1. Introduction
Interleukin-6 (IL-6) is a pleiotropic cytokine of major importance for human health and well-being. Due to its signal transduction mechanisms, it has the capacity to modulate the activity of numerous cells in various organs [
1,
2]. It is thereby involved in the pathogenesis of a large number of different conditions such as chronic inflammatory and autoimmune diseases, cancer, severe infections, cardiovascular disease, the metabolic syndrome, diabetes 2 and neurodegenerative diseases. These conditions are usually characterized by over-production of IL-6 with an enhanced serum concentration.
Different types of cells, e.g., immune cells, mesenchymal cells, endothelial cells, fibroblasts, and tumor cells have the capacity to produce IL-6. Its synthesis is induced by various stimuli such as pathogen or damage associated molecular patterns (PAMPs or DAMPs). The detailed mechanisms for initiation and maintenance of enhanced IL-6 synthesis in chronic inflammation and cancer have, however, still to be clarified [
1]. The inflammatory process can start in different types of tissue injury as discussed by Hirano in “The Local Initiation Model” [
3]. IL-6 synthesis is carefully regulated by transcriptional and post-transcriptional mechanisms (recently reviewed by Hirano [
3]) including stimulation by IL-1β, IL17, TNF-α, prostaglandins, and by cross-linking of Fc-receptors [
4].
IL-6 plays a fundamental role in the cytokine network, pivotal for initiating the normal immune response. However, in specific regulatory contexts, it becomes a central player in immune system dysregulation. IL-6 is of critical importance whether driving over-activation, as seen in inflammatory diseases and cytokine storm, or inducing down-regulation, leading to immunosuppression as in chronic inflammation and cancer [
5,
6]. In addition, it has the capacity to promote tumor progression by stimulating angiogenesis, metastasis, and proliferation of tumor cell [
7]. Furthermore, it acts as an autocrine growth factor in some malignancies, e.g., myeloma and renal carcinoma. Binding of IL-6 to its receptor results in activation of signal transducer and activator of transcription 3 (STAT3) [
8]. Activation of STAT3 is of central importance in many resistance mechanisms [
9,
10] involving chemotherapy, immunotherapy, and radiotherapy [
11]. In particular blockade of IL-6 is suggested in order to overcome resistance to check-point inhibitors [
12,
13].
Not in all cases, could a diagnostic and prognostic correlation of the IL-6 serum concentration in colorectal cancer be demonstrated. A meta-analysis of the IL-6 serum concentration and prognosis including more than 11 000 patients with 23 different types of cancer in 100 studies concluded that the IL-6 serum concentration correlates to the prognosis in later stages, independent of cancer type [
14]. In this analysis the results on colorectal cancer were, however, considered to be inconclusive. In a systematic review on gastrointestinal cancer a high serum concentration of IL-6 was found to correlate with a poor prognosis in gastric, bile duct, and pancreatic cancer, but not in colorectal cancer [
15]. However, in the meta-analysis by Xu et al. it was concluded that a high serum concentration of IL-6 was associated with poor prognosis of colorectal cancer [
16].
Tissue expression of IL-6 in colorectal cancer correlated with lymph node metastasis, venous invasion, advanced stage and was a poor prognosis predictor [
17,
18]. Similarly, tumor expression of IL-6 and IL-6 receptor (IL-6R) were prognostic factors for over-all survival and metastasis-free survival in soft tissue sarcoma patients [
19]. Tumor expression of IL-6 was also found to be a marker of poor prognosis in cervical cancer [
20,
21]. However, tumor infiltrating interleukin-6 positive immune cells at the invasive front were associated with a significantly longer survival in early-stage colorectal cancer patients [
22].
PBMC co-cultured with ovarian cancer cells from advanced patients were found to produce significantly more IL-6 compared to PBMC co-cultured with early-stage cancer cells [
23]. IL-6 synthesis by PBMC from patients with hepatocellular carcinoma [
24] and breast cancer [
25] was found to correlate to prognosis. To further increase the diagnostic efficacy and to discriminate between different clinical stages by analyzing the production of IL-6 in PBMC cultures, a very low concentration of LPS was added to cell cultures from colorectal cancer patients. Indeed, patients with lymph node metastases and normal IL-6 production had good prognosis whereas patients with such metastases and an enhanced production of IL-6 were found to have progressive disease [
26]. LPS stimulated PBMC from advanced pancreatic cancer patients produced significantly more IL-6 compared to healthy controls [
27]. The value of this type of culture set up was also demonstrated in a group of patients with head and neck squamous cell carcinoma, where low IL-6 production in LPS stimulated monocyte cultures predicted long term survival [
28]. The dysregulation of the immune system was further explored by analyzing the signaling response to IL-6 [
29] or IFN-γ [30] by PBMC from breast cancer patients. The IL-6 results in both these studies correlated to prognosis.
The cellular response to IL-6 is mediated by the IL-6R which is expressed only by a limited number of cells (lymphocytes, myeloid cells, and hepatocytes). The signal transduction of IL-6 bound to IL-6R is mediated by binding to glycoprotein 130 (gp130), resulting in classic signaling. Alternatively, IL-6R undergoes proteolytic shedding from receptor-positive cells. Upon binding IL-6, this complex binds to gp130, initiating what is known as trans-signaling [
31]. Gp130 is widely expressed on various cells, the function of which then can be modulated. Classic signaling induce anti-inflammatory and reparative activities whereas trans-signaling is proinflammatory. The latter mechanism is involved in several inflammatory conditions and its blockade by sgp130fc (Olamkicept) has therapeutic activity [
2].
Thus, IL-6 is playing a fundamental role not only for the normal function of the immune system, but also for its dysregulation with over-activity in chronic inflammatory and autoimmune diseases or for immunosuppression in for example cancer and sepsis. These diverse functions complicate the possibility to use IL-6 as a therapeutic target in conditions where disease control at least partly depends on the normal function of the immune system [
32]. In rheumatoid arthritis and Castelman´s disease, inhibition of IL-6 significantly contributes to disease control. Cytokine storm related to CAR T-cell treatment in blood malignancies is efficiently inhibited by IL-6 blockade [
33]. Probably because the immune system is no longer involved in disease control in these conditions. In cancer, IL-6 inhibitors have been described to counteract various pro-tumorigenic activities, but clinical cancer control in humans has not been reported. Siltuximab, a monoclonal antibody binding to and inhibiting the function of IL-6, as a single drug, did not achieve cancer control as shown in multiple studies on various solid tumors [
34,
35,
36]. The lack of effect in these cancers might be due to the fact that in some of these patients the immune system is still of importance for disease control. In addition, blockade of IL-6 carries the risk of infectious diseases as observed during Siltuximab treatment of multiple myeloma [
37,
38,
39].
Although IL-6 mediated trans-signaling can be selectively inhibited by sgp130fc, the uncontrolled overproduction of IL-6 in pathological conditions is still not fully understood [
1]. It would thus be important to find initiators of IL-6 production specific for pathological conditions leaving the normal regulation of IL-6 intact.
In the search for new so far unexplored immunosuppressor mechanisms in cancer, it was found that albumin neo-structures generated by proteolytic fragmentation had immunoregulatory activities such as stimulation of IL-6 synthesis. This mechanism is further described in the present paper.
2. Material and Methods
2.1. Preparation of Peripheral Blood Mononuclear Cells
Venous blood was drawn in glass vaccum tubes with acid dextrose citrate solution A as anti-coagulant (Vacutainer, Becton Dickinson, Franklin Lakes, NJ). PBMC were isolated by Ficoll- paque Plus (GE Healthcare Bio-Sciences AB, Uppsala, Sweden) density gradient centrifugation after removal of erythrocytes by sedimentation in 2% dextran T500 solution (Amersham Pharmacia Biotech AB, Uppsala, Sweden) in 0.9% NaCI.. PBMCs were suspended in RPMI/2% human serum albumin HSA and the cell concentration adjusted to 5 x 105 lymphocytes/ml.
2.2. Preparation of Serum Samples
Sera (collected in Vacutainer, Becton Dickinson, Franklin Lakes, NJ were heat-inactivated for 30 minutes at 56oC and frozen at -70oC. After thawing, sera were diluted in RPMI1640 to a concentration of 20% and used in co-culture experiments for cytokine-induction with control PBMC. 100 μl of diluted sera (20%) or ultra-filtered serum fractions were added to microtiter plates together with 100 μl PBMC suspension (5 x 105/ml). Cell-free supernatants (SNs) were harvested after over-night incubation and tested for monokine activity by ELISA.
2.3. Ultra Filtration
Filtrates from 100 000 mw (molecular weight) filters, retentates or filtrates from 50000 mw filters and fractions concentrated on a 3000 mw cut-off filter were used (Amicon Centriplus centrifugal filters, Millipore Co. MA, US). Retentates were reconstituted in RPMI1640 with 200 IU/ml penicillin, 200 μg/ml streptomycin, 4 mM L-glutamine (Sigma) to their original volume.
2.4. Preparation of Urine Samples
Urine samples from renal cell carcinoma or malignant melanoma patients or healthy individuals were centrifuged for 10 minutes at 3000 x g followed by filtration with a 0.45 μm Millex-HV syringe filter (Millipore) and filtered at 50 000 mw and concentrated on a 3000 mw filter. Before co-culture with normal PBMC, the samples were buffer exchange to RPMI1640 with 200 IU/ml penicillin, 200 μg/ml streptomycin, 4 mM L-glutamine (Sigma) by gel filtration over a Sephadex-G25 (PD-10) desalting column (Pharmacia, SE).
2.5. Albumin Peptides
Synthetic albumin peptides, > 95% pure, were prepared by Schafer-N (Copenhagen, Denmark). They were reconstituted in sterile water H2O (Sigma) for use in ELISA or in RPM11640 (GIBCO) and sterile filtered for use in cell cultures.
2.6. Generation of Cell Culture Supernatants for Cytokine Determination
100 μl of culture medium consisting of RPMI1640 supplemented with 200 IU/ml penicillin, 200 μg/ml streptomycin, 4 mM L-glutamine (Sigma Chemical, MO, US) and 20% fresh heat-inactivated autologous serum were added to un-coated or -pre-coated microtiter plates followed by 100 μl of PBMC suspension (5 x 104 lymphocytes) in RPMI/2%HSA. PBMCs from healthy donors were also cultured in synthetic AIM V Medium (Thermo Fisher). In some experiments Lipopolysaccharide (LPS, Sigma Chemical Co, MO, US) was added at a concentration of 0.05 ng/ml. SNs were harvested after 24 hrs and residual cells were removed by centrifugation (refrigerated Beckman) at 2600 x g for 5 minutes. SNs were frozen and stored at -70oC until cytokine concentrations were measured by ELISA.
2.7. Cyto- / MONOKINE ELISA
Cytokines in culture supernatants were assessed by ELISA using the DuoSet ELISA development system for human IL-6 (R&D Systems Europe Ltd., Abingdon, UK) following the manufacturer's recommended procedures. Samples were analyzed as mean of triplicate wells.
2.8. Autoantibody ELISA
This protocol describes the detection method for assaying relative amounts of total autoantibodies that bind to the IL-6IF-structure, which are present in human sera /plasma. The ELISA Maxisorp 96 well plate (Nunc; #442404) was coated with a solution of 1x Phosphate Buffered Saline (PBS) pH 7.4 containing 5 ug/ml Peptide-IL-6IF, 100 ul per well. The plate was incubated overnight at 4°C. The next day, healthy and cancer patient sera / plasma were defrosted at 37°C for 30 minutes, then diluted to 2% in a solution of PBS containing 1% fish gelatin (Sigma; #G7041) as a buffering and blocking agent fish gelatin-PBS (FG-PBS). The 2% diluted samples were heated at 56°C for 30 minutes. The ELISA plate was washed 3x with phosphate buffer saline Tween (PBST) and 200 ul per well of FG-PBS was added for blocking, with a 60-minute incubation at room temperature. Following blocking, the plate was washed 3x with PBST and 100 ul per well of 2% diluted serum or plasma was added to relevant wells. The plate was incubated for 2 hours at room temperature. Following 3x washes with PBST, the detection antibody was added at 100 ul per well. This was a horseradish peroxidase (HRP)-conjugated sheep-anti-human-IgG antibody (GE; #NA933) diluted 1/3000 in FG-PBS. The plate was incubated for 60 minutes at room temperature and then washed 5x with PBST over 10 minutes. The TMB developer solution (Sigma; #T4444) was added to the wells (100 ul) and the plate was incubated for 30 minutes at room temperature in darkness. The development was stopped with 100 ul per well of 1 molar sulfuric acid. Absorbance readings were taken at 450nm and 570nm in a FluoStar Omega plate spectrophotometer (BMG LabTech).
2.9 Inhibition ELISA
Healthy sera were defrosted at 37°C for 30 minutes, then diluted to 2% in a solution of PBS containing 10% Superblock in PBS (Thermo Scientific; #37515). The 2% diluted control samples were heated at 56°C for 30 minutes in a water bath in order to release autoantibodies (anti modified protein antibodies). Pierce™ Protein G Coated 96 well Plates were then coated with 0.2% autoantibody solution in PBS-T, 100µL/well at room temperature (RT) during 120min on shaker. Plates were then washed 3X using 200uL PBS-T and blocked with 10% Superblock in PBS, 200µL/well at RT during 60min. 100µL/well diluted probe (biotinylated IL-6IF peptide) were added +/- inhibition with serum factors in 1/10 superblock in PBS-0.1% Tween were added and incubated at 37°C during 60min. Plates were then washed 3X using 200uL PBS-T. Rabbit anti-Streptavidin-Alkaline Phosphatase, AKP (diluted 1:1000) in Superblock 1:10 in PBS-0.1% Tween were added (100µL/well) and are incubated for 60 minutes at RT. Plates were then washed 3X using 200µL PBS-T. The PNPP Substrate was dissolved in diethanolamine Substrate Buffer (1X), 100µL were added per well. Samples were incubated for 30-90 minutes at RT. The color development was monitored, and the absorbance were measured at 405nm and the reference wavelength at 570nm was used to compensate for plate impurities. Absorbance was measured using the SpectraMax i3x..
2.10. Adsorption of Human Sera with Protein G-Coupled Sepharose
Heat-inactivated human serum was incubated with protein-G Sepharose. 20% original serum or 20% protein G non-binding serum was added to the cell culture medium.
2.11. Sample Preparation for 2-D Gel Electrophoresis
Urine samples (100 – 450 ml) from cancer patients or healthy controls were ultra centrifuged on Jumbosep centrifugal devices (Pall Life Science, MI, US) using a 30 K membrane insert or alternatively, with a Proflux M12 system using a 30 K Pellicon 2 mini filter (Millipore, MA, US) followed by concentration on Jumbosep with a 3K membrane insert. The urine fraction, 3-30 KD, was tested for cytokine-inducing activity as previously described.
2.12. PBMC-Adsorbed Urine Fractions from Cancer Patients
Cytokine production by the PBMCs in response to urine fractions from cancer patients was verified.
Approximately 50 x 106 PBMC were added to 2.7 ultrafiltered (3-30 KD) urine fractions pooled from two patients with renal cell carcinoma. Unabsorbed urine fractions, used as controls, received the equivalent volume of PBS without PBMC. The urine fractions were incubated for 1½ hour at 4oC. The adsorbed urine fractions were tested for cytokine-inducing activity.
2.13. 2-D Gel Electrophoresis and Mass Spectrometry
2-D was performed in a horizontal 2-D set-up (Multiphore/IPGphore, Pharmacia Biotech, SE) as described based on isoelectric focusing (IEF) in the first dimension and molecular mass in the second dimension [
40]. Briefly, samples (230µg, 350µg, 600µg) were applied to IPG gels, pH 4-7, (Amersham Pharmacia Biotech, SE) and focused overnight for 48000Vh. SDS-PAGE was then carried out with 16% T/1% C polyacrylamide casted slab gels. Molecular weight standards were included in each run. Separated proteins were detected by Coomassie blue staining or SYPRO Ruby staining. The protein patterns in the gels were analyzed as digitized images using a CCD (Charged-Coupled Device) camera (1340 x 1040 pixels) in combination with a computerized imaging 12-bit system, PDQuest Version 6.1.0, in the case of fluorescent stained gels using UV scanning illumination mode (Fluor-S Multi-imager, Bio-Rad). The amount of protein in a spot was assessed as background-corrected optical density, integrated over all pixels in the spot and expressed as integrated optical density (IOD). Tryptic digests of excised protein spots were performed using MALDI_TOF MS (Voyager-DE PRO, Applied Biosystems, CA, US) as previously described [
41].
Electrotransfer and N-terminal sequence analysis: Selected protein spots were electro transferred to PVDF membranes and subjected to N-terminal sequence analysis by Edman degradation in a Procise cLC or a Procise HT sequencer (PE-Applied Biosystems) at the Protein Analysis Center, Karolinska Institute, Stockholm, Sweden.
2.14. Fragmentation of IgG and Serum Albumin Using Matrix Metalloproteinases (MMPs)
MMP -1, -2, –13 (R&D Systems) and MMP-3 and -7 (Chemicon, UK) were activated according to instructions of the manufacturer and were incubated with 1 mg/ml of either human serum albumin (HSA, Octapharma, SE) or pooled human IgG (IvIg, Gammagard, Baxter, DK) The mixtures were incubated for 5-20 hours at 37°C. and buffer exchanged to RPMI and tested for cytokine inducing activity.
2.15. Fragmentation of IgG and Serum Albumin Using Homogenized Tumor Biopsies
Human, frozen, tumor biopsies from patients with renal cell carcinoma, malignant melanoma or colon carcinoma were suspened in 3-10 ml cold RPMI/PEST (Penicillin Streptomycin) or PBS/PEST and homogenized using a Mikro-Dismembrator U (B. Braun Biotech International, GE). The tissue was transferred to a PTFE shaking flask together with 1 ml RPMI/PEST or PBS/PEST and a tungsten carbide grinding ball and homogenized during 15-20 seconds with a shaking frequency of 1500-2000 RPM. The homogenized tumor tissue with 20 mg/ml HSA in RPMI/PEST, was incubated for 18-21.5 h at 37°C. The supernatants were buffer exchanged to RPMI and tested for cytokine inducing activity with normal PBMC.
2.16. Identification of Immune Cell Binding Albumin Sequences—Artificial Cell Surface Chromatography
PBMC were prepared from 4 x 450 ml blood and suspended in PBS containing Ca and Mg (GIBCO) at a concentration of 10 x 106 /ml. EZ Link Sulfo-NHS-biotin (Pierce USA) was added at a final concentration of 0.2 mg/ml and the mixture incubated on a shaker at room temperature for 10 min. Biotinylated PBMC were lysed by adding 1,0 ml ice-cold lysing buffer (50 mM Tris-HCL, pH 7.5, with 0.15 M NaCI, 5 mM MgCI2 containing 100 mM Octyl glucoside and 1 mM Phenylmethylsulfonyl fluoride) per 2 x 107 pelleted cells with gentle shaking, then incubated for 30 min. on ice. Debris was removed by centrifugation.
2.17. Preparation of Affinity Column with Biotinylated Cell Surface Proteins from Mononuclear Cells Coupled to Streptavidin-Sepharose.
Biotinylated cell lysate, corresponding to 36 x 107 mononuclear cells, in lysate buffer was diluted 1/10 in binding buffer (20 mM NaH2PO4, 0,15 M NaCI, pH 7.5) It was added to a 1 ml Hitrap Streptavidin HP affinity column (Amersham Biosciences). The column was carefully washed with PBS and stored in PBS with 0,1% NaN3 at 4°C until use.
2.18. Proteolytic Fragmentation of Denatured Human Serum Albumin with Trypsin
Freeze dried HSA (0.5 mg) was reconstituted in 25 mM NH4HCO3, pH 8, containing 10 mg sequencing grade modified trypsin (Promega Corporation, WI) and incubated at 37 °C overnight. Unfragmented albumin and enzyme was removed by ultrafiltration. The filtrate, containing fragmented HSA without enzyme was collected and diluted with PBS with Ca and Mg (GIBCO).
2.19. Adsorption of Trypsin-Fragmented dHSA Using an Affinity Column with Biotinylated Cell Surface Proteins (ACS)
Two ml of trypsin-fragmented dHSA in PBS, corresponding to a total of 0.2 mg protein, was passaged over the ACS column. The flow-through was collected in small portions of 0.2 ml. Thirty µl of each sample, including a control sample that has not been adsorbed, were dried in a Speed-Vac centrifuge.
2.20. Mass Spectrometry
Dried samples were reconstituted in 10 µl of 0.1 % TFA. Zip Tip pipette tips (Millipore, USA) containing C18 reversed-phase media were used for desalting reconstituted samples. For analysis of samples in the mass range 700 - 3600 Da, one μl of each Zip Tip eluted sample was mixed with 1 μl of a saturated solution of α-cyano-4-hydroxycinamic acid (0.02 mg/ml) in 70%acetonitrile/0.3% trifluoro acetic acid. For the analysis of samples in the mass range 1500 - 9000 Da, 1 μl of each Zip Tip eluted sample was mixed with 1 μl of sinapinic acid (3-methoxy-4-hydroxycinnamic acid). 1 μl of the mixture was spotted on the MALDI plate and analysed using MALDI-TOF MS (Voyager-DE PRO, Applied Biosystems, CA, US). Mass identity search for resulting spectra was performed in the SwissProt or NCBI databases using MS-Fit.
2.21. Generation of Rabbit Antiserum Specific for Albumin Peptide IL-6IF
Peptide IL-6IF was synthesized and conjugated with keyhole limpet hemocyanin (Schafer-N, Copenhagen, Denmark). Polyclonal antisera were generated by immunizations of rabbits with the conjugate and Freund's adjuvants (Agrisera AB, Umeå, Sweden). Rabbit antibodies were prepared using affinity chromatography over protein-A Sepharose and columns with bound IL-6IF peptide (Ultralink lodoacetyl gels (Pierce Biotechnology Inc.). For cell cultures, buffer was exchanged to RPMI and sterile fltered.
2.22. IHC Using anti-IL-6IF Rabbit Antibodies
Frozen tissue sections were fixed in fresh ice-cold acetone for 5 min. and dried at room temperature. The slides were hydrated in TBS for 2 min with added protease inhibitor, washed in TBS for 5 min (x 3), incubated with primary antibody in 2.5 % ready to use horse serum with added protease inhibitor (150 µl/slide) for 1h at room temperature, and washed in TBS for 5 min (x 3). Rabbit specific AP conjugate 150 µl/slide was applied and incubated for 30 minutes at room temperature, then slides were washed with TBS for 5 min (x 4). Next,10 drops of Levamisole were added to 80 μl Impact Vector Red reagent 1 and 60 μl reagent 2 per 5 mL and 150 µl/slide were applied onto the slides, which were incubated for 5 minutes at room temperature, then washed in TBS (x 4) followed by Millipore water before counter-staining with Haematoxylin for 8 min. and mounting with CYTOSEAL XYL. Abcam antibody (ab6672) was used as primary antibody in staining for IL-6.
The specificity of the staining using oligoclonal rabbit antibodies directed against the IL-6IF peptide was demonstrated as the binding of the antibodies to tumors was generally completely inhibited by preincubation of the antibodies with the IL-6IF peptide. In some tumors a strong staining of a few cells with macrophage morphology was observed also after peptide blockade of the antibodies. This background staining was very limited and did never interfere with the evaluation of the staining pattern.
2.23. Statistical Analysis
Data were analysed with the GraphPad Prism v10 or Excel v16.85. Comparisons of the means between different patient groups or different test occasions were performed using an unpaired t-test or Mann Whitney. Comparison between multiple groups were analysed with one-way ANOVA + Dunnett's multiple comparisons test. Pearson Correlation test was used to analyse serum IL-6 concentration and autoantibodies. Time to progression and survival was analysed using the Kaplan-Meier method and Logrank test. Standard error of the mean (SEM) or Standard Deviation (SD) are used. Specific test method is indicated in figure legends.
3. Results
3.1. Production of IL-6 by PBMCs
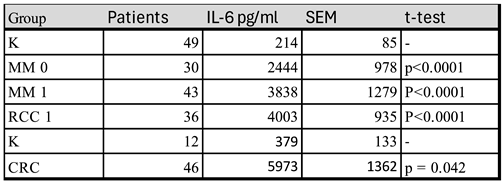
In the search for a model to further explore regulation of IL-6 synthesis. PBMC from cancer patients were found to produce large amounts of IL-6 in short term cultures with autologous serum in the medium (
Table 1). In a first series, patients with radically resected stage III melanoma and untreated patients with metastatic melanoma or renal cell carcinoma were compared to healthy controls and in a second series primary colorectal cancer patients were compared with such controls.
The serum concentration of IL-6 in these patients was generally below the detection limit of the ELISA-technique used. Thus, it is likely that serum IL-6 levels are not only derived from the tumor but is also to a considerable degree produced by PBMCs. This means that mononuclear blood cells in vitro are somehow induced to produce the cytokine. Furthermore, IL-6 production is not restricted to patients with advanced disease as PBMCs from patients with primary colorectal cancers (Figure 5A) and radically resected stage III melanoma (MM 0) also showed enhanced production.
3.2. Occurrence and characterisation of a serum factor inducing IL-6 production by PBMCs
Production of IL-6 by PBMCs from cancer patients in vitro, means that either were the producing cells triggered in vivo and maintained this status in cultures or there is a serum factor which stimulates production of IL-6. Sera from cancer patients with a high IL-6 production induced IL-6 in cultures with normal PBMC in five out of five cultures. (S1,
Table 1, patient 1-5) demonstrating the presence of an IL-6 inducing serum factor. In contrast, sera from patients who did not produce IL-6 in autologous cultures (patient 6-7) did not induce IL-6 in healthy control PBMC (S1,
Table 1).
3.3. Characterization of IL-6IF in serum and urine by ultrafiltration
Ultrafiltration of sera revealed that Il-6 inducing activity generally has a molecular weight of less than 50 kD, in some sera of sometimes it was found in the more than 50 kD but it was not found in the less than 3kD fraction. fraction but not in the less than 3 kD fraction. These results indicate either that the IL-6 inducing activity can depend on molecules of different sizes or that a low molecular weight factor is bound to other serum proteins. If the IL-6IF is a small fragment it is likely that it is bound to other serum proteins or cells as it would otherwise be excreted in the urine. IL-6 inducing activity was actually found in the 3-50 kD fraction in urine from five out of five patients. Urine from two healthy controls had only low IL-6 inducing activity.
3.4. Identification of Interleukin-6 Inducing Activity
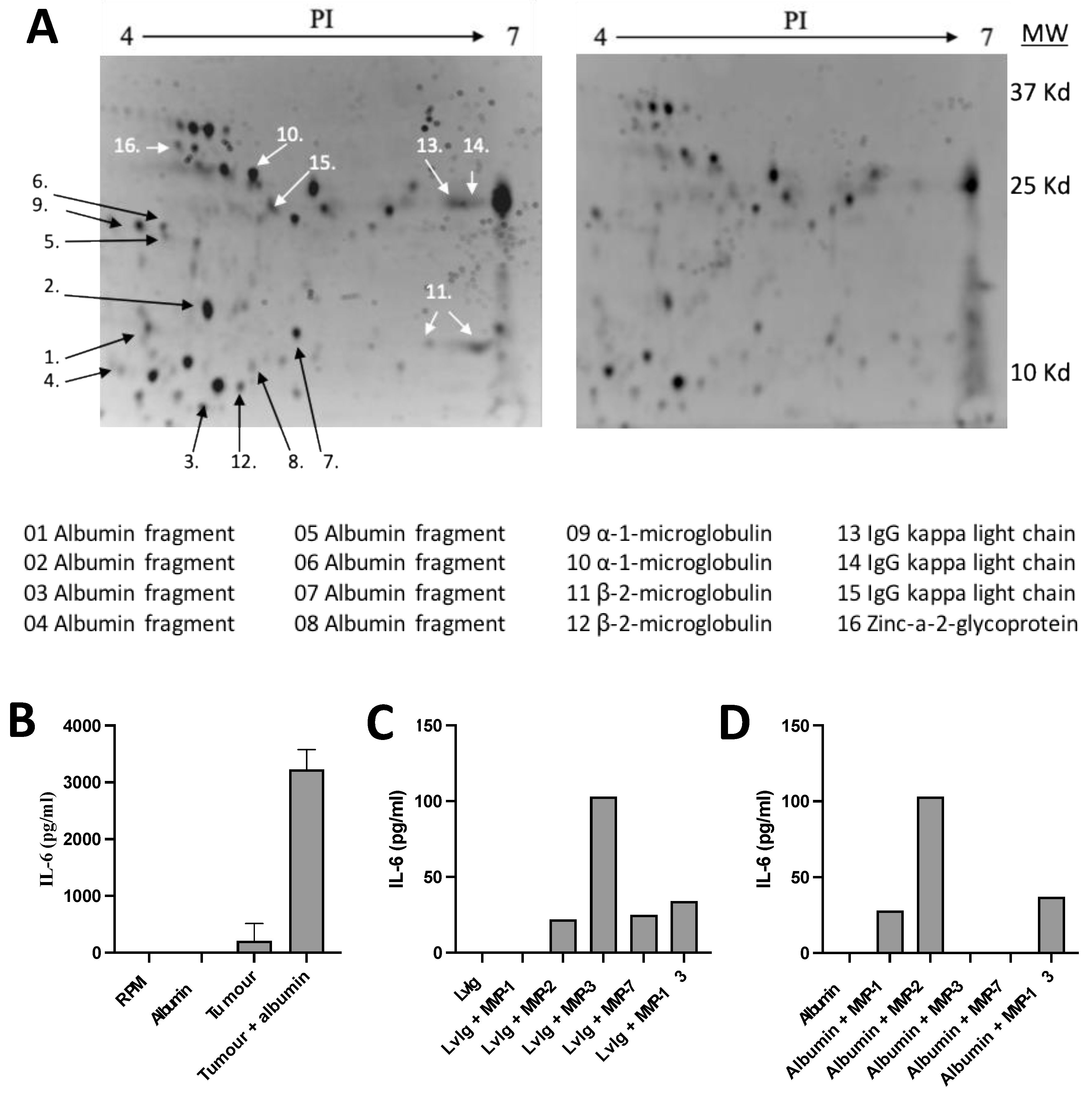
The nature of IL-6 inducing factors was further explored by identifying protein fractions in cancer patient urine. Urine fractions with the capacity to produce high amounts of IL-6 were adsorbed by incubation with a surplus of mononuclear blood cells. The IL-6 inducing activity was thereby significantly reduced. Protein fractions in unadsorbed and adsorbed urine were then compared in 2D-gel electrophoresis. Reduction in the amount of the proteins after adsorption was recorded visually and confirmed using densitometry (
Figure 1A).
Adsorbed proteins were identified using MALDI-TOF MS or amino acid sequencing according to the Edman technique (9 proteins). The identified proteins are shown at the bottom of
Figure 1A. The majority of these fractions were found to be fragments of normally occurring proteins, such as serum albumin, immunoglobulins and microglobulins. Indicating that IL-6 inducing substances might be in the group of albumin fragments with a molecular weight below 25kD or immunoglobulin related fragments with a molecular weight below 37 kD.
These fragments were identified based on their binding to PBMC, indicating the occurrence of receptors for these protein fragments on normal PBMC sensitive to IL-6 inducing factors. As normal albumin does not bind to these cells and induce IL-6, it can be anticipated that fragmentation of albumin results in conformational changes exposing new structures, neo-structures, with a specific binding to receptors on PBMC.
3.5. Production of IL-6 Inducing Activity By Adding Serum Albumin To Washed Tumor Homogenates Or Serum Albumin Or Igg To Activated Matrix Metalloproteases (MMPs)
Tumor homogenates were thoroughly washed to make sure that they did not contain any IL-6 inducing activity. Such homogenates or activated MMPs were incubated with serum albumin or IgG in order to study if tumor tissue (
Figure 1B) or MMPs (Figure C and D) have the capacity to generate active fragments from these proteins.
It was then found that addition of IgG and albumin markedly increased the production of IL-6 inducing activity. The cytokine inducing activity was analyzed in cultures with normal PBMCs and IL-6 production was determined using the ELISA technique. These results indicate that IL-6 inducing fragments of serum albumin or IgG can be produced in the intra-tumoral milieu.
3.5. Identification of the IL-6 Inducing Albumin Sequence – IL-6IF
The results described above indicate that albumin fragments have the capacity to induce production of IL-6. Therefore, a more systematic search for immunoregulatory albumin sequences was started. An artificial cell surface (ACS) was prepared by selectively biotinylating cell surface structures of PBMCs. The cells were dissolved, and the biotinylated proteins were bound to streptavidin columns. A mixture of peptides obtained after trypsination of albumin was adsorbed on such ACS-columns. The binding peptides were then eluted and identified by using the MALDI TOF MS technique. One of the identified peptides, a 24 mere close to the C-terminal of albumin, peptide IL-6IF, was found to induce production of IL-6 when added to PBMC from healthy individuals in short term cultures. A representative experiment is shown in Figure 4A).
3.7. Programming of PBMCs in vivo
Increasing amounts of albumin were added to PBMC cultures and supernatants were collected after 24 hours. The amount of albumin neo-structure was determined using ELISA where plates were coated with rabbit antibodies directed to the IL-6IF-structure. Interestingly, more of these albumin neo-structures were generated in PBMC cultures from advanced cancer patients compared to healthy controls (S1,
Figure 2). This indicates, that not only is there an enhanced amount of the IL-6 inducing neo-structure present in cancer patient sera, but such neo-structures are produced selectively in PBMC cultures from cancer patients, probably due to a unique set up of proteases.
3.8. Distribution of IL-6IF in Tumors
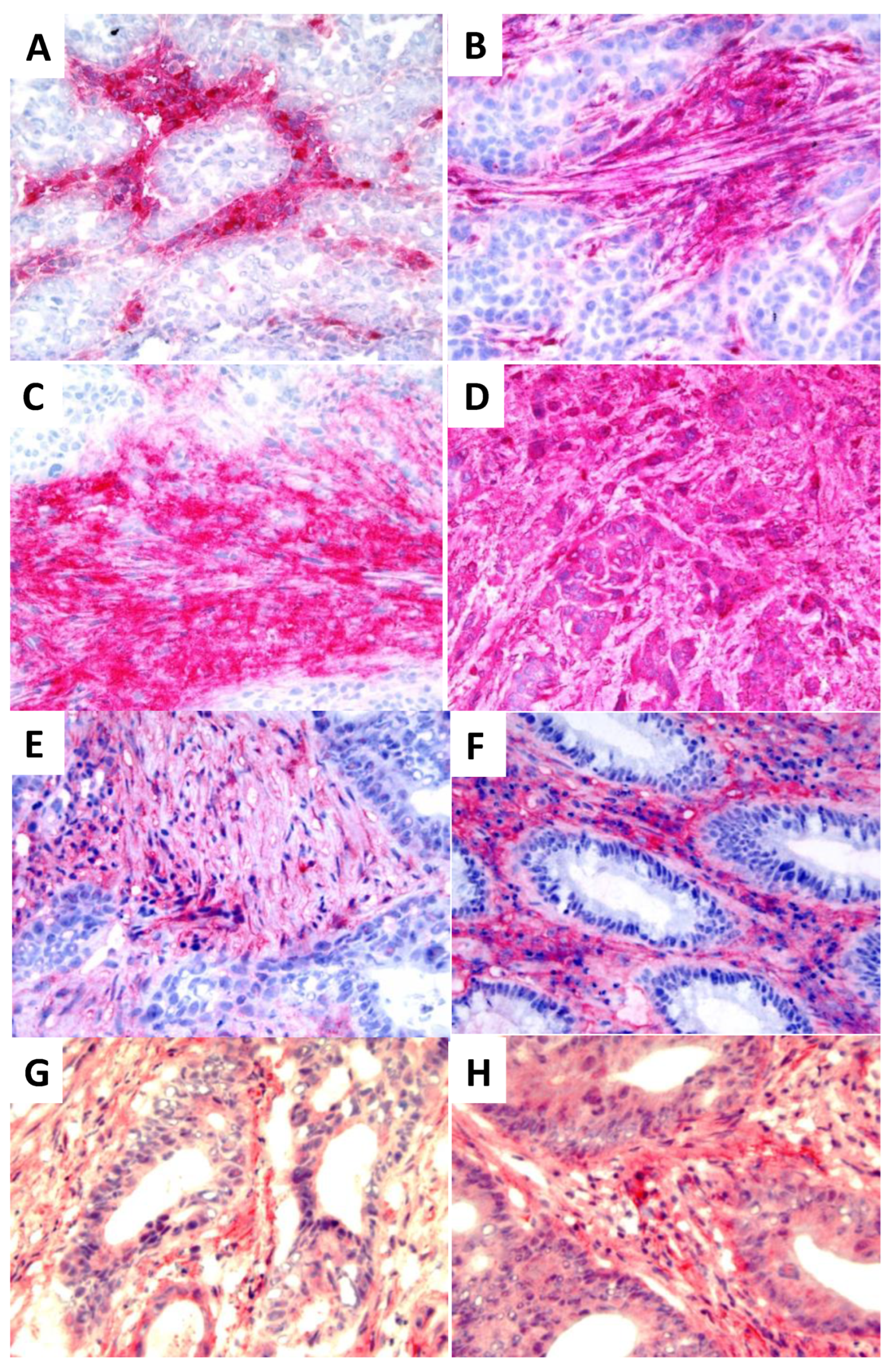
The distribution of the IL-6IF neo-structure in human tumors was explored by histochemical staining using an affinity purified rabbit antibody directed against IL-6IF. As demonstrated in
Figure 2 A -D different staining patterns were obtained in four different human breast cancers.
A similar pattern was found in colon cancer. Two out of ten representative tumors are shown (
Figure 2 E and F). In tumor (2E), IL-6IF is mainly confined to non-cellular stromal areas and some inflammatory cells, in tumor (2F) a strong expression is found in inflammatory cells localized in the stroma. Some tumor cells are faintly stained but the majority do not express IL-6IF. The specificity of the staining was demonstrated as incubation of the antibody with the specific peptide, IL-6IF, blocked the staining.
As an IL-6 inducing albumin neo-structure was found intratumorally, these tumors were also stained for the occurrence of IL-6. Expression of this cytokine was found both in stroma and tumor cells. Examples of two tumors out of ten are shown in
Figure 2 G and H. However, no correlation between occurrence of IL-6IF in the tumors and expression of IL-6 was found, probably because of extensive tumor heterogeneity.
3.9. Immunogenicity of albumin neo-structures – Autoantibodies (Anti-Modified Protein Antibodies) against the neo-structure IL-6IF
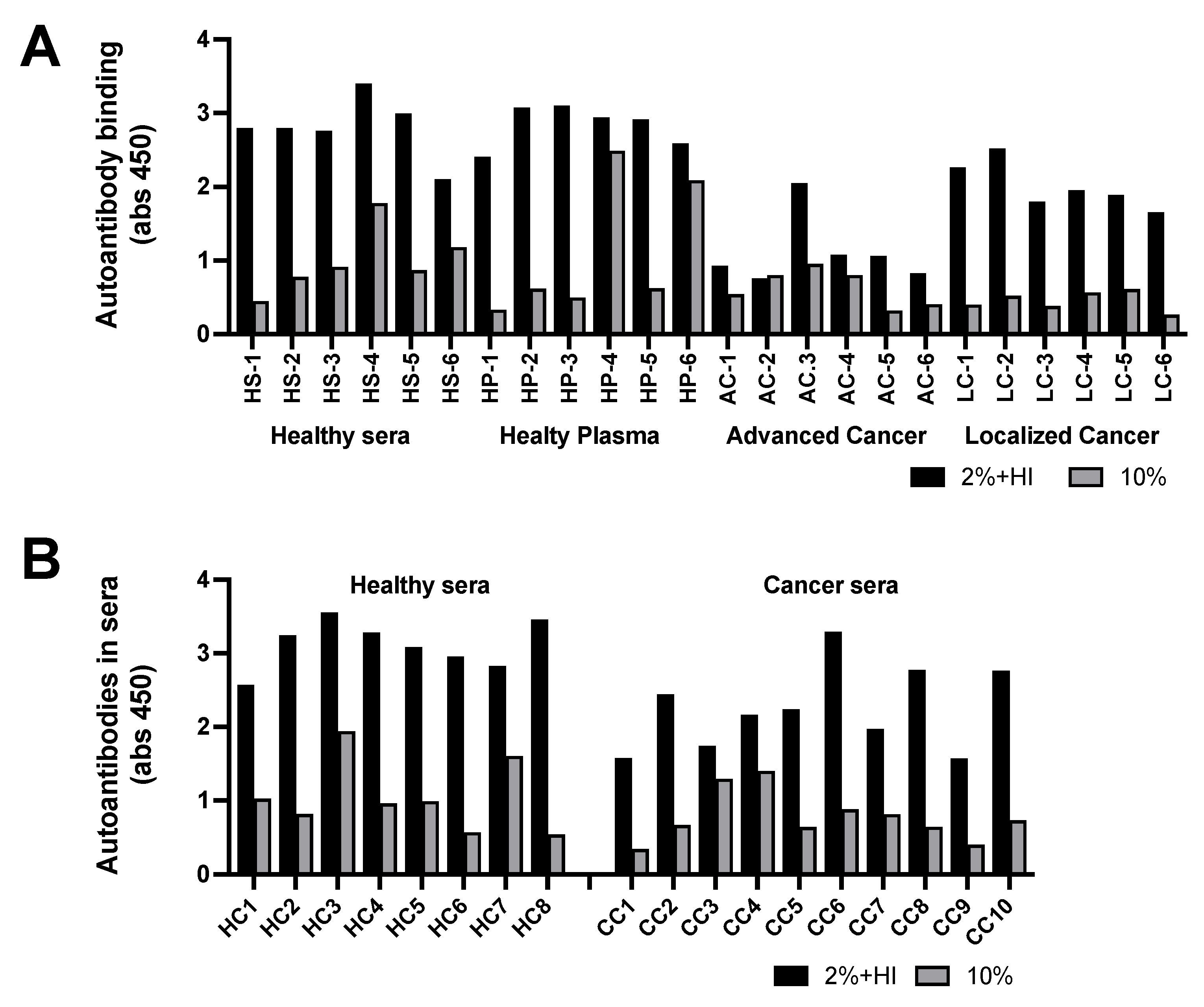
Immunoregulation by albumin neo-structures is further complicated as some of these neo-structures including IL-6IF are immunogenic, resulting in the production of autoantibodies (
Figure 3). The presence of both the antigen, that is IL-6IF, and the autoantibodies (anti-modified protein antibodies) in vivo results in development of immune complexes. Consequently, the albumin neo-structures and autoantibodies exist in immune complexes and as free antigen and antibody depending on their ratio. In order to enable proper determination and clinical evaluation, the constituents of these complexes have to be further characterized.
Using the ELISA technique, autoantibodies in sera were bound to plates coated with the IL-6IF-peptide followed by determination of antibodies using an anti-IgG antibody.
The analyses of two types of samples are shown in
Figure 3 A and B. Serum or plasma was diluted to 10% (grey bars) or diluted to 2% and heat inactivated at 56
oC for 30 minutes (black bars). It was found that standard heat inactivation of plasma or sera resulted in release of a large amount of free antibody. Presumably due to aggregation of albumin neo-structures, which blocks antibody binding sites, resulting in release of autoantibodies. Interestingly, the antibody titer against IL-6IF in sera diluted to 2% (five times more) and heat inactivated contained significantly more free antibodies than sera just diluted to 10 % in both healthy controls and in patients with localized cancer (p<0,0001) but not in patients with advanced cancer, indicating the presences of IL-6IF in high concentration in advanced disease. This is also demonstrated by the fact that serum titer of such autoantibodies after heat inactivation was significantly lower in advanced cancer (p<0.0001) or localized cancer (p<0.0002) disease compared to healthy controls, indicating an increased elimination of immune complexes due to a high production of the antigen. An additional cohort of samples from patients with colon cancer is shown in
Figure 3B. The serum titer of IL-6IF autoantibodies after heat inactivation was significantly lower in colon cancer compared to healthy controls (p<0.0016). The heat inactivation released significantly more autoantibodies in both healthy control and in cancer serum (p<0.0001).
3.10. Effect of Antibodies on IL-6 Synthesis Induced by the IL-6IF Peptide or Serum Factors
The IL-6IF peptide has the capacity to induce IL-6 synthesis by PBMC (
Figure 4A). In another experiment, in order to avoid any influence by serum factors the cultures were set up with a synthetic medium. The IL-6 synthesis was efficiently inhibited by incubation of the inducing medium with affinity purified autoantibodies directed against the IL-6IF-structure bound to protein-G beads (
Figure 4B). This result strongly supports the regulatory effect of autoantibodies on IL-6 synthesis in vivo.
The impact of IL-6IF-structures in sera on IL-6 synthesis was further explored in PBMC cultures where cells from healthy donors were exposed to sera from cancer patients or healthy donors. Rabbit antibodies directed against the IL-6IF were added to the cultures in order to inhibit the IL-6IF-structure in the sera (
Figure 4C and D).
These antibodies had no effect on IL-6 synthesis in cultures with sera from two controls and one cancer patient (Figure4C). As described above IgG and immune complexes can have a regulatory effect on IL-6 production by PBMC (4). Therefore, IgG and CIC was, in another set of cultures, removed from the sera by adsorption using protein-G Dynabeads. This procedure significantly enhanced the inhibitory effect of specific rabbit antibodies blocking the IL-6IF-structure (
Figure 4D) in all cultures indicating that cross-linking of the Fc-receptor (FcR) or cross-linking of the FcR and the cellular receptor of IL-6IF might play an important role in regulation of IL-6 synthesis. As the antibodies in this experiment significantly reduced IL-6 production, the IL-6IF -structure is the main inducing factor and LPS, at this concentration, plays a minor role for IL-6 synthesis.
3.11. Tumor Stage Influences IL-6 Production by PBMCs from Colorectal Cancer Patients
Production of IL-6 by PBMC in autologous cultures depends on the tumor burden. In preoperative patients IL-6 production increases from T
2 to T
3-4 tumors and further in cultures from patients with lymph node metastases (
Figure 5A).
3.12. Correlation between Autoantibody Titer and Serum Concentration IL-6
A possible correlation between the serum concentration of IL-6 and the occurrence of autoantibodies against the IL-6IF-structure was investigated. The serum concentration of IL-6 and autoantibodies are presented in S1, Table 3. A low concentration of these auto-antibodies is considered to be due to a high in vivo production of the IL-6IF-struture binding the antibodies in immune complexes which are eliminated. A low titer of autoantibodies thus indicates a high in vivo exposure to the IL-6IF-structure.
As shown in
Figure 5B, a significant correlation was found between the titer of the autoantibodies and the IL-6 serum concentration in these colon cancer patients (p=0.031). Thus, the serum titer of autoantibodies was inversely correlated to the serum concentration of IL-6 in colon cancer, indicating that a high titer of autoantibodies blocks the activity of IL-6IF.
3.13. Determination of the IL-6IF-structure in sera, using biotinylated IL-6IF peptide as a probe (probe IL-6IF)
Next an inhibition ELISA for determination of the IL-6IF-structure in sera from healthy controls and cancer patients was set up. Antibodies directed against the IL-6IF-structure released from immune complexes by heat inactivation of control sera as described above were bound to protein-G coated ELISA plates. The biotinylated IL-6IF peptide was used as a probe binding to these ELISA plates. Simultaneous incubation with sera containing the IL-6 inducing structure will compete with the binding of the probes and reduce their binding. Thereby giving a measure of the amount of the IL-6IF-structure in serum.
The concentration of the IL-6IF-structure competing with the IL-6IF probe is significantly higher in patients with more advanced cancer. This is in good agreement with the higher production of IL-6 in autologous PBMC cultures from patients with more advanced cancer stages (see
Figure 5A). The difference in concentration of the IL-6IF-structure in colon cancer patients, stadium I – IV is shown in
Figure 5C. Comparison of stages for inhibition of probe IL-6IF, t-test: Stage I versus IV (P<0.0001), I versus III (P=0.0021), I versus II (NS), II versus IV (P=0.0027), II versus III (P=0.0276),
3.14. Prognostic Significance of the IL-6IF-Structure in Serum from Colon Cancer Patients – Survival Analysis
Protein-G plates were prepared as described above, incubated with the serum samples to be analyzed and then incubated with the probe. IL-6IF-structures present in sera compete with the probe and inhibit its binding to the autoantibodies bound to the protein-G plate.
The serum concentration (S1, Table 4) of these neo-structures was significantly enhance, inhibiting the binding of the probe, resulting in low values in sera from colon cancer patients with poor cancer specific survival. The discriminatory level was set to be 0.7. Eight stage III patients in the high IL-6IF group (low probe binding group) had died from their cancer, four were still alive after five years. In the group with low IL-6IF seven stage III patients were still alive after five years. The Kaplan Meier analysis is shown in
Figure 5D. A high serum concentration of IL-6IF was significantly associated with poor survival (p<0.0080, Logrank test).
4. Discussion
IL-6 is of major importance in regulation of the normal immune system and during the pathological function of the immune system, it is a key player in both hyperstimulation and suppression. In addition to IL-6 related immunosuppression and poor prognosis in most types of cancers, this cytokine has often been found to be involved in resistance to treatment including all non-surgical modalities: chemo-, radio- and immunotherapy [
11]. Currently there is a great interest in combining check-point inhibitor therapies with various types of IL-6 inhibitors.
The IL-6 serum concentration is found to be associated with poor prognosis of many types of cancer, particularly in later stages. This, however, does not seem to be the case in colorectal cancer [
14,
15]. The production rate of cytokines such as IL-6 in relation to distribution, binding to cells, receptors, therapeutic antibodies
cellular binding and elimination influences the serum concentration. Consequently, appearance of cytokines in sera from pathological conditions can be expected only at a relatively high production rate. IL-6 can seriously impact and dysregulate the function of the immune system despite a low serum concentration. There are several reports on the discrepancy between the presence of IL-6 in tumor tissue and serum. A study on Hodgkin´s disease found no correlation between the IL-6 expression in Reed Sternberg cells or leukocytes and the serum concentration [
42]. Similarly, the study by Piancatelli et al. did not find any correlation between IL-6 serum concentration and the expression of IL-6 mRNA in colorectal cancer tissue [
43].
In this paper, a large amount of IL-6 was found to be produced by PBMC in cultures from cancer patients. Untreated melanoma and renal cell carcinoma patients with newly detected metastases had in general a serum IL-6 concentration within the normal range, but PBMC from these patients produced large amounts of IL-6. PBMC cultures is a sensitive method to detect an enhanced production of IL-6, also in patients with localized disease, such as preoperative colorectal cancer or radically resected Stage III melanoma. In a previous paper this type of IL-6 production by PBMC from pre-operative colorectal cancer patients was found to be associated with a poor over-all survival [
26]. A high production of IL-6 by PBMC from cancer patients was reported previously [
24,
25]. Similarly, dysregulation of the immune system has been reported for localized breast cancer [
29]. Disease related IL-6 production by PBMC can be a useful diagnostic method to detect subclinical disease after radical surgery. Identification of such patients for adjuvant treatment would most likely enhance the therapeutic efficacy. Obviously, determination of IL-6 production by PBMC in autologous cultures is a more sensitive method to investigate IL-6 related dysregulation of the immune system than just analyzing the serum concentration.
In these in vitro cultures, PBMC were either programmed in vivo to produce IL-6 or, possibly, stimulated by serum factors in vitro. Interestingly, as demonstrated in this study, sera from cancer patients were found to stimulate IL-6 production by PBMC from healthy donors, creating a basis for further analyses of such serum factors. IL-6 inducing activity, which was also found in urine from cancer patients, could be adsorbed by PBMC. 2D-gel electrophoresis of samples before and after this procedure, demonstrated binding of albumin, IgG and β2-microglobulin fragments to the cells, indicating that albumin fragments could have the capacity to induce IL-6 production. In this context, generation of albumin fragments by proteolytic degradation of albumin in urine has to be considered [
44]. However, as demonstrated here the IL-6 inducing fragments are present also in sera and are thus not the result of urine proteolytic activity.
The hypothesis that albumin neo-structures generated in cancer might have an IL-6 inducing activity was analyzed by incubating tumor homogenates with albumin. This resulted in fragments which when tested on normal PBMC were found to induce production of IL-6. The impact of proteolytic degradation was then confirmed by incubation of albumin or IgG with recombinant matrix metalloproteinases followed by analysis of the IL-6 stimulating activity of fragmented albumin on normal PBMC. These structures were then identified using Artificial Cell Surface chromatography and synthesized.
Interestingly, full-sized normal albumin does not have any known immunomodulatory activity. Thus, it was postulated that fragmentation generated conformational changes resulting in neo-structures with immune cell binding properties and immunoregulatory capacity.
These results are compatible with the observation that conformational changes in denatured normal proteins such as human serum albumin, ovalbumin, transferrin, fibronectin etc. expose structures binding to receptors on a monocyte cell line [
45,
46]. It has furthermore been demonstrated that binding of the denatured proteins could be efficiently inhibited by several monoclonal antibodies directed to β2-integrins [
45].
The IL-6 inducing capacity of an albumin neo-structure constitutes a completely new mechanism for initiating IL-6 synthesis. A new IL-6 inducing factor, IL-6IF, was identified. Its generation in pathological conditions characterized by an enhanced proteolytic activity such as malignant tumors and chronic inflammation is in good agreement with the high IL-6 production in these conditions. It also fits very well in “The local initiation model” described by Wilkin [
47] and Hirano [
3] where a tissue injury, including an enhanced proteolytic activity, initiates an inflammatory process.
Many albumin sequences with various biological activities were recently summarized [
48]. Conformational changes of albumin resulting in neo-structures with the capacity to bind to immune cells and induce production of IL-6 has been described. Shacter et al.. [
49] reported that polymers of albumin stimulated peritoneal macrophages to secret interleukin-6 and prostaglandin E2 [
49]. Several papers describe that albumin modified by glycation has the capacity to induce IL-6 synthesis [
50].
The IL-6 inducing structure, IL-6IF was found to be immunogenic resulting in autoantibody production. Consequently, determination of the IL-6IF in sera from healthy controls, cancer patients and patients with inflammatory diseases has to take into account that this structure to a variable degree is bound in immune complexes. In addition to the IL-6IF, antibodies to this structure and immune complexes containing these constituents are involved in regulation of IL-6 synthesis. These results highlight an additional level of immunoregulation, not only are there regulatory albumin neo-structures, but their activity is further modulated by the presence of autoantibodies. In cancer, the serum titer of such autoantibodies is significantly lower in advanced disease compared to healthy controls presumably due to a high production of the antigens, the albumin neo-structures, including IL-6IF. These antigens bind autoantibodies in immune complexes which are then eliminated. Consequently, a high systemic exposure to IL-6IF correlates with a low concentration of specific autoantibodies.
The autoantibodies can be released from immune complexes by “standard” heat inactivation of serum (56oC for 30 minutes). The antigens, the conformationally changed albumin neo-structures, are presumably aggregated by this procedure, which results in blockade and reduced number of antibody binding sites and thereby release of free antibodies. This procedure shows that immune complexes in sera from healthy controls contains significantly more antibodies than sera from advanced cancer patients. In sera from healthy controls with a high titer of free autoantibodies the immune complexes exist in antibody excess whereas in advanced cancer with a low titer of free antibodies a situation with antigen excess prevails. This means that the immunoregulatory function of free albumin neo-structures is exerted when the autoantibody titer is low. Interestingly, “standard” heat inactivation, a procedure frequently used in in vitro immunological experiments, significantly alters the immune status of sera by releasing autoantibodies directed to such immunoregulatory structures.
The impact of the systemic exposure to IL-6IF was further demonstrated by analyzing the correlation between autoantibodies to this structure and the serum concentration of IL-6 in colorectal cancer patients. Interestingly, a low concentration of specific autoantibodies is significantly correlated to a high serum concentration of IL-6. This demonstrates the importance of IL-6IF for pathological production of IL-6 in vivo. These results are further supported by the observation that the serum concentration of IL-6IF, determined in an inhibition ELISA gradually increased with the stage of colon cancer and was significantly higher in sera from advanced stages III and IV compared to stages I and II. It was also found that a high serum concentration of IL-6IF significantly correlated to a poor over-all survival of the patients.
As mentioned above, IL-6 is of major importance in the pathogenesis of a large number of severe diseases such as cancer and chronic inflammation. Therefore, multiple attempts have been made to inhibit its pathological activity by antibodies directed to this cytokine or its receptor or by inhibiting the signal transduction pathways by for example JAK/STAT inhibitors. While IL-6 mediated trans-signaling can be selectively inhibited by sgp130fc (Olamkicept), effective treatment for the typically uncontrolled pathological overproduction of IL-6 in numerous conditions is still not available.
IL-6 production by PBMC from healthy donors incubated with the IL-6IF-structure can be inhibited by specific autoantibodies directed against this structure. Thus, specific autoantibodies which selectively inhibit the pathological IL-6 production stimulated by a protease generated albumin neo-structure were identified. Thereby, the specific epitope of a therapeutic, human monoclonal antibody with the capacity to inhibit pathological production of IL-6 was identified.
IL-6 activity in rheumatoid arthritis and Castleman´s disease can be efficiently inhibited by antibodies in contrast to the situation in cancer. Treating hyperstimulation of the immune system, e.g., cytokine storm elicited by immunotherapy using CAR-T cells with antibodies directed against IL-6 or its receptor has been successful. In this situation IL-6 most likely does not contribute to the control of the disease. In cancer and infectious diseases, however, the situation is more complicated as IL-6 might still be of importance for the immune mediated disease control. Treatment with antibodies directed against IL-6 or its receptor unselectively block the activity of this cytokine. In contrast, antibodies directed against IL-6IF the IL-6 inducing factor described here selectively block pathological IL-6 synthesis related to an enhanced proteolytic activity in diseases such as cancer. Furthermore, these antibodies are developed based on the specificity of autoantibodies normally present in healthy persons who thereby are protected from a potential pathological effect of IL-6IF. Therapeutic administration of such antibodies should therefore not cause any adverse events. Consequently, a substantial therapeutic advantage arises from the selective blockade of pathological IL-6 production, allowing the crucial functions of IL-6 in regulating the immune system's normal response to remain intact, thereby contributing significantly to disease control.
5. Conclusions
PBMC from cancer patients produce large amounts of IL-6 in cultures with autologous serum despite a normal serum concentration of IL-6 in these patients. Using this model pathological activation of IL-6 synthesis was found also in patients with localized disease (colon cancer, T2N0) and patients with clinically undetectable disease (radically resected melanoma Stage III). It was then found that proteolytic degradation of normal serum albumin resulted in conformational changes of albumin generating an immune cell binding albumin neo-structure with the capacity to induce IL-6. An inducer of pathological IL-6 production related to an enhanced proteolytic activity was found, the IL-6 Inducing Factor, IL-6IF. This IL-6 inducing peptide sequence was synthesized and further characterized.
IL-6IF was found to be immunogenic with production of specific autoantibodies with the capacity to inhibit the activity of IL-6IF. Thus, an additional immunoregulatory level was discovered, not only does IL-6IF stimulate IL-6 synthesis but this synthesis can be inhibited by specific autoantibodies. The immune balance, ratio between antigen and antibody determines the IL-6 immune status of these patients. In advanced cancer, a high production of IL-6IF results in production of immune complexes in antigen excess with elimination of autoantibodies. In contrast, a high antibody titer in healthy persons block the activity of IL-6IF normally produced in benign inflammation, and protects the individual from IL-6 production and related symptoms. Actually, a significant inverse correlation between the serum concentrations of IL-6 and autoantibodies in colon cancer patients was demonstrated in this project.
The specific autoantibodies directed against IL-6IF were also used to set up a diagnostic test where the serum concentration of IL-6IF could be determined. It was then found that the serum concentration of IL-6IF was significantly increased in advanced stages of cancer and was significantly correlated to poor cancer specific survival.
Based on the immunoregulatory activity of the autoantibodies, B-cells producing these antibodies can be identified and used to produce recombinant human monoclonal antibodies with this specificity. Such antibodies with a specificity normally present in healthy people are not supposed to cause any adverse events. In contrast to currently available IL-6 inhibitors, these antibodies can be used therapeutically for specific inhibition of pathological IL-6 production in patients, leaving physiological IL-6 regulation needed for the normal function of the immune system, intact.
Author Contributions
Conceptualization, L.H., A.H., and B.C.; Methodology, L.H., B.C., Bi.G.. and P.D.; Investigation, P.D., E.B., Bi.G., and B.C.; Writing – Original Draft, L.H., E.B., and P.D.; Writing – Review & Editing, L.H., P.D., A.H., Y.W.; Funding Acquisition, L.H., A.H., Y.W., and B.G.; Resources, A.H., Y.W., and B.G.; Supervision, L.H., and P.D.
Funding
Funding for this study was provided by: the Swedish state under the LUA/ALF agreement (ALFGBG-966007) and the Swedish Cancer Society (CAN 22 2370), County Council of Östergötland and the Health Research Council in the South East of Sweden. Canimguide Therapeutics AB, Therim Diagnostica / Research AB, Sweden.
Institutional Review Board Statement
The studies were conducted according to the principles of the Declaration of Helsinki and were approved by the Ethical Committees at the University Hospital of Linköping, Sweden, Sahlgrenska University Hospital, Gothenburg, Sweden (No. 118-15, 2023-02974-02 and 2014-81713) and Skane University Hospital, Sweden (No. 2017-928 and 2019-02234).
Informed Consent statement
Patients gave informed consent and studies were approved by their respective ethical review committees.
Data availability statement:
The original contributions presented in the study are included in the study / supplementary material, further inquires can be directed to the corresponding author.
Acknowledgments
We thank the patients for supporting this study. Drs Ben Duell, Rebecca Rosberg are acknowledged for excellent technical assistance and prof. Lennart Greiff for patient sera.
Conflicts of interest
LH is the founder of Therim Diagnostica AB and Therim Research AB and is shareholder of Therim Diagnostica AB and owner of a patent application. AH is shareholder of Therim Diagnostica AB. Additional affiliation of PD: Redoxis AB Medicon Village, Lund, Sweden. P.D., E.B., B.G, Y.W. Bi.G. and BC declare no competing interests.
References
- Tanaka, T.; Kishimoto, T. The Biology and Medical Implications of Interleukin-6. Cancer Immunology Research 2014, 2, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Rose-John, S.; Jenkins, B.J.; Garbers, C.; Moll, J.M.; Scheller, J. Targeting IL-6 Trans-Signalling: Past, Present and Future Prospects. Nat Rev Immunol 2023, 23, 666–681. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T. IL-6 in Inflammation, Autoimmunity and Cancer. International Immunology 2021, 33, 127–148. [Google Scholar] [CrossRef]
- Berger, S.; Balló, H.; Stutte, H.J. Immune Complex-induced Interleukin-6, Interleukin-10 and Prostaglandin Secretion by Human Monocytes: A Network of Pro- and Anti-inflammatory Cytokines Dependent on the Antigen: Antibody Ratio. Eur J Immunol 1996, 26, 1297–1301. [Google Scholar] [CrossRef] [PubMed]
- Fleming, V.; Hu, X.; Weber, R.; Nagibin, V.; Groth, C.; Altevogt, P.; Utikal, J.; Umansky, V. Targeting Myeloid-Derived Suppressor Cells to Bypass Tumor-Induced Immunosuppression. Front. Immunol. 2018, 9, 398. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, H.; Fujieda, K.; Senju, S.; Ikeda, T.; Oshiumi, H.; Nishimura, Y. Immune-suppressive Effects of Interleukin-6 on T-cell-mediated Anti-tumor Immunity. Cancer Science 2018, 109, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Rašková, M.; Lacina, L.; Kejík, Z.; Venhauerová, A.; Skaličková, M.; Kolář, M.; Jakubek, M.; Rosel, D.; Smetana, K.; Brábek, J. The Role of IL-6 in Cancer Cell Invasiveness and Metastasis—Overview and Therapeutic Opportunities. Cells 2022, 11, 3698. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; He, Z.; Ye, J.; Liu, Z.; She, X.; Gao, X.; Liang, R. Progress in Understanding the IL-6/STAT3 Pathway in Colorectal Cancer. OncoTargets and therapy 2020, 13, 13023–13032. [Google Scholar] [CrossRef]
- Rébé, C.; Ghiringhelli, F. STAT3, a Master Regulator of Anti-Tumor Immune Response. Cancers 2019, 11, 1280. [Google Scholar] [CrossRef]
- Matsuda, T. The Physiological and Pathophysiological Role of IL-6/STAT3-Mediated Signal Transduction and STAT3 Binding Partners in Therapeutic Applications. Biological and Pharmaceutical Bulletin 2023, 46, 364–378. [Google Scholar] [CrossRef]
- Singh, S.; Gomez, H.J.; Thakkar, S.; Singh, S.P.; Parihar, A.S. Overcoming Acquired Drug Resistance to Cancer Therapies through Targeted STAT3 Inhibition. International Journal of Molecular Sciences 2023, 24, 4722. [Google Scholar] [CrossRef] [PubMed]
- Huseni, M.A.; Wang, L.; Klementowicz, J.E.; Yuen, K.; Breart, B.; Orr, C.; Liu, L.; Li, Y.; Gupta, V.; Li, C.; et al. CD8+ T Cell-Intrinsic IL-6 Signaling Promotes Resistance to Anti-PD-L1 Immunotherapy. Cell Reports Medicine 2023, 4, 100878. [Google Scholar] [CrossRef]
- Hailemichael, Y.; Johnson, D.H.; Abdel-Wahab, N.; Foo, W.C.; Daher, M.; Haymaker, C.; Wani, K.M.; Saberian, C.M.; Ogata, D.; Bentebibel, S.E.; et al. Interleukin-6 Blockade Abrogates Immunotherapy Toxicity and Promotes Tumor Immunity. SSRN Journal 2021. [Google Scholar] [CrossRef]
- Lippitz, B.E.; Harris, R.A. Cytokine Patterns in Cancer Patients: A Review of the Correlation between Interleukin 6 and Prognosis. OncoImmunology 2016, 5, e1093722. [Google Scholar] [CrossRef]
- Vainer, N.; Dehlendorff, C.; Johansen, J.S. Systematic Literature Review of IL-6 as a Biomarker or Treatment Target in Patients with Gastric, Bile Duct, Pancreatic and Colorectal Cancer. Oncotarget 2018, 9, 29820–29841. [Google Scholar] [CrossRef]
- Xu, J.; Ye, Y.; Zhang, H.; Szmitkowski, M.; Mäkinen, M.; Li, P.; Xia, D.; Yang, J.; Wu, Y.; Wu, H. Diagnostic and Prognostic Value of Serum Interleukin-6 in Colorectal Cancer. Medicine 2016, 95, e2502. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.-C.; Chaen, Y.-L.; Hsu, C.-P. Clinical Significance of Tissue Expression of Interleukin-6 in Colorectal Carcinoma. Anticancer Res 2006, 26, 3905–3911. [Google Scholar] [PubMed]
- Zeng, J.; Tang, Z.-H.; Liu, S.; Guo, S.-S. Clinicopathological Significance of Overexpression of Interleukin-6 in Colorectal Cancer. World Journal of Gastroenterology 2017, 23, 1780–1786. [Google Scholar] [CrossRef]
- Nakamura, K.; Nakamura, T.; Iino, T.; Hagi, T.; Kita, K.; Asanuma, K.; Sudo, A. Expression of Interleukin-6 and the Interleukin-6 Receptor Predicts the Clinical Outcomes of Patients with Soft Tissue Sarcomas. Cancers 2020, 12, 585. [Google Scholar] [CrossRef]
- Domenici, L.; Tonacci, A.; Aretini, P.; Garibaldi, S.; Perutelli, A.; Bottone, P.; Muzii, L.; Benedetti Panici, P. Inflammatory Biomarkers as Promising Predictors of Prognosis in Cervical Cancer Patients. Oncology 2021, 99, 571–579. [Google Scholar] [CrossRef]
- Song, Z.; Lin, Y.; Ye, X.; Feng, C.; Lu, Y.; Yang, G.; Dong, C. Expression of IL-1α and IL-6 Is Associated with Progression and Prognosis of Human Cervical Cancer. Med Sci Monit 2016, 22, 4475–4481. [Google Scholar] [CrossRef] [PubMed]
- Gulubova, M.; Chonov, D.; Aleksandrova, E.; Ivanova, K.; Ignatova, M.M.; Vlaykova, T. Interleukin-6-Positive Immune Cells as a Possible New Immunologic Marker Associated with the Colorectal Cancer Prognosis. Applied Immunohistochemistry & Molecular Morphology 2024, 32, 233–243. [Google Scholar] [CrossRef]
- Nowak, M.; Klink, M.; Glowacka, E.; Sulowska, Z.; Kulig, A.; Szpakowski, M.; Szyllo, K.; Tchorzewski, H. Production of Cytokines During Interaction of Peripheral Blood Mononuclear Cells with Autologous Ovarian Cancer Cells or Benign Ovarian Tumour Cells. Scandinavian Journal of Immunology 2010, 71, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Kang, B.; Ha, Y.; Lee, S.H.; Kim, I.; Kim, H.; Lee, W.S.; Kim, G.; Jung, S.; Rha, S.Y.; et al. High Serum IL-6 Correlates with Reduced Clinical Benefit of Atezolizumab and Bevacizumab in Unresectable Hepatocellular Carcinoma. JHEP Reports 2023, 5, 100672. [Google Scholar] [CrossRef] [PubMed]
- Zare Moaiedi, M.; Ahmadpoor, F.; Rashidi, M.; Ahmadzadeh, A.; Salmasi, A.A.; Mohammadzadeh, G. The Association between mRNA Expression of Resistin, TNF- α, IL-6, IL-8, and ER-α in Peripheral Blood Mononuclear Cells and Breast Cancer. Turk J Med Sci 2021, 51, 1345–1353. [Google Scholar] [CrossRef] [PubMed]
- Clinchy, B.; Fransson, A.; Druvefors, B.; Hellsten, A.; Håkansson, A.; Gustafsson, B.; Sjödahl, R.; Håkansson, L. Preoperative Interleukin-6 Production by Mononuclear Blood Cells Predicts Survival after Radical Surgery for Colorectal Carcinoma. Cancer 2007, 109, 1742–1749. [Google Scholar] [CrossRef] [PubMed]
- Moses, A.G.W.; Maingay, J.; Sangster, K.; Fearon, K.C.H.; Ross, J.A. Pro-Inflammatory Cytokine Release by Peripheral Blood Mononuclear Cells from Patients with Advanced Pancreatic Cancer: Relationship to Acute Phase Response and Survival. Oncology Reports 2009, 21, 1091–1095. [Google Scholar] [CrossRef]
- Aarstad, H.H.; Vintermyr, O.K.; Ulvestad, E.; Kross, K.; Heimdal, J.H.; Aarstad, H.J. In Vitro-Stimulated IL-6 Monocyte Secretion and In Vivo Peripheral Blood T Lymphocyte Activation Uniquely Predicted 15-Year Survival in Patients with Head and Neck Squamous Cell Carcinoma. PLOS ONE 2015, 10, e0129724. [Google Scholar] [CrossRef]
- Wang, L.; Miyahira, A.K.; Simons, D.L.; Lu, X.; Chang, A.Y.; Wang, C.; Suni, M.A.; Maino, V.C.; Dirbas, F.M.; Yim, J.; et al. IL6 Signaling in Peripheral Blood T Cells Predicts Clinical Outcome in Breast Cancer. Cancer Research 2017, 77, 1119–1126. [Google Scholar] [CrossRef]
- Wang, L.; Simons, D.L.; Lu, X.; Tu, T.Y.; Avalos, C.; Chang, A.Y.; Dirbas, F.M.; Yim, J.H.; Waisman, J.; Lee, P.P. Breast Cancer Induces Systemic Immune Changes on Cytokine Signaling in Peripheral Blood Monocytes and Lymphocytes. EBioMedicine 2020, 52, 102631. [Google Scholar] [CrossRef]
- Rose-John, S.; Heinrich, P.C. Soluble Receptors for Cytokines and Growth Factors: Generation and Biological Function. Biochemical Journal 1994, 300, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Nejad, E.B.; Labrie, C.; van Elsas, M.J.; Kleinovink, J.W.; Mittrücker, H.-W.; Franken, K.L.M.C.; Heink, S.; Korn, T.; Arens, R.; Hall, T. van; et al. IL-6 Signaling in Macrophages Is Required for Immunotherapy-Driven Regression of Tumors. J Immunother Cancer 2021, 9, e002460. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, T.; Kang, S. IL-6 Revisited: From Rheumatoid Arthritis to CAR T Cell Therapy and COVID-19. Annu. Rev. Immunol. 2022, 40, 323–348. [Google Scholar] [CrossRef]
- Dorff, T.B.; Goldman, B.; Pinski, J.K.; Mack, P.C.; Lara, P.N.; Van Veldhuizen, P.J.; Quinn, D.I.; Vogelzang, N.J.; Thompson, I.M.; Hussain, M.H.A. Clinical and Correlative Results of SWOG S0354: A Phase II Trial of CNTO328 (Siltuximab), a Monoclonal Antibody against Interleukin-6, in Chemotherapy-Pretreated Patients with Castration-Resistant Prostate Cancer. Clinical Cancer Research 2010, 16, 3028–3034. [Google Scholar] [CrossRef]
- Rossi, J.-F.; Négrier, S.; James, N.D.; Kocak, I.; Hawkins, R.; Davis, H.; Prabhakar, U.; Qin, X.; Mulders, P.; Berns, B. A Phase I/II Study of Siltuximab (CNTO 328), an Anti-Interleukin-6 Monoclonal Antibody, in Metastatic Renal Cell Cancer. Br J Cancer 2010, 103, 1154–1162. [Google Scholar] [CrossRef] [PubMed]
- Angevin, E.; Tabernero, J.; Elez, E.; Cohen, S.J.; Bahleda, R.; Van Laethem, J.-L.; Ottensmeier, C.; Lopez-Martin, J.A.; Clive, S.; Joly, F.; et al. A Phase I/II, Multiple-Dose, Dose-Escalation Study of Siltuximab, an Anti-Interleukin-6 Monoclonal Antibody, in Patients with Advanced Solid Tumors. Clinical Cancer Research 2014, 20, 2192–2204. [Google Scholar] [CrossRef]
- Brighton, T.A.; Khot, A.; Harrison, S.J.; Ghez, D.; Weiss, B.M.; Kirsch, A.; Magen, H.; Gironella, M.; Oriol, A.; Streetly, M.; et al. Randomized, Double-Blind, Placebo-Controlled, Multicenter Study of Siltuximab in High-Risk Smoldering Multiple Myeloma. Clinical Cancer Research 2019, 25, 3772–3775. [Google Scholar] [CrossRef]
- Orlowski, R.Z.; Gercheva, L.; Williams, C.; Sutherland, H.; Robak, T.; Masszi, T.; Goranova-Marinova, V.; Dimopoulos, M.A.; Cavenagh, J.D.; Špička, I.; et al. A Phase 2, Randomized, Double-blind, Placebo-controlled Study of Siltuximab (anti-IL-6 mAb) and Bortezomib versus Bortezomib Alone in Patients with Relapsed or Refractory Multiple Myeloma. American J Hematol 2015, 90, 42–49. [Google Scholar] [CrossRef]
- San-Miguel, J.; Bladé, J.; Shpilberg, O.; Grosicki, S.; Maloisel, F.; Min, C.-K.; Polo Zarzuela, M.; Robak, T.; Prasad, S.V.S.S.; Tee Goh, Y.; et al. Phase 2 Randomized Study of Bortezomib-Melphalan-Prednisone with or without Siltuximab (Anti–IL-6) in Multiple Myeloma. Blood 2014, 123, 4136–4142. [Google Scholar] [CrossRef]
- Lindahl, M.; Ståhlbom, B.; Svartz, J.; Tagesson, C. Protein Patterns of Human Nasal and Bronchoalveolar Lavage Fluids Analyzed with Two-dimensional Gel Electrophoresis. Electrophoresis 1998, 19, 3222–3229. [Google Scholar] [CrossRef] [PubMed]
- Ghafouri, B.; Ståhlbom, B.; Tagesson, C.; Lindahl, M. Newly Identified Proteins in Human Nasal Lavage Fluid from Non-Smokers and Smokers Using Two-Dimensional Gel Electrophoresis and Peptide Mass Fingerprinting. PROTEOMICS 2002, 2, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Gholiha, A.R.; Hollander, P.; Glimelius, I.; Hedstrom, G.; Molin, D.; Hjalgrim, H.; Smedby, K.E.; Hashemi, J.; Amini, R.-M.; Enblad, G. Revisiting IL-6 Expression in the Tumor Microenvironment of Classical Hodgkin Lymphoma. Blood Advances 2021, 5, 1671–1681. [Google Scholar] [CrossRef]
- Piancatelli, D.; Romano, P.; Sebastiani, P.; Adorno, D.; Casciani, C.U. Local Expression of Cytokines in Human Colorectal Carcinoma: Evidence of Specific Interleukin-6 Gene Expression. Journal of Immunotherapy 1999, 22, 25–32. [Google Scholar] [CrossRef]
- Kania, K.; Byrnes, E.A.; Beilby, J.P.; Webb, S.A.R.; Strong, K.J. Urinary Proteases Degrade Albumin: Implications for Measurement of Albuminuria in Stored Samples. Ann Clin Biochem 2010, 47, 151–157. [Google Scholar] [CrossRef]
- Davis, G.E. The Mac-1 and P150,95 Β2 Integrins Bind Denatured Proteins to Mediate Leukocyte Cell-Substrate Adhesion. Experimental Cell Research 1992, 200, 242–252. [Google Scholar] [CrossRef]
- Davis, G.E.; Thomas, J.S.; Madden, S. The A4β1 Integrin Can Mediate Leukocyte Adhesion to Casein and Denatured Protein Substrates. Journal of Leukocyte Biology 1997, 62, 318–328. [Google Scholar] [CrossRef]
- Wilkin, T.J. The Primary Lesion Theory of Autoimmunity: A Speculative Hypothesis. Autoimmunity 1990, 7, 225–235. [Google Scholar] [CrossRef]
- Kragh-Hansen, U. Possible Mechanisms by Which Enzymatic Degradation of Human Serum Albumin Can Lead to Bioactive Peptides and Biomarkers. Front. Mol. Biosci. 2018, 5. [Google Scholar] [CrossRef]
- Shacter, E.; Arzadon, G.; Williams, J. Stimulation of Interleukin-6 and Prostaglandin E2 Secretion from Peritoneal Macrophages by Polymers of Albumin. Blood 1993, 82, 2853–2864. [Google Scholar] [CrossRef]
- Wang, H.-J.; Lo, W.-Y.; Lin, L.-J. Angiotensin-(1–7) Decreases Glycated Albumin-Induced Endothelial Interleukin-6 Expression via Modulation of miR-146a. Biochemical and Biophysical Research Communications 2013, 430, 1157–1163. [Google Scholar] [CrossRef]
Figure 1.
Identification of immune cell binding protein fragments and IL-6 inducing albumin neo-structures. (A). 2D gel electrophoresis of pooled urine fractions (3-30kD) from two patients with renal cell carcinoma, un-adsorbed urine is shown to the left and the fraction adsorbed by purified normal PBMC to the right. Adsorbed proteins / fragments are identified by numbers and their identity is shown at the bottom of the figure. (B). Generation of IL-6 inducing factors by incubation of serum albumin with tumor homogenate. (C) Generation of IL-6 inducing factors by incubation of IvIg with activated MMPs, in particular, MMP-2, -3, -7 and –13 released active fragments from IgG. (D). Generation of IL-6 inducing factors by incubation of serum albumin with activated MMPs, in particular, MMP- 1, -2, -13 released active fragments from albumin.
Figure 1.
Identification of immune cell binding protein fragments and IL-6 inducing albumin neo-structures. (A). 2D gel electrophoresis of pooled urine fractions (3-30kD) from two patients with renal cell carcinoma, un-adsorbed urine is shown to the left and the fraction adsorbed by purified normal PBMC to the right. Adsorbed proteins / fragments are identified by numbers and their identity is shown at the bottom of the figure. (B). Generation of IL-6 inducing factors by incubation of serum albumin with tumor homogenate. (C) Generation of IL-6 inducing factors by incubation of IvIg with activated MMPs, in particular, MMP-2, -3, -7 and –13 released active fragments from IgG. (D). Generation of IL-6 inducing factors by incubation of serum albumin with activated MMPs, in particular, MMP- 1, -2, -13 released active fragments from albumin.
Figure 2.
Expression of IL-6IF in breast and colon cancer and IL-6 in colon cancer. (A-D) Expression of IL-6IF in four human breast cancers. Four different staining patterns are shown. In tumor (A) the IL-6IF structure is mainly expressed in inflammatory cells localized in the stroma, in tumor (B) and (C) this neo-structure is widely present in stromal areas and in tumor (D) the stroma and tumor cells show a strong expression of the IL-6IF structure. (E and F) Occurrence of IL-6IF in two different colon cancers. Inflammatory cells in the stroma and stromal matrix areas clearly express the IL-6IF structure whereas tumor cells are mainly negative. (G and H) Expression of IL-6 in two colon cancers.
Figure 2.
Expression of IL-6IF in breast and colon cancer and IL-6 in colon cancer. (A-D) Expression of IL-6IF in four human breast cancers. Four different staining patterns are shown. In tumor (A) the IL-6IF structure is mainly expressed in inflammatory cells localized in the stroma, in tumor (B) and (C) this neo-structure is widely present in stromal areas and in tumor (D) the stroma and tumor cells show a strong expression of the IL-6IF structure. (E and F) Occurrence of IL-6IF in two different colon cancers. Inflammatory cells in the stroma and stromal matrix areas clearly express the IL-6IF structure whereas tumor cells are mainly negative. (G and H) Expression of IL-6 in two colon cancers.
Figure 3.
Occurrence of autoantibodies directed against IL-6IF. (A). Occurrence of autoantibodies directed against the IL-6IF-structure in sera or plasma from healthy controls and advanced or localized cancer patients. Serum and plasma from 6 controls were compared and the antibody titers were found to be very similar. The antibody titers in sera from one group with advanced, metastatic cancer and one with localized head and neck cancer are shown. Grey bars, samples diluted to 10% and black bars, samples diluted to 2% and heat inactivated for 30 minutes at 56oC. Sera from healthy controls (HS 1-6), plasma from healthy controls (HP 1-6), sera from advanced cancer (AC 1-6) and sera from localized cancer (LC 1-6). (B). Occurrence of autoantibodies against the IL-6IF-structure in sera from a group of colon cancer patients compared to healthy controls. Sera from healthy controls (HC 1-8) and sera from preoperative colon cancer (CC 1-10).
Figure 3.
Occurrence of autoantibodies directed against IL-6IF. (A). Occurrence of autoantibodies directed against the IL-6IF-structure in sera or plasma from healthy controls and advanced or localized cancer patients. Serum and plasma from 6 controls were compared and the antibody titers were found to be very similar. The antibody titers in sera from one group with advanced, metastatic cancer and one with localized head and neck cancer are shown. Grey bars, samples diluted to 10% and black bars, samples diluted to 2% and heat inactivated for 30 minutes at 56oC. Sera from healthy controls (HS 1-6), plasma from healthy controls (HP 1-6), sera from advanced cancer (AC 1-6) and sera from localized cancer (LC 1-6). (B). Occurrence of autoantibodies against the IL-6IF-structure in sera from a group of colon cancer patients compared to healthy controls. Sera from healthy controls (HC 1-8) and sera from preoperative colon cancer (CC 1-10).
Figure 4.
IL-6 synthesis induced by IL-6IF or serum factors and its inhibition by autoantibodies or rabbit antibodies directed against this factor. (A). Production of IL-6 in short term cultures of PBMC from healthy individuals after addition of the IL-6IF peptide (albumin sequence 512-536). The concentration of IL-6 in culture supernatants after 24 hours was measured using the ELISA technique. Bars represents mean +/- SD, *** p < 0.001, t-test. (B). Inhibition of the IL-6 inducing activity of peptide IL-6IF by affinity purified autoantibodies in PBMCs cultured with synthetic medium without serum. Bars represents mean +/- SD, * p<0.05, t-test. (C). IL-6 production by control PMBC in the presence of healthy or cancer sera diluted to 10% with addition of LPS, 0.05 ng/ml. Rabbit IgG or anti- IL-6IF antibody at a concentration of 20 µg/mL were added to half of the cultures (C). Sera from healthy controls, (HC) 1-4 and cancer patients (CP) 1-4. (D). Same experimental set up us Figure C but after depletion of sera by protein-G beads, the effect of the anti-IL-6IF antibody is enhanced.
Figure 4.
IL-6 synthesis induced by IL-6IF or serum factors and its inhibition by autoantibodies or rabbit antibodies directed against this factor. (A). Production of IL-6 in short term cultures of PBMC from healthy individuals after addition of the IL-6IF peptide (albumin sequence 512-536). The concentration of IL-6 in culture supernatants after 24 hours was measured using the ELISA technique. Bars represents mean +/- SD, *** p < 0.001, t-test. (B). Inhibition of the IL-6 inducing activity of peptide IL-6IF by affinity purified autoantibodies in PBMCs cultured with synthetic medium without serum. Bars represents mean +/- SD, * p<0.05, t-test. (C). IL-6 production by control PMBC in the presence of healthy or cancer sera diluted to 10% with addition of LPS, 0.05 ng/ml. Rabbit IgG or anti- IL-6IF antibody at a concentration of 20 µg/mL were added to half of the cultures (C). Sera from healthy controls, (HC) 1-4 and cancer patients (CP) 1-4. (D). Same experimental set up us Figure C but after depletion of sera by protein-G beads, the effect of the anti-IL-6IF antibody is enhanced.
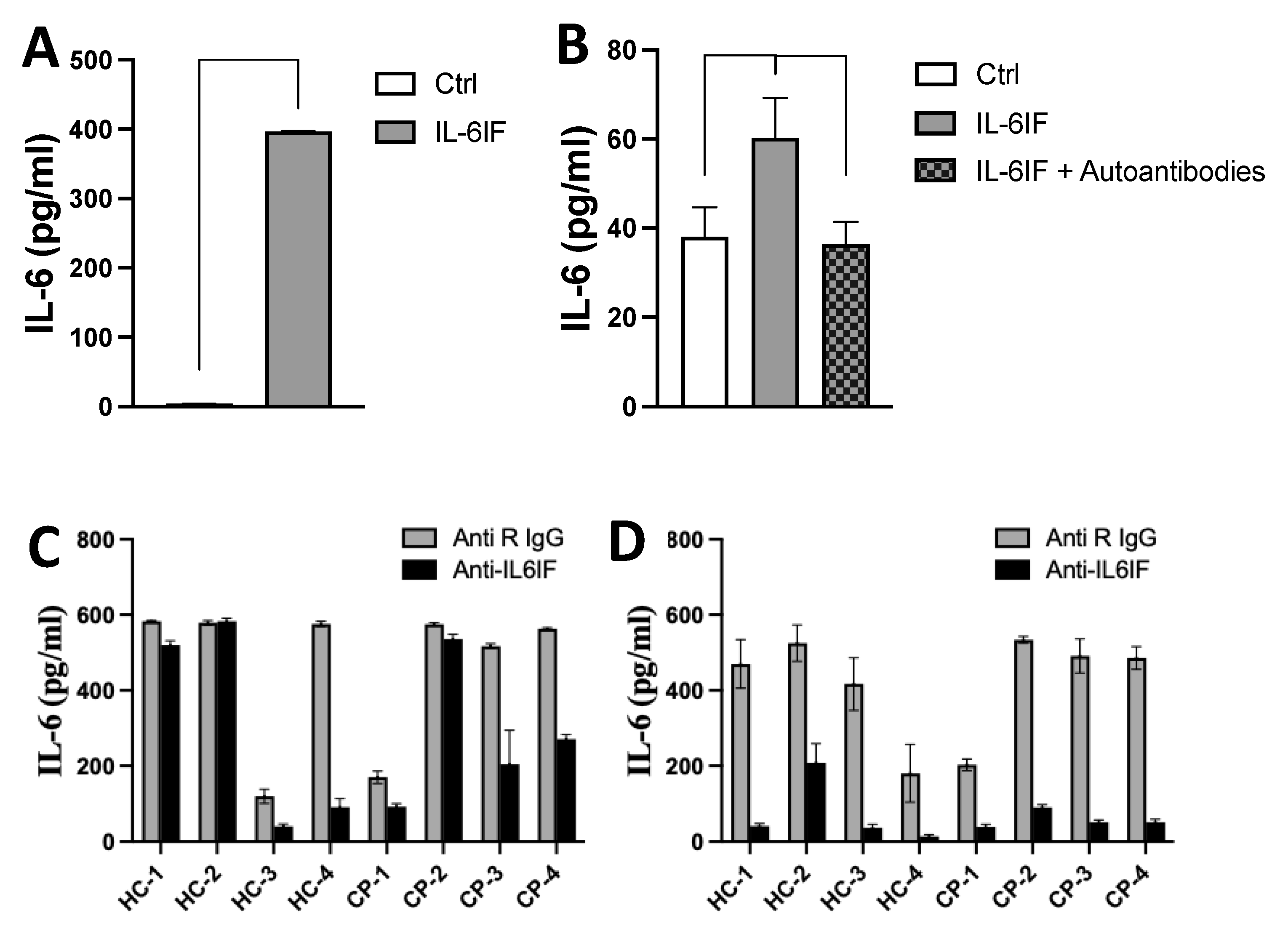
Figure 5.
IL-6 correlation to cancer stage and serum concentration of autoantibodies and association of serum concentration of IL-6IF and cancer stage and cancer specific survival. (A). Impact of tumor burden on IL-6 production by PBMC from colorectal cancer patients. Even patients with localized disease have significant production of IL-6. Bars represents groups (mean +/- SD), *p < 0.05 (Mann Whitney). (B). Correlation between the serum concentration of autoantibodies directed against IL-6IF-structures and the serum concentration of IL-6. Pearson Correlation test, p=0.031, R2 = 0.5694. (C). Serum concentration of IL-6IF according to colon cancer stage. Comparison of stages for probe IL-6IF, t-test: Stage I versus IV (P<0.0001), I versus III (P=0.0021), I versus II (NS), II versus IV (P=0.0027), II versus III (P=0.0276). Base signal was compared with all stages with one-way ANOVA + Dunnett's multiple comparisons test, **** p < 0.0001. (D). Over-all survival, Kaplan Meier analysis of colon cancer patients, stage III, showing a significantly reduced survival of patients with a high production of the IL-6IF-structure. The discriminatory level was set to 0.7. Logrank test (p=0.0080).
Figure 5.
IL-6 correlation to cancer stage and serum concentration of autoantibodies and association of serum concentration of IL-6IF and cancer stage and cancer specific survival. (A). Impact of tumor burden on IL-6 production by PBMC from colorectal cancer patients. Even patients with localized disease have significant production of IL-6. Bars represents groups (mean +/- SD), *p < 0.05 (Mann Whitney). (B). Correlation between the serum concentration of autoantibodies directed against IL-6IF-structures and the serum concentration of IL-6. Pearson Correlation test, p=0.031, R2 = 0.5694. (C). Serum concentration of IL-6IF according to colon cancer stage. Comparison of stages for probe IL-6IF, t-test: Stage I versus IV (P<0.0001), I versus III (P=0.0021), I versus II (NS), II versus IV (P=0.0027), II versus III (P=0.0276). Base signal was compared with all stages with one-way ANOVA + Dunnett's multiple comparisons test, **** p < 0.0001. (D). Over-all survival, Kaplan Meier analysis of colon cancer patients, stage III, showing a significantly reduced survival of patients with a high production of the IL-6IF-structure. The discriminatory level was set to 0.7. Logrank test (p=0.0080).
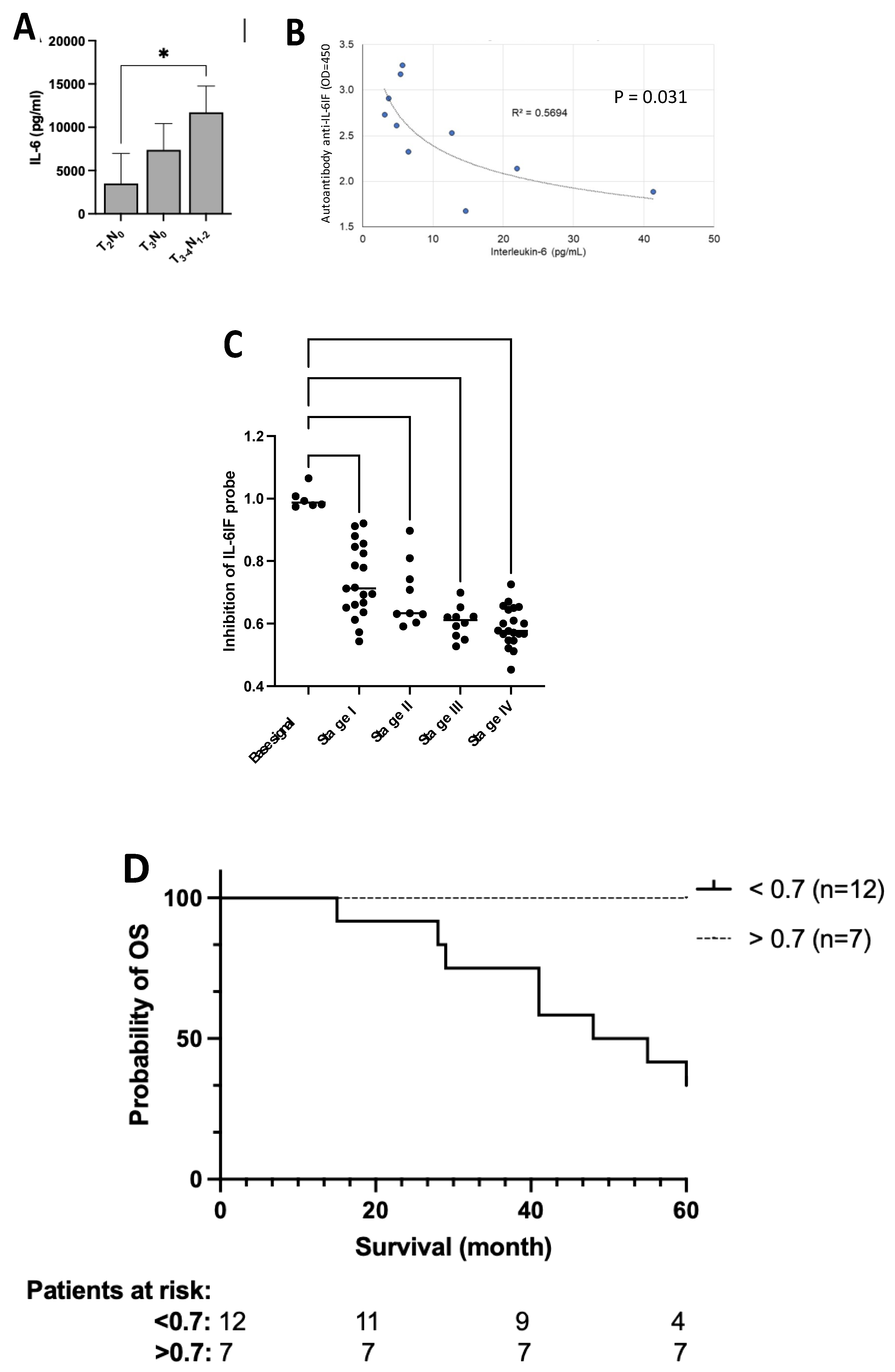
Table 1.
IL-6 production by PBMCs from patients with radically resected stage III melanoma (MM0), previously untreated metastatic melanoma (MM1), previously untreated metastatic renal cell carcinoma (RCC1) and primary colorectal cancer (CRC). IL-6 production is compared to that of healthy controls (K). The table display the mean values +/- SEM and p values are calculated using t-test.
Table 1.
IL-6 production by PBMCs from patients with radically resected stage III melanoma (MM0), previously untreated metastatic melanoma (MM1), previously untreated metastatic renal cell carcinoma (RCC1) and primary colorectal cancer (CRC). IL-6 production is compared to that of healthy controls (K). The table display the mean values +/- SEM and p values are calculated using t-test.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).