Submitted:
09 August 2024
Posted:
21 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. Ground Level Ozone and Wheat Yields
1.2. Wheat Breeding for Improved Yields
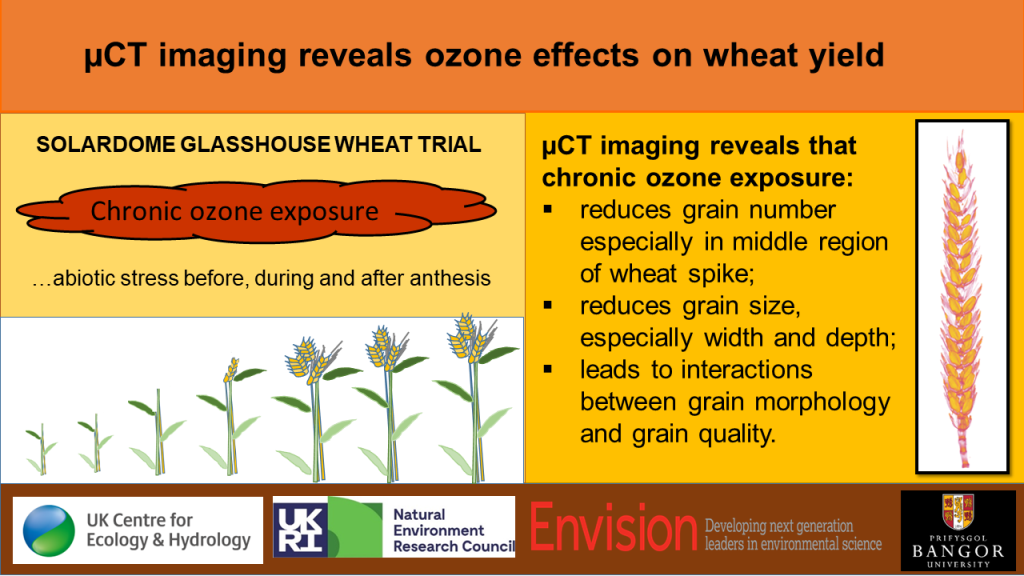
1.3. Use of µCT Imaging
1.4. Aims of the Study
2. Materials and Methods
2.1. Plant Establishment
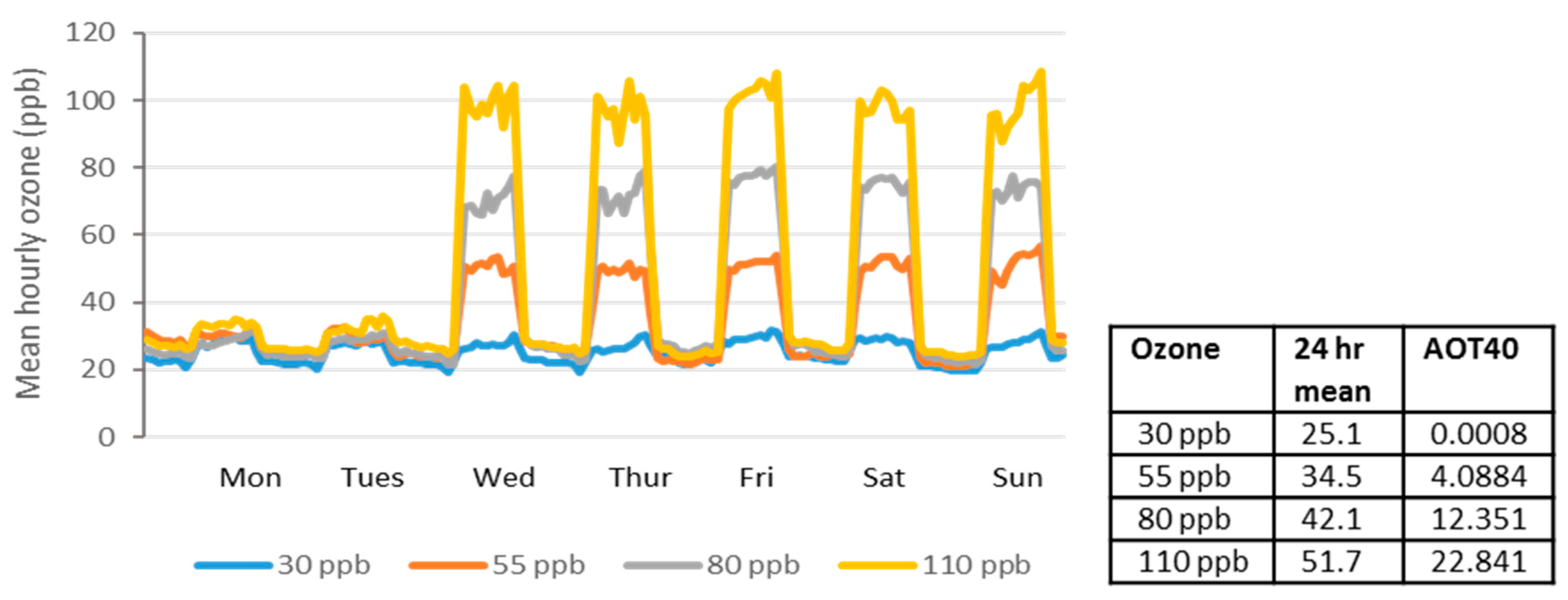
2.2. Ozone Treatment
2.3. Chlorophyll Index
2.4. Biomass and Yield Measurements
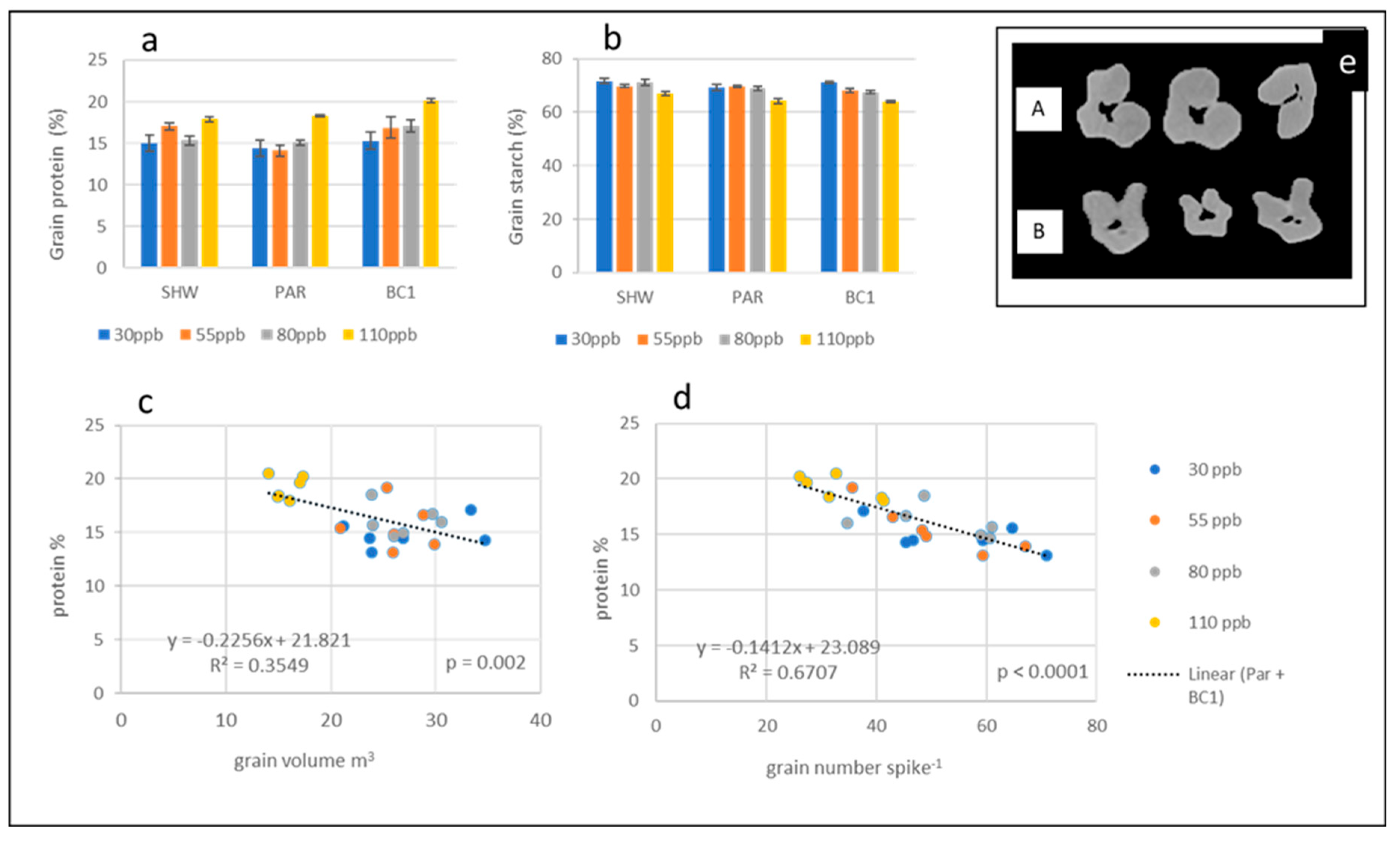
2.5. Grain Protein and Starch
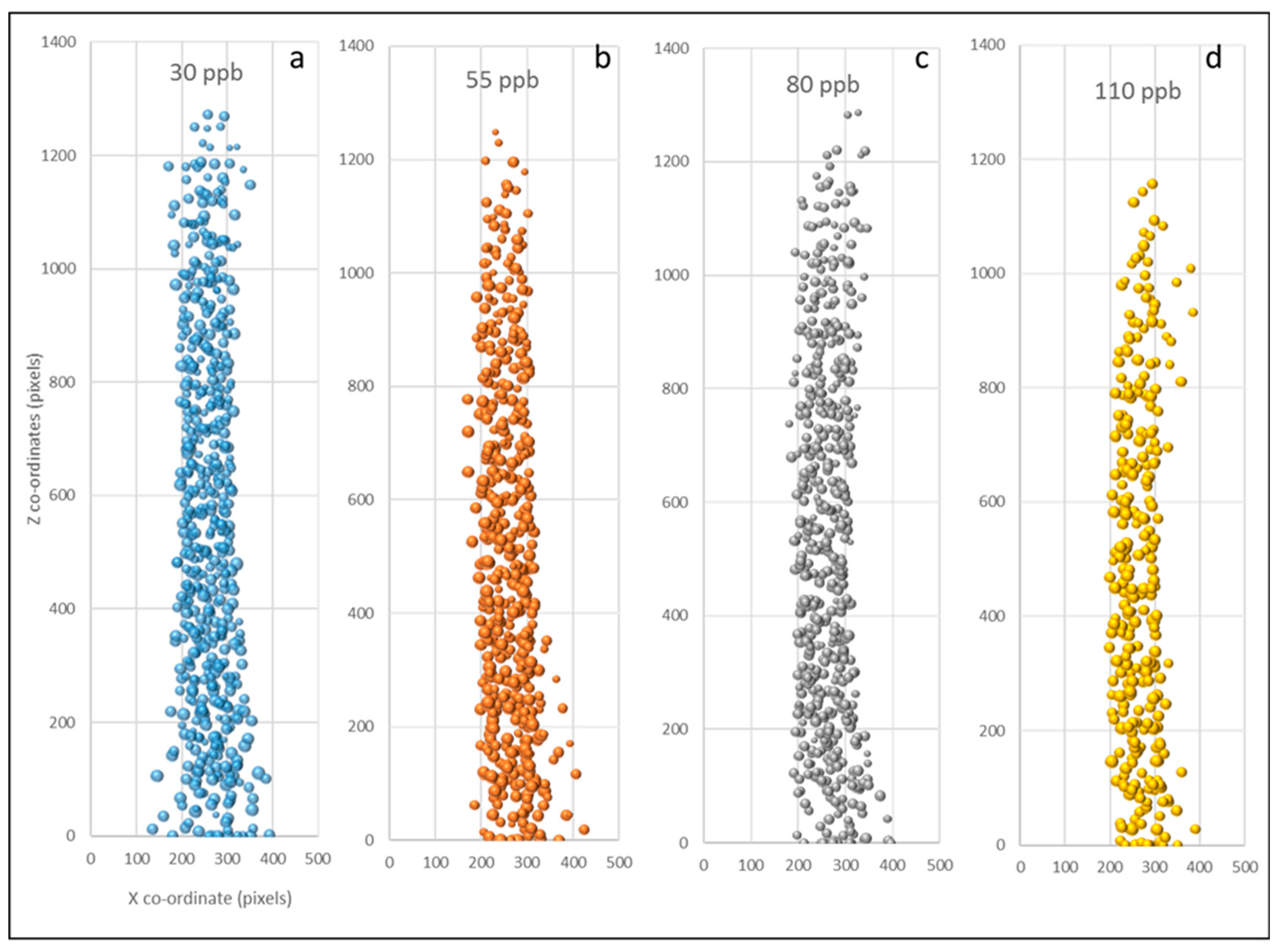
2.6. X-ray Microcomputed Tomography (µCT) Imaging
2.7. Statistical Tests
3. Results
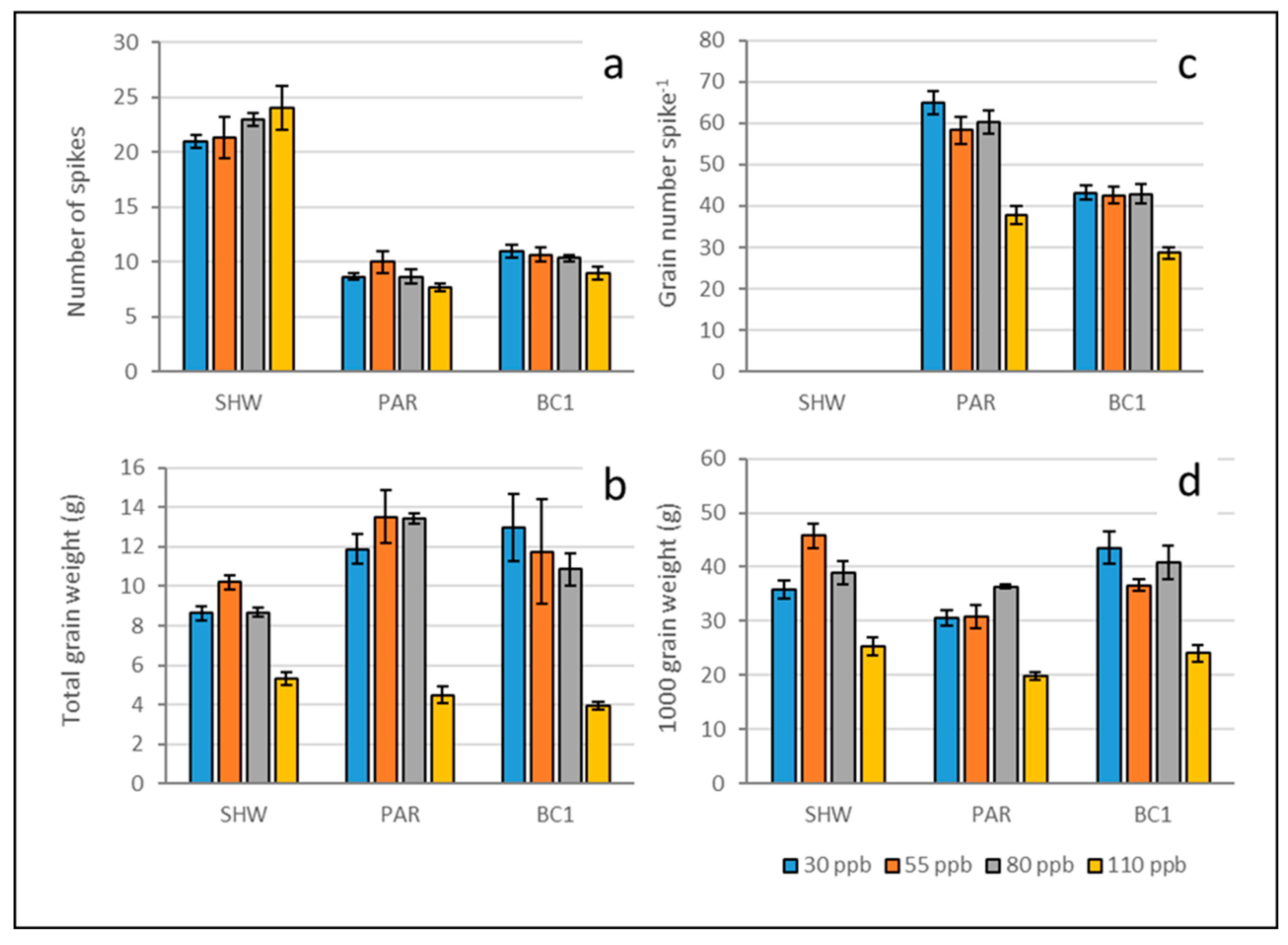
3.1. Ozone Reduces Spike Number, Total Grain Number and TGW
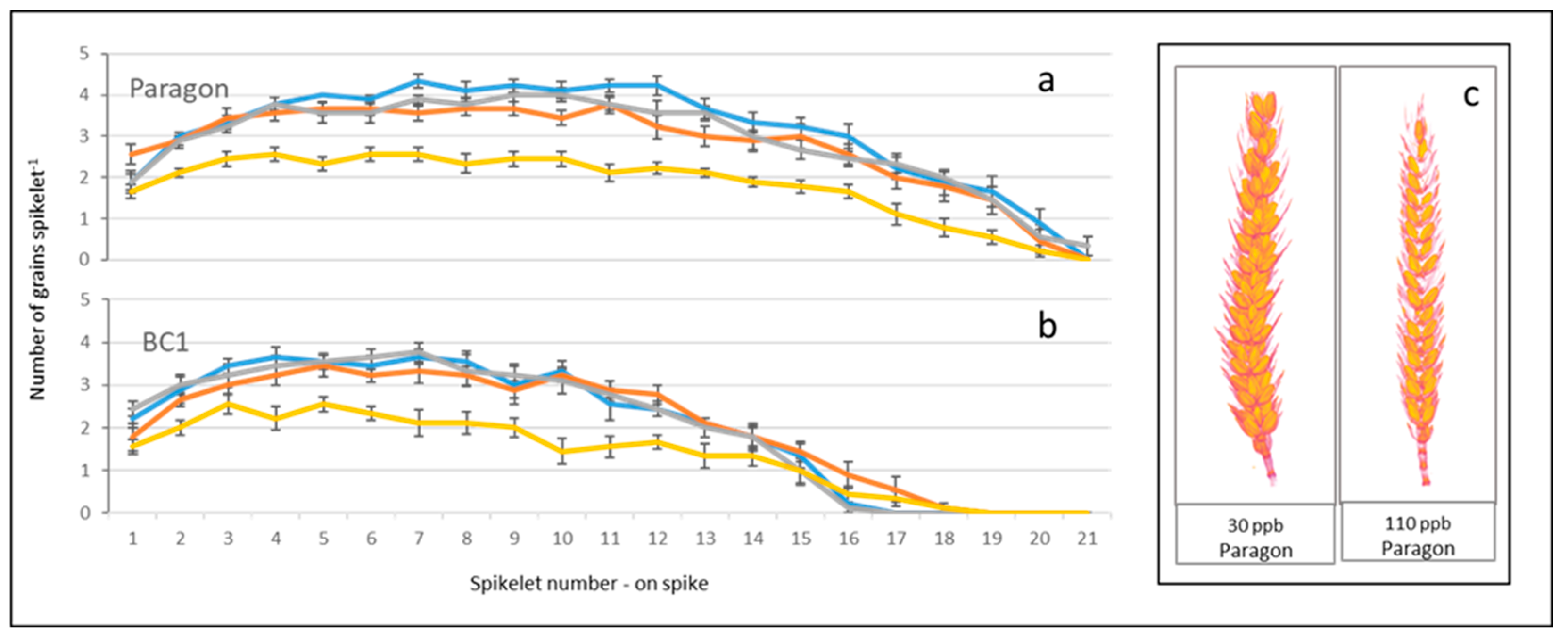
3.2. Ozone Reduces the Number of Grains per Spikelet
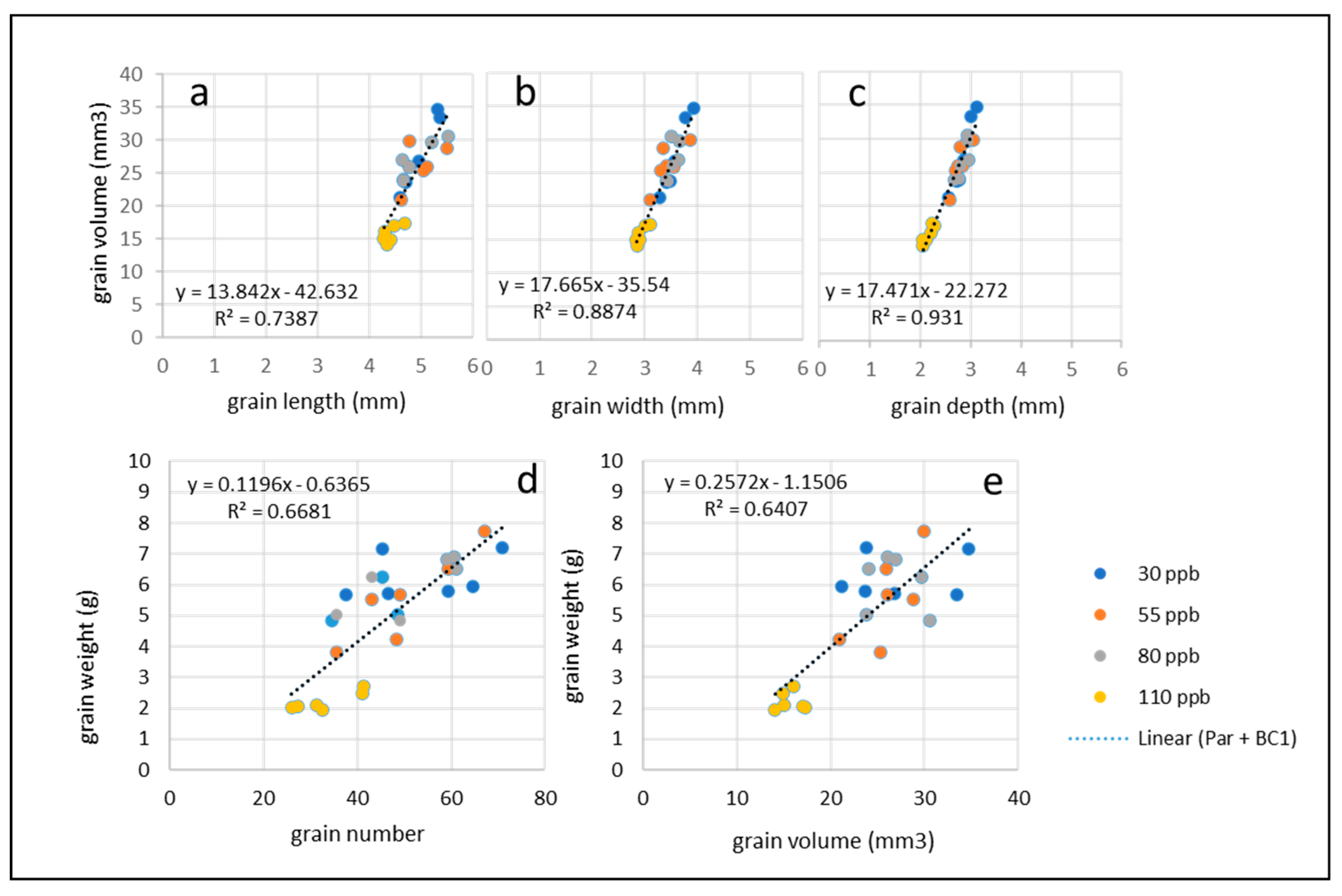
3.3. Ozone Changes Grain Morphology
3.4. Correlations between Ozone, Grain Morphology and Grain Quality
3.5. Ozone Reduces Chlorophyll Levels
4. Discussion
4.1. Effects on Grain Number
4.2. Effects on Grain Size and Quality
4.3. Potential Use of Synthetic Wheat and µCT Digital Imaging for Plant Breeding
5. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Acknowledgements
Declaration of interests
| 1 | Pulse Width Modulation involves an ozone delivery solenoid valve being opened and closed once per second. It may be opened all that period to deliver 100% or maximum dosage, or 0% to give zero delivery. Regulation is achieved by adjusting the 'on' period within each one second time interval, so a 50% dosage represents a square control waveform ('on' for 500 milliseconds and 'off' for 500 milliseconds). |
References
- Agriculture and Horticulture Development Board. 2021. “Wheat Growth Guide AHDB.” Agriculture and Horticulture Development Board. 2021. https://ahdb.org.uk/wheatgg.
- Ainsworth, E. A., P. Lemonnier, and J. M. Wedow. 2020. “The Influence of Rising Tropospheric Carbon Dioxide and Ozone on Plant Productivity.” Plant Biology 22 (S1): 5–11. [CrossRef]
- Ainsworth, Elizabeth A., Craig R. Yendrek, Stephen Sitch, William J. Collins, and Lisa D. Emberson. 2012. “The Effects of Tropospheric Ozone on Net Primary Productivity and Implications for Climate Change.” Annual Review of Plant Biology 63 (1): 637–61. [CrossRef]
- Ali, Ahmad, Zahid Ullah, Naveed Alam, S. M.Saqlan Naqvi, Muhammad Jamil, Hadi Bux, and Hassan Sher. 2020. “Genetic Analysis of Wheat Grains Using Digital Imaging and Their Relationship to Enhance Grain Weight.” Scientia Agricola 77 (6): 1–10. [CrossRef]
- Altenbach, Susan B. 2012. “New Insights into the Effects of High Temperature, Drought and Post-Anthesis Fertilizer on Wheat Grain Development.” Journal of Cereal Science 56 (1): 39–50. [CrossRef]
- Backhaus, Anna Elisabeth, Cara Griffiths, Angel Vergara-Cruces, James Simmonds, Rebecca Lee, Richard J. Morris, and Cristobal Uauy. 2023. “Delayed Development of Basal Spikelets in Wheat Explains Their Increased Floret Abortion and Rudimentary Nature.” Journal of Experimental Botany 74 (17): 5088–5103. [CrossRef]
- Bailey-Serres, Julia, Jane E. Parker, Elizabeth A. Ainsworth, Giles E.D. Oldroyd, and Julian I. Schroeder. 2019. “Genetic Strategies for Improving Crop Yields.” Nature 575 (7781): 109–18. [CrossRef]
- Barnabás, Beáta, Katalin Jäger, and Attila Fehér. 2008. “The Effect of Drought and Heat Stress on Reproductive Processes in Cereals.” Plant, Cell and Environment 31 (1): 11–38. [CrossRef]
- Barnes, J. D., D. Velissariou, A. W. Davison, and C. D. Holevas. 1990. “Comparative Ozone Sensitivity of Old and Modern Greek Cultivars of Spring Wheat.” New Phytologist 116 (4): 707–14. [CrossRef]
- Black, V. J., C. R. Black, J. A. Roberts, and C. A. Stewart. 2000. “Impact of Ozone on the Reproductive Development of Plants.” New Phytologist 147 (3): 421–47. [CrossRef]
- Borrill, Philippa, Brendan Fahy, Alison M. Smith, and Cristobal Uauy. 2015. “Wheat Grain Filling Is Limited by Grain Filling Capacity Rather than the Duration of Flag Leaf Photosynthesis: A Case Study Using NAM RNAi Plants.” PLoS ONE 10 (8): 1–14. [CrossRef]
- Brewster, Clare, Felicity Hayes, and Nathalie Fenner. 2019. “Ozone Tolerance Found in Aegilops Tauschii and Synthetic Hexaploid Wheat.” Plants 8 (195): 1–12.
- Broberg, Malin C., Sara Daun, and Håkan Pleijel. 2020. “Ozone Induced Loss of Seed Protein Accumulation Is Larger in Soybean than in Wheat and Rice.” Agronomy 10 (3): 1–13. [CrossRef]
- Broberg, Malin C., Zhaozhong Feng, Yue Xin, and Håkan Pleijel. 2015. “Ozone Effects on Wheat Grain Quality - A Summary.” Environmental Pollution 197:203–13. [CrossRef]
- Calderini, Daniel F., and Ivan Ortiz-Monasterio. 2003. “Crop Physiology & Metabolism: Grain Position Affects Grain Macronutrient and Micronutrient Concentrations in Wheat.” Crop Science 43 (1): 141–51.
- Caporaso, Nicola, Martin B. Whitworth, and Ian D. Fisk. 2018. “Near-Infrared Spectroscopy and Hyperspectral Imaging for Non-Destructive Quality Assessment of Cereal Grains.” Applied Spectroscopy Reviews 53 (8): 667–87. [CrossRef]
- Cohen, Itay, Sara I. Zandalinas, Clayton Huck, Felix B. Fritschi, and Ron Mittler. 2021. “Meta-Analysis of Drought and Heat Stress Combination Impact on Crop Yield and Yield Components.” Physiologia Plantarum 171 (1): 66–76. [CrossRef]
- Dolferus, Rudy, Xuemei Ji, and Richard A. Richards. 2011. “Abiotic Stress and Control of Grain Number in Cereals.” Plant Science 181 (4): 331–41. [CrossRef]
- Emberson, Lisa D., Håkan Pleijel, Elizabeth A. Ainsworth, Maurits van den Berg, Wei Ren, Stephanie Osborne, Gina Mills, et al. 2018. “Ozone Effects on Crops and Consideration in Crop Models.” European Journal of Agronomy 100:19–34. [CrossRef]
- Feng, Fan, Yunliang Han, Shengnan Wang, Shaojing Yin, Zhenyu Peng, Min Zhou, Wenqi Gao, Xiaoxia Wen, Xiaoliang Qin, and Kadambot H.M. Siddique. 2018. “The Effect of Grain Position on Genetic Improvement of Grain Number and Thousand Grain Weight in Winter Wheat in North China.” Frontiers in Plant Science 9 (February): 1–9. [CrossRef]
- Feng, Zhao Zhong, Kazuhiko Kobayashi, Xiao Ke Wang, and Zong Wei Feng. 2009. “A Meta-Analysis of Responses of Wheat Yield Formation to Elevated Ozone Concentration.” Chinese Science Bulletin 54 (2): 249–55. [CrossRef]
- Feng, Zhaozhong, and Kazuhiko Kobayashi. 2009. “Assessing the Impacts of Current and Future Concentrations of Surface Ozone on Crop Yield with Meta-Analysis.” Atmospheric Environment 43 (8): 1510–19. [CrossRef]
- Feng, Zhaozhong, Liang Wang, Håkan Pleijel, Jianguo Zhu, and Kazuhiko Kobayashi. 2016. “Differential Effects of Ozone on Photosynthesis of Winter Wheat among Cultivars Depend on Antioxidative Enzymes Rather than Stomatal Conductance.” Science of the Total Environment 572:404–11. [CrossRef]
- Fischer, R. A. 2011. “Wheat Physiology: A Review of Recent Developments.” Crop and Pasture Science 62 (2): 95–114. [CrossRef]
- Fleming, Zoë L., Ruth M. Doherty, Erika Von Schneidemesser, Christopher S. Malley, Owen R. Cooper, Joseph P. Pinto, Augustin Colette, et al. 2018. “Tropospheric Ozone Assessment Report: Present-Day Ozone Distribution and Trends Relevant to Human Health.” Elem Sci Anth 6:1–41. [CrossRef]
- Flintham, J. E., A. Börner, A. J. Worland, and M. D. Gale. 1997. “Optimizing Wheat Grain Yield: Effects of Rht (Gibberellin-Insensitive) Dwarfing Genes.” Journal of Agricultural Science 128 (1): 11–25. [CrossRef]
- Fox, J, and S Weisberg. 2011. An R Companion to Applied Regression. 2nd ed. Sage Publications.
- Gelang, Johanna, Håkan Pleijel, Ebe Sild, Helena Danielsson, Suhaila Younis, and Gun Selldén. 2000. “Rate and Duration of Grain Filling in Relation to Flag Leaf Senescence and Grain Yield in Spring Wheat (Triticum Aestivum) Exposed to Different Concentrations of Ozone.” Physiologia Plantarum 110 (3): 366–75. [CrossRef]
- González, Fernanda G., Daniel J. Miralles, and Gustavo A. Slafer. 2011. “Wheat Floret Survival as Related to Pre-Anthesis Spike Growth.” Journal of Experimental Botany 62 (14): 4889–4901. [CrossRef]
- Gooding, Mike J., R. H. Ellis, P. R. Shewry, and J. D. Schofield. 2003. “Effects of Restricted Water Availability and Increased Temperature on the Grain Filling, Drying and Quality of Winter Wheat.” Journal of Cereal Science 37 (3): 295–309. [CrossRef]
- Griffiths, Simon, Luzie Wingen, Julian Pietragalla, Guillermo Garcia, Ahmed Hasan, Daniel Miralles, Daniel F. Calderini, et al. 2015. “Genetic Dissection of Grain Size and Grain Number Trade-Offs in CIMMYT Wheat Germplasm.” PLoS ONE 10 (3): 1–18. [CrossRef]
- Grulke, N. E., and R. L. Heath. 2020. “Ozone Effects on Plants in Natural Ecosystems.” Plant Biology 22 (S1): 12–37. [CrossRef]
- Guo, Zifeng, and Thorsten Schnurbusch. 2015. “Variation of Floret Fertility in Hexaploid Wheat Revealed by Tiller Removal.” Journal of Experimental Botany 66 (19): 5945–58. [CrossRef]
- Harmens, Harry, Felicity Hayes, Katrina Sharps, Alan Radbourne, and Gina Mills. 2019. “Can Reduced Irrigation Mitigate Ozone Impacts on an Ozone-Sensitive African Wheat Variety?” Plants 8 (7): 1–17. [CrossRef]
- Hawkesford, Malcolm J., Jose Luis Araus, Robert Park, Daniel Calderini, Daniel Miralles, Tianmin Shen, Jianping Zhang, and Martin A.J. Parry. 2013. “Prospects of Doubling Global Wheat Yields.” Food and Energy Security 2 (1): 34–48. [CrossRef]
- Hewitt, D. K.L., G. Mills, F. Hayes, and W. Davies. 2016. “The Climate Benefits of High-Sugar Grassland May Be Compromised by Ozone Pollution.” Science of the Total Environment 565:95–104. [CrossRef]
- Ho, Lim C., and Roger M. Gifford. 1984. “Accumulation and Conversion of Sugars by Developing Wheat Grains: V. The Endosperm Apoplast and Apoplastic Transport.” Journal of Experimental Botany 35 (1): 58–73. [CrossRef]
- Hothorn, Torsten, Frank Bretz, and Peter Westfall. 2008. “Simultaneous Inference in General Parametric Models.” Biometrical Journal 50 (3): 346–63. [CrossRef]
- Hughes, Nathan, Karen Askew, Callum P. Scotson, Kevin Williams, Colin Sauze, Fiona Corke, John H. Doonan, and Candida Nibau. 2017. “Non-Destructive, High-Content Analysis of Wheat Grain Traits Using X-Ray Micro Computed Tomography.” Plant Methods 13 (1): 1–16. [CrossRef]
- Ji, Xuemei, Baodi Dong, Behrouz Shiran, Mark J. Talbot, Jane E. Edlington, Trijntje Hughes, Rosemary G. White, Frank Gubler, and Rudy Dolferus. 2011. “Control of Abscisic Acid Catabolism and Abscisic Acid Homeostasis Is Important for Reproductive Stage Stress Tolerance in Cereals.” Plant Physiology 156 (2): 647–62. [CrossRef]
- Ji, Xuemei, Behrouz Shiran, Jianlin Wan, David C. Lewis, Colin L.D. Jenkins, Anthony G. Condon, Richard A. Richards, and Rudy Dolferus. 2010. “Importance of Pre-Anthesis Anther Sink Strength for Maintenance of Grain Number during Reproductive Stage Water Stress in Wheat.” Plant, Cell and Environment 33 (6): 926–42. [CrossRef]
- Jiang, Yi, Marcus D. Hanwell, Elliot Padgett, Shawn Waldon, David A. Muller, and Robert Hovden. 2016. “Advanced Platform for 3D Visualization, Reconstruction, and Segmentation with Electron Tomography.” Microscopy and Microanalysis 22 (S3): 2070–71. [CrossRef]
- Johnston, A.E., and P.R. Poulton. 2018. “The Importance of Long-term Experiments in Agriculture: Their Management to Ensure Continued Crop Production and Soil Fertility; the Rothamsted Experience.” European Journal of Soil Science 69:113–25.
- Knox, Jerry, Andre Daccache, Tim Hess, and David Haro. 2016. “Meta-Analysis of Climate Impacts and Uncertainty on Crop Yields in Europe.” Environmental Research Letters 11 (11): 1–10. [CrossRef]
- Le, Thang Duong Quoc, Camille Alvarado, Christine Girousse, David Legland, and Anne-Laure Chateigner-Boutin. 2019. “Use of X-Ray Micro Computed Tomography Imaging to Analyze the Morphology of Wheat Grain through Its Development.” Plant Methods 15 (1): 1–19. [CrossRef]
- Leisner, Courtney P., and Elizabeth A. Ainsworth. 2012. “Quantifying the Effects of Ozone on Plant Reproductive Growth and Development.” Global Change Biology 18 (2): 606–16. [CrossRef]
- Microsoft Corporation. 2016. “Microsoft Excel 2016.”.
- Millet, E., and M. J. Pinthus. 1984. “The Association between Grain Volume and Grain Weight in Wheat.” Journal of Cereal Science 2 (1): 31–35. [CrossRef]
- Mills, Gina, Katrina Sharps, David Simpson, William J Davies, Malin Broberg, Johan Uddling, Fernando Jaramillo, et al. 2018. “Ozone Pollution Will Compromise Efforts to Increase Global Wheat Production.” Global Change Biology 24 (8): 3560–74. [CrossRef]
- Parish, Roger W., Huy A. Phan, Sylvana Iacuone, and Song F. Li. 2012. “Tapetal Development and Abiotic Stress: A Centre of Vulnerability.” Functional Plant Biology 39 (7): 553–59. [CrossRef]
- Philipp, Norman, Heiko Weichert, Utkarsh Bohra, Winfriede Weschke, Albert Wilhelm Schulthess, and Hans Weber. 2018. “Grain Number and Grain Yield Distribution along the Spike Remain Stable despite Breeding for High Yield in Winter Wheat.” PLoS ONE 13 (10): 1–17. [CrossRef]
- Pinheiro, J, D Bates, S DebRoy, D Sarkar, and & R Core Team. 2017. “Nlme: Linear and Non Linear Mixed Effect Models. R Package Version 3.1-131.”.
- Pleijel, H, H Danielsson, J Gelang, E Sild, and G Selldén. 1998. “Growth Stage Dependence of the Grain Yield Response to Ozone in Spring Wheat (Triticum Aestivum L.).” Agriculture, Ecosystems & Environment 70 (1): 61–68. [CrossRef]
- Pleijel, H., A. Berglen Eriksen, H. Danielsson, N. Bondesson, and G. Selldén. 2006. “Differential Ozone Sensitivity in an Old and a Modern Swedish Wheat Cultivar - Grain Yield and Quality, Leaf Chlorophyll and Stomatal Conductance.” Environmental and Experimental Botany 56:63–71. [CrossRef]
- Pleijel, H., L. Mortensen, J. Fuhrer, K. Ojanperä, and H. Danielsson. 1999. “Grain Protein Accumulation in Relation to Grain Yield of Spring Wheat (Triticum Aestivum L.) Grown in Open-Top Chambers with Different Concentrations of Ozone, Carbon Dioxide and Water Availability.” Agriculture, Ecosystems and Environment 72 (3): 265–70. [CrossRef]
- Pleijel, Håkan, Malin C. Broberg, Johan Uddling, and Gina Mills. 2018. “Current Surface Ozone Concentrations Significantly Decrease Wheat Growth, Yield and Quality.” Science of the Total Environment 613–614:687–92. [CrossRef]
- Pleijel, Håkan, and Johan Uddling. 2012. “Yield vs. Quality Trade-Offs for Wheat in Response to Carbon Dioxide and Ozone.” Global Change Biology 18 (2): 596–605. [CrossRef]
- R Core Team. 2020. “R: A Language and Environment for Statistical Computing.” R Foundation for Statistical Computing, Vienna, Austria. 2020. http://www.r-project.org.
- Rasheed, Awais, Francis C. Ogbonnaya, Evans Lagudah, Rudi Appels, and Zhonghu He. 2018. “The Goat Grass Genome’s Role in Wheat Improvement.” Nature Plants 4:56–58. [CrossRef]
- Rasheed, Awais, Xianchun Xia, Francis Ogbonnaya, Tariq Mahmood, Zongwen Zhang, Abdul Mujeeb-Kazi, and Zhonghu He. 2014. “Genome-Wide Association for Grain Morphology in Synthetic Hexaploid Wheats Using Digital Imaging Analysis.” BMC Plant Biology 14 (1): 1–21. [CrossRef]
- Reynolds, Matthew, David Bonnett, Scott C. Chapman, Robert T. Furbank, Yann Manés, Diane E. Mather, and Martin A.J. Parry. 2011. “Raising Yield Potential of Wheat. I. Overview of a Consortium Approach and Breeding Strategies.” Journal of Experimental Botany 62 (2): 439–52. [CrossRef]
- Reynolds, Matthew P., Janet M. Lewis, Karim Ammar, Bhoja R. Basnet, Leonardo Crespo-Herrera, José Crossa, Kanwarpal S. Dhugga, et al. 2021. “Harnessing Translational Research in Wheat for Climate Resilience.” Journal of Experimental Botany 72 (14): 5134–57. [CrossRef]
- Royal Society. 2008. Ground-Level Ozone in the 21st Century: Future Trends, Impacts and Policy Implications. Science Policy Report - The Royal Society. London: The Royal Society.
- Sarkar, Abhijit, and S. B. Agrawal. 2010. “Elevated Ozone and Two Modern Wheat Cultivars: An Assessment of Dose Dependent Sensitivity with Respect to Growth, Reproductive and Yield Parameters.” Environmental and Experimental Botany 69 (3): 328–37. [CrossRef]
- Schmidt, Jessica, Joelle Claussen, Norbert Wörlein, Anja Eggert, Delphine Fleury, Trevor Garnett, and Stefan Gerth. 2020. “Drought and Heat Stress Tolerance Screening in Wheat Using Computed Tomography.” Plant Methods 16 (1): 1–12. [CrossRef]
- Sehgal, Akanksha, Kumari Sita, Kadambot H.M. Siddique, Rakesh Kumar, Sailaja Bhogireddy, Rajeev K. Varshney, Bindumadhava HanumanthaRao, Ramakrishnan M. Nair, P. V.Vara Prasad, and Harsh Nayyar. 2018. “Drought or/and Heat-Stress Effects on Seed Filling in Food Crops: Impacts on Functional Biochemistry, Seed Yields, and Nutritional Quality.” Frontiers in Plant Science 871 (November): 1–19. [CrossRef]
- Singh, Aditya Abha, Adeeb Fatima, Amit Kumar Mishra, Nivedita Chaudhary, Arideep Mukherjee, Madhoolika Agrawal, and Shashi Bhushan Agrawal. 2018. “Assessment of Ozone Toxicity among 14 Indian Wheat Cultivars under Field Conditions: Growth and Productivity.” Environmental Monitoring and Assessment 190:1–14. [CrossRef]
- Soja, G., J. D. Barnes, M. Posch, K. Vandermeiren, H. Pleijel, and G. Mills. 2000. “Phenological Weighting of Ozone Exposures in the Calculation of Critical Levels for Wheat, Bean and Plantain.” Environmental Pollution 109 (3): 517–24. [CrossRef]
- Sreenivasulu, Nese, and Thorsten Schnurbusch. 2012. “A Genetic Playground for Enhancing Grain Number in Cereals.” Trends in Plant Science 17 (2): 91–101. [CrossRef]
- Strange, Harry, Reyer Zwiggelaar, Craig Sturrock, Sacha J. Mooney, and John H. Doonan. 2015. “Automatic Estimation of Wheat Grain Morphometry from Computed Tomography Data.” Functional Plant Biology 42 (5): 452–59. [CrossRef]
- Tomás, Diana, José Carlos Rodrigues, Wanda Viegas, and Manuela Silva. 2020. “Assessment of High Temperature Effects on Grain Yield and Composition in Bread Wheat Commercial Varieties.” Agronomy 10 (4): 1–12. [CrossRef]
- Tracy, Saoirse R., José Fernández Gómez, Craig J. Sturrock, Zoe A. Wilson, and Alison C. Ferguson. 2017. “Non-Destructive Determination of Floral Staging in Cereals Using X-Ray Micro Computed Tomography (ΜCT).” Plant Methods 13 (1): 1–12. [CrossRef]
- Ullah, Aman, Faisal Nadeem, Ahmad Nawaz, Kadambot H.M. Siddique, and Muhammad Farooq. 2021. “Heat Stress Effects on the Reproductive Physiology and Yield of Wheat.” Journal of Agronomy and Crop Science 00 (January): 1–17. [CrossRef]
- United States Department of Agriculture - Economic Research Service. 2022. “Wheat Data.” 2022. https://www.ers.usda.gov/data-products/wheat-data/.
- Wang, Wenbo, and Jitendra Paliwal. 2007. “Near-Infrared Spectroscopy and Imaging in Food Quality and Safety.” Sensing and Instrumentation for Food Quality and Safety 1 (4): 193–207. [CrossRef]
- Wang, Yunxia, and Michael Frei. 2011. “Stressed Food - The Impact of Abiotic Environmental Stresses on Crop Quality.” Agriculture, Ecosystems and Environment 141 (3–4): 271–86. [CrossRef]
- Zadoks, J.C., T.T. Chang, and C.F. Konzak. 1974. “A Decimal Code for the Growth Stages of Cereals.” Weed Research 14:415–21.
- Zhou, Hu, Andrew B. Riche, Malcolm J. Hawkesford, William R. Whalley, Brian S. Atkinson, Craig J. Sturrock, and Sacha J. Mooney. 2021. “Determination of Wheat Spike and Spikelet Architecture and Grain Traits Using X-Ray Computed Tomography Imaging.” Plant Methods 17 (1): 1–9. [CrossRef]

| Line | Name used in text | Source | Datasets presented |
| T. aestivum L., cv. Paragon (spring); released 1999 | Paragon (Par) | RAGT Seeds | Yield data, grain number per spikelet, grain morphology, grain quality, leaf chlorophyll |
| NIAB – SHW – BC- 181 – 1 | BC1 | NIAB | |
| NIAB – SHW – 027 | Primary synthetic hexaploid wheat (SHW) | NIAB | Yield data, grain quality, leaf chlorophyll |
| NIAB – SHW – BC- 181 - 5 | BC5 | NIAB |
Shoot/Root and seedhead biomass only (See supplementary files) |
| NIAB – SHW – BC- 181 - 7 | BC7 | NIAB | |
| NIAB – SHW – BC- 181 - 3 | BC3 | NIAB | |
| NIAB – SHW – BC- 181 - 4 | BC4 | NIAB | |
| NIAB – SHW – BC- 181 - 6 | BC6 | NIAB | |
| NIAB – SHW – BC- 181 - 8 | BC8 | NIAB | |
| NIAB – SHW – BC- 181 - 9 | BC9 | NIAB | |
| NIAB – SHW – BC- 181 - 10 | BC10 | NIAB |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





