Submitted:
21 August 2024
Posted:
22 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
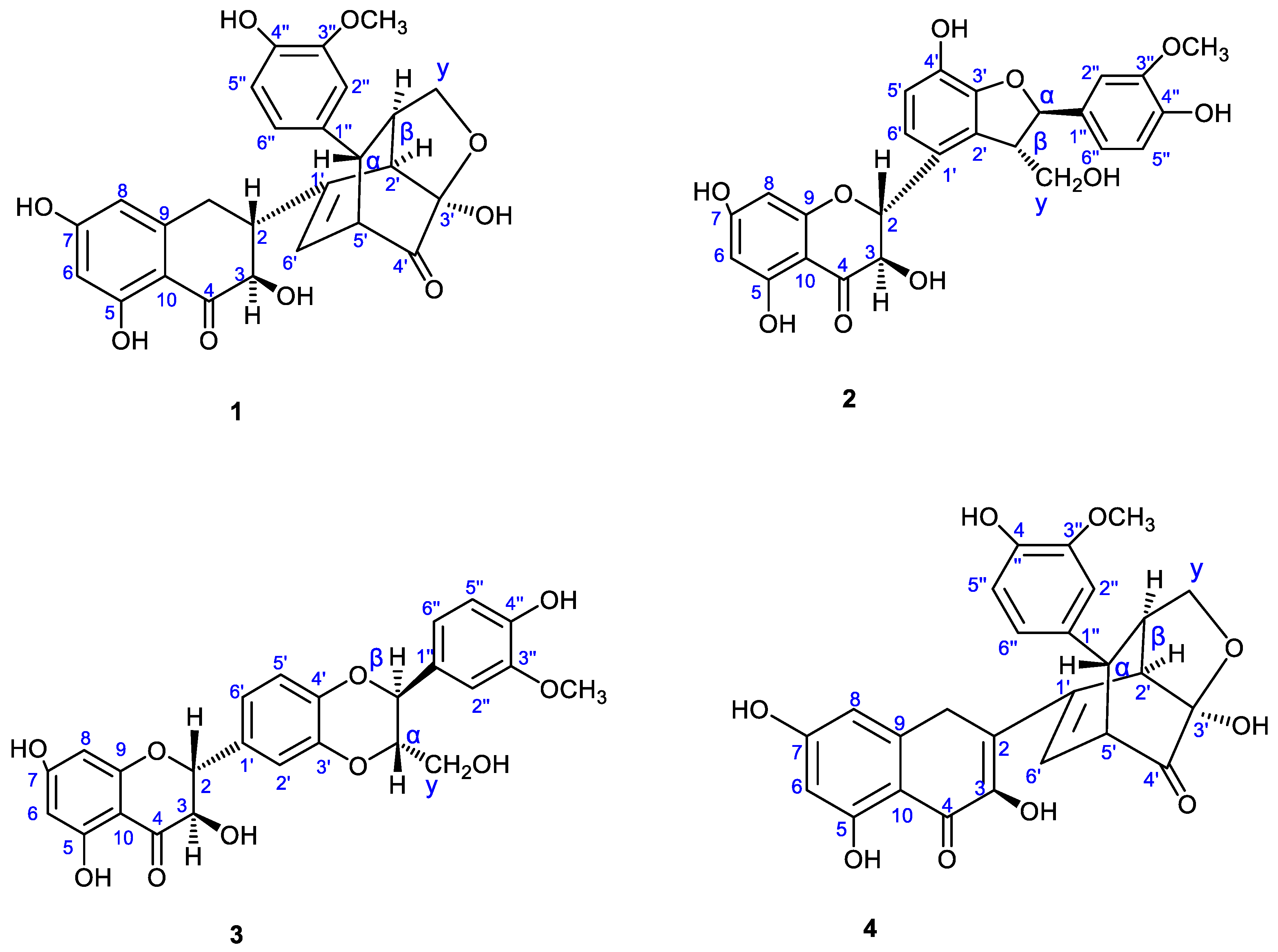
2.1. Characterization of Silybum marianum Extract Using NMR Spectroscopy
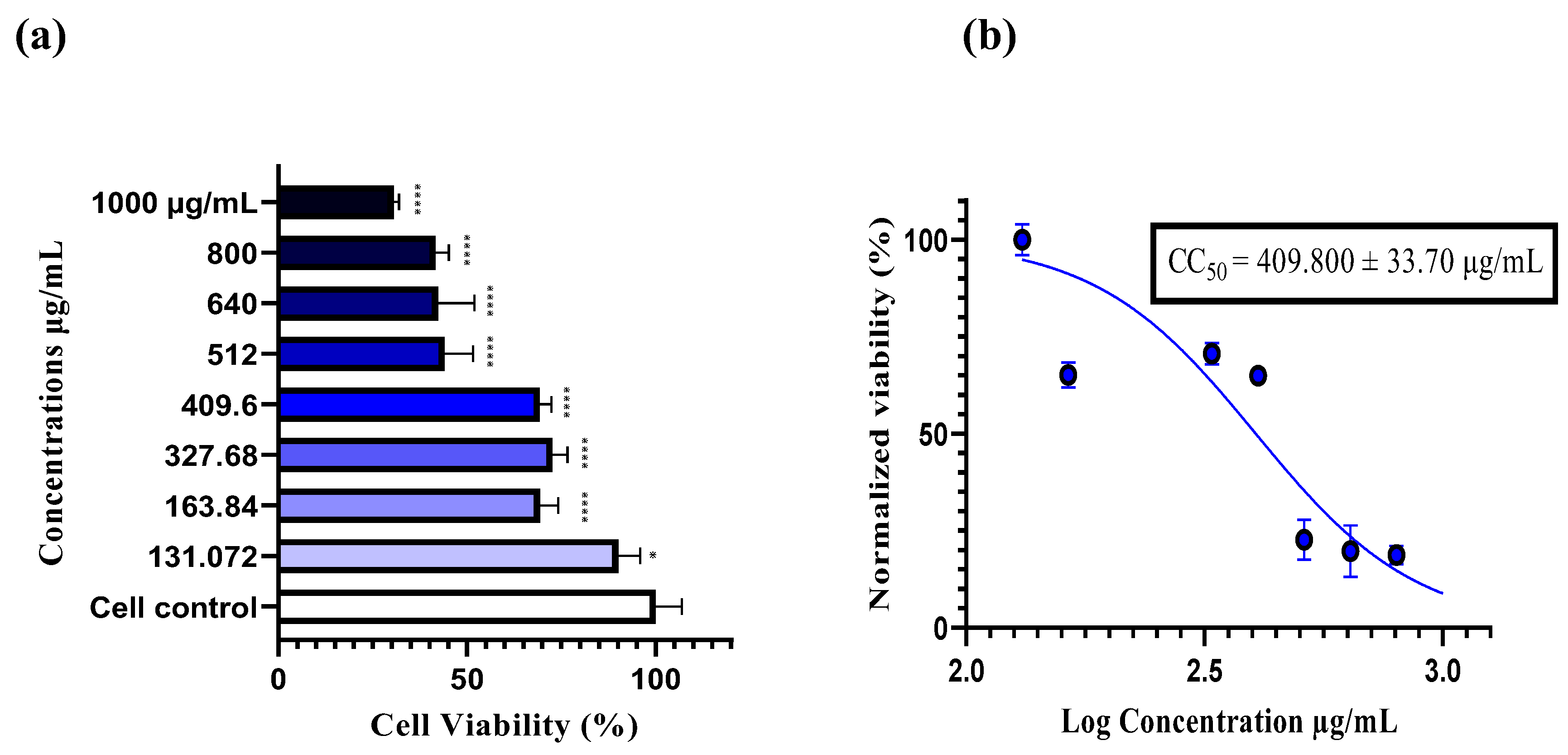
2.2. Cytotoxicity of Sylibium Marianum Extract on RD Cells
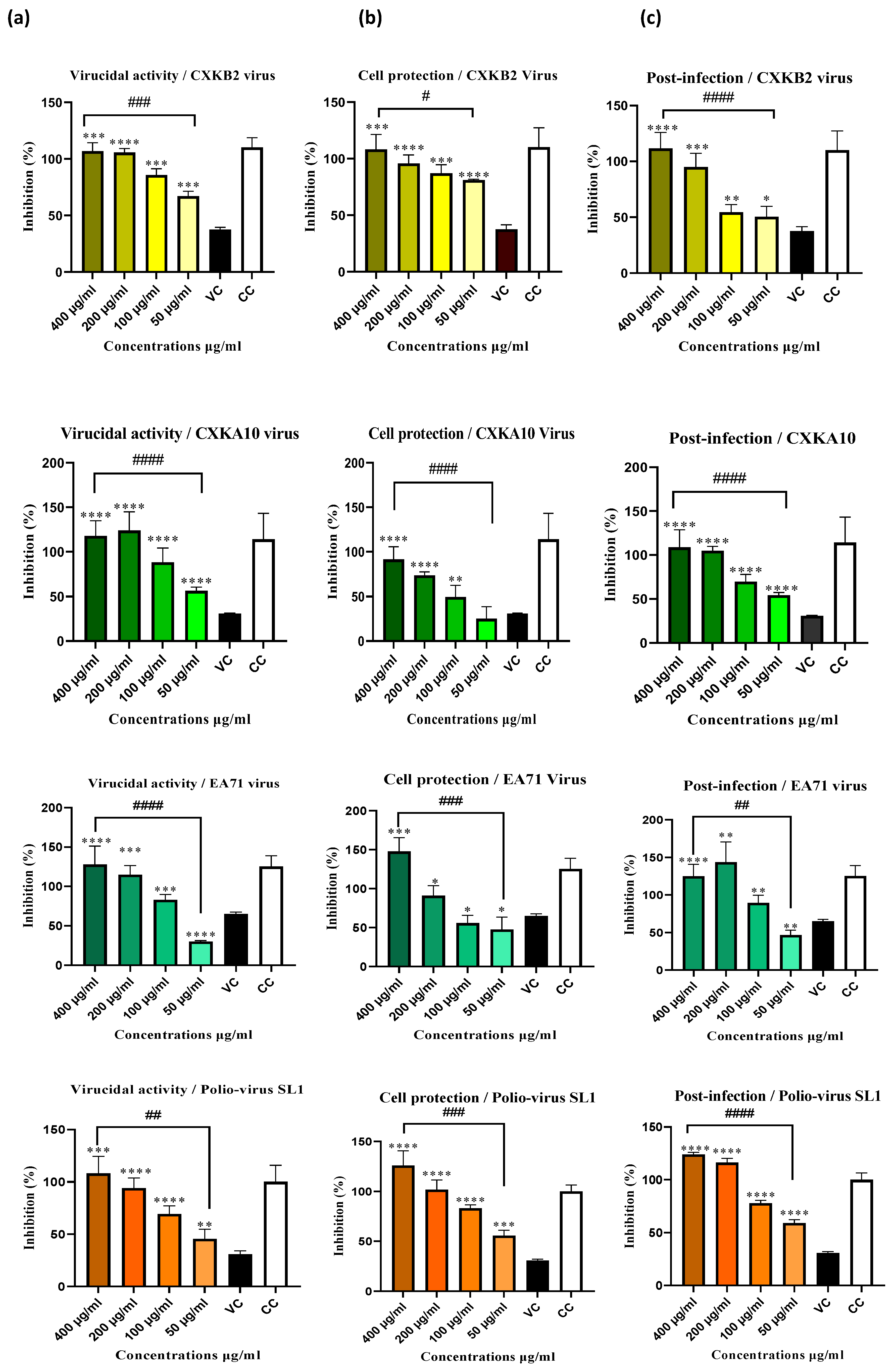
2.3. The Antiviral Assays by the Neutral Red Uptake
2.4. Real-Time RT-PCR Assay
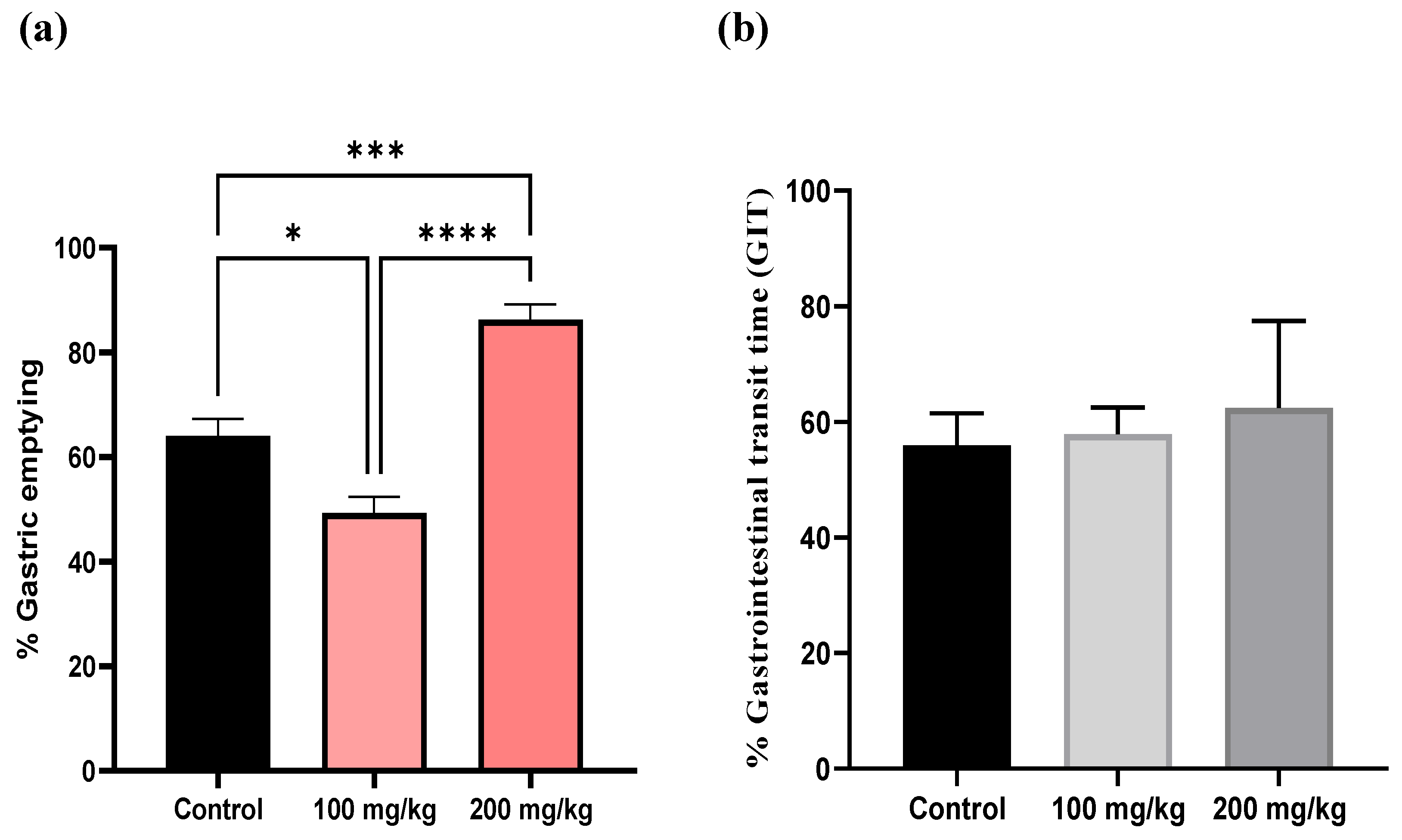
2.5. Effect of Extract on Gastric Emptying In Vivo
3. Discussion
4. Materials and Methods
4.1. Plant Materiel
4.2. Extraction
4.3. Biological Assay In Vitro
4.3.1. Virus and Cell Line
4.3.2. Titration of Viruses (TCID50)
4.3.3. Cytotoxicity Assay
4.3.4. Viral RNA Extraction and Quantitative Real-Time RT-PCR (rRT-PCR)
4.3.5. Antiviral Activity Profiles Using Neutral Red Assay and Quantitative Real-Time RT-PCR
4.3.5.1. Virucidal Activity
4.3.5.2. Cell Protection Activity
4.3.5.3. Post-Infection Activity
4.4. Biological Assay In Vivo
4.4.1. Animals
4.4.2. Determination of Gastric Emptying by Phenol Red Method
4.5. Characterization of Silybum marianum Hydro-Methanolic Extract
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shen, S.; Xu, J.; Lamm, V.; Vachaparambil, C.T.; Chen, H.; Cai, Q. Diabetic Gastroparesis and Nondiabetic Gastroparesis. Gastrointest. Endosc. Clin. North Am. 2019, 29, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Mandarino, F.V.; Sinagra, E.; Barchi, A.; Verga, M.C.; Brinch, D.; Raimondo, D.; Danese, S. Gastroparesis: The Complex Interplay with Microbiota and the Role of Exogenous Infections in the Pathogenesis of the Disease. Microorganisms 2023, 11, 1122. [Google Scholar] [CrossRef] [PubMed]
- Barkin, J.A.; Czul, F.; Barkin, J.S.; Klimas, N.G.; Rey, I.R.; Moshiree, B. Gastric Enterovirus Infection: A Possible Causative Etiology of Gastroparesis. Dig. Dis. Sci. 2016, 61, 2344–2350. [Google Scholar] [CrossRef]
- M. V. Yates, “Enterovirus,” in Microbiology of Waterborne Diseases, Elsevier, 2014, pp. 493–504. [CrossRef]
- Solomon, T.; Lewthwaite, P.; Perera, D.; Cardosa, M.J.; McMinn, P.; Ooi, M.H. Virology, epidemiology, pathogenesis, and control of enterovirus 71. Lancet Infect. Dis. 2010, 10, 778–790. [Google Scholar] [CrossRef]
- Yee, P.T.I.; Tan, K.O.; Othman, I.; Poh, C.L. Identification of molecular determinants of cell culture growth characteristics of Enterovirus 71. Virol. J. 2016, 13, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Ahmad, A. Targeting calpains: A novel immunomodulatory approach for microbial infections. Eur. J. Pharmacol. 2017, 814, 28–44. [Google Scholar] [CrossRef]
- J. L. Melnick, “Enteroviruses,” 1984.
- Heim, A.; Pfetzing, U.; Müller, G.; Grumbach, I.M. Antiviral activity of WIN 54954 in coxsackievirus B2 carrier state infected human myocardial fibroblasts. Antivir. Res. 1998, 37, 47–56. [Google Scholar] [CrossRef]
- Bowers, J.R.; Readler, J.M.; Sharma, P.; Excoffon, K.J. Poliovirus Receptor: More than a simple viral receptor. Virus Res. 2017, 242, 1–6. [Google Scholar] [CrossRef]
- Lemrabet, S.; Filali-Maltouf, A.; Medraoui, L.; Oumzil, H. A case of vaccine associated paralytic poliomyelitis in an immune competent child in Morocco. J. Med Pharm. Allied Sci. 2023, 12, 6059–6061. [Google Scholar] [CrossRef]
- Lemrabet, S.; El Qazoui, M.; Azzouzi, L.M.I.; Rguig, A.; Elhamdaoui, M.; Filali-Maltouf, A.; Medraoui, L.; Oumzil, H. Evaluation of the Acute Flaccid Paralysis Virological Surveillance System in Polio-Free Morocco, 2010–2018. Adv. Public Heal. 2022, 2022, 1–6. [Google Scholar] [CrossRef]
- AbouZid, S.F.; Ahmed, H.S.; Moawad, A.S.; Owis, A.I.; Chen, S.-N.; Nachtergael, A.; McAlpine, J.B.; Friesen, J.B.; Pauli, G.F. Chemotaxonomic and biosynthetic relationships between flavonolignans produced by Silybum marianum populations. Fitoterapia 2017, 119, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Li, M.; Yang, Y.; Zhang, Z.; Zhu, Q.; Liu, J.; Wang, A. Natural flavonolignans as potential therapeutic agents against common diseases. J. Pharm. Pharmacol. 2022, 74, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Csupor, D.; Csorba, A.; Hohmann, J. Recent advances in the analysis of flavonolignans of Silybum marianum. J. Pharm. Biomed. Anal. 2016, 130, 301–317. [Google Scholar] [CrossRef]
- Marmouzi, I.; Bouyahya, A.; Ezzat, S.M.; El Jemli, M.; Kharbach, M. The food plant Silybum marianum (L.) Gaertn.: Phytochemistry, Ethnopharmacology and clinical evidence. J. Ethnopharmacol. 2020, 265, 113303. [Google Scholar] [CrossRef]
- Biedermann, D.; Moravcová, V.; Valentová, K.; Kuzma, M.; Petrásková, L.; Císařová, I.; Křen, V. Oxidation of flavonolignan silydianin to unexpected lactone-acid derivative. Phytochem. Lett. 2019, 30, 14–20. [Google Scholar] [CrossRef]
- Di Carlo, G.; Autore, G.; A Izzo, A.; Maiolino, P.; Mascolo, N.; Viola, P.; Diurno, M.V.; Capasso, F. Inhibition of Intestinal Motility and Secretion by Flavonoids in Mice and Rats: Structure-activity Relationships. J. Pharm. Pharmacol. 1993, 45, 1054–1059. [Google Scholar] [CrossRef]
- Çağlayan, T.; Özakin, E.; Özdemi̇r, A. .; Acar, N.; Çanakçi, M.E.; Arslan, E.; Dolgun, H.; Baloğlu, F.K. Hemorajik Ve İskemik Serebrovasküler Hastalık Tanısı Alan Hastalarda Kan Laktat Düzeyinin Prognoz Üzerine Etkisi. Osman. J. Med. 2020, 00. [Google Scholar] [CrossRef]
- G. K~rber., “Beitrag zur kollektiven Behandlung pharmakologischer Reihenversuche”.
- Repetto, G.; del Peso, A.; Zurita, J.L. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat. Protoc. 2008, 3, 1125–1131. [Google Scholar] [CrossRef]
- Monpoeho, S.; Dehée, A.; Mignotte, B.; Schwartzbrod, L.; Marechal, V.; Nicolas, J.-C.; Billaudel, S.; Férré, V. Quantification of Enterovirus RNA in Sludge Samples Using Single Tube Real-Time RT-PCR. BioTechniques 2000, 29, 88–93. [Google Scholar] [CrossRef]
- Bertaccini, G.; Scarpignato, C. HISTAMINE H2-ANTAGONISTS MODIFY GASTRIC EMPTYING IN THE RAT. Br. J. Pharmacol. 1982, 77, 443–448. [Google Scholar] [CrossRef]
- Qin, N.; Sasaki, T.; Li, W.; Wang, J.; Zhang, X.; Li, D.; Li, Z.; Cheng, M.; Hua, H.; Koike, K. Identification of flavonolignans from Silybum marianum seeds as allosteric protein tyrosine phosphatase 1B inhibitors. J. Enzym. Inhib. Med. Chem. 2018, 33, 1283–1291. [Google Scholar] [CrossRef]
- Kim, N.-C.; Graf, T.N.; Sparacino, C.M.; Wani, M.C.; Wall, M.E. Complete isolation and characterization of silybins and isosilybins from milk thistle (Silybum marianum). Org. Biomol. Chem. 2003, 1, 1684–1689. [Google Scholar] [CrossRef] [PubMed]
- DETECTION OF ENTEROVIRUSES AND ADENOVIRUS BY REAL- TIME POLYMERASE CHAIN REACTION IN CELL CULTURE NEGATIVE STOOL SPECIMENS OF PATIENTS WITH ACUTE FLACCID PARALYSIS IN MOROCCO. Int. J. Biol. Pharm. Allied Sci. 2023, 12. [CrossRef]
- S. Shahmahmoodi et al., “Enteroviruses and Adenoviruses in stool specimens of paralytic children-can they be the cause of paralysis?” [Online]. Available: www.megasoftware.net.
- Lalani, S.S.; Anasir, M.I.; Poh, C.L. Antiviral activity of silymarin in comparison with baicalein against EV-A71. BMC Complement. Med. Ther. 2020, 20, 1–12. [Google Scholar] [CrossRef]
- da Silva, T.F.; Ferraz, A.C.; Almeida, L.T.; Caetano, C.C.d.S.; Camini, F.C.; Lima, R.L.S.; Andrade, A.C.d.S.P.; de Oliveira, D.B.; Rocha, K.L.S.; Silva, B.d.M.; et al. Antiviral effect of silymarin against Zika virus in vitro. Acta Trop. 2020, 211, 105613. [Google Scholar] [CrossRef] [PubMed]
- Lani, R.; Hassandarvish, P.; Chiam, C.W.; Moghaddam, E.; Chu, J.J.H.; Rausalu, K.; Merits, A.; Higgs, S.; Vanlandingham, D.; Abu Bakar, S.; et al. Antiviral activity of silymarin against chikungunya virus. Sci. Rep. 2015, 5, 11421. [Google Scholar] [CrossRef] [PubMed]
- J. C. G. V. W. T. A. Desplaces, “The effects of silymarin on experimental phalloidine poisoning,” Arzneimittel-Forschung/Drug Research, vol. 25, no. 1, pp. 89–96, 1975.
- F. C. Camini et al., “Antiviral activity of silymarin against Mayaro virus and protective effect in virus-induced oxidative stress,” Antiviral Res, vol. 158, pp. 8–12, Oct. 2018. [CrossRef]
- Song, J.; Choi, H. Silymarin efficacy against influenza A virus replication. Phytomedicine 2011, 18, 832–835. [Google Scholar] [CrossRef]
- Wagoner, J.; Negash, A.; Kane, O.J.; Martinez, L.E.; Nahmias, Y.; Bourne, N.; Owen, D.M.; Grove, J.; Brimacombe, C.; McKeating, J.A.; et al. Multiple effects of silymarin on the hepatitis C virus lifecycle. Hepatology 2010, 51, 1912–1921. [Google Scholar] [CrossRef]
- S. Ihsan, S. M. S. Ihsan, S. M. Hasany, S. Akram, A. Adnan, and M. Mushtaq, “Silybins: Antiviral liver analeptics,” in A Centum of Valuable Plant Bioactives, Elsevier, 2021, pp. 445–465. [CrossRef]
- Cardile, A.P.; Mbuy, G.K. Anti-herpes virus activity of silibinin, the primary active component of Silybum marianum. J. Herb. Med. 2013, 3, 132–136. [Google Scholar] [CrossRef]
- Biedermann, D.; Moravcová, V.; Valentová, K.; Kuzma, M.; Petrásková, L.; Císařová, I.; Křen, V. Oxidation of flavonolignan silydianin to unexpected lactone-acid derivative. Phytochem. Lett. 2019, 30, 14–20. [Google Scholar] [CrossRef]
- L. R. Fitzpatrick and T. Woldemariam, “Small-Molecule Drugs for the Treatment of Inflammatory Bowel Disease,” in Comprehensive Medicinal Chemistry III, Elsevier, 2017, pp. 495–510. [CrossRef]
- Biedermann, D.; Moravcová, V.; Valentová, K.; Kuzma, M.; Petrásková, L.; Císařová, I.; Křen, V. Oxidation of flavonolignan silydianin to unexpected lactone-acid derivative. Phytochem. Lett. 2019, 30, 14–20. [Google Scholar] [CrossRef]
- Wen, Y.-J.; Zhou, Z.-Y.; Zhang, G.-L.; Lu, X.-X. Metal coordination protocol for the synthesis of-2,3-dehydrosilybin and 19-O-demethyl-2,3-dehydrosilybin from silybin and their antitumor activities. Tetrahedron Lett. 2018, 59, 1666–1669. [Google Scholar] [CrossRef]
- Jaichand, J.; Sabu, K.K.; Iyer, T.V. Cytotoxicity Studies and Antiviral Activity of Sesbania grandiflora. Res. J. Pharm. Technol. 2024, 2839–2845. [Google Scholar] [CrossRef]
- Zhang, G.-J.; Li, Y.-H.; Jiang, J.-D.; Yu, S.-S.; Qu, J.; Ma, S.-G.; Liu, Y.-B.; Yu, D.-Q. Anti-Coxsackie virus B diterpenes from the roots of Illicium jiadifengpi. Tetrahedron 2013, 69, 1017–1023. [Google Scholar] [CrossRef]
- Morokutti-Kurz, M.; Graf, C.; Prieschl-Grassauer, E. Amylmetacresol/2,4-dichlorobenzyl alcohol, hexylresorcinol, or carrageenan lozenges as active treatments for sore throat. Int. J. Gen. Med. 2017, ume 10, 53–60. [Google Scholar] [CrossRef]
- W. X., A. In vitro antiviral activity of medicinal mushroom Ganoderma neo-japonicum Imazeki against enteroviruses that caused hand, foot and mouth disease. Trop. Biomed. 2021, 38, 239–247. [Google Scholar] [CrossRef]
- Dowdle, W.R.; De Gourville, E.; Kew, O.M.; Pallansch, M.A.; Wood, D.J. Polio eradication: the OPV paradox. Rev. Med Virol. 2003, 13, 277–291. [Google Scholar] [CrossRef]
- G. D. Rizea, I. G. D. Rizea, I. Crisan, C. C. Diaconu, and D. Mihele, “EVALUATION OF ANTIVIRAL AND CYTOTOXIC ACTIVITIES OF TAMARIX GALLICA AND SILYBUM MARIANUM EXTRACTS DANIELA IONESCU 1#, IOANA MADALINA ALDEA 2#, CORALIA BLEOTU 2, MIHAELA CHIVU ECONOMESCU 2, LILIA MATEI,” 2014.
- Chia, J.K.; Chia, A.Y.; Wang, D.; El-Habbal, R. Functional Dyspepsia and Chronic Gastritis Associated with Enteroviruses. Open J. Gastroenterol. 2015, 05, 21–27. [Google Scholar] [CrossRef]
- L.-F. A. O. S. et al Khetsuriani N, “Enterovirus surveillance—United States, 1970–2005. MMWR Surveill Summ..,” in Enterovirus surveillance—United States, 1970–2005. MMWR Surveill Summ.., 2006, pp. 55:1–20.
- Illuri, R.; Venkataramana, S.H.; Daguet, D.; Kodimule, S. Sub-acute and acute toxicity of Ferula asafoetida and Silybum marianum formulation and effect of the formulation on delaying gastric emptying. BMC Complement. Altern. Med. 2019, 19, 1–11. [Google Scholar] [CrossRef]
| Infections assay | Virus | |||||||
| Coxsackie B2 | Coxsackie A10 | EA71 | Poliovirus SL-1 | |||||
| EC50 (µg/mL) | SI | EC50 (µg/mL) | SI | EC50 (µg/mL) | SI | EC50 (µg/mL) | SI | |
| Virucidal activity | 109.86±29.04 | 3.73 | 112.40±20.36 | 3.64 | 100.31±2.70 | 4.08 | 110.78±13.90 | 3.69 |
| Cell protection | 169.26±41.61 | 2.42 | 120.88±43.62 | 3.39 | 186.100±45.47 | 2.20 | 173.36±54.33 | 2.36 |
| Post-Infection | 169.40±58.76 | 2.41 | 105.06±1.96 | 3.90 | 107.67±6.04 | 3.82 | 120.96±19.92 | 3.38 |
| CC50(µg/mL) | 409.80±33.70 | |||||||
| Infections assays | Concentraions (µg/mL) | ΔCt values | |||
| CXKB2 Virus | EA71 Virus | CXKA10 Virus | Polio Virus -SL1 | ||
| Virucidal activity | 200 | - | 14.167±0.707b | - | - |
| 100 | 8.833 ±0.707a | 13.167±0.707a | - | - | |
| 50 | 0.833±0.707a | 9.667±1.414ab | - | - | |
| Cell protection | 200 | 8.333±1.414ns | 3.667±1.414b | - | - |
| 100 | 5.333±1.414a | 3.667±1.414a | - | - | |
| 50 | 1.333±1.414a | 1.167±0.707ab | 2.167±0.707 | - | |
| Post-infection | 200 | - | 6.667±2.828b | 3.667±1.414ns | - |
| 100 | 8.833±0.707a | 4.167±2.121a | 3.167±0.707ns | - | |
| 50 | 2.333±2.828a | 1.167±2.121a | 1.767±1.414ns | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





