1. Introduction
Bisabolol is a naturally occurring bioactive compound (BAC) with a structure of monocyclic sesquiterpene alcohol. First derived from
Matricaria chamomilla, it is today identified as a main constituent of several essential oils of aromatic plants belonging to the
Asteraceae and the
Lamiaceae family (e.g.,
Vanillosmopsis sp.,
Salvia runcinata,
Stachys lavandulifolia) [
1,
2]. The most active and prevalent α (-) diastereoisomer of bisabolol is also known as levomenol. It presents as a colorless oily liquid with a low density (0.93 g/cm
3) and a delicate sweet scent. Being a strictly lipophilic compound, α-bisabolol possesses negligible solubility in water (1.688 mg/L at 25°C) but is however soluble in ethanol and other polar organic solvents [
1,
2,
3]. In cosmetics and topical pharmaceuticals, α-bisabolol and rich in it essential oils find a broad application as an anti-inflammatory, anti-oxidant, and anti-allergic active components; furthermore, it exhibits antimicrobial, insecticidal, and permeation enhancing properties [
3].
Micellar solubilization is a technique for increasing the water solubility of slightly soluble or practically insoluble substances by including them in micelles of surfactants [
4]. As a result, a thermodynamically stable and isotropic system is formed [
5]. The method is highly relevant for the preparation of aqueous dispersions of lipophilic ingredients, such as bisobolol and many other BACs, for cosmetic and pharmaceutical purposes [
6,
7,
8,
9].
The ability to solubilize is inherent to surfactants with a high hydrophilic-lipophilic balance (HLB) value (>10). Upon reaching their critical micelle concentration (CMC) in aqueous solutions, they tend to self-aggregate spontaneously and form distinct structures comprised of a hydrophobic core and a hydrophilic crown, called micelles. The micellar inclusion of strictly hydrophobic substances takes place in the micellar core, whereas partially water-soluble substances with low to moderate hydrophobicity may also get solubilized in the micellar crown among the hydrophilic chains [
10,
11]. The resulting dispersion is referred to as microemulsion and is characterized by the presence of nano-sized micelles (most often within the range of 5-20 nm). In contrast to classic emulsions (typically with a droplet size of 1-100 µm), microemulsions possess thermo-dynamic stability and transparency [
7,
12,
13]. The most commonly used solubilizers in cosmetic products are sodium oleate (HLB 12), Polysorbate 20 (HLB 17), Polysorbate 80 (HLB 15), some PEG-ylated vegetable oils (olive, castor, etc.) and Poloxamers (e.g. subspecies 124, 188, 407, etc., with HLB in the range 18-23). Generally, the effective solubilization requires a high amount of surfactant/s above 3-5 times the amount of the water-insoluble phase [
14,
15].
Poloxamers are nonionic copolymers with a triblock architecture of a repeating structural unit comprising two hydrophilic ethylene oxide (EO) blocks and a transitional hydrophobic propylene oxide (PO) block between them. The various subtypes of Poloxamers differ in molecular weight, content of poly (propylene oxide) (PPO), and consistency. Due to their unique properties as emulsifiers, solubilizers, wetting agents, and thermo-sensitive gelling agents, the Poloxamers are widely used in the cosmetic and pharmaceutical industries, with subtypes 188 and 407 being the most universally utilized [
16,
17].
According to the European Pharmacopoeia, Poloxamer 407 is characterized by a molecular mass of 9 840-14 600 g/mol, HLB 22, and a content of 95-105 EO and 54-60 PO units. It appears as white or almost white, waxy granules or flakes and is well soluble in water and ethanol and practically insoluble in oils [
18]. Poloxamer 407 exhibits a temperature-dependent dissolution and gelation due to dehydration of the PPO blocks at elevated temperatures. The CMC of Poloxamer 407 decreases with the increase in temperature; values of 0.7% w/w at 25°C, 0.1% w/w at 30°C, and 0.025% w/w at 35°C have been reported [
19]. A gelation occurs upon reaching the critical gelation concentration (CGT) due to a tight "packing" of the Poloxamer micelles, wherefore more concentrated solutions (>13-15% w/w) find a broad application in biomedicine. As gelation is determined by the temperature as well, critical gelation temperature (CGT) is also defined; variations in CGT at a given Poloxamer concentration are observed in the presence of active ingredients or excipients [
20]. In a gel state, Poloxamer 407 is used as a viscosity-enhancing and thickening ingredient; however, in the role of emulsifier and solubilizer in liquid bases, Poloxamer 407 is used in concentrations lower than its CGT/CGC in order to preserve the fluidity and easy application of the composition on extensive skin areas. In this regard, micellar solutions with concentrations in the range of 1-5% are reported to possess a good washing effect and are thus eligible as cosmetic bases with a cleansing effect [
7,
21,
22]; in the presence of a water-insoluble phase for solubilization in the Poloxamer solution, the upper limit of this range is to be targeted.
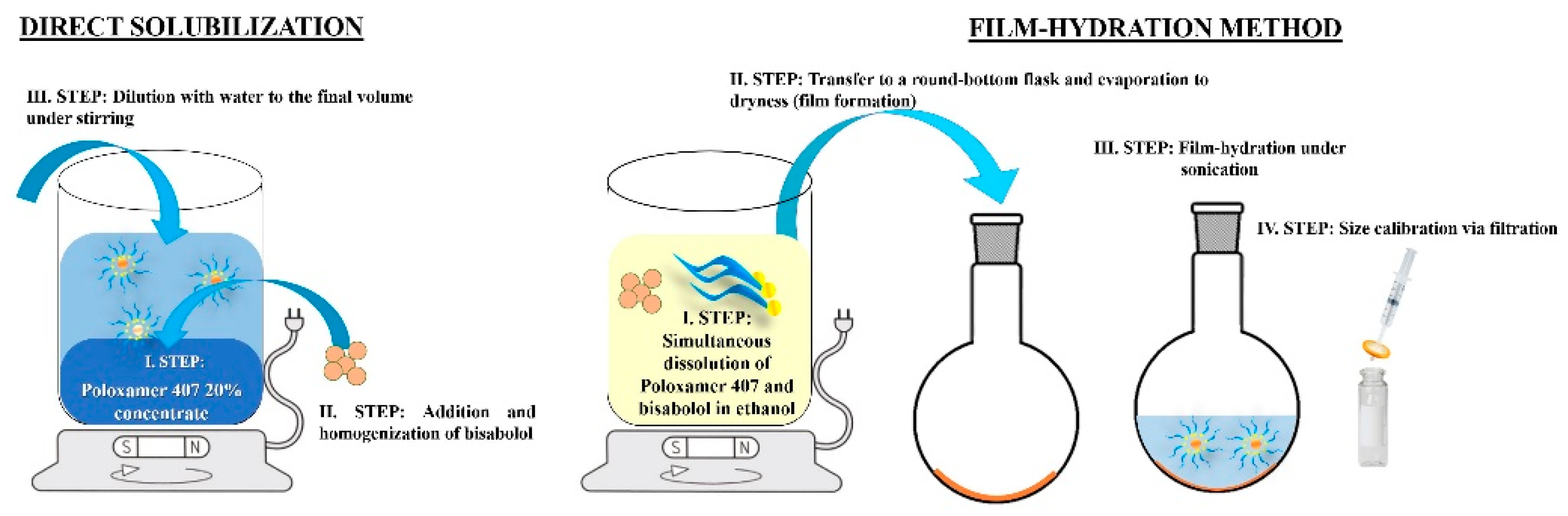
Several techniques are applied for solubilization with the use of Poloxamers, among which are direct solubilization, solubilization via film-hydration method, solubilization by melting, and solubilization by a precipitation technique followed by evaporation/diffusion of an organic solvent [
19]. Direct solubilization (DS) is considered the simplest approach and is based on the spontaneous micelle formation in aqueous Poloxamer solutions above the CMC. Depending on the active compound’s solubility, DS could be carried out by dilution of a Poloxamer concentrate containing the substance to be solubilized or by a simultaneous dissolution of the polymer and the active ingredient in cold water (5-10°C) and subsequent tempering at the room temperature, during which the solubility of Poloxamer decreases and a micro-heterogeneous system is formed [
19]. The film-hydration method (FH) is based on the co-dissolution of the polymer and the active substance in an organic solvent (e.g., ethanol, acetone, chloroform) followed by evaporation so that a thin film is formed. The next steps of this technique include hydration of the film and following filtration aiming micellar size calibration [
23]. Solubilization by melting could be carried out by obtaining a melt of the polymer and the active substance (at a temperature above the melting point of the substance and above the glass transition of Poloxamer) followed by rapid cooling and homogenization of the mixture. This method is only suitable for solubilization of thermally stable substances. Additionally, varieties of precipitation techniques with subsequent solvent evaporation and/or diffusion are applied in order to obtain nano-sized actives-loaded micelles in an aqueous dispersion [
19].
2. Materials and Methods
Materials
Alpha-bisabolol 85% 10 ml, extracted from
Matricaria chamomilla, was ordered from an online trader on
www.ekomama.net; Kolliphor
® P407 (Poloxamer 407) and Ethyl alcohol 96% 2.5 l were purchased from Sigma-Aldrich, USA; all microbial strains and growth media were supplied by Ridacom, Bulgaria.
Methods
Preparation of Test Samples
Bisabolol-loaded micellar solutions were prepared at a constant Poloxamer concentration of 5% and varying concentrations of the active substance – 0.5%, 1.0%, and 1.5%. The techniques of direct solubilization and solubilization by film-hydration method were applied (
Figure 1).
For the purposes of direct solubilization, a 20% Poloxamer concentrate was prepared by dissolving the polymer in cold water and storing the so-obtained stock solution in a refrigerator for at least 24 h before use. Then, a calculated amount of the Poloxamer concentrate was added α-bisabolol under continuous stirring of 1000 rpm on IKA® magnet stirrer model C-MAG HS 4, Germany. Dilution of the resulting primary solubilizate was performed with distilled water in a 1:4 ratio and the mixture was allowed to homogenize at the same rotation speed for 10 min. A reference 5% Poloxamer 407 solution (not loaded with bisabolol), was prepared accordingly and used for comparative purposes during the analyses.
Sample preparation via the film-hydration method was carried out by a simultaneous dissolution of accurately weighted quantities of bisabolol and Poloxamer 407 in ethanol 96% and following vacuum-assisted evaporation at 50°C and 55 rpm on a rotary evaporator Buchi, Germany, until a thin film was formed [
23]. The film was re-hydrated with the required amount of distilled water under sonication at 35°C for 30 min. The resulting solution was carefully withdrawn using a syringe and a needle and then filtered through a 0.22 µm syringe filter.
All samples were stored in hermetically sealed vials at room temperature and protected from light. The composition of each test formulation is presented in
Table 1.
Accelerated Stability Testing
An accelerated physical stability study was conducted via centrifugation at 15.000 rpm for 30 minutes [
24,
25]; all samples were applied in a volume of 0.5 ml. At the end of the test, the visual appearance of the dispersions was observed for phase separation and opalescence. Photographs were taken before and after centrifugation in order to follow any changes arising from the applied forces. A test for resuspendability was performed thereafter on the affected samples.
DLS
DLS analysis of the samples was carried out on a Zetasizer Ultra Red (λ = 632.8 nm) Malvern Panalytical Ltd., Malvern, UK, on the day of preparation and after performing the accelerated stability test followed by re-homogenization. The multi-angle DLS (MADLS) technique was applied, based on which data with the highest significance and best repeatability were extracted. All measurements were repeated in triplicate.
ELS
ELS analysis for zeta potential measurement was performed on a Zetasizer Ultra Red (λ = 632.8 nm) Malvern Panalytical Ltd., Malvern, UK, on the day of sample preparation at 25°C by using DTS1070 type of cuvettes. The samples were filtered through a 0.22 µm pore size syringe filter prior to each measurement. All measurements were repeated in triplicate.
Foamability
A foamability test was conducted as 0.5 mL of each sample was vigorously shaken for 30 seconds and placed to rest on a stand. The height of the foam was measured right after shaking and at chosen times thereafter [
26].
Viscosimetry
Poloxamer 407 5% micellar solution (bisabolol-free) and the optimal bisabolol-loaded test sample were subjected to Viscosimetry on IKA® Rotavisc lo-vi viscometer, IKA®-Werke GmbH & Co. KG, Germany with the aid of Spindle №SP-1. The measurements were carried out at a gradient increase in temperature within 20-40°C. As a result, temperature-viscosity curves were plotted.
Antimicrobial Activity
The antimicrobial activity of alpha-bisabolol was assessed against Escherichia coli ATCC 25922, Staphylococcus aureus ATCC 25923, and Candida albicans ATCC 10231 reference strains. Two-fold serial dilutions of a bisabolol-loaded sample of 2% (obtained by a film-hydration method) were made in 1.0 mL Mueller-Hinton broth. Each tube was inoculated with 0.1 mL of standardized microbial suspension (McFarland 0.5), after which the samples were aerobically cultivated under the standard conditions for each strain: 24 hours at 37°C for E. coli and S. aureus and 48 hours for 35°C for C. albicans. The minimal inhibitory concentrations (MICs) were determined as the lowest active concentrations by which no visible turbidity is observed. Positive controls (PC) of all microbial strains were set.
The minimal bactericidal and fungicidal concentrations (MBC/MFC) were determined by a single bacterial-loop-volume transfer of the test suspensions onto blood agar. The so-obtained specimens were incubated once again under the aforementioned conditions. The lowest concentration at which bacterial or fungal growth was 99.9% inhibited was reported as MBC/MFC.
In addition, the cup-plate technique was applied to evaluate the zones of inhibition of alpha-bisabolol. For this purpose, dense seeds of all microbial strains were made on Muller-Hinton agar. By using a sterile cork-borer, a cylindrical hole with a diameter of 7 mm was drilled in the center of each petri dish, into which 100 μL of the test sample was placed. The Petri dishes were left for aerobic cultivation under the standard conditions for each strain: 24 hours at 37°C for E. coli and S. aureus and 48 hours at 35°C for C. albicans. Thereafter, the diameter of the manifested zones of inhibition was measured.
All antimicrobial studies were repeated twice.
3. Results and Discussion
3.1. Visual Appearance and Physical Stability
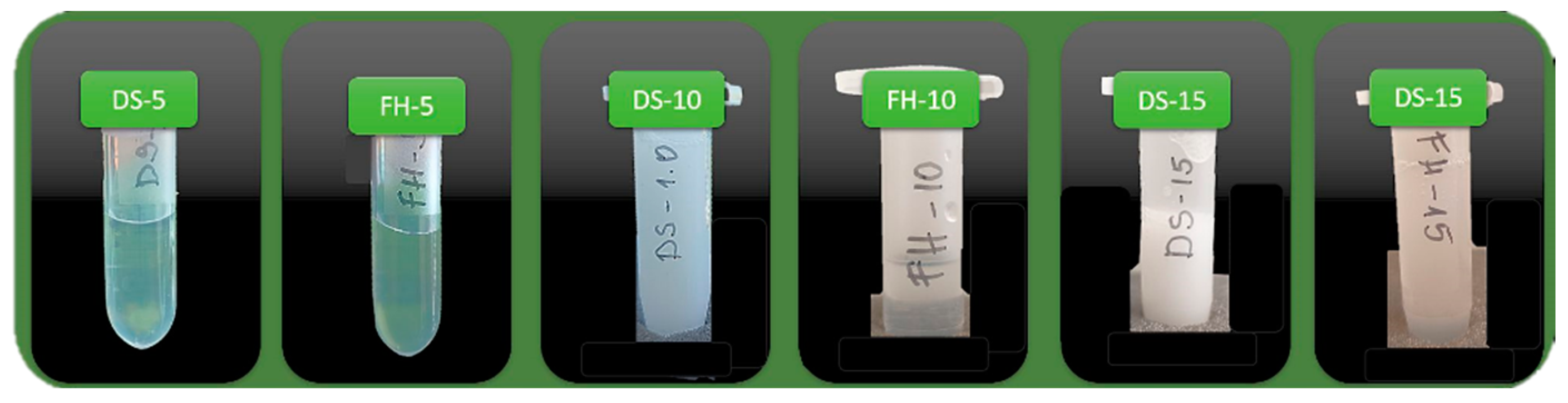
All test samples, except for the pure micellar solution (B-5) and the sample obtained by film-hydration with 0.5% bisabolol (FH-5), appeared as low-viscous dispersions with a slight to notable opalescence (
Figure 2). In contrast, B-5 and FH-5 were observed as clear and colorless solutions. In the case of FH-5, this result testifies to a complete solubilization of the oil phase and a successful formation of a microemulsion. The presence of a weak to moderate turbidity by the rest of the formulations is likely a consequence of the presence of a non-solubilized excess of alpha-bisabolol that remains emulsified in the aqueous medium, or of a larger size of the bisabolol-loaded micelles.
Samples DS-5, FH-5, and FH-10 showed a sustainable appearance over 30 days of storage under standard conditions and after centrifugation for accelerated stability testing. By samples DS-10, DS-15, and FH-15, creaming was observed after centrifugation, as well as after the second day of storage under standard conditions (
Figure 3). All affected samples were easily resuspended after a gentle shaking and a complete recovery of the original appearance was achieved.
3.2. Average Size and Size Distribution
MADLS revealed the highest significance and repeatability of the front-angle scans (12.78
o) data, wherefore these measurements were taken into account for the comparative purposes of the DLS analysis.
Table 2 summarizes the most important parameters derived from the DLS study.
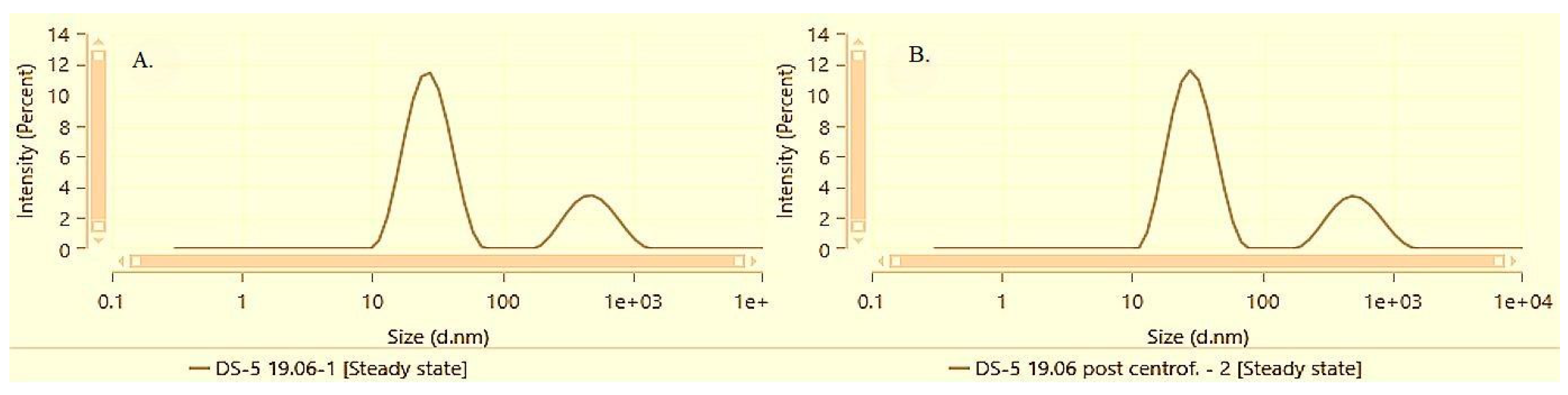
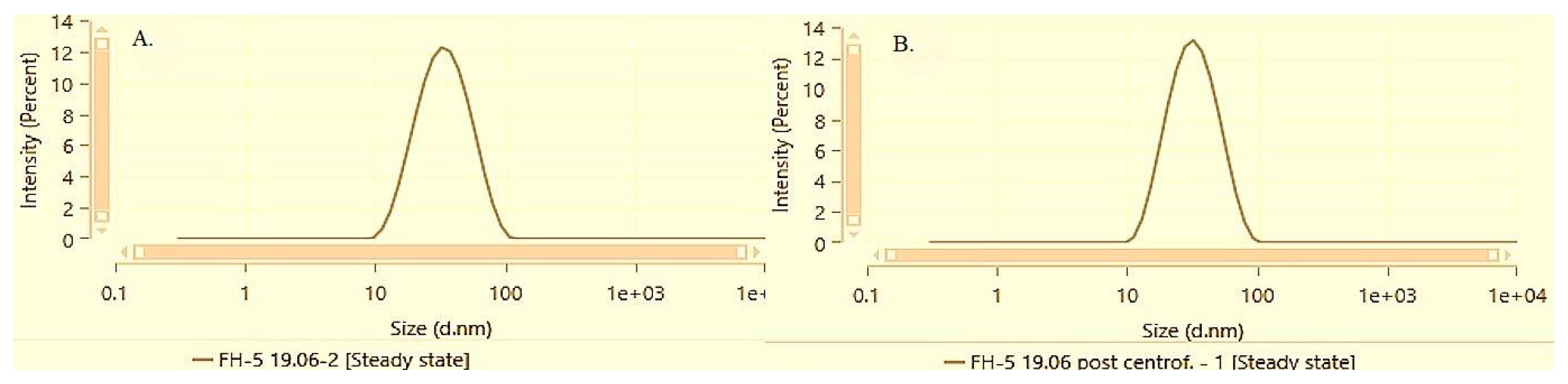
At the lowest bisabolol concentration of 0.5%, both solubilization methods demonstrated effective micellar solubilization and preservation of physical stability given the minor deviations in PDI and Z-average reported before and after centrifugation. However, the film-hydration method results in a smaller average micellar size and lower PDI as compared to direct solubilization.
Figure 4 and
Figure 5 illustrate the particle size distribution of samples DS-5 and FH-5 before and after performing the accelerated stability test by centrifugation followed by a subsequent resuspension.
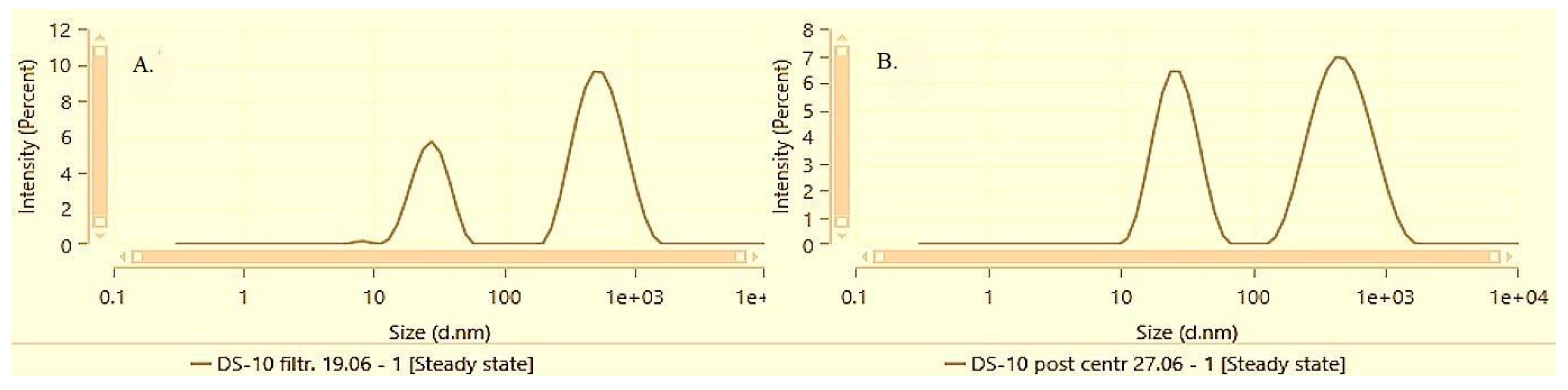
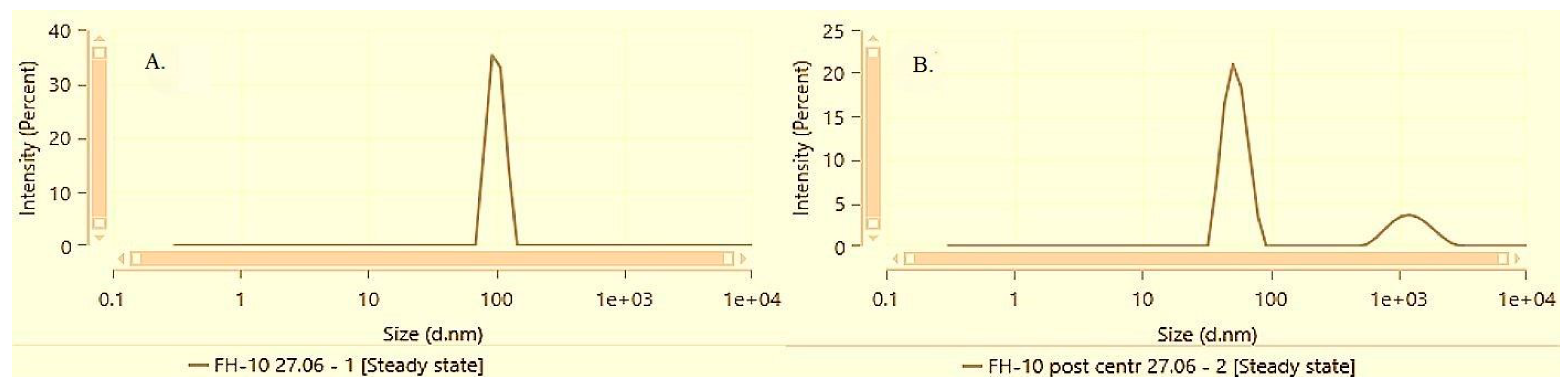
The differences between the solubilization techniques and the respective solubilization capacity of Poloxamer 407 for alpha-bisabolol became more pronounced at the next active concentration level of 1%. According to the DLS data, both FH-10 and DS-10 recover their dispersity after centrifugation, but FH-10 distincts with significantly lower average size and polydispersity.
Figure 6 and
Figure 7 illustrate the particle size distribution of samples DS-10 and FH-10 before and after performing the accelerated stability test by centrifugation followed by a subsequent resuspension.
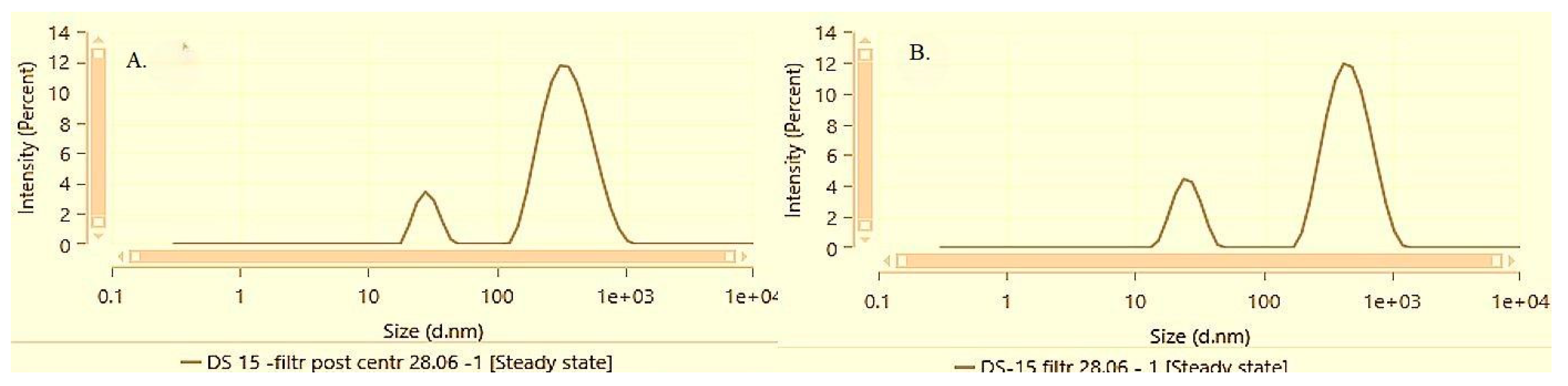
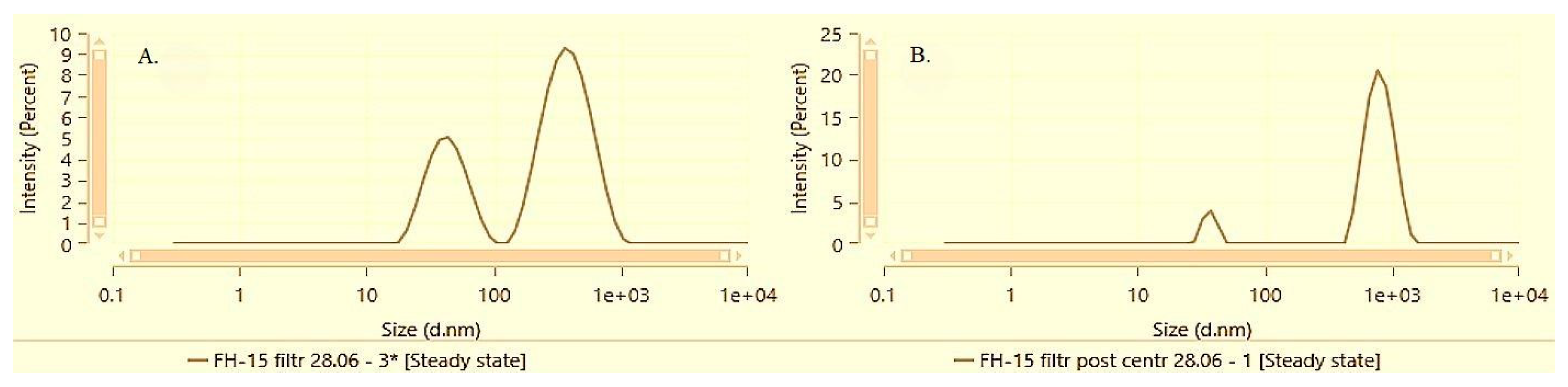
At the highest alpha-bisabolol concentration of 1.5%, both solubilization methods demonstrated an increase in the mean hydrodynamic size and polydispersity, as well as notable differences in the dispersion characteristics before and after centrifugation and resuspension.
Figure 8 and
Figure 9 illustrate the particle size distribution of samples DS-15 and FH-15 before and after performing the accelerated stability test by centrifugation followed by a subsequent resuspension.
3.3. Zeta-Potential
The zeta-potential values obtained, although low, show a clear tendency for theoretical destabilization with increasing the oil concentration and a greater absolute value for the samples obtained by direct solubilization at all concentration levels (
Table 3).
Although the zeta potential values fall in the unfavorable range of -30 to +30 mV, in reality, we observed satisfactory physical stability of all samples obtained with 0.5-1.0% alpha-bisabolol [
27]. It could be claimed that in this particular case, a prevailing role for the colloidal stability is played by the smaller size and narrow size distribution.
3.4. Foamability
A foamability test was conducted to evaluate the washing potential of the bisabolol-loaded micellar solutions in comparison to the free micellar water. The ability to form a foam is well-known to be tightly related to the presence of surfactants and the cleansing properties of the product [
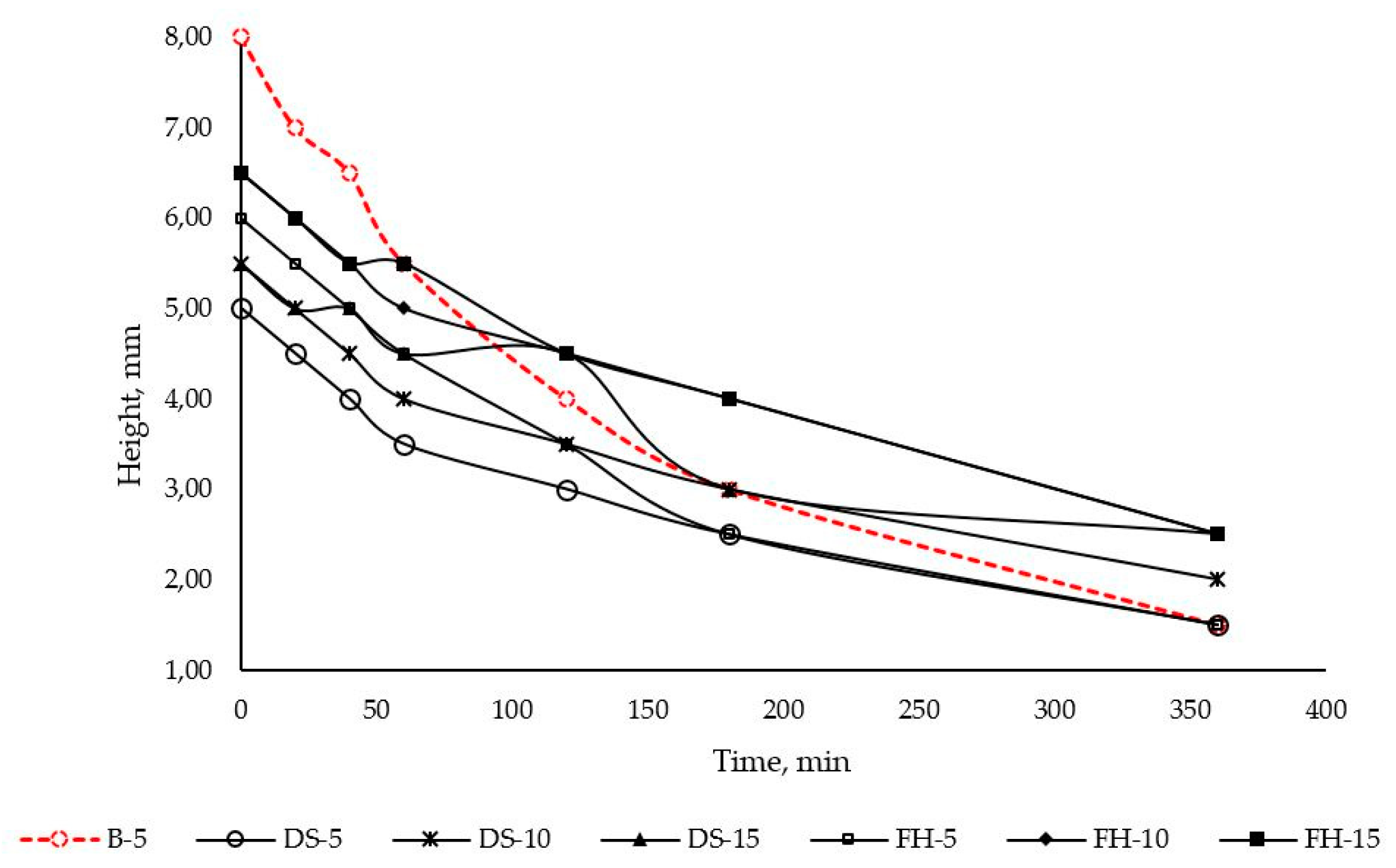
28]. Under the chosen experimental conditions, all test samples demonstrated the formation of a stable foam persisting for at least 6 hours. The presence of solubilized alpha-bisabolol was shown to slightly reduce the initial height of the foam, but stabilize it over time. The latter effect became more evident as the concentration of bisabolol increased (
Figure 10).
3.5. Viscosity
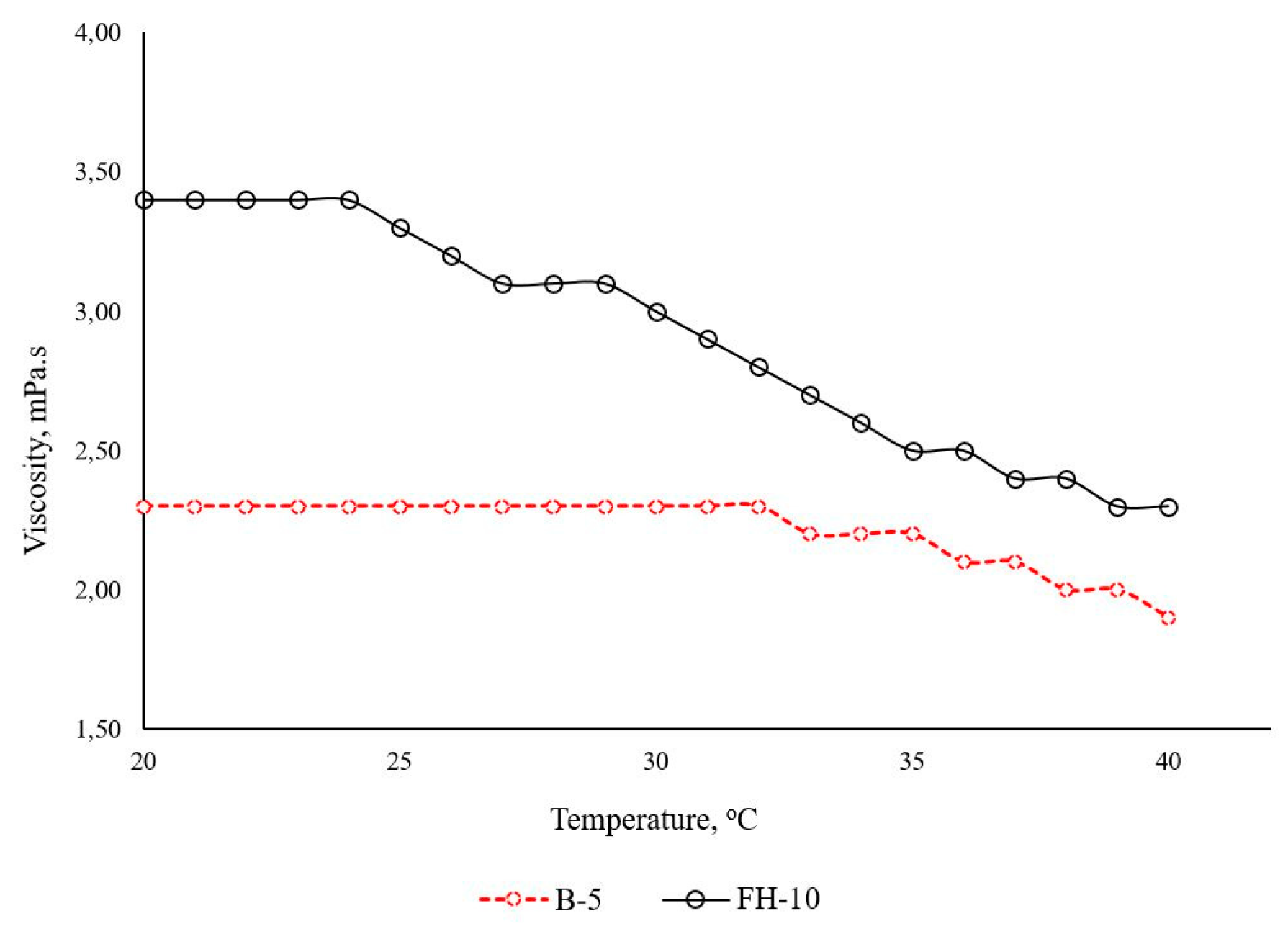
At the chosen Poloxamer 407 concentration of 5%, both the pure polymeric solution (reference) and the 1% alpha-bisabolol-loaded solubilizate (test) showed low viscosity and liquid consistency with no signs of thermo-geleation up until 40°C (
Figure 11). The viscosity was slightly increased in the presence of solubilized oil, whereas both samples demonstrated a decrease in viscosity with the rise in temperature. Thus, the optimized test formulation could be considered convenient for application on large skin areas if stored under the recommended conditions.
3.6. Antimicrobial Activity
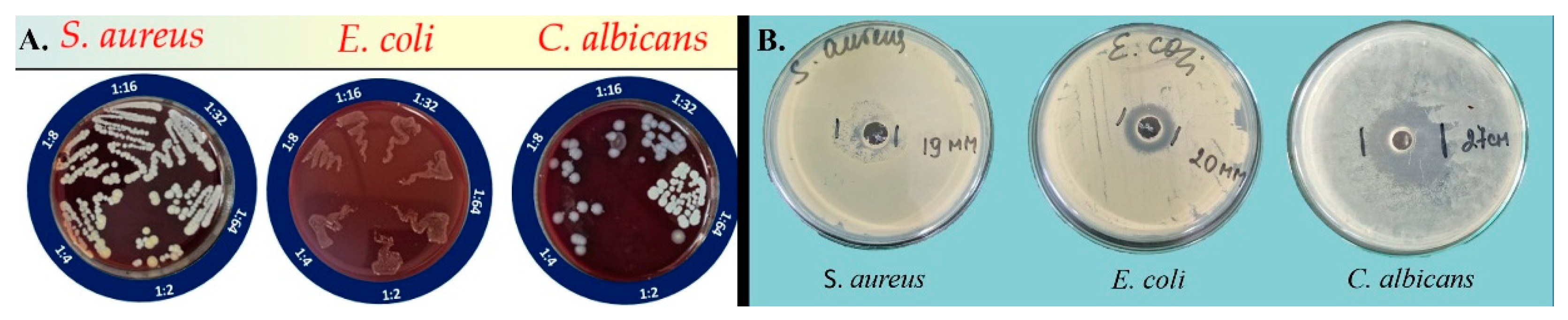
The antimicrobial study revealed the inhibitory activity of the alpha-bisabolol-loaded micellar solution against all microbial strains tested. The minimum inhibitory concentrations (MICs) of alpha-bisabolol against S. aureus, E. coli, and C. albicans were determined (
Table 4). At the highest affordable concentration with respect to physical stability – 1%, the compound demonstrated microbicidal activity only against C. albicans; bactericidal activity against S. aureus and E. coli was not established (
Figure 12A). However, clear zones of inhibition were reported for both C. albicans and E. coli, while the lack of a sterile zone of inhibition by S. aureus confirmed the absence of bactericidal activity against this pathogen (
Figure 12B).
4. Conclusions
The study revealed a better solubilization capacity of the 5% Poloxamer 407 solution with respect to alpha-bisabolol when the film-hydration method is applied as compared to the direct solubilization approach. The composition obtained by the film-hydration technique with 1% bisabolol (FH-10) was found most favorable with respect to fungicidal activity, satisfactory micellar encapsulation, transparency, and physical stability. The optimized formulation was characterized by a suitable for a cleansing micellar solution viscosity and a preserved foaming- and washing ability not regarded by the presence of a solubilized phase of bisabolol. The research carried out might be taken as a successful first step in the development of a cosmetic composition with alpha-bisabolol for facial skin hygiene providing washing and antimicrobial effect.
Author Contributions
Conceptualization, N.I.; methodology, N.I. and N.E; software, N.I.; validation, V.A.; formal analysis, N.I.; investigation, N.I. and N.E; resources, N.I.; data curation, N.I.; writing—original draft preparation, N.I.; writing—review and editing, V.A.; visualization, N.I.; supervision, V.A.; project administration, V.A.; funding acquisition, V.A. All authors have read and agreed to the published version of the manuscript.
Funding
This study is financed by the European Union—Next Generation EU—through the National Recovery and Resilience Plan of the Republic of Bulgaria, project № BG-RRP-2.004-0009-C02.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
Special thanks to the Medical University of Varna and the European Union for the financial support provided for the publication of this paper.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Eddin, L. B.; Jha, N. K.; Goyal, S. N.; Agrawal, Y. O.; Subramanya, S. B.; Bastaki, S. M. A.; Ojha, S. Health Benefits, Pharmacological Effects, Molecular Mechanisms, and Therapeutic Potential of α-Bisabolol. Nutrients 2022, 14, 1370. [Google Scholar] [CrossRef]
- Souza, F. C. B. de; Souza, R. F. B. De; Moraes, Â. M. Incorporation and release kinetic of alpha-bisabolol from PCL and chitosan/guar gum membranes. Braz. J. Chem. Eng. 2016, 33, 453–467. [Google Scholar] [CrossRef]
- Kamatou, G. P. P.; Viljoen, A. M. A Review of the Application and Pharmacological Properties of α-Bisabolol and α-Bisabolol-Rich Oils. J. Am. Oil. Chem. Soc. 2009, 87, 1–7. [Google Scholar] [CrossRef]
- Christian, S. D.; Scamehorn, J. F. Solubilization in Surfactant Aggregates; CRC press: Boca Raton, USA, 2020; pp. 31–111. [Google Scholar] [CrossRef]
- Ohshima, H.; Makino, K. Colloid and Interface Science in Pharmaceutical Research and Development; Elsivier: Amsterdam, Netherlands, 2014; pp. 1–54. [Google Scholar]
- Andrei, F. Dermatopharmacy and Cosmetology Practical Study Guide for Students of the English Section of the Faculty of Pharmacy; Victor Babes: Timisoara, Romania, 2023. [Google Scholar]
- Sakamoto, K.; Lochhead, R. Y.; Maibach, H. I.; Yamashita, Y. Cosmetic Science and Technology; Elsevier: Amsterdam, Netherlands, 2017. [Google Scholar]
- Sarkic, A.; Stappen, I. Essential Oils and Their Single Compounds in Cosmetics—A Critical Review. Cosmetics 2018, 5, 11. [Google Scholar] [CrossRef]
- Gugleva, V.; Ivanova, N.; Sotirova, Y.; Andonova, V. Dermal Drug Delivery of Phytochemicals with Phenolic Structure via Lipid-Based Nanotechnologies. Pharmaceuticals 2021, 14, 837. [Google Scholar] [CrossRef] [PubMed]
- Zakharova, L. Ya.; Vasilieva, E. A.; Mirgorodskaya, A. B.; Zakharov, S. V.; Pavlov, R. V.; Kashapova, N. E.; Gaynanova, G. A. Hydrotropes: Solubilization of nonpolar compounds and modification of surfactant solutions. J. Mol. Liq. 2023, 370, 120923. [Google Scholar] [CrossRef]
- Chen, P. Molecular Interfacial Phenomena of Polymers and Biopolymers; Woodhead Publishing: Sawston, 2005. [Google Scholar]
- Kumar, P.; Mittal, K. L. Handbook of Microemulsion Science and Technology; CRC press: Boca Raton, USA, 2018. [Google Scholar]
- Karunaratne, D. N.; Pamunuwa, G.; Ranatunga, R. J. K. U. Properties and Uses of Microemulsions. InTech: London, UK, 2017. [Google Scholar]
- Mahdi, E. S.; Sattar, M.; Sakeena, M. H. F.; Abdulkarim, M.; Noor, A. M.; Abdullah, G. Effect of surfactant and surfactant blends on pseudoternary phase diagram behavior of newly synthesized palm kernel oil esters. Drug Des. Devel. Ther. 2011, 311. [Google Scholar] [CrossRef] [PubMed]
- Khan, K. U.; Minhas, M. U.; Badshah, S. F.; Suhail, M.; Ahmad, A.; Ijaz, S. Overview of nanoparticulate strategies for solubility enhancement of poorly soluble drugs. Life Sci. 2022, 291, 120301. [Google Scholar] [CrossRef] [PubMed]
- Zarrintaj, P.; Ramsey, J. D.; Samadi, A.; Atoufi, Z.; Yazdi, M. K.; Ganjali, M. R.; Amirabad, L. M.; Zangene, E.; Farokhi, M.; Formela, K.; Saeb, M. R.; Mozafari, M.; Thomas, S. Poloxamer: A versatile tri-block copolymer for biomedical applications. Acta Biomater. 2020, 110, 37–67. [Google Scholar] [CrossRef] [PubMed]
- Dumortier, G.; Grossiord, J. L.; Agnely, F. , Chaumeil, J. C. A Review of Poloxamer 407 Pharmaceutical and Pharmacological Characteristics. Pharm. Res. 2006, 23, 2709–2728. [Google Scholar] [CrossRef]
- European Directorate for the Quality of Medicines & HealthCare of the Council of Europe (EDQM). European Pharmacopoeia. Strasbourg: Council of Europe, 10.0(1), 2019; pp. 3052 – 3054.
- Bodratti, A.; Alexandridis, P. Formulation of Poloxamers for Drug Delivery. J. Funct. Biomater. 2018, 9, 11. [Google Scholar] [CrossRef]
- Ivanova, N.; Ermenlieva, N.; Simeonova, L.; Vilhelmova-Ilieva, N.; Bratoeva, K.; Stoyanov, G.; Andonova, V. In Situ Gelling Behavior and Biopharmaceutical Characterization of Nano-Silver-Loaded Poloxamer Matrices Designed for Nasal Drug Delivery. Gels 2024, 10, 385. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.; Villa, C. Poloxamer Hydrogels for Biomedical Applications. Pharmaceutics 2019, 11, 671. [Google Scholar] [CrossRef] [PubMed]
- Cui, N.; Dai, C.-Y.; Mao, X.; Lv, X.; Gu, Y.; Lee, E.-S.; Jiang, H.-B.; Sun, Y. Poloxamer-Based Scaffolds for Tissue Engineering Applications: A Review. Gels 2022, 8, 360. [Google Scholar] [CrossRef]
- Sedlarikova, J.; Janalikova, M.; Egner, P.; Pleva, P. Poloxamer-Based Mixed Micelles Loaded with Thymol or Eugenol for Topical Applications. ACS Omega 2024, 9, 23209–23219. [Google Scholar] [CrossRef] [PubMed]
- Estanqueiro, M.; Conceição, J.; Amaral, M. H.; Santos, D.; Silva, J. B.; Lobo, J. M. S. Characterization and stability studies of emulsion systems containing pumice. Braz. J. Pharm. Sci. 2014, 50, 361–369. [Google Scholar] [CrossRef]
- Navarro-Pérez, Y. M.; Cedeño-Linares, E.; Norman-Montenegro, O.; Ruz-Sanjuan, V.; Mondeja-Rivera, Y.; Hernández-Monzón, A. M.; González-Bedia, M. M. Prediction of the physical stability and quality of O/W cosmetic emulsions using full factorial design. J. pharm. pharmacogn. res. 2021, 9, 98–112. [Google Scholar] [CrossRef]
- Petkova, B.; Tcholakova, S.; Denkov, N. Foamability of surfactant solutions: Interplay between adsorption and hydrodynamic conditions. Coll. Surf. A Colloid. Surf. A Physicochem. Eng. Asp. 2021, 626, 127009. [Google Scholar] [CrossRef]
- Malvern® Panalytical. Zetasizer advance series user guide, 2022.
- Yorke, K.; Amin, S. High Performance Conditioning Shampoo with Hyaluronic Acid and Sustainable Surfactants. Cosmetics 2021, 8, 71. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).