Submitted:
20 August 2024
Posted:
22 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
- There will be no alterations to the enamel surface following treatment with or without laser and after the pH cycle.
- The percentage of chemical elements in the measured regions will remain unchanged following treatment with or without laser and after the pH cycling.
- The three wavelengths will not have a distinct impact on the chemical structure of the enamel.
2. Materials and Methods
2.1. Tooth Selection and Preparation Phase
2.2. Experminetal Techniques
2.2.1. DIAGNOdent® Pen
2.2.2. Applied Treatments
| Group (G) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| FV-Varnish | - | X | - | - | - | - | - | - |
| L 980 nm | - | - | X | - | - | X | - | - |
| L 808 nm | - | - | - | X | - | - | X | - |
| L 450 nm | - | - | - | - | X | - | - | X |
| FV-After Laser | - | - | - | - | - | X | X | X |
2.3. Evaluation Methods
2.3.1. The DIAGNOdent®
2.3.2. Scanning Electron Microscopy (SEM) and Energy Dispersive X-Ray Spectroscopy (EDX) Analysis
2.4. Statistical Analysis
3. Results
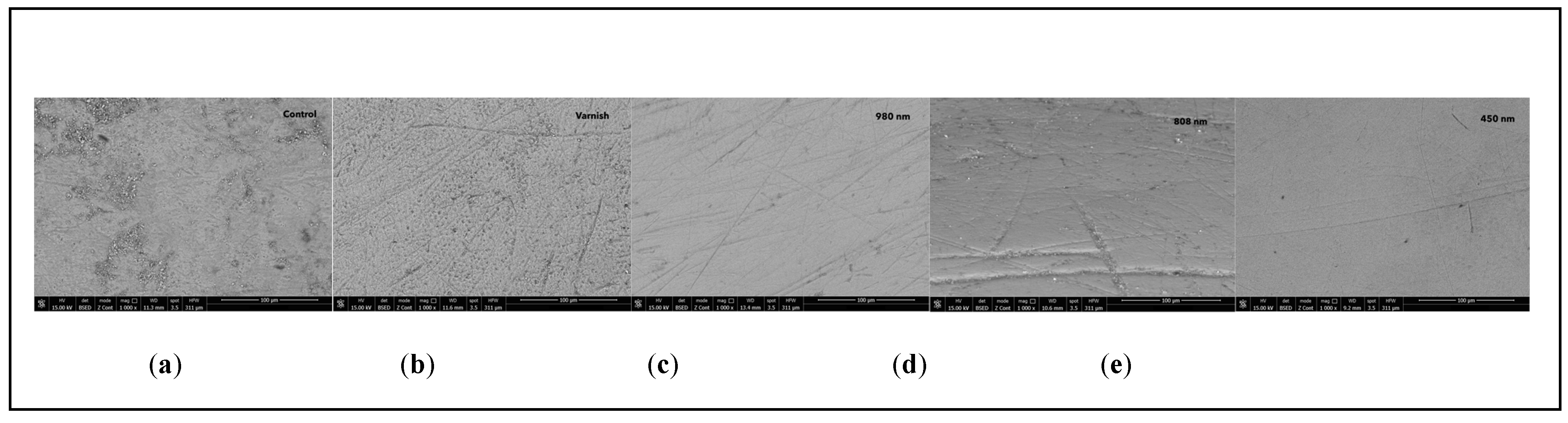
3.1. Scanning Electron Microscopy (SEM)
3.2. DIAGNOdent® Analysis
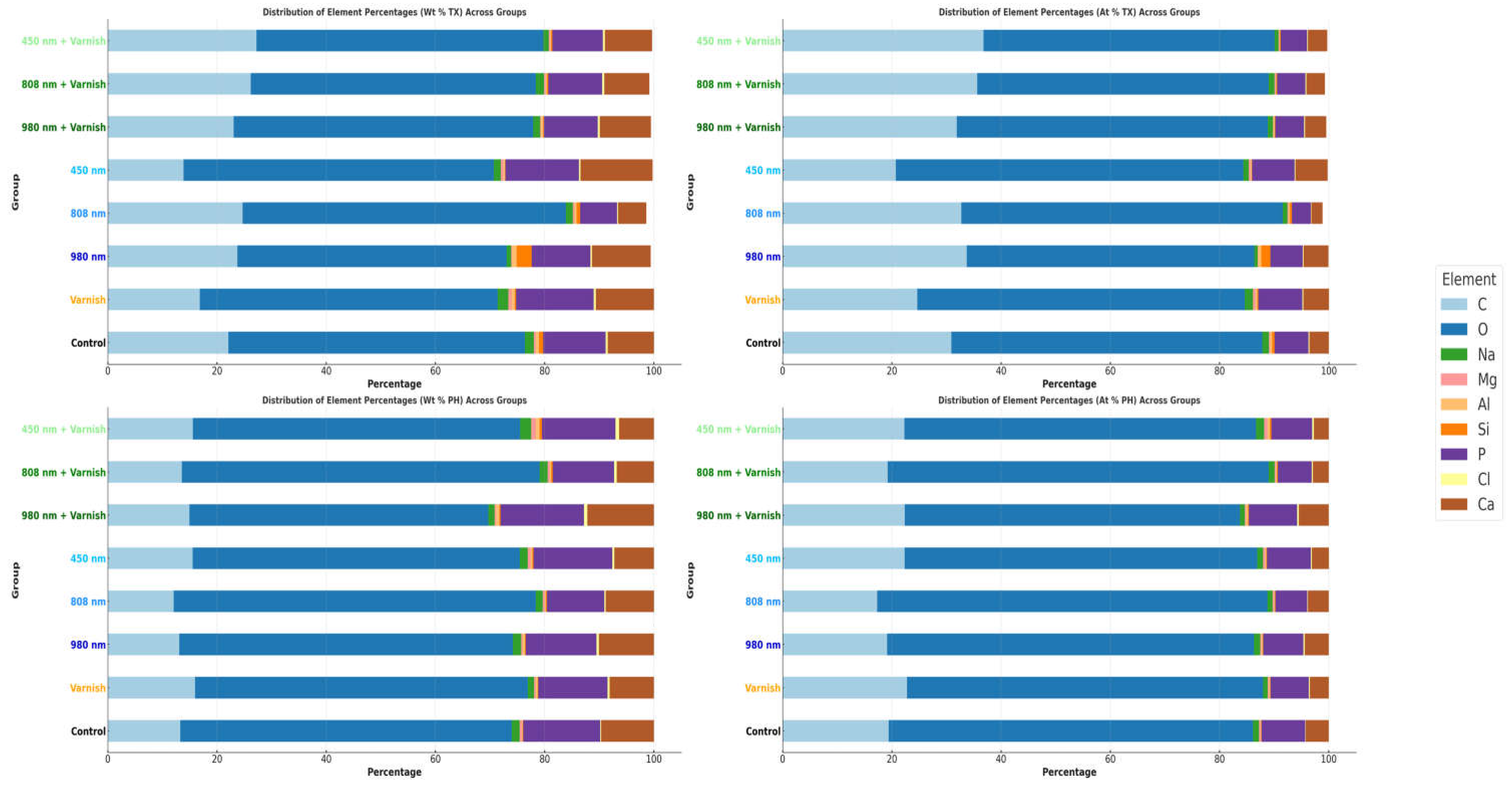
3.3. EDX Chemical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bahrololoomi, Z.; Zarebidoki, F.; Mostafalu, N. The effect of different re-mineralizing agents and diode laser irradiation on the microhardness of primary molar enamel: An in vitro study. Laser Ther. 2019, 28, 187–192. [Google Scholar] [CrossRef]
- Gao, S.S.; Zhang, S.; Mei, M.L.; Lo, E.C.-M.; Chu, C.-H. Caries remineralisation and arresting effect in children by professionally applied fluoride treatment – a systematic review. BMC Oral Heal. 2016, 16, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Nozari, A. Impact of Nano Hydroxyapatite, Nano Silver Fluoride and Sodium Fluoride Varnish on Primary Enamel Remineralization: An In Vitro Study. J. Clin. Diagn. Res. 2017, 11, ZC97–ZC100. [Google Scholar] [CrossRef]
- dos Santos, V.E., Jr.; Filho, A.V.; Targino, A.G.R.; Flores, M.A.P.; Galembeck, A.; Caldas, A.F.; Rosenblatt, A. A New “Silver-Bullet” to treat caries in children – Nano Silver Fluoride: A randomised clinical trial. J. Dent. 2014, 42, 945–951. [Google Scholar] [CrossRef] [PubMed]
- A Bagramian, R.; Garcia-Godoy, F.; Volpe, A.R. The global increase in dental caries. A pending public health crisis.. 2009, 22, 3–8. [Google Scholar]
- Curylofo-Zotti FA, Tanta GS, Zugliani AL, Milori SA. The combined use of sodium fluoride and Er: YAG laser to control the progression of enamel caries. European Journal of Pharmaceutical and Medical Research. 2016;3(9):1-5.
- Lata, S.; Varghese, N.; Varughese, J.M. Remineralization potential of fluoride and amorphous calcium phosphate-casein phospho peptide on enamel lesions: Anin vitrocomparative evaluation. J. Conserv. Dent. 2010, 13, 42–46. [Google Scholar] [CrossRef]
- Joshi, S.R.; Patil, N.; Choudhari, S.; Kulkarni, S. Comparative evaluation of remineralizing potential of three agents on artificially demineralized human enamel: An in vitro study. J. Conserv. Dent. 2013, 16, 116–20. [Google Scholar] [CrossRef] [PubMed]
- Tschoppe, P.; Zandim, D.L.; Martus, P.; Kielbassa, A.M. Enamel and dentine remineralization by nano-hydroxyapatite toothpastes. J. Dent. 2011, 39, 430–437. [Google Scholar] [CrossRef]
- Vitiello, F.; Tosco, V.; Monterubbianesi, R.; Orilisi, G.; Gatto, M.L.; Sparabombe, S.; Memé, L.; Mengucci, P.; Putignano, A.; Orsini, G. Remineralization Efficacy of Four Remineralizing Agents on Artificial Enamel Lesions: SEM-EDS Investigation. Materials 2022, 15, 4398. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, D.; Bai, Y.; Li, S. Casein phosphopeptide–amorphous calcium phosphate remineralization of primary teeth early enamel lesions. J. Dent. 2014, 42, 21–29. [Google Scholar] [CrossRef]
- Poosti, M.; Ahrari, F.; Moosavi, H.; Najjaran, H. The effect of fractional CO2 laser irradiation on remineralization of enamel white spot lesions. Lasers Med Sci. 2013, 29, 1349–1355. [Google Scholar] [CrossRef]
- Pitts, N.B.; Zero, D.T.; Marsh, P.D.; Ekstrand, K.; Weintraub, J.A.; Ramos-Gomez, F.; Tagami, J.; Twetman, S.; Tsakos, G.; Ismail, A. Dental caries. Nat. Rev. Dis. Primers 2017, 3, 17030. [Google Scholar] [CrossRef]
- Stookey, G.K. The effect of saliva on dental caries. J. Am. Dent. Assoc. 2008, 139, 11S–17S. [Google Scholar] [CrossRef] [PubMed]
- Orsini, G.; Tosco, V.; Monterubbianesi, R.; Orilisi, G.; Putignano, A. A. A New Era in Restorative Dentistry. In The First Outstanding 50 Years of “Università Politecnica delle Marche”; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Neel, E.A.A.; Aljabo, A.; Strange, A.; Ibrahim, S.; Coathup, M.; Young, A.M.; Bozec, L.; Mudera, V. Demineralization–remineralization dynamics in teeth and bone. Int. J. Nanomed. 2016, ume 11, 4743–4763. [Google Scholar] [CrossRef]
- Bandekar, S.; Patil, S.; Dudulwar, D.; Moogi, P.P.; Ghosh, S.; Kshirsagar, S. Remineralization potential of fluoride, amorphous calcium phosphate-casein phosphopeptide, and combination of hydroxylapatite and fluoride on enamel lesions: An in vitro comparative evaluation. J. Conserv. Dent. 2019, 22, 305–309. [Google Scholar] [CrossRef]
- Marinho, V.C.; Worthington, H.V.; Walsh, T.; E Clarkson, J. Fluoride varnishes for preventing dental caries in children and adolescents. Cochrane Database Syst. Rev. 2013, 2014, CD002279. [Google Scholar] [CrossRef]
- Ten Cate, J.M. Current Concepts on the Theories of the Mechanism of Action of Fluoride. Acta Odontol. Scand. 1999, 57, 325–329.) Article 24 (15) (Reynolds, E.C. Calcium Phosphate-Based Remineralization Systems: Scientific Evidence? Aust. Dent. J. 2008, 53, 268–273. [Google Scholar]
- Reema, S.D.; Lahiri, P.K.; Roy, S.S. Review of casein phosphopeptides-amorphous calcium phosphate. . 2014, 17, 7–14. [Google Scholar] [PubMed]
- Farooq, I.; Bugshan, A. The role of salivary contents and modern technologies in the remineralization of dental enamel: a narrative review. F1000Research 2021, 9, 171. [Google Scholar] [CrossRef]
- Philip, N. State of the Art Enamel Remineralization Systems: The Next Frontier in Caries Management. Caries Res. 2018, 53, 284–295. [Google Scholar] [CrossRef]
- Pinelli, M.; Catelan, A.; De Resende, L.; Soares, L.; Aguiar, F.; Liporoni, P. Chemical composition and roughness of enamel and composite after bleaching, acidic beverages and toothbrushing. J. Clin. Exp. Dent. 2019, 11, e1175–e1180. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-J.; Lee, M.-J.; Kim, K.-M.; Yang, S.-Y.; Seo, J.-Y.; Choi, S.-H.; Kwon, J.-S. Enamel Demineralization Resistance and Remineralization by Various Fluoride-Releasing Dental Restorative Materials. Materials 2021, 14, 4554. [Google Scholar] [CrossRef]
- Singal, K.; Sharda, S.; Gupta, A.; Malik, V.S.; Singh, M.; Chauhan, A.; Agarwal, A.; Pradhan, P.; Singh, M. Effectiveness-of Calcium Phosphate derivative agents on the prevention and remineralization of caries among children- A systematic review & meta-analysis of randomized controlled trials. J. Évid. Based Dent. Pr. 2022, 22, 101746. [Google Scholar] [CrossRef]
- de Oliveira, P.R.A.; Barboza, C.M.; Barreto, L.S.d.C.; Tostes, M.A. Effect of CPP-ACP on remineralization of artificial caries-like lesion: an in situ study. Braz. Oral Res. 2020, 34, e061. [Google Scholar] [CrossRef]
- Imani, M.; Safaei, M.; Afnaniesfandabad, A.; Moradpoor, H.; Sadeghi, M.; Golshah, A.; Sharifi, R.; Mozaffari, H. Efficacy of CPP-ACP and CPP-ACPF for Prevention and Remineralization of White Spot Lesions in Orthodontic Patients: a Systematic Review of Randomized Controlled Clinical Trials. Acta Inform. Medica 2019, 27, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Somasundaram, P.; Mandke, L.G.; Vimala, N. Protective potential of casein phosphopeptide amorphous calcium phosphate containing paste on enamel surfaces. J. Conserv. Dent. 2013, 16, 152–6. [Google Scholar] [CrossRef]
- Moharam, L.; Sadony, D.; Nagi, S. Evaluation of diode laser application on chemical analysis and surface microhardness of white spots enamel lesions with two remineralizing agents. J. Clin. Exp. Dent. 2020, 12, e271–e276. [Google Scholar] [CrossRef]
- Chen, L.; Hontsu, S.; Komasa, S.; Yamamoto, E.; Hashimoto, Y.; Matsumoto, N. Hydroxyapatite Film Coating by Er:YAG Pulsed Laser Deposition Method for the Repair of Enamel Defects. Materials 2021, 14, 7475. [Google Scholar] [CrossRef]
- Heravi, F.; Ahrari, F.; Mahdavi, M.; Basafa, S. Comparative evaluation of the effect of Er:YAG laser and low level laser irradiation combined with CPP-ACPF cream on treatment of enamel caries. J. Clin. Exp. Dent. 2014, 6, e121–6. [Google Scholar] [CrossRef] [PubMed]
- Bahrololoomi, Z.; Lotfian, M. Effect of Diode Laser Irradiation Combined with Topical Fluoride on Enamel Microhardness of Primary Teeth. 2015, 12, 85–89.
- El Mansy, M.M.; Gheith, M.; El Yazeed, A.M.; Farag, D.B.E. Influence of Er, Cr: YSGG (2780 nm) and Nanosecond Nd: YAG Laser (1064 nm) Irradiation on Enamel Acid Resistance: Morphological and Elemental Analysis. Open Access Maced. J. Med Sci. 2019, 7, 1828–1833. [Google Scholar] [CrossRef]
- Al-Shaker SM, Nayif MM, Al-Sabawi NA. Microhardness of Artificially Demineralized Enamel treated with Different Regimes of ACP-CPP and Fluoride Agents. Int. J. Enhan Res. Sci. Tech. Eng. 2014, 3, 102–107.
- Ana, P.A.; Bachmann, L.; Zezell, D.M. Lasers effects on enamel for caries prevention. Laser Phys. 2006, 16, 865–875. [Google Scholar] [CrossRef]
- Belcheva, A.; El Feghali, R.; Nihtianova, T.; Parker, S. Effect of the carbon dioxide 10,600-nm laser and topical fluoride gel application on enamel microstructure and microhardness after acid challenge: an in vitro study. Lasers Med Sci. 2018, 33, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Scatolin, R.S.; Colucci, V.; Lepri, T.P.; Alexandria, A.K.; Maia, L.C.; Galo, R.; Borsatto, M.C.; Corona, S.A.M. Er:YAG laser irradiation to control the progression of enamel erosion: an in situ study. Lasers Med Sci. 2014, 30, 1465–1473. [Google Scholar] [CrossRef]
- Jorge, A.C.T.; Cassoni, A.; de Freitas, P.M.; Reis, A.F.; Junior, A.B.; Rodrigues, J.A. Influence of Cavity Preparation with Er,Cr:YSGG Laser and Restorative Materials on In Situ Secondary Caries Development. Photomed. Laser Surg. 2015, 33, 98–103. [Google Scholar] [CrossRef]
- de Oliveira, R.M.; de Souza, V.M.; Esteves, C.M.; Lima-Arsati, Y.B.d.O.; Cassoni, A.; Rodrigues, J.A.; Junior, A.B. Er,Cr:YSGG Laser Energy Delivery: Pulse and Power Effects on Enamel Surface and Erosive Resistance. Photomed. Laser Surg. 2017, 35, 639–646. [Google Scholar] [CrossRef]
- Stangler, L.P.; Romano, F.L.; Shirozaki, M.U.; Galo, R.; Afonso, A.M.C.; Borsatto, M.C.; Matsumoto, M.A.N. Microhardness of Enamel Adjacent to Orthodontic Brackets After CO2 Laser Irradiation and Fluoride Application. Braz. Dent. J. 2013, 24, 508–512. [Google Scholar] [CrossRef]
- Hicks, M.J.; Flaitz, C.M.; Westerman, G.H.; Blankenau, R.J.; Powell, G.L. Root caries in vitro after low fluence argon laser and fluoride treatment. . 1997, 18, 543. [Google Scholar]
- Ana, P.; Tabchoury, C.; Cury, J.; Zezell, D. Effect of Er,Cr:YSGG Laser and Professional Fluoride Application on Enamel Demineralization and on Fluoride Retention. Caries Res. 2012, 46, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Vieira, K.A.; Steiner-Oliveira, C.; Soares, L.E.S.; Rodrigues, L.K.A.; Nobre-Dos-Santos, M. In vitro evaluation of enamel demineralization after several overlapping CO2 laser applications. Lasers Med Sci. 2013, 30, 901–907. [Google Scholar] [CrossRef]
- Soltanimehr, E.; Bahrampour, E.; Yousefvand, Z. Efficacy of diode and CO2 lasers along with calcium and fluoride-containing compounds for the remineralization of primary teeth. BMC Oral Heal. 2019, 19, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Eymirli, P.S.; İleri, T.; Ergin, E.; Turgut, M.D. Evaluation of ER;CR:YSGG Laser and Remineralization Agents on Mineral Density and Ion Levels of Primary and Permanent Enamel. Photobiomodulation, Photomedicine, Laser Surg. 2024, 42, 81–89. [Google Scholar] [CrossRef]
- de Freitas, P.M.; Rapozo-Hilo, M.; Eduardo, C.d.P.; Featherstone, J.D.B. In vitro evaluation of erbium, chromium:yttrium–scandium–gallium–garnet laser-treated enamel demineralization. Lasers Med Sci. 2008, 25, 165–170. [Google Scholar] [CrossRef]
- Cate, J.T.; Duijsters, P. Alternating Demineralization and Remineralization of Artificial Enamel Lesions. Caries Res. 1982, 16, 201–210. [Google Scholar] [CrossRef]
- Baecker, D.; Guenther, S. General Applicability of High-Resolution Continuum-Source Graphite Furnace Molecular Absorption Spectrometry to the Quantification of Oligopeptides Using the Example of Glutathione. Analytica 2022, 3, 24–35. [Google Scholar] [CrossRef]
- Featherstone JDB, Lussi A. Understanding the chemistry of dental erosion. Monogr Oral Sci. 2006;20:66-76. [CrossRef]
- Borzabadi-Farahani, A. The Adjunctive Soft-Tissue Diode Laser in Orthodontics. . 2017, 38, e18–e31. [Google Scholar] [PubMed]
- Moghadam, N.C.Z.; Seraj, B.; Chiniforush, N.; Ghadimi, S. Effects of Laser and Fluoride on the Prevention of Enamel Demineralization: An In Vitro Study. J. Lasers Med Sci. 2018, 9, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Barbosa, P.; da Ana, P.A.; Poiate, I.A.; Zezell, D.M.; de Sant’ Anna, G.R. Dental enamel irradiated with a low- intensity infrared laser and photoabsorbing cream: A study of microhardness, surface, and pulp temperature. Photomed. Laser Surg. 2013, 31, 439–446. [Google Scholar] [CrossRef]
- Rodríguez-Vilchis, L.E.; Contreras-Bulnes, R.; Mejìa, O.F.O.L.; Sánchez-Flores, I.; Centeno-Pedraza, C. Morpho- logical and structural changes on human dental enamel after Er:YAG laser irradiation: AFM, SEM, and EDS evaluation. Photomed. Laser Surg. 2011, 29, 493–500. [Google Scholar] [CrossRef]
- Lara-Carrilloa, E.; Doroteo-Chimalb, C.; Lopez-Gonzaleza, S.; Morales-Luckiec, R.; Olea-Mejiac, O.F.; Kubodera-Itoa, T.; Medina-Solisd, C. Remineralization effect of low-level laser and amorphous sodium–calcium–phosphosilicate paste in teeth with fixed orthodontic appliances. Tanta Dent. J. 2016, 13, 55. [Google Scholar] [CrossRef]
- Gan, J.; Liu, S.; Zhou, L.; Wang, Y.; Guo, J.; Huang, C. Effect of Nd:YAG Laser Irradiation Pretreatment on the Long-Term Bond Strength of Etch-and-Rinse Adhesive to Dentin. Oper. Dent. 2017, 42, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Neto, W.R.; Lepri, C.P.; Romano, J.J.F.; Fernandes, F.S.; Raucci, L.M.S.D.C.; Bachmann, L.; Dibb, R.G.P. Chemical and Morphological Changes of Primary Teeth Irradiated with Nd:YAG Laser: AnEx VivoLong-Term Analysis. Photomed. Laser Surg. 2015, 33, 266–273. [Google Scholar] [CrossRef]
- Dilber, E.; Malkoc, M.A.; Ozturk, A.N.; Ozturk, F. Effect of various laser irradiations on the mineral content of dentin. 2013, 7, 74–80.
- Paes Leme AF, Tabchoury CP, Zero DT, Cury JA (2003) Effect of fluoridated dentifrice and acidulated phosphate fluoride application on early artificial carious lesions. Am J Dent 16:91–95.
- Featherstone JDB (2000) The science and practice of caries prevention. J Am Dent Assoc 131:887–899.
- Hossain, M.; Kimura, Y.; Nakamura, Y.; Yamada, Y.; Kinoshita, J.-I.; Matsumoto, K. A Study on Acquired Acid Resistance of Enamel and Dentin Irradiated by Er,Cr:YSGG Laser. J. Clin. Laser Med. Surg. 2001, 19, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Kantorowitz Z, Featherstone JDB, Fried D (1998) Caries prevention by CO2 laser treatment: dependency of the number of pulses used. J Am Dental Assoc 129:585–591.
- Esteves-Oliveira, M.; Pasaporti, C.; Heussen, N.; Eduardo, C.; Lampert, F.; Apel, C. Rehardening of acid-softened enamel and prevention of enamel softening through CO2 laser irradiation. J. Dent. 2011, 39, 414–421. [Google Scholar] [CrossRef]
- Santaella, M.R.L.A.; Braun, A.; Matson, E.; Frentzen, M. Effect of diode laser and fluoride varnish on initial surface demineralization of primary dentition enamel: an in vitro study. Int. J. Paediatr. Dent. 2004, 14, 199–203. [Google Scholar] [CrossRef]
- González-Rodríguez, A.; de Dios López-González, J.; de Dios Luna del Castillo, J.; Villalba-Moreno, J. Comparison of effects of diode laser and CO2 laser on human teeth and their usefulness in topical fluoridation. Lasers Med. Sci. 2011, 26, 317–324. [Google Scholar] [CrossRef]
- Vitale, M.C.; Zaffe, D.; Botticell, A.R.; Caprioglio, C. Diode laser irradiation and fluoride uptake in human teeth. Eur. Arch. Paediatr. Dent. 2011, 12, 90–92. [Google Scholar] [CrossRef] [PubMed]
- Kato, I.T.; Kohara, E.K.; Sarkis, J.E.; Wetter, N.U. Effects of 960-nm Diode Laser Irradiation on Calcium Solubility of Dental Enamel: Anin VitroStudy. Photomed. Laser Surg. 2006, 24, 689–693. [Google Scholar] [CrossRef]
- Apel, C.; Meister, J.; Schmitt, N.; Gräber, H.; Gutknecht, N. Calcium solubility of dental enamel following sub-ablative Er:YAG and Er:YSGG laser irradiation in vitro. Lasers Surg. Med. 2002, 30, 337–341. [Google Scholar] [CrossRef]
- Rodrigues LK, Nobre Dos Santos M, Featherstone JD. In situ mineral loss inhibition by CO2 laser and fluoride. J Dent Res. 2006;85:617–21.
- Featherstone, J.; Barrett-Vespone, N.; Fried, D.; Kantorowitz, Z.; Seka, W. CO2 Laser Inhibition of Artificial Caries-like Lesion Progression in Dental Enamel. J. Dent. Res. 1998, 77, 1397–1403. [Google Scholar] [CrossRef]
- Mocuta(Bojoga), D.-E.; Grad(Buriac), O.; Mateas, M.; Luca, R.; Todea, D.C. Comparative Evaluation of Influence of Nd:YAG Laser (1064 nm) and 980 nm Diode Laser on Enamel around Orthodontic Brackets: An In Vitro Study. Medicina 2022, 58, 633. [Google Scholar] [CrossRef] [PubMed]
- Hibst, R.; Keller, U. Experimental studies of the application of the Er:YAG laser on dental hard substances: I. Measurement of the ablation rate. Lasers Surg. Med. 1989, 9, 338–344. [Google Scholar] [CrossRef]
- Ana PA, Zezzel DM, Blay CC, Blay A, Eduardo CP, Miyakawa W (2004) Thermal analysis of dental enamel following Er,Cr: YSGG laser irradiation at low fluencies. Lasers Surg Med 16:53–63.
- Umana, M.; Heysselaer, D.; Tielemans, M.; Compere, P.; Zeinoun, T.; Nammour, S. Dentinal Tubules Sealing by Means of Diode Lasers (810 and 980 nm): A Preliminary In Vitro Study. Photomed. Laser Surg. 2013, 31, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Nandkumar, A.; Iyer, V.H. In vitro analysis comparing efficacy of lasers and desensitizing agents on dentin tubule occlusion: A scanning electron microscope study. Int. J. Laser Dent. 2014, 4, 1–7. [Google Scholar]
- Pereira, D.L.; Freitas, A.Z.; Bachmann, L.; Benetti, C.; Zezell, D.M.; Ana, P.A. Variation on Molecular Structure, Crystallinity, and Optical Properties of Dentin Due to Nd:YAG Laser and Fluoride Aimed at Tooth Erosion Prevention. Int. J. Mol. Sci. 2018, 19, 433. [Google Scholar] [CrossRef] [PubMed]



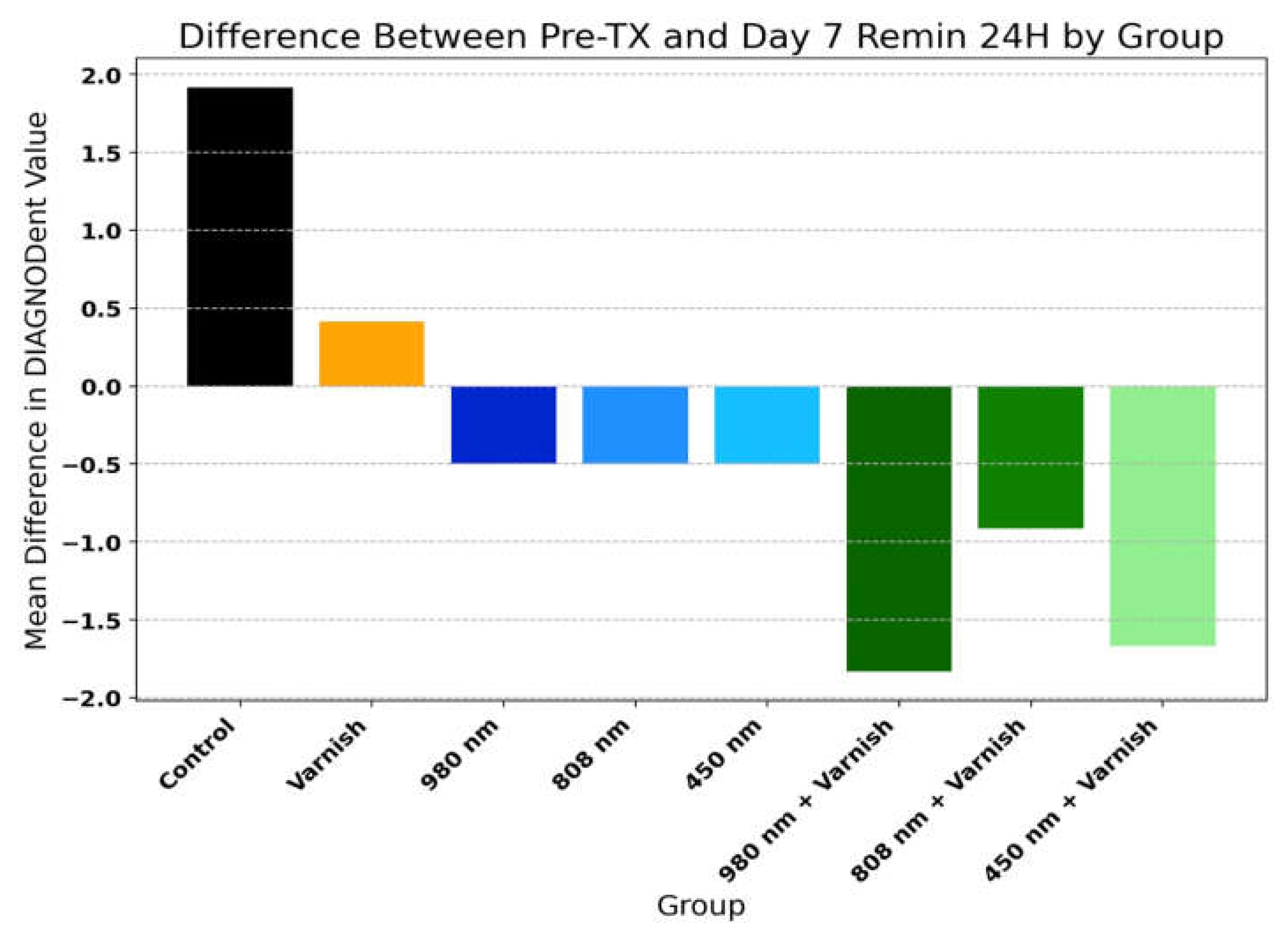
| Group | Pre-TX Mean | Day 7 Remin Mean | Mean Difference | T-test p-value |
|---|---|---|---|---|
| Control | 2,83 | 4,75 | 1,92 | <0.001*** |
| Varnish | 3,67 | 4,08 | 0,42 | 0.053 |
| 980 nm | 3,33 | 2,83 | -0,50 | 0.052 |
| 808 nm | 3,33 | 2,83 | -0,50 | 0.026* |
| 450 nm | 3,00 | 2,50 | -0,50 | 0.026* |
| 980 nm + Varnish | 5,08 | 3,25 | -1,83 | <0.001*** |
| 808 nm + Varnish | 3,92 | 3,00 | -0,92 | 0.019* |
| 450 nm + Varnish | 5,08 | 3,42 | -1,67 | <0.001*** |
| Comparison | Mean Difference | Adjusted p-value | Significance |
|---|---|---|---|
| 450 nm vs 450 nm + Varnish | -1,1667 | 0.018 | * |
| 450 nm vs Control | 2,4167 | <0.001 | ** |
| 450 nm + Varnish vs Control | 3,5833 | <0.001 | ** |
| 450 nm + Varnish vs Varnish | 2,0833 | <0.001 | ** |
| 808 nm vs 808 nm + Varnish | -0,4167 | 0.919 | - |
| 808 nm vs Control | 2,4167 | <0.001 | ** |
| 808 nm + Varnish vs Control | 2,8333 | <0.001 | ** |
| 808 nm + Varnish vs Varnish | 1,3333 | 0.003 | ** |
| 980 nm vs 980 nm + Varnish | -1,3333 | 0.003 | ** |
| 980 nm vs Control | 2,4167 | <0.001 | ** |
| 980 nm + Varnish vs Control | 3,75 | <0.001 | ** |
| 980 nm + Varnish vs Varnish | 2,25 | <0.001 | ** |
| Control vs Varnish | -1,5 | <0.001 | ** |
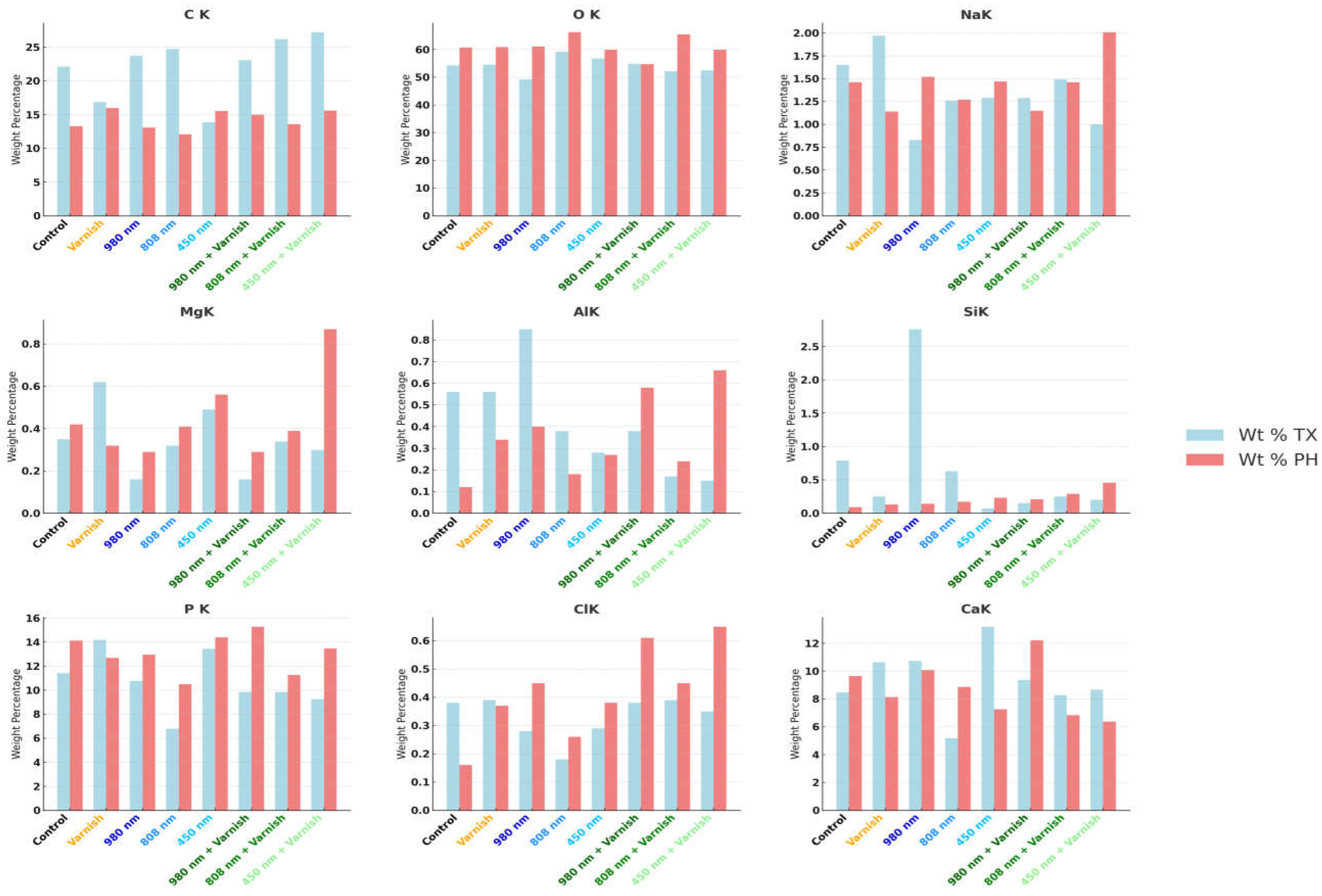
| Element | Control Group | Varnish Group | 980 nm | 808 nm | 450 nm | 980 nm + Varnish | 808 nm + Varnish | 450 nm + Varnish | ||||||||
| Wt% TX | Wt% PH | Wt% TX | Wt% PH | Wt% TX | Wt% PH | Wt% TX | Wt% PH | Wt% TX | Wt% PH | Wt% TX | Wt% PH | Wt% TX | Wt% PH | Wt% TX | Wt% PH | |
| Mean ± SD | ||||||||||||||||
| Ca | 8.48 ± 1.16 | 9.65 ± 1.32 | 10.65 ± 1.76 | 8.13 ± 1.35 | 10.74 ± 1.58 | 10.08 ± 1.49 | 5.18 ± 0.74 | 8.86 ± 1.26 | 13.20 ± 1.91 | 7.27 ± 1.05 | 9.36 ± 1.68 | 12.21 ± 2.19 | 8.27 ± 1.42 | 6.84 ± 1.18 | 8.67 ± 1.52 | 6.38 ± 1.12 |
| P | 11.43 ± 1.76 | 14.12 ± 2.17 | 14.20 ± 2.65 | 12.68 ± 2.37 | 10.76 ± 1.79 | 12.96 ± 2.15 | 6.78 ± 1.09 | 10.5 ± 1.69 | 13.44 ± 2.19 | 14.39 ± 2.35 | 9.85 ± 1.99 | 15.29 ± 3.08 | 9.86 ± 1.91 | 11.26 ± 2.18 | 9.26 ± 1.83 | 13.46 ± 2.66 |
| C | 22.1 ± 2.93 | 13.27 ± 1.76 | 16.85 ± 2.71 | 15.98 ± 2.57 | 23.72 ± 3.4 | 13.08 ± 1.87 | 24.7 ± 3.42 | 12.07 ± 1.67 | 13.88 ± 1.95 | 15.54 ± 2.19 | 23.04 ± 4.00 | 14.96 ± 2.6 | 26.17 ± 4.37 | 13.57 ± 2.27 | 27.22 ± 4.63 | 15.59 ± 2.65 |
| O | 54.27 ± 6.15 | 60.69 ± 6.88 | 54.51 ± 7.49 | 60.91 ± 8.37 | 49.29 ± 6.03 | 61.08 ± 7.48 | 59.17 ± 7.00 | 66.29 ± 7.84 | 56.79 ± 6.83 | 59.89 ± 7.20 | 54.83 ± 8.14 | 54.7 ± 8.12 | 52.22 ± 7.46 | 65.5 ± 9.36 | 52.51 ± 7.64 | 59.91 ± 8.71 |
| Na | 1.65 ± 0.12 | 1.46 ± 0.10 | 1.97 ± 0.17 | 1.14 ± 0.10 | 0.83 ± 0.06 | 1.52 ± 0.12 | 1.26 ± 0.09 | 1.27 ± 0.10 | 1.29 ± 0.10 | 1.47 ± 0.11 | 1.29 ± 0.12 | 1.15 ± 0.11 | 1.49 ± 0.13 | 1.46 ± 0.13 | 1.00 ± 0.09 | 2.01 ± 0.19 |
| Mg | 0.35 ± 0.03 | 0.42 ± 0.03 | 0.62 ± 0.06 | 0.32 ± 0.03 | 0.16 ± 0.01 | 0.29 ± 0.03 | 0.32 ± 0.03 | 0.41 ± 0.04 | 0.49 ± 0.04 | 0.56 ± 0.05 | 0.16 ± 0.02 | 0.29 ± 0.03 | 0.34 ± 0.04 | 0.39 ± 0.04 | 0.30 ± 0.03 | 0.87 ± 0.09 |
| Al | 0.56 ± 0.05 | 0.12 ± 0.01 | 0.56 ± 0.06 | 0.34 ± 0.04 | 0.85 ± 0.08 | 0.40 ± 0.04 | 0.38 ± 0.04 | 0.18 ± 0.02 | 0.28 ± 0.03 | 0.27 ± 0.03 | 0.38 ± 0.05 | 0.58 ± 0.07 | 0.17 ± 0.02 | 0.24 ± 0.03 | 0.15 ± 0.02 | 0.66 ± 0.08 |
| Si | 0.79 ± 0.04 | 0.09 ± 0.00 | 0.25 ± 0.02 | 0.13 ± 0.01 | 2.76 ± 0.16 | 0.14 ± 0.01 | 0.63 ± 0.04 | 0.17 ± 0.01 | 0.07 ± 0.00 | 0.23 ± 0.01 | 0.15 ± 0.01 | 0.21 ± 0.01 | 0.25 ± 0.02 | 0.29 ± 0.02 | 0.20 ± 0.01 | 0.46 ± 0.03 |
| Cl | 0.38 ± 0.02 | 0.16 ± 0.01 | 0.39 ± 0.02 | 0.37 ± 0.02 | 0.28 ± 0.01 | 0.45 ± 0.02 | 0.18 ± 0.01 | 0.26 ± 0.01 | 0.29 ± 0.01 | 0.38 ± 0.02 | 0.38 ± 0.02 | 0.61 ± 0.03 | 0.39 ± 0.02 | 0.45 ± 0.02 | 0.35 ± 0.02 | 0.65 ± 0.03 |
| Comparison | Mean Difference | Adjusted p-value | Significance |
|---|---|---|---|
| Ca/P ratio TX | |||
| 450 nm vs 808 nm | -0.4295 | 0.0127 | * |
| Ca/P ratio pH | |||
| 450 nm vs 808 nm | 0.3333 | 0.0055 | ** |
| 450 nm vs 980 nm | 0.2857 | 0.0265 | * |
| 450 nm vs 980 nm + Varnish | 0.3160 | 0.0099 | ** |
| 450 nm + Varnish vs 808 nm | 0.3610 | 0.0021 | ** |
| 450 nm + Varnish vs 980 nm | 0.3134 | 0.0108 | ** |
| 450 nm + Varnish vs 980 nm + Varnish | 0.3437 | 0.0038 | ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





