1. Introduction
African swine fever (ASF) is a contagious fatal hemorrhagic disease of domestic and European wild pigs [
1]. It continues to spread globally with serious implications on swine health, trade, and economy. The causative agent, ASF virus (ASFV), is a large DNA virus with complex structure and pathogenicity [
2,
3]. Historically, based on the partial sequence of the gene B646L encoding ASFV p72 protein, ASFV strains have been grouped into 24 genotypes [
4]. Recently, based on the full-length B646 sequence, a new classification dividing ASFV strains into six p72 genotypes (1-6) has been proposed [
5].
Until 1957, ASF was restricted to Sub-Saharan Africa. In 1957, an ASFV p72 genotype I strain from Angola spread to Portugal, and subsequently to the neighboring countries in Europe [
6]. Later, the virus spread to Malta, Cuba, Brazil, Haiti, and the Dominican Republic. This outbreak was successfully eradicated from Europe and the Americas by 1995, except from the Island of Sardinia [
7]. In June 2007, an ASF outbreak caused by an ASFV p72 genotype II strain was confirmed in the Caucasus region of Georgia [
8]. The virus quickly spread to Armenia (August 2007), and to the Russian Federation (November 2007), and slowly but steadily spread to the neighboring countries [
9]. In 2018, the virus entered China, the country with the largest pig population in the world [
10]. From China, ASFV p72 genotype II rapidly spread to Vietnam and the neighboring countries in Asia killing millions of pigs [
11].
In 2021, China reported detection of low-virulent ASFV p72 genotype I strains, circulating in domestic pigs [
12]. These strains showed high genetic similarity to the non-hemadsorbing ASFV strains isolated in Portugal in 1968 and 1988. Despite low virulence, they spread efficiently causing mild onset and chronic disease characterized by necrotic skin lesions and joint swellings. In 2023, highly virulent ASFV strains containing genetic elements of both genotypes I and II strains emerged in three provinces of China [
13]. The recombinant ASFV (rASFV I/II) strains contained ~44% of the genome, including p72 gene, from the low-virulent genotype I strains, and the remaining 56% of the genome from genotype II strains previously from China. The live-attenuated p72 genotype II vaccine strain HLJ/18-7GD was not able to protect pigs against the new rASFV I/II.
Vietnam reported its first ASFV outbreak in February 2019 in the Northern Hung Yen province [
14]. The virus was similar to the p72 genotype II strains circulating in China, and it spread throughout the entire country within 5 months [
15]. Since then, p72 genotype II ASFV strains have been circulating in Vietnam killing millions of pigs and causing severe economic losses to the farmers [
16]. In September 2023, rASFV I/II was detected in family-run backyard farms in Northern Vietnam [
17]. The rASFV I/II from Vietnam were genetically similar to those reported from China, indicating a recent spread of these viruses from China to Vietnam.
In 2023, Vietnam licensed two live-attenuated ASFV vaccines, AVAC ASF LIVE
® (AVAC Viet Nam JSC, Hung Yen, Vietnam) and NAVET-ASFVAC
® (Navetco Central Veterinary Medicine Company, Hanoi, Vietnam) to control the ongoing ASFV outbreaks. The master seed in the AVAC ASF LIVE
® was produced by multiple passaging and cloning of ASFV-G-ΔMGF strain [
18] in a cell line (DMAC) developed by the AVAC Viet Nam JSC [
19]. NAVET-ASFVAC
® vaccine contains the ASFV-G-ΔI177L strain [
20] propagated in porcine peripheral leukocyte cultures.
Both these vaccines are recommended for fattening pigs 4 weeks and older, and a single dose of induces protection against the ASFV p72 genotype II strains circulating in Vietnam [
19,
21]. This study was conducted to evaluate the protective efficacy of the two vaccine strains, ASFV-G-ΔI177L and ASFV-G-ΔMGF against the emerging rASFV I/II strain in Vietnam.
2. Materials and Methods
2.1. Viruses
The ASFV-G-ΔMGF strain propagated and tittered on the DMAC (Diep’s Macrophage cell) cell line was provided by AVAC JSC. The ASFV-G-ΔI177L strain [
20], propagated and tittered on porcine primary leukocyte (PPL) cultures was obtained from the USDA. The rASFV I/II isolate VNUA/rASFV/TN1/23 [
17] was propagated and titered on PPL cultures.
2.2. Animal Experiment
A total of 18 healthy, eight-week-old, crossbred (Yorkshire-Landrace-Duroc) pigs obtained from a high-health commercial pig farm in Bac Giang, Vietnam were used in this study. All pigs were confirmed negative for ASF, porcine circovirus-2, foot-and-mouth disease, classical swine fever, and porcine reproductive and respiratory syndrome viruses by real-time PCR and real-time RT-PCR. Pigs were also tested negative for anti-ASFV antibodies by enzyme-linked immunosorbent assay (VDPro® ASFV Ab i-ELISA Version 2.0 Kit, Median Diagnostics, Seoul, Republic of Korea). Upon arrival, pigs were randomly assigned into three groups (6 pigs per group), ear-tagged and moved into three separate pens at the animal facility at AVAC JSC. Pigs were provided ad libitum feed and water and observed at last once daily before vaccination and twice a day after.
After one week of acclimatization, pigs in the first group (pigs #5-10) were inoculated with 1 ml sterile PBS per pig IM. The second group (pigs #11-16) was vaccinated intramuscularly (IM) with ASFV-G-ΔI177L (104 HAD50 in 1 ml per pig). The third group (pigs #17, 18, 20, 21, 22 and 23) was vaccinated IM with ASFV-G-ΔMGF strain (104 HAD50 IM in 1 ml per pig). Please note that there was no pig #19. After vaccination, pigs were monitored twice daily for clinical signs, and their rectal temperatures were measured in the morning using a digital thermometer, while the pigs were eating. Nasal swabs, serum, and whole blood samples from each pig were collected on 0-, 7-, 14-, 21-, and 28 days post-vaccination (dpv). For sample collection, each pig was restrained using a snare. After the sample collection on 28 dpv, all pigs were challenged IM with rASFV I/II at 103 HAD50 in 1 ml per pig.
After the challenge, pigs continued to be monitored twice daily for clinical signs (fever, anorexia, depression, skin discoloration, staggering gait, cough, constipation, and diarrhea), and rectal temperatures were measured daily throughout the experiment. Nasal swabs, serum, and whole blood samples were collected on 4, 7-, 14-, 21- and 28 days post-challenge (dpc). Rectal temperatures above 40 degrees for two consecutive days were considered fever. Any pig reaching the humane endpoint was euthanized using 10 ml of sodium pentobarbital (240 mg/ml) intravenous injection. Any animal found dead or euthanized was subjected to a full postmortem examination and tissues were collected for virus detection. A clinical scoring system developed in-house (
Supplementary Table S1) was used to record the daily clinical findings for each animal.
2.3. DNA Extraction and Real-Time PCR (RT-PCR)
Total DNA from whole blood, nasal swabs, and tissue samples were extracted using DNeasy Blood & Tissue Kit (Qiagen, Hilden, Germany), following the manufacturer’s instructions. ASFV genomic material was detected using the VDx ASFV qPCR kit (Median Diagnostics) according to the instructions in a CFX96 Touch Re-al-Time PCR Detection System (Bio-Rad Laboratories Ltd., Hercules, CA, USA).
To differentiate the vaccine strains from the rASFV I/II strains, two RT-PCR assays, Phan G1 and Phan G2 were developed. Both assays target the ASFV p72 gene and use the same forward and reverse primers, but two different probes (
Supplementary Table S2A). The Phan G1 probe is specific for ASFV p72 gene encoded by genotype I viruses, and the rASFV I/II strains. The Phan G2 probe is specific for p72 genotype II viruses. The reaction conditions were, 50°C for 5 min, 95°C for 20 sec, followed by 40 cycles of 95 °C for 3 seconds and 60 °C for 30 seconds. Samples with a Ct (cycle threshold) value less than 40 were considered positive for ASFV genomic material. The sensitivity of the Phan G1 and G2 assays were compared to the pan-ASFV real-time PCR assay Tignon [
22] and ASFV Genotype I specific real-time PCR assay G1-ACDP [
23] (
Supplementary Table S2B).
2.4. ELISA
Anti-ASFV antibodies in the serum samples were detected using VDPro® ASFV Ab i-ELISA V 2.0 kit (Median Diagnostics) according to the instructions from the manufacturer. Samples with result interpretation, S/P ratios ≥ 0.25 were considered positive, while those with S/P ratios < 0.25 were considered negative.
3. Results
3.1. Clinical Signs
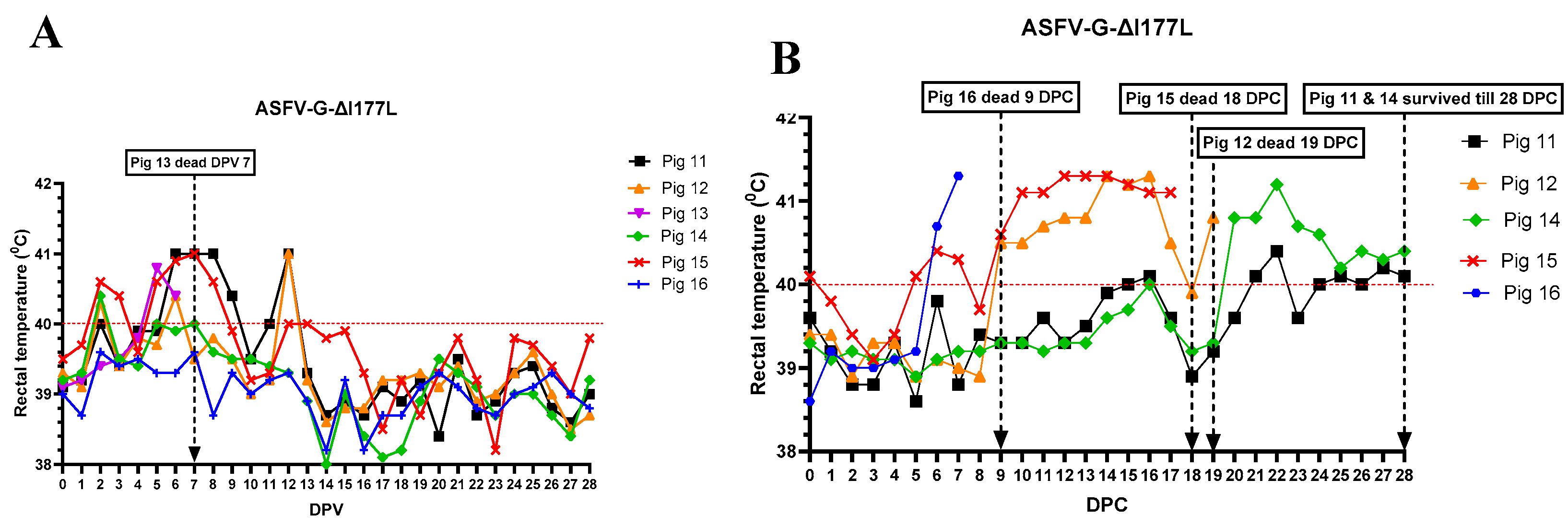
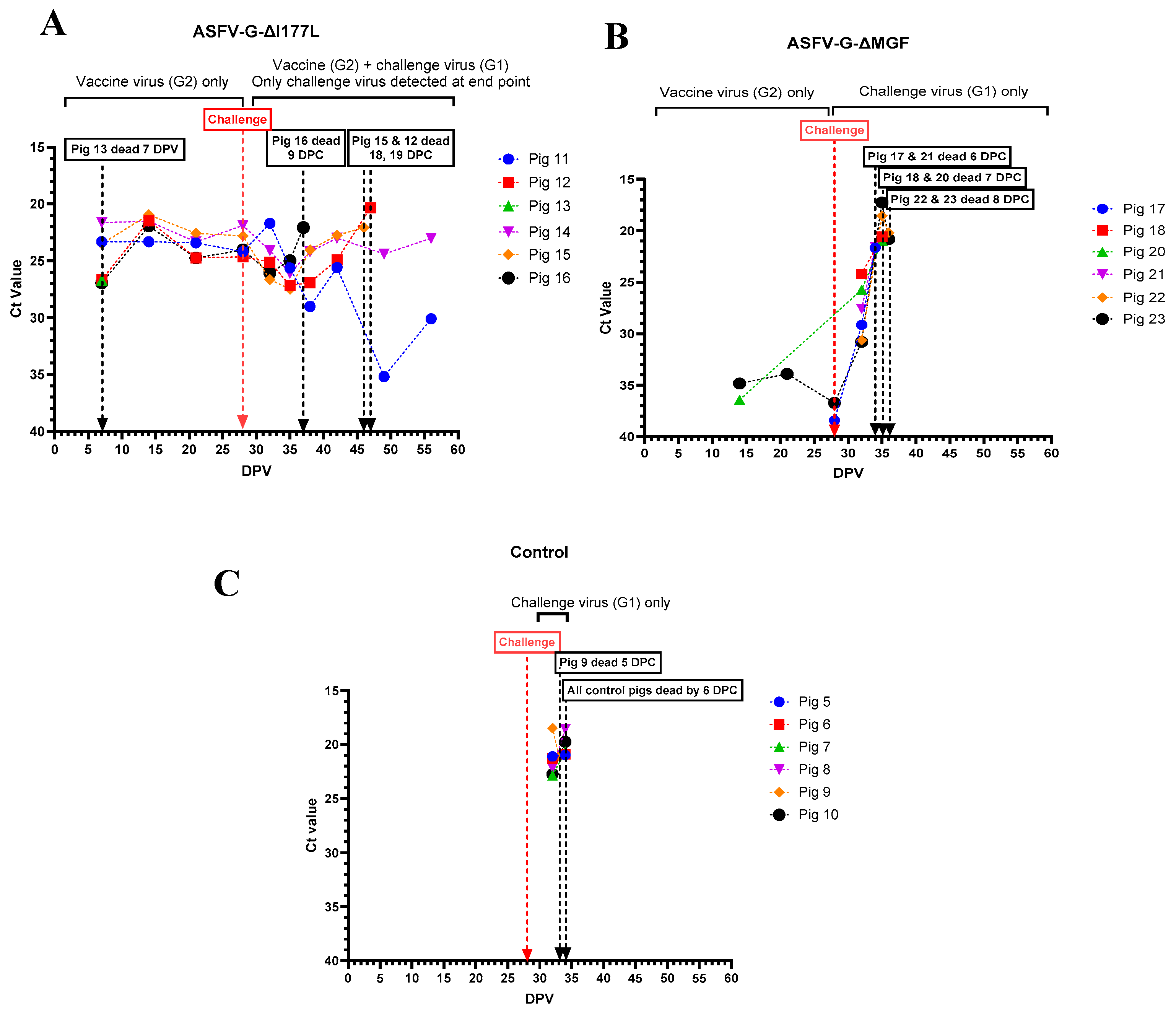
Of the six pigs vaccinated with ASFV-G-ΔI177L, three (pigs #11, 13 & 15) developed a mild fever between 2- 5 dpv (
Figure 1A), and pigs #16 and #11 were lethargic and not interested in feed between 5 - 7 dpv.
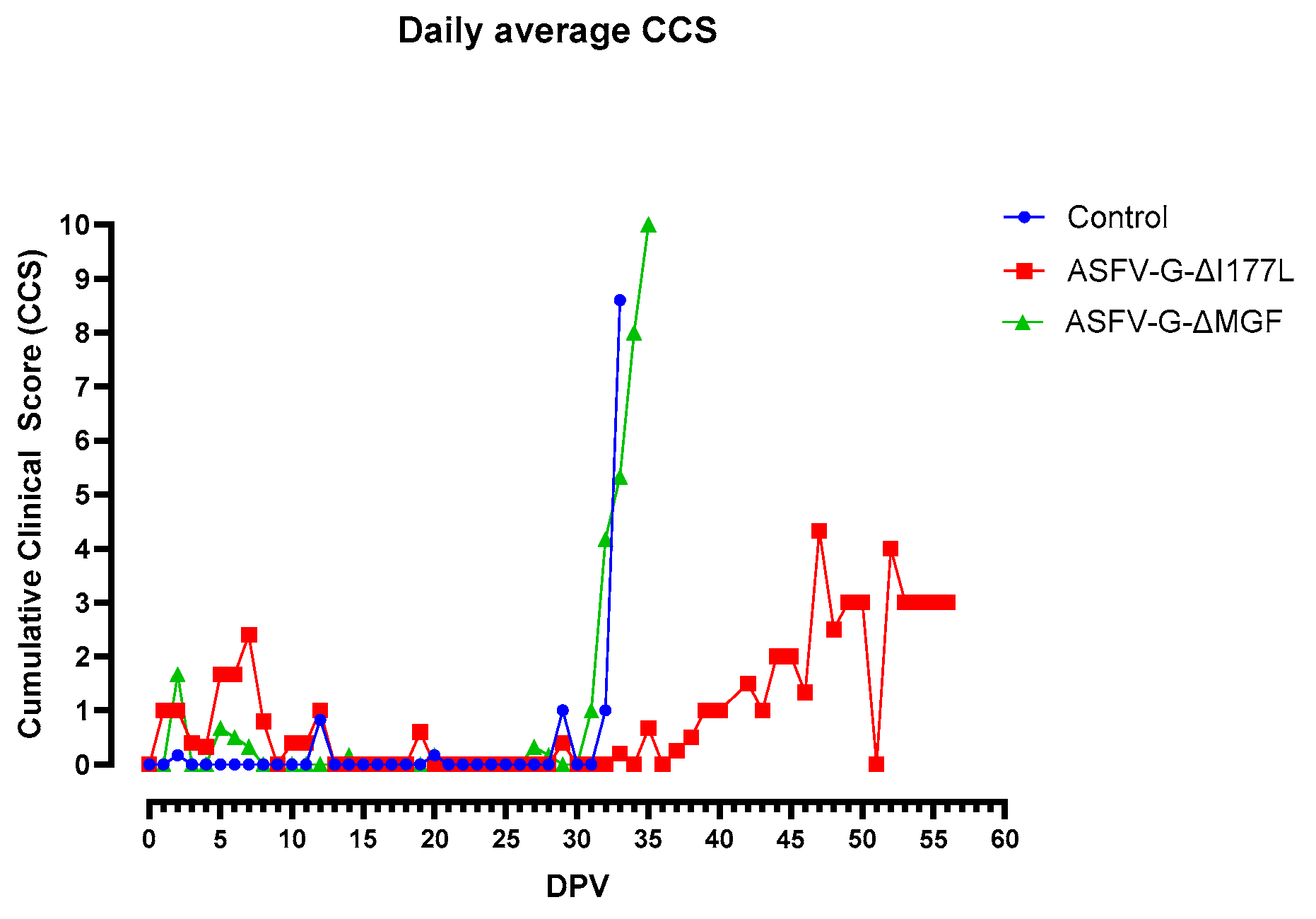
As a result, the daily cumulative clinical score (CCS) of this group increased slightly (
Figure 2).
On 7 dpv, pig #13 was found dead. After challenge with rASFVI/II, pig #15 developed a mild fever on 5 dpc, but the rectal temperature returned to normal by 8 dpc. Starting 9 dpc, rectal temperature of pig #15 increased again, reached its peak by 12 dpc, and remained high until it was found dead on 18 dpc. Pig #16 developed fever on 6 dpc, reached 41.5 ℃ by 7 dpc and it was found dead on 9 dpc. Pig # 12 developed fever on 9 dpc, peaked on 14 dpc, returned to normal on 18 dpc, developed fever again on 19 dpc, and was euthanized on the same day as it reached the humane endpoint. Pigs #14 and 11 developed a mild fever on 20 and 21 dpc respectively, and the fever remained until the end of the study. As the pigs developed clinical signs post-challenge, the CCS of the group increased gradually (
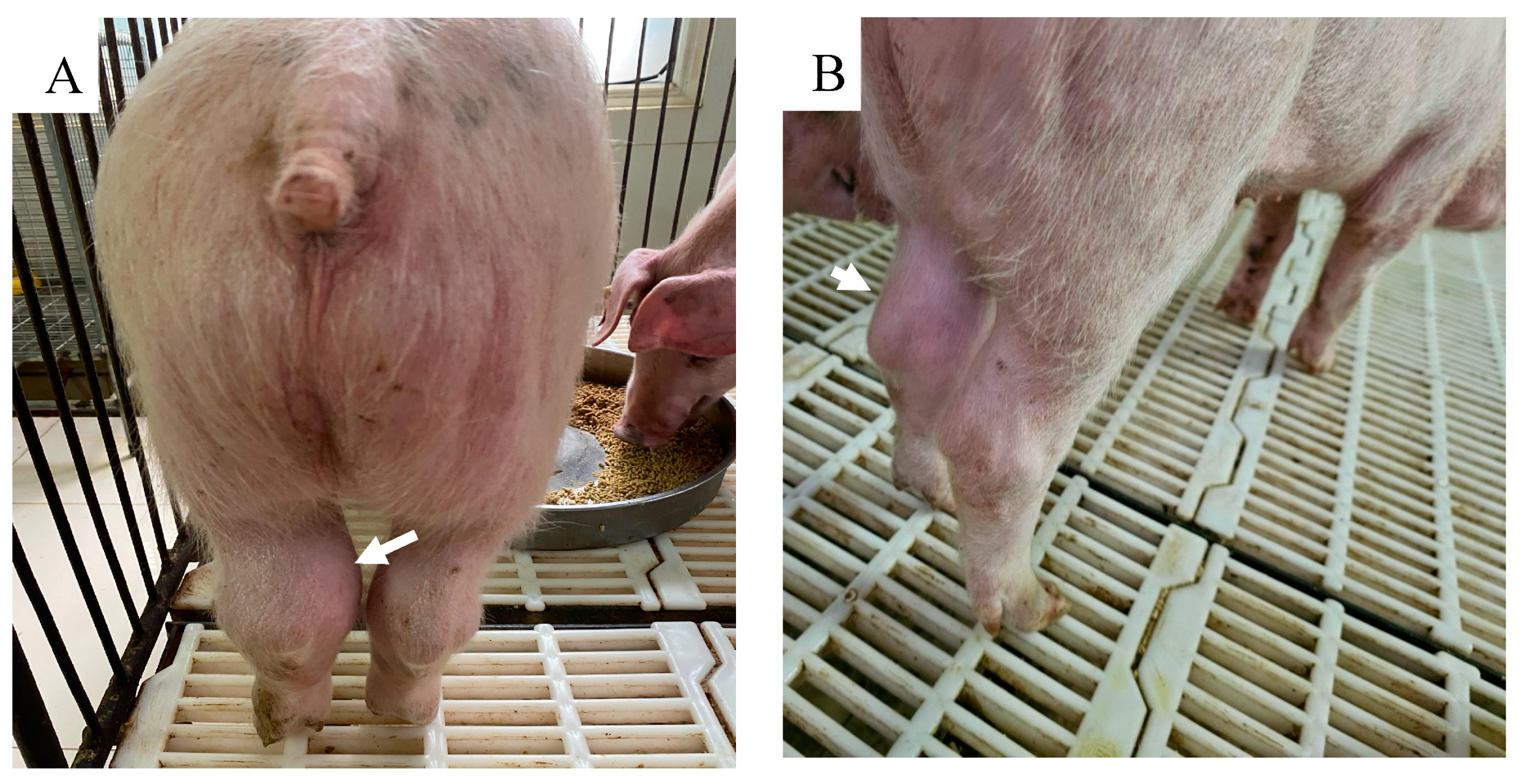
Figure 2). Starting 20 dpc, pig #14 started limping and a swelling in its left hock (tarsal) joint was observed. It became lethargic and depressed, and the swelling and limping worsened over time. By 28 dpc, a mild swelling in the right hock joint, and a large subcutaneous hemorrhage with denuded skin were observed under the neck. Pig #11 stayed active until the end of the study but started to develop a mild swelling in the left hock joint around 27 dpc (
Figure 3). Pigs #14 and 11 were euthanized on 28 DPC inorder to conclude the study.
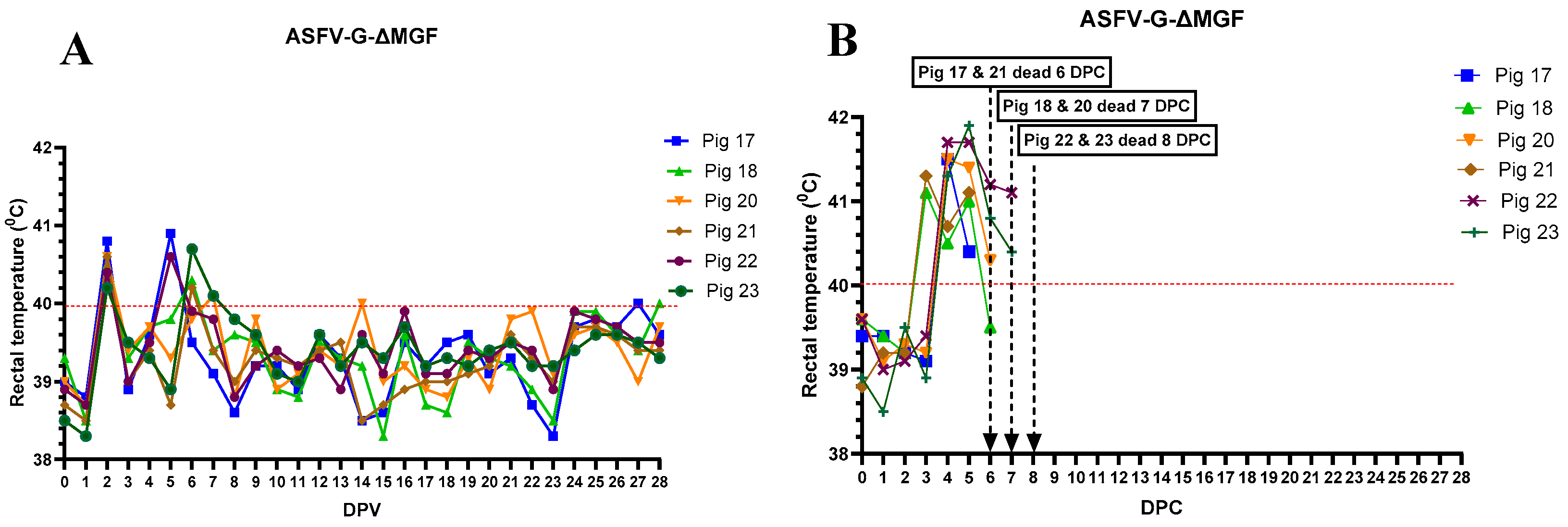
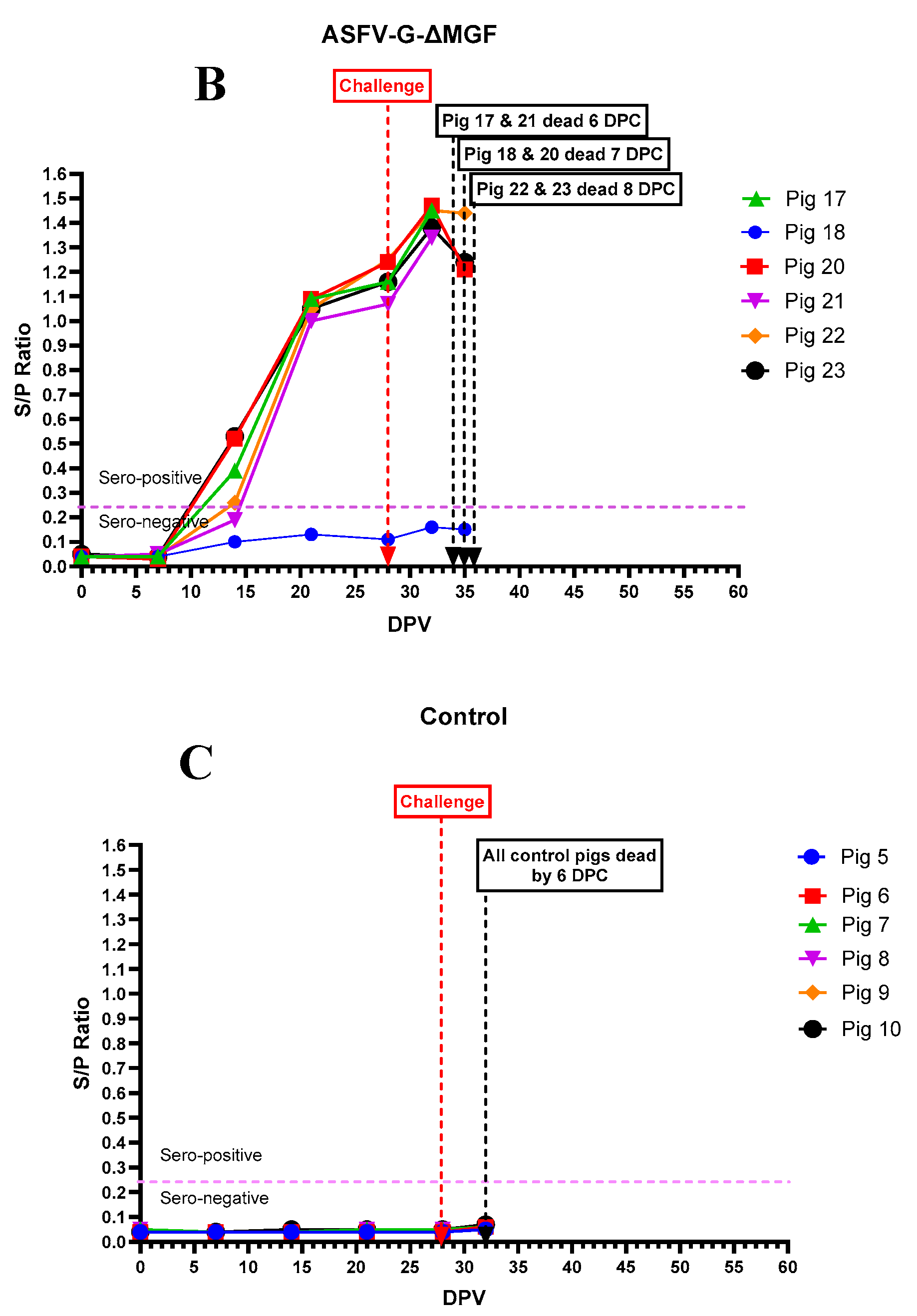
In the ASFV-G-ΔMGF group, one pig (#23) developed a mild fever for 2 days (
Figure 4A). All pigs remained active and alert until they were challenged on 28 dpv, hence the CCS of these animals remained low (
Figure 2). After the challenge, all six pigs developed high fever, anorexia, lethargy, and skin hemorrhages, and were humanly euthanized by 8 dpc (
Figure 4B).
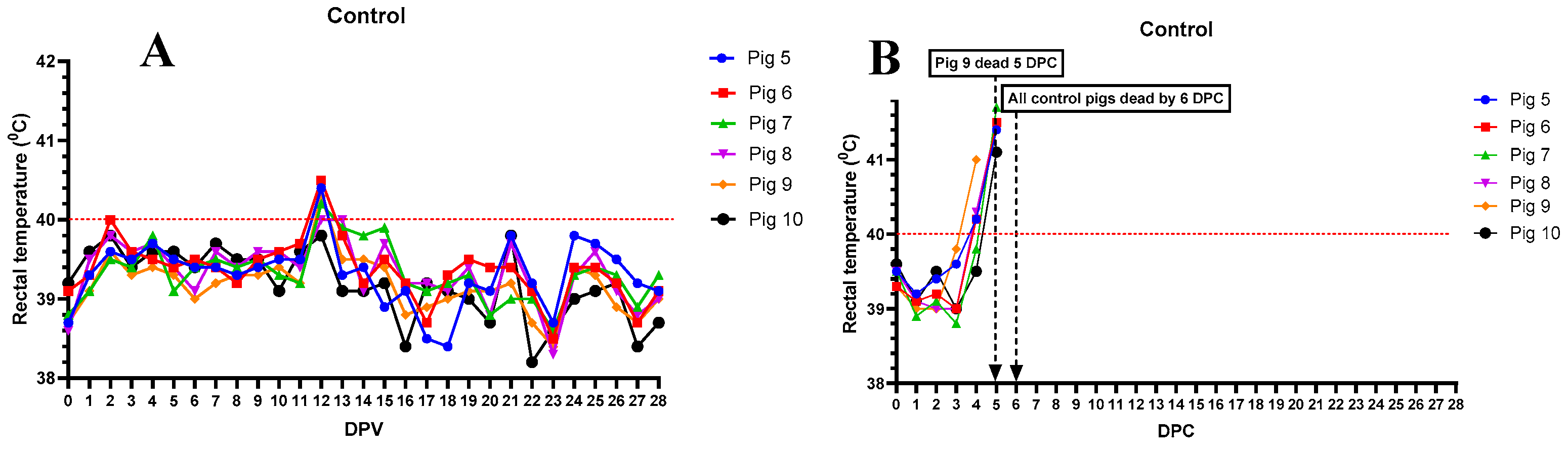
As expected, all pigs in the control group, (pigs #5-10) remained bright and alert and exhibited normal appetite and developed no fever before the challenge (
Figure 5A). After the challenge, all 6 pigs developed high fever (
Figure 5B), severe anorexia, lethargy and were found dead or humanly euthanized within 6 days. No skin lesions, respiratory (labored breathing) or digestive (constipation, diarrhea or blood in stools) signs were observed before they succumbed to ASF.
3.2. Viremia and Virus Shedding
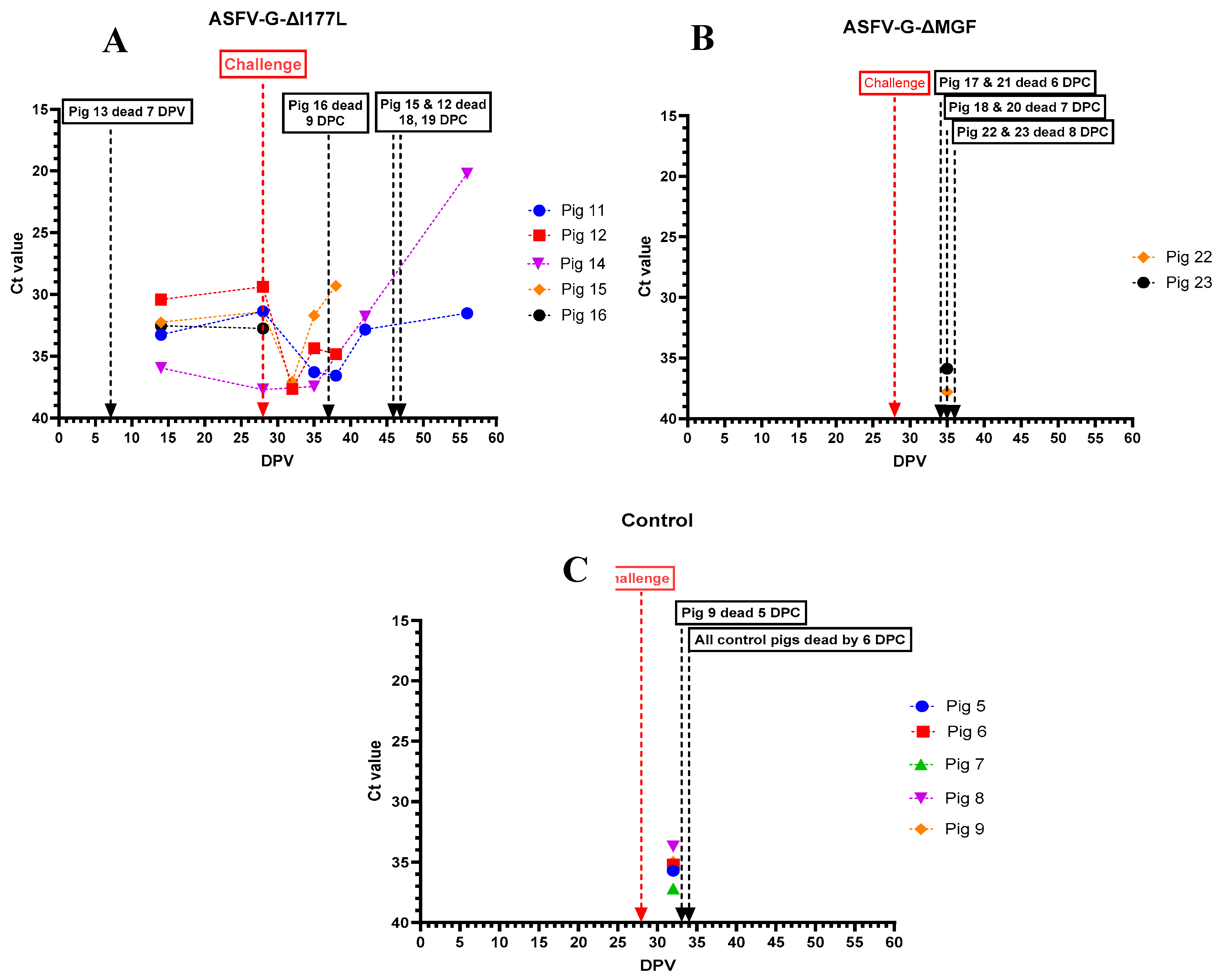
All pigs that received the ASFV-G-ΔI177L developed viremia, and remained viremic until they were challenged with rASFVI/II (
Figure 6 A). Pigs # 12, 15, and 16 that succumbed to ASF after the challenge developed high viremia. Pig #14 which had fever, lethargy and swollen hock joints also had a high level of viremia until the end of the study. In contrast, pig #11, had low levels of viremia by the end of the study. Only 2 (#20 and 23) out of 6 pigs that received ASFV-G-ΔMGF developed a detectable level of viremia before the challenge (
Figure 6B). After the challenge, all 6 pigs developed viremia within 4 days, and the viremia continued to increase until they were found dead/humanely euthanized. All pigs in the control group developed high levels of viremia and succumbed to ASF by 6 dpc (
Figure 6C).
In order to determine if the viremia developed after the challenge was caused by the vaccine strains (p72 genotype II) or rASFV I/II (p72 genotype I), all ASFV RT-PCR positive samples were subjected to Phan G1 and G2 qRT-PCR. Phan G1 and G2 qRT-PCR assays were specific but slightly less sensitive than the Tignon assay (
Supplementary Table S2). Whole blood samples from pigs that received ASFV-G-ΔI177L tested positive by both Phan G1 and G2 RT-PCR assays, except those collected on the last day of the study. Whole blood from pigs #11 and 14 collected before euthanasia tested positive only in the Phan G1 assay.
ASFV genomic material was detected in nasal swabs collected from pigs that received ASFV-G-ΔI177L, starting at 15 dpv. The highest amount of ASFV genome was detected in the nasal swab collected from pig # 14 on the day it was euthanized (
Figure 7A). No ASFV genome was detected in nasal swabs collected from pigs that received the AVAC ASFV-G-ΔMGF strain, until they were challenged with rASFV I/II (
Figure 7B). Similarly, the ASFV genome was positive (low levels) in nasal swabs collected from control pigs only after they were challenged with rASFV I/II (
Figure 7C).
3.3. Anti-ASFV Antibodies
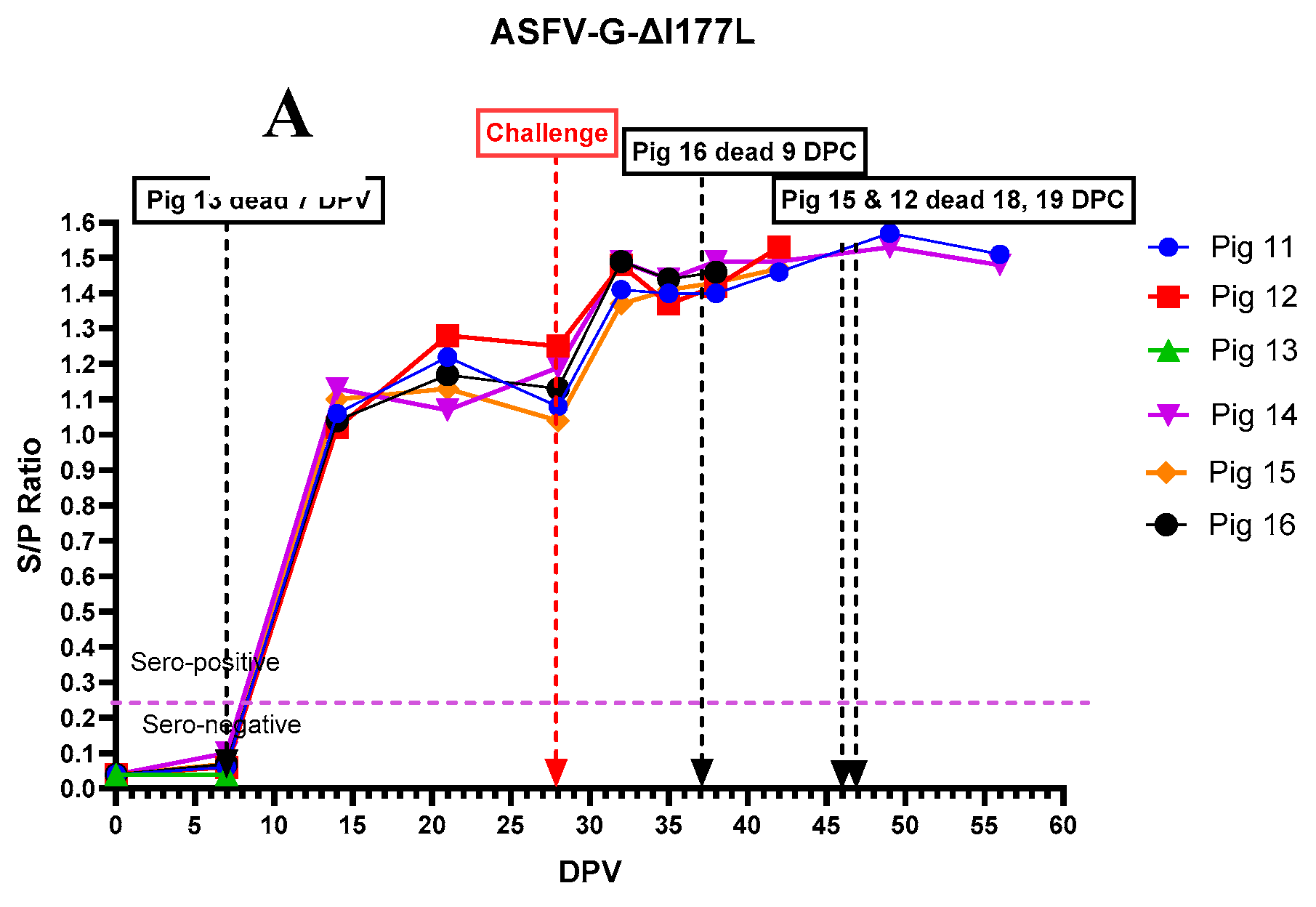
All pigs that received ASFV-G-ΔI177L, except pig # 13 that died on 7 dpv, developed anti-ASFV antibodies by 14 dpv (
Figure 8A). Anti-ASFV antibodies continued to increase in those pigs and plateaued around 20 dpv. Following rASFVI/II challenge on 29 dpv, the antibodies increased again and remained high until the end of the experiment. In contrast, pigs that received the AVAC ASFV-G-ΔMGF vaccine strain developed anti-ASFV antibodies relatively slow compared to those received ASFV-G-ΔI177L (
Figure 8B). Interestingly, pig #18, did not develop detectable levels of anti-ASFV antibodies even after the challenge. As expected, none of the pigs in the control group developed detectable levels of anti-ASFV antibodies until they succumbed to the rASFV I/II challenge.
3.4. Post-Mortem Findings
Pig #13 was the first to die in this study at 7 dpv. Other than the mucosal ulceration at the gastroesophageal junction (
Figure 9 E), and hemorrhagic submandibular lymph nodes, no other gross lesions were observed in this pig. Pig #12, 15 and 16 that died after the rASFV I/II challenge had subcutaneous hemorrhages in the skin (
Figure 9A) and lymphoid tissues, splenomegaly (
Figure 9 D), consolidated lung lobes (9B) and congested gastric mucosa (
Figure 9 C).
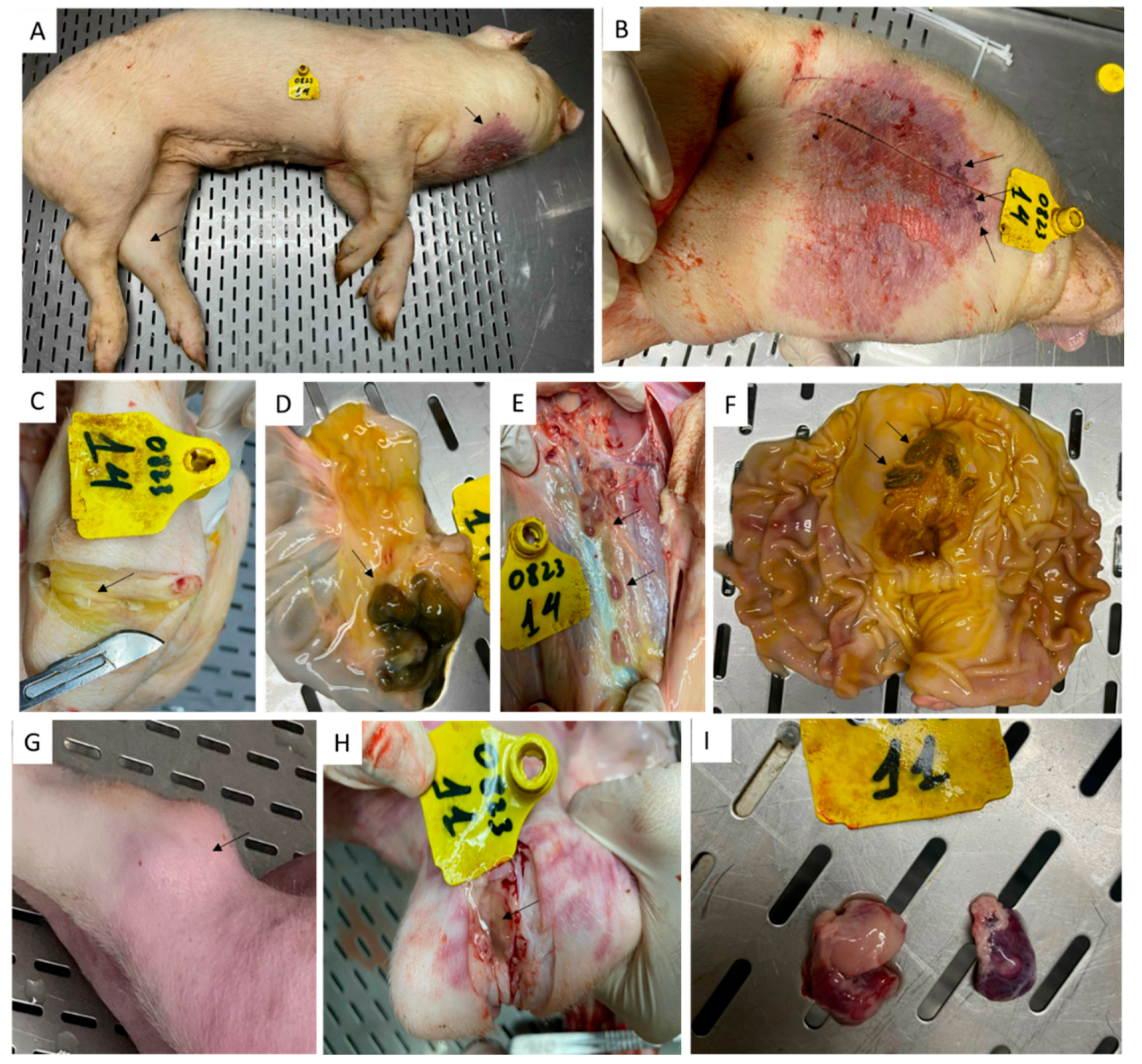
Among the two pigs that survived the rASFV I/II challenge, pig #14 had the most gross pathological lesions (
Figure 10). Under the neck, it had severe dermatitis with denuded and necrotic skin lesions (10A and 10B). During the postmortem, gastric ulcers (10F), hemorrhagic lymph nodes (10E) necrotic ileocecal lymph nodes (10D) and increased synovial fluid in the swollen hock joints (10C) were observed. In contrast, pig #11 only had a swollen hock joint with increased synovial fluid (10G and 10H) and hemorrhagic submandibular lymph nodes (10I). A selected number of tissues from pigs # 11 and 14 was tested by RT- PCR and pig #14 had the highest amount of viral genomic material in the tissues compared to that in pig # 11 (
Table 1). When used Phan G1 and G2 assays, both vaccine (G2) and challenge (G1) viruses were detected in lymphoid tissues collected from the two pigs.
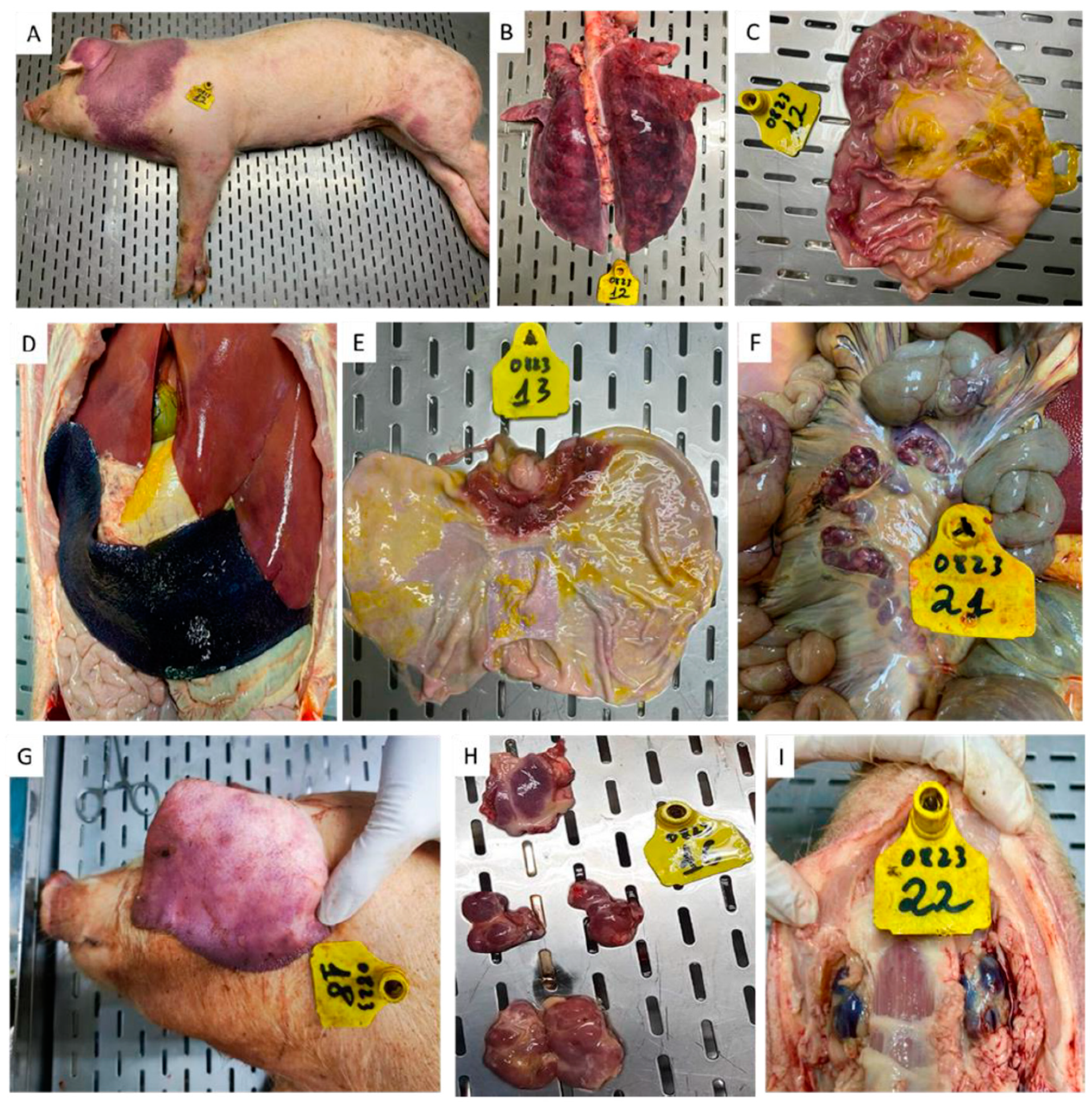
In controls (pigs #5-10) and those that received ASFV-G-ΔMGF (pigs #17-23), hemorrhages on the skin (
Figure 9 G), and in internal lymphoid organs (
Figure 9F, H and I) were observed.
4. Discussion
To date there is no globally approved vaccine available for ASF, however, live-attenuated ASFV strains are considered the most promising vaccine candidates available to date. Several live attenuated ASFV strains including HLJ/18-7GD [
24], ASFV-G-ΔI177L [
20], and ASFV-G-ΔMGF [
18] have been shown to induce complete protection against homologous high virulent ASFV challenge under experimental conditions. Of those, ASFV-G-ΔI177L and ASFV-G-ΔMGF strains have been licensed as NAVET-ASFVAC
® and AVAC ASF LIVE
® in Vietnam, respectively. In this study, we showed that ASFV-G-ΔI177L and ASFV-G-ΔMGF strains are not able to effectively protect the pigs from the emerging rASFV I/II in Vietnam.
In this study, we used 18 specific pathogen-free weaned piglets. They were inoculated with ASFV-G-ΔI177L (6 pigs) or DMAC passaged ASFV-G-ΔMGF (6 pigs). Six pigs were used as controls, and they received sterile PBS intramuscularly. Out of 6 pigs that received DMAC passaged ASFV-G-ΔMGF, none showed ASF-related clinical signs, and 2 developed low levels of viremia. There was no virus shedding observed in nasal secretions. The findings were different from those reported by O’Donnell et al. [
18] and Deutschmann et al. [
25] with the original ASFV-G-ΔMGF strain. In both studies, all pigs that received the ASFV-G-ΔMGF (IM at 10
4 HAD
50/pig) developed viremia, and some shed the virus in secretions and excretions. The difference observed could be due to further attenuation of the ASFV-G-ΔMGF strain because of multiple passages in the DMAC cell line. All pigs that received the ASFV-G-ΔI177L strain developed viremia and shed virus in their nasal secretions. Four pigs including pig #13 that died on 7 dpv developed a fever. Pig #13 had the highest level of viremia, but there were no ASF-related lesions other than gastric erosions observed at the postmortem. Therefore, it was not clear if the pig died of ASF or due to another cause.
When challenged, the control pigs developed severe clinical signs and succumbed to ASF by 6 dpc, confirming the highly pathogenic nature of the rASFV I/II. Similarly, pigs vaccinated with the AVAC ASFV-G-ΔMGF strain when challenged, died within 8 dpc, despite high antibody levels observed in 5 out of 6 pigs. In contrast, in pigs that received the ASFV-G-ΔI177L strain, the development of ASF clinical signs was delayed. Only three pigs (#12, 15, and 16) developed acute clinical signs and succumbed to ASF on 19, 18, and 9 dpc, respectively. The two remaining pigs (#11 and 14) survived, but started to show signs of chronic ASF infection as they reached the end of the study. The lymphoid tissues from pigs #11 and 14 contained ASFV genomes from the vaccine and challenge viruses. Chronic ASF was characterized by necrotic dermatitis and swollen joints and has been reported exclusively in pigs infected with low-virulent ASFV genotype I viruses. Therefore, one can speculate that these lesions could be caused by the proteins encoded by ASFV genotype I genes in the rASFV I/II strain.
The findings from this study are in line with those reported by Zhao et al. [
13]. They used HLJ/18-7GD vaccine strain which lacks seven ASFV genes to vaccinate the pigs and challenged them with one of the three rASFV I/II viruses isolated from China. The vaccine was not able to protect the pigs against rASFV I/II as all vaccinated pigs developed fever and succumbed to ASF 10 days post-challenge.
To our knowledge, this is the first study that directly compared ASFV-G-ΔI177L and ASFV-G-ΔMGF vaccine strains in an in vivo experiment. Both strains were inoculated to pigs at the same dose (104 HAD50 per pig) for direct comparison. The AVAC ASF LIVE® single dose contains ~ 104 HAD50 ASFV-G-ΔMGF master seed, however, the NAVET-ASFVAC® contains only ~103 HAD50 ASFV-G-ΔI177L. Therefore, the viremia, clinical signs and better immune response and enhanced protection observed in pigs that received ASFV-G-ΔI177L could be due to the 10 times the recommended dose of ASFV-G-ΔI177L used in this experiment. Compared to the AVAC ASFV-G-ΔMGF vaccine strain, The NAVET-ASFVAC®, when used as recemented, might not be able to delay the clinical signs and induce chronic ASF in some of the vaccinated pigs as observed in this study.
ASF attenuated vaccine strains mimic the natural infection and therefore create a strong and long-lasting immune response. They need to replicate enough to induce protective immunity but not too much to cause a cytokine storm that leads to pathological lesions eventually killing the pig. Therefore, increasing the ASFV-G-ΔMGF dose or boosting the pigs might help to increase resistance to the rASFV I/II challenge. However, this might not prevent the development of chronic ASF in the vaccinated animals as observed in pigs that received the ASFV-G-ΔI177L strain.
Due to the highly virulent nature and resistance to the licensed vaccines in Vietnam, the emerging rASFV I/II strains soon could replace the genotype II pandemic strain circulating in the region derailing the ongoing efforts to control the ASF pandemic. Hence, there is an urgent need to develop a novel vaccine based on rASFV I/II for effective control of ASF in Asia. Targeted deletion of I177L or MGF genes from the rASFV I/II, might result in an effective vaccine strain, however, due to the presence of genotype I genetic elements, such a vaccine could give rise to complications such as arthritis and skin lesions as observed when p72 genotype I vaccines were used to control the ASF genotype I outbreak in Europe. Little is known about the pathogenesis of chronic ASF. The ability to induce chronic ASF in pigs in a short time by ASFV-G-ΔI177L vaccination followed by rASF I/II challenge might provide a model for those studies.
In conclusion, the findings from this study show that both vaccine strains licensed in Vietnam are unlikely to protect pigs from the emerging highly virulent rASFV I/II strains. This complicates the ongoing efforts to control ASF in Asia and globally and emphasizes the urgent need for a novel vaccine that can protect pigs from the rASFV I/II strains.
Supplementary Materials
The following supporting information can be downloaded at: preprints.org, Tables S1 and S2.
Author Contributions
For research articles with several authors, a short paragraph specifying their individual contributions must be provided. The following statements should be used “Conceptualization, A.A., N.V.P and V.P.L; methodology, N.V.D., N.T.N., V.X.D., T.N.T., V.D.N., T.T.T. and D.M.; writing—original draft preparation, A.A.; writing—review and editing, N.V.P, K.G. and V.P.L.; visualization, K.G.; supervision, N.V.P and V.P.L and A.A.; project administration, N.V.P.; funding acquisition, N.V.P.
Funding
This study was supported by the AVAC JSC.
Institutional Review Board Statement
All animal experiments were performed in the animal facilities at AVAC in compliance with the the 2011 Guide for Care and Use of Laboratory Animals (eighth edition), and the guidelines of good experimental practices approved by the Committee on Animal Research and Ethics at the of the AVA JSC (Approval Number: AA-2308).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We like to thank AVAC animal care staff for their support during the animal experiment. Authors like to thank, Shawn Babiuk, Kathleen Hooper McGrevy and Charles Nfon at the Canadian Food inspection Agency for their insightful input during the review process.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- OIE African swine fever - World Organization for Animal Health [Internet]. WOAH - World Organisation for Animal Health. [cited 2023 Jan 12]. Available from: https://www.woah.org/en/disease/african-swine-fever/.
- Dixon, L.K. Molecular Cloning and Restriction Enzyme Mapping of an African Swine Fever Virus Isolate from Malawi. J. Gen. Virol. 1988, 69, 1683–1694. [Google Scholar] [CrossRef] [PubMed]
- de Villiers, E.P.; Gallardo, C.; Arias, M.; da Silva, M.; Upton, C.; Martin, R.; Bishop, R.P. Phylogenomic analysis of 11 complete African swine fever virus genome sequences. Virology 2010, 400, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Bastos, A.D.S.; Penrith, M.-L.; Crucière, C.; Edrich, J.L.; Hutchings, G.; Roger, F.; Couacy-Hymann, E.; R. Thomson, G. Genotyping field strains of African swine fever virus by partial p72 gene characterisation. Arch. Virol. 2003, 148, 693–706. [Google Scholar] [CrossRef] [PubMed]
- Spinard, E.; Dinhobl, M.; Tesler, N.; Birtley, H.; Signore, A.V.; Ambagala, A.; Masembe, C.; Borca, M.V.; Gladue, D.P. A Re-Evaluation of African Swine Fever Genotypes Based on p72 Sequences Reveals the Existence of Only Six Distinct p72 Groups. Viruses 2023, 15, 2246. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.; Boinas, F.S.; Iacolina, L.; Ruíz-Fons, F.; Gavier-Widén, D. 1. African swine fever (ASF), the pig health challenge of the century. In Understanding and Combatting African Swine Fever; Iacolina, L., Penrith, M.-L., Bellini, S., Chenais, E., Jori, F., Montoya, N., Ståhl, K., Gavier-Widén, D., Eds.; Wageningen Publishers: Wageningen, The Netherlands, 2021. [Google Scholar]
- Danzetta, M.L.; Marenzoni, M.L.; Iannetti, S.; Tizzani, P.; Calistri, P.; Feliziani, F. African Swine Fever: Lessons to Learn From Past Eradication Experiences. A Systematic Review. Front. Veter- Sci. 2020, 7, 296. [Google Scholar] [CrossRef] [PubMed]
- Rowlands, R.J.; Michaud, V.; Heath, L.; Hutchings, G.; Oura, C.; Vosloo, W.; Dwarka, R.; Onashvili, T.; Albina, E.; Dixon, L.K. African Swine Fever Virus Isolate, Georgia, 2007. Emerg. Infect. Dis. 2008, 14, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Vizcaíno, J.M.; Mur, L.; Martínez-López, B. African swine fever (ASF): Five years around Europe. Veter- Microbiol. 2013, 165, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Li, N.; Luo, Y.; Liu, Y.; Miao, F.; Chen, T.; Zhang, S.; Cao, P.; Li, X.; Tian, K.; et al. Emergence of African Swine Fever in China, 2018. Transbound. Emerg. Dis. 2018, 65, 1482–1484. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Kawaguchi, N.; Bosch, J.; Aguilar-Vega, C.; Sánchez-Vizcaíno, J.M. What can we learn from the five-year African swine fever epidemic in Asia? Front Vet Sci. 2023 Sep 28;10:1273417.
- Sun, E.; Huang, L.; Zhang, X.; Zhang, J.; Shen, D.; Zhang, Z.; Wang, Z.; Huo, H.; Wang, W.; Huangfu, H.; et al. Genotype I African swine fever viruses emerged in domestic pigs in China and caused chronic infection. Emerg. Microbes Infect. 2021, 10, 2183–2193. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Sun, E.; Huang, L.; Ding, L.; Zhu, Y.; Zhang, J.; Shen, D.; Zhang, X.; Zhang, Z.; Ren, T.; et al. Highly lethal genotype I and II recombinant African swine fever viruses detected in pigs. Nat. Commun. 2023, 14, 3096. [Google Scholar] [CrossRef] [PubMed]
- Le, V.P.; Jeong, D.G.; Yoon, S.-W.; Kwon, H.-M.; Trinh, T.B.N.; Nguyen, T.L.; Bui, T.T.N.; Oh, J.; Kim, J.B.; Cheong, K.M.; et al. Outbreak of African Swine Fever, Vietnam, 2019. Emerg. Infect. Dis. 2019, 25, 1433–1435. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Thi, T.; Pham-Thi-Ngoc, L.; Nguyen-Ngoc, Q.; Dang-Xuan, S.; Lee, H.S.; Nguyen-Viet, H.; Padungtod, P.; Nguyen-Thi, T.; Tran-Cong, T.; Rich, K.M. An Assessment of the Economic Impacts of the 2019 African Swine Fever Outbreaks in Vietnam. Front. Veter- Sci. 2021, 8. [Google Scholar] [CrossRef] [PubMed]
- Shao, Q.; Li, R.; Han, Y.; Han, D.; Qiu, J. Temporal and Spatial Evolution of the African Swine Fever Epidemic in Vietnam. Int. J. Environ. Res. Public Heal. 2022, 19, 8001. [Google Scholar] [CrossRef]
- Le, V.P.; Nguyen, V.T.; Le, T.B.; Mai, N.T.A.; Nguyen, V.D.; Than, T.T.; Lai, T.N.H.; Cho, K.H.; Hong, S.-K.; Kim, Y.H.; et al. Detection of Recombinant African Swine Fever Virus Strains of p72 Genotypes I and II in Domestic Pigs, Vietnam, 2023. Emerg. Infect. Dis. 2024, 30, 991–994. [Google Scholar] [CrossRef] [PubMed]
- O'Donnell, V.; Holinka, L.G.; Gladue, D.P.; Sanford, B.; Krug, P.W.; Lu, X.; Arzt, J.; Reese, B.; Carrillo, C.; Risatti, G.R.; et al. African Swine Fever Virus Georgia Isolate Harboring Deletions of MGF360 and MGF505 Genes Is Attenuated in Swine and Confers Protection against Challenge with Virulent Parental Virus. J. Virol. 2015, 89, 6048–6056. [Google Scholar] [CrossRef] [PubMed]
- Ta, H.L. Quality Control of A Live—Attenuated African Swine Fever Vaccine in Viet Nam; WOAH Regional Representation for Asia and the Pacific, Tokyo, Japan, 2022.
- Borca, M.V.; Ramirez-Medina, E.; Silva, E.; Vuono, E.; Rai, A.; Pruitt, S.; Holinka, L.G.; Velazquez-Salinas, L.; Zhu, J.; Gladue, D.P. Development of a Highly Effective African Swine Fever Virus Vaccine by Deletion of the I177L Gene Results in Sterile Immunity against the Current Epidemic Eurasia Strain. J. Virol. 2020, 94. [Google Scholar] [CrossRef] [PubMed]
- Tran, X.H.; Le, T.T.P.; Nguyen, Q.H.; Do, T.T.; Nguyen, V.D.; Gay, C.G.; Borca, M.V.; Gladue, D.P. African swine fever virus vaccine candidate ASFV-G-ΔI177L efficiently protects European and native pig breeds against circulating Vietnamese field strain. Transbound Emerg Dis. 2022 Jul;69(4):e497-e504.
- Tignon, M.; Gallardo, C.; Iscaro, C.; Hutet, E.; Van der Stede, Y.; Kolbasov, D.; De Mia, G.M.; Le Potier, M.-F.; Bishop, R.P.; Arias, M.; et al. Development and inter-laboratory validation study of an improved new real-time PCR assay with internal control for detection and laboratory diagnosis of African swine fever virus. J. Virol. Methods 2011, 178, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Addressing African Swine Fever Protocols and Guidelines for Laboratory Diagnosis. May 2024https://www.woah.org/app/uploadss/2024/06/woah-asf-lab-guidelines-170524-en.pdf.
- Wang, Z.; Zhang, J.; Li, F.; Zhang, Z.; Chen, W.; Zhang, X.; Sun, E.; Zhu, Y.; Liu, R.; He, X.; et al. The attenuated African swine fever vaccine HLJ/18-7GD provides protection against emerging prevalent genotype II variants in China. Emerg. Microbes Infect. 2024, 13, 2300464. [Google Scholar] [CrossRef] [PubMed]
- Deutschmann, P.; Carrau, T.; Sehl-Ewert, J.; Forth, J.H.; Viaplana, E.; Mancera, J.C.; Urniza, A.; Beer, M.; Blome, S. Taking a Promising Vaccine Candidate Further: Efficacy of ASFV-G-ΔMGF after Intramuscular Vaccination of Domestic Pigs and Oral Vaccination of Wild Boar. Pathogens 2022, 11, 996. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Daily rectal temperatures of pigs (#11-16) inoculated with ASFV-G-ΔI177L (104 HAD50 IM per pig) before (A) and after (B) challenge with rASFV I/II (103 HAD50 IM per pig). The dates pigs found dead or euthanized as they reached humane end point are marked with an arrow. DPV = days post-vaccination. DPC = days post-challenge.
Figure 1.
Daily rectal temperatures of pigs (#11-16) inoculated with ASFV-G-ΔI177L (104 HAD50 IM per pig) before (A) and after (B) challenge with rASFV I/II (103 HAD50 IM per pig). The dates pigs found dead or euthanized as they reached humane end point are marked with an arrow. DPV = days post-vaccination. DPC = days post-challenge.
Figure 2.
Daily average cumulative clinical scores (CCS) of the pigs that received ASFV-G-∆I177L, ASFV-G-ΔMGF or PBS before and after the challenge with the rASFV I/II strain. DPV = days post-vaccination.
Figure 2.
Daily average cumulative clinical scores (CCS) of the pigs that received ASFV-G-∆I177L, ASFV-G-ΔMGF or PBS before and after the challenge with the rASFV I/II strain. DPV = days post-vaccination.
Figure 3.
Swelling of tarsal joints (arrows) of pigs # 14 (A) and # 11 (B) that survived the rASFV I/II challenge.
Figure 3.
Swelling of tarsal joints (arrows) of pigs # 14 (A) and # 11 (B) that survived the rASFV I/II challenge.
Figure 4.
Daily rectal temperatures of pigs (#17-23) inoculated with ASFV-G-ΔMGF (104 HAD50 IM per pig) before (A) and after (B) the challenge with rASFV I/II (103 HAD50 IM per pig). The dates pigs were euthanized are marked with an arrow. DPV = days post-vaccination. DPC = days post-challenge.
Figure 4.
Daily rectal temperatures of pigs (#17-23) inoculated with ASFV-G-ΔMGF (104 HAD50 IM per pig) before (A) and after (B) the challenge with rASFV I/II (103 HAD50 IM per pig). The dates pigs were euthanized are marked with an arrow. DPV = days post-vaccination. DPC = days post-challenge.
Figure 5.
Daily rectal temperatures of control pigs (#5-10) inoculated IM with a sterile PBS (A) and challenged with rASFV I/II (103 HAD50 IM per pig) (B). The dates pigs found dead or euthanized are marked with an arrow. DPV = days post-vaccination. DPC = days post-challenge.
Figure 5.
Daily rectal temperatures of control pigs (#5-10) inoculated IM with a sterile PBS (A) and challenged with rASFV I/II (103 HAD50 IM per pig) (B). The dates pigs found dead or euthanized are marked with an arrow. DPV = days post-vaccination. DPC = days post-challenge.
Figure 6.
Viremia was detected in individual pigs following inoculation with ASFV-G-ΔI177L (A), ASFV-G-ΔMGF (B) or sterile PBS (C) followed by the rASFV I/II challenge. The samples with detectable levels of ASFV viral genome were further tested to differentiate the genotype II (G2) vaccinated strains vs recombinant challenge virus strain (G1). DPV = days post-vaccination.
Figure 6.
Viremia was detected in individual pigs following inoculation with ASFV-G-ΔI177L (A), ASFV-G-ΔMGF (B) or sterile PBS (C) followed by the rASFV I/II challenge. The samples with detectable levels of ASFV viral genome were further tested to differentiate the genotype II (G2) vaccinated strains vs recombinant challenge virus strain (G1). DPV = days post-vaccination.
Figure 7.
Detection of ASFV genomic material in nasal swabs collected from individual pigs following inoculation with ASFV-G-ΔI177L (A), ASFV-G-ΔMGF (B) or sterile PBS (C) followed by the rASFV I/II challenge. DPV = days post-vaccination.
Figure 7.
Detection of ASFV genomic material in nasal swabs collected from individual pigs following inoculation with ASFV-G-ΔI177L (A), ASFV-G-ΔMGF (B) or sterile PBS (C) followed by the rASFV I/II challenge. DPV = days post-vaccination.
Figure 8.
Serum anti-ASFV antibody levels in individual pigs following inoculation with ASFV-G-ΔI177L (A), ASFV-G-ΔMGF (B) or sterile PBS (C) followed by the rASFV I/II challenge.
Figure 8.
Serum anti-ASFV antibody levels in individual pigs following inoculation with ASFV-G-ΔI177L (A), ASFV-G-ΔMGF (B) or sterile PBS (C) followed by the rASFV I/II challenge.
Figure 9.
Gross pathological lesions were observed in pigs that received ASFV-G-ΔI177L (A, B.C, D, E), ASFV-G-ΔMGF (F, G, H, I) and died/euthanized after the rASFV I/II challenge. A -Subcutaneous hemorrhage and hematoma in the left pinna, B -Consolidated lungs, C -Congestion in the gastric mucosa, D -Speenomagaly, E -Uulceration at the gastroesophageal junction, F-Hemorrhagic mesenteric lymph nodes, G-hemorrhages in the ear, H-Hemorrhages in the tonsil, submandibular lymph nodes and superficial inguinal lymph nodes, I-Hemorrhagic submandibular lymph nodes.
Figure 9.
Gross pathological lesions were observed in pigs that received ASFV-G-ΔI177L (A, B.C, D, E), ASFV-G-ΔMGF (F, G, H, I) and died/euthanized after the rASFV I/II challenge. A -Subcutaneous hemorrhage and hematoma in the left pinna, B -Consolidated lungs, C -Congestion in the gastric mucosa, D -Speenomagaly, E -Uulceration at the gastroesophageal junction, F-Hemorrhagic mesenteric lymph nodes, G-hemorrhages in the ear, H-Hemorrhages in the tonsil, submandibular lymph nodes and superficial inguinal lymph nodes, I-Hemorrhagic submandibular lymph nodes.
Figure 10.
Gross pathological lesions were observed in pigs #14 (A-F) and 11 (G-I) that received ASFV-G-ΔI177L and survived the rASFV I/II challenge. A-Swollen left hock joint (arrow) and dermatitis under the neck, B- Dermatitis with necrotic and denuded skin, C– Increased synovial fluid in the left hock joint, D- Necrotic lymphoid tissues of the ilea-cecal valve, E– Hemorrhagic lymph nodes, F Gastric ulcers (arrows), G- Swollen hock joint, H- Increased synovial fluid in the hock joint, I- hemorrhagic submandibular lymph nodes.
Figure 10.
Gross pathological lesions were observed in pigs #14 (A-F) and 11 (G-I) that received ASFV-G-ΔI177L and survived the rASFV I/II challenge. A-Swollen left hock joint (arrow) and dermatitis under the neck, B- Dermatitis with necrotic and denuded skin, C– Increased synovial fluid in the left hock joint, D- Necrotic lymphoid tissues of the ilea-cecal valve, E– Hemorrhagic lymph nodes, F Gastric ulcers (arrows), G- Swollen hock joint, H- Increased synovial fluid in the hock joint, I- hemorrhagic submandibular lymph nodes.
Table 1.
Detection of ASFV genomic material in tissues collected from pigs # 11 and 14 that survived the rASFV I/II challenge using the Median Diagnostics VDx ASFV qPCR kit, Phan G1 and Phan G2 RT-PCR assays.
Table 1.
Detection of ASFV genomic material in tissues collected from pigs # 11 and 14 that survived the rASFV I/II challenge using the Median Diagnostics VDx ASFV qPCR kit, Phan G1 and Phan G2 RT-PCR assays.
| Sample |
Pig # 11 |
Pig #14 |
| VDx ASFV |
Phan G1 |
Phan G2 |
VDx ASFV |
Phan G1 |
Phan G2 |
| Joint fluid |
25.01 |
29.66 |
35.33 |
18.75 |
23.18 |
- |
| Tonsils |
37.35 |
- |
- |
22.36 |
27.55 |
33.30 |
| Thoracic lymph node |
30.13 |
35.61 |
- |
22.54 |
29.27 |
30.70 |
| Submandibular lymph node |
32.12 |
- |
- |
19.16 |
23.44 |
28.12 |
| Mesenteric lymph node |
32.49 |
- |
- |
25.67 |
30.40 |
- |
| Renal lymph node |
26.30 |
30.56 |
- |
24.05 |
30.08 |
30.50 |
| Liver |
35.76 |
- |
- |
27.15 |
31.94 |
- |
| Kidney |
36.02 |
- |
- |
30.66 |
34.53 |
- |
| Spleen |
30.48 |
34.95 |
- |
27.39 |
32.66 |
- |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).