Submitted:
21 August 2024
Posted:
23 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
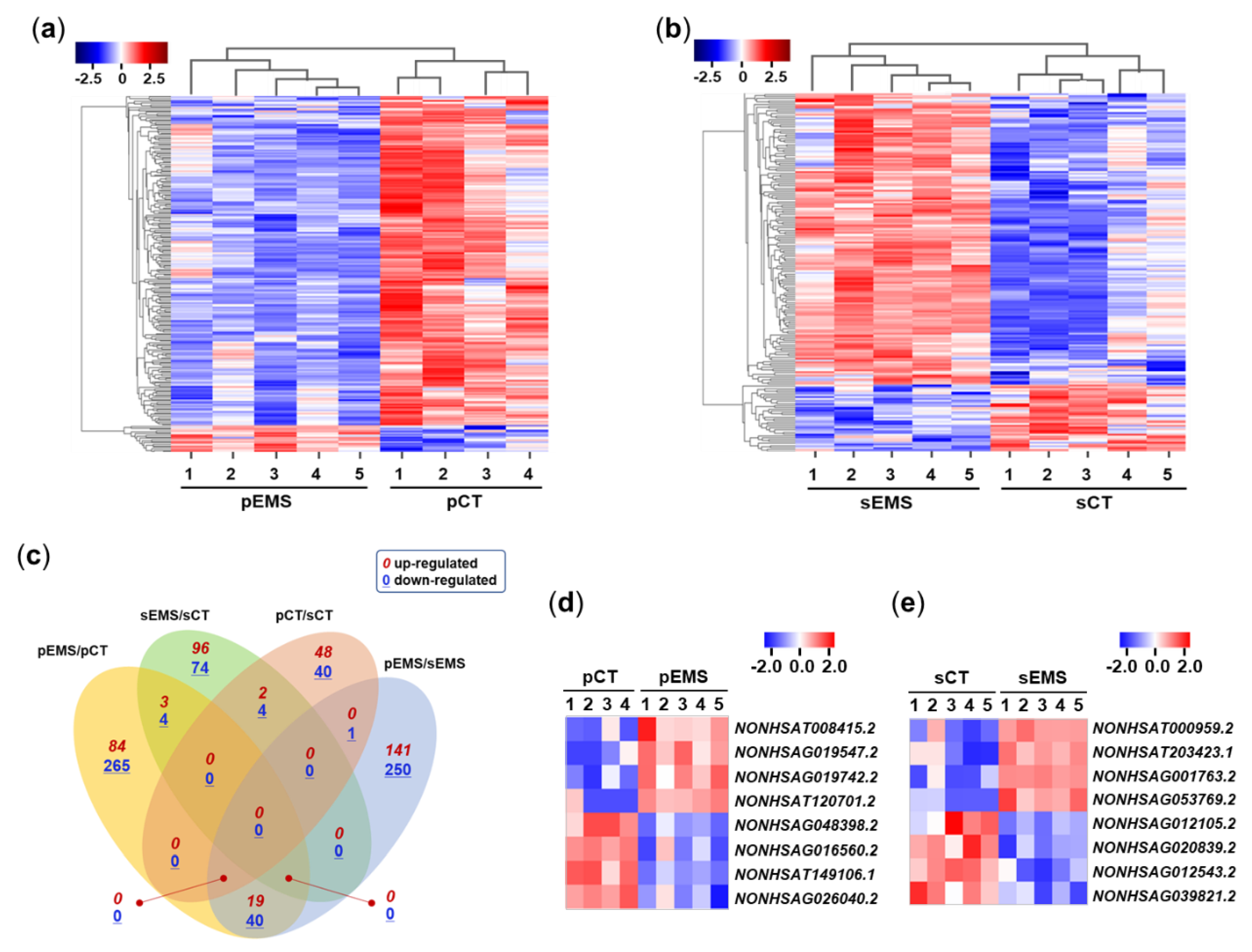
2.1. Identification of DElncRNAs in the Control and EMS Groups during the Menstrual Cycle
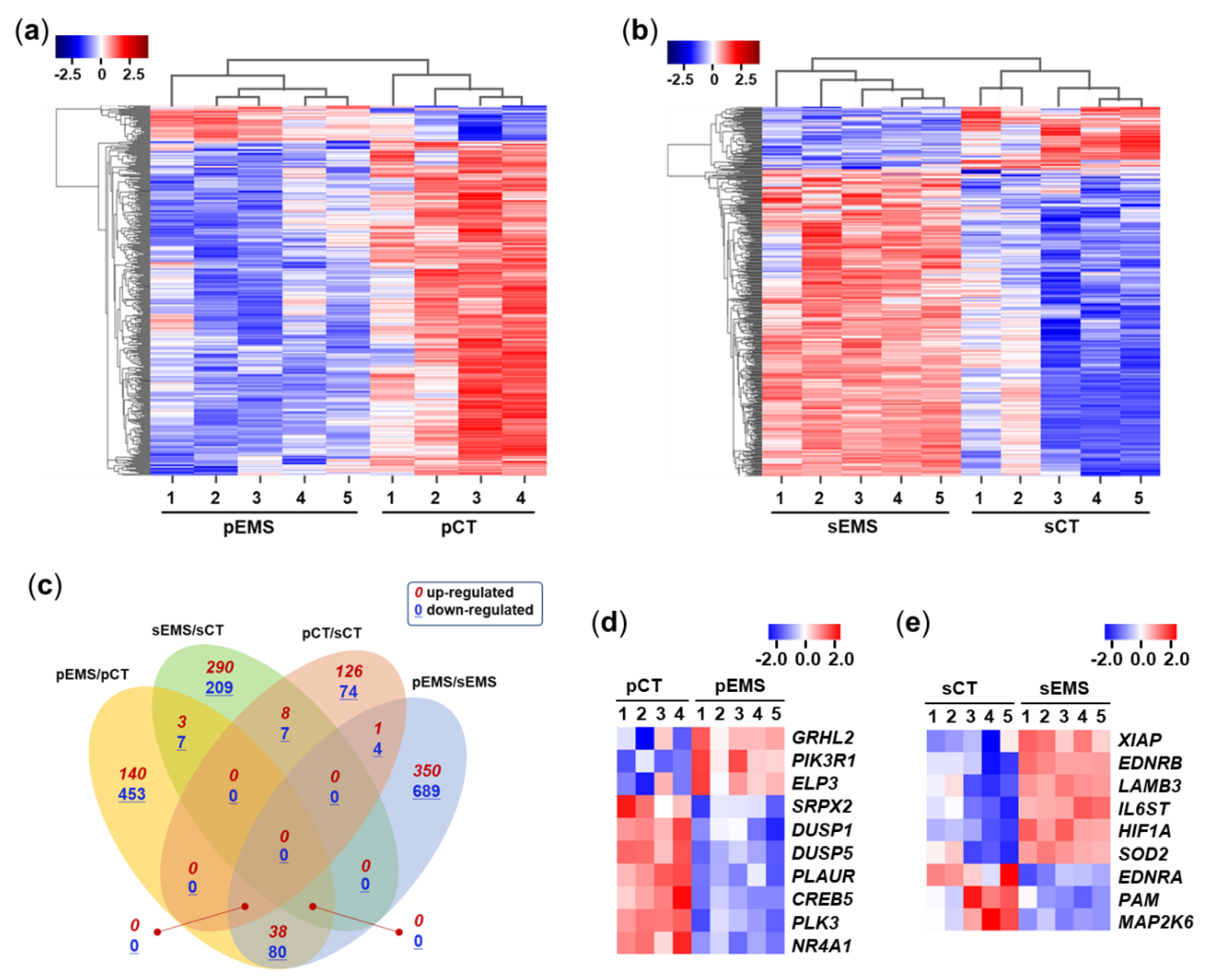
2.2. Identification of DEmRNAs between NC and EMS Groups during the Menstrual Cycle
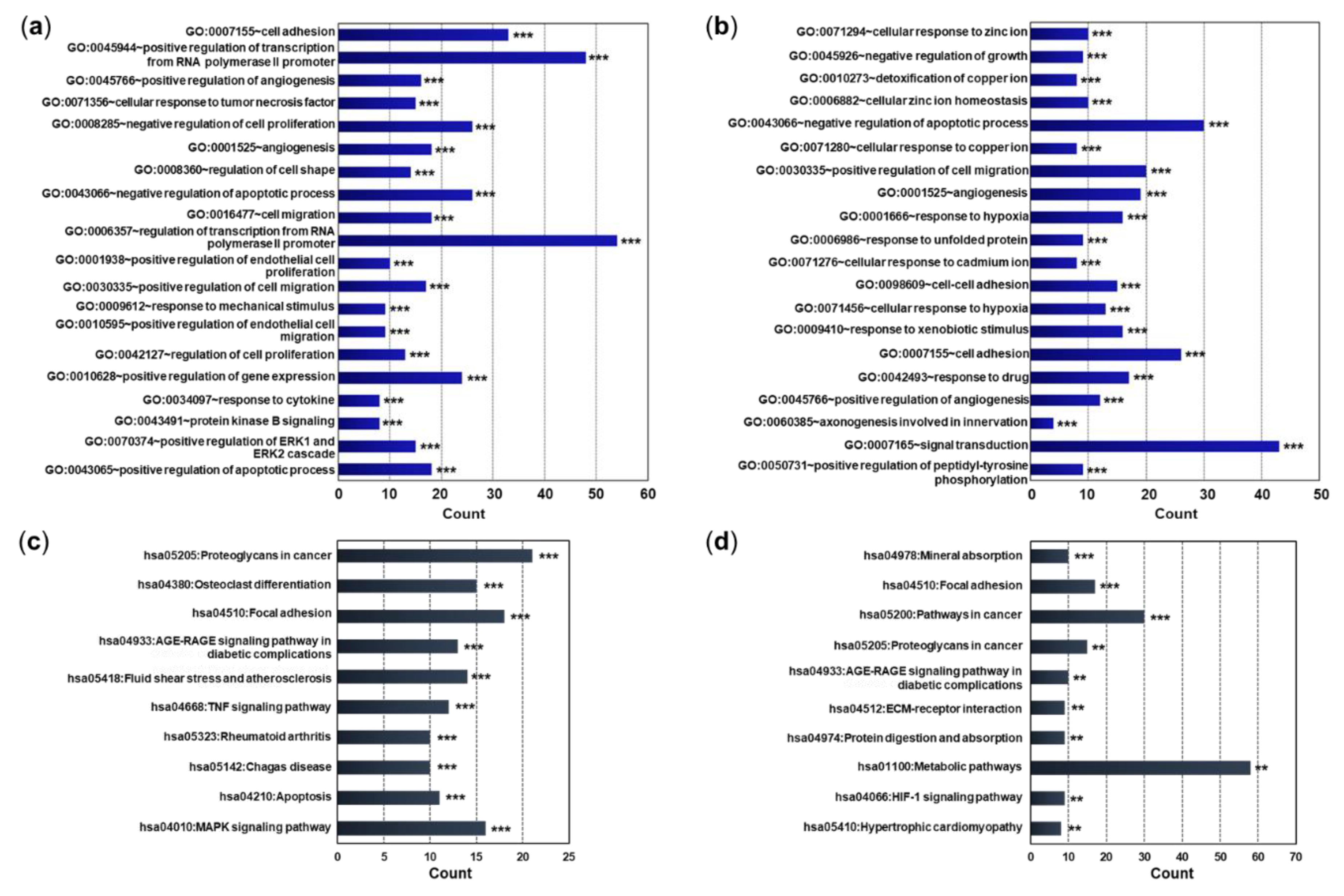
2.3. Functional Annotation and Pathway Networks of mRNAs
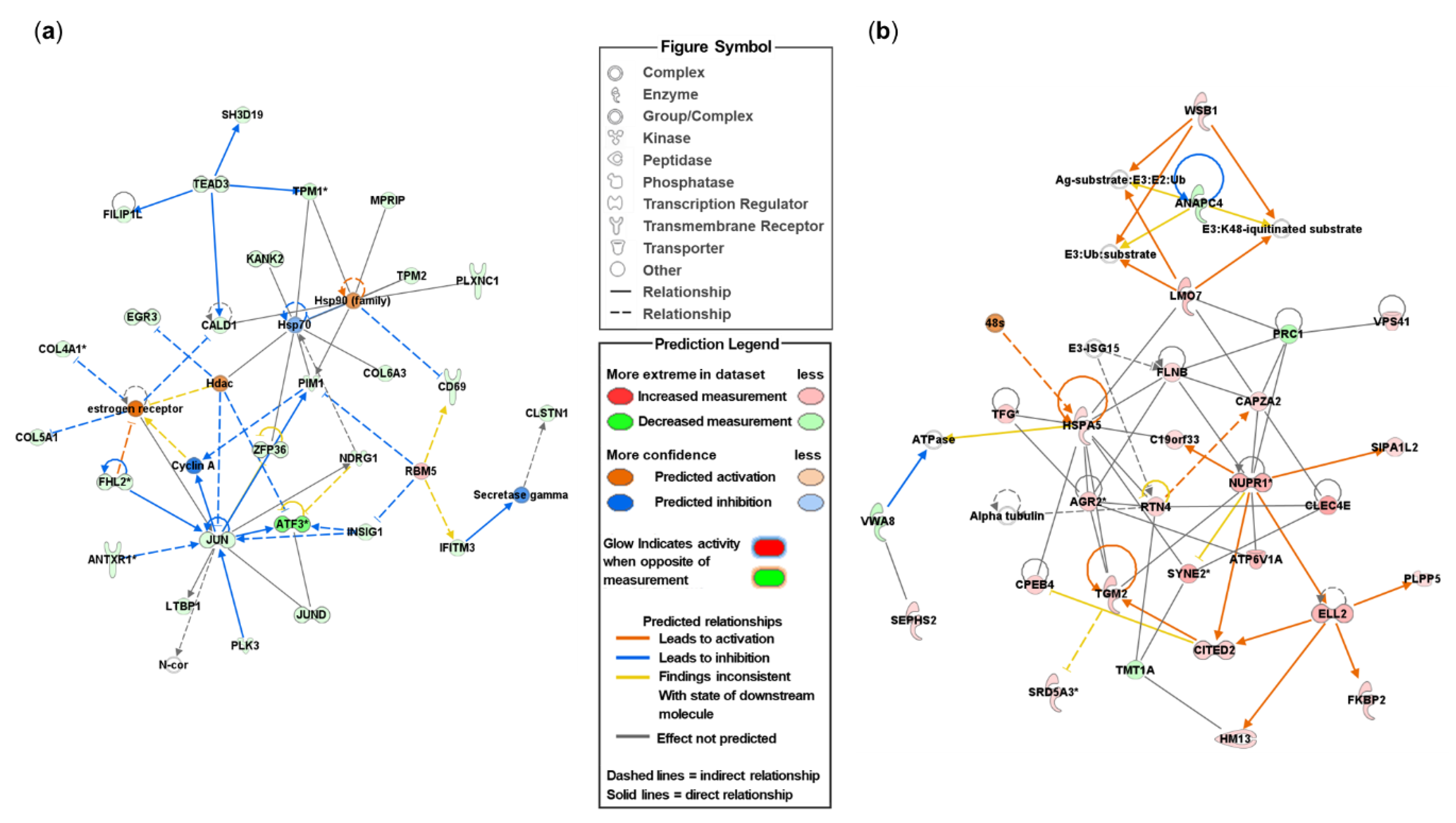
2.4. Pathway Network Identification of DEmRNAs
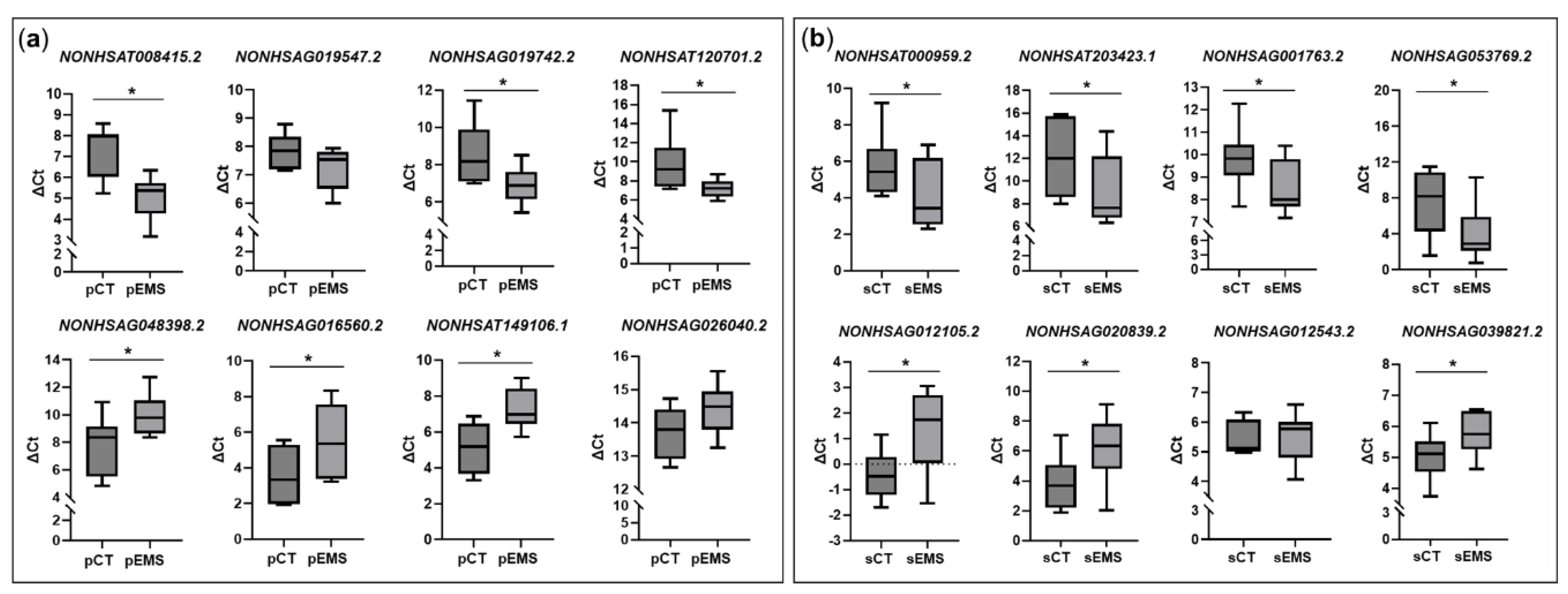
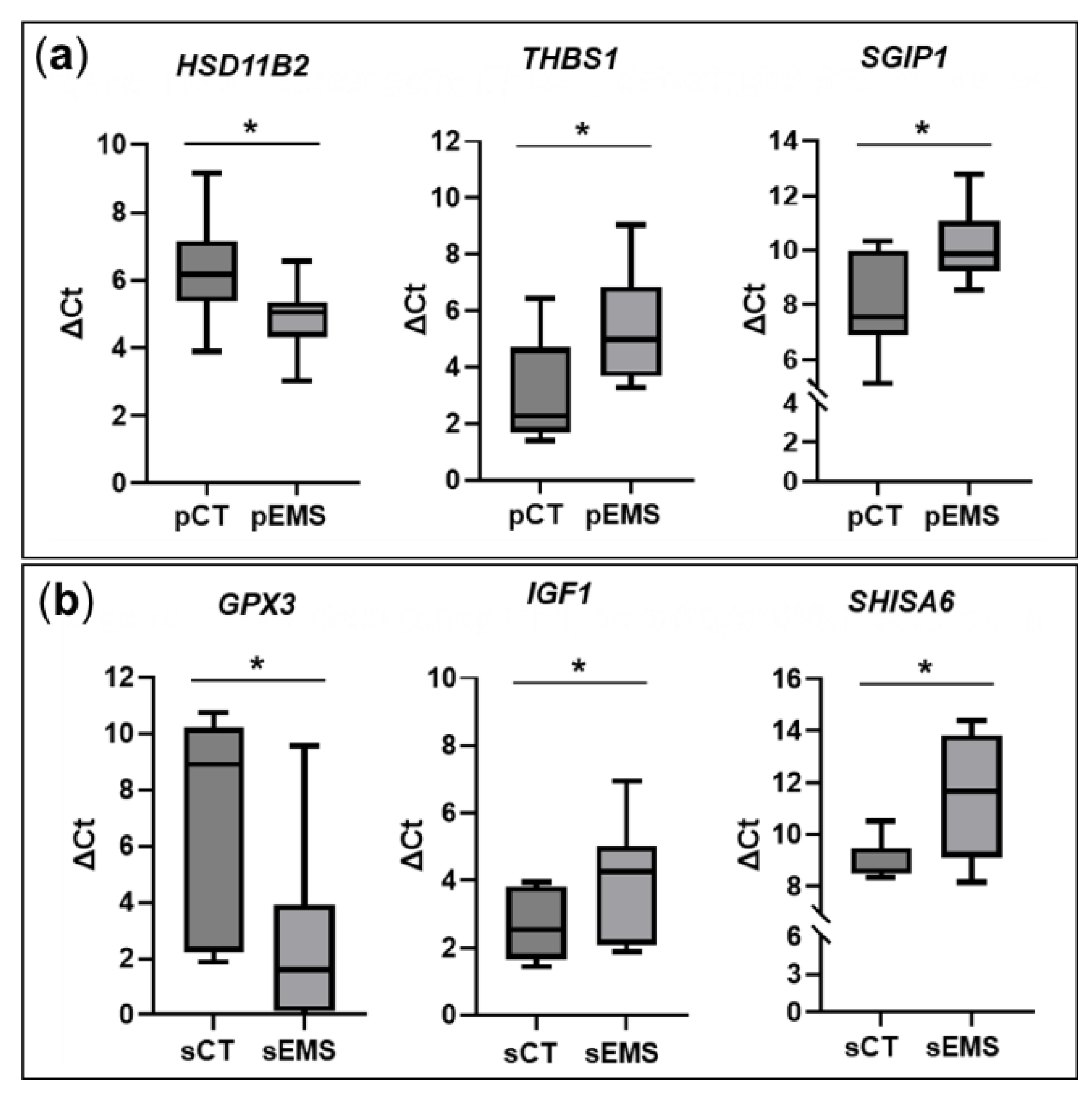
2.5. Validation of lncRNAs and Neighboring Genes Based on qRT-PCR
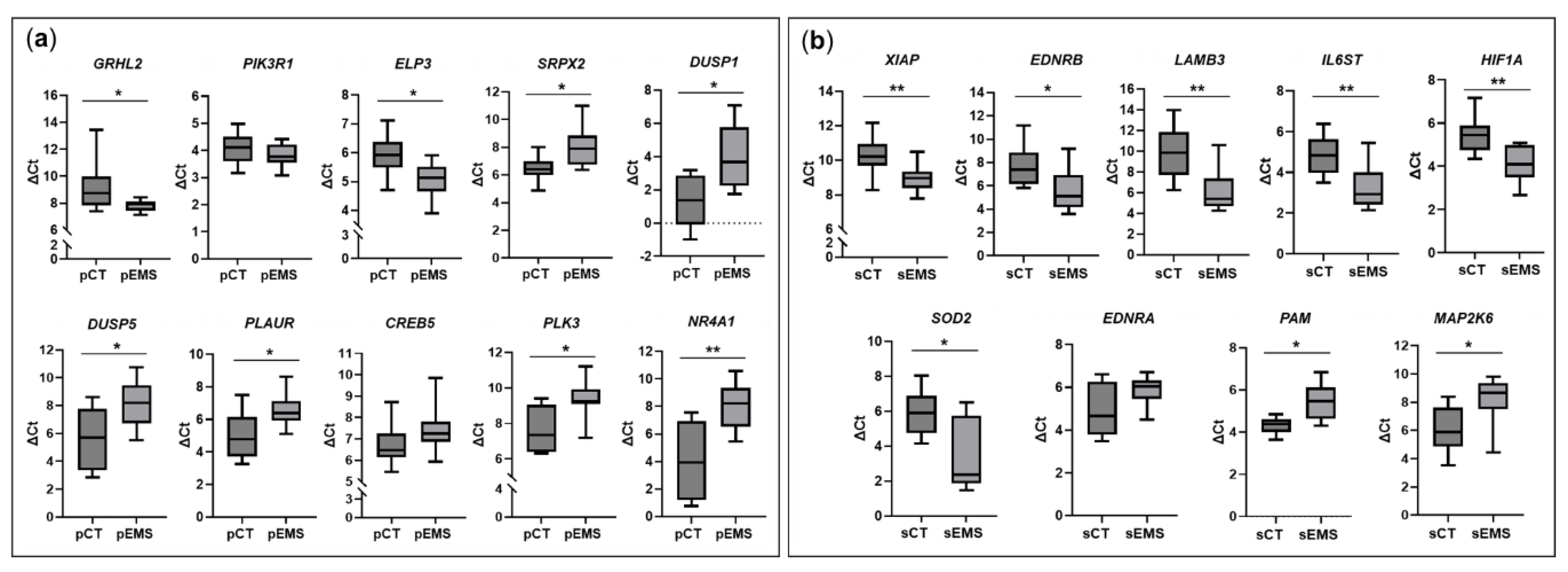
2.6. Validation of mRNAs Based on qRT-PCR
3. Discussion
4. Materials and Methods
4.1. Sample Collection
4.2. Library Preparation and Sequencing
4.3. Differerential Gene Expression Analysis and Functional Annotation
4.4. qRT-PCR Analysis
4.5. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giudice, L.C.; Kao, L.C. Endometriosis. Lancet 2004, 364, 1789–1799. [Google Scholar] [CrossRef]
- Ozkan, S.; Murk, W.; Arici, A. Endometriosis and infertility: epidemiology and evidence-based treatments. Ann N Y Acad Sci 2008, 1127, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Wingfield, M.; Macpherson, A.; Healy, D.L.; Rogers, P.A. Cell proliferation is increased in the endometrium of women with endometriosis. Fertil. Steril. 1995, 64, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Esteller, M. Non-coding RNAs in human disease. Nat. Rev. Genet. 2011, 12, 861–874. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.R.; Jin, Y.B.; Kim, H.N.; Lee, H.; Chai, Y.G.; Lee, S.R.; Kim, D.J. Differential Gene Expression in the Hippocampi of Nonhuman Primates Chronically Exposed to Methamphetamine, Cocaine, or Heroin. Psychiatry Investig 2022, 19, 538–550. [Google Scholar] [CrossRef]
- Choi, M.R.; Cho, S.; Kim, D.J.; Choi, J.S.; Jin, Y.B.; Kim, M.; Chang, H.J.; Jeon, S.H.; Yang, Y.D.; Lee, S.R. Effects of Ethanol on Expression of Coding and Noncoding RNAs in Murine Neuroblastoma Neuro2a Cells. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.R.; Jin, Y.B.; Kim, H.N.; Chai, Y.G.; Im, C.N.; Lee, S.R.; Kim, D.J. Gene expression in the striatum of cynomolgus monkeys after chronic administration of cocaine and heroin. Basic Clin Pharmacol Toxicol 2021, 128, 686–698. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.R.; Jin, Y.B.; Bang, S.H.; Im, C.N.; Lee, Y.; Kim, H.N.; Chang, K.T.; Lee, S.R.; Kim, D.J. Age-related Effects of Heroin on Gene Expression in the Hippocampus and Striatum of Cynomolgus Monkeys. Clin Psychopharmacol Neurosci 2020, 18, 93–108. [Google Scholar] [CrossRef]
- Khatoon, Z.; Figler, B.; Zhang, H.; Cheng, F. Introduction to RNA-Seq and its applications to drug discovery and development. Drug Dev. Res. 2014, 75, 324–330. [Google Scholar] [CrossRef]
- Fang, Y.; Fullwood, M.J. Roles, Functions, and Mechanisms of Long Non-coding RNAs in Cancer. Genomics Proteomics Bioinformatics 2016, 14, 42–54. [Google Scholar] [CrossRef]
- Hudson, Q.J.; Proestling, K.; Perricos, A.; Kuessel, L.; Husslein, H.; Wenzl, R.; Yotova, I. The role of long non-coding RNAs in endometriosis. Int. J. Mol. Sci. 2021, 22, 11425. [Google Scholar] [CrossRef]
- Zhang, M.; He, P.; Bian, Z. Long Noncoding RNAs in Neurodegenerative Diseases: Pathogenesis and Potential Implications as Clinical Biomarkers. Front Mol Neurosci 2021, 14, 685143. [Google Scholar] [CrossRef]
- Sun, P.R.; Jia, S.Z.; Lin, H.; Leng, J.H.; Lang, J.H. Genome-wide profiling of long noncoding ribonucleic acid expression patterns in ovarian endometriosis by microarray. Fertil. Steril. 2014, 101, 1038–1046. [Google Scholar] [CrossRef] [PubMed]
- Bi, J.; Wang, D.; Cui, L.; Yang, Q. RNA sequencing-based long non-coding RNA analysis and immunoassay in ovarian endometriosis. Am J Reprod Immunol 2021, 85, e13359. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Luo, Y.; Wang, G.; Yang, Q. Circular RNA expression profiles and bioinformatics analysis in ovarian endometriosis. Mol Genet Genomic Med 2019, 7, e00756. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Zhai, L.; Wang, J.; Yu, Q.; Li, T.; Xu, X.; Guo, X.; Mao, X.; Zhou, J.; Zhang, X. Comprehensive Analysis of RNA-Seq in Endometriosis Reveals Competing Endogenous RNA Network Composed of circRNA, lncRNA and mRNA. Front Genet 2022, 13, 828238. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Song, X. The Working Modules of Long Noncoding RNAs in Cancer Cells. Adv Exp Med Biol 2016, 927, 49–67. [Google Scholar] [CrossRef]
- Yang, G.; Lu, X.; Yuan, L. LncRNA: a link between RNA and cancer. Biochim. Biophys. Acta 2014, 1839, 1097–1109. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Q.; Wang, H.; Duan, E. Uterine Fluid in Pregnancy: A Biological and Clinical Outlook. Trends Mol Med 2017, 23, 604–614. [Google Scholar] [CrossRef]
- Anderson, K.M.; Anderson, D.M.; McAnally, J.R.; Shelton, J.M.; Bassel-Duby, R.; Olson, E.N. Transcription of the non-coding RNA upperhand controls Hand2 expression and heart development. Nature 2016, 539, 433–436. [Google Scholar] [CrossRef]
- Joung, J.; Engreitz, J.M.; Konermann, S.; Abudayyeh, O.O.; Verdine, V.K.; Aguet, F.; Gootenberg, J.S.; Sanjana, N.E.; Wright, J.B.; Fulco, C.P.; et al. Genome-scale activation screen identifies a lncRNA locus regulating a gene neighbourhood. Nature 2017, 548, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Lu, J.Y.; Liu, L.; Yin, Y.; Chen, C.; Han, X.; Wu, B.; Xu, R.; Liu, W.; Yan, P.; et al. Divergent lncRNAs regulate gene expression and lineage differentiation in pluripotent cells. Cell Stem Cell 2016, 18, 637–652. [Google Scholar] [CrossRef]
- Wang, Y.; Nicholes, K.; Shih, I.M. The Origin and Pathogenesis of Endometriosis. Annu Rev Pathol 2020, 15, 71–95. [Google Scholar] [CrossRef] [PubMed]
- Nasiadek, M.; Stragierowicz, J.; Klimczak, M.; Kilanowicz, A. The role of zinc in selected female reproductive system disorders. Nutrients 2020, 12, 2464. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Yue, X.; Zhang, Y.; Shen, L.; Zhang, H.; Wang, X.; Yin, T.; Zhang, H.; Peng, J.; Wang, X.; et al. Elevated levels of Zn, Cu and Co are associated with an increased risk of endometriosis: Results from a casecontrol study. Ecotoxicol Environ Saf 2024, 271, 115932. [Google Scholar] [CrossRef] [PubMed]
- Salmena, L.; Poliseno, L.; Tay, Y.; Kats, L.; Pandolfi, P.P. A ceRNA hypothesis: the Rosetta Stone of a hidden RNA language? Cell 2011, 146, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Villegas, V.E.; Zaphiropoulos, P.G. Neighboring gene regulation by antisense long non-coding RNAs. Int. J. Mol. Sci. 2015, 16, 3251–3266. [Google Scholar] [CrossRef]
- Zhang, X.; Nie, X.; Yuan, S.; Li, H.; Fan, J.; Li, C.; Sun, Y.; Zhao, Y.; Hou, H.; Wang, D.W.; et al. Circulating Long Non-coding RNA ENST00000507296 Is a Prognostic Indicator in Patients with Dilated Cardiomyopathy. Mol Ther Nucleic Acids 2019, 16, 82–90. [Google Scholar] [CrossRef]
- Cai, B.; Cai, J.; Yin, Z.; Jiang, X.; Yao, C.; Ma, J.; Xue, Z.; Miao, P.; Xiao, Q.; Cheng, Y.; et al. Long non-coding RNA expression profiles in neutrophils revealed potential biomarker for prediction of renal involvement in SLE patients. Rheumatology (Oxford) 2021, 60, 1734–1746. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hu, S.; Yao, G.; Zhu, Q.; He, Y.; Lu, Y.; Qi, J.; Xu, R.; Ding, Y.; Li, J.; et al. A Novel Molecule in Human Cyclic Endometrium: LncRNA TUNAR Is Involved in Embryo Implantation. Front Physiol 2020, 11, 587448. [Google Scholar] [CrossRef]
- Krozowski, Z.; MaGuire, J.A.; Stein-Oakley, A.N.; Dowling, J.; Smith, R.E.; Andrews, R.K. Immunohistochemical localization of the 11 beta-hydroxysteroid dehydrogenase type II enzyme in human kidney and placenta. J. Clin. Endocrinol. Metab. 1995, 80, 2203–2209. [Google Scholar] [CrossRef] [PubMed]
- Monsivais, D.; Bray, J.D.; Su, E.; Pavone, M.E.; Dyson, M.T.; Navarro, A.; Kakinuma, T.; Bulun, S.E. Activated glucocorticoid and eicosanoid pathways in endometriosis. Fertil. Steril. 2012, 98, 117–125. [Google Scholar] [CrossRef]
- Tokue, M.; Ikami, K.; Mizuno, S.; Takagi, C.; Miyagi, A.; Takada, R.; Noda, C.; Kitadate, Y.; Hara, K.; Mizuguchi, H.; et al. SHISA6 Confers Resistance to Differentiation-Promoting Wnt/β-Catenin Signaling in Mouse Spermatogenic Stem Cells. Stem Cell Reports 2017, 8, 561–575. [Google Scholar] [CrossRef]
- Woodward, E.; Schlingmann, K.; Tobias, J.; Turner, R. Characterisation of the testicular transcriptome in stallions with age-related testicular degeneration. Equine Vet J 2023, 55, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Ouyang, C.; Huang, C.; Zhang, J.; Xiao, Q.; Zhang, F.; Wang, H.; Lin, F.; Wang, J.; Wang, Z.; et al. ELP3 stabilizes c-Myc to promote tumorigenesis. J. Mol. Cell. Biol. 2024, 15, mjad059. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ikeda, J.I.; Rahadiani, N.; Mamat, S.; Ueda, Y.; Tian, T.; Enomoto, T.; Kimura, T.; Aozasa, K.; Morii, E. Prognostic significance of elongator protein 3 expression in endometrioid adenocarcinoma. Oncol Lett 2012, 3, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Kalma, Y.; Granot, I.; Gnainsky, Y.; Or, Y.; Czernobilsky, B.; Dekel, N.; Barash, A. Endometrial biopsy-induced gene modulation: first evidence for the expression of bladder-transmembranal uroplakin Ib in human endometrium. Fertil. Steril. 2009, 91, 1042–1049. [Google Scholar] [CrossRef] [PubMed]
- Safe, S.; Karki, K. The Paradoxical Roles of Orphan Nuclear Receptor 4A (NR4A) in Cancer. Mol Cancer Res 2021, 19, 180–191. [Google Scholar] [CrossRef]
- Zeng, X.; Yue, Z.; Gao, Y.; Jiang, G.; Zeng, F.; Shao, Y.; Huang, J.; Yin, M.; Li, Y. NR4A1 is Involved in Fibrogenesis in Ovarian Endometriosis. Cell Physiol Biochem 2018, 46, 1078–1090. [Google Scholar] [CrossRef]
- Tang, X.; Zheng, H.; Xu, H.; Wang, M.; Kang, X.; Wu, Z. NR4A1 Affects endometrial receptivity by participating in mesenchymal–epithelial transition of endometrial stromal cells. Reprod. Sci. 2022, 29, 133–142. [Google Scholar] [CrossRef]
- Ii, M.; Yamamoto, H.; Taniguchi, H.; Adachi, Y.; Nakazawa, M.; Ohashi, H.; Tanuma, T.; Sukawa, Y.; Suzuki, H.; Sasaki, S.; et al. Co-expression of laminin β3 and γ2 chains and epigenetic inactivation of laminin α3 chain in gastric cancer. Int. J. Oncol. 2011, 39, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.M.; Li, J.; Yan, M.X.; Liu, L.; Jia, D.S.; Geng, Q.; Lin, H.C.; He, X.H.; Li, J.J.; Yao, M. Integrative analyses identify osteopontin, LAMB3 and ITGB1 as critical pro-metastatic genes for lung cancer. PLoS One 2013, 8, e55714. [Google Scholar] [CrossRef]
- Diao, B.; Yang, P. Comprehensive Analysis of the Expression and Prognosis for Laminin Genes in Ovarian Cancer. Pathol Oncol Res 2021, 27, 1609855. [Google Scholar] [CrossRef] [PubMed]
- Guirguis, A.; Elishaev, E.; Oh, S.H.; Tseng, G.C.; Zorn, K.; DeLoia, J.A. Use of gene expression profiles to stage concurrent endometrioid tumors of the endometrium and ovary. Gynecol. Oncol. 2008, 108, 370–376. [Google Scholar] [CrossRef]
- Kumari, P.; Sharma, I.; Saha, S.C.; Srinivasan, R.; Sharma, A. Promoter methylation status of key genes and its implications in the pathogenesis of endometriosis, endometrioid carcinoma of ovary and endometrioid endometrial cancer. J Cancer Res Ther 2022, 18, S328–s334. [Google Scholar] [CrossRef]
- Sivridis, E.; Giatromanolaki, A.; Gatter, K.C.; Harris, A.L.; Koukourakis, M.I.; Tumor and Angiogenesis Research Group. Association of hypoxia-inducible factors 1α and 2α with activated angiogenic pathways and prognosis in patients with endometrial carcinoma. Cancer 2002, 95, 1055–1063. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.M.; Yang, W.X. Epithelial-to-mesenchymal transition in the development of endometriosis. Oncotarget 2017, 8, 41679–41689. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, X.L.; Wang, W.; Dong, H.L.; Xia, Y.F.; Ruan, L.P.; Liu, L.P. Expression of MMIF, HIF-1α and VEGF in Serum and Endometrial Tissues of Patients with Endometriosis. Curr Med Sci 2018, 38, 499–504. [Google Scholar] [CrossRef]
- Simon, A. Babraham Bioinformatics. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc (accessed on 24 May 2023).
- Hannon Lab. FASTX-Toolkit. Available online: http://hannonlab.cshl.edu/fastx_toolkit/index.html (accessed on 24 April 2023).
- Bushnell, B. BBMap. Available online: https://sourceforge.net/projects/bbmap (accessed on 3 May 2023).
- Trapnell, C.; Pachter, L.; Salzberg, S.L. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 2009, 25, 1105–1111. [Google Scholar] [CrossRef]
- Roberts, A.; Trapnell, C.; Donaghey, J.; Rinn, J.L.; Pachter, L. Improving RNA-Seq expression estimates by correcting for fragment bias. Genome Biol 2011, 12, R22. [Google Scholar] [CrossRef]
- Choi, M.R.; Jung, K.H.; Park, J.H.; Das, N.D.; Chung, M.K.; Choi, I.G.; Lee, B.C.; Park, K.S.; Chai, Y.G. Ethanol-induced small heat shock protein genes in the differentiation of mouse embryonic neural stem cells. Arch Toxicol 2011, 85, 293–304. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




