired t test was used for testing statistical significance between data of women and men. Simple linear regression was used to analyse correlation of DR expression with sex hormone levels. One-Way ANOVA with Geisser-Greenhouse correction and Dunnett multiple comparisons test was used for statistical testing of SHR stimulation using three different concentrations of E2 and DHT. To compare effects of SHR stimulation between women and men using the same concentration of E2 or DHT Mann-Whitney test was used; *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001.
Figure 2.
Higher DRD1 and DRD3 expression on B cells from men was partly regulated by estradiol and testosterone. A, B) Basal expression of DRD1, DRD2, DRD3 and DRD4 on monocytes (A) and B cells (B) from women and men measured via flow cytometry; n=17-19 each group. C, E) Expression of DRD1 (C) and DRD3 (E) on B cells from women and men after 24 h stimulation with E2 (10-8, 10-9, 10-10 M) in mixed PBMC culture. Samples were measured via flow cytometry and normalized to DMSO control; n=8 each condition. D, F) Correlation of expression of DRD1 (D) and DRD3 (F) on B cells from women (pink) and men (grey) with basal estrogen level in plasma; n=17-18 each group. G, I) Expression of DRD1 (G) and DRD3 (I) on B cells from women and men after 24 h in cell culture of mixed PBMCs after stimulation with DHT (10-7, 10-8, 10-9 M) for 24 h normalized to DMSO control measured via flow cytometry; n=8 each condition. H, J) Correlation of expression of DRD1 (H) and DRD3 (J) on B cells from women (pink) and men (grey) with basal testosterone level in plasma; n=17-18 each group. Unpaired t test was used for testing statistical significance between data of women and men. Simple linear regression was used to analyse correlation of DR expression with sex hormone levels. One-Way ANOVA with Geisser-Greenhouse correction and Dunnett multiple comparisons test was used for statistical testing of SHR stimulation using three different concentrations of E2 and DHT. To compare effects of SHR stimulation between women and men using the same concentration of E2 or DHT Mann-Whitney test was used; *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001.
Figure 3.
DR stimulation increased activation of female B cells. A) Expression of CD86 on B cells from women and men after 24 h in cell culture of mixed PBMCs without stimulation (left) and after stimulation with A68930 (10-7 M) and Ropinirole (10-6 M) for 24 h normalized to unstimulated control (right) measured via flow cytometry; n=13-14 each group. B) Representative flow cytometry plots of complete PBMCs and PBMCs after depletion of CD14+ monocytes (“monocyte-depleted PBMCs”). C) Expression of CD86 on B cells from women and men after 24 h in cell culture of monocyte-depleted PBMCs without stimulation (left) and after stimulation with A68930 (10-7 M) and Ropinirole (10-6 M) for 24 h normalized to unstimulated control (right) measured via flow cytometry; n=7-8 each group. D) Representative scheme of gating strategy for B cell subsets (1, naïve B cells: IgD+CD27-; 2, marginal zone-like B cells: IgD+CD27+; switched memory B cells: IgD-CD27+) obtained by flow cytometry. E) Expression of CD86 on naïve, marginal zone-like and switched memory B cells from women and men after stimulation of mixed PBMCs with A68930 (10-7 M) and Ropinirole (10-6 M) for 24 h normalized to unstimulated control measured via flow cytometry; n=13-14 each group. Mann-Whitney test was used for testing statistical significance between unpaired data of women and men. For comparison of paired data including unstimulated vs. stimulated samples, Wilcoxon test was used.; *p ≤ 0.05, ***p ≤ 0.001.
Figure 3.
DR stimulation increased activation of female B cells. A) Expression of CD86 on B cells from women and men after 24 h in cell culture of mixed PBMCs without stimulation (left) and after stimulation with A68930 (10-7 M) and Ropinirole (10-6 M) for 24 h normalized to unstimulated control (right) measured via flow cytometry; n=13-14 each group. B) Representative flow cytometry plots of complete PBMCs and PBMCs after depletion of CD14+ monocytes (“monocyte-depleted PBMCs”). C) Expression of CD86 on B cells from women and men after 24 h in cell culture of monocyte-depleted PBMCs without stimulation (left) and after stimulation with A68930 (10-7 M) and Ropinirole (10-6 M) for 24 h normalized to unstimulated control (right) measured via flow cytometry; n=7-8 each group. D) Representative scheme of gating strategy for B cell subsets (1, naïve B cells: IgD+CD27-; 2, marginal zone-like B cells: IgD+CD27+; switched memory B cells: IgD-CD27+) obtained by flow cytometry. E) Expression of CD86 on naïve, marginal zone-like and switched memory B cells from women and men after stimulation of mixed PBMCs with A68930 (10-7 M) and Ropinirole (10-6 M) for 24 h normalized to unstimulated control measured via flow cytometry; n=13-14 each group. Mann-Whitney test was used for testing statistical significance between unpaired data of women and men. For comparison of paired data including unstimulated vs. stimulated samples, Wilcoxon test was used.; *p ≤ 0.05, ***p ≤ 0.001.
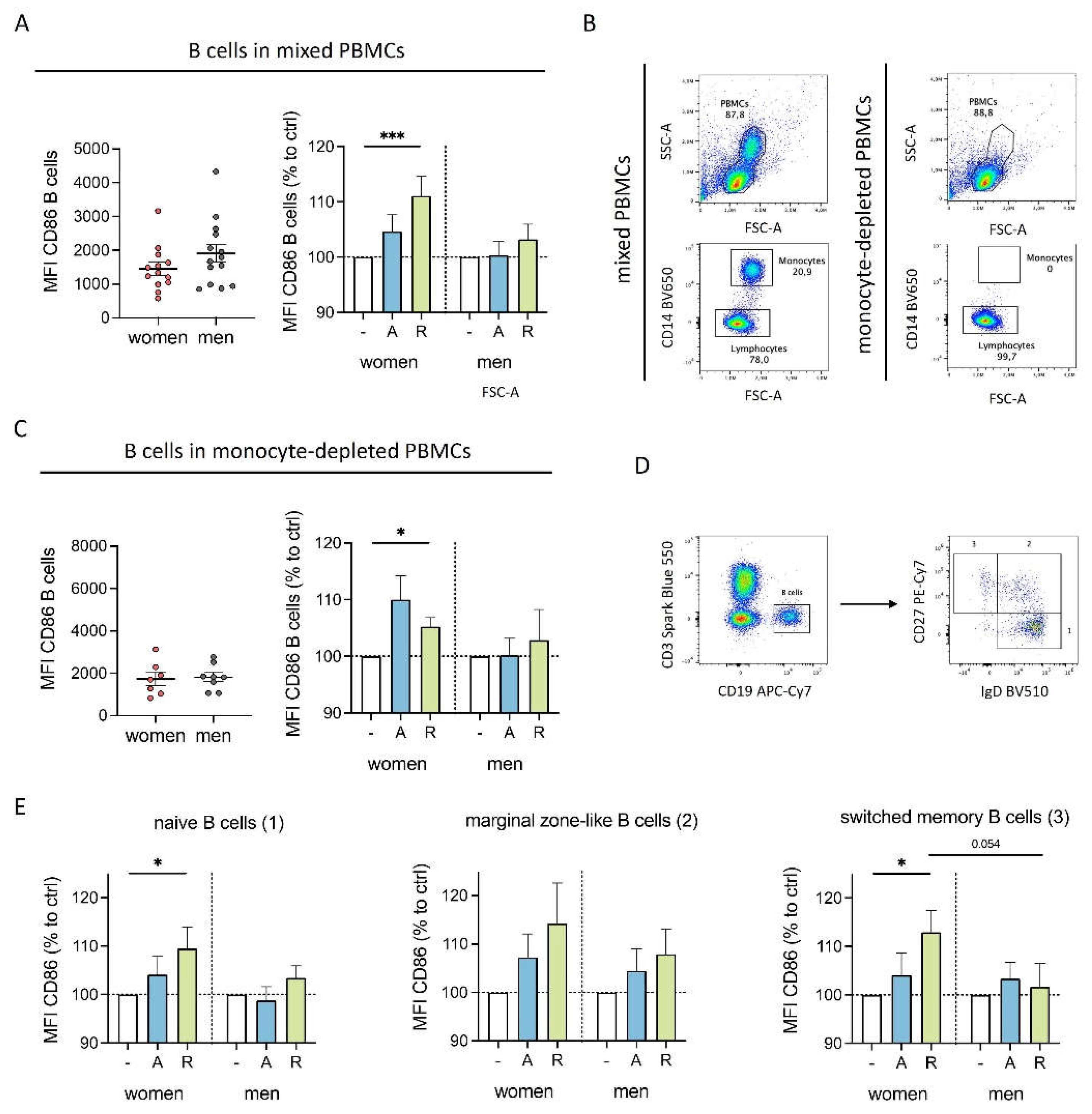
Figure 4.
Sex-specific cytokine secretion of monocytes after DR stimulation is dependent of B cells. A) Representative flow cytometry plots of complete PBMCs and PBMCs after depletion of CD19
+ B cells (“B cell-depleted PBMCs”).
B, C) Secreted IL8 in supernatant from PBMCs and B cell-depleted PBMCs from women (B) and men (C) after 24 h in cell culture without stimulation (left) and secreted IL8 after
in vitro stimulation of PBMCs and B cell-depleted PBMCs with A68930 (10
-7 M) and Ropinirole (10
-6 M) for 24 h normalized to unstimulated control (right) measured via ELISA; n=8-9 each group.
D, E) Secreted MCP1 in supernatant from PBMCs and B cell-depleted PBMCs from women (D) and men (E) after 24 h in cell culture without stimulation (left) and secreted MCP1 after
in vitro stimulation of PBMCs and B cell-depleted PBMCs with A68930 (10
-7 M) and Ropinirole (10
-6 M) for 24 h normalized to unstimulated control (right) measured via ELISA; n=8 each group.
F) Graphical scheme of the interplay between B cells and monocytes after DR stimulation showing effects on activation markers and cytokine secretion for cells from women (left) and men (right); created with
BioRender.com. For comparison of paired data including PBMCs vs. B cells depleted as well as unstimulated vs. stimulated samples, Wilcoxon test was used. Mann-Whitney test was used for testing statistical significance between unpaired data of women and men; *p ≤ 0.05.
Figure 4.
Sex-specific cytokine secretion of monocytes after DR stimulation is dependent of B cells. A) Representative flow cytometry plots of complete PBMCs and PBMCs after depletion of CD19
+ B cells (“B cell-depleted PBMCs”).
B, C) Secreted IL8 in supernatant from PBMCs and B cell-depleted PBMCs from women (B) and men (C) after 24 h in cell culture without stimulation (left) and secreted IL8 after
in vitro stimulation of PBMCs and B cell-depleted PBMCs with A68930 (10
-7 M) and Ropinirole (10
-6 M) for 24 h normalized to unstimulated control (right) measured via ELISA; n=8-9 each group.
D, E) Secreted MCP1 in supernatant from PBMCs and B cell-depleted PBMCs from women (D) and men (E) after 24 h in cell culture without stimulation (left) and secreted MCP1 after
in vitro stimulation of PBMCs and B cell-depleted PBMCs with A68930 (10
-7 M) and Ropinirole (10
-6 M) for 24 h normalized to unstimulated control (right) measured via ELISA; n=8 each group.
F) Graphical scheme of the interplay between B cells and monocytes after DR stimulation showing effects on activation markers and cytokine secretion for cells from women (left) and men (right); created with
BioRender.com. For comparison of paired data including PBMCs vs. B cells depleted as well as unstimulated vs. stimulated samples, Wilcoxon test was used. Mann-Whitney test was used for testing statistical significance between unpaired data of women and men; *p ≤ 0.05.
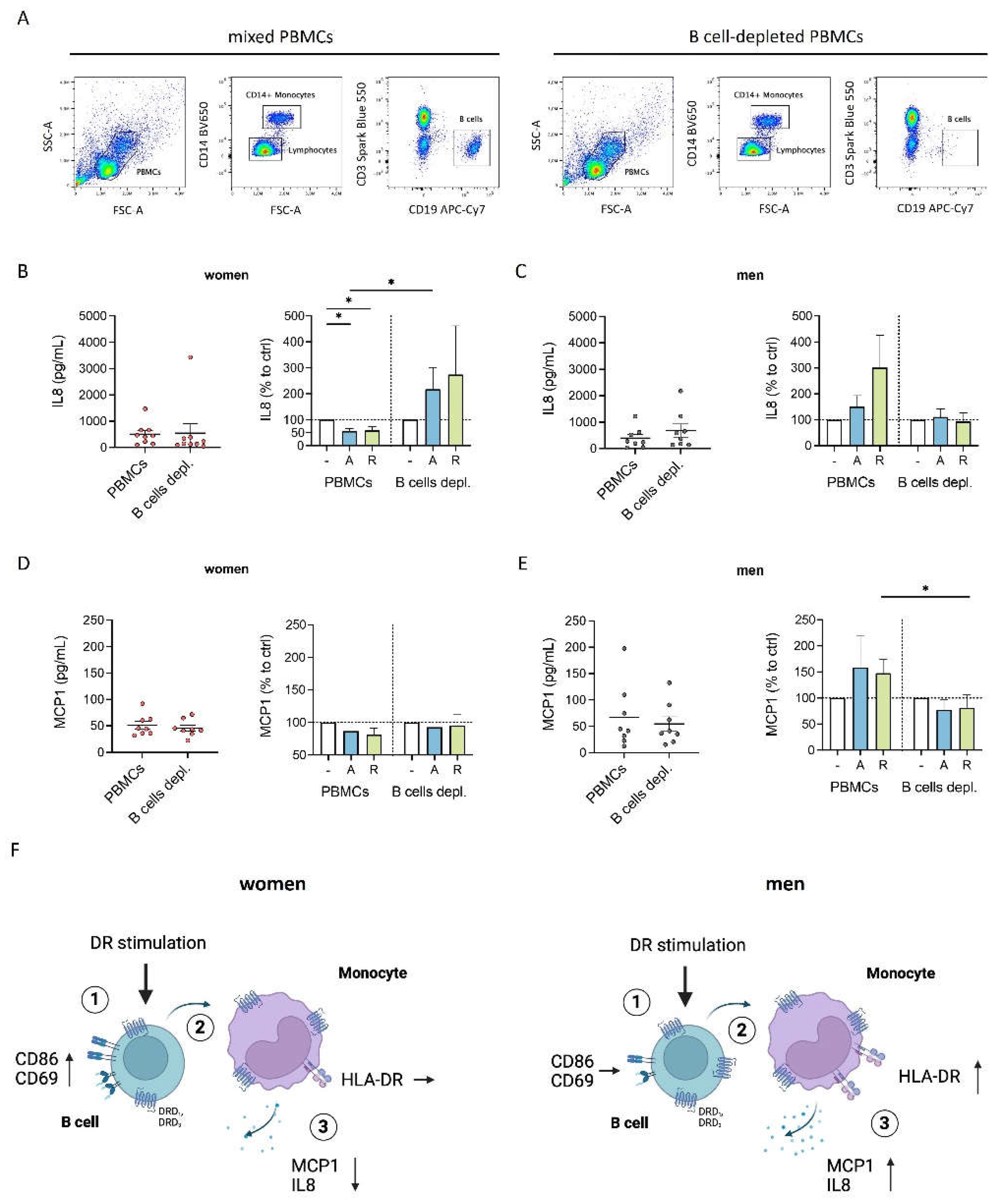
Figure 5.
Inflammatory condition turned DR stimulation-induced characteristics of female monocytes into proinflammatory phenotype. A) Percentage of MCP1+ B cells, monocytes, T cells and NK cells after 24 h in cell culture of mixed PBMCs with or without CpG (0.195 μM) stimulation measured via flow cytometry; n=12 each condition. B) Secreted MCP1 in supernatant from mixed PBMCs and Monocyte-depleted PBMCs after 24 h of CpG (0.195 μM) stimulation measured via ELISA; n=14 each condition. C) Secreted MCP1 in supernatant from PBMCs from women and men after 24 h in cell culture with or without CpG (0.195 μM) stimulation (left) and secreted MCP1 after in vitro stimulation of PBMCs with CpG (0.195 μM) + A68930 (10-7 M) and CpG (0.195 μM) + Ropinirole (10-6 M) for 24 h normalized to CpG control (right) measured via ELISA; n=11-13 each group. D-G) Percentage of CD69+ monocytes (D) and expression of HLA-DR (E), CD86 (F) and CD38 (G) on monocytes from women and men after 24 h in cell culture of mixed PBMCs with or without CpG (0.195 μM) stimulation (left) and after stimulation with CpG (0.195 μM) + A68930 (10-7 M) and CpG (0.195 μM) + Ropinirole (10-6 M) for 24 h normalized to CpG control (right) measured via flow cytometry; n=14 each group. For comparison of paired data including unstimulated vs. stimulated samples as well as PBMCs vs. monocytes depleted, Wilcoxon test was used. Mann-Whitney test was used for testing statistical significance between unpaired data of women and men; *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001.
Figure 5.
Inflammatory condition turned DR stimulation-induced characteristics of female monocytes into proinflammatory phenotype. A) Percentage of MCP1+ B cells, monocytes, T cells and NK cells after 24 h in cell culture of mixed PBMCs with or without CpG (0.195 μM) stimulation measured via flow cytometry; n=12 each condition. B) Secreted MCP1 in supernatant from mixed PBMCs and Monocyte-depleted PBMCs after 24 h of CpG (0.195 μM) stimulation measured via ELISA; n=14 each condition. C) Secreted MCP1 in supernatant from PBMCs from women and men after 24 h in cell culture with or without CpG (0.195 μM) stimulation (left) and secreted MCP1 after in vitro stimulation of PBMCs with CpG (0.195 μM) + A68930 (10-7 M) and CpG (0.195 μM) + Ropinirole (10-6 M) for 24 h normalized to CpG control (right) measured via ELISA; n=11-13 each group. D-G) Percentage of CD69+ monocytes (D) and expression of HLA-DR (E), CD86 (F) and CD38 (G) on monocytes from women and men after 24 h in cell culture of mixed PBMCs with or without CpG (0.195 μM) stimulation (left) and after stimulation with CpG (0.195 μM) + A68930 (10-7 M) and CpG (0.195 μM) + Ropinirole (10-6 M) for 24 h normalized to CpG control (right) measured via flow cytometry; n=14 each group. For comparison of paired data including unstimulated vs. stimulated samples as well as PBMCs vs. monocytes depleted, Wilcoxon test was used. Mann-Whitney test was used for testing statistical significance between unpaired data of women and men; *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001.
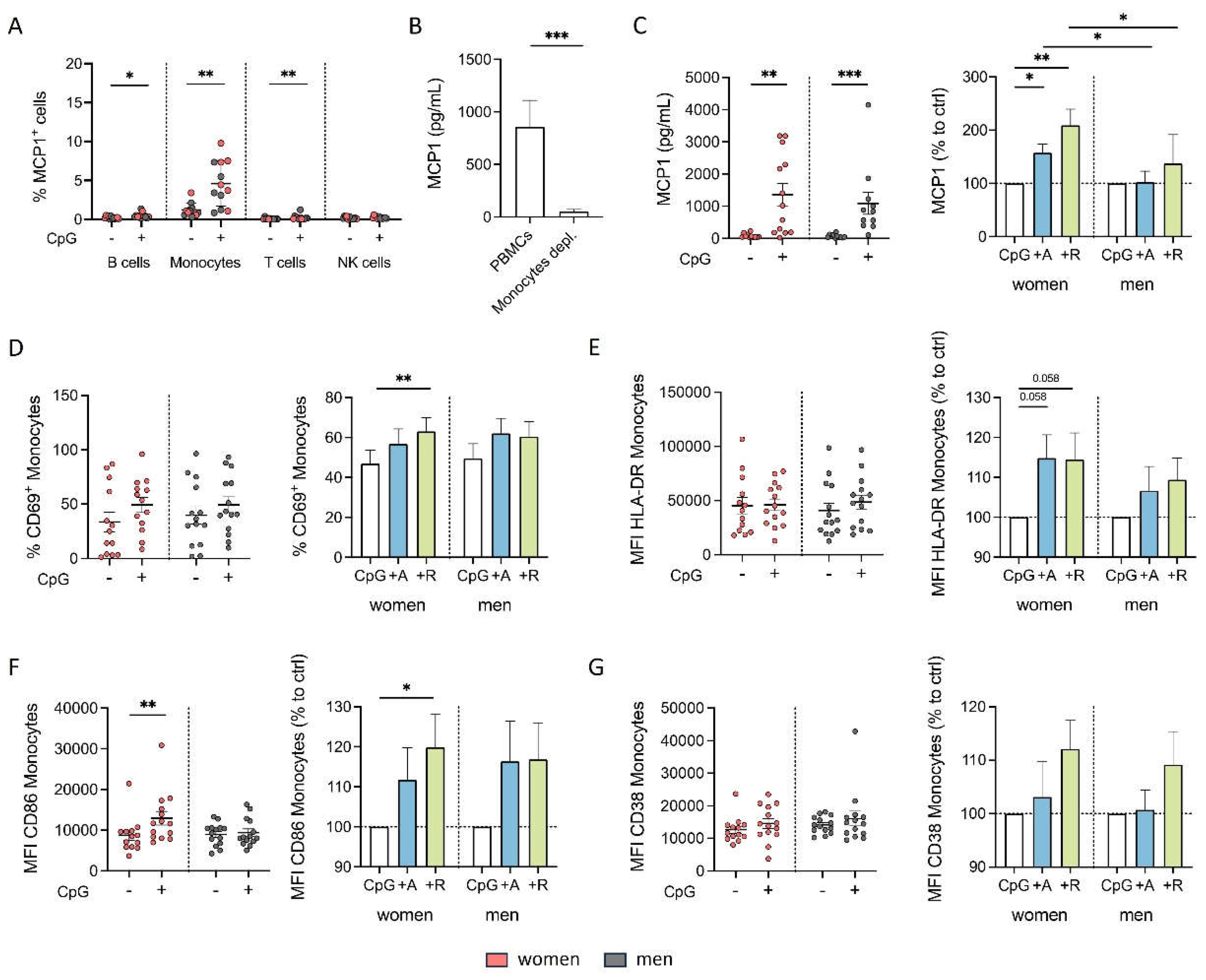
Figure 6.
Proinflammatory phenotype of monocytes from women after DR stimulation is independent of B cells under inflammatory condition. A, B) Secreted MCP1 in supernatant from PBMCs and B cell-depleted PBMCs from women (B) and men (C) after 24 h in cell culture with or without CpG (0.195 μM) stimulation (left) and secreted MCP1 after in vitro stimulation of PBMCs with CpG (0.195 μM) + A68930 (10-7 M) and CpG (0.195 μM) + Ropinirole (10-6 M) for 24 h normalized to CpG control (right) measured via ELISA; n=7-8 each group. C, D) Percentage of CD69+ monocytes from women (E) and men (F) after 24 h in cell culture of mixed PBMCs and B cell-depleted PBMCs with CpG (0.195 μM) stimulation (left) and after stimulation with CpG (0.195 μM) + A68930 (10-7 M) and CpG (0.195 μM) + Ropinirole (10-6 M) for 24 h normalized to CpG control (right) measured via flow cytometry; n=7 each group. E, F) Expression of HLA-DR on monocytes from women (H) and men (I) after 24 h in cell culture of mixed PBMCs and B cell-depleted PBMCs with CpG (0.195 μM) stimulation (left) and after stimulation with CpG (0.195 μM) + A68930 (10-7 M) and CpG (0.195 μM) + Ropinirole (10-6 M) for 24 h normalized to CpG control (right) measured via flow cytometry; n=7 each group. G, H) Expression of CD86 on monocytes from women (K) and men (L) after 24 h in cell culture of mixed PBMCs and B cell-depleted PBMCs with CpG (0.195 μM) stimulation (left) and after stimulation with CpG (0.195 μM) + A68930 (10-7 M) and CpG (0.195 μM) + Ropinirole (10-6 M) for 24 h normalized to CpG control (right) measured via flow cytometry; n=7 each group. I, J) Expression of CD38 on monocytes from women (N) and men (O) after 24 h in cell culture of mixed PBMCs and B cell-depleted PBMCs with CpG (0.195 μM) stimulation (left) and after stimulation with CpG (0.195 μM) + A68930 (10-7 M) and CpG (0.195 μM) + Ropinirole (10-6 M) for 24 h normalized to CpG control (right) measured via flow cytometry; n=7 each group. For comparison of paired data including unstimulated vs. stimulated samples as well as PBMCs vs. B cells depleted, Wilcoxon test was used. Mann-Whitney test was used for testing statistical significance between unpaired data of women and men; *p ≤ 0.05.
Figure 6.
Proinflammatory phenotype of monocytes from women after DR stimulation is independent of B cells under inflammatory condition. A, B) Secreted MCP1 in supernatant from PBMCs and B cell-depleted PBMCs from women (B) and men (C) after 24 h in cell culture with or without CpG (0.195 μM) stimulation (left) and secreted MCP1 after in vitro stimulation of PBMCs with CpG (0.195 μM) + A68930 (10-7 M) and CpG (0.195 μM) + Ropinirole (10-6 M) for 24 h normalized to CpG control (right) measured via ELISA; n=7-8 each group. C, D) Percentage of CD69+ monocytes from women (E) and men (F) after 24 h in cell culture of mixed PBMCs and B cell-depleted PBMCs with CpG (0.195 μM) stimulation (left) and after stimulation with CpG (0.195 μM) + A68930 (10-7 M) and CpG (0.195 μM) + Ropinirole (10-6 M) for 24 h normalized to CpG control (right) measured via flow cytometry; n=7 each group. E, F) Expression of HLA-DR on monocytes from women (H) and men (I) after 24 h in cell culture of mixed PBMCs and B cell-depleted PBMCs with CpG (0.195 μM) stimulation (left) and after stimulation with CpG (0.195 μM) + A68930 (10-7 M) and CpG (0.195 μM) + Ropinirole (10-6 M) for 24 h normalized to CpG control (right) measured via flow cytometry; n=7 each group. G, H) Expression of CD86 on monocytes from women (K) and men (L) after 24 h in cell culture of mixed PBMCs and B cell-depleted PBMCs with CpG (0.195 μM) stimulation (left) and after stimulation with CpG (0.195 μM) + A68930 (10-7 M) and CpG (0.195 μM) + Ropinirole (10-6 M) for 24 h normalized to CpG control (right) measured via flow cytometry; n=7 each group. I, J) Expression of CD38 on monocytes from women (N) and men (O) after 24 h in cell culture of mixed PBMCs and B cell-depleted PBMCs with CpG (0.195 μM) stimulation (left) and after stimulation with CpG (0.195 μM) + A68930 (10-7 M) and CpG (0.195 μM) + Ropinirole (10-6 M) for 24 h normalized to CpG control (right) measured via flow cytometry; n=7 each group. For comparison of paired data including unstimulated vs. stimulated samples as well as PBMCs vs. B cells depleted, Wilcoxon test was used. Mann-Whitney test was used for testing statistical significance between unpaired data of women and men; *p ≤ 0.05.
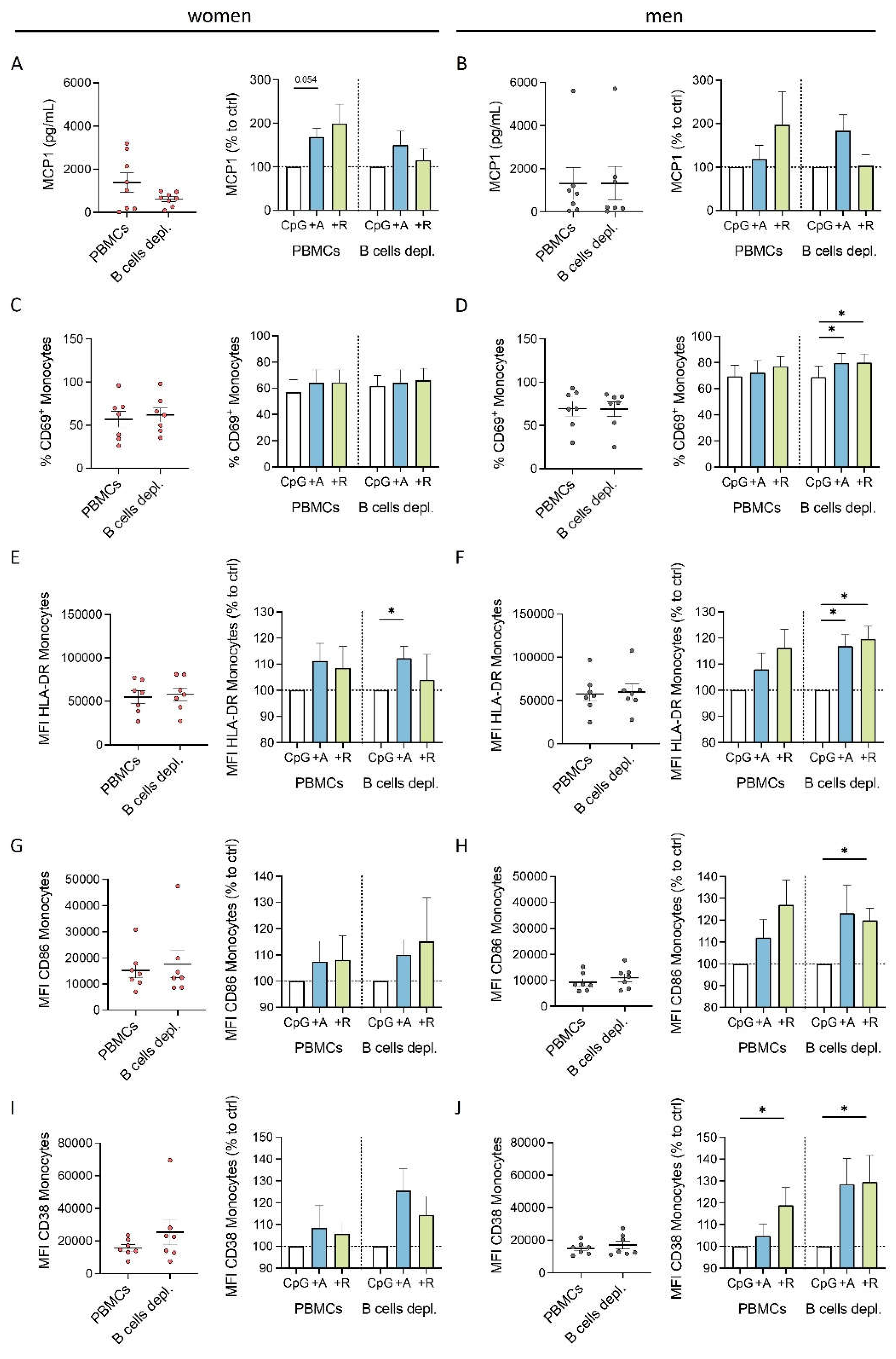
Figure 7.
Effects of DR stimulation on monocytes from women under physiological (left) and acute inflammatory condition induced by CpG (right). DR stimulation leads to a dampened cytokine secretion of monocytes under physiological condition which is dependent on activated B cells. In contrast, acute inflammatory stimulation via CpG leads to a switch into a proinflammatory phenotype of monocytes from women induced by DR stimulation independent of B cells; created with
BioRender.com.
Figure 7.
Effects of DR stimulation on monocytes from women under physiological (left) and acute inflammatory condition induced by CpG (right). DR stimulation leads to a dampened cytokine secretion of monocytes under physiological condition which is dependent on activated B cells. In contrast, acute inflammatory stimulation via CpG leads to a switch into a proinflammatory phenotype of monocytes from women induced by DR stimulation independent of B cells; created with
BioRender.com.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).