1. Introduction
Advancements in artificial insemination (AI) within the swine industry have led to enhancements in the quantitative assessment of boar-produced spermatozoa quality, aiding in the prediction of seminal doses fertility potential [
1]. The enhancing sperm quality holds economic advantages for insemination centers [
2] and contributes to the profitability of swine-production industry [
3]. The consistent production of high-quality semen by genetically superior animals is a critical determinant of success for centers of artificial insemination (AI) [
4,
5]. In contemporary swine management practices, the elimination of breeding boars showing inferior semen quality is routine to uphold optimal reproductive outcomes.
The semen analyses may determine the semen quality, male fertility potential, , and potential causes of infertility are determined; using conventional methods such as spermatozoa counts [
6] and assessing sperm swimming patterns [
7] such as total and progressive motility and sperm kinematics [
8,
9,
10] by CASA systems [
11,
12].
Sexual maturity in boars can be affected by factors such as breed and environment [
13,
14,
15,
16]. Studies indicate that maximum semen quality is typically achieved in boars aged 24–29 months in temperate zones [
17]. Age or season variation influence in hormone levels and have been observed to impact boar semen quality [
14,
16,
18], and sperm production [
19]. Some studies in farm animals have explored and have highlighted the effect of male age on semen characteristics [
20,
21,
22,
23], consequently, male age could influence of semen quality. For example, in canine semen, older males often show reduced sperm motility [
24]. Similarly, in older bulls, the age has been correlated with decreases on ejaculates volume, semen concentration, and total sperm production [
25,
26].
Advanced male age is consistently linked with a notable decline in various semen parameters, including semen volume, concentration, motility, morphology, and viability [
27,
28]. Among the mechanisms proposed to understand how sperm motility varies include a dysfunction of the accessory sex glands and epididymis leading to impaired semen motility [
29]. Collectively, these factors contribute to impaired spermatogenesis in older animals, potentially resulting in swimming patterns abnormalities in their sperm [
30]. Age and seasonal changes significantly impact the quality of boar semen [
14,
18,
31]. Reproductive seasonality, influenced by factors such as photoperiod and temperature, profoundly affects boar semen quality. Numerous studies have explored the impact of seasonal variations on boar semen quality [
32,
33,
34,
35,
36,
37].
The CASA systems use sperm counting chamber for assessments [
38]. However, variations in results that may lead to errors can occur, such as when using different types of sperm counting chambers for analysis [
39,
40,
41,
42]. There are various types of counting sperm chamber available for use with CASA systems [
43,
44,
45]. Additionally, there is variability in the design, shape, and size of the chambers [
39,
46]. These instruments may influence the eventual semen analysis, affecting sperm dynamics in different ways, leading to divergent output values.
To improve understanding of the physiological characteristics of sperm, and swimming patterns of spermatozoa, the effect of breed, genetic lines, seasonal changes [
47] and intrinsic boar traits, is essential for optimizing reproductive efficiency and perpetuating the most favorable traits in semen quality [
48]. Therefore, the aim of this investigation was to evaluate the seminal quality associated with age, season, breed, and sperm counting chamber in boar ejaculates.
2. Materials and Methods
The study was conducted in compliance with laws and regulations for conducting experiments on live animals in Costa Rica. In this investigation, animals were handled with care to avoid any unnecessary stress and conformed with the animal welfare guidelines of the Costa Rica Institute of Technology. This study was conducted following ethical principles and with the approval of the Committee of the Research and Development Center for Sustainable Agriculture in the Humid Tropics of the Costa Rica Institute of Technology (CIDASTH-ITCR) Section 22/2022, Article 6.0, DAGSC-262-2022. All experiments were conducted in accordance with relevant guidelines and regulations. The study was carried out in compliance with the ARRIVE guidelines (
https://arriveguidelines.org/).
2.1. Study Site
The experiment was carried on two pig farms (commercial) located in Alajuela and Heredia (provinces in Costa Rica). Agropecuaria Los Sagitarios S.A., located in Alajuela, Costa Rica, is situated in the Northwest region of the country (Río Cuarto, 10°20′32″ N, 84°12′55″ W) and the farm Mejoramiento Porcino S.A., located in Heredia, Costa Rica, is also in the Northwest region (San José de la Montaña, 10°3′0'' N, 84°7′0'' W, Barva, Heredia, Costa Rica). In this area, the influence of the rainy season is from May to October and the dry season is from November to April.
At the time of research, both farms had a valid veterinary operation certificate (CVO), and animal health was monitored through vaccinations and deworming treatments. Comprehensive production records were maintained for all animals. The study was conducted from June 2022 to June 2023.
2.2. Animals
In this study, 22 sexually mature and healthy boars of the Duroc (n=5), Landrace (n=2), Pietrain (n=6), and Yorkshire(n=1) breeds, as well as boars from a commercial sire line (LT: Duroc x Pietrain; n=8), with an average age of 21.0 ± 7.2 months at the start of the experiment and known fertility, were used as semen donors. At least two ejaculates per boar were utilized (collected), totaling 45 ejaculates. The boars were grouped according to age into three categories: <12 months (n=5), 12-24 months (n=8), and >24 months (n=9).
Animals were individually housed in well-ventilated pens with an average temperature range of 17.5 to 23.6 °C throughout the experiment. They were fed a standard breeder mix containing soybean meal, corn, mineral mix, and common salt as ingredients to meet nutrient requirements of Swine [
49]. The boars had water ad libitum.
2.3. Semen Collection and Processing
Semen doses were collected using the "double-glove" technique [
50] and diluted 1:1 (v:v) with Androstar Plus
®, commercial diluent (Minitübe GmbH, Germany) after each extraction. Earlier each ejaculation, boars were stimulated by taking them to a separate extraction pen, which contained the extraction dummy, and semen was obtained by manual manipulation of the penis after the boar mounted the extraction dummy. The last three fractions of semen from each ejaculation were collected in graduated semen collection containers and filtered through 3 layers of sterilized gauze to separate the bulbourethral gland secretions from other semen constituents. After each semen collection, routine macroscopic evaluation was performed (volume, color, and consistency). A spectrophotometer was used for sperm concentration measurement following established protocols [
6]. An aliquot of diluted semen (1:1, v:v) less than 1 ml was taken using a Pasteur pipette and placed in a micro cuvette before measurement. After this evaluation, the filtered fraction was placed in a water bath (37°C), where the doses remained during the packaging and semen density determination period. Following the sperm concentration evaluation, the ejaculate was processed to prepare semen doses. One seminal dose from each ejaculate was transported to the laboratory under refrigeration conditions (17 °C) for analysis in a Dometic
® cooler (Lane Manufacturing Inc, Denver, CO, USA) and without exposure to light. Upon arrival at the laboratory, the samples were stored horizontally for 24 hours at 17 °C. Subsequently, the samples were to acclimate for 30 minutes in room laboratory (24°C), then placed in a 1.5 ml microtube (Eppendorf
®, Germany) on a heating plate at 37 °C for 30 minutes. Then, realized sperm analysis.
2.4. Semen Evaluation
Seminal doses from each ejaculation were assessed to determine motility and morphology, and only ejaculates with >75% morphologically normal spermatozoa were utilized. The evaluation of sperm morphology was conducted by the same technician, following strict criteria outlined by the WHO (World Health Organization) [
51]. For the analysis , 200 spermatozoa per slide were evaluated, with 100 spermatozoa assessed from each of two different areas on the slide. If the variation in the evaluation of morphology between the two areas was 5% or less, the mean value was calculated.
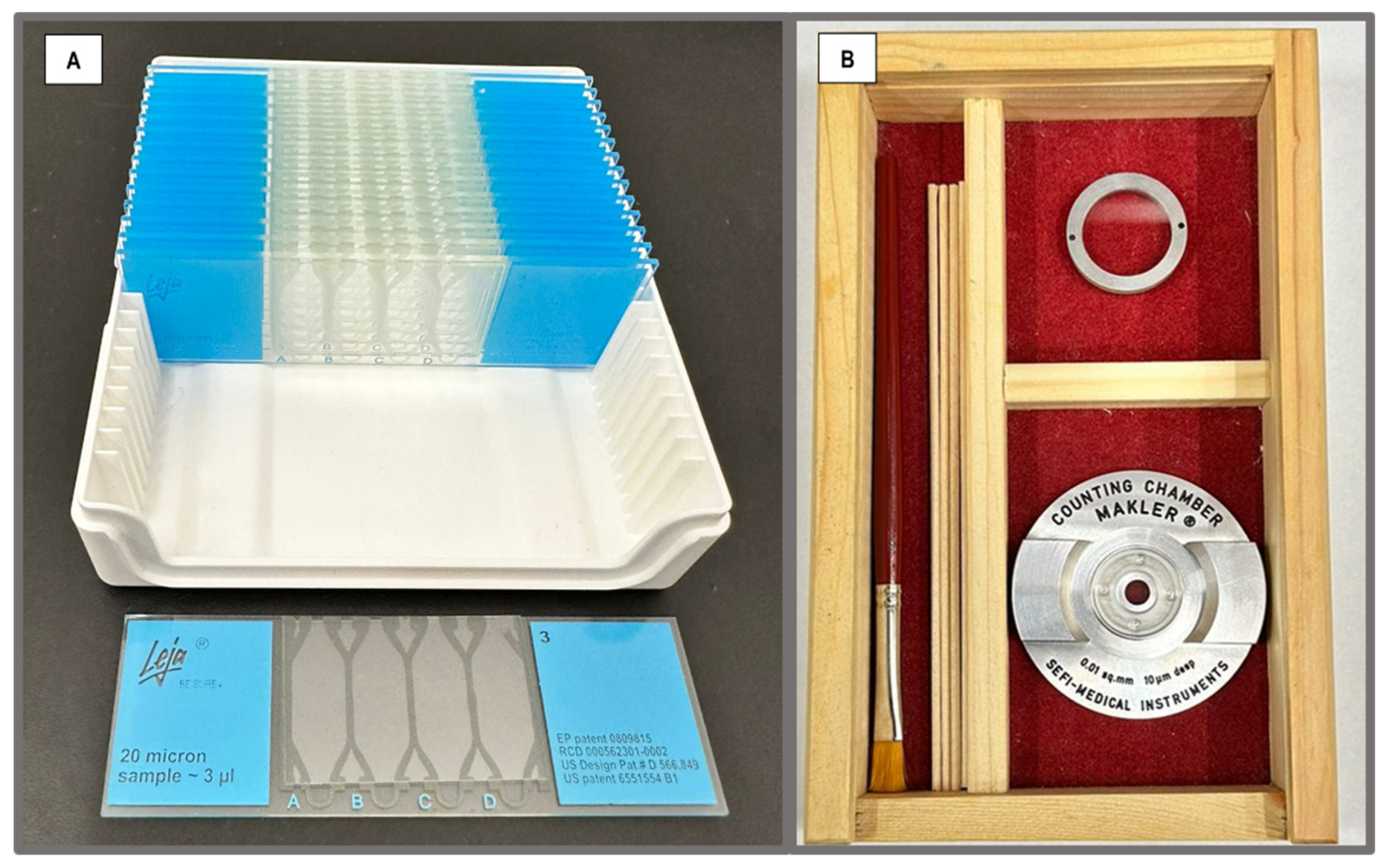
Sperm motility analysis was performed using disposable counting chambers: Leja
® (Microoptic, Barcelona, Spain), 20 μm deep with 4 counting areas (
Figure 1-A), and the reusable chamber; Makler
® (Sefi-Medical Instruments Ltd., Israel), 10 μm deep (
Figure 1-B); both preheated (37°C). After thorough mixing of diluted semen doses, in the Leja
® chamber, semen volume (2.7 µl) was capillary distributed along the counting chamber fields until completely filled, while in the Makler
® chamber, the diluted semen was drop-dispersed with an equivalent volume of 2.7 µl. Semen evaluation were conducted using the CASA-Mot (ISAS
® v1 system, Integrated Semen Analysis System, Proiser I+D company, Paterna, Spain), equipped with a video camera (Proiser 782M, Proiser I+D), capturing 25 frames per field at a frame rate of 50 Hz and a final resolution of 768 x 576 pixels. The camera was connected to a UB203 microscope (UOP/Proiser R+D) with a 1x eyepiece and a negative phase contrast objective of 10x (AN 0.25), and an integrated heated stage maintained at a constant temperature of 37.0 ± 0.5°C.
The percentage of total motile cells (TM) was defined as the percentage of motile cells exhibiting a curvilinear velocity (VCL) > 10 μm·s-1 within the sample. Progressive motility (PM, %) corresponded to spermatozoa exhibiting a fast forward swimming pattern in a straight line. The following parameters defined progressive motility: straightness (STR, linearity index) ≥45%, and average path velocity (VAP) ≥25 µm·s-1, defined as the mean velocity along the smoothed cell path. Non-progressive motile sperm (NPM) were motile spermatozoa with a prevalence of circular movements. Additionally, the percentages of static spermatozoa were determined. Progressive movement was defined as the percentage of spermatozoa exhibiting movement with a straightness index (STR) ≥ 75% within the sample. Static spermatozoa corresponded to cells exhibiting a curvilinear velocity (VCL) < 10 μm·s-1. Within motile spermatozoa, the percentage of cells with movement classified as fast, medium, and slow was determined according to the curvilinear velocity criterion (μm·s-1): 10 < slow < 25 < medium < 45 fast. Seminal analyses were performed in the Animal Reproduction Laboratory of the Center for Research and Development in Sustainable Agriculture for the Humid Tropics (CIDASTH), School of Agronomy at San Carlos Local Campus, Costa Rica Institute of Technology, Alajuela, Costa Rica.
2.5. Analysis of Sperm Kinematics Variables Using a CASA-Mot System
Sperm kinetic analysis was conducted by acquiring seven microscopic fields along the sperm counting chambers to achieve an average of six hundred spermatozoa per field. The variables evaluated by CASA-Mot system were: VSL (straight-line velocity, µm·s-1), corresponding to the speed of the sperm head along a straight line from the start to the end of the detected cell position; VCL (curvilinear velocity, µm·s-1), measured along the actual, point-by-point trajectory followed by the spermatozoon; VAP (average path velocity, µm·s-1), the average velocity along the smoothed cell path calculated as an interpolation between points corresponding to the VCL trajectory; ALH (lateral head displacement amplitude, µm), defined as the maximum (or mean) height of the head oscillation while the spermatozoon moves in a curvilinear trajectory; BCF (beat-cross frequency, Hz), defined as the frequency at which the actual (curvilinear) trajectory crosses the smoothed linear trajectory in any direction; motility (%), defined as the percentage of total mobile cells; and progressive motility (%), corresponding to spermatozoa rapidly moving forward in a straight line. Three progression ratios, expressed as percentages, were calculated from the velocity measurements described above: linearity of forward progression [LIN = (VSL/VCL) ·100], straightness index [STR = (VSL/VAP) ·100], and wobble index [WOB = (VAP/VCL) ·100]. The CASA software configuration was adjusted for boar sperm analysis, with a particle area ranging from 10 to 80 μm2 for the sperm head area and a connectivity of 11 μm.
2.6. Statistical Analysis
The assumptions of normality and homogeneity of variance were assessed using the Shapiro-Wilks and Levene tests, respectively. Normal probability plots were utilized to evaluate the normal distribution of all analyzed sperm variables. Analysis of variance (ANOVA) and generalized linear models were employed to ascertain the impact of age, genetic composition, and type of sperm counting chamber on sperm quality variables. The total sample space comprised 22 experimental units. Mean differences for the effects of age, genetic composition, and type of sperm counting chamber were analyzed using the Bonferroni test (P < 0.05). Results were expressed as mean ± standard error of the mean (SEM). Data analysis was performed using IBM SPSS version 23.0 for Windows (SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Motile and Swimming Patterns of Spermatozoa
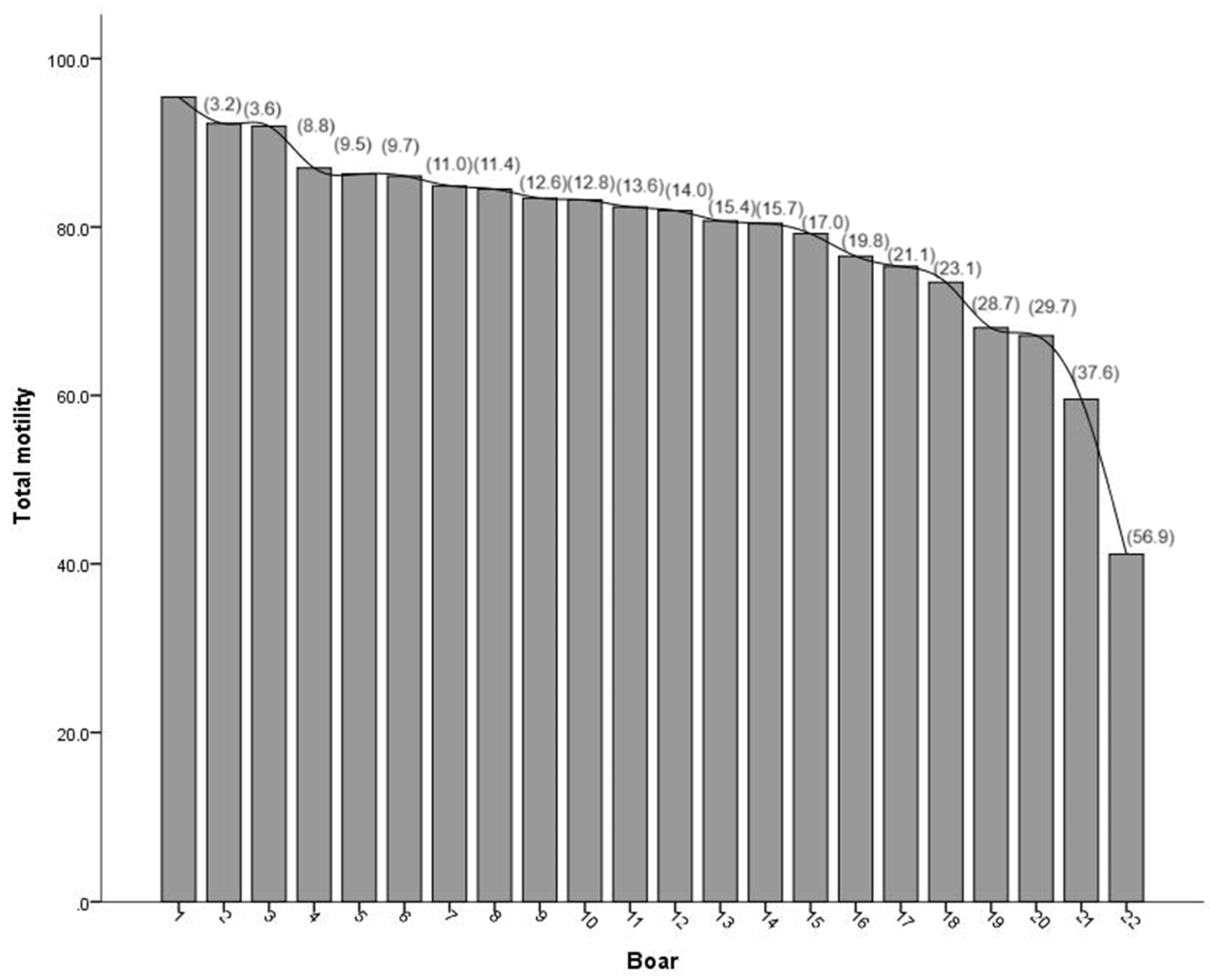
Total motility of boar ejaculates of different breeds was shown in
Figure 2. The highest values corresponded mostly to boars of the terminal sire lines (Duroc, Pietrain; boars: 1-7, 9-16, 19-22 ) compared to the maternal line males (Landrace and Yorkshire; boars: 8, 17, 18).
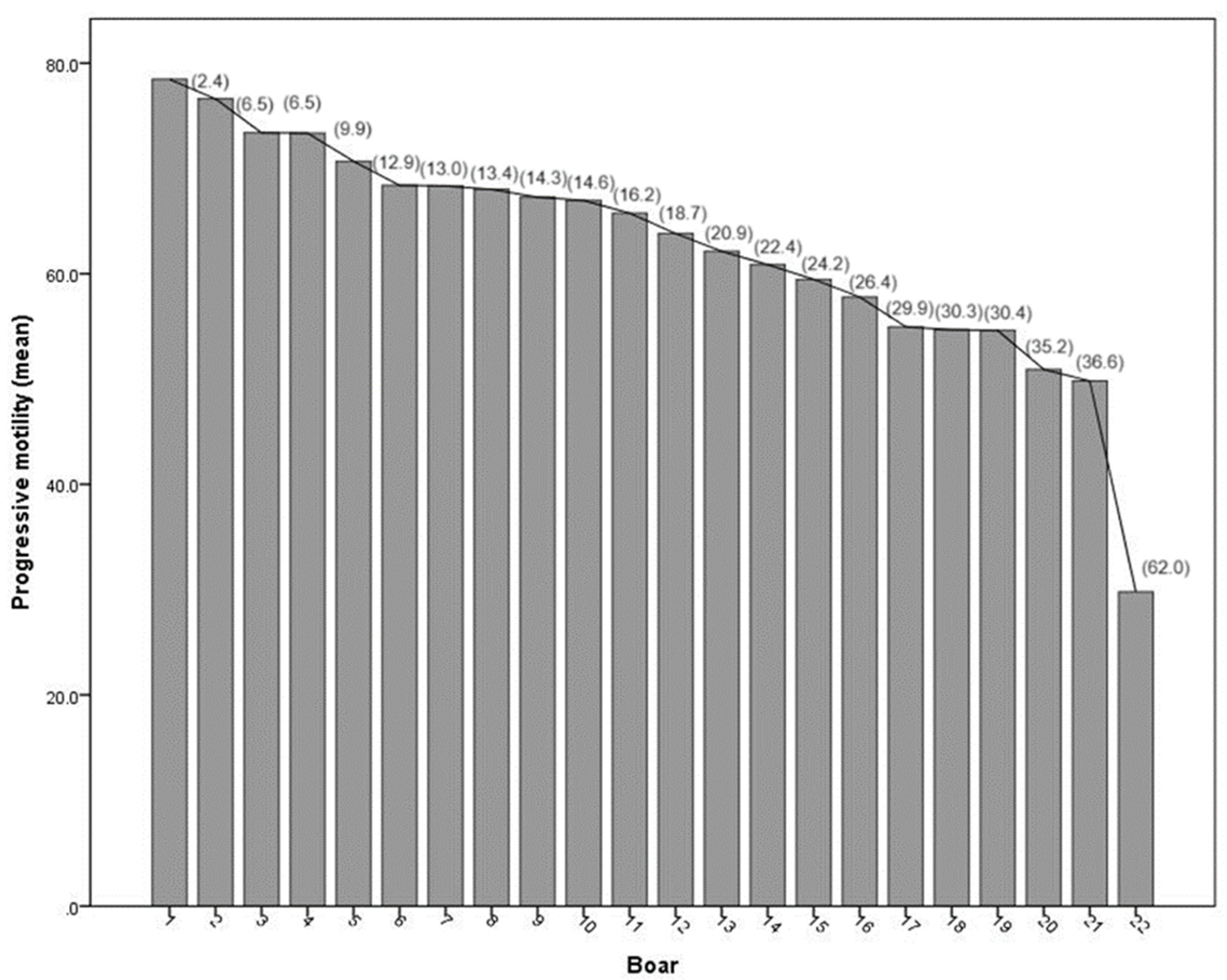
The progressive sperm motility values showed how younger boars had higher values than older boars (
Table 1). In addition, boars from sire lines, such as Duroc, Pietrain and LT (boars: 1, 2, 4-14, 17-22) had higher progressive motility values (
p < 0.05) than boars from maternal lines, such as Landrace and Yorkshire (boars: 15-16). However, maternal line boar 3 is an exception, showing higher values compared to other boars from both sire and maternal lines, excluding sire line boars 1 and 2 (
Figure 3).
Differences on total sperm motility and swimming patterns of boar age was observed (
p < 0.05;
Table 1). The ejaculates of youngest boars under 12 months old had a greater total motility and progressive motility, also the proportion of spermatozoa with fast movement than in older ages. Regarding the swimming patterns categories, boars aged under 12 months old exhibited the highest percentage of fast sperm (76.37 ± 1.47%). However, as age increased, there was a decrease in the value (%) of total and progressive motility (
Table 1).
Total sperm motility and swimming patterns were affected by season of the year (
p < 0.05). In the rainy season boar ejaculates had a greater total motility and proportion of spermatozoa classified with fast movement. No differences were found (
p > 0.05) between dry or rainy season, or age for progressive motility (
Table 1).
There was an effect of sperm counting chamber on the swimming patterns (
p < 0.05;
Table 2). Semen samples analyzed with the Makler
® chamber presented a greater total motility (%) compared with the semen samples analyzed with the Leja
® counting chamber. The progressive motility was higher in the Makler
® chamber (63.31 ± 1.21%) than in Leja
® Chamber (61.57 ± 1.09 %). Non-progressive motility was also higher in the Makler
® chamber (20.00% ± 0.41) than in the Leja
® chamber (15.53% ± 0.37). There were no significant differences between the two chambers for fast, average, and slow swimming patterns of sperm.
An effect of breed composition on seminal characteristics was observed (
p < 0.05). The Duroc and Landrace boars presented a greater total motility rate compared with the other breeds (
Table 3). The total motility was lower in the Yorkshire breed than in Duroc breed and Landrace breed (
p < 0.05). The sperm swimming characteristics indicated a higher proportion of fast spermatozoon in terminal sire breeds (Duroc, Pietrain) in comparison to maternal lines (Landrace and Yorkshire). No differences were found (
p >0.05) between boar breeds for the progressive motility of the ejaculates. There was a 28.4 % of difference in non-progressive motility of breed Duroc in relation to the Yorkshire breed.
3.2. Overall Kinematic Variables
There were differences (
p < 0.05) in the pairwise comparison by age category on these kinematic variables. Also, the interaction for breed*season*age was significant (
p < 0.05) for kinematics boar semen variables. The velocity variables (curvilinear velocity and average path velocity) were different (
p < 0.05) in all age groups (
Table 4). For curvilinear velocity, boars with >24 months age presented higher values than the other ages groups analyzed, and for linearity (LIN), boars in the <12 months age group exhibited the lowest value (44.00 ± 0.08 %) in relation to the boar with >24 months age (49.32 ± 0.08 %) and boars with 12-24 months (59.15 ± 0.18 %). Percentage of straightness index (STR) was higher in the 12-24 months group compared to other age groups <12 and >24 months old. There were differences between age groups for the WOB (sperm oscillation, %), where intermediate age group (12-24 months) was greater than <12 months (73.72 ± 0.13 %; 61.70 ± 0.05 % respectively). For the amplitude of the sperm head (ALH) the intermediate age groups presented lower values compared to the other groups of age. The crossover frequency (BCF), the boar group with more than 24 months presented lower values of Hz, compared to the group less than 12 months and intermediate age group although these differences were not considered biologically relevant. Found an effect of season on kinematics sperm variables analyzed (
p < 0.05). In the rainy season boar ejaculates had a lower (
p < 0.05) velocities, progressiveness, and oscillation of spermatozoa.
There was an effect of sperm counting chamber on the kinematic variables (
Table 5). The sperm analyzed with the Makler
® chamber showcased the highest VCL (69.73 ± 0.12 μm·s
-1) compared to the Leja
® chamber (63.90 ± 0.13 μm·s
-1). For VSL there were no differences (
p > 0.05) between sperm counting chamber. A superior average path velocity with the Makler
® chamber was determined for VAP kinematic variable compared to the Leja
® chamber (45.54 ± 0.08 μm·s
-1; 42.00 ± 0.09 μm·s
-1, respectively). For linearity (LIN), the Leja
® chamber exhibited the highest value (51.98 ± 0.08 %) compared to the Makler
® chamber (47.40 ± 0.08 %). The straightness index (% of STR) was higher in the sperm analyzed with Leja
® chamber (74.05 ± 0.10 %) compared to Duroc breed (65.75 ± 0.10 %). There were differences between all sperm counting chambers for the sperm oscillation (WOB), amplitude of the sperm head (ALH) and BCF, where the higher values were determined with de Makler
® sperm counting chamber.
There was an effect of genetic breed composition on the kinematic variables (
Table 6). Sire line breed Duroc x Pietrain (LT) showcased the highest VCL (72.80 ± 0.10 μm·s
-1), followed closely by Duroc (71.50 ± 0.13 μm·s
-1). The Landrace breed recorded the lowest VCL (55.96 ± 0.32 μm·s
-1), with Pietrain and Yorkshire manifesting intermediate velocities. For VSL and VAP kinematic variables the Pietrain breed exhibited the most accelerated linear progression (36.23 ± 0.10 μm·s
-1 and 48.50 ± 0.10 μm·s
-1 respectively). By contrast, Duroc displayed the most sluggish values of VSL and VAP (28.01 ± 0.08 μm·s
-1 and 41.60 ± 0.09 μm·s
-1, respectively). For linearity (LIN), the Landrace boars exhibited the highest value (64.35 ± 0.21 %). Duroc breed presented the least linear progression (40.87 ± 0.08 %), while LT and Yorkshire breeds presented intermediary linearity indices. The straightness index (STR) was higher in the Landrace breed (80.20 ± 0.24 %) compared to Duroc breed (65.75 ± 0.10 %). There were differences between all boars breeds for the sperm oscillation (WOB), where Landrace breed was greater than Duroc (77.86 ± 0.15 %; 59.31 ± 0.06 % respectively). For the amplitude of the sperm head (ALH) the Duroc breed presented higher values compared to the other breeds.
4. Discussion
In our study, we estimated the swimming patterns for multiple breeds (commercial dam and sire breeds) in different age cohorts, seasons and two sperm counting chambers. Studies of age on sperm quality in boar studs have covered a short timeframe, spanning from 8 months to 3 years of age, and generally, boars are replaced [
18,
52]. The high turnover rate of boars is attributed to various factors including the necessity for genetic diversity, suboptimal semen quality, foot and leg issues, as well as diminished health and libido beyond the age of 3-years-old [
14]. From our previous studies on the evaluation of boar breed composition on semen traits, it was found that there were no remarkable differences in the boar breeds. It is important to note that when studying the impact of male age on sperm production and quality in boars, several environmental variables such as season, breed, and nutrition can influence the results. Numerous studies, such as those by Banaszewska and Kondracki [
53] and Czubaszek [
54] have attempted to correlate these factors with sperm quality across different age groups. However, these studies often overlook the interaction between age and other influencing factors.
The CASA-mot system has enabled a comprehensive exploration into the motility characteristics of boar sperm and the tools used in the evaluation of semen in the laboratory such as sperm counting chambers under investigation [
12,
55,
56]. Several works have performed a detailed boar semen evaluation and artifacts such as, frame rate [
57], video capture time [
58] and sperm counting chamber height [
39,
45] analyzed by CASA systems. It was found that there were remarkable differences in the semen parameters of commercial breeds. This fact highlights the importance to define optimal conditions for boar semen analysis in the laboratory. The findings regarding total and progressive motile spermatozoa in boars seem to be inconsistent, as various studies have reported either no significant difference concerning the age of the boar or higher sperm motility in young or middle-aged boars [
59,
60]. Our work has demonstrated that in younger boars, sperm motility is better than in older animals; however, when analyzing sperm kinematics, boars of intermediate ages (12-24 months) exhibit better values of progressiveness than younger or older animals over 24 months of age.
Several studies highlighted that assessing sperm motility remains a crucial aspect in determining sperm fertility potential [
61,
62,
63,
64,
65,
66]. Our study unveiled that advanced age correlated with diminished sperm motility, contrasting the observed in younger counterparts. The assessment of spermatozoa progression and velocity offers precise insights into progressively rapid sperm, widely recognized as a key indicator when using sperm motility to predict fertilizing capacity [
8,
10,
67,
68,
69]. Furthermore, the analysis of sperm kinematic characteristics underscores the potential for objectively evaluating the quality profile of boar spermatozoa in different treatments and conditions [
61,
70]. Regarding sperm kinematics, significant differences were observed, particularly in velocity parameters (VCL, VSL, VAP) and progressiveness (LIN, STR), albeit primarily in younger boars regarding older boars. Sperm kinematics were higher in boar ejaculates of sire lines than maternal sire lines, with additional effects attributed to the age of boars used. These motility outcomes align with prior studies on the impact of different laboratory conditions on boar sperm analysis [
10,
71].
Additionally, elder boars exhibited compromised swimming patterns, evidenced by low levels of fast spermatozoa. While these findings may indicate the sperm quality of the boar reduces with age, differences could be attributed to variations in semen microbiota [
72]. Recent studies in microbiota analysis have unveiled that semen is not sterile, hinting at potential variations in semen microbiota across boars of varying reproductive ages [
73,
74]. These microbial variances carry the potential to influence semen antioxidant capacity, thereby impacting semen quality [
72,
73,
74,
75]. Some studies have been demonstrated presence of detrimental bacteria in the semen of aging boars, contributing to the observed decline in semen antioxidant capacity and overall semen quality, consequently impairing the fertility of the semen dose [
76,
77,
78].
Most disposable sperm counting chambers available in the market are based on the principle of capillary action loading and commonly include a sliding cover that is fixed with different types of adhesives [
38]. Other chambers are based on the principle of drop dispersion [
79]. In our study, we evaluated both types of counting sperm chambers and determined that motile and kinematics were higher when the Makler
® chamber (drop dispersion) was used compared to the Leja
® chamber (capillary action). However, when analyzing sperm progressive motility, higher values were observed with the Leja
® analysis chamber. Several instances have demonstrated improved analysis outcomes using drop-displacement and capillary chambers, as seen in studies conducted on rams [
80] and goats [
46]. These studies noted that drop-displacement chambers yielded better results in terms of motile parameters compared to capillary chambers [
46,
80] which coincides with results obtained in our work in boar semen. In terms of kinematics variables, our study has reported variation for drop- or capillarity-loaded chambers across the microscopic fields. Conversely, some studies have not reported significant differences between the two types of counting chambers, such as in boars [
81], fish [
82] and eels [
83,
84].
When CASA analysis is utilized, typically advocate for selecting the determination of semen quality that yields the highest sperm motility, kinematics, or straightest movement [
40]. Given that motility remains the primary criterion for assessing sperm quality in production facilities, we emphasize the importance of recognizing the dependency of CASA output on the type of viewing chamber [
43,
85]. If different chambers produce different values for the same sample, the spermatozoa movement may be somewhat inhibited by the chamber with lower values [
86]. Based on this premise and considering the two chambers we assessed, the Makler
® chamber yields superior results immediately after filling. However, for longer duration examinations on progressiveness or those conducted a few minutes after filling, the Leja
® chambers are recommended. Therefore, it is advisable for examiners to standardize their methods for the species they are evaluating before assessing semen samples.
5. Conclusions
A positive effect of younger age of boars was observed on the semen parameters of motility and faster swimming patterns, while seasonal variations also influenced motility positively. Duroc and Landrace breeds showed higher sperm motility, with sire line breeds displaying accelerated linear progression. The advanced age correlated with diminished sperm motility, highlighting the need for thorough evaluation, and monitoring of boar semen quality over time. The standardization of laboratory practices is necessary, particularly in assessing motility and kinematic variables using different sperm counting chambers.
Author Contributions
Conceptualization, A.V., M.A.S; methodology, F.S., L.M., A.V.; software, F.S., L.M., I.A.Z.; validation, F.S, A.V.; formal analysis, A.V.; investigation, F.S., L.M., I.A.Z.; resources, A.V.; data curation, L.M., F.S., I.A.Z; writing—original draft preparation, A.V., F.S.; writing—review and editing, A.V., F.S., A.S., M.A.S. B.V.; visualization, A.V.; supervision, A.V.; project administration, A.V.; funding acquisition, A.V., M.A.S.. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Costa Rica Institute of Technology (Vice-Chancellor’s office of Research and Extension; VIE (Vicerrectoría de Investigación y Extensión); Project 2151083) and Postgraduate Office of ITCR. The APC was funded by Costa Rica Institute of Technology (Vice-Chancellor’s office of Research and Extension; VIE (Vicerrectoría de investigación y Extensión) and by AGROALNEXT programme (Agroalnext/2022/063; European Union NextGenerationEU (PRTR-C17.I1), Generalitat Valenciana).
Institutional Review Board Statement
The use and care of animals in experimental treatments complied with the Costa Rica Institute of Technology animal welfare guidelines. Ethical approval has been given by the Committee of Centro de Investigación y Desarrollo de la Agricultura Sostenible para el Trópico Húmedo at the Costa Rica Institute of Technology (CIDASTH-ITCR) according to Section 08/2023, article 5.0, DAGSC-075-2023.
Informed Consent Statement
Not applicable for studies not involving humans.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Acknowledgments
The authors thank the Costa Rica Institute of Technology (ITCR) for financing this study. This work was supported Costa Rica Institute of Technology [Vice-Chancellor’s office of Research and Extension; VIE (Vicerrectoría de investigación y Extensión; Project 2151083)] and Postgraduate Office of ITCR. The funders had no role in the study’s design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Waberski, D.; Petrunkina, A.M.; Töpfer-Petersen, E. Can External Quality Control Improve Pig AI Efficiency? Theriogenology 2008, 70, 1346–1351. [Google Scholar] [CrossRef]
- Krupa, E.; Wolfová, M.; Krupová, Z.; Žáková, E. Estimation of Economic Weights for Number of Teats and Sperm Quality Traits in Pigs. Journal of Animal Breeding and Genetics 2020, 137, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Pena, D.; Knox, R. V.; Rodriguez-Zas, S.L. Contribution of Semen Trait Selection, Artificial Insemination Technique, and Semen Dose to the Profitability of Pig Production Systems: A Simulation Study. Theriogenology 2016, 85, 335–344. [Google Scholar] [CrossRef]
- Gonzalez-Peña, D.; Knox, R. V.; Pettigrew, J.; Rodriguez-Zas, S.L. Impact of Pig Insemination Technique and Semen Preparation on Profitability1. J Anim Sci 2014, 92, 72–84. [Google Scholar] [CrossRef]
- Okere, C.; Joseph, A.; Ezekwe, M. Seasonal and Genotype Variations in Libido, Semen Production and Quality in Artificial Insemination Boars. Journal of Animal and Veterinary Advances 2005, 4, 885–888. [Google Scholar]
- Sevilla, F.; Soler, C.; Araya-Zúñiga, I.; Barquero, V.; Roldan, E.R.S.; Valverde, A. Are There Differences between Methods Used for the Objective Estimation of Boar Sperm Concentration and Motility? Animals 2023, 13, 1622. [Google Scholar] [CrossRef]
- Calderón-Calderón, J.; Sevilla, F.; Roldan, E.R.S.; Barquero, V.; Valverde, A. Influence of Fat-Soluble Vitamin Intramuscular Supplementation on Kinematic and Morphometric Sperm Parameters of Boar Ejaculates. Front Vet Sci 2022, 9. [Google Scholar] [CrossRef]
- Barquero, V.; Roldan, E.R.S.; Soler, C.; Vargas-Leitón, B.; Sevilla, F.; Camacho, M.; Valverde, A. Relationship between Fertility Traits and Kinematics in Clusters of Boar Ejaculates. Biology (Basel) 2021, 10, 595. [Google Scholar] [CrossRef]
- Fair, S.; Romero-Aguirregomezcorta, J. Implications of Boar Sperm Kinematics and Rheotaxis for Fertility after Preservation. Theriogenology 2019, 137, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Barquero, V.; Soler, C.; Sevilla, F.; Calderón-Calderón, J.; Valverde, A. A Bayesian Analysis of Boar Spermatozoa Kinematics and Head Morphometrics and Their Relationship with Litter Size Fertility Variables. Reproduction in Domestic Animals 2021, 56, 1024–1033. [Google Scholar] [CrossRef] [PubMed]
- Madrigal-Valverde, M.; Bittencourt, R.F.; Brito, L.S.; Lents, M.P.; Santos, E.S.; Valverde-Abarca, A. Analysis of Testicular Variables, Semen Motility and Kinematics-derived Indexes in Boar Using a CASA-Mot System. Reproduction in Domestic Animals 2020, 55, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Yeste, M.; Bonet, S.; Rodríguez-Gil, J.E.; Álamo, M.M.R. Del; Rivera Del Álamo, M.M. Evaluation of Sperm Motility with CASA-Mot: Which Factors May Influence Our Measurements? Reprod Fertil Dev 2018, 30, 789–798. [Google Scholar] [CrossRef]
- Kunavongkrit, A.; Suriyasomboon, A.; Lundeheim, N.; Heard, T.W.; Einarsson, S. Management and Sperm Production of Boars under Differing Environmental Conditions. In Proceedings of the Theriogenology; Elsevier Inc., January 15 2005; Vol. 63; pp. 657–667. [Google Scholar]
- Huang, Y.H.; Lo, L.L.; Liu, S.H.; Yang, T.S. Age-related Changes in Semen Quality Characteristics and Expectations of Reproductive Longevity in Duroc Boars. Animal Science Journal 2010, 81, 432–437. [Google Scholar] [CrossRef]
- Yeste, M.; Sancho, S.; Briz, M.; Pinart, E.; Bussalleu, E.; Bonet, S. A Diet Supplemented with L-Carnitine Improves the Sperm Quality of Piétrain but Not of Duroc and Large White Boars When Photoperiod and Temperature Increase. Theriogenology 2010, 73, 577–586. [Google Scholar] [CrossRef]
- Kumaresan, A.; Bujarbaruah, K.M.; Kadirvel, G.; Khargharia, G.; Sarma, R.G.; Goswami, J.; Basumatary, R.; Palaniappan, K.; Bardoloi, R.K. Early Sexual Maturity in Local Boars of Northeastern India: Age-Related Changes in Testicular Growth, Epididymal Sperm Characteristics and Peripheral Testosterone Levels. Theriogenology 2011, 75, 687–695. [Google Scholar] [CrossRef]
- Kennedy, B.W.; Wilkins, J.N. BOAR, BREED AND ENVIRONMENTAL FACTORS INFLUENCING SEMEN CHARACTERISTICS OF BOARS USED IN ARTIFICIAL INSEMINATION. Can J Anim Sci 1984, 64, 833–843. [Google Scholar] [CrossRef]
- Tsakmakidis, I.A.; Khalifa, T.A.; Boscos, C.M. Age-Related Changes in Quality and Fertility of Porcine Semen. Biol Res 2012, 45, 381–386. [Google Scholar] [CrossRef]
- Park, C.S.; Yi, Y.J. Comparison of Semen Characteristics, Sperm Freezability and Testosterone Concentration between Duroc and Yorkshire Boars during Seasons. Anim Reprod Sci 2002, 73, 53–61. [Google Scholar] [CrossRef]
- Hallap, T.; Jaakma, Ü.; Rodriguez-Martinez, H. Changes in Semen Quality in Estonian Holstein AI Bulls at 3, 5 and 7 Years of Age. Reproduction in Domestic Animals 2006, 41, 214–218. [Google Scholar] [CrossRef]
- Hoflack, G.; Opsomer, G.; Rijsselaere, T.; Van Soom, A.; Maes, D.; De Kruif, A.; Duchateau, L. Comparison of Computer-assisted Sperm Motility Analysis Parameters in Semen from Belgian Blue and Holstein–Friesian Bulls. Reproduction in Domestic Animals 2007, 42, 153–161. [Google Scholar] [CrossRef]
- Carreira, J.T.; Trevizan, J.T.; Carvalho, I.R.; Kipper, B.; Rodrigues, L.H.; Silva, C.; Perri, S.H. V.; Drevet, J.R.; Koivisto, M.B. Does Sperm Quality and DNA Integrity Differ in Cryopreserved Semen Samples from Young, Adult, and Aged Nellore Bulls? Basic Clin Androl 2017, 27, 12. [Google Scholar] [CrossRef] [PubMed]
- Long, J.A.; Bongalhardo, D.C.; Pelaéz, J.; Saxena, S.; Settar, P.; O’Sullivan, N.P.; Fulton, J.E. Rooster Semen Cryopreservation: Effect of Pedigree Line and Male Age on Postthaw Sperm Function. Poult Sci 2010, 89, 966–973. [Google Scholar] [CrossRef]
- Goericke-Pesch, S.; Failing, K. Retrospective Analysis of Canine Semen Evaluations with Special Emphasis on the Use of the Hypoosmotic Swelling (HOS) Test and Acrosomal Evaluation Using Spermac ®. Reproduction in Domestic Animals 2013, 48, 213–217. [Google Scholar] [CrossRef]
- Kelso, K.A.; Redpath, A.; Noble, R.C.; Speake, B.K. Lipid and Antioxidant Changes in Spermatozoa and Seminal Plasma throughout the Reproductive Period of Bulls. Reproduction 1997, 109, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.M.; Kelly, A.K.; O’Meara, C.; Eivers, B.; Lonergan, P.; Fair, S. Influence of Bull Age, Ejaculate Number, and Season of Collection on Semen Production and Sperm Motility Parameters in Holstein Friesian Bulls in a Commercial Artificial Insemination Centre. J Anim Sci 2018, 96, 2408–2418. [Google Scholar] [CrossRef]
- Winkle, T.; Rosenbusch, B.; Gagsteiger, F.; Paiss, T.; Zoller, N. The Correlation between Male Age, Sperm Quality and Sperm DNA Fragmentation in 320 Men Attending a Fertility Center. J Assist Reprod Genet 2009, 26, 41–46. [Google Scholar] [CrossRef]
- Kipper, B.H.; Trevizan, J.T.; Carreira, J.T.; Carvalho, I.R.; Mingoti, G.Z.; Beletti, M.E.; Perri, S.H.V.; Franciscato, D.A.; Pierucci, J.C.; Koivisto, M.B. Sperm Morphometry and Chromatin Condensation in Nelore Bulls of Different Ages and Their Effects on IVF. Theriogenology 2017, 87, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Dowsett, K.F.; Knott, L.M. The Influence of Age and Breed on Stallion Semen. Theriogenology 1996, 46, 397–412. [Google Scholar] [CrossRef]
- Mandal, D.K.; Kumar, M.; Tyagi, S. Effect of Age on Spermiogram of Holstein Friesian × Sahiwal Crossbred Bulls. Animal 2010, 4, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Zasiadczyk, L.; Fraser, L.; Kordan, W.; Wasilewska, K. Individual and Seasonal Variations in the Quality of Fractionated Boar Ejaculates. Theriogenology 2015, 83, 1287–1303. [Google Scholar] [CrossRef] [PubMed]
- Flowers, W.L. Genetic and Phenotypic Variation in Reproductive Traits of AI Boars. Theriogenology 2008, 70, 1297–1303. [Google Scholar] [CrossRef]
- Ciereszko, A.; Ottobre, J.S.; Glogowski, J. Effects of Season and Breed on Sperm Acrosin Activity and Semen Quality of Boars. Anim Reprod Sci 2000, 64, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Knecht, D.; Środoń, S.; Duziński, K. The Influence of Boar Breed and Season on Semen Parameters. South African Journal of Animal Sciences 2014, 44, 1–9. [Google Scholar] [CrossRef]
- Knecht, D.; Jankowska-Mąkosa, A.; Duziński, K. The Effect of Age, Interval Collection and Season on Selected Semen Parameters and Prediction of AI Boars Productivity. Livest Sci 2017, 201, 13–21. [Google Scholar] [CrossRef]
- Fraser, L.; Strzeżek, J.; Filipowicz, K.; Mogielnicka-Brzozowska, M.; Zasiadczyk, L. Age and Seasonal-Dependent Variations in the Biochemical Composition of Boar Semen. Theriogenology 2016, 86, 806–816. [Google Scholar] [CrossRef] [PubMed]
- Savic, R.; Petrovic, M.; Radojkovic, D.; Radovic, C.; Parunovic, N. The Effect of Breed, Boar and Season on Some Properties of Sperm. Biotechnology in Animal Husbandry 2013, 29, 299–310. [Google Scholar] [CrossRef]
- Bompart, D.; García-Molina, A.; Valverde, A.; Caldeira, C.; Yániz, J.; Núñez de Murga, M.; Soler, C. CASA-Mot Technology: How Results Are Affected by the Frame Rate and Counting Chamber. Reprod Fertil Dev 2018, 30, 810. [Google Scholar] [CrossRef]
- Soler, C.; Picazo-Bueno, J.Á.; Micó, V.; Valverde, A.; Bompart, D.; Blasco, F.J.; Álvarez, J.G.; García-Molina, A. Effect of Counting Chamber Depth on the Accuracy of Lensless Microscopy for the Assessment of Boar Sperm Motility. Reprod Fertil Dev 2018, 30, 924. [Google Scholar] [CrossRef]
- Lenz, R.W.; Kjelland, M.E.; VonderHaar, K.; Swannack, T.M.; Moreno, J.F. A Comparison of Bovine Seminal Quality Assessments Using Different Viewing Chambers with a Computer-Assisted Semen Analyzer1. J Anim Sci 2011, 89, 383–388. [Google Scholar] [CrossRef]
- Hoogewijs, M.K.; De Vliegher, S.P.; Govaere, J.L.; De Schauwer, C.; De Kruif, A.; Van Soom, A. Influence of Counting Chamber Type on CASA Outcomes of Equine Semen Analysis. Equine Vet J 2012, 44, 542–549. [Google Scholar] [CrossRef]
- Gloria, A.; Carluccio, A.; Contri, A.; Wegher, L.; Valorz, C.; Robbe, D. The Effect of the Chamber on Kinetic Results in Cryopreserved Bull Spermatozoa. Andrology 2013, 1, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Nanni Peng, X.Z.; Li, L. Comparison of Different Counting Chambers Using a Computer-Assisted Semen Analyzer. Syst Biol Reprod Med 2015, 61, 307–313. [Google Scholar] [CrossRef]
- Bompart, D.; Vázquez, R.F.; Gómez, R.; Valverde, A.; Roldán, E.R.S.; García-Molina, A.; Soler, C. Combined Effects of Type and Depth of Counting Chamber, and Rate of Image Frame Capture, on Bull Sperm Motility and Kinematics. Anim Reprod Sci 2019, 209, 106169. [Google Scholar] [CrossRef]
- Valverde, A.; Madrigal Valverde, M.; Madrigal-Valverde, M. Assessment of Counting Chambers on Boar Sperm Parameters Analyzed by a CASA-Mot System. Agronomy Mesoamerican 2019, 30, 447–458. [Google Scholar] [CrossRef]
- Del Gallego, R.; Sadeghi, S.; Blasco, E.; Soler, C.; Yániz, J.L.; Silvestre, M.A. Effect of Chamber Characteristics, Loading and Analysis Time on Motility and Kinetic Variables Analysed with the CASA-Mot System in Goat Sperm. Anim Reprod Sci 2017, 177, 97–104. [Google Scholar] [CrossRef]
- Peña, S.T.; Gummow, B.; Parker, A.J.; Paris, D.B.B.P. Antioxidant Supplementation Mitigates DNA Damage in Boar (Sus Scrofa Domesticus) Spermatozoa Induced by Tropical Summer. PLoS One 2019, 14, e0216143. [Google Scholar] [CrossRef] [PubMed]
- Žaja, I.Ž.; Samardžija, M.; Vince, S.; Majić-Balić, I.; Vilić, M.; Đuričić, D.; Milinković-Tur, S. Influence of Boar Breeds or Hybrid Genetic Composition on Semen Quality and Seminal Plasma Biochemical Variables. Anim Reprod Sci 2016, 164, 169–176. [Google Scholar] [CrossRef] [PubMed]
- National Research Council Nutrient Requirements of Swine; National Academies Press, 2012;
- Hancock, J.; Hovell, G. The Collection of Boar Semen. Vet Rec 1959, 71, 664–665. [Google Scholar]
- World Health Organization Laboratory Manual for the Examination and Processing of Human Semen; Geneva, Ed.; Sixth Edit.; ISBN 978 92 4 0030787, 2021; 0030.
- Tsakmakidis, I.A.; Lymberopoulos, A.G.; Khalifa, T.A.A. Relationship between Sperm Quality Traits and Field-Fertility of Porcine Semen. J Vet Sci 2010, 11, 151. [Google Scholar] [CrossRef]
- Banaszewska, D.; Kondracki, S. An Assessment of the Breeding Maturity of Insemination Boars Based on Ejaculate Quality Changes. Folia Biol (Praha) 2012, 60, 151–162. [Google Scholar] [CrossRef]
- Czubaszek, M.; Andraszek, K.; Banaszewska, D. Influence of the Age of the Individual on the Stability of Boar Sperm Genetic Material. Theriogenology 2020, 147, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Yániz, J.L.; Silvestre, M.A.; Santolaria, P.; Soler, C. CASA-Mot in Mammals: An Update. Reprod Fertil Dev 2018, 30, 799. [Google Scholar] [CrossRef]
- Gallagher, M.T.; Smith, D.J.; Kirkman-Brown, J.C. CASA: Tracking the Past and Plotting the Future. Reprod Fertil Dev 2018, 30, 867. [Google Scholar] [CrossRef] [PubMed]
- Valverde, A.; Madrigal, M.; Caldeira, C.; Bompart, D.; de Murga, J.N.; Arnau, S.; Soler, C. Effect of Frame Rate Capture Frequency on Sperm Kinematic Parameters and Subpopulation Structure Definition in Boars, Analysed with a <scp>CASA</Scp> -Mot System. Reproduction in Domestic Animals 2019, 54, 167–175. [Google Scholar] [CrossRef]
- Valverde, A.; Madrigal-Valverde, M.; Lotz, J.; Bompart, D.; Soler, C. Effect of Video Capture Time on Sperm Kinematic Parameters in Breeding Boars. Livest Sci 2019, 220, 52–56. [Google Scholar] [CrossRef]
- Kondracki, S.; Bonaszewska, D.; Mielnicka, C. The Effect of Age on the Morphometric Sperm Traits of Domestic Pigs (Sus Scrofa Domestica). Cell Mol Biol Lett 2005, 10, 3–13. [Google Scholar] [PubMed]
- Wolf, J.; Smital, J. Quantification of Factors Affecting Semen Traits in Artificial Insemination Boars from Animal Model Analyses1. J Anim Sci 2009, 87, 1620–1627. [Google Scholar] [CrossRef]
- Holt, C.; Holt, W. V; Moore, H.D.; Reed, H.C.; Curnock, R.M. Objectively Measured Boar Sperm Motility Parameters Correlate with the Outcomes of On-Farm Inseminations: Results of Two Fertility Trials. J Androl 1997, 18, 312–323. [Google Scholar] [CrossRef]
- Tardif, S.; Laforest, J.-P.; Cormier, N.; Bailey, J.L. The Importance of Porcine Sperm Parameters on Fertility in Vivo. Theriogenology 1999, 52, 447–459. [Google Scholar] [CrossRef]
- De Ambrogi, M.; Ballester, J.; Saravia, F.; Caballero, I.; Johannisson, A.; Wallgren, M.; Andersson, M.; Rodriguez-Martinez, H. Effect of Storage in Short- and Long-term Commercial Semen Extenders on the Motility, Plasma Membrane and Chromatin Integrity of Boar Spermatozoa. Int J Androl 2006, 29, 543–552. [Google Scholar] [CrossRef]
- Maes, D.; Lopez Rodriguez, A.; Rijsselaere, T.; Vyt, P.; Van Soom, A. Artificial Insemination in Pigs. In Artificial Insemination in Farm Animals; InTech, 2011.
- Vyt, P.; Maes, D.; Quinten, C.; Rijsselaere, T.; Deley, W.; Aarts, M.; de Kruif, A.; Van Soom, A. Detailed Motility Evaluation of Boar Semen and Its Predictive Value for Reproductive Performance in Sows. Vlaams Diergeneeskd Tijdschr 2008, 77. [Google Scholar] [CrossRef]
- Vyt, P.; Maes, D.; Rijsselaere, T.; Dejonckheere, E.; Castryck, F.; Van Soom, A. Motility Assessment of Porcine Spermatozoa: A Comparison of Methods. Reproduction in Domestic Animals 2004, 39, 447–453. [Google Scholar] [CrossRef]
- Tremoen, N.H.; Gaustad, A.H.; Andersen-Ranberg, I.; van Son, M.; Zeremichael, T.T.; Frydenlund, K.; Grindflek, E.; Våge, D.I.; Myromslien, F.D. Relationship between Sperm Motility Characteristics and ATP Concentrations, and Association with Fertility in Two Different Pig Breeds. Anim Reprod Sci 2018, 193, 226–234. [Google Scholar] [CrossRef]
- Gadea, J.; Sellés, E.; Marco, M.A. The Predictive Value of Porcine Seminal Parameters on Fertility Outcome under Commercial Conditions. Reproduction in Domestic Animals 2004, 39, 303–308. [Google Scholar] [CrossRef]
- Santolaria, P.; Vicente-Fiel, S.; Palacín, I.; Fantova, E.; Blasco, M.E.; Silvestre, M.A.; Yániz, J.L. Predictive Capacity of Sperm Quality Parameters and Sperm Subpopulations on Field Fertility after Artificial Insemination in Sheep. Anim Reprod Sci 2015, 163, 82–88. [Google Scholar] [CrossRef]
- Boe-Hansen, G.B.; Satake, N. An Update on Boar Semen Assessments by Flow Cytometry and CASA. Theriogenology 2019, 137, 93–103. [Google Scholar] [CrossRef]
- Schulze, M.; Henning, H.; Rüdiger, K.; Wallner, U.; Waberski, D. Temperature Management during Semen Processing: Impact on Boar Sperm Quality under Laboratory and Field Conditions. Theriogenology 2013, 80, 990–998. [Google Scholar] [CrossRef]
- Li, D.; Xu, Y.; Wang, M.; Fang, S.; Li, S.H.; Cui, Y. Differences of Semen Microbiota among Breeding Boars with Different Reproductive Ages. J Anim Sci 2023, 101. [Google Scholar] [CrossRef] [PubMed]
- Baud, D.; Pattaroni, C.; Vulliemoz, N.; Castella, V.; Marsland, B.J.; Stojanov, M. Sperm Microbiota and Its Impact on Semen Parameters. Front Microbiol 2019, 10. [Google Scholar] [CrossRef]
- Dalmutt, A.C.; Moreno, L.Z.; Gomes, V.T.M.; Cunha, M.P. V.; Barbosa, M.R.F.; Sato, M.I.Z.; Knöbl, T.; Pedroso, A.C.; Moreno, A.M. Characterization of Bacterial Contaminants of Boar Semen: Identification by MALDI-TOF Mass Spectrometry and Antimicrobial Susceptibility Profiling. J Appl Anim Res 2020, 48, 559–565. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, L.; Li, J.; Li, H.; Hong, Z.; Xie, M.; Chen, S.; Yao, B. The Semen PH Affects Sperm Motility and Capacitation. PLoS One 2015, 10, e0132974. [Google Scholar] [CrossRef] [PubMed]
- Fraczek, M.; Szumala-Kakol, A.; Jedrzejczak, P.; Kamieniczna, M.; Kurpisz, M. Bacteria Trigger Oxygen Radical Release and Sperm Lipid Peroxidation in in Vitro Model of Semen Inflammation. Fertil Steril 2007, 88, 1076–1085. [Google Scholar] [CrossRef] [PubMed]
- McAnally, B.E.; Smith, M.S.; Wiegert, J.G.; Palanisamy, V.; Chitlapilly Dass, S.; Poole, R.K. Characterization of Boar Semen Microbiome and Association with Sperm Quality Parameters. J Anim Sci 2023, 101. [Google Scholar] [CrossRef]
- R. M. Jones; J. W. Mercante; A. S. Neish Reactive Oxygen Production Induced by the Gut Microbiota: Pharmacotherapeutic Implications. Curr Med Chem 2012, 19, 1519–1529. [Google Scholar] [CrossRef]
- Mrkun, J.; Kosec, M.; Zakosek, M.; Zrimsek, P. Method Agreement between Measuring of Boar Sperm Concentration Using Makler Chamber and Photometer. Acta Vet Brno 2007, 57, 563–572. [Google Scholar] [CrossRef]
- Palacín, I.; Vicente-Fiel, S.; Santolaria, P.; Yániz, J.L. Standardization of CASA Sperm Motility Assessment in the Ram. Small Ruminant Research 2013, 112, 128–135. [Google Scholar] [CrossRef]
- Gączarzewicz, D. Influence of Chamber Type Integrated with Computer-Assisted Semen Analysis (CASA) System on the Results of Boar Semen Evaluation. Pol J Vet Sci 2015, 18, 817–824. [Google Scholar] [CrossRef]
- Gallego, V.; Asturiano, J.F. Sperm Motility in Fish: Technical Applications and Perspectives through CASA-Mot Systems. Reprod Fertil Dev 2018, 30, 820. [Google Scholar] [CrossRef] [PubMed]
- Caldeira, C.; Hernández-Ibáñez, S.; Valverde, A.; Martin, P.; Herranz-Jusdado, J.G.; Gallego, V.; Asturiano, J.F.; Dzyuba, B.; Pšenička, M.; Soler, C. Standardization of Sperm Motility Analysis by Using CASA-Mot for Atlantic Salmon (Salmo Salar), European Eel (Anguilla Anguilla) and Siberian Sturgeon (Acipenser Baerii). Aquaculture 2019, 502, 223–231. [Google Scholar] [CrossRef]
- Gallego, V.; Carneiro, P.C.F.; Mazzeo, I.; Vílchez, M.C.; Peñaranda, D.S.; Soler, C.; Pérez, L.; Asturiano, J.F. Standardization of European Eel (Anguilla Anguilla) Sperm Motility Evaluation by CASA Software. Theriogenology 2013, 79, 1034–1040. [Google Scholar] [CrossRef]
- Valverde, A.; Areán, H.; Fernández, A.; Bompart, D.; García-Molina, A.; López-Viana, J.; Soler, C. Combined Effect of Type and Capture Area of Counting Chamber and Diluent on Holstein Bull Sperm Kinematics. Andrologia 2019, 51, e13223. [Google Scholar] [CrossRef] [PubMed]
- Ibănescu, I.; Leiding, C.; Ciornei, Ş.G.; Roșca, P.; Sfartz, I.; Drugociu, D. Differences in CASA Output According to the Chamber Type When Analyzing Frozen-Thawed Bull Sperm. Anim Reprod Sci 2016, 166, 72–79. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).