1. Introduction
Colossoma macropomum has significant economic value in Latin America, despite facing challenges in the intensification of its production [
1]. Among the major issues related to the production of this fish species, bacterial diseases can highlighted, with infections caused by
Aeromonas hydrophila [
2],
Aeromonas jandaei [
3],
Edwardsiella tarda [
4] and
Flavobacterium oreochromis [
3] having already been reported.
Columnaris-causing bacteria drastically affect tropical freshwater fish like
C. macropomum [
5]. These bacteria reside in water, ponds, mud and sediments, and can survive for extended periods in various environmental conditions [
6]. Fish affected by these pathogens may present clinical signs such as anorexia, lethargy, erratic swimming, rapid opercular movements, gill lesions, tegument erosion, fin erosion, melanosis and saddleback lesions [
3]. High mortality rates associated with columnaris have been reported in both natural and experimental infections [
7,
8]; However. vaccines have been developed as an effective prophylactic measure against
F. oreochromis [
9]. Through vaccination, the immune system is stimulated to develop memory antibodies, thus enhancing the acquired immune response upon exposure to the pathogen [
10].
To develop vaccination protocols, it is necessary to validate the immunological memory against the target-pathogen through laboratory analyses such as the indirect enzyme-linked immunosorbent assay (iELISA). This method involves coating a microplate with an antigen, followed by the sequential addition of a primary antibody and a secondary antibody linked to a revealing enzyme [
11]. The ELISA assay requires specific antibodies for its standardization, which are used as biotechnological tools [
12]. Immunoglobulin G (IgG) produced by mammals is widely used in research and laboratory diagnostics, but its extraction is invasive, often involving bloodletting and euthanasia of animals like mice and rabbits to obtain large blood volumes [
13]. As an alternative to IgG, the immunoglobulin Y (IgY), which is produced by chickens, provides immune support during the embryonic and post-natal phases [
14]. IgY is abundantly produced in the egg yolk and serum of birds, can be generated rapidly with minimal environmental impact, requires low investment in infrastructure and has low production and handling costs when obtained from chickens [
15]. IgY has been used in aquaculture with good results for diagnostic purposes in various aquatic animals [
16], and has already been applied in immunological assays for the detection of infection by
Aeromonas hydrophila in fish [
17].
Combining the potential use of IgY with emerging vaccinations against columnaris and considering the scarcity of information on the immunity and antibody titers in C. macropomum, this study aimed to standardize an iELISA using IgY extracted from chicken plasma to validate an experimental vaccination protocol applied to C. macropomum against F. oreochromis.
2. Materials and Methods
2.1. Hen Immunization
A Dekalb White laying hen was maintained in an individual cage with drinking water and food provided
ad libitum. The hen was immunized via multiple pectoral muscle injections on days 0, 14, and 21 with an inoculum containing 9.11 μL of
C. macropomum immunoglobulin extract purified with caprylic acid [
18] and 300 μL of Montanide
TM ISA adjuvant (Seppic, France), which was topped up to 600 μL with phosphate-buffered saline (PBS). Immunizations were carried out by the Ezscience company (Manaus, Brazil). Blood samples were collected from the hen’s ulnar vein on days 0, 7, 14, and 21. The aliquots were kept in the refrigerator for 2 h and then centrifuged at 2,000 x
g at 4 °C for 12 min. The obtained plasma was stored at -80 °C until use in the ELISA standardization.
2.2. Bacterial Inoculum and Immunization of Fish against Flavobacterium oreochromis
The
Flavobacterium oreochromis strain (AM-FO14) was previously obtained from diseased
Colossoma macropomum and ascertained according pathogenicity (complete Koch’s Postulate) and identity (PCR-multiplex, REP-PCR and complete genome sequencing) [
19]. The strain (stored at -80 °C in G broth enriched with glycerol 15%) was thawed and streaked onto G agar at 28 °C for 48 h. A colony was picked, inoculated into G broth, and incubated under the same conditions, at 100 rpm. The bacteria were resuspended in sterile PBS at a concentration of 3.3 x 10
9 colony-forming units (CFU)/mL, which were determined using the plating-count method. The bacterial inoculum was inactivated with 1% formalin and incubated at 4 °C for 24 h. Bacterin synthesis was confirmed by the absence of bacterial growth on G agar (28 °C for 48 h). To produce the oil-adjuvanted vaccine, antigen (bacterin) and adjuvant (Montanide
TM) were mixed at a ratio of 27:73 (v/v), respectively, [
20] and emulsified.
For the immunization, fifty-four juvenile C. macropomum (average weight of 30 g) with no history of diseases were acquired from a population maintained in tanks at Nilton Lins University. Prior to the experimental assay, six fish were randomly selected and submitted to bacteriological examination to ensure they were free from bacterial infections. The remaining fish were acclimated for 10 days and later randomly distributed into the following groups: G1 – group inoculated with bacterin + adjuvant, G2 – group inoculated with bacterin, G3 – group inoculated with adjuvant, and G4 – control group (inoculated with sterile PBS). The fish were kept in polyethylene tanks (80 L) at a density of 6 fish per tank, with a supplementary aeration system, daily partial water exchange at a rate of 70%, and a temperature of 28 °C. Commercial fish feed (Nutripiscis, Brazil, 36% protein) was provided twice per day throughout the experimental and maintaince period. Immunization was carried out on days 0 and 21 via intraperitoneal injection (i.p.), after anesthesia (benzocaine, 100 mg/L, immersion bath), with 0.2 mL of inoculum per fish. On day 30, the experimental groups were inoculated with bacterin via i.p. to stimulate the humoral response, except G4, which was inoculated with sterile PBS. The experiment was conducted in duplicate. In order to evaluate humoral responses, blood samples (0.5 mL) were collected from all fish via puncture of the caudal vein after anesthesia at 0, 21, 30, 40, 50, and 60-days post-inoculation (dpi).
2.3. Standardization of the iindirect Enzyme-Linked Immunosorbent Assay (iELISA)
For the standardization of the iELISA, polyethylene microplates (Kasvi, Brazil) were coated with inactivated F. oreochromis diluted in carbonate/bicarbonate buffer (1:1,000). A volume of 50 µL of concentrated antigen (0.5 x 102 CFU/mL) was added per well and the microplates were incubated in a humid chamber for 1 h at 37 °C. For blocking, 150 µL of ELISA Blocking Buffer (Scienco Biotech, Brazil) were added to each well, followed by incubation under the same conditions described above. Next, the plate was washed four times with 200 µL per well of 1x PBS with 0.05% Tween (PBST). After the washes, 50 µL of the primary antibody (anti-F. oreochromis from fish) were tested at the following dilutions: 1:50, 1:100, 1:200, and 1:400. Then, the blocking buffer was added to each well and incubated at 37 °C for 1 h. Five further washes with 200 µL PBST were performed. Then, 50 µL of the unmarked secondary antibody (anti-C. macropomum IgY), diluted at 1:100 in ELISA blocking buffer were added in each well to recognize the primary antibody bound to the antigen fixed to the plate. The plate was again incubated in a humid chamber at 37 °C for 1 h, followed by five washes with PBST. Then, 50 µL of the labeled secondary antibody, goat anti-chicken IgY (Sigma-Aldrich, Germany, catalog number: SAB3700226), diluted at 1:10,000 in ELISA blocking buffer, were added to each well, followed by incubation (37 °C for 1 h) and five washes with PBST. The reaction step was carried out using One Step – TMB Linear (Scienco) chromogenic substrate, added at 50 µL/well, with a 2-minute reaction time. The reaction was stopped with 25 µL of 1M sulfuric acid in each well. The plates were read in an ELISA plate reader (LMR-96, Loccus, Brazil) at a wavelength of 450 nm to assess optical density.
2.4. Statistical Analysis
After standardizing the technique, the comparison between experimental groups was performed using linear mixed models, which were implemented according to the experiment’s longitudinal dependency. The cut-off point was defined using the mean optical density readings from negative samples (G3 and G4) multiplied by three times the standard deviation [
21]. Groups with antibody levels equal to or greater than the cut-off were considered immunized. Those with lower results were considered unimmunized. Statistical analysis was conducted using R software v.4.3.1 [
22], with various packages (tidyverse, lme4, sjPlot, and hnp), considering a significance level of p < 0.05.
3. Results
The iELISA was performed under the following conditions: 50 μL of antigen per well, 50 μL of
C. macropomum plasma diluted at 1:400 (best dilution of the primary antibody), 50 μL of anti-
C. macropomum IgY at 1:100 as the unlabeled secondary antibody, and 50 μL of anti-IgY at 1:10,000 as the labeled secondary antibody. Under these conditions, the temporal samples from the experimental groups were subjected to absorbance testing and analysis.
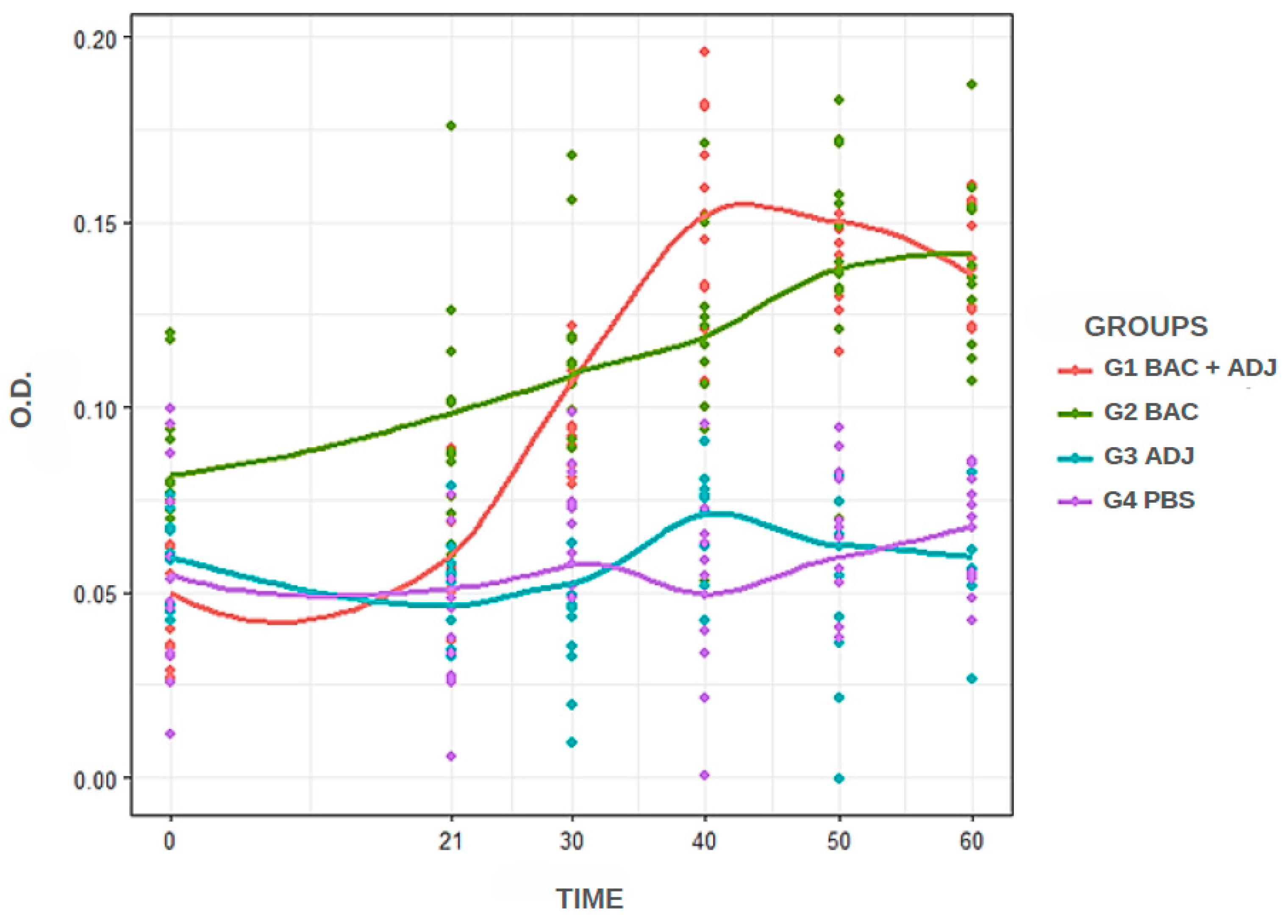
Figure 1 shows the distribution of antibody titers among the experimental groups. In general, groups G1 and G2 exhibited higher antibody titer values, while the groups injected with adjuvant (G3) and PBS (G4) inoculum showed lower titer values. When analyzing absorbance over time, it is evident that the G2 group had a stronger reaction at day 0 compared to the other groups, but it was surpassed by the G1 at 40 and 50 days. The G1, G3 and G4 groups showed similar titration levels at 0 and 21 days, but G1 increased antibody titers from day 30, the day on which the fish were re-inoculated with bacterin.
The linear regression (mixed model) showed that G1 and G2 were similar and both differed from groups G3 and G4 (
Table 1).
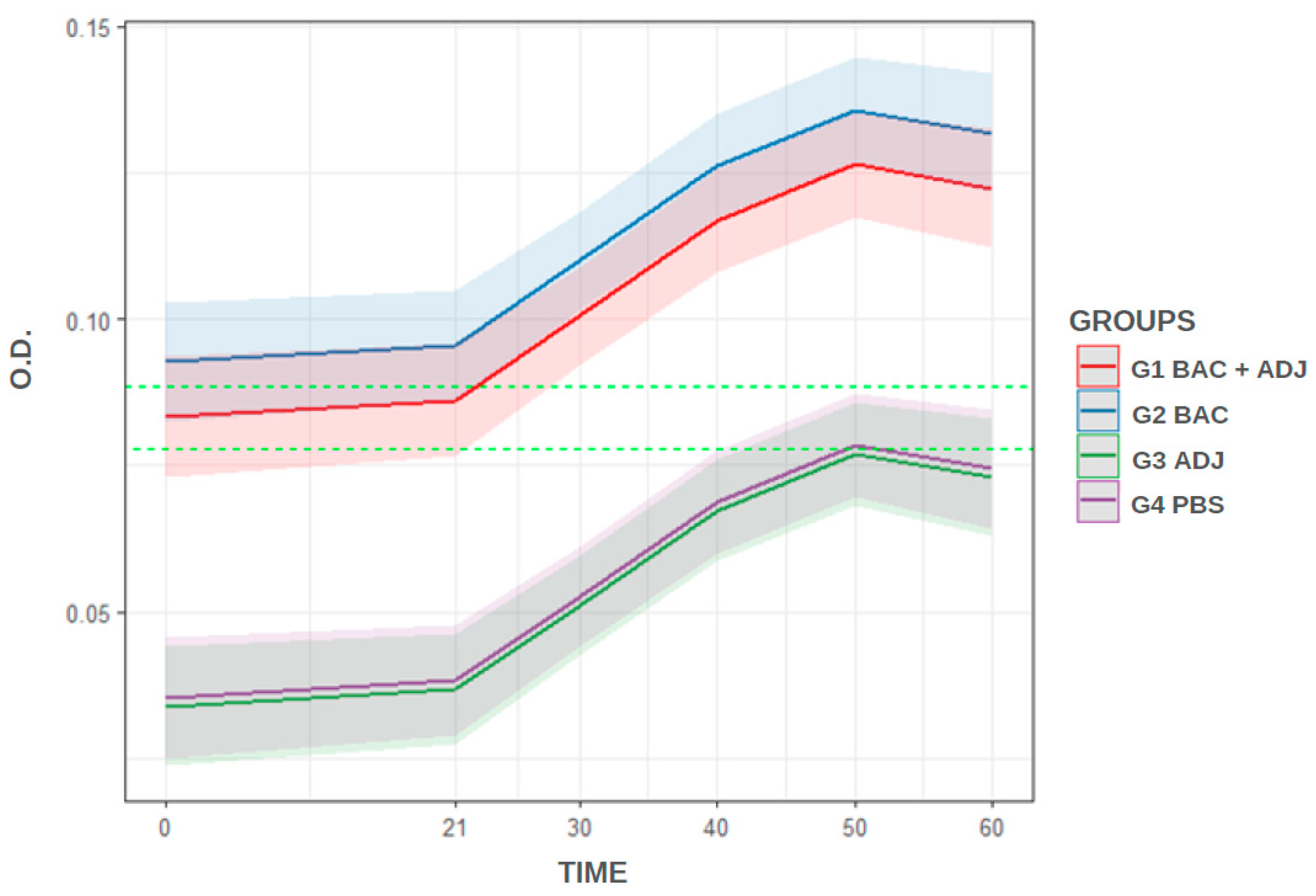
According to the cut-off that was determined (0.088 OD
450), G1 and G2 were considered immunized from 21 dpi (exceeded cut-off value). Groups G3 and G4 did not reach the cut-off throughout the analysis and are therefore considered unimmunized. The model’s prediction (
Figure 2) shows the overlap of confidence bands between G1 and G2, demonstrating the statistical similarity between those immunized with bacterin + adjuvant and bacterin. The figure also shows the overlap of bands between G3 and G4, demonstrating the statistical similarity between those that are unimmunized. According to the regression analysis (
Table 1), the immunized groups differ (p<0.05) from unimmunized groups.
4. Discussion
This study demonstrated the potential of anti-
C. macropomum IgY in recognizing the fish’s humoral immunity, ensuring the reliability of the developed standardization of the iELISA. iELISA has already been established to evaluate humoral immunity in fish species such as
Ictalurus punctatus [
23],
Oreochromis niloticus [
24] and
Piaractus mesopotamicus [
25]. Despite the imminent risks that
Flavobacterium sp. can pose to fish production, to our knowledge, to date, there is no description of this fundamental tool for the detection of antibodies against
Flavobacterium oreochromis that infects
C. macropomum.
For the standardization of the iELISA, the implementation of IgY technology is an ethical and cost-effective alternative [
26]. Unlike the production of antibodies in mammals, the use of IgY eliminates the need for bleeding and killing animals and produces a high yield of antibodies [
27]. Additionally, it offers practical material collection and ease of handling [
28], especially when extracted from chicken eggs. The use of IgY has already been described for the immunodetection of
Aeromonas hydrophila [
29] and
Streptococcus agalactiae [
30] in
Oreochromis niloticus. Despite evidence of promising applicability in fish farming, to our knowledge, this study is the first to implement a methodology for IgY production using an Amazonian fish species.
The standardization of the iELISA developed in this study demonstrated that IgY specific to C. macropomum (anti-C. macropomum IgY) was successfully recognized by the fish serum, indicating that the immune response or the presence of the target-protein in the serum can be successfully detected by IgY antibodies. Therefore, it allowed the antibody titration in fish immunized against columnaris. The results achieved support a standardized technique that, despite being performed at an experimental level, could be used in the market as a laboratory method to verify the health and/or immunization status of C. macropomum.
In our study, it was observed that, on day 0, there was a similarity in antibody titers between groups G1, G3 and G4, while G2 had a greater abundance. Operational factors can influence the accuracy of the test and result in a false positive, such as the use of a polyclonal antibody, which has less specificity compared to a monoclonal antibody and has the capacity to recognize different epitopes, enabling cross-reactivity in the ELISA [
31]. Furthermore, there is also the possibility of the prior exposure of individuals in G2 to
Flavobacterium oreochromis, since the bacteria are present in the aquatic environment and healthy fish may be infected [
32]; however, the disease could manifest itself when associated with predisposing factors such as poor water quality, injuries and prolonged transportation [
3].
The fish were immunized on day 0 and received a booster on day 21. The increase in antibody abundance between these days, especially in G1 and G2, was relatively low compared to the increase observed between days 21 and 40 (
Figure 1). During this period, a safe challenge (day 30) was performed with bacterin to assess the humoral response of
C. macropomum. The increase in antibody abundance demonstrates that the vaccine booster is an important factor for enhancing fish immunity against
F. oreochromis, as the absorbance values of groups G1 and G2 remained high until day 60 (higher than the calculated cut-off,
Figure 2), with the peak antibody level recorded on day 40 after the first immunization. In contrast, a study evaluating
Rhamdia quelen vaccinated against
Aeromonas hydrophila observed that the peak antibody titer occurred on day 21 after vaccination with a single dose [
33]. The humoral immunity of
Oreochromis spp. against TiLV showed a significant increase in antibody levels between day 0 and day 14 after challenge, with levels remaining elevated for up to six weeks. The research evaluated the individual immune response and demonstrated variations in antibody titers between individuals [
34]. The interpretation of these results suggests that the pattern of humoral immune response is associated with the fish species and pathogens studied, which is reflected in the processing of immunological analyses and the standardization of the immunoassay.
5. Conclusions
In conclusion, the IgY obtained from hen serum in this study was able to react specifically against epitopes present in the serological material of Colossoma macropomum and can be used in the standardized iELISA protocol to detect antibodies from this fish species immunized against Flavobacterium oreochromis. The application of the iELISA will enable the analysis of antibody titration and the validation of vaccine protocols in future studies.
Author Contributions
Conceptualization: MVLC, JCG, RLS, ALGC, LAMM, SUG, GCT; Investigation: MVLC, ECP, TFB, JCG, ALGC, LAMM, SUG, GCT; Methodology: MVLC, ECP, TFB, JCG, GCT; Formal analyses: MVLC, AASB; Data curation: MVLC, JCG, ALGC, LAMM, AASB; Validation: MVLC, JCG; Visualization: MVLC; Resources: RLS, LAMM, SUG, GCT; Funding acquisition: LAMM, SUG, GCT; Project administration: LAMM, SUG, GCT; Supervision: LAMM, SUG, GCT; Writing – original draft: MVLC, GCT; Writing – review & editing: MVLC, ECP, TFB, JCG, RLS, ALGC, AASB, LAMM, SUG, GCT.
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES), through the PROCAD/Amazônia (grant number 88881.200614/2018-01), PDPG- and PROSUP-CAPES; Fundação de Amparo à Pesquisa do Estado do Amazonas (FAPEAM, grant numbers 01.02.016301.02785/2021-21 and 01.02.016301.03247/2021-54); and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, grant numbers 422010/2021-9 and 405706/2022-7)
Ethical Approval
All in vivo protocols were approved by the Ethics Committee for Animal Use at the Nilton Lins University (protocol No. 005/2023).
Data Availability Statement
The data will be made available on request.
Acknowledgments
The authors gratefully acknowledge the support provided by Instituto Leônidas e Maria Deane (ILMD), Fundação Oswaldo Cruz (FIOCRUZ).
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
- Valladão, G.M.R.; Gallani, S.U.; Pilarski, F. South American Fish for Continental Aquaculture. Rev. Aquac. 2018, 10, 351–369. [CrossRef]
- Gallani, S.U.; Valladão, G.M.R.; Assane, I.M.; Alves, L. de O.; Kotzent, S.; Hashimoto, D.T.; Pilarski, F. Motile Aeromonas Septicemia in Tambaqui Colossoma macropomum: Pathogenicity, Lethality and New Insights for Control and Disinfection in Aquaculture. Microb. Pathog. 2020, 149, 104512. [CrossRef]
- Mielke, T.D.; Francisco, C.J.; Dorella, F.A.; Figueiredo, H.C.P.; Tavares, G.C.; Gallani, S.U. The Strategic Use of Water Additives for Tambaqui Colossoma macropomum Transport: New Insights of Bacteriosis and Productivity Approach. Aquaculture 2022, 558, 738406. [CrossRef]
- Reis, F.Y.; Rocha, V.P.; Janampa-Sarmiento, P.C.; Costa, H.L.; Egger, R.C.; Passos, N.C.; de Assis, C.H.; Carneiro, S.P.; Santos, Á.F.; Silva, B.A.; et al. Edwardsiella tarda in Tambaqui (Colossoma macropomum): A Pathogenicity, Antimicrobial Susceptibility, and Genetic Analysis of Brazilian Isolates. Animals 2023, 13.
- Hilsdorf, A.W.S.; Hallerman, E.; Valladão, G.M.R.; Zaminhan-Hassemer, M.; Hashimoto, D.T.; Dairiki, J.K.; Takahashi, L.S.; Albergaria, F.C.; Gomes, M.E. de S.; Venturieri, R.L.L.; et al. The Farming and Husbandry of Colossoma macropomum: From Amazonian Waters to Sustainable Production. Rev. Aquac. 2022, 14, 993–1027. [CrossRef]
- Mohammed, H.; Arias, C. Epidemiology of Columnaris Disease Affecting Fishes within the Same Watershed. Dis. Aquat. Organ. 2014, 109, 201–211.
- Shoemaker, C.A.; Olivares-Fuster, O.; Arias, C.R.; Klesius, P.H. Flavobacterium columnare Genomovar Influences Mortality in Channel Catfish (Ictalurus punctatus). Vet. Microbiol. 2008, 127, 353–359. [CrossRef]
- Barony, G.; Tavares, G.; Assis, G.; Luz, R.; Figueiredo, H.; Leal, C. New Hosts and Genetic Diversity of Flavobacterium columnare Isolated from Brazilian Native Species and Nile Tilapia. Dis. Aquat. Organ. 2015, 117, 1–11.
- Bunnoy, A.; Thangsunan, P.; Chokmangmeepisarn, P.; Yata, T.; Klongklaew, N.; Pirarat, N.; Kitiyodom, S.; Srisapoome, P.; Rodkhum, C. Mucoadhesive Cationic Lipid-Based Flavobacterium oreochromis Nanoencapsulation Enhanced the Efficacy of Mucoadhesive Immersion Vaccination against Columnaris Disease and Strengthened Immunity in Asian Sea Bass (Lates calcarifer). Fish Shellfish Immunol. 2022, 127, 633–646. [CrossRef]
- Ma, J.; Bruce, T.J.; Jones, E.M.; Cain, K.D. A Review of Fish Vaccine Development Strategies: Conventional Methods and Modern Biotechnological Approaches. Microorganisms 2019, 7.
- Sakamoto, S.; Putalun, W.; Vimolmangkang, S.; Phoolcharoen, W.; Shoyama, Y.; Tanaka, H.; Morimoto, S. Enzyme-Linked Immunosorbent Assay for the Quantitative/Qualitative Analysis of Plant Secondary Metabolites. J. Nat. Med. 2018, 72, 32–42. [CrossRef]
- Aydin, S. A Short History, Principles, and Types of ELISA, and Our Laboratory Experience with Peptide/Protein Analyses Using ELISA. Peptides 2015, 72, 4–15. [CrossRef]
- Barati, B.; Ebrahimi, F.; Nazarian, S. Production of Chicken Egg Yolk Antibody (IgY) against Recombinant Cholera Toxin B Subunit and Evaluation of Its Prophylaxis Potency in Mice. Iran. J. Immunol. 2018, 15, 47–58.
- Lanzarini, N.M.; Bentes, G.A.; Volotão, E. de M.; Pinto, M.A. Use of Chicken Immunoglobulin Y in General Virology. J. Immunoass. Immunochem. 2018, 39, 235–248. [CrossRef]
- Amro, W.A.; Al-Qaisi, W.; Al-Razem, F. Production and Purification of IgY Antibodies from Chicken Egg Yolk. J. Genet. Eng. Biotechnol. 2018, 16, 99–103. [CrossRef]
- Zhang, L.; Lin, L.; Qin, Z. A Review on the Application of Chicken Immunoglobulin Y in Aquaculture. Rev. Aquac. 2024, 16, 536–551. [CrossRef]
- Fernandes, D.C.; Eto, S.F.; Claudiano, G.S.; Marcusso, P.F.; Ramos-Espinoza, F.C.; Moraes, J.R.E. Production and Use of Immunoglobulin Y in the Diagnosis of Haemorrhagic Septicemia Caused by Aeromonas hydrophila in Piaractus mesopotamicus. Aquac. Res. 2022, 53, 2930–2936. [CrossRef]
- Dias, C.A.C. de V.; Góes Filho, J.D. de; Oliveira, R.R.C.O.; Sousa, R.L. de; Duncan, W.L.P.; Ono, E.; Mariúba, L.A.; Lameiras, J.L.V.; Santos, M.C. dos. Caracterização Parcial Das Imunoglobulinas de Plesiotrygon iwamae (Chondrichtyes - Potamotrygonidae) e de Colossoma macropomum (Osteichtyes Characidae) Isoladas Com Ácido Caprílico. Sci. Amaz. 2015, 2, 1–9.
- Pereira, E.C.; Botinelly, T.F.; Nogueira, L.F.F.; Kotzent, S.; Pilarski, F.; Figueiredo, H.C.P.; Valladão, G.M.R.; Tavares, G.C.; Gallani, S.U. Identification, Virulence and Genetic Diversity of Flavobacterium oreochromis of Tambaqui (Colossoma macropomum) in Different Brazilian States. In Proceedings of the I Integrative International Congress on Animal and Environmental Health; Manaus, 2024; pp. 1–2.
- Wangkaghart, E.; Deville, S.; Wang, B.; Srisapoome, P.; Wang, T.; Secombes, C.J. Immune Response and Protective Efficacy of Two New Adjuvants, MontanideTM ISA 763B VG and MontanideTM GEL02, Administered with a Streptococcus agalactiae Ghost Vaccine in Nile Tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2021, 116, 19–29. [CrossRef]
- Crowther, J.R. Basic Immunology BT - ELISA: Theory and Practice. In; Crowther, J.R., Ed.; Humana Press: Totowa, NJ, 1995; pp. 1–34 ISBN 978-1-59259-529-7.
- R Core Team R: A Language and Environment for Statistical Computing 2024.
- Shoemaker, C.A.; Shelby, R.A.; Klesius, P.H. Development of an Indirect ELISA to Detect Humoral Response to Flavobacterium columnare Infection of Channel Catfish, Ictalurus punctatus. J. Appl. Aquac. 2003, 14, 43–52. [CrossRef]
- Leal, C.A.G.; Carvalho-Castro, G.A.; Sacchetin, P.S.C.; Lopes, C.O.; Moraes, A.M.; Figueiredo, H.C.P. Oral and Parenteral Vaccines against Flavobacterium columnare: Evaluation of Humoral Immune Response by ELISA and in Vivo Efficiency in Nile Tilapia (Oreochromis niloticus). Aquac. Int. 2010, 18, 657–666. [CrossRef]
- Farias, T.H. V; Silva, K.R.; Mariguela, V.C.; Montassier, H.J.; Pilarski, F. Development of an Indirect ELISA Assay to Evaluation of the Adaptive Immune Response of Pacu (Piaractus mesopotamicus). An. Acad. Bras. Cienc. 2018, 90, 3327–3335.
- Glória, J.C.; Chaves, Y.O.; Figueiredo, A.M. de; Souza, C.C. de; Silva, E.R.D. da; Batista, J.C.L.; Nogueira, P.A.; Mariúba, L.A.M. Standardization of a Cytometric Bead Assay Based on Egg-Yolk Antibodies. JoVE 2023, e65123, doi:doi:10.3791/65123.
- Li, X.; Wang, L.; Zhen, Y.; Li, S.; Xu, Y. Chicken Egg Yolk Antibodies (IgY) as Non-Antibiotic Production Enhancers for Use in Swine Production: A Review. J. Anim. Sci. Biotechnol. 2015, 6, 40. [CrossRef]
- Michael, A.; Meenatchisundaram, S.; Parameswari, G.; Subbraj, T.; Selvakumaran, R.; Ramalingam, S. Chicken Egg Yolk Antibodies (IgY) as an Alternative to Mammalian Antibodies. Indian J. Sci. Technol. 2010, 3, 468–474. [CrossRef]
- Fernandes, D.C.; Eto, S.F.; Funnicelli, M.I.G.; Fernandes, C.C.; Charlie-Silva, I.; Belo, M.A.A.; Pizauro, J.M. Immunoglobulin Y in the Diagnosis of Aeromonas hydrophila Infection in Nile Tilapia (Oreochromis niloticus). Aquaculture 2019, 500, 576–585. [CrossRef]
- Eto, S.F.; Fernandes, D.C.; Moraes, A.C.; Prado, E.J.R.; Baldassi, A.C.; Manrique, W.G.; Silva, I.C.; Medeiros, A.S.R.; Belo, M.A.A.; Balbuena, T.S.; et al. Validation of IgY for the Diagnosis of Streptococcus agalactiae-Caused Endocarditis and Bacterial Meningitis in Nile Tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2018, 76, 153–160. [CrossRef]
- Kieliszewski, M.; Lamport, D.T.A. Cross-Reactivities of Polyclonal Antibodies against Extensin Precursors Determined via ELISA Techniques. Phytochemistry 1986, 25, 673–677. [CrossRef]
- Suomalainen, L.-R.; Tiirola, M.A.; Valtonen, E.T. Influence of Rearing Conditions on Flavobacterium columnare Infection of Rainbow Trout, Oncorhynchus mykiss (Walbaum). J. Fish Dis. 2005, 28, 271–277. [CrossRef]
- Soveral, L. de F.; de Almeida, P.A.; Kreutz, Y.; Ribeiro, V.A.; Frandoloso, R.; Kreutz, L.C. Modulation of Expression of Proinflammatory Genes and Humoral Immune Response Following Immunization or Infection with Aeromonas hydrophila in Silver Catfish (Rhamdia quelen). Fish Shellfish Immunol. Reports 2022, 3, 100053. [CrossRef]
- Tattiyapong, P.; Dechavichitlead, W.; Waltzek, T.B.; Surachetpong, W. Tilapia Develop Protective Immunity Including a Humoral Response Following Exposure to Tilapia Lake Virus. Fish Shellfish Immunol. 2020, 106, 666–674. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).