Submitted:
20 August 2024
Posted:
22 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Gull Sampling
2.2. Influenza A Virus RNA and Antibody Detection
2.3. Generalized Linear Models
3. Results
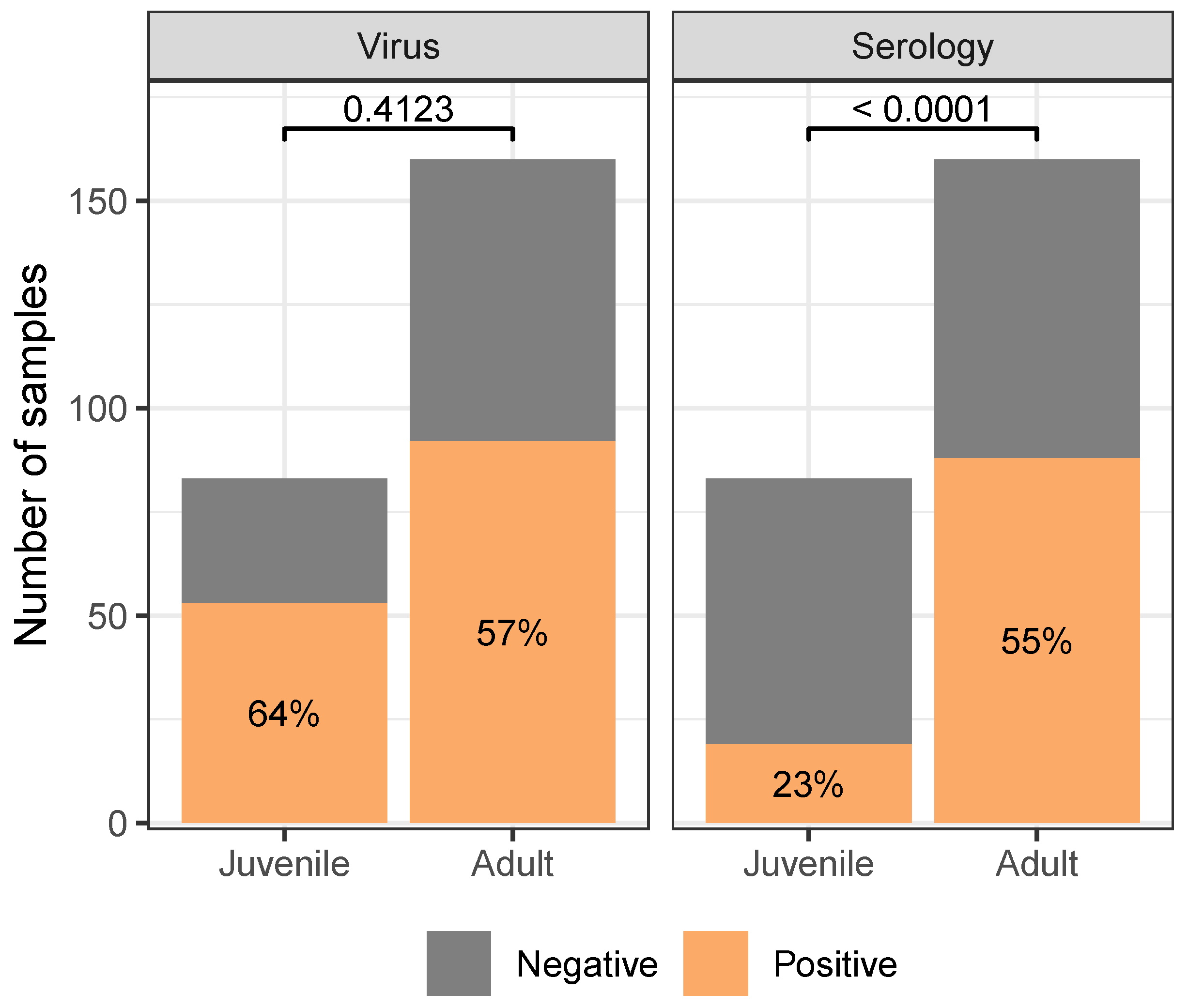
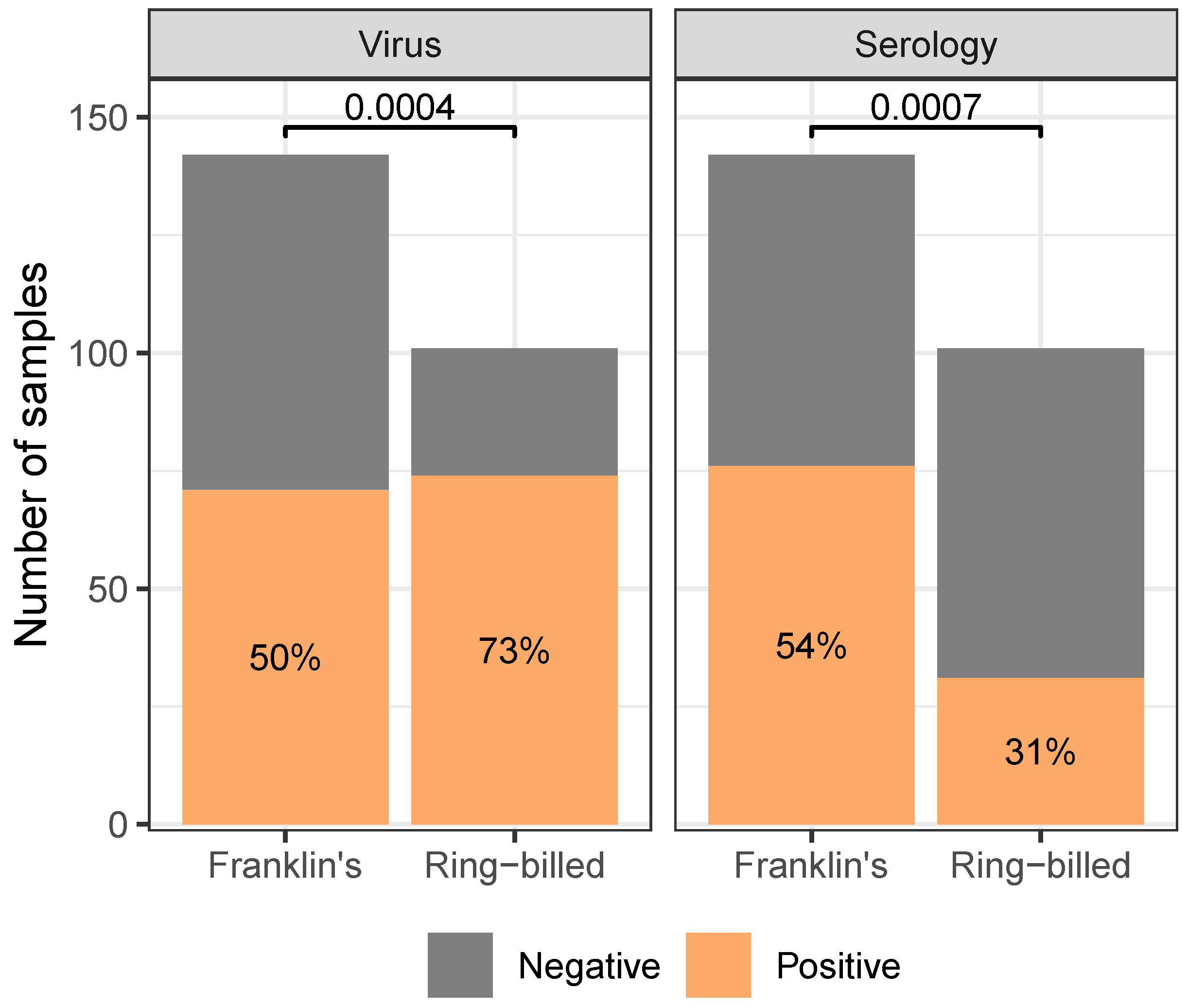
3.1. Sampling Distribution
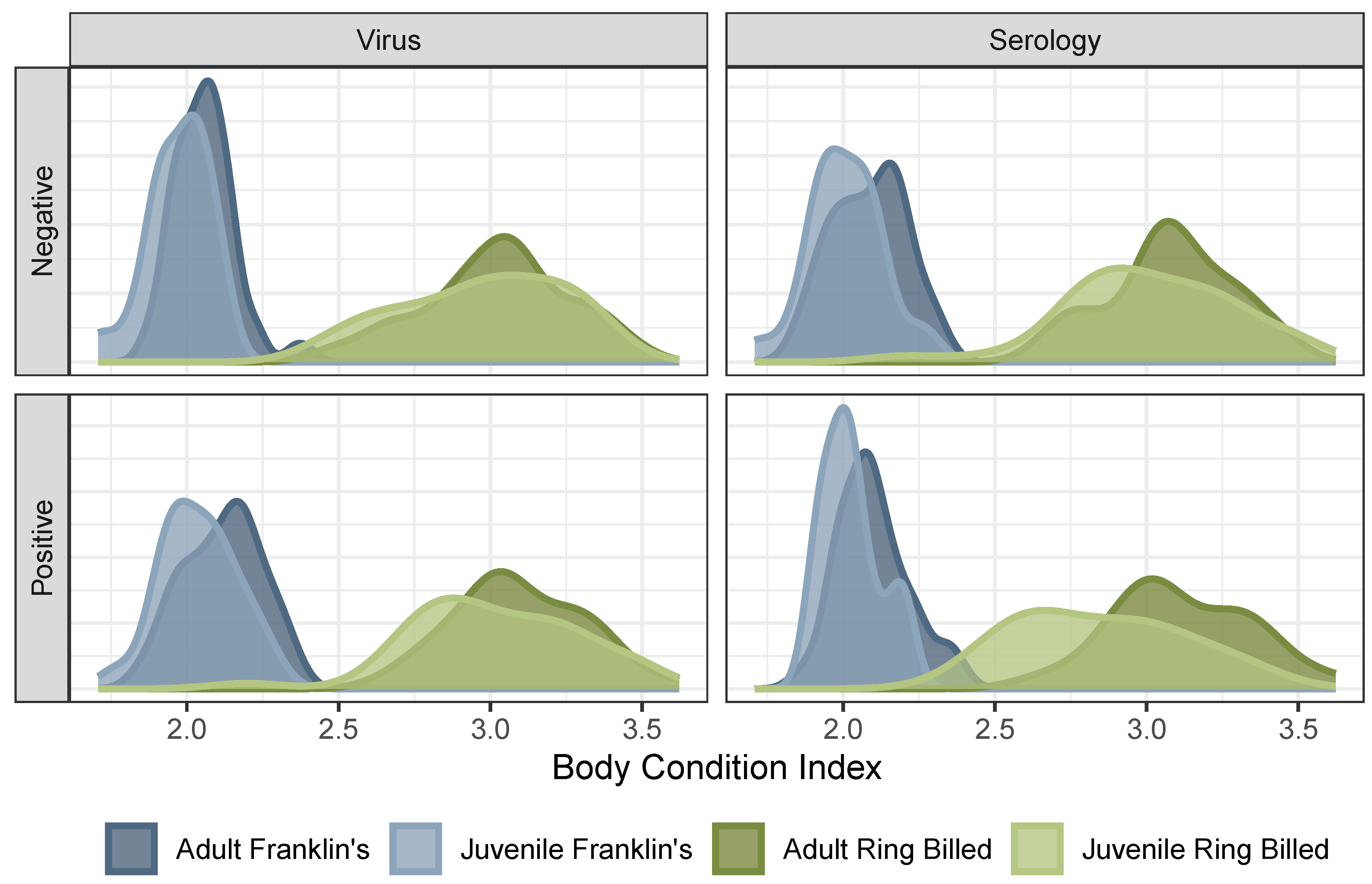
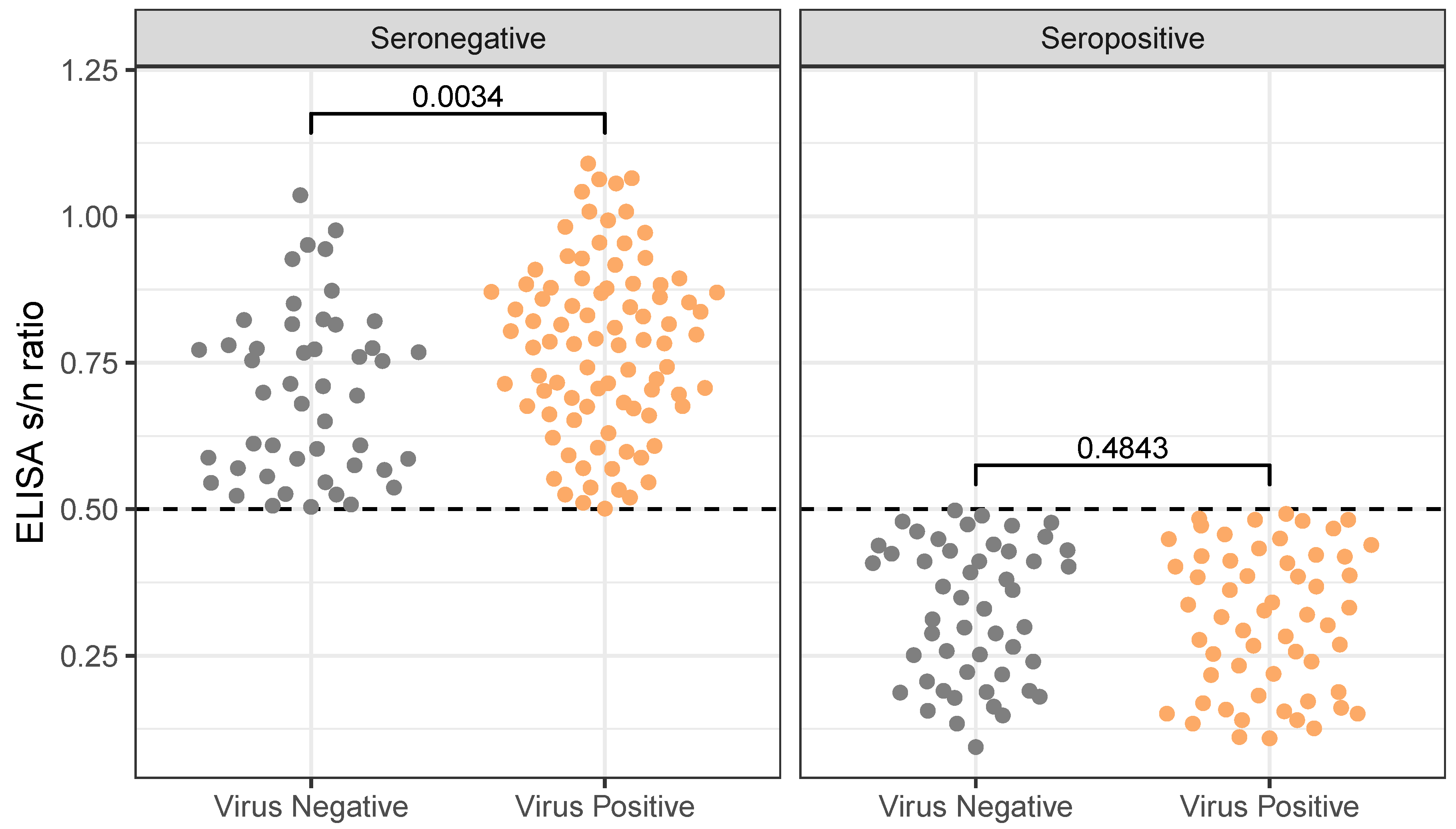
3.2. Observational Results
3.3. Model Results
4. Discussion
4.1. Serological Sampling Can Complement Current Surveillance
4.2. The Role of the Surrounding Environment
4.3. Insights into Influenza Reservoirs
4.4. Limitations of BCI in Cross-Species Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IAV | Influenza A Virus |
| RNA | Ribonucleic acid |
| PCR CT | Polymerase Chain Reaction cycle threshold |
| ELISA S/N | Enzyme-linked Immunosorbent Assay sample result-to-negative control absorbance ratio |
| HPAI | High Pathogenic Avian Influenza |
| LPAI | Low Pathogenic Avian Influenza |
| BIC | Body Condition Index |
References
- Daszak, P.; Cunningham, A.A.; Hyatt, A.D. Emerging Infectious Diseases of Wildlife—Threats to Biodiversity and Human Health. Science 2000, 287, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.E.; Patel, N.G.; Levy, M.A.; Storeygard, A.; Balk, D.; Gittleman, J.L.; Daszak, P. Global trends in emerging infectious diseases. Nature 2008, 451, 990–993. [Google Scholar] [CrossRef] [PubMed]
- Carlson, C.J.; Albery, G.F.; Merow, C.; Trisos, C.H.; Zipfel, C.M.; Eskew, E.A.; Olival, K.J.; Ross, N.; Bansal, S. Climate change increases cross-species viral transmission risk. Nature 2022, 607, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Meadows, A.J.; Stephenson, N.; Madhav, N.K.; Oppenheim, B. Historical trends demonstrate a pattern of increasingly frequent and severe spillover events of high-consequence zoonotic viruses. BMJ Global Health 2023, 8, e012026. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.F.; Acevedo-Whitehouse, K.; Pedersen, A.B. The role of infectious diseases in biological conservation. Animal Conservation 2009, 12, 1–12. [Google Scholar] [CrossRef]
- Musoke, J.; Hlokwe, T.; Marcotty, T.; du Plessis, B.J.; Michel, A.L. Spillover of Mycobacterium bovis from Wildlife to Livestock, South Africa. Emerging Infectious Diseases 2015, 21, 448–451. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miller, R.S.; Farnsworth, M.L.; Malmberg, J.L. Diseases at the livestock-wildlife interface: Status, challenges, and opportunities in the United States. Preventive Veterinary Medicine 2013, 110, 119–132. [Google Scholar] [CrossRef]
- Ruiz-Aravena, M.; McKee, C.; Gamble, A.; Lunn, T.; Morris, A.; Snedden, C.E.; Yinda, C.K.; Port, J.R.; Buchholz, D.W.; Yeo, Y.Y.; Faust, C.; Jax, E.; Dee, L.; Jones, D.N.; Kessler, M.K.; Falvo, C.; Crowley, D.; Bharti, N.; Brook, C.E.; Aguilar, H.C.; Peel, A.J.; Restif, O.; Schountz, T.; Parrish, C.R.; Gurley, E.S.; Lloyd-Smith, J.O.; Hudson, P.J.; Munster, V.J.; Plowright, R.K. Ecology, evolution and spillover of coronaviruses from bats. Nature Reviews Microbiology 2022, 20, 299–314. [Google Scholar] [CrossRef]
- Plowright, R.K.; Parrish, C.R.; McCallum, H.; Hudson, P.J.; Ko, A.I.; Graham, A.L.; Lloyd-Smith, J.O. Pathways to zoonotic spillover. Nature Reviews Microbiology 2017, 15, 502–510. [Google Scholar] [CrossRef]
- Viana, M.; Mancy, R.; Biek, R.; Cleaveland, S.; Cross, P.C.; Lloyd-Smith, J.O.; Haydon, D.T. Assembling evidence for identifying reservoirs of infection. Trends in Ecology & Evolution 2014, 29, 270–279. [Google Scholar] [CrossRef]
- Haydon, D.T.; Cleaveland, S.; Taylor, L.; Laurenson, M.K. Identifying Reservoirs of Infection: A Conceptual and Practical Challenge. Emerging Infectious Diseases 2002, 8, 1468–1473. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wilber, M.Q.; Yang, A.; Boughton, R.; Manlove, K.R.; Miller, R.S.; Pepin, K.M.; Wittemyer, G. A model for leveraging animal movement to understand spatio-temporal disease dynamics. Ecology Letters 2022, 25, 1290–1304. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.K.; Craft, M.E.; Zuk, M.; Binning, S.A. Host migration strategy is shaped by forms of parasite transmission and infection cost. Journal of Animal Ecology 2019, 88, 1601–1612. [Google Scholar] [CrossRef] [PubMed]
- Taubenberger, J.K.; Morens, D.M. The 1918 Influenza Pandemic and Its Legacy. Cold Spring Harbor Perspectives in Medicine 2020, 10, a038695. [Google Scholar] [CrossRef] [PubMed]
- Bourret, V. Avian influenza viruses in pigs: An overview. The Veterinary Journal 2018, 239, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Loeb, J. Scottish seabirds hit by avian influenza. Veterinary Record 2022, 190, 488–488. [Google Scholar] [CrossRef]
- Garber, L.; Bjork, K.; Patyk, K.; Rawdon, T.; Antognoli, M.; Delgado, A.; Ahola, S.; McCluskey, B. Factors Associated with Highly Pathogenic Avian Influenza H5N2 Infection on Table-Egg Layer Farms in the Midwestern United States, 2015. Avian Diseases 2016, 60, 460–466. [Google Scholar] [CrossRef]
- Ssematimba, A.; Malladi, S.; Hagenaars, T.J.; Bonney, P.J.; Weaver, J.T.; Patyk, K.A.; Spackman, E.; Halvorson, D.A.; Cardona, C.J. Estimating within-flock transmission rate parameter for H5N2 highly pathogenic avian influenza virus in Minnesota turkey flocks during the 2015 epizootic. Epidemiology & Infection 2019, 147, e179. [Google Scholar] [CrossRef]
- van Dijk, J.G.B.; Hoye, B.J.; Verhagen, J.H.; Nolet, B.A.; Fouchier, R.A.M.; Klaassen, M. Juveniles and migrants as drivers for seasonal epizootics of avian influenza virus. Journal of Animal Ecology 2014, 83, 266–275. [Google Scholar] [CrossRef]
- Elbers, A.R.W.; Gonzales, J.L. Quantification of visits of wild fauna to a commercial free-range layer farm in the Netherlands located in an avian influenza hot-spot area assessed by video-camera monitoring. Transboundary and Emerging Diseases 2020, 67, 661–677. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Spackman, E. A Brief Introduction to Avian Influenza Virus. In Animal Influenza Virus: Methods and Protocols; Spackman, E., Ed.; Methods in Molecular Biology, Springer US: New York, NY, 2020; pp. 83–92. [Google Scholar] [CrossRef]
- Chen, H.; Yuan, H.; Gao, R.; Zhang, J.; Wang, D.; Xiong, Y.; Fan, G.; Yang, F.; Li, X.; Zhou, J.; Zou, S.; Yang, L.; Chen, T.; Dong, L.; Bo, H.; Zhao, X.; Zhang, Y.; Lan, Y.; Bai, T.; Dong, J.; Li, Q.; Wang, S.; Zhang, Y.; Li, H.; Gong, T.; Shi, Y.; Ni, X.; Li, J.; Zhou, J.; Fan, J.; Wu, J.; Zhou, X.; Hu, M.; Wan, J.; Yang, W.; Li, D.; Wu, G.; Feng, Z.; Gao, G.F.; Wang, Y.; Jin, Q.; Liu, M.; Shu, Y. Clinical and epidemiological characteristics of a fatal case of avian influenza A H10N8 virus infection: a descriptive study. The Lancet 2014, 383, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Reid, A.H.; Taubenberger, J.K. . The origin of the 1918 pandemic influenza virus: a continuing enigma. Journal of General Virology 2003, 84, 2285–2292. [Google Scholar] [CrossRef]
- Arnal, A.; Vittecoq, M.; Pearce-Duvet, J.; Gauthier-Clerc, M.; Boulinier, T.; Jourdain, E. Laridae: A neglected reservoir that could play a major role in avian influenza virus epidemiological dynamics. Critical Reviews in Microbiology 2015, 41, 508–519. [Google Scholar] [CrossRef] [PubMed]
- Postnikova, Y.; Treshchalina, A.; Boravleva, E.; Gambaryan, A.; Ishmukhametov, A.; Matrosovich, M.; Fouchier, R.A.M.; Sadykova, G.; Prilipov, A.; Lomakina, N. Diversity and Reassortment Rate of Influenza A Viruses in Wild Ducks and Gulls. Viruses 2021, 13, 1010. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ineson, K.M.; Hill, N.J.; Clark, D.E.; MacKenzie, K.G.; Whitney, J.J.; Laskaris, Y.; Ronconi, R.A.; Ellis, J.C.; Giroux, J.F.; Lair, S.; Stevens, S.; Puryear, W.B.; Runstadler, J.A. Age and season predict influenza A virus dynamics in urban gulls: consequences for natural hosts in unnatural landscapes. Ecological Applications 2022, 32, e2497. [Google Scholar] [CrossRef]
- Hill, N.J.; Takekawa, J.Y.; Cardona, C.J.; Meixell, B.W.; Ackerman, J.T.; Runstadler, J.A.; Boyce, W.M. Cross-Seasonal Patterns of Avian Influenza Virus in Breeding and Wintering Migratory Birds: A Flyway Perspective. Vector-Borne and Zoonotic Diseases 2012, 12, 243–253. [Google Scholar] [CrossRef]
- Anderson, C.M.; Gilchrist, H.G.; Ronconi, R.A.; Shlepr, K.R.; Clark, D.E.; Fifield, D.A.; Robertson, G.J.; Mallory, M.L. Both short and long distance migrants use energy-minimizing migration strategies in North American herring gulls. Movement Ecology 2020, 8, 26. [Google Scholar] [CrossRef]
- Rasmussen, E.A.; Czaja, A.; Cuthbert, F.J.; Tan, G.S.; Lemey, P.; Nelson, M.I.; Culhane, M.R. Influenza A viruses in gulls in landfills and freshwater habitats in Minnesota, United States. Frontiers in Genetics 2023, 14, 2024-02–14]. [Google Scholar] [CrossRef]
- Arriero, E.; Müller, I.; Juvaste, R.; Martínez, F.J.; Bertolero, A. Variation in Immune Parameters and Disease Prevalence among Lesser Black-Backed Gulls (Larus fuscus sp.) with Different Migratory Strategies. PLOS ONE 2015, 10, e0118279. [Google Scholar] [CrossRef]
- Dusek, R.J.; Hallgrimsson, G.T.; Ip, H.S.; Jónsson, J.E.; Sreevatsan, S.; Nashold, S.W.; TeSlaa, J.L.; Enomoto, S.; Halpin, R.A.; Lin, X.; Fedorova, N.; Stockwell, T.B.; Dugan, V.G.; Wentworth, D.E.; Hall, J.S. North Atlantic Migratory Bird Flyways Provide Routes for Intercontinental Movement of Avian Influenza Viruses. PLOS ONE 2014, 9, e92075. [Google Scholar] [CrossRef]
- Huang, Y.; Wille, M.; Benkaroun, J.; Munro, H.; Bond, A.L.; Fifield, D.A.; Robertson, G.J.; Ojkic, D.; Whitney, H.; Lang, A.S. Perpetuation and reassortment of gull influenza A viruses in Atlantic North America. Virology 2014, 456-457, 353–363. [Google Scholar] [CrossRef]
- Guinn, K.; Fojtik, A.; Davis-Fields, N.; Poulson, R.L.; Krauss, S.; Webster, R.G.; Stallknecht, D.E. Antibodies to Influenza A Viruses in Gulls at Delaware Bay, USA. Avian Diseases 2016, 60, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Reeves, A.B.; Hall, J.S.; Poulson, R.L.; Donnelly, T.; Stallknecht, D.E.; Ramey, A.M. Influenza A virus recovery, diversity, and intercontinental exchange: A multi-year assessment of wild bird sampling at Izembek National Wildlife Refuge, Alaska. PLOS ONE 2018, 13, e0195327. [Google Scholar] [CrossRef]
- Bevins, S.N.; Pedersen, K.; Lutman, M.W.; Baroch, J.A.; Schmit, B.S.; Kohler, D.; Gidlewski, T.; Nolte, D.L.; Swafford, S.R.; DeLiberto, T.J. Large-Scale Avian Influenza Surveillance in Wild Birds throughout the United States. PLOS ONE 2014, 9, e104360. [Google Scholar] [CrossRef]
- Krauss, S.; Stucker, K.M.; Schobel, S.A.; Danner, A.; Friedman, K.; Knowles, J.P.; Kayali, G.; Niles, L.J.; Dey, A.D.; Raven, G.; Pryor, P.; Lin, X.; Das, S.R.; Stockwell, T.B.; Wentworth, D.E.; Webster, R.G. Long-term surveillance of H7 influenza viruses in American wild aquatic birds: are the H7N3 influenza viruses in wild birds the precursors of highly pathogenic strains in domestic poultry? Emerging Microbes & Infections 2015, 4, 1–9. [Google Scholar] [CrossRef]
- Criado, M.F.; Moresco, K.A.; Stallknecht, D.E.; Swayne, D.E. Low-pathogenicity influenza viruses replicate differently in laughing gulls and mallards. Influenza and Other Respiratory Viruses 2021, 15, 701–706. [Google Scholar] [CrossRef]
- USDA-APHIS. Epidemiologic and other analyses of HPAI affected poultry flocks: , 2022 Interim Report. Technical report, USDA:APHIS:VS: Center for Epidemiology and Animal Health, Fort Collins, CO, 2022. [Online; accessed 2024-02-14]. 1 July.
- Froberg, T.; Cuthbert, F.; Jennelle, C.S.; Cardona, C.; Culhane, M. Avian Influenza Prevalence and Viral Shedding Routes in Minnesota Ring-Billed Gulls (Larus delawarensis). Avian Diseases 2018, 63, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Howell, S.N.G.; Dunn, J. A Reference Guide to Gulls of the Americas; Houghton Mifflin Harcourt, 2007. Google-Books-ID: J7lQsufWfdkC.
- Pyle, P. Identification Guide to North American Birds. Part II: Anatidae to Alcidae; Slate Creek Press: Point Reyes Station Cal, 2008. [Google Scholar]
- Pollet, I.L.; Shutler, D.; Chardine, J.W.; Ryder, J.P. Ring-billed Gull (Larus delawarensis), version 2.0. Birds of North America. [CrossRef]
- Velarde, R.; Calvin, S.E.; Ojkic, D.; Barker, I.K.; Nagy, E. Avian Influenza Virus H13 Circulating in Ring-Billed Gulls (Larus delawarensis) in Southern Ontario, Canada. Avian Diseases 2010, 54, 411–419. [Google Scholar] [CrossRef]
- Boersma, D.; Ryder, J.P. Reproductive Performance and Body Condition of Earlier and Later Nesting Ring-Billed Gulls. Journal of Field Ornithology 1983, 54, 374–380. [Google Scholar]
- Brown, J.D.; Stallknecht, D.E.; Berghaus, R.D.; Luttrell, M.P.; Velek, K.; Kistler, W.; Costa, T.; Yabsley, M.J.; Swayne, D. Evaluation of a Commercial Blocking Enzyme-Linked Immunosorbent Assay To Detect Avian Influenza Virus Antibodies in Multiple Experimentally Infected Avian Species. Clinical and Vaccine Immunology 2009, 16, 824–829. [Google Scholar] [CrossRef]
- of Natural Resources, M.D. National Wetland Inventory for Minnesota, 2019. [Online; accessed 2024-07-11].
- McKelvey, R.D.; Zavoina, W. A statistical model for the analysis of ordinal level dependent variables. The Journal of Mathematical Sociology 1975, 4, 103–120. [Google Scholar] [CrossRef]
- Veall, M.R.; Zimmermann, K.F. Evaluating Pseudo-R2’s for binary probit models. Quality and Quantity 1994, 28, 151–164. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing, 2024.
- Robinson, D.; Hayes, A.; Couch, S. broom: Convert Statistical Objects into Tidy Tibbles, 2024. R package version 1.0.6.
- Robinson, D. fuzzyjoin: Join Tables Together on Inexact Matching, 2020. R package version 0.1.6.
- Schloerke, B.; Cook, D.; Larmarange, J.; Briatte, F.; Marbach, M.; Thoen, E.; Elberg, A.; Crowley, J. GGally: Extension to ’ggplot2’, 2024. R package version 2.2.1.
- Clarke, E.; Sherrill-Mix, S.; Dawson, C. ggbeeswarm: Categorical Scatter (Violin Point) Plots, 2023. R package version 0.7.2.
- Kahle, D.; Wickham, H. ggmap: Spatial Visualization with ggplot2. The R Journal 2013, 5, 144–161. [Google Scholar] [CrossRef]
- Kassambara, A. ggpubr: ’ggplot2’ Based Publication Ready Plots, 2023. R package version 0.6.0.
- Rizopoulos, D. GLMMadaptive: Generalized Linear Mixed Models using Adaptive Gaussian Quadrature, 2023. R package version 0.9-1.
- Firke, S. janitor: Simple Tools for Examining and Cleaning Dirty Data, 2023. R package version 2.2.0.
- Zhu, H. kableExtra: Construct Complex Table with ’kable’ and Pipe Syntax, 2024. R package version 1.4.0.
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. Journal of Statistical Software 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Grolemund, G.; Wickham, H. Dates and Times Made Easy with lubridate. Journal of Statistical Software 2011, 40, 1–25. [Google Scholar] [CrossRef]
- Bache, S.M.; Wickham, H. magrittr: A Forward-Pipe Operator for R, 2022. R package version 2.0.3.
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biometrical Journal 2008, 50, 346–363. [Google Scholar] [CrossRef]
- Pedersen, T.L. patchwork: The Composer of Plots, 2024. R package version 1.2.0.
- Robin, X.; Turck, N.; Hainard, A.; Tiberti, N.; Lisacek, F.; Sanchez, J.C.; Müller, M. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics 2011, 12, 77. [Google Scholar] [CrossRef]
- Yu, G. scatterpie: Scatter Pie Plot, 2024. R package version 0.2.3.
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; Kuhn, M.; Pedersen, T.L.; Miller, E.; Bache, S.M.; Müller, K.; Ooms, J.; Robinson, D.; Seidel, D.P.; Spinu, V.; Takahashi, K.; Vaughan, D.; Wilke, C.; Woo, K.; Yutani, H. Welcome to the tidyverse. Journal of Open Source Software 2019, 4, 1686. [Google Scholar] [CrossRef]
- Michel, A.L.; Van Heerden, H.; Crossley, B.M.; Al Dahouk, S.; Prasse, D.; Rutten, V. Pathogen detection and disease diagnosis in wildlife: challenges and opportunities. Revue Scientifique Et Technique (International Office of Epizootics) 2021, 40, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.D.; Luttrell, M.P.; Berghaus, R.D.; Kistler, W.; Keeler, S.P.; Howey, A.; Wilcox, B.; Hall, J.; Niles, L.; Dey, A.; Knutsen, G.; Fritz, K.; Stallknecht, D.E. Prevalence of Antibodies to Type A Influenza Virus in Wild Avian Species Using Two Serologic Assays. Journal of Wildlife Diseases 2010, 46, 896–911. [Google Scholar] [CrossRef]
- Gilbert, A.T.; Fooks, A.R.; Hayman, D.T.S.; Horton, D.L.; Müller, T.; Plowright, R.; Peel, A.J.; Bowen, R.; Wood, J.L.N.; Mills, J.; Cunningham, A.A.; Rupprecht, C.E. Deciphering Serology to Understand the Ecology of Infectious Diseases in Wildlife. EcoHealth 2013, 10, 298–313. [Google Scholar] [CrossRef] [PubMed]
- Salewski, V.; Bruderer, B. The evolution of bird migration—a synthesis. Naturwissenschaften 2007, 94, 268–279. [Google Scholar] [CrossRef]
- Johnson, J.A.; DeCicco, L.H.; Ruthrauff, D.R.; Krauss, S.; Hall, J.S. Avian Influenza Virus Antibodies in Pacific Coast Red Knots (Calidris canutus roselaari). Journal of Wildlife Diseases 2014, 50, 671–675. [Google Scholar] [CrossRef]
- Verhagen, J.H.; Munster, V.J.; Majoor, F.; Lexmond, P.; Vuong, O.; Stumpel, J.B.G.; Rimmelzwaan, G.F.; Osterhaus, A.D.M.E.; Schutten, M.; Slaterus, R.; Fouchier, R.A.M. Avian Influenza A Virus in Wild Birds in Highly Urbanized Areas. PLOS ONE 2012, 7, e38256. [Google Scholar] [CrossRef] [PubMed]
- Wilson, H.M.; Hall, J.S.; Flint, P.L.; Franson, J.C.; Ely, C.R.; Schmutz, J.A.; Samuel, M.D. High Seroprevalence of Antibodies to Avian Influenza Viruses among Wild Waterfowl in Alaska: Implications for Surveillance. PLOS ONE 2013, 8, e58308. [Google Scholar] [CrossRef]
- Lu, L.; Lycett, S.J.; Leigh Brown, A.J. Reassortment patterns of avian influenza virus internal segments among different subtypes. BMC Evolutionary Biology 2014, 14, 16. [Google Scholar] [CrossRef]
- Morse, S.S. Public Health Surveillance and Infectious Disease Detection. Biosecurity and Bioterrorism: Biodefense Strategy, Practice, and Science 2012, 10, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Fuller, T.L.; Gilbert, M.; Martin, V.; Cappelle, J.; Hosseini, P.; Njabo, K.Y.; Abdel Aziz, S.; Xiao, X.; Daszak, P.; Smith, T.B. Predicting Hotspots for Influenza Virus Reassortment. Emerging Infectious Diseases 2013, 19, 581–588. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fereidouni, S. Ecology of Avian Influenza Viruses in Wild Birds. In Ecology of Wild Bird Diseases; CRC Press, 2024. number-of-pages: 18.
- Ljubojević, D.; Radosavljević, V.; Milanov, D. The role of gulls (Laridae) in the emergence and spreading of antibiotic resistance in the environment. World’s Poultry Science Journal 2016, 72, 853–864. [Google Scholar] [CrossRef]
- Endo, A.; Nishiura, H. The Role of Migration in Maintaining the Transmission of Avian Influenza in Waterfowl: A Multisite Multispecies Transmission Model along East Asian-Australian Flyway. Canadian Journal of Infectious Diseases and Medical Microbiology 2018, 2018, 3420535. [Google Scholar] [CrossRef]
- Raven, S.; Coulson, J. The distribution and abundance of Larus gulls nesting on buildings in Britain and Ireland. Bird Study 1997, 44, 13–34. [Google Scholar] [CrossRef]
- Zhang, Y.; Aevermann, B.D.; Anderson, T.K.; Burke, D.F.; Dauphin, G.; Gu, Z.; He, S.; Kumar, S.; Larsen, C.N.; Lee, A.J.; Li, X.; Macken, C.; Mahaffey, C.; Pickett, B.E.; Reardon, B.; Smith, T.; Stewart, L.; Suloway, C.; Sun, G.; Tong, L.; Vincent, A.L.; Walters, B.; Zaremba, S.; Zhao, H.; Zhou, L.; Zmasek, C.; Klem, E.B.; Scheuermann, R.H. Influenza Research Database: An integrated bioinformatics resource for influenza virus research. Nucleic Acids Research 2017, 45, D466–D474. [Google Scholar] [CrossRef]
- Hatch, J.J. Threats to public health from gulls (Laridae). International Journal of Environmental Health Research 1996, 6, 5–16. [Google Scholar] [CrossRef]
- Ahlstrom, C.A.; van Toor, M.L.; Woksepp, H.; Chandler, J.C.; Reed, J.A.; Reeves, A.B.; Waldenström, J.; Franklin, A.B.; Douglas, D.C.; Bonnedahl, J.; Ramey, A.M. Evidence for continental-scale dispersal of antimicrobial resistant bacteria by landfill-foraging gulls. Science of The Total Environment 2021, 764, 144551. [Google Scholar] [CrossRef] [PubMed]
- Bevins, S.N.; Shriner, S.A.; Cumbee, J.C.; Dilione, K.E.; Douglass, K.E.; Ellis, J.W.; Killian, M.L.; Torchetti, M.K.; Lenoch, J.B. Intercontinental Movement of Highly Pathogenic Avian Influenza A(H5N1) Clade 2.3.4.4 Virus to the United States, 2021. Emerging Infectious Diseases 2022, 28, 1006–1011. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Labocha, M.K.; Hayes, J.P. Morphometric indices of body condition in birds: a review. Journal of Ornithology 2012, 153, 1–22. [Google Scholar] [CrossRef]
| Virus Name | Subtype | Pathogenicity | Abbreviation | Lab designator |
|---|---|---|---|---|
| A/Chicken/Mexico/1433-2/2008 | H5N2 | Low | MX/08/H5N2/LP | none |
| A/Turkey/Wisconsin/1968 | H5N9 | Low | WI/68/H5N9/LP | none |
| A/Turkey/Oregon/1971 | H7N3 | Low | OR/71/H7N3/LP | none |
| A/Turkey/Minnesota/9845-4/2015 | H5N2 | High | MN/15/H5N2/HP | 133 ADV 1501 |
| 1/4/2005 to 7/3/2013 | 7/4/2013 to 12/31/2021 | |||||
|---|---|---|---|---|---|---|
| Positive | Tested | Positivity | Positive | Tested | Positivity | |
| Anatidae | 14,624 | 164,215 | 8.9% | 29,584 | 258,631 | 11.4% |
| Laridae | 1,396 | 28,225 | 4.9% | 3,206 | 31,134 | 10.3% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





