1. Introduction
After the invasion of Iraq from August 1990 to February 1991, it was estimated that 25 to 50 million barrels of unburned oil were spilled over from damaged facilities, creating about 300 oil lakes. Approximately 40 million tons of sand and soil were contaminated [
1,
2]. Over time, the oil would continue to sink into the sand, potentially contaminating Kuwait’s groundwater resources. With more than 30 years of weathering, the huge volume of spilled oil formed several types of contamination: dry oil lakes, wet and dry oil lakes, and contaminated piles [
3,
4,
5]. Several technologies, including solvent extraction [
6,
7], thermal treatment [
8], pyrolysis [
9], surfactants [
10,
11], and soil washing and flushing [
12] were employed to remediate the contaminated soil on the oil lakes. In the dry oil lakes, the crude oil components were transformed through weathering due to exposure to sunlight and high temperatures. On average, the crude oil penetrated the soil up to 1 m, but the upper 30 cm contained more than 40% of total petroleum hydrocarbons (TPH) while forming an oily sludge-like oil ball. Below the oily sludge layer, the TPH concentration is less than 20% [
1,
13].
Physical and chemical remediation technologies as mentioned above were recommended to treat soils with over 40% TPH. Bioremediation, including in-situ and ex-situ remediations, was often employed for lightly contaminated soil with less than 5% TPH [
13,
14,
15]. Bioremediation approaches were considered efficient cleaning technologies for the crude oil-contaminated soil of low TPH levels because microorganisms were able to degrade a variety of petroleum hydrocarbon compounds, including aliphatic, aromatic alkanes, and polycyclic aromatic hydrocarbons (PAH) [
14,
16,
17,
18,
19,
20]. However, although bioremediation methods such as landfarming had a high percentage (82.5%) of TPH
reduction (40 to 7.0 g TPH/kg soil) after 12 months, a weak point was observed since 7,000 ppm of TPH remained after treatment. The concentration of 7,000 ppm was still very high for safe ecological exposure [
21]. In addition, the shortcoming of bioremediation technologies such as landfarming is the time required for application processes. In such sense, a reactor treatment system in the present study might provide the advantage of a speedy treatment process.
In our previous study, the hemoglobin (Hb) application to Kuwait crude oil-contaminated soil enhanced the population of surfactant-producing bacteria among the indigenous bacterial community, while promoting TPH degradation. The catalytic role of Hb was not as significant as the nutrient role of Hb [
22]. This result suggested that the application of Hb as a cheap and rich nutrient source is one of the best solutions to enhance TPH removal. The price of Hb is about
$1.0/kg in the market. In another study, Kuwaiti soil contaminated with crude oil was cleaned up using the biowashing and biopile steps [
23]. The biowashing step used hemoglobin as a nutrient source to enrich the indigenous bacterial community for the desorption and emulsification of crude oil from soil samples. The 4 day-biowashing step removed 75% of TPH and the 16 day-biopile step removed an additional 11% of TPH. Thus, if one process needs to be chosen for an enhanced and fast remediation with a pilot-scale reactor, the biowashing process could be more useful for the efficient remediation of the aged crude oil-contaminated soil. In the present study, we aimed to develop a 200-liter pilot-scale biowashing process for TPH degradation of crude oil-contaminated Kuwaiti soil.
2. Materials and Methods
2.1. Soil Sample and Chemicals
Crude oil-contaminated soil with a TPH greater than 8% and moisture content less than 1% from the Burgan oil-contaminated site was obtained from Kuwait Oil Company and stored in a 4oC refrigerator until experimental setup. The loamy soil sample had 81% sand, 19% silt, and 1% clay. The initial concentrations of TPH, 16 PAH, and alkylated PAH in the contaminated soil were determined after removing the crude oil through hexane and were 84,000 ± 15,230, 42.3 ± 2.0, and 3,174 ± 66.0 mg/kg soil, respectively. Hemoglobin powder was purchased from Shenzhen Taier Biotechnology (Shenzhen, Guangdong, China). It was dissolved in 50 mM phosphate buffer (pH 7.0).
2.2. Setting Up of Biowashing Pilot Reactor
Ten 250-mL Erlenmeyer flasks, each containing 10 g of Kuwait crude oil-contaminated soil and 1 g of hemoglobin in 100 mL of 50 mM phosphate buffer (pH 7.0), were cultured at 30oC for a week. Then, the culture was repeated in a 500-mL Erlenmeyer flask in 200 mL of 50 mM phosphate buffer (pH 7.0) at the same condition. The whole cultured content was equally poured into the three reactors, which received 50 L of 50 mM phosphate buffer (pH 7.0). Fifteen kilograms of Kuwait crude oil-contaminated soil were sieved through a 2 mm wire screen and fed into the lower biowashing reactor. The reactor was stirred with a corn impeller (~180 rpm) to have dissolved oxygen of 2.5~3.5 mg/L, so that the soil mixture was treated under aerobic conditions for the biowashing and microbial biodegradation. Other operating procedures for biowashing pilot reactor are explained below.
2.3. Operation of Biowashing Pilot Reactor
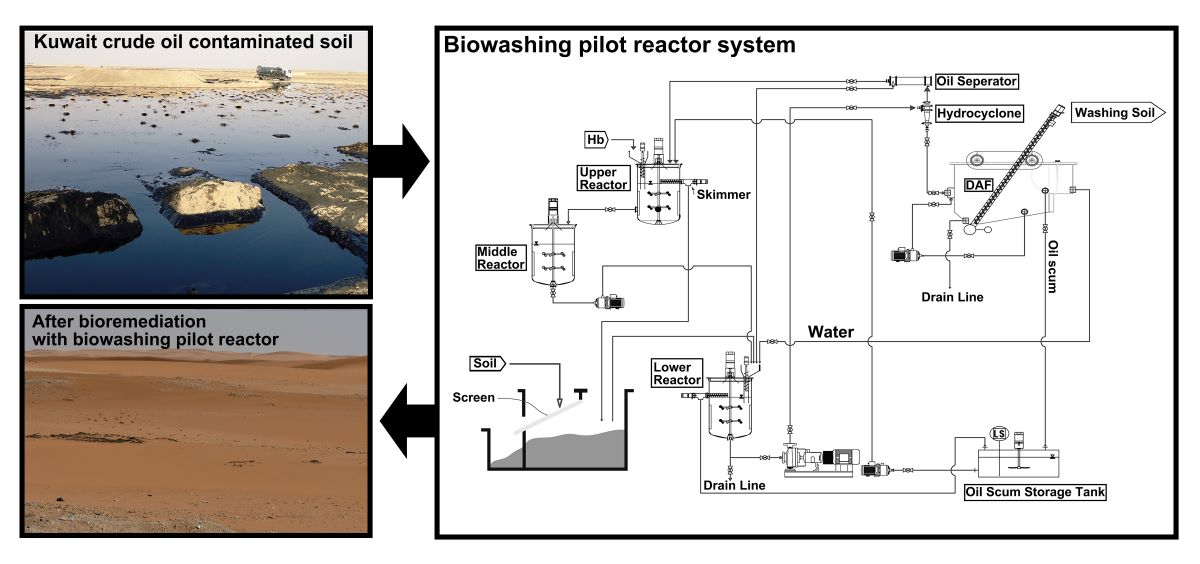
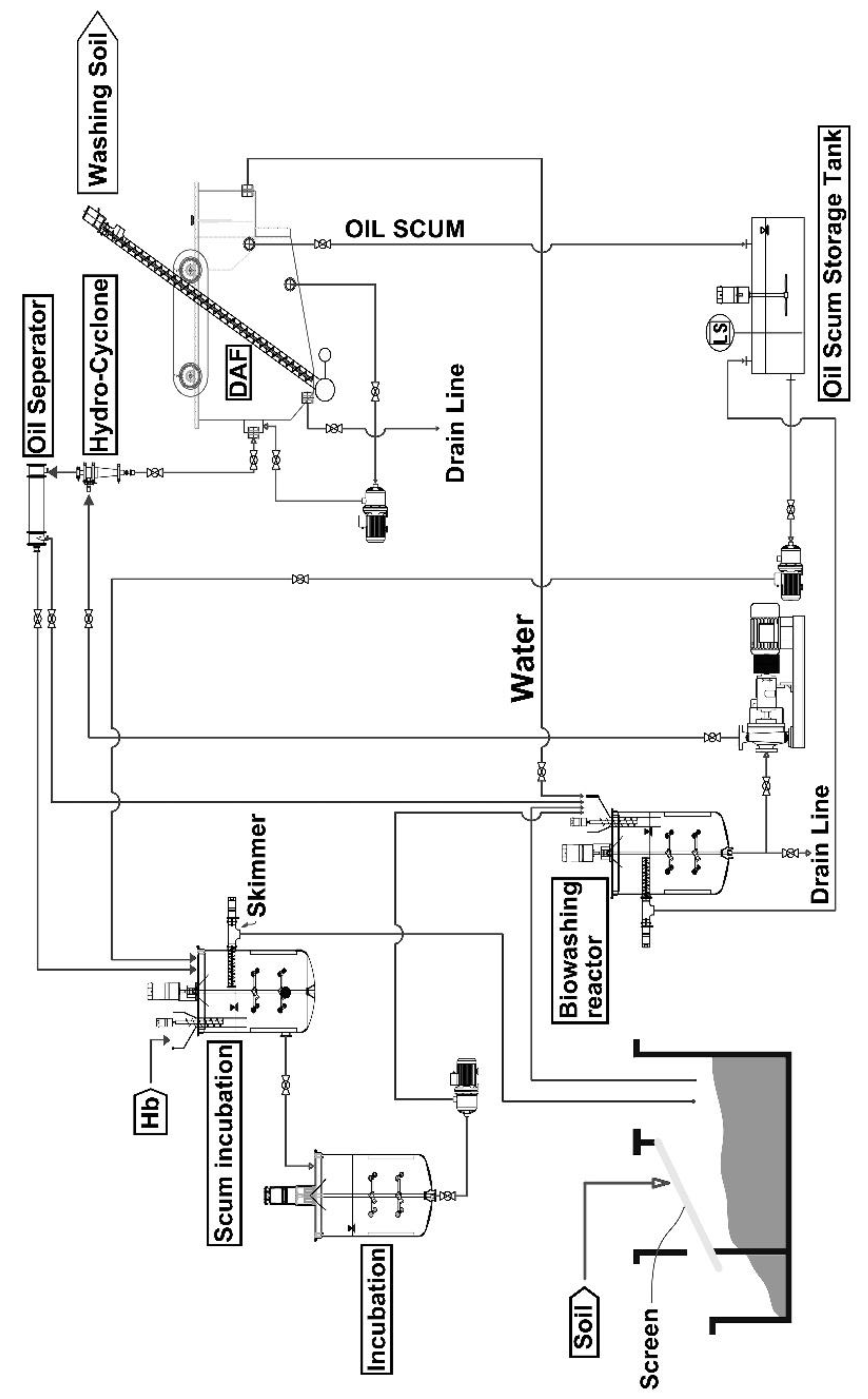
In the biological washing process system, three reactors had cone-type impellers that were installed on the rotating shafts in two stages, together with a hydrocyclone for the separation of oil scum and soil, an oil separator for further separation of oil scum and fine soil, and a dissolved air flotation (DAF), as shown in
Figure 1. The stainless steel 304 reactor vessels had a dimension of 45 cm outer diameter (OD) by 80 cm height and an effective capacity of 50 L. The lower biowashing reactor was for major microbiological washing treatment of contaminated soil. The upper scum reactor received hemoglobin at 1 g/day to accelerate the microbial decomposition of residual oil from the oil separator. The middle incubation reactor was employed for microbial growth stabilization, after which the incubator content was returned to the lower biowashing reactor. The washed soil in the lower biowashing reactor was fed into the hydrocyclone to separate scum oil from the soil. Then, the scum oil from the hydrocyclone was sent to the oil separator for further separation of scum oil. Scum oil from the oil separator was fed into the upper scum reactor. However, a fine soil fraction from the oil separator was sent into the lower biowashing reactor. One kg of the separated soil fraction from the hydrocyclone was sent daily into the DAF. DAF received an initial 50 L of 50 mM phosphate buffer (pH 7.0) and performed further washing of the scum soil for 5 days. Samples for analysis were collected through a drain line at the bottom of the DAF. Then, the soil scum from DAF was collected in the oil scum storage tank and fed to the upper scum reactor.
2.4. Analytical Methods
Oils from contaminated soil and other samples were extracted using n-hexane (95%, J. T. Baker, NJ, USA) using a Soxhlet apparatus for 18 h. The extracted solvent was concentrated by evaporation with a nitrogen concentrator (Turbo Vap II, Biotage, Uppsala, Sweden). After washing, a gas chromatograph equipped with a flame ionization detector (GC-FID; Agilent 6890 N, Agilent Technologies, DE, USA), which had the split mode (1:10) injection on a bonded phase fused silica capillary column DB-5 (Agilent J&W, 30 m ✕ 0.25 mm internal diameter ✕ 0.25 μm film thickness), was used to investigate the residual TPH and unresolved complex mixture (UCM) concentrations in the extracts. Sixteen PAH, alkylated PAH, and n-alkanes were analyzed by GC/MS (Agilent 6890/HP 5973) with splitless mode injection on a DB5-MS (30 m length ✕ 0.25 mm inner diameter ✕ 0.25 µm film thickness). The operation condition of GC-FID and GC/MS was described in our previous study [
24].
2.5. Bacterial Quantification
The PowerSoil DNA Isolation Kit (MoBio Laboratories, CA, USA) was used to extract DNA from processed samples according to the manufacturer’s protocol. The bacterial 16S rRNA gene copy number was estimated using real-time polymerase chain reaction analysis with SYBR Premix Ex Taq reagent (Takara, Shiga, Japan) and bacterial universal primer sets of EUB338F [
25] and BAC515R [
26]. SYBR Green I and ROX were used as reporter and passive reference dyes, respectively. The real-time PCR method used in this study has been described in detail in our previous study [
22].
3. Results and Discussion
3.1. The Degradation Rate of TPH, UCM, PAH, and Alkylated PAH by Biowashing Pilot Reactor Process
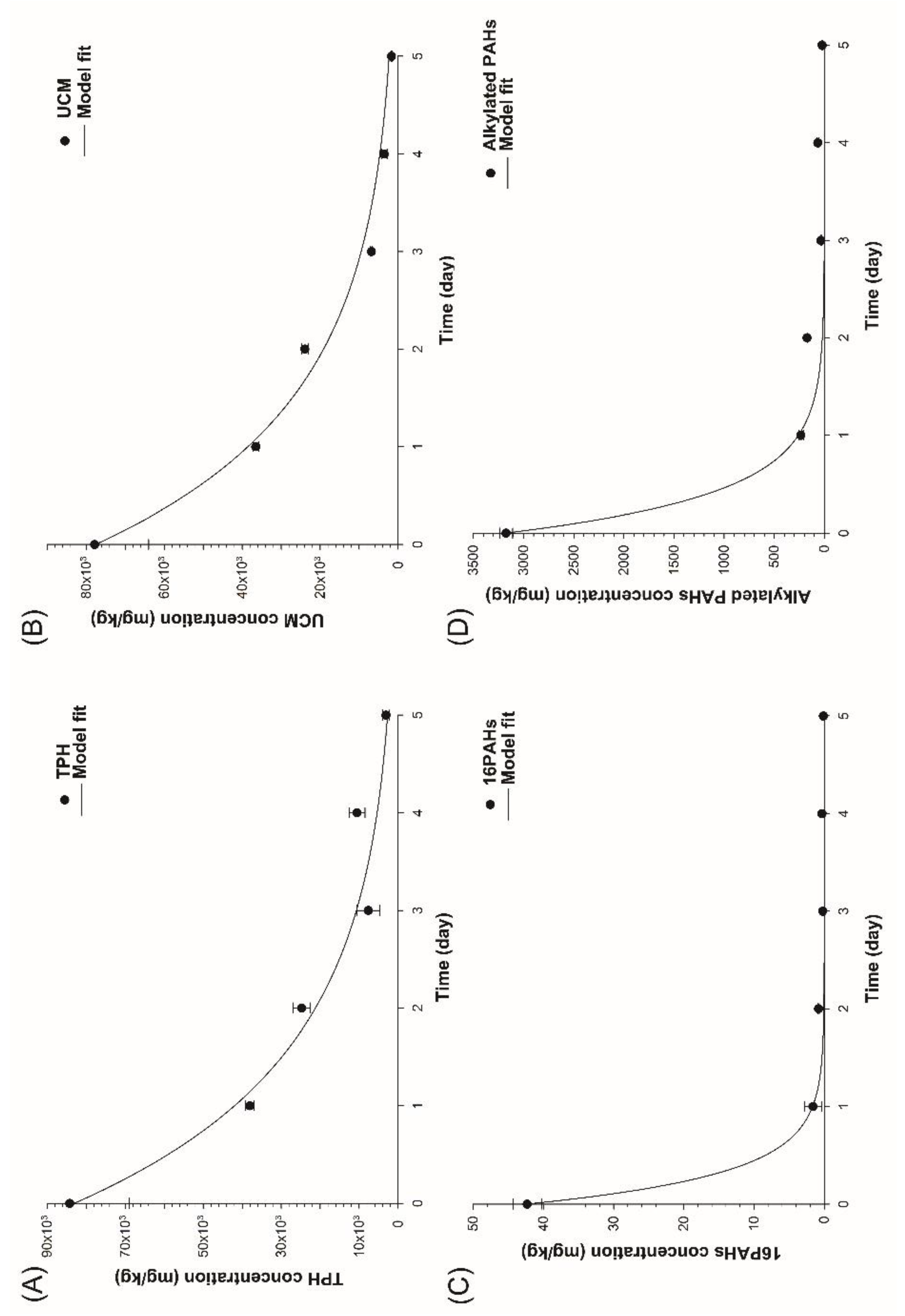
The biowashing pilot reactor process was evaluated for its speedy treatment processes. TPH concentration decreased rapidly. TPH concentration decreased from about 84,000 mg/kg on day 0 to about 38,000 mg/kg on day 1 (
Figure 2A). TPH concentration decreased further to 25,000 mg/kg, 7,600 mg/kg, and 3,100 mg/kg, respectively, on day 2, day 3, and day 5. Thus, the removal extents of TPH were about 55%, 71%, 91%, and 96% on day 1, day 2, day 3, and day 5, respectively. When TPH degradation was considered a first-order reaction, the first-order rate constant was estimated to be 0.682±0.0004/day. UCM concentration also decreased rapidly. UCM concentration decreased from about 78,000 mg/kg on day 0 to about 36,500 mg/kg on day 1 (
Figure 2B).
UCM concentration decreased further to about 24,000 mg/kg, 6,800 mg/kg, and 1,700 mg/kg, respectively, on day 2, day 3, and day 5. Thus, the removal extents of UCM were about 53%, 70%, 91%, and 98% on day 1, day 2, day 3, and day 5, respectively. When UCM degradation was considered the first-order reaction, the first-order rate constant was estimated to be 0.699+0.0002/day. These results showed that the biowashing pilot reactor was very efficient for the degradation of both TPH and UCM.
The 16 different PAH are well-known components of soil contaminated with TPH. The overall degradation extent of PAH with time might show the degradation efficiency of PAH in the biowashing pilot reactor. The total concentration of 16 PAH decreased rapidly from about 42.3 mg/kg on day 0 to about 1.6 mg/kg on day 1 (
Figure 2C). The overall removal extent of PAHs was 96.3% on day 1. Then, the overall concentration of PAH decreased slowly to 0.1 mg/kg at day 5, which corresponded to the overall removal extent of 99.7%. This indicates that most of the PAHs were degraded by the biowashing pilot reactor. When PAH degradation was considered a first-order reaction, the first-order rate constant was estimated to be 3.238±0.0002/day.
The alkylated PAH is also a major component of soil contaminated with TPH. The degradation efficiency of overall alkylated PAH was also investigated with the biowashing pilot reactor (
Figure 2D). The degradation of alkylated PAH had almost the same trend as that of 16 PAH. The concentration of alkylated PAH decreased rapidly from about 3,174 mg/kg on day 0 to about 234 mg/kg on day 1. Thus, the removal extent of alkylated PAH on day 1 was about 92.6%. The concentration of alkylated PAHs decreased slowly to 21.8 mg/kg, indicating that the degradation efficiency was about 99.3% at 5 days. When alkylated PAH degradation was considered a first-order reaction, the first-order rate constant was estimated to be 2.504±0.0015/day.
3.2. Change in the Components of PAHs, Alkylated PAHs, and n-Alkane during Biowashing Pilot Reactor Process
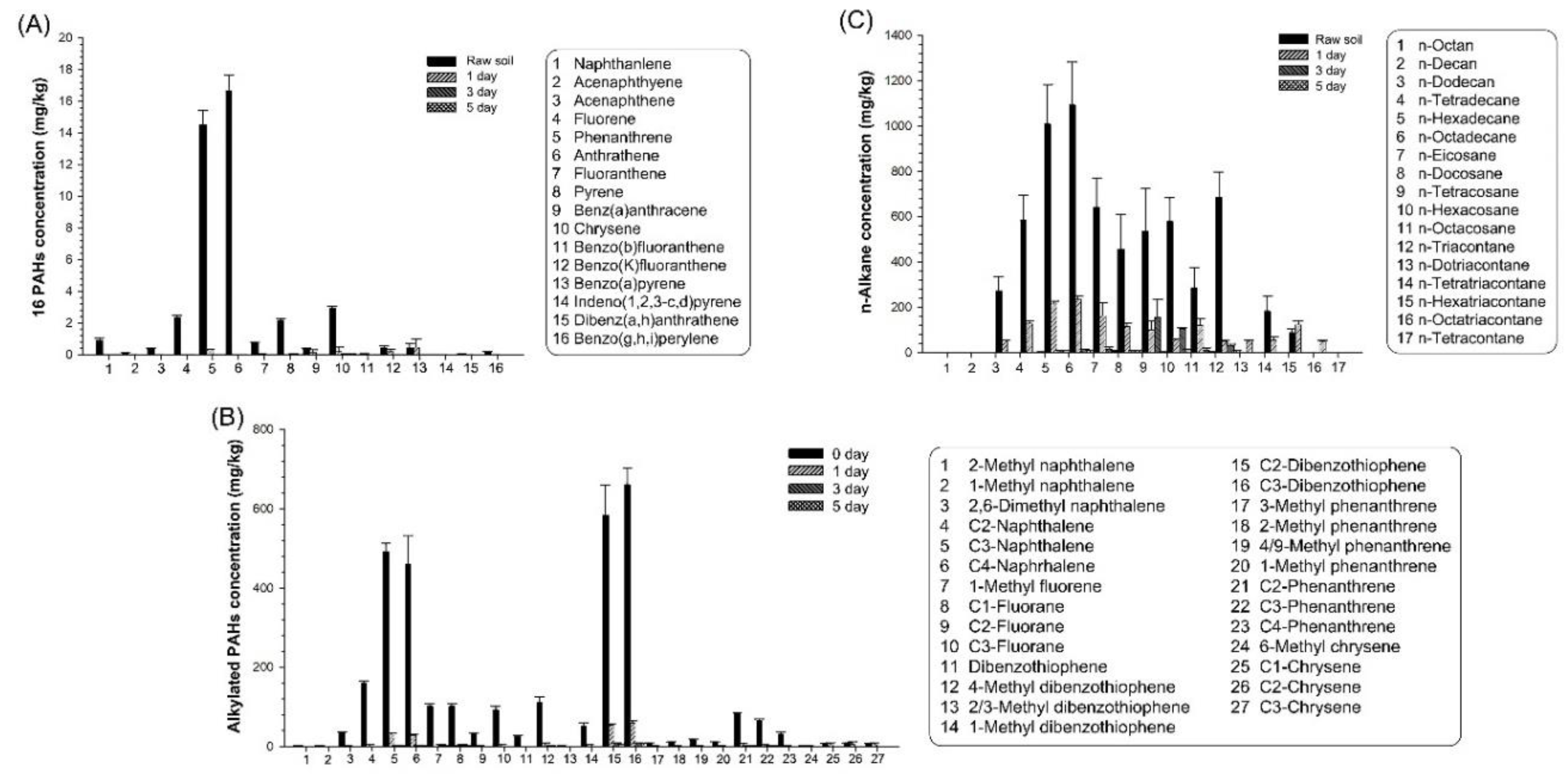
The degradation of 16 individual PAH was examined, as shown in
Figure 3A. The initial concentration of phenanthrene and anthracene was higher than the concentration of the other individual PAH. The concentration of anthracene decreased very rapidly and was about 16.7 mg/kg, 0.02 mg/kg, 0.00 mg/kg, and 0.00 mg/kg, respectively, on day 0, day 1, day 3, and day 5. Thus, the concentration of anthracene was 0% at day 5. The degradation trend of most individual PAH was very similar to that of anthracene. It was noticed that benzo(
a)pyrene concentration did not decrease much on day 1 (about 0.46 mg/kg) compared to day 0 (about 0.41 mg/kg). However, the benzo(
a)pyrene concentration decreased to 0.01 mg/kg and 0.00 mg/kg, respectively, on day 3 and day 5.
Then, the degradation of individual alkylated PAH was observed, as shown in
Figure 3B. The highest concentration of alkylated PAH (69.1 %) was explained by 4 alkylated PAH (i.e., C2-phenanthrene, C4-phenanthrene, C2-dibenzothiophene, and C3-dibenzothiophene). The concentration of C3-dibenzothiophene decreased rapidly and was 662.0 mg/kg, 60.0 mg/kg, 8.80 mg/kg, and 6.8 mg/kg, respectively, on day 0, day 1, day 3, and day 5. Thus, the removal extents of C3-dibenzothiophene were about 90.9%, 98.7%, and 99.0% on day 1, day 3, and day 5, respectively. The degradation trend of other individual alkylated PAH was like that of C3-dibenzothiophene. Although the concentration of 3 chrysenes was low, their degradation was not rapid until day 1, and then their concentrations decreased very rapidly.
The degradation of individual
n-alkanes was also observed, as shown in
Figure 3C. The highest concentration of
n-alkane (86.51 %) was explained by 8
n-alkanes (i.e.,
n-hexadecane,
n-octadecane,
n-eicosane,
n-docosane,
n-tetracosane,
n-hexacosane,
n-cotacosane, and
n-dotricoacontane). The concentration of
n-eicosane decreased rapidly and was 1090.0 mg/kg, 234.0 mg/kg, 14.4 mg/kg, and 8.0 mg/kg on day 0, day 1, day 3, and day 5, respectively. Thus, the removal extents of
n-eicosane were about 78.5%, 98.7%, and 99.3% on day 1, day 3, and day 5, respectively. The degradation trend of the other individual
n-alkane was like that of
n-eicosane.
3.3. Change in Bacterial Number during Operation of Biowashing Pilot Reactor
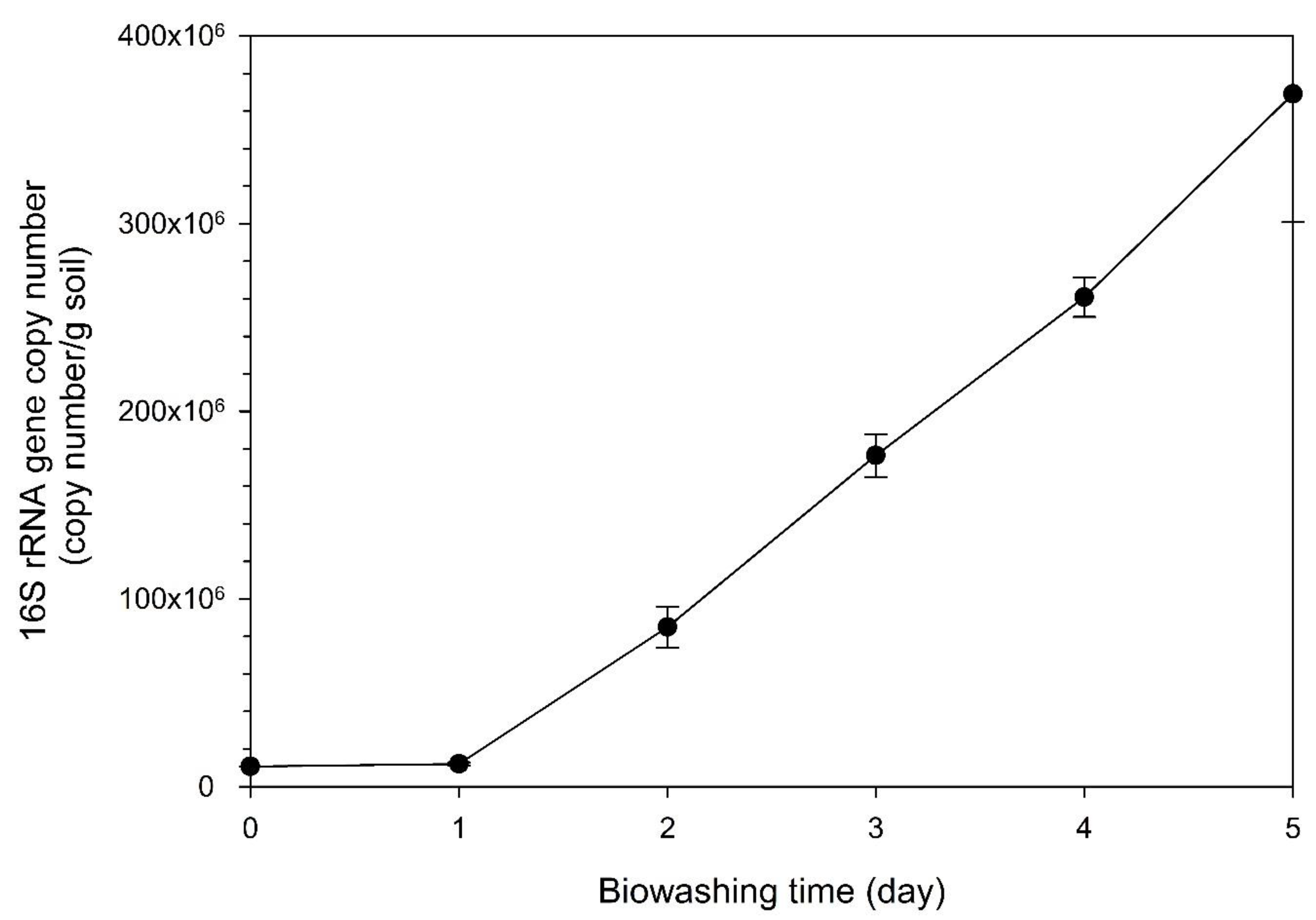
The bacterial number was expected to increase with the operation of the biowashing pilot reactor, as indicated by the increased degradation of various compounds mentioned above. 16S rRNA gene copy number was measured to estimate the bacterial number during the operation of the biowashing pilot reactor (
Figure 4). The 16S rRNA gene copy numbers were 1.1 ✕ 10
7 and 1.2 ✕ 10
7, respectively, on day 0 and day 1, indicating that there was not much increase in bacterial population for a day of operation. However, the copy number increased from day 2 (8.5 ✕ 10
7) and up to day 5 (3.8 ✕ 10
8). Thus, the copy number was increased by about 34 times in 5 days. The results suggest that although there is a delay in bacterial number increase from day 0 to day 1, bacteria in the biowashing pilot reactor actively degraded the various compounds of TPH during the first 1 day. This fact suggests that the copy number was increased after day 1 by using the degraded compounds and hemoglobin as nutrients for continued growth up to day 5. This result was supported by our previous study, in which the population of surfactant-producing bacteria was shown to be increased among the indigenous bacterial community by the application of Hb to Kuwait crude oil-contaminated soil (Vidonish et al., 2016). In the study, a greater recovery of the microbial community structure was observed with a double Hb injection than with a single Hb injection. All these results suggest that the application of Hb is one of the best solutions to enhance TPH removal. It needs to be mentioned that the previous experiment was performed with a 250-mL micro-reactor of Pyrex glass.
4. Conclusions
In our previous study, the 20-day sequential processes were employed to remediate the crude oil-contaminated Kuwaiti soil using an Erlenmeyer flask for the biowashing step and a small-scale reactor for the biopile steps (Hong et al., 2018). The biowashing step used an enrichment culture of the indigenous soil bacterial communities using Hb as a nutrient source, and the biopile step included hemoglobin-catalyzed oxidation. The results showed that the biowashing step was more efficient due to the promoted emulsification of crude oil from the soil sample compared to the biopile step. The results supported the development of a large-scale pilot reactor using a biowashing step for TPH degradation of crude oil-contaminated Kuwait soil.
In the present study, a 200-liter pilot-scale reactor using the biowashing process employed hemoglobin as a key nutrient ingredient and the adapted bacterial community as a potential surfactant producer. In the pilot-scale reactor, a high level of bioremediation was obtained for TPH (~96.3%) and UCM (~97.8) in 5 days. All these results suggest that the biowashing pilot reactor is very efficient for bioremediation of the crude oil-contaminated Kuwaiti soil. These results also indicate that further development might be needed for an upscaled reactor to remediate the aged crude oil-contaminated soil in Kuwait for an efficient and fast remediation process.
Author Contributions
D.J.: Data curation, formal analysis, investigation, writing–original draft. S.J.L.: Project administration, methodology, resources, software, validation. G.K.: Conceptualization, funding acquisition. M.P.: Writing–editing review (support). Y.H.J.: Visualization. N.C.: Supervision, writing–editing review (lead).
Funding
This work was supported by the GAIA project (No. 2016000550004) of the Ministry of Environment, South Korea, and the 2020 Hankuk University of Foreign Studies Research Fund.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- ElNawawy, A.S.; Al-Daher, R.; Yateem, A.; Al-Awadhi, N. Bioremediation of oil-contaminated soil in Kuwait; Environmental Biotechnology; Springer: Dordrecht, Netherland, 1995; pp. 249–258. [Google Scholar] [CrossRef]
- Elgibaly, A.A.M. Cleanup of oil-contaminated soils of Kuwaiti oil lakes by retorting. Energy Sources 1999, 21, 547–565. [Google Scholar] [CrossRef]
- Al-Awadhi, N.; Al-Daher, R.; Balba, M.; Chino, H.; Tsuji, H. Bioremediation of oil-contaminated desert soil: the Kuwait experience. Environ. Int. 1998, 24, 163–173. [Google Scholar] [CrossRef]
- Almutairi, M.S. An assessment of remediation strategies for Kuwaiti oil lakes. Environ. Geotech. 2018, 5, 345–355. [Google Scholar] [CrossRef]
- Bruckberger, M.C.; Morgan, M.J.; Bastow, T.P.; Walsh, T.; Prommer, H.; Mukhopadhyay, A.; Kaksonen, A.H.; Davis, G.B.; Puzon, G.J. Investigation into the microbial communities and associated crude oil-contamination along a Gulf War impacted groundwater system in Kuwait. Water Res. 2020, 170, 115314. [Google Scholar] [CrossRef] [PubMed]
- Gan, S.; Lau, E.V.; Ng, H.K. Remediation of soils contaminated with polycyclic aromatic hydrocarbons (PAH). J. Hazard. Mater. 2009, 172, 532–549. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.; Park, M.; Hur, M.; Kang, G.; Kim, Y.H.; Kim, S. Molecular-level investigation of soils contaminated by oil spilled during the Gulf War. J. Hazard. Mater. 2019, 373, 271–277. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, P.L.; DeSutter, T.M.; Casey, F.X.M.; Khan, E.; Wick, A.F. Thermal remediation alters soil properties - a review. J. Environ. Manage. 2018, 206, 826–835. [Google Scholar] [CrossRef] [PubMed]
- Vidonish, J.E.; Zygourakis, K.; Masiello, C.A.; Gao, X.; Mathieu, J.; Alvarez, P.J. Pyrolytic treatment and fertility enhancement of soils contaminated with heavy hydrocarbons. Environ. Sci. Technol. 2016, 50, 2498–2506. [Google Scholar] [CrossRef]
- Mao, X.; Jiang, R.; Xiao, W.; Yu, J. Use of surfactants for the remediation of contaminated soils: a review. J. Hazard. Mater. 2015, 285, 419–435. [Google Scholar] [CrossRef]
- Cheng, M.; Zeng, G.; Huang, D.; Yang, C.; Lai, C.; Zhang, C.; Liu, Y. Advantages and challenges of Tween 80 surfactant-enhanced technologies for the remediation of soils contaminated with hydrophobic organic compounds. Chemical Engineering Journal 2017, 314, 98–113. [Google Scholar] [CrossRef]
- Trellu, C.; Mousset, E.; Pechaud, Y.; Huguenot, D.; van Hullebusch, E.D.; Giovanni, E.; Oturan, M.A. Removal of hydrophobic organic pollutants from soil washing/flushing solutions: a critical review. J. Hazard. Mater. 2016, 306, 149–174. [Google Scholar] [CrossRef] [PubMed]
- Al-Awadhi, N.; Al-Daher, R.; EINawawy, A.; Salba, M. Bioremediation of oil-contaminated soil in Kuwait. I. landfarming to remediate oil-contaminated soil. Soil J. Sediment Contam. 1996, 5, 243–260. [Google Scholar] [CrossRef]
- Al-Gounaim, M.; Diab, A.; Al-Hilali, A.; Abu-Shady, A.S. Aromatic hydrocarbons: degrading bacteria in the desert soil of Kuwait. Arab Gulf J. Sci. Res. 2005, 23, 28–36. [Google Scholar]
- Gallego, J.L.R.; Peña-Álvarez, V.; Lara, L.M.; Baragaño, D.; Forján, R.; Colina, A.; Prosenkov, A.; Peláez, A. I. Effective bioremediation of soil from the Burgan oil field (Kuwait) using compost: A comprehensive hydrocarbon and DNA fingerprinting study. Ecotoxicol. Environ. Saf. 2022, 2022. 247, 114267. [Google Scholar] [CrossRef]
- Wang, X.; Yu, X.; Bartha, R. Effect of bioremediation on polycyclic aromatic hydrocarbon residues in soil. Environ. Sci. Technol. 1990, 24, 1086–1089. [Google Scholar] [CrossRef]
- Alsaleh, E.; Drobiova, H.; Obuekwe, C. Predominant culturable crude oil degrading bacteria in the coast of Kuwait. Int. Biodeterior. Biodegrad. 2009, 63, 400–406. [Google Scholar] [CrossRef]
- Al-Wasify, R.S.; Hamed, S.R. Bacterial biodegradation of crude oil using local isolates. Int. J. Bacteriol. 2014, 863272. [Google Scholar] [CrossRef] [PubMed]
- Suja, F.; Rahim, F.; Taha, M.R.; Hambali, N.; Razali, M.R.; Khalid, A.; Hamzah, A. Effects of local microbial bioaugmentation and biostimulation on the bioremediation of total petroleum hydrocarbons (TPH) in crude oil contaminated soil based on laboratory and field observations. Int. Biodeterior. Biodegrad. 2014, 90, 115–122. [Google Scholar] [CrossRef]
- Ali, N.; Khanafer, M.; Al-Awadhi, H. Indigenous oil-degrading bacteria more efficient in soil bioremediation than microbial consortium and active even in super oil-saturated soils. Front. Microbiol. 2022, 13, 950051. [Google Scholar] [CrossRef]
- Balba, M.T.; Al-Awadhi, N.; Al-Daher, R. Bioremediation of oil-contaminated soil: microbiological methods for feasibility assessment and field evaluation. J. Microbiol. Methods 1998, 32, 155–164. [Google Scholar] [CrossRef]
- Hong, J.-K.; Jho, E.H.; Choi, H.S.; Kang, G. Role of hemoglobin in hemoglobin-based remediation of the crude oil-contaminated soil. Sci. Total Environ. 2018, 627, 1174–1181. [Google Scholar] [CrossRef]
- Kim, T.; Hong, J.-K.; Jho, E.H.; Kang, G.; Yang, D.J.; Lee, S.-J. Sequential biowashing-biopile processes for remediation of crude oil contaminated soil in Kuwait. J. Hazard. Mater. 2019, 378, 120710. [Google Scholar] [CrossRef]
- Keum, H.; Kim, J.; Joo, Y.H.; Kang, G.; Chung, N. Hemoglobin peroxidase reaction of hemoglobin efficiently catalyzes oxidation of benzo[a]pyrene. Chemosphere 2021, 268, 128795. [Google Scholar] [CrossRef]
- Nadkarni, M.A.; Martin, F.E.; Jacques, N.; Hunter, N. Determination of bacterial load by real-time PCR using a broad-range (universal) probe and primers set. Microbiology 2002, 148, 257–266. [Google Scholar] [CrossRef]
- Stahl, D.A.; Amann, R.I. Development and application of nucleic acid probes; Nucleic Acid Techniques in Bacterial Systematics; John Wiley & Sons: New York, USA, 1991; pp. 205–248. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).