1. Introduction
The development and widespread use of effective vaccines play a fundamental role in controlling numerous serious, and potentially lethal, contagious diseases worldwide. The impact of vaccines is significant, with estimates suggesting that global immunization programs save two to three million lives annually. Despite their high safety profiles, adverse events following immunization (AEFI) were frequently encountered, although most were mild and resolved spontaneously within a short period. Serious adverse events (SAEs), while rare, can be associated with severe morbidity, long-term sequelae, or even death. Establishing a causal relationship between SAEs and vaccination on an individual case basis can be challenging, leading to legal conflicts among affected individuals, vaccine manufacturers, healthcare institutions, and governments. Moreover, safety concerns and misinformation about vaccines have emerged as critical issues contributing to vaccine hesitancy, suboptimal vaccine coverage, and the resurgence of vaccine-preventable diseases. Data concerning the types and incidence of AEFIs, with their causality linked to vaccines (hereafter referred to as adverse reactions, or ARs), are crucial for building public trust in vaccines and promoting vaccine uptake. However, such information is lacking in many countries.
In Taiwan, several systems are available for reporting AEFI. These include the Adverse Drug Reaction (ADR) reporting system operated by the Taiwan Drug Relief Foundation and the Taiwan National ADR Reporting Center, which is affiliated with the Taiwan Food and Drug Administration. Additionally, the Vaccine Adverse Event Reporting System (VAERS) is managed by the Taiwan Centers for Disease Control (CDC), and there is also the national Vaccine Injury Compensation Program (VICP). All of these systems utilize passive reporting from individuals, healthcare providers, or pharmacists.
The VICP is considered a potential resource for post-marketing surveillance of vaccine ARs. The first vaccine injury compensation system was established by the Federal Republic of Germany in 1961 [
1]. In the United States, the surge in litigation related to vaccination in the 1970s led to vaccine shortages and a decline in national vaccination rates as manufacturers withdrew production [
2]. Concerns about a resurgence of vaccine-preventable diseases prompted the establishment of the national VICP between 1986 and 1988 as a public health safeguard in the states [
2,
3]. In Taiwan, the VICP was introduced in 1988 following a serious adverse event where a boy developed polio-like symptoms after receiving the oral polio vaccine [
4]. The program aims to raise awareness of vaccine safety, encourage immunization, and maintain high vaccination coverage. It operates under a "no-fault" compensation scheme, compensating injured patients or their families through government funding once a causal link between an adverse event and immunization is established [
5,
6,
7].
The Vaccine Adverse Event Reporting System (VAERS) is another channel for reporting AEFIs in Taiwan. However, the national VICP offers several advantages over the VAERS database for analyzing vaccine ARs in Taiwan. Firstly, the VICP was established in 1988, predating VAERS. Secondly, due to public debates over high-profile AEFI cases adjudicated as ‘unrelated’ by the VICP during the 2009 influenza A H1N1 pandemic, the VICP is more familiar to the Taiwanese public. Thirdly, while VAERS reporting is restricted to healthcare providers, the VICP is open to the public, allowing any Taiwanese citizen to file a claim of vaccine injury. Such claims are encouraged by the Taiwanese government and can be easily submitted via an online application form (
https://www.cdc.gov.tw/Vicp/Fill). Finally, after registration, a series of processes ensue, including the collection of adverse event-related medical records by local health bureaus and causality determination by an expert committee. The causal relationship between AEFI and vaccination is not determined in VAERS. Thus, the VICP represents the most comprehensive database for post-marketing surveillance of vaccine ARs in Taiwan. In this study, we aim to analyze the incidence and types of ARs associated with various vaccines using the VICP database from 2014 to 2019 before the COVID-19 pandemic.
2. Materials and Methods
Ethical approval
This study was approved by the Research and Ethics Committee of Chang Gung Memorial Hospital (IRB: 202002607B1).
Claims to the Vaccine Injury Compensation Program (VICP) [
4,
8,
9]
The national VICP in Taiwan was established under Article 30 of the Communicable Disease Control Act. This program covers all officially approved vaccines used for disease prevention, regardless of whether they are publicly funded or self-paid. If a vaccine-related injury is suspected, claims can be filed within two years of becoming aware of the AEFI or within five years of the first occurrence of any AEFI symptoms. Claims can be submitted by the injured individuals, guardians of individuals under 20 years old, or heirs of deceased individuals.
The local official health bureau manages the claim process, responsible for compiling all available medical records pertaining to the adverse event, including clinical manifestations, laboratory data, examination reports, medical history, disease progression, treatment outcomes, and vaccine types involved. At least two medical experts from the VICP working group independently review these medical records and relevant literature to assess AEFI causality. If the two experts disagree, a third expert is consulted. The final causality decision is made collectively during a review conference by all members of the VICP working group, which includes clinical physicians, pharmacists, pathologists, legal professionals, and impartial community members [
4,
8].
AEFI causality is classified as ‘related,’ ‘indeterminate,’ or ‘unrelated’ to vaccination. Financial compensation is provided for cases adjudicated as ‘related’ or with ‘indeterminate’ causality. Compensation is also available for unrelated cases involving deceased individuals who underwent autopsy, those who had medical procedures to determine causality, and pregnant women who experienced stillbirth or miscarriage [
9]. On average, the resolution process from claim filing to adjudication takes approximately six months, with claimants having the right to appeal within 30 days if dissatisfied with the outcome.
Following Wang's publication of updates to Taiwan's VICP in 2014 [
4], this study collected data from claims adjudicated between 2014 and 2019. The VICP database records include claimants' age, sex, and residence; types of vaccines involved; dates of immunization; reported symptoms; clinical diagnoses; duration from vaccination to symptom onset; adjudication results; and compensation amounts. For this study, AEFIs adjudicated as ‘related’ or ‘indeterminate’ were classified as ARs to the vaccines. Adjudication outcomes are periodically published online by the Taiwan Centers for Disease Control [
10]. Claims adjudicated from 2014 to 2019 were reviewed and analyzed for this study.
Definitions
Data on the total administered doses of each vaccine during the study period, as provided by the Taiwan CDC (Supplementary Table), was used to estimate AR incidence for each vaccine. If multiple vaccine types were administered simultaneously and the specific offending vaccine could not be precisely identified, the AEFI was attributed to all administered vaccines. The interval from vaccination to AEFI onset was calculated in days, with symptoms occurring on the day of vaccination counted as 0.5 days.
Statistics
Statistical analysis was performed using GraphPad Prism and Microsoft Excel. Medians were compared using the Mann-Whitney U test, while categorical variables were compared using the Chi-square test and Fisher’s exact test. A p-value of < 0.05 was considered statistically significant.
3. Results
From 2014 to 2019, the Vaccine Injury Compensation Program (VICP) of Taiwan received and adjudicated a total of 491 claims, with an annual range of 42 to 122 claims (mean: 81.8 claims). Of these, 327 claims (66.6%) were adjudicated as either ‘related’ (231 claims, 47.0%) or ‘indeterminate’ (96 claims, 19.6%), while the remaining 164 claims (33.4%) were adjudicated as ‘unrelated’ (
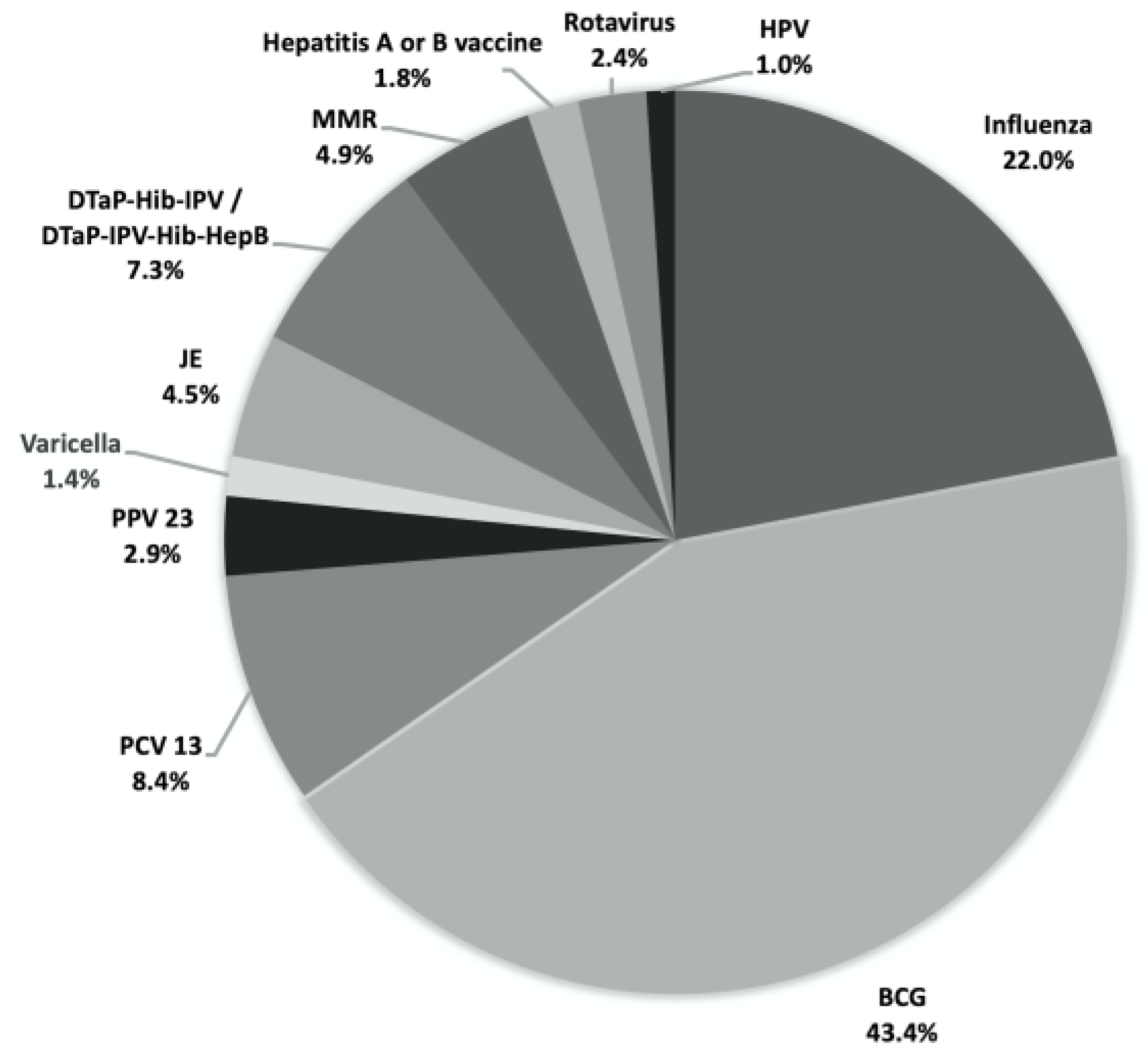
Table 1). The total compensation disbursed for all claims amounted to approximately 30 million New Taiwan Dollars (~1 million USD) during the 2014-2019 period. Bacillus Calmette-Guerin (BCG) vaccine was the most frequently identified offending vaccine with 213 claims, followed by the influenza vaccine with 108 claims (
Figure 1 and
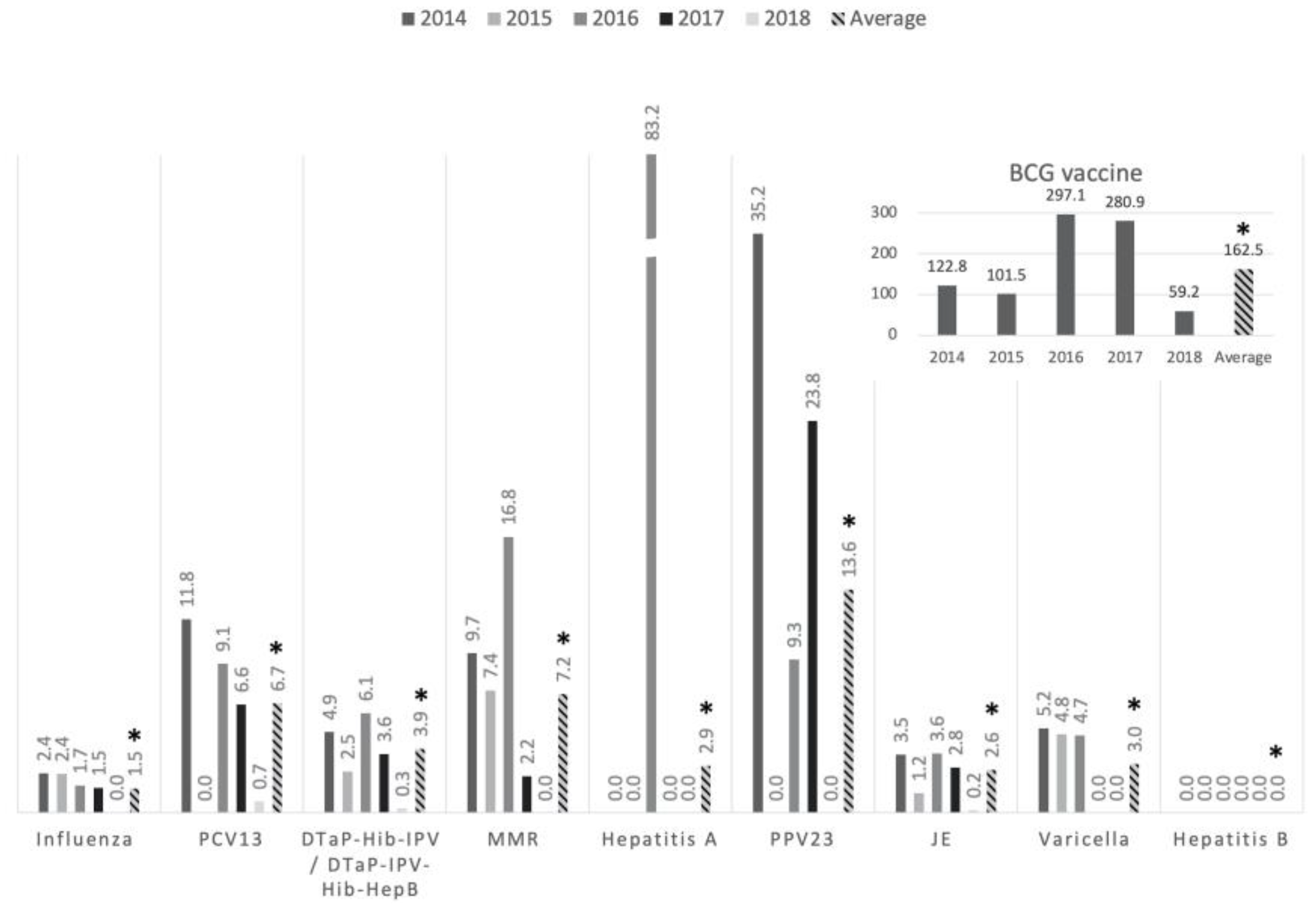
Table 1). The incidence of adverse reactions (ARs) was generally low (
Figure 2), averaging 6.0 ARs per million vaccine doses, except for the BCG vaccine, which showed an incidence of 162.5 ARs per million doses.
4. Discussion
The BCG vaccine has been extensively used in regions where tuberculosis is prevalent and has shown optimal protection against severe manifestations such as meningitis and disseminated disease [
11]. This study found that among EPI vaccines, BCG was associated with the highest incidence of ARs. In 2007, active surveillance by the Taiwan CDC led to a marked increase in reported BCG-related AEFI, particularly osteitis and osteomyelitis, with incidence rates rising approximately tenfold from 3.68 to 30.1 per million doses between 2002–2006 and 2008–2012 [
12,
13]. Osteomyelitis symptoms typically emerged 6 to 12 months post-vaccination, with diagnosis taking an average of 16.4 months [
14]. Fortunately, BCG osteomyelitis generally has a good prognosis, with long-term sequelae being rare following adequate anti-tuberculosis therapy [
14,
15,
16].
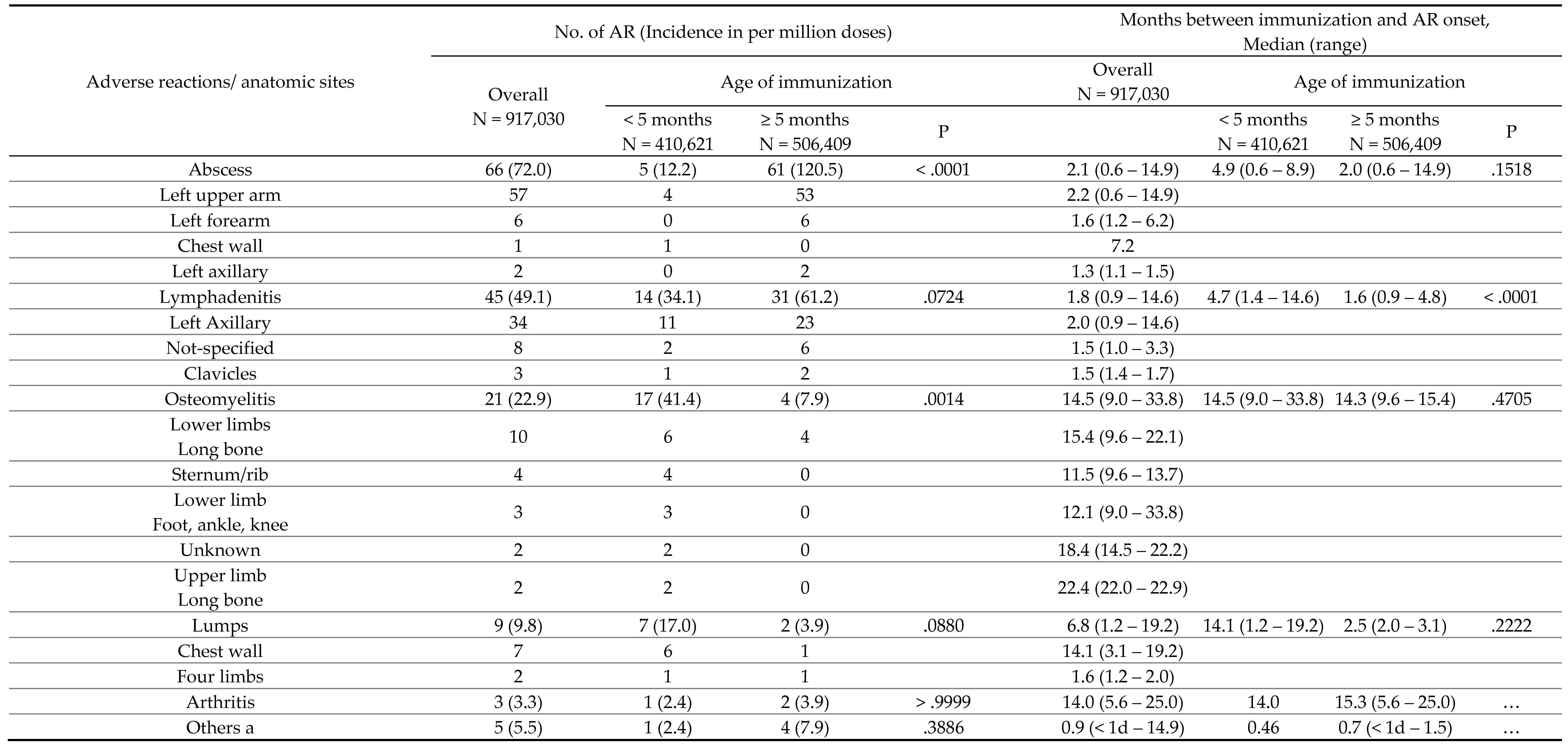
The relatively high incidence of BCG osteomyelitis in Taiwan prompted a change in the national immunization policy in 2016, delaying BCG administration from 24 hours after birth to 5 to 8 months of age. Huang et al. observed a decrease in the incidence of BCG-related osteitis/osteomyelitis in the birth cohort from 2012 to 2017 following this policy change [
17]. Our study supports these findings, noting approximately a fivefold reduction in BCG-related osteitis and osteomyelitis while the timing after vaccination and affected skeletal regions remained consistent. These data suggest that delaying BCG vaccination could reduce the incidence of these serious bone infections. However, potential disadvantages include the uncertainty of increased severe tuberculosis cases during the vaccination delay window, warranting further surveillance in infants under 5 months. Also, abscesses and lymphadenitis became more frequent with older vaccination age, developing sooner post-vaccination, causing prolonged and troublesome symptoms for caregivers. In response, the Taiwan CDC issued guidelines to better manage these ARs [
18].
Along with the policy change of vaccination age of BCG, the manufacture of BCG vaccines was also temporary changed from the National Health Research Institutes (NHRI) in Taiwan to the Japan BCG Laboratory© during July 2016 and August 2020 due to the shortage of stockpile. However, both vaccines contained the same Tokyo-172 strains fulfilling WHO requirements. The intradermal route of administration did not change, either. The manufacture was changed back to the NHRI after August 2020. Continuing observation of the incidences of BCG abscess and osteomyelitis will be mandatory to clarify the role of different vaccine manufacture in the occurrence of the AR of BCG vaccine. Like the finding in this study, BCG was also the vaccine being most frequently compensated for ARs in many other countries [
19,
20]. However, the estimated incidences of AE following BCG vaccination varied greatly in different countries and ranged from 3.4 to 1540 per million vaccine doses [
21,
22,
23]. Of note, an investigation into the AE following BCG immunization between year 2013 to 2018 in Korea indicated that the incidence was as high as 260 per million vaccine doses [
24]. Consistent with our finding, abscess and lymphadenitis were the major disease entities caused by BCG in Australia, Korea, Poland and Oman [
20,
22,
23,
24].
Although the influenza vaccine was the second most frequent in AR-related claims, its overall AR incidence was extremely low. Severe ARs like neurological disorders, anaphylactic shock, and ITP were observed at rates below one per million doses, underscoring the influenza vaccine's excellent safety profile in post-marketing surveillance. Given its widespread use across populations globally, injury claims remain common across regions and demographics [
19,
25], with a notable spike in 2010 due to extensive social media coverage during the 2009 H1N1 pandemic [
4]. The low AR incidence data from this study are crucial for bolstering public trust and encouraging influenza vaccine uptake.
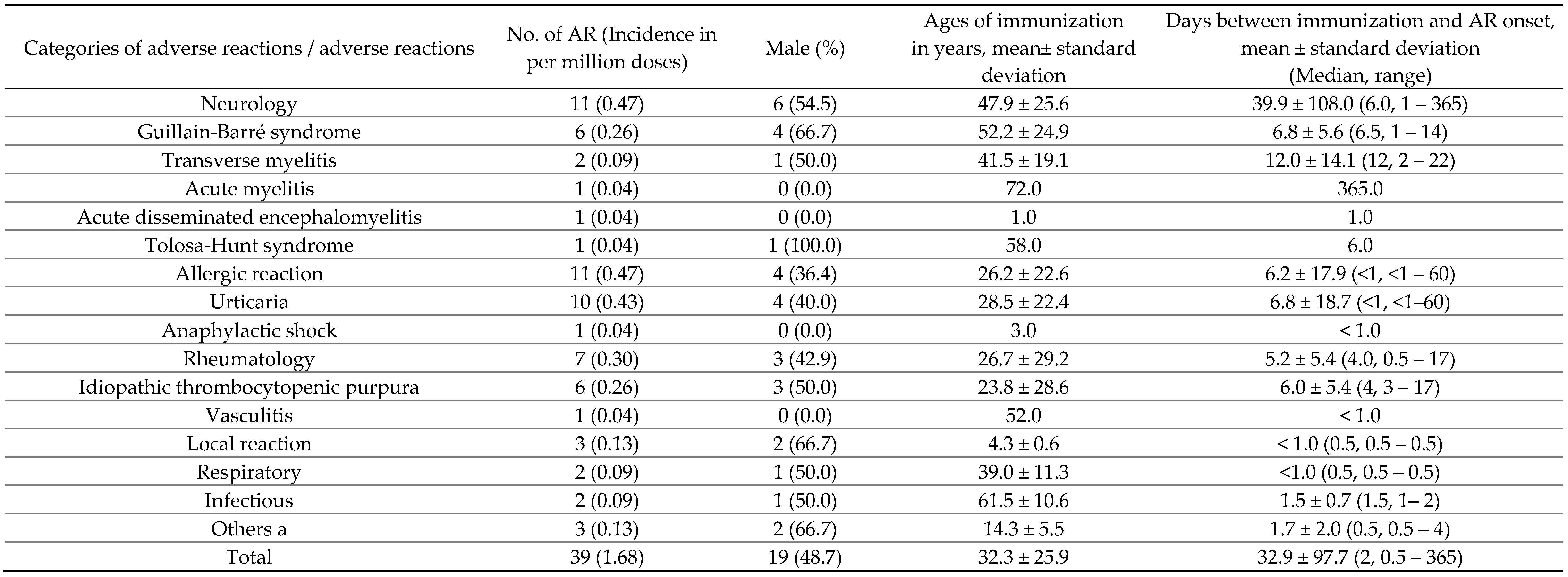
Guillain-Barré syndrome (GBS) is historically considered a significant AR of influenza vaccines, linked notably to the 1976 swine-origin influenza vaccine [
26,
27,
28,
29,
30]. There remain public concerns regarding GBS and its association with influenza vaccination, notably during the 2009 H1N1 pandemic [
31,
32,
33,
34], although recent meta-analyses have not identified such associations [
35]. Influenza vaccine-associated GBS is viewed as an immune-mediated condition, generally manifesting within six weeks post-vaccination [
36]. This study observed GBS occurrences within two weeks post-immunization, reinforcing that the benefits of influenza vaccination considerably outweigh potential AR risks.
Limitations
This study has several limitations. First, identifying the specific vaccine responsible for systemic adverse reactions was challenging, particularly when multiple vaccines were administered simultaneously or in combination forms. Second, there was significant variability in the reported adverse event entities, which lacked standardized terminology, potentially leading to misclassification. For example, severe local reactions with extensive limb swelling were often reported as cellulitis, which might not accurately reflect the nature of the reaction. Third, while adjudications were largely based on available scientific evidence, there were no established quality criteria for the evidence used by the expert committee. In complex cases, this could result in subjective adjudications or reliance on evidence of lower quality. Fourth, because the VICP operates as a passive reporting system, there was a potential for underreporting of adverse reactions, particularly mild ones. However, the Taiwanese government encourages reporting through VICP, supported by the possibility of financial compensation, so adverse reactions of significant severity and clinical importance were likely captured. Lastly, the time limitations for claim submission—either within two years of awareness of an adverse event or within five years of symptom onset—make it challenging to accurately estimate incidences of adverse events with long intervals post-immunization, such as BCG osteomyelitis.
5. Conclusions
Between 2014 and 2019, the BCG and influenza vaccines were the primary vaccines associated with ARs requiring compensation in Taiwan. The EPI vaccines used during this period demonstrated an excellent safety profile, with very low incidences of ARs. The BCG vaccine was an exception, as it was associated with more serious reactions, including osteomyelitis and osteitis. Delaying BCG immunization to when infants are 5 to 8 months old may effectively reduce the occurrence of these severe adverse reactions.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
W.-C.L. and C.-J.C. conceived the study. C.-J.C designed the study and produced the protocol. Y.-C.H. and N.-C.C. assisted analysis of the study. C.-H.Y. collected the Data on the total administered doses. W.-C.L. and C.-J.C. wrote the first draft of manuscript. All authors read and approved the manuscript.
Funding
This research was funded by by grants from Chang Gung Memorial Hospital [grant number CORPG3L0171, CORPG3L0172].
Institutional Review Board Statement
This study was approved by the Research and Ethics Committee of Chang Gung Memorial Hospital (IRB: 202002607B1).
Informed Consent Statement
Patient consent was waived due to the retrospective analysis of the de-identified VICP database, which contains no personally identifiable information, thereby protecting the privacy and confidentiality of all individuals involved. Due to the de-identified nature of the data, the research was conducted in accordance with ethical guidelines that allow for the use of such information without the need for obtaining individual informed consent. This approach enables the study to contribute valuable insights while maintaining strict adherence to privacy standards.
Data Availability Statement
Raw data of the study can be obtained by contacting the corresponding author.
Acknowledgments
We thanked the committee of the Vaccine Injury Compensation Program (VICP) in Taiwan for providing the electronic records of the adjudication results. We also thanked the Centers for Disease Control in Taiwan for providing the statistics of annual administered doses of EPI vaccines in Taiwan.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Evans, G. Vaccine injury compensation programs worldwide. Vaccine 1999, 17 (Suppl 3), S25–S35. [Google Scholar] [PubMed]
- Meissner HC, Nair N, Plotkin SA. The National Vaccine Injury Compensation Program: Striking a balance between individual rights and community benefit. JAMA.
- Cook KM, Evans G. The National Vaccine Injury Compensation Program. Pediatrics, 1.
- Wang, PC. Updates on Vaccine Injury Compensation Program in Taiwan and program evaluation. Taiwan Epidemiology Bulletin, 2015, 31, 149–158. [Google Scholar]
- Halabi SF, Omer SB. A global vaccine injury compensation system. JAMA, 2017, 317, 471–2. [Google Scholar]
- Looker C, Kelly H. No-fault compensation following adverse events attributed to vaccination: a review of international programmes. Bull World Health Organ, 2011, 89, 371–8. [Google Scholar]
- Ridgway, D. No-fault vaccine insurance: lessons from the National Vaccine Injury Compensation Program. J Health Polit Policy Law 1999, 24, 59–90. [Google Scholar] [PubMed]
- The manual of the vaccine injury compensation program by Taiwan CDC (in Traditional Chinese) Available online:. Available online: https://www.cdc.gov.tw/vicp (accessed on 16 August 2024).
- Regulations governing collection and review of relief fund for victims of immunization, 2004 Available online:. Available online: https://law.moj.gov.tw/ENG/LawClass/LawAll.aspx?pcode=L0050021 (accessed on 16 August 2024).
- Taiwan Center of Disease Control. Available online: https://www.cdc.gov.tw/Category/Page/OM4QgMRlmzYg5UDLHlnXRw (accessed on 16 August 2024).
- Colditz GA, Berkey CS, Mosteller F, Brewer TF, Wilson ME, Burdick E, et al. The efficacy of bacillus Calmette-Guérin vaccination of newborns and infants in the prevention of tuberculosis: meta-analyses of the published literature. Pediatrics, 1995, 96, 29–35. [Google Scholar]
- Chan, P. , Huang, W., Wang, K., Ma, C., Lu, B., Lin, F., et al. The Active surveillance of BCG-related adverse events. Taiwan Epidemiology Bulletin 2012, 28, 13–22. [Google Scholar]
- Chiu N, Lin M, Lin W, et al. Mycobacterium bovis BCG–associated osteomyelitis/osteitis, Taiwan. Emerg Infect Dis 2015, 21, 539–40. [Google Scholar]
- Yang TL, Lee CM, Lee KL, et al. Clinical features of tuberculosis and Bacillus Calmette-Guérin (BCG) -associated adverse effects in children: A 12-year study. J Formos Med Assoc, 2021, 120, 443–51. [Google Scholar]
- Huang CY, Chiu NC, Chi H, Huang FY, Chang PH. Clinical manifestations, management, and outcomes of osteitis/osteomyelitis caused by Mycobacterium bovis Bacillus Calmette-Guérin in children: comparison by site(s) of affected bones. J Pediatr, 2019, 207, 97–102. [Google Scholar]
- Tsai YL, Chen YJ, Lai YC. Long-term follow-up of osteomyelitis caused by Bacille Calmette-Guérin vaccination in immuno-competent children. Sci Rep, 2020, 10, 4072. [Google Scholar]
- Wei Huang, Chiu N-C, Chi H, Huang FY, Huang CY. Inoculation Age of Bacillus Calmette-Guérin Tokyo-172 Strain and Vaccine-related Adverse Reactions in Taiwan Birth Cohort of 2012–2017. Clin Infect Dis, 2021, 73, e1554–9. [Google Scholar] [CrossRef]
- Taiwan Centers for Disease Control. Recommendations for BCG injection site (in traditional Chinese). Available online: https://www.cdc.gov.tw/Category/ListContent/4T8vDeSxOpmz0ILvF_D8Yw?uaid=QsqXMEzXYCw6Gp1n7YLSDA (accessed on 16 August 2024).
- Kim MK, Lee YK, Kim TE, Kong I, Yang HJ, Suh ES. Surveillance and compensation claims for adverse events following immunization from 2011 to 2016 in the Republic of Korea. Clin Exp Vaccine Res, 2017, 6, 146–55. [Google Scholar]
- Patel PK, Al-Rawahi B, Al-Jawari A, Al-Abaidani I, Al-Abri S. Surveillance of adverse events following immunization in Oman, 2006-2015. East Mediterr Health J, 2018, 24, 119–26. [Google Scholar]
- Guo B, Page A, Wang H, Taylor R, McIntyre P. Systematic review of reporting rates of adverse events following immunization: an international comparison of post-marketing surveillance programs with reference to China. Vaccine, 2013, 31, 603–17. [Google Scholar]
- Clothier HJ, Hosking L, Crawford NW, Russell M, Easton ML, Quinn JA, et al. Bacillus Calmette-Guérin (BCG) vaccine adverse events in Victoria, Australia: analysis of reports to an enhanced passive surveillance system. Drug Saf, 2015, 38, 79–86. [Google Scholar]
- Krysztopa-Grzybowska K, Paradowska-Stankiewicz I, Lutyńska A. The rate of adverse events following BCG vaccination in Poland. Przegl Epidemiol, 2012, 66, 465–9. [Google Scholar]
- Roh EJ, Lee YK, Lee MH, Kim MK, Kim TE, Lee SG, et al. Investigation of adverse events following bacille Calmette-Guérin immunization using immunization safety surveillance system in Korea Centers for Disease Control and Prevention. Clin Exp Vaccine Res, 2020, 9, 133–145. [Google Scholar]
- Thompson KM, Orenstein WA, Hinman AR. Performance of the United States Vaccine Injury Compensation Program (VICP): 1988-2019. Vaccine, 2020, 38, 2136–43. [Google Scholar]
- Ehrengut W, Allerdist H. Uber neurologische Komplikationen nach der Influenza-schutzimpfung [Neurological complications after influenza vaccination]. MMW Munch Med Wochenschr, 1977, 119, 705–10. [Google Scholar]
- Ehrengut, W. Guillain-Barré syndrome and influenza vaccine. Br Med J, 1977, 1, 1662–3. [Google Scholar]
- Schonberger LB, Bregman DJ, Sullivan-Bolyai JZ, Keenlyside RA, Ziegler DW, Retailliau HF, et al. Guillain-Barre syndrome following vaccination in the National Influenza Immunization Program, United States, 1976-1977. Am J Epidemiol.
- Langmuir AD, Bregman DJ, Kurland LT, Nathanson N, Victor M. An epidemiologic and clinical evaluation of Guillain-Barré syndrome reported in association with the administration of swine influenza vaccines. Am J Epidemiol, 1984, 119, 841–79. [Google Scholar]
- Marks JS, Halpin TJ. Guillain-Barré syndrome in recipients of A/New Jersey influenza vaccine. JAMA, 2490.
- Martín Arias LH, Sanz R, Sáinz M, Treceño C, Carvajal A. Guillain-Barré syndrome and influenza vaccines: A meta-analysis. Vaccine, 2015, 33, 3773–78. [Google Scholar]
- Principi N, Esposito S. Vaccine-preventable diseases, vaccines, and Guillain-Barre syndrome. Vaccine, 2019, 37, 5544–50. [Google Scholar]
- Dieleman J, Romio S, Johansen K, Weibel D, Bonhoeffer J, Sturkenboom M; VAESCO-GBS case-control study group. Guillain-Barre syndrome and adjuvanted pandemic influenza A (H1N1) 2009 vaccine: multinational case-control study in Europe. BMJ, 2011, 343, d3908. [Google Scholar]
- Salmon DA, Proschan M, Forshee R, Gargiullo P, Bleser W, Burwen DR, et al. ; H1N1 GBS meta-analysis working group. Association between Guillain-Barré syndrome and influenza A (H1N1) 2009 monovalent inactivated vaccines in the USA: a meta-analysis. Lancet, 2013, 381, 1461–8. [Google Scholar]
- Petráš M, Lesná IK, Dáňová J, Čelko AM. Is an increased risk of developing Guillain-Barré syndrome associated with seasonal influenza vaccination? A systematic review and meta-analysis. Vaccines, 2020, 8, 150. [Google Scholar]
- Vellozzi C, Iqbal S, Broder K. Guillain-Barre syndrome, influenza, and influenza vaccination: the epidemiologic evidence. Clin Infect Dis, 2014, 58, 1149–55. [Google Scholar]
Figure 1.
Distribution of alleged vaccines in the claims received by national vaccine injury compensation program in Taiwan during 2014 and 2019. Abbreviations: BCG, Bacillus Calmette–Guérin; PCV13, 13-valent pneumococcal conjugate vaccine; PPV23, 23-valent polysaccharide pneumococcal vaccine; JE, Japanese encephalitis vaccine; DTaP-Hib-IPV, diphtheria, tetanus, acellular pertussis, Hemophilus influenzae type b and inactivated poliovirus vaccine; DTaP-IPV-Hib-HepB, diphtheria, tetanus, acellular pertussis, Hemophilus influenzae type b, inactivated poliovirus and hepatitis B; MMR, measles-mumps-rubella vaccine; HPV, human papillomavirus vaccine.
Figure 1.
Distribution of alleged vaccines in the claims received by national vaccine injury compensation program in Taiwan during 2014 and 2019. Abbreviations: BCG, Bacillus Calmette–Guérin; PCV13, 13-valent pneumococcal conjugate vaccine; PPV23, 23-valent polysaccharide pneumococcal vaccine; JE, Japanese encephalitis vaccine; DTaP-Hib-IPV, diphtheria, tetanus, acellular pertussis, Hemophilus influenzae type b and inactivated poliovirus vaccine; DTaP-IPV-Hib-HepB, diphtheria, tetanus, acellular pertussis, Hemophilus influenzae type b, inactivated poliovirus and hepatitis B; MMR, measles-mumps-rubella vaccine; HPV, human papillomavirus vaccine.
Figure 2.
The incidence of the adverse reactions in per million doses of vaccines. The average incidence is indicated on the right column with an asterisk mark. Abbreviations: BCG, Bacillus Calmette–Guérin; PCV13, 13-valent pneumococcal conjugate vaccine; PPV23, 23-valent polysaccharide pneumococcal vaccine; JE, Japanese encephalitis vaccine; DTaP-Hib-IPV, diphtheria, tetanus, acellular pertussis, Hemophilus influenzae type b and inactivated poliovirus vaccine; DTaP-IPV-Hib-HepB, diphtheria, tetanus, acellular pertussis, Hemophilus influenzae type b, inactivated poliovirus and hepatitis B; MMR, measles-mumps-rubella
Figure 2.
The incidence of the adverse reactions in per million doses of vaccines. The average incidence is indicated on the right column with an asterisk mark. Abbreviations: BCG, Bacillus Calmette–Guérin; PCV13, 13-valent pneumococcal conjugate vaccine; PPV23, 23-valent polysaccharide pneumococcal vaccine; JE, Japanese encephalitis vaccine; DTaP-Hib-IPV, diphtheria, tetanus, acellular pertussis, Hemophilus influenzae type b and inactivated poliovirus vaccine; DTaP-IPV-Hib-HepB, diphtheria, tetanus, acellular pertussis, Hemophilus influenzae type b, inactivated poliovirus and hepatitis B; MMR, measles-mumps-rubella
Table 1.
Claims of vaccine injury and adjudication results in Taiwan vaccine injury compensation program from 2014 to 2019.
Table 1.
Claims of vaccine injury and adjudication results in Taiwan vaccine injury compensation program from 2014 to 2019.
| Vaccine type |
No. of events (%) |
| Adverse reactions |
Unrelated events |
| Related |
Indeterminate |
Total |
|
| BCG vaccine (n=213) |
188 (88.3) |
18 (8.5) |
206 (96.7) |
7 (3.3) |
| Influenza vaccine (n=108) |
4 (3.7) |
35 (32.4) |
39 (36.1) |
69 (63.9) |
| PCV13 (n=41) |
14 (34.1) |
9 (22.0) |
23 (56.1) |
18 (43.9) |
| DTaP-Hib-IPV/ DTaP-IPV-Hib-HepB vaccine (n=36) |
12 (33.3) |
5 (13.9) |
17 (47.2) |
19 (52.8) |
| MMR vaccine (n=24) |
1 (4.2) |
15 (62.5) |
16 (66.7) |
8 (33.3) |
| JE vaccine (n=22) |
5 (22.7) |
5 (22.7) |
10 (45.5) |
12 (54.5) |
| PPV23 (n=14) |
7 (50.0) |
0 (0) |
7 (50.0) |
7 (50.0) |
| Rotavirus vaccine (n=12) |
0 (0) |
3a (25.0) |
3 (25.0) |
9 (75.0) |
| Hepatitis A or B vaccine (n=9) |
0 (0) |
2 (22.2) |
2 (22.2) |
7 (77.8) |
| Varicella vaccine (n=7) |
0 (0) |
3 (42.9) |
3 (42.9) |
4 (57.1) |
| HPV vaccine (n=5) |
0 (0) |
1 (20.0) |
1 (20.0) |
4 (80.0) |
| Total claims (n=491) |
231 (47.0) |
96 (19.6) |
327 (66.6) |
164 (33.4) |
Table 2.
Incidences and durations post-immunization for various adverse reactions (ARs) of BCG vaccine in different ages of immunization, 2014-2018.
Table 2.
Incidences and durations post-immunization for various adverse reactions (ARs) of BCG vaccine in different ages of immunization, 2014-2018.
Table 3.
Incidences and demographics of various adverse reactions (AR) of influenza vaccine and intervals between immunization and onset of AR.
Table 3.
Incidences and demographics of various adverse reactions (AR) of influenza vaccine and intervals between immunization and onset of AR.
Table 4.
Incidence, demographics of various adverse reactions, and intervals between immunization and onset of AR of selected EPI vaccines.
Table 4.
Incidence, demographics of various adverse reactions, and intervals between immunization and onset of AR of selected EPI vaccines.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).