Introduction
The reproductive study of animals used in the livestock industry is part of the success of these systems (Madhusoodan et al., 2019). Bull fertility is important in the development of the productive systems (Waberski et al. 2021, Barth 2018), and their evaluation allows one to select those animals with better reproductive conditions (Petrunkina et al., 2007) or discard animals or ejaculates with poor quality parameters that could be infertile or subfertile. A bull’s seminal quality could be influenced by multiple factors, such as season (Barth, 2018), health (Taylor et al., 2018), nutrition (Cheah & Yang, 2011), animal species (Castellini et al., 2011), and semen conservation (Awad, 2011).
Bull semen preservation is usually associated with assisted reproduction techniques in which the spermatozoon is generally frozen. However, subjecting the cells to this process can affect their characteristics, thus limiting their functionality when they are subsequently thawed (Awad, 2011). Sperm morphometry is a cell characteristic that can be used as an indicator for potential freezability or male fertility (Maroto-Morales et al., 2016; Villaverde-Morcillo et al., 2017).
Sperm morphometry is usually determined by performing the CASA-Morph module (Gallagher et al., 2018; Mortimer & De Jonge, 2018; Valverde et al., 2019), which allows one to analyze characteristics related to the size and shape of the head, acrosome, and intermediate piece of the sperm (Vicente-Fiel et al., 2013; Yániz et al., 2015). These characteristics may differ according to the method of cell fixation (Soler et al., 2015), staining (Banaszewska et al., 2015; Boersma et al., 1999), and evaluation systems (Vicente-Fiel et al., 2013). Morphometry is relevant because it could be used to predict the fertility potential of males (Franken, 2015; Toner et al., 1995) and to explain alterations of cells caused by the spermiogenesis process (Valverde et al., 2016), as well as explain some relationships with other sperm variables (Giaretta et al., 2017; Hook & Fisher, 2020). Despite the importance of this analysis, its use has been characterized by variability in determinations (Garcia-Herreros et al., 2006).
For this reason, these variations in the morphometry of sperm cells have been analyzed and distinct characteristics have been determined in the ejaculate of the same animal (Buchelly Imbachí et al., 2022; Martínez-Pastor, 2022). Therefore, using cluster analysis allowed us to identify different subpopulations with an ejaculate based on the data from the CASA systems (Martínez-Pastor, 2022; Valverde et al., 2016; Yániz et al., 2018). These new analyses have favored the explanation of the conformation of the ejaculates and their relationship with the potential fertility of the animals analyzed (Santolaria et al., 2015).
On the other hand, sperm morphometric characterization could be used to distinguish animal species (Sampaio et al., 2017). Differences in sperm morphometry have been observed when comparing different species of foxes (Andraszek et al., 2020), ovine (Martínez-Fresneda et al., 2019), neotropical primate (Sampaio et al., 2017), and even dog breeds (Soler, Alambiaga, et al., 2017). In the literature, there are separate studies of sperm morphometry for both B. taurus and B. indicus, but there are only a few that compare them within the same study (Beletti et al., 2005) .To our knowledge, no study has compared sperm morphometry between B. taurus taurus and B. taurus indicus using a CASA-morph system and subpopulation analyses.
Therefore, the aim of this study was to determine different sperm subpopulations based on morphometry parameters of frozen-thawed semen in two bovine subspecies using a CASA-morph system.
Methodology
This study was performed following ethical principles and with the approval of the Committee of Centro de Investigación y Desarrollo de la Agricultura Sostenible para el Trópico Húmedo at the Costa Rica Institute of Technology (CIDASTH-ITCR) according to Section 08/2023, article 5.0, DAGSC-075-2023. The study was conducted using cryopreserved straws. The cryopreserved semen was supplied by Avance Genético S.A. (Zapote, Costa Rica). The present study was conducted from May to December 2023, at the Animal Reproduction Laboratory (AndroTEC), located at the Campus Tecnológico Local San Carlos, in Santa Clara, Florencia, San Carlos, Alajuela, Costa Rica (CRTM05; X: 444296 Y: 1146016).
Semen Collection and Processing: Five Bos taurus (Simmental) bulls and five Bos indicus (Brahman) bulls with an average age of 5.7±2.8 and 3.6±1.6 years, respectively, were used. Semen from bulls was collected using an artificial vagina. The bulls were under a collection program and routinely used for commercial purposes two times per week. The sampling approach involved collecting a single ejaculate from ten randomly selected bulls, which had been previously assessed for acceptable post-thaw characteristics during an evaluation for standard breeding soundness and fertility. A few minutes after semen collection, the volume of the ejaculate was assessed by employing a conical tube with 0.1 mL gradations at 530 nm wavelength measurement using a bovine photometer Accucell (IMV, L’Aigle, France).
The OptiXcell® (IMV, L’Aigle, France; Triladyl®, Minitube, Tiefenbach, Germany) extender was used to dilute the raw semen. The commercial extender was used following the manufacturer’s instructions. The dilution ratio is dependent on the ejaculate volume; the final concentration given with the diluent was 25×106 cells/straw. Diluted semen was slowly cooled in a refrigerator at a linear rate of −0.3°C min−1 until 4 °C, and the semen was maintained at the same temperature for 4–5 hours to equilibrate. Then, an automatic filling and sealing machine (MRS1, IMV Technologies, L’Aigle, France) was used to package the semen in 0.25 mL straws, which were frozen in a programmable freezer, (Digitcool 5300, IMV, L’Aigle, France) at the following rates: 4 to −10°C at 5°C min−1, −10 to −100°C at 40°C min−1, and −110 to −140°C at 20°C min−1. Finally, the straws were stored by plunging into liquid nitrogen. For the morphometric analysis, samples were coded so that the technician would not know information about the bull or to which bull the ejaculate belonged.
Sample preparation for morphometric analysis: Morphometric analysis was carried out with the samples prepared from straws for each bull. For analysis, the straw contents were mixed and a subsample of 10 μL extended on a slide. The slides were identified for staining. The Diff-Quik® kit (Medion Diagnostics, Düdingen, Switzerland) was used for staining following all the manufacturer’s instructions step by step. The slides were then kept for the subsequent double-blind scheme analysis.
Assessment of sperm variables: ISAS® v1 (Integrated Semen Analysis System, Proiser R+D, Valencia, Spain) was used for morphometric assessment. The system consists of a UB203 microscope (UOP/Proiser R + D) with a 100× bright-field objective and an attached 3.3× photo-ocular. Above the microscope, a digital video camera (Proiser 782 m, Proiser R + D) captured all the images, which were saved on the linked computer. The video frame grabber array size was 746 × 578 × 8 bit, making a 0.084 μm/pixel resolution available in both axes of the analysis images and 256 gray levels. In both axes, the image resolution was 0.08 μm per pixel. Different fields were randomly selected for the capture of spermatozoa, and overlapping cells or background particles interfering with the posterior processing of the image were rejected. The system allowed us to vary the analysis factor in case of an erroneous definition of the sperm head boundary, and when it was not possible to analyze a correct boundary, the cell was deleted to avoid errors in the subsequent data analysis.
Morphometric analysis: To obtain an adequate value for each of the eight morphometric variables, a minimum of 200 images of different spermatozoa were captured and analyzed. The technician followed the Boersma criteria (Boersma et al., 1999) and measured the main parameters of head size (length [L, μm], width [W, μm], area [A, μm2], and perimeter [P, μm]). Four additional dimensionless variables derived from the head size were obtained, namely head shape (ellipticity [L/W], rugosity [4πA/P2], elongation [(L−W)/(L+W)], and regularity [πLW/4A]). Data obtained from each spermatozoon were saved in an Excel® (Microsoft Corporation, Redmond, Washington, USA) compatible database for further analysis.
Statistical analysis: Data obtained for the analysis of all sperm variables were assessed for homoscedasticity using Levene’s tests. Furthermore, a normal probability plot was used to assess distribution. Normal distribution with an identity link function was assumed for all sperm morphometric response variables. ANOVA was further applied to evaluate statistical differences between treatments for all morphometric variables. Pairwise comparisons between species and extender means were performed using the Tukey–Kramer test.
Multivariate procedures: Multivariate procedures were performed to identify sperm clusters from this subset of sperm morphometric data. All values for morphometric variables were standardized to avoid any scaling effect. Principal factor analysis (PFA) was performed on the data to derive a small number of linear combinations while still retaining as much information as possible from the original variables. Prior communalities for this analysis were estimated from the maximum absolute correlation coefficient between each variable and any other. The number of principal factors (PFs) to be extracted was determined from the Kaiser criterion, namely by selecting only those with an eigenvalue >1. The KMO (Kaiser–Meyer–Olkin) statistic was also determined (Spencer, 2013) as a measure of dataset adequacy for factor extraction. We used the varimax method with Kaiser normalization (Kaiser, 1958) as a rotation method.
Furthermore, we performed a two-step cluster procedure with the sperm-derived indices obtained after the PCA for analysis. All sperm morphometric measurements within each ejaculate were clustered according to shape and size parameters using a non-hierarchical K-means clustering procedure (K-means model and Euclidean distance) (Kaufman & Rousseuw, 1991). In doing so, the spermatozoa of the dataset are classified into a small number of cluster subpopulations according to their head dimensions, as has been previously described (Barquero et al., 2021). This analysis allowed us to identify sperm subpopulations and the detect outliers. ANOVA was further applied to evaluate statistical differences between clusters for all morphometric variables. The threshold for significance was defined as P < 0.05. Pairwise comparison between cluster means was performed via the Tukey–Kramer test. Results are presented as mean ± standard deviation of the mean. All data were analyzed using the IBM SPSS statistical program, version 23.0, for Windows (SPSS Inc., Chicago, IL, USA).
Analysis and Results
Some previous research has demonstrated the adverse effects of cryopreservation on semen quality (Maroto-Morales et al., 2016). The cryopreservation process has an important impact on sperm morphology (Awad, 2011) and potential fertilization capacity (Franken, 2015), so it has a very specific effect on the proportion of spermatozoa that make up the different subpopulations (Rubio-Guillén et al., 2007). The decrease in the proportion of sperm with large and long sperm heads, and the increase in the number of cells in the smaller sperm subpopulation is a known consequence of cryopreservation (Gravance et al., 2009). This effect may directly condition the results observed in this work, based on the evidence that European breeds may have greater tolerance to cryopreservation processes (Saranholi et al., 2021). However, this factor would also imply that the decrease in the morphometric parameters of one subspecies compared to another causes an effect on the distribution of subpopulations, since, when evaluating other variables such as kinetics, some changes are observed as reported in previous studies (Víquez et al., 2020).
It has been reported that sperm morphometry can be affected by different factors, such as osmotic pressure, staining techniques, freezing and thawing (Caldeira et al., 2022; García-Molina et al., 2022, 2023; Soler, Alambiaga, et al., 2017; Soler, Contell, et al., 2017; Soler et al., 2005, 2016; Soler, Valverde, et al., 2017; Valverde et al., 2019; Yániz et al., 2016). These variables can have a significant impact not only on sperm dimensions (Awad, 2011), which could lead to inaccurate assessment, but also on chromatic structure, which can have direct negative implications on the individual's fertility potential (Banaszewska et al., 2015). Our work allows us to understand more about the variations that could occur in the morphometric characteristics of spermatozoa between the different bovine subspecies.
Differences between species indicated that genotype composition influenced the value of each sperm head size and shape variable. When the sperm head length was studied, samples from
B. taurus (9.007 ± 0.037 µm) were the longest (p < 0.05) compared to those obtained from
B. indicus (8.969 ± 0.026 µm). Width values differed (p < 0.05) according to species, in decreasing order, of
B. taurus (4.886 ± 0.016 µm), and
B. indicus (4.756 ± 0.011 µm). The head area of spermatozoa in both
B. taurus or
B. indicus was not different (p > 0.05). There were differences in head sperm perimeters (p < 0.05) when comparing the use of
B. taurus and
B. indicus species. In relation to head shape variables, the values oscillated differently in both species (
Table 1).
Heterogeneity in mammalian ejaculates could explain the behavior of species and their evolution according to the conditions in which they have developed (Holt & Van Look, 2004; Ramón et al., 2014) as this has allowed us to understand how different types of spermatozoa different functions within the ejaculate could have to achieve successful reproduction of the species (Ramón et al., 2014). Morphometric evaluations between the subspecies B. taurus and B. indicus showed differences in sperm size and shape characteristics. This coincides with previous research that has determined that sperm from zebu bulls tend to be smaller in size compared to other breeds, including European breeds (Beletti et al., 2005).
For sperm subpopulation, Principal Component (PC) analysis revealed the existence of three PC factors. In B. indicus, PC1 was found to be positively associated with ellipticity and elongation, and negatively related with the rugosity parameter. PC2 was related to perimeter and area. PC3 was related to shape variable regularity. In the
B. taurus, PC1 was found to be related to head size (area, width, and perimeter, in decreasing order) and PC2 was associated with shape head (ellipticity, elongation and regularity and, to a lesser degree, length). PC3 was negatively related with regularity. The total variance explained was 95.39%, and 90.798% for
B. indicus and
B. taurus, respectively (
Table 2).
This discovery underscores the importance of further investigating the genetic mechanisms responsible for these differences for a thorough understanding of how breed genetics can shape sperm characteristics as a measure of adaptation. These results suggest that the cryopreserved materials of Bos indicus and Bos taurus could have three sperm subpopulations according to their shape and size. Other researchers have studied the relationship that the shape and size of sperm could have with evolutionary mechanisms of different species (Kramer et al., 2024). In addition, other studies found in the literature comparing the morphometric characteristics of Bos taurus and Bos indicus and suggest that the differences could be associated with individual characteristics of the animals and their fertility (Beletti et al., 2005) and that they could be related as a function of membrane and acrosome quality (Palacin et al., 2020).
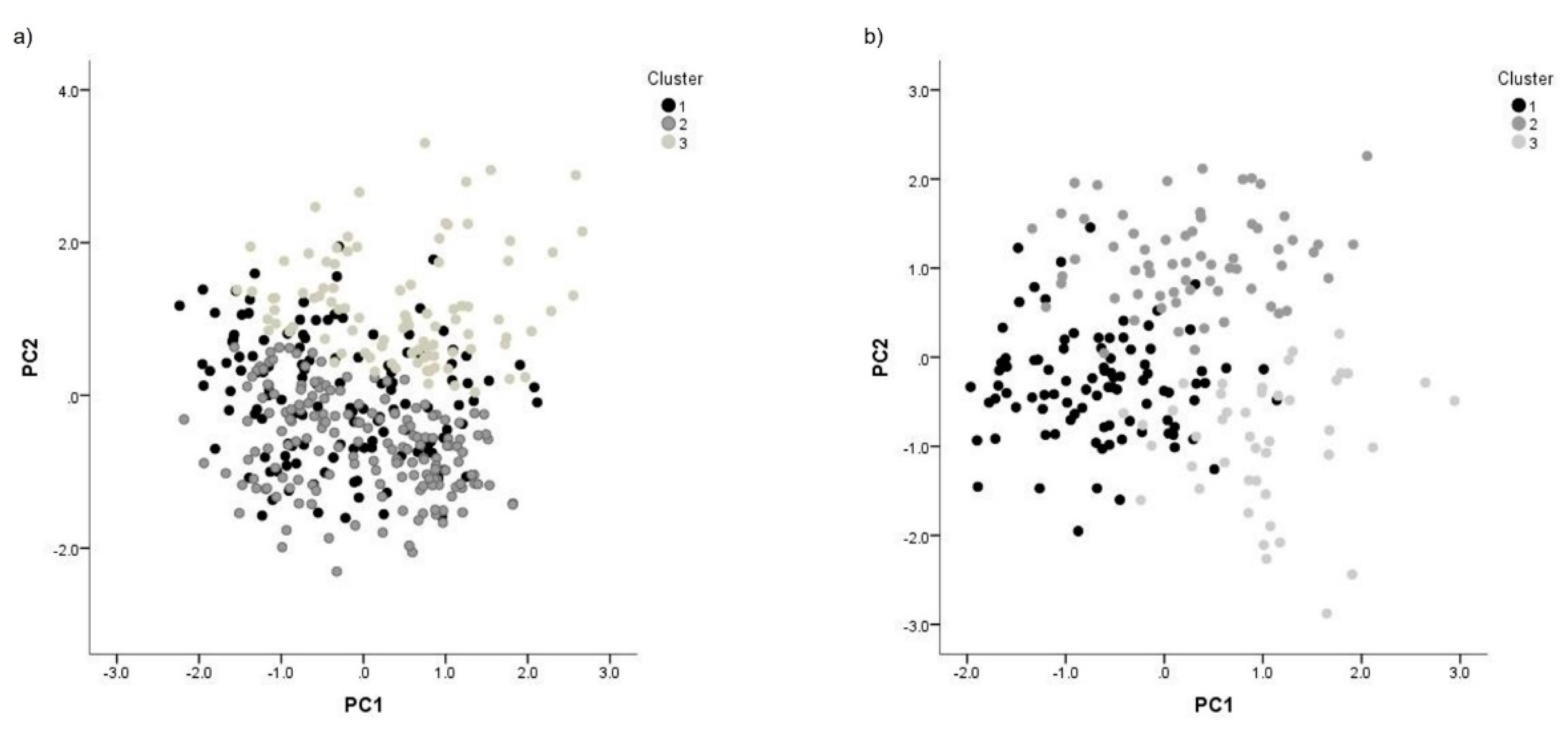
The principal component analysis allowed us to identify three subpopulations in
B. indicus: SP1 with 32.9% of the total spermatozoa, SP2 with 43.0% of the cell analyzed in this species, and SP3 with 24.1% of the total spermatozoa. For
B. taurus, three subpopulations were found: SP1 contained 46.0% of the sperm cells. SP2 had a total of 32.0% of the male gamete. The last subpopulation (SP3) was comprised of 22.0% cells (
Figure 1).
There were differences between sperm subpopulations within each species (p<0.05), and the distribution was not the same for each species. Results from B. indicus indicated differences between subpopulations upon comparison. SP1 was characterized by the medium size (length and area), while SP2 was characterized by the smallest cells and SP3 showed the highest spermatozoa size. Similarly, results described in B. taurus indicate that sperm subpopulations were different (p < 0.05). SP1 was characterized by the smallest cells, and the head shape was the intermediate for all variables, except for the rugosity value, which was the highest. SP2 and SP3 were high, with the highest values of each variable oscillating from one to the other. In both species, a different composition was observed, with SP3 having the highest head size values, except for the length. The shape variables in B. taurus indicate that SP1 and SP2 values were different to SP2 and SP3 for B. indicus (
Table 3).
Cluster analysis has shown that bull ejaculates are not homogeneous in terms of sperm population (Martínez-Pastor, 2022), and the distribution of spermatozoa also varied between subspecies. Our results showed the presence of three subpopulations with wide variability in both B. taurus and B. indicus bulls. These findings suggest that the differences could be related to genetics between these two subspecies, evidence that points towards the possibility that race-associated genetics could exert a significant influence on the observed variations in sperm morphometry (Terán et al., 2021; Thurston et al., 2002). Despite this, it is necessary to further investigate the possible implications of these genetic differences and their relevance on other sperm variables as an adaptation mechanism of each subspecies to conditions that affect the reproductive success of livestock.
Conclusions
The variability in sperm morphometry assessments underscores the need to standardize semen evaluation protocols. This approach has revealed differences in sperm characteristics between subspecies, such as B. taurus and B. indicus, indicating potential genetic influences. Furthermore, the cryopreservation process itself introduces changes in sperm morphology, which begs for a comprehensive understanding of its effects on different sperm subpopulations.
Author Contributions
Conceptualization, A.V., M.A.S; methodology, F.S., L.V., A.V.; software, F.S., L.V., J.S., I.A.Z.; validation, F.S, A.V.; formal analysis, A.V.; investigation, F.S., L.V., I.A.Z.; resources, A.V.; data curation, F.S., L.V., , I.A.Z; writing—original draft preparation, A.V., F.S., I.A.Z.; writing—review and editing, A.V., F.S., J.S., M.B., M.A.S., C.D.C.; visualization, A.V.; supervision, A.V.; project administration, A.V.; funding acquisition, A.V., M.A.S.. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Costa Rica Institute of Technology (Vice-Chancellor’s office of Research and Extension; VIE (Vicerrectoría de investigación y Extensión); Project VIE-5402-2151-1016. The APC was funded by Costa Rica Institute of Technology (Vice-Chancellor’s office of Research and Extension; VIE (Vicerrectoría de investigación y Extensión) and by AGROALNEXT programme (Agroalnext/2022/063; European Union NextGenerationEU (PRTR-C17.I1), Generalitat Valenciana).
Data Availability Statement
The data supporting the results of this study will be made available by the corresponding author, [A.V. ], upon reasonable request.
Acknowledgments
The authors thank the Costa Rica Institute of Technology (ITCR) for financing this study. This work was supported Costa Rica Institute of Technology [Vice-Chancellor’s office of Research and Extension; VIE (Vicerrectoría de investigación y Extensión; Project 2151083)] and Postgraduate Office of ITCR. The funders had no role in the study’s design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Andraszek, K.; Banaszewska, D.; Szeleszczuk, O.; Kuchta-Gładysz, M.; Grzesiakowska, A. Morphometric Characteristics of the Spermatozoa of Blue Fox (Alopex lagopus) and Silver Fox (Vulpes vulpes). Animals 2020, 10, 1927. [Google Scholar] [CrossRef] [PubMed]
- Awad, M. Effect of some permeating cryoprotectants on CASA motility results in cryopreserved bull spermatozoa. Anim. Reprod. Sci. 2011, 123, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Banaszewska, D.; Andraszek, K.; Czubaszek, M.; Biesiada–Drzazga, B. The effect of selected staining techniques on bull sperm morphometry. Anim. Reprod. Sci. 2015, 159, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Barquero, V.; Roldan, E.R.S.; Soler, C.; Yániz, J.L.; Camacho, M.; Valverde, A. Predictive Capacity of Boar Sperm Morphometry and Morphometric Sub-Populations on Reproductive Success after Artificial Insemination. Animals 2021, 11, 920. [Google Scholar] [CrossRef]
- Barth, A.D. Review: The use of bull breeding soundness evaluation to identify subfertile and infertile bulls. Animal 2018, 12, s158–s164. [Google Scholar] [CrossRef]
- Beletti, M.E.; Costa, L.d.F.; Viana, M.P. A comparison of morphometric characteristics of sperm from fertile Bos taurus and Bos indicus bulls in Brazil. Anim. Reprod. Sci. 2005, 85, 105–116. [Google Scholar] [CrossRef]
- Boersma, A.; Braun, J.; Stolla, R. Influence of Random Factors and Two Different Staining Procedures on Computer-assisted Sperm Head Morphometry in Bulls. Reprod. Domest. Anim. 1999, 34, 77–82. [Google Scholar] [CrossRef]
- Imbachí, F.B.; Zalazar, L.; Pastore, J.I.; Nicolli, A.; Ledesma, A.; Hozbor, F.A.; Cesari, A.; Ballarin, V. Clustering and classification software for sperm subpopulation analysis. Comput. Methods Biomech. Biomed. Eng. Imaging Vis. 2022, 10, 585–598. [Google Scholar] [CrossRef]
- Caldeira, C.; Hernández-Ibánez, S.; Vendrell, A.; Valverde, A.; García-Molina, A.; Gallego, V.; Asturiano, J.F.; Soler, C. Characterisation of European eel (Anguilla anguilla) spermatozoa morphometry using Trumorph tool in fixed and non-fixed samples. Aquaculture 2022, 553. [Google Scholar] [CrossRef]
- Castellini, C.; Bosco, A.D.; Ruggeri, S.; Collodel, G. What is the best frame rate for evaluation of sperm motility in different species by computer-assisted sperm analysis? Fertil. Steril. 2011, 96, 24–27. [Google Scholar] [CrossRef]
- Cheah, Y.; Yang, W. Functions of essential nutrition for high quality spermatogenesis. Adv. Biosci. Biotechnol. 2011, 02, 182–197. [Google Scholar] [CrossRef]
- Franken, D.R. How accurate is sperm morphology as an indicator of sperm function? Andrologia 2015, 47, 720–723. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, M.T.; Smith, D.J.; Kirkman-Brown, J.C. CASA: tracking the past and plotting the future. Reprod. Fertil. Dev. 2018, 30, 867–874. [Google Scholar] [CrossRef]
- García-Herreros, M.; Aparicio, I.M.; Barón, F.J.; García-Marín, L.J.; Gil, M.C. Standardization of sample preparation, staining and sampling methods for automated sperm head morphometry analysis of boar spermatozoa. Int. J. Androl. 2006, 29, 553–563. [Google Scholar] [CrossRef]
- García-Molina, A.; Navarro, N.; Cerveró, C.; Sadeghi, S.; Valverde, A.; Roldan, E.R.; Bompart, D.; Garrido, N.; Soler, C. Effect of incubation and analysis temperatures on sperm kinematics and morphometrics during human semen analysis. Rev. Int. De Androl. 2022, 21, 100350. [Google Scholar] [CrossRef]
- García-Molina, A.; Navarro, N.; Cerveró, C.; Sadeghi, S.; Valverde, A.; Roldan, E.R.; Bompart, D.; Garrido, N.; Soler, C. Effect of incubation and analysis temperatures on sperm kinematics and morphometrics during human semen analysis. Rev. Int. De Androl. 2023, 21, 100350. [Google Scholar] [CrossRef] [PubMed]
- Giaretta, E.; Munerato, M.; Yeste, M.; Galeati, G.; Spinaci, M.; Tamanini, C.; Mari, G.; Bucci, D. Implementing an open-access CASA software for the assessment of stallion sperm motility: Relationship with other sperm quality parameters. Anim. Reprod. Sci. 2017, 176, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Gravance, C.; Casey, M.; Casey, P. Pre-freeze bull sperm head morphometry related to post-thaw fertility. Anim. Reprod. Sci. 2009, 114, 81–88. [Google Scholar] [CrossRef]
- Holt, W.; Van Look, K. Concepts in sperm heterogeneity, sperm selection and sperm competition as biological foundations for laboratory tests of semen quality. Reproduction 2004, 127, 527–535. [Google Scholar] [CrossRef]
- Hook, K.A.; Fisher, H.S. Methodological considerations for examining the relationship between sperm morphology and motility. Mol. Reprod. Dev. 2020, 87, 633–649. [Google Scholar] [CrossRef]
- Kaiser, H.F. The varimax criterion for analytic rotation in factor analysis. Psychometrika 1958, 23, 187–200. [Google Scholar] [CrossRef]
- Gentle, J.E. Finding Groups in Data: An Introduction to Cluster Analysis. Biometrics 1991, 47, 788. [Google Scholar] [CrossRef]
- Kramer, E.M.; Enelamah, J.; Fang, H.; Tayjasanant, P.A. Karyotype depends on sperm head morphology in some amniote groups. Front. Genet. 2024, 15, 1396530. [Google Scholar] [CrossRef]
- Parameshwarappa, M.A.; Veerasamy, S.; Pandarathil, R.V.; Tulasiramu, S.S.; Madiajagan, B.; Govindan, K.; Raghavendra, B. Resilient capacity of cattle to environmental challenges – An updated review. J. Anim. Behav. Biometeorol. 2019, 7, 104–118. [Google Scholar] [CrossRef]
- Garde, J.; Maroto-Morales, A.; García-Álvarez, O.; Ramón, M.; Martínez-Pastor, F.; Fernández-Santos, M.; Soler, A. Current status and potential of morphometric sperm analysis. Asian J. Androl. 2016, 18, 863–870. [Google Scholar] [CrossRef]
- Martínez-Fresneda, L.; O'Brien, E.; Velázquez, R.; Toledano-Díaz, A.; Martínez-Cáceres, C.M.; Tesfaye, D.; Schellander, K.; García-Vázquez, F.A.; Santiago-Moreno, J. Seasonal variation in sperm freezability associated with changes in testicular germinal epithelium in domestic (Ovis aries) and wild (Ovis musimon) sheep. Reprod. Fertil. Dev. 2019, 31, 1545–1557. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Pastor, F. What is the importance of sperm subpopulations? Anim. Reprod. Sci. 2021, 246, 106844. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, S. T. , & De Jonge, C. J. (2018). CASA—Computer-Aided Sperm Analysis. Encyclopedia of Reproduction, 59-63. [CrossRef]
- Yániz, J.; Palacin, I.; Santolaria, P.; Alquezar-Baeta, C.; Soler, C.; Silvestre, M. Relationship of sperm plasma membrane and acrosomal integrities with sperm morphometry in Bos taurus. Asian J. Androl. 2020, 22, 578–582. [Google Scholar] [CrossRef]
- Petrunkina, A.M.; Waberski, D.; Günzel-Apel, A.R.; Töpfer-Petersen, E. Determinants of sperm quality and fertility in domestic species. Reproduction 2007, 134, 3–17. [Google Scholar] [CrossRef]
- Ramón, M.; Jiménez-Rabadán, P.; García-Álvarez, O.; Maroto-Morales, A.; Soler, A.; Santos, F.; Guzmán, P.; Garde, J. Understanding Sperm Heterogeneity: Biological and Practical Implications. Reprod. Domest. Anim. 2014, 49, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Guillén, J.; González, D.; Garde, J.; Esteso, M.; Santos, F.; Rodríguez-Gíl, J.; Madrid-Bury, N.; Quintero-Moreno, A. Effects of Cryopreservation on Bull Spermatozoa Distribution in Morphometrically Distinct Subpopulations. Reprod. Domest. Anim. 2007, 42, 354–357. [Google Scholar] [CrossRef]
- Sampaio, W.V.; Oliveira, K.G.; Leão, D.L.; Caldas-Bussiere, M.C.; Queiroz, H.L.; Paim, F.P.; Santos, R.R.; Domingues, S.F. Morphologic analysis of sperm from two neotropical primate species: comparisons between the squirrel monkeysSaimiri collinsiandSaimiri vanzolinii. Zygote 2017, 25, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Santolaria, P.; Vicente-Fiel, S.; Palacín, I.; Fantova, E.; Blasco, M.; Silvestre, M.; Yániz, J. Predictive capacity of sperm quality parameters and sperm subpopulations on field fertility after artificial insemination in sheep. Anim. Reprod. Sci. 2015, 163, 82–88. [Google Scholar] [CrossRef]
- Saranholi, D.A.C.; de Paula, R.R.; Pytilak, E.; Afonso, F.; Canela, L.F.; de Almeida, A.B.M.; Hidalgo, M.M.T.; Martins, M.I.M.; Blaschi, W.; Barreiros, T.R.R. Comparison of seminal characteristics of Aberdeen Angus, Holstein and Nelore bulls before and after cryopreservation. Res. Soc. Dev. 2021, 10. [Google Scholar] [CrossRef]
- Soler, C.; Alambiaga, A.; Martí, M.; García-Molina, A.; Valverde, A.; Contell, J.; Campos, M. Dog sperm head morphometry: its diversity and evolution. Asian J. Androl. 2017, 19, 149–153. [Google Scholar] [CrossRef]
- Soler, C.; Contell, J.; Bori, L.; Sancho, M.; García-Molina, A.; Valverde, A.; Segarvall, J. Sperm kinematic, head morphometric and kinetic-morphometric subpopulations in the blue fox (Alopex lagopus). Asian J. Androl. 2017, 19, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Soler, C.; Cooper, T.; Valverde, A.; Yániz, J. Afterword to Sperm morphometrics today and tomorrow special issue in Asian Journal of Andrology. Asian J. Androl. 2016, 18, 895–897. [Google Scholar] [CrossRef]
- Soler, C.; Gadea, B.; Soler, A.; Fernández-Santos, M.; Esteso, M.; Núñez, J.; Moreira, P.; Núñez, M.; Gutiérrez, R.; Sancho, M.; et al. Comparison of three different staining methods for the assessment of epididymal red deer sperm morphometry by computerized analysis with ISAS®. Theriogenology 2005, 64, 1236–1243. [Google Scholar] [CrossRef] [PubMed]
- Soler, C.; García-Molina, A.; Contell, J.; Silvestre, M.; Sancho, M. The Trumorph℗® system: The new univ the morphology of living sperm. Anim. Reprod. Sci. 2015, 158, 1–10. [Google Scholar] [CrossRef]
- Soler, C. , Valverde, A., Bompart, D., Fereidounfar, S., Sancho, M., Yániz, J., Garcia-Molina, A., & Korneenko- Zhilyaev, Yu. A. New methods of semen analysis by casa. Sel'skokhozyaistvennaya Biol. 2017, 52, 232–241. [Google Scholar] [CrossRef]
- Spencer, N.H. Essentials of Multivariate Data Analysis; Taylor & Francis: London, United Kingdom, 2013. [Google Scholar]
- Taylor, J.F.; Schnabel, R.D.; Sutovsky, P. Review: Genomics of bull fertility. Animal 2018, 12, S172–S183. [Google Scholar] [CrossRef] [PubMed]
- Terán, E.; Azcona, F.; Ramón, M.; Molina, A.; Dorado, J.; Hidalgo, M.; Ross, P.; Goszczynski, D.; Demyda-Peyrás, S. Sperm morphometry is affected by increased inbreeding in the Retinta cattle breed: A molecular approach. Mol. Reprod. Dev. 2021, 88, 416–426. [Google Scholar] [CrossRef]
- Thurston, L.M.; Siggins, K.; Mileham, A.J.; Watson, P.F.; Holt, W.V. Identification of Amplified Restriction Fragment Length Polymorphism Markers Linked to Genes Controlling Boar Sperm Viability Following Cryopreservation1. Biol. Reprod. 2002, 66, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Toner, J.P.; Mossad, H.; Grow, D.R.; Morshedi, M.; Swanson, R.; Oehninger, S. Value of sperm morphology assessed by strict criteria for prediction of the outcome of artificial (intrauterine) insemination. Andrologia 1995, 27, 143–148. [Google Scholar] [CrossRef]
- Soler, C.; Valverde, A.; Arenán, H.; Sancho, M.; Contell, J.; Yániz, J.; Fernández, A. Morphometry and subpopulation structure of Holstein bull spermatozoa: variations in ejaculates and cryopreservation straws. Asian J. Androl. 2016, 18, 851–857. [Google Scholar] [CrossRef]
- Valverde, A.; Madrigal-Valverde, M.; Castro-Morales, O.; Gadea-Rivas, A.; Johnston, S.; Soler, C. Kinematic and head morphometric characterisation of spermatozoa from the Brown Caiman (Caiman crocodilus fuscus). Anim. Reprod. Sci. 2019, 207, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Abarca, A.V.; Castro-Morales, O.; Mdrigal-Valverde, M. Sperm kinematics and morphometric subpopulations analysis with CASA systems: a review. Rev. De Biol. Trop. 2019, 67. [Google Scholar] [CrossRef]
- Vicente-Fiel, S.; Palacín, I.; Santolaria, P.; Hidalgo, C.; Silvestre, M.; Arrebola, F.; Yániz, J. A comparative study of the sperm nuclear morphometry in cattle, goat, sheep, and pigs using a new computer-assisted method (CASMA-F). Theriogenology 2013, 79, 436–442. [Google Scholar] [CrossRef]
- Villaverde-Morcillo, S.; Soler, A.; Esteso, M.; Castaño, C.; Miñano-Berna, A.; Gonzalez, F.; Santiago-Moreno, J. Immature and mature sperm morphometry in fresh and frozen-thawed falcon ejaculates. Theriogenology 2017, 98, 94–100. [Google Scholar] [CrossRef]
- Víquez, L.; Barquero, V.; Soler, C.; Roldan, E.R.; Valverde, A. Kinematic Sub-Populations in Bull Spermatozoa: A Comparison of Classical and Bayesian Approaches. Biology 2020, 9, 138. [Google Scholar] [CrossRef]
- Waberski, D.; Suarez, S.S.; Henning, H. Assessment of sperm motility in livestock: Perspectives based on sperm swimming conditions in vivo. Anim. Reprod. Sci. 2021, 246, 106849. [Google Scholar] [CrossRef] [PubMed]
- Yániz, J.L.; Palacín, I.; Caycho, K.S.; Soler, C.; Silvestre, M.A.; Santolaria, P. Determining the relationship between bull sperm kinematic subpopulations and fluorescence groups using an integrated sperm quality analysis technique. Reprod. Fertil. Dev. 2018, 30, 919–923. [Google Scholar] [CrossRef]
- Yániz, J.L.; Soler, C.; Santolaria, P. Computer assisted sperm morphometry in mammals: A review. Anim. Reprod. Sci. 2015, 156, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yániz, J.; Vicente-Fiel, S.; Soler, C.; Recreo, P.; Carretero, T.; Bono, A.; Berné, J.; Santolaria, P. Comparison of different statistical approaches to evaluate morphometric sperm subpopulations in men. Asian J. Androl. 2016, 18, 819–823. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).