Submitted:
21 August 2024
Posted:
22 August 2024
You are already at the latest version
Abstract
Keywords:
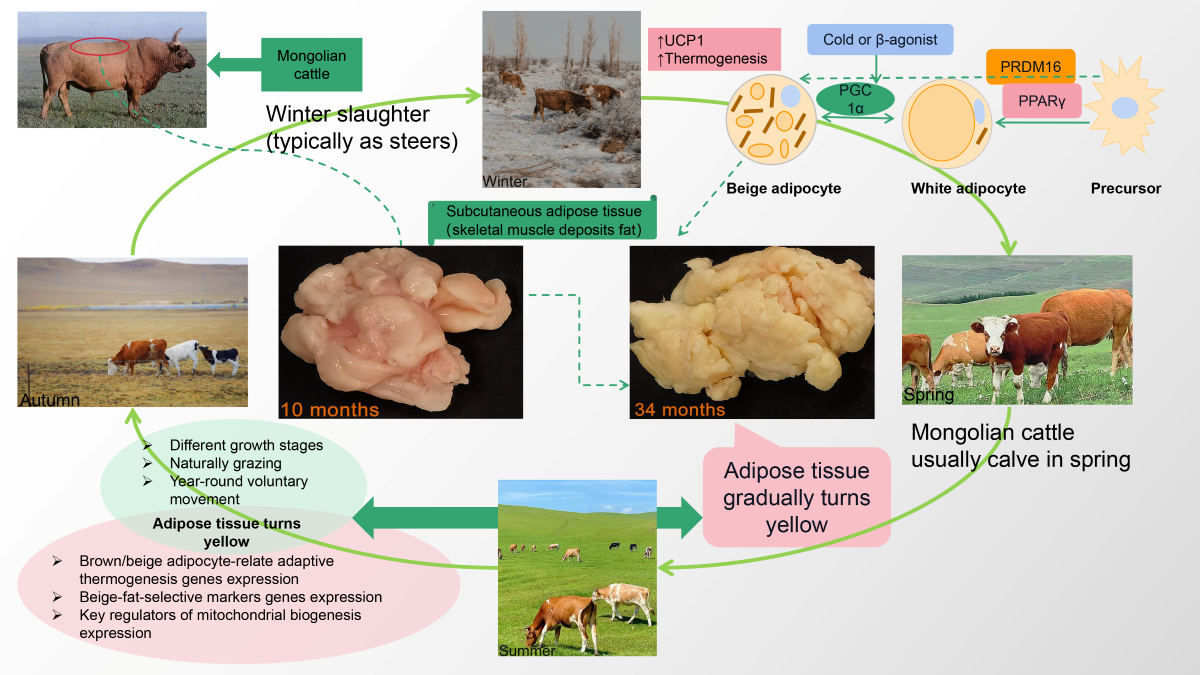
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Tissue Color Measurement
2.3. Measurement of Mitochondrial DNA Copy Number
2.4. Quantitative Real-Time RT-qPCR Analysis
2.5. Lipid Extraction and Lipidomic Analysis
2.6. Statistical and Bioinformatics Analyses
3. Results and Discussion
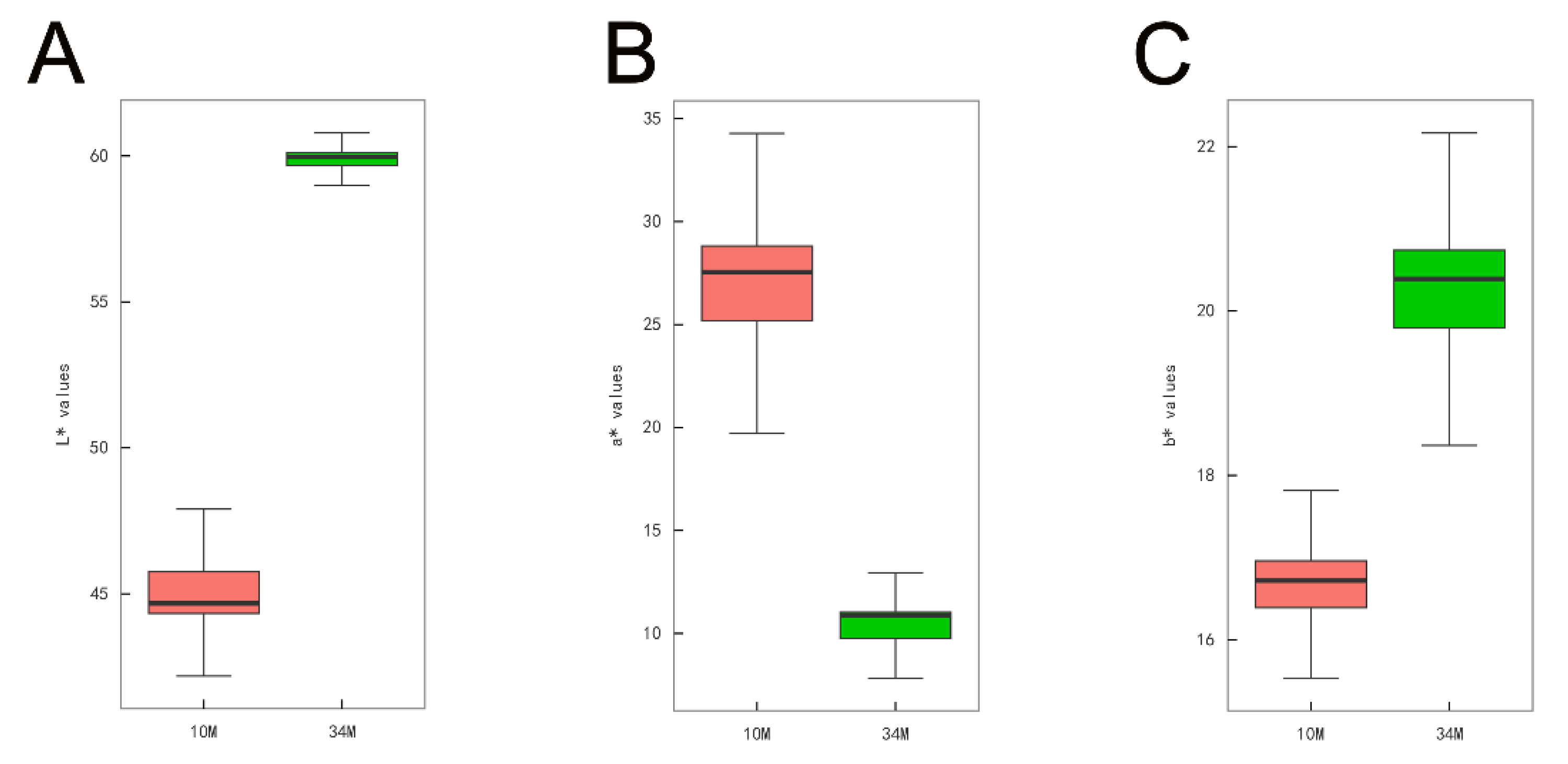
3.1. Adipose Tissue Color Analysis
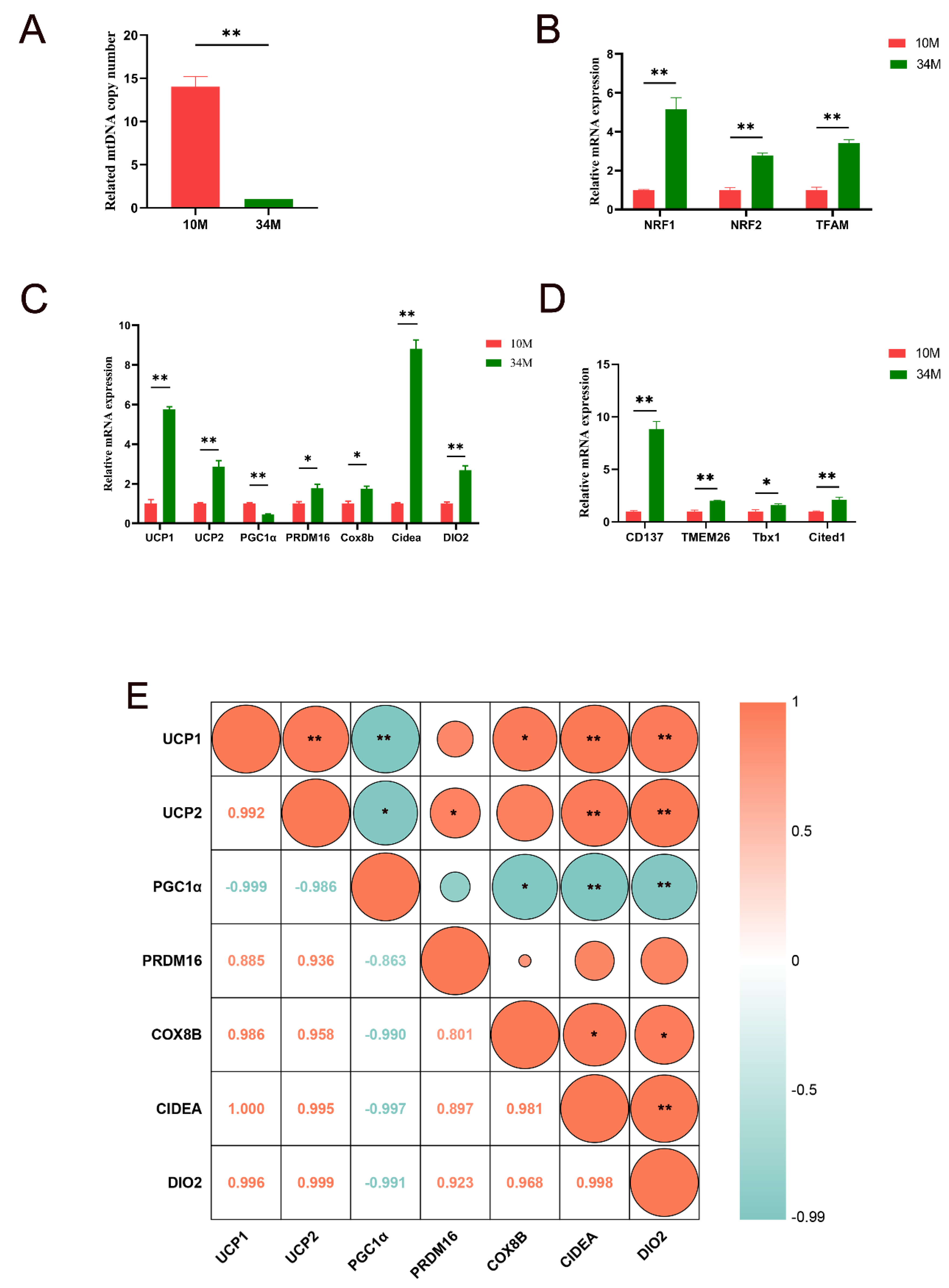
3.2. Expression of mtDNA Copy Numbers, Mitochondrial Biogenesis–Specific Markers, and Browning-Related Factor
3.3. Lipid Metabolomics Analysis
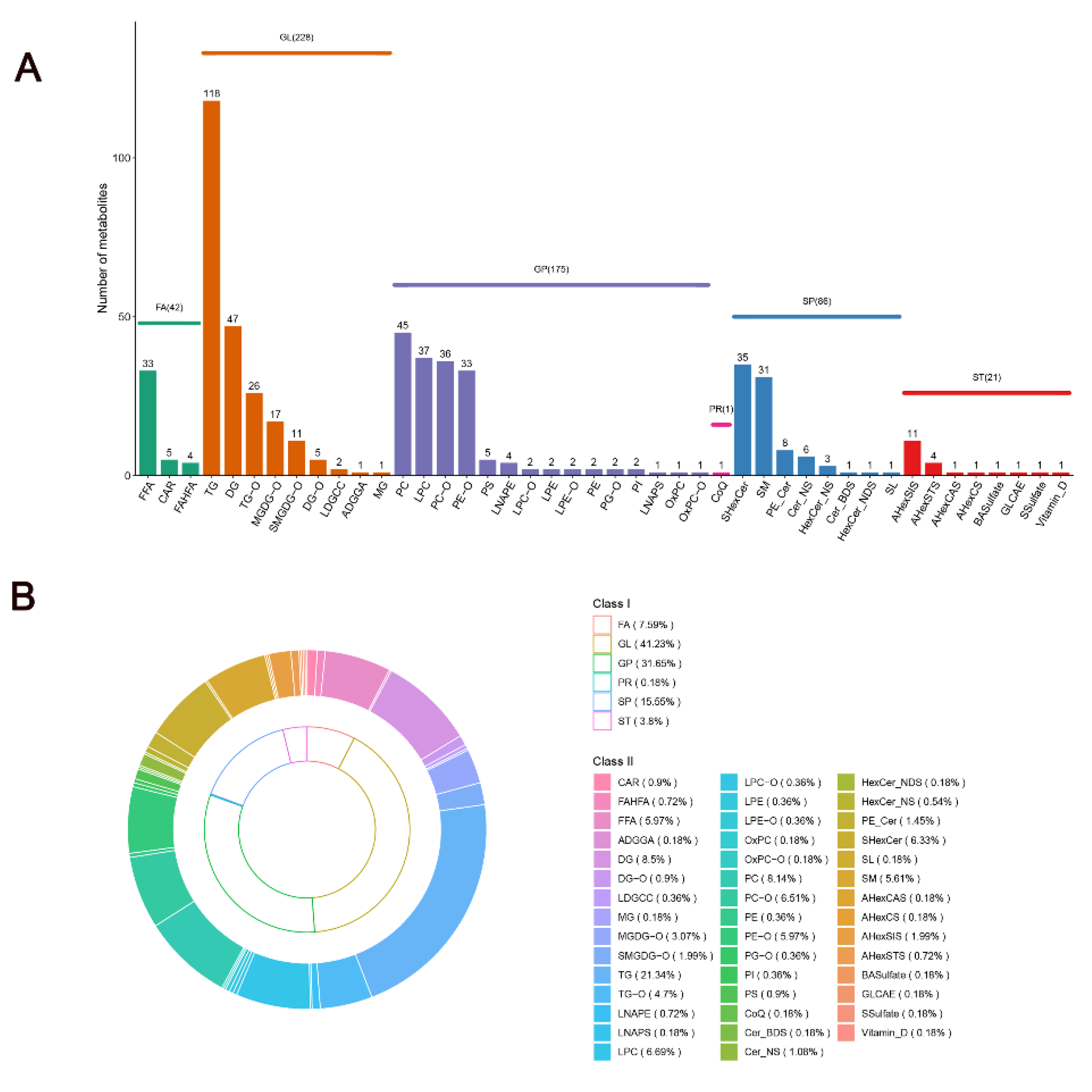
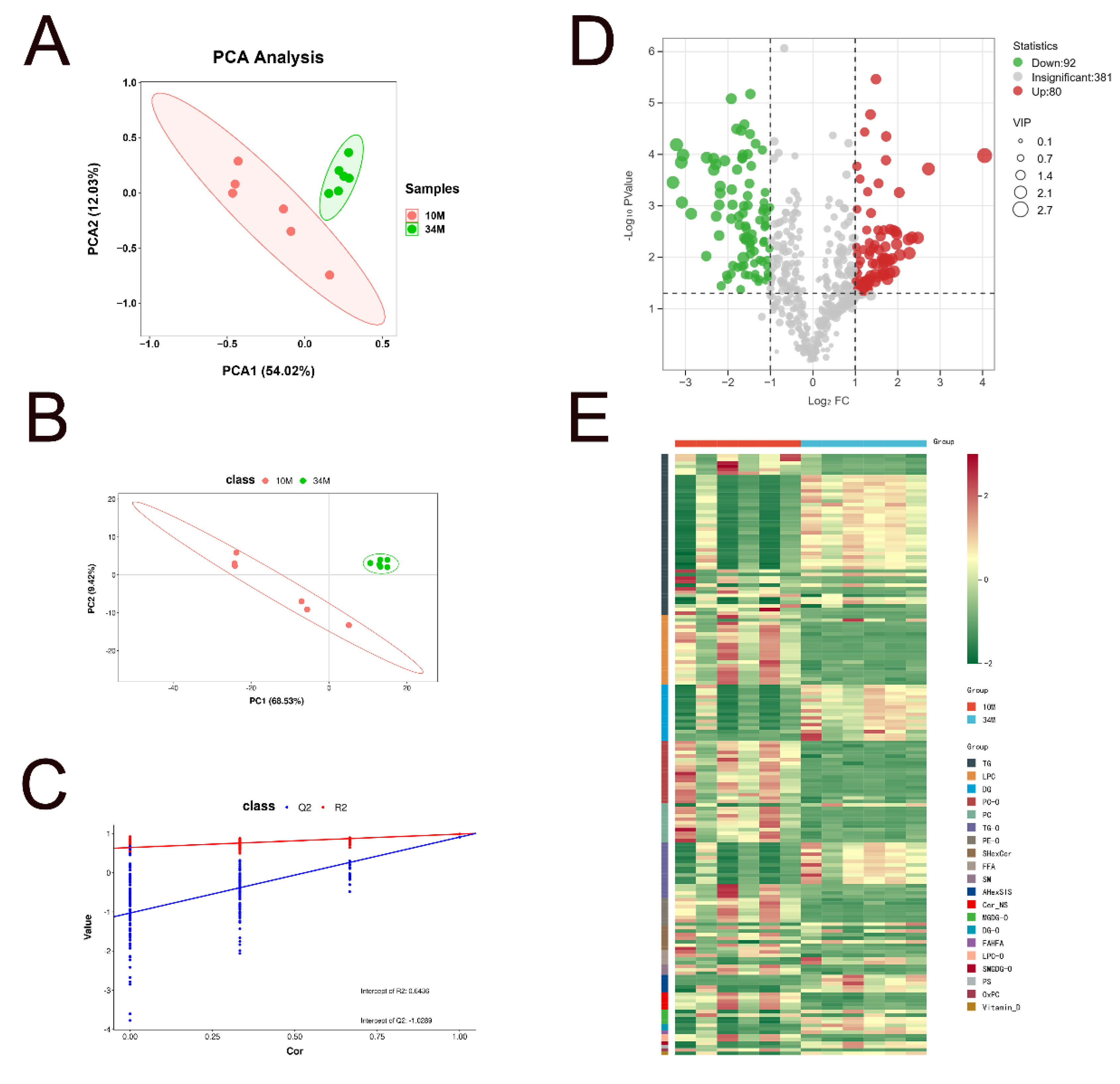
3.3.1. Overview of Lipid Metabolomics Results
3.3.2. Identification of Characteristic Lipids
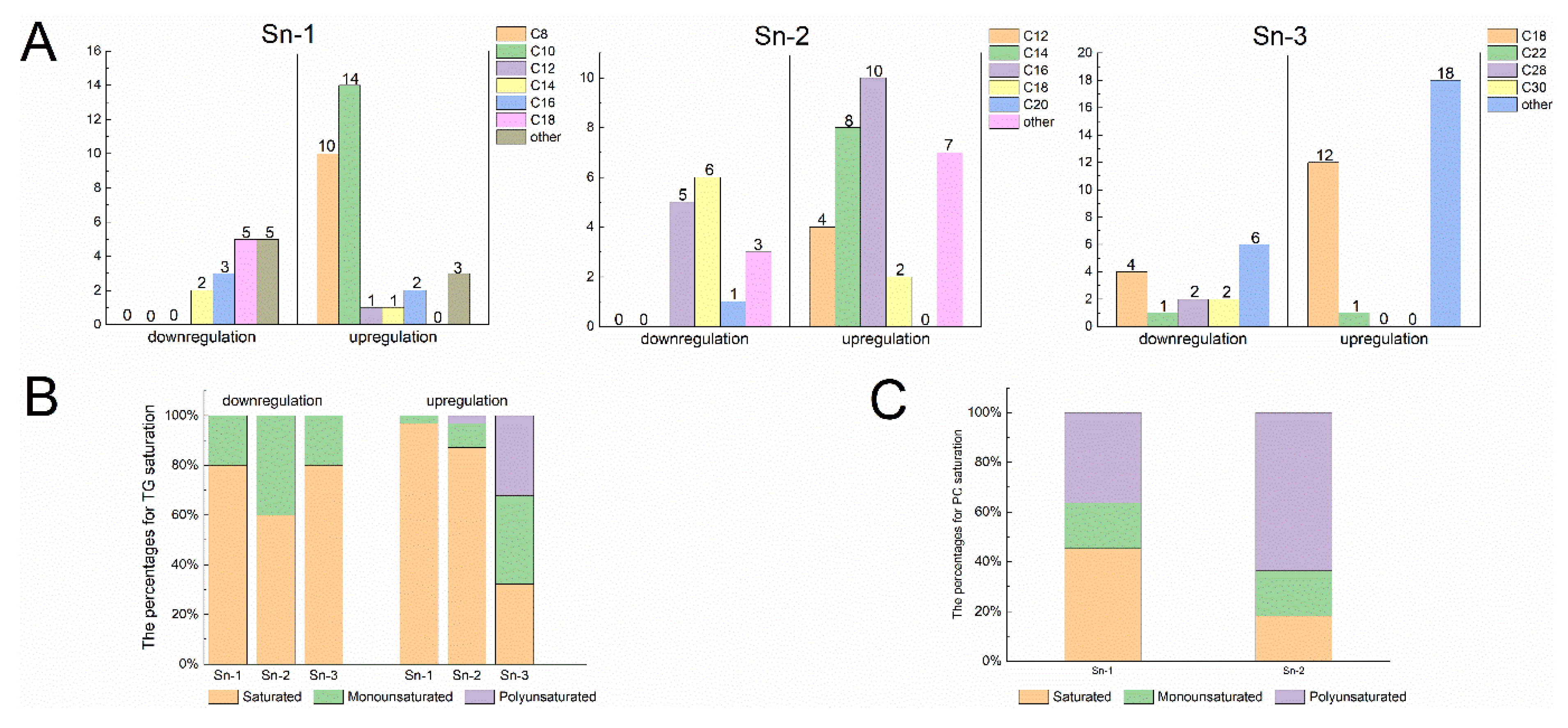
3.3.3. Distribution and Composition of Fatty Acids in Lipid Molecules
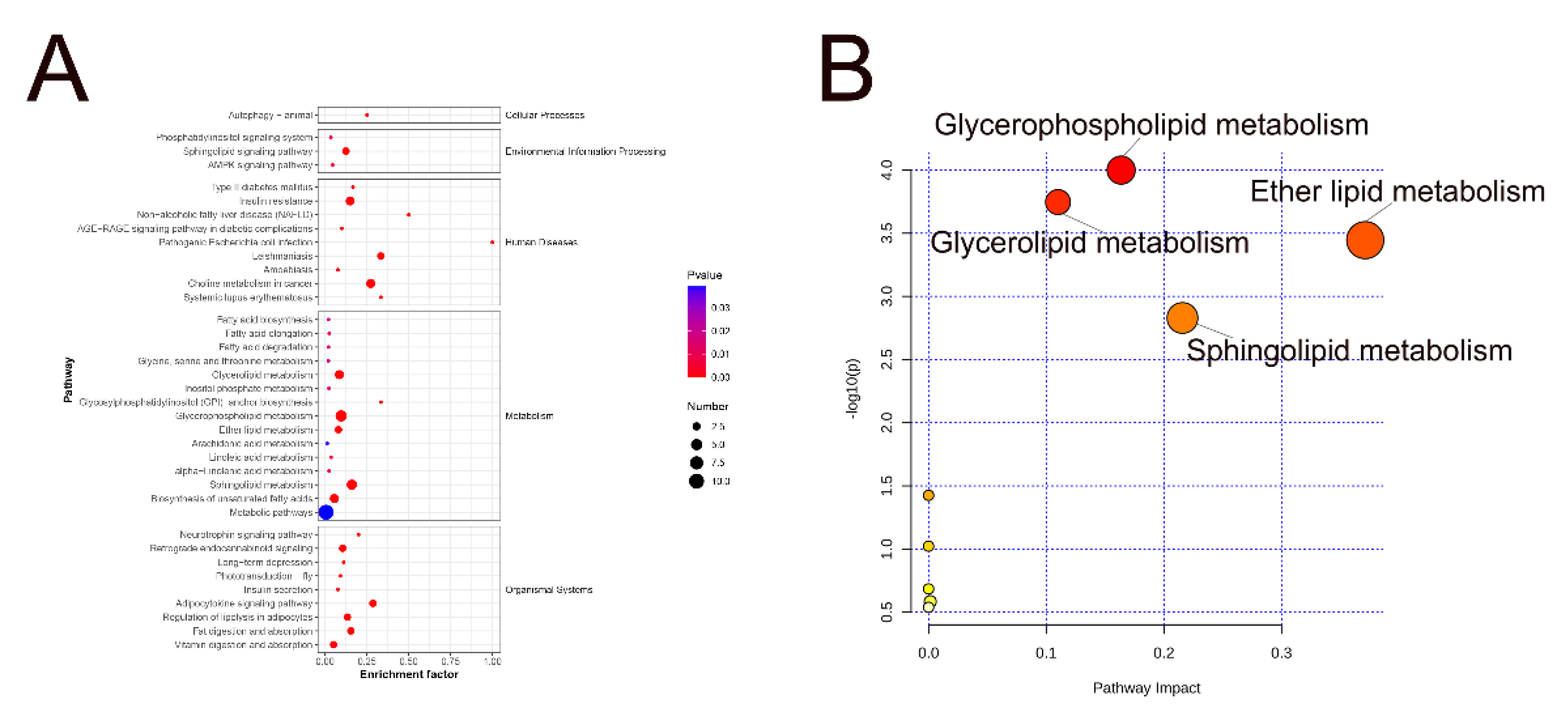
3.3.4. Metabolic Pathway Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leiria, L.O.; Tseng, Y.H. Lipidomics of brown and white adipose tissue: Implications for energy metabolism. BBA - Gen Subjects. 2020, 1865, 158788–158788. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.; Lee, D.; Berry, D.C. Thermogenic adipose tissue in energy regulation and metabolic health. Front. Endocrinol. 2023, 14. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Maretich, P.; Kajimura, S. The Common and Distinct Features of Brown and Beige Adipocytes. Trends Endocrinol Metab. 2018, 29, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Barbatelli, G.; Murano, I.; Madsen, L.; Hao, Q.; Jimenez, M.; Kristiansen, K.; Giacobino, J.P.; De, M.R.; Cinti, S. The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am J Physiol Endocrinol Metab. 2010, 298, E1244–1253. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Bai, H.; Yang, L.; Wang, H.; Wei, S.; Yan, P. Cold exposure induces browning of bovine subcutaneous white fat in vivo and in vitro. J. Therm. Biol. 2023, 112, 103446–103446. [Google Scholar] [CrossRef]
- Du, L.; Chang, T.; An, B.; Liang, M.; Deng, T.; Li, K.; Cao, S.; Du, Y.; Gao, X.; Xu, L.; et al. Transcriptomics and Lipid Metabolomics Analysis of Subcutaneous, Visceral, and Abdominal Adipose Tissues of Beef Cattle. Genes. 2022, 14. [Google Scholar] [CrossRef]
- Xiong, L.; Pei, J.; Bao, P.; Wang, X.; Guo, S.; Cao, M.; Kang, Y.; Yan, P.; Guo, X. The Effect of the Feeding System on Fat Deposition in Yak Subcutaneous Fat. Int. J. Mol. Sci. 2023, 24. [Google Scholar] [CrossRef]
- Gu, X.; Sun, W.; Yi, K.; Yang, L.; Chi, F.; Luo, Z.; Wang, J.; Zhang, J.; Wang, W.; Yang, T.; et al. Comparison of muscle lipidomes between cattle-yak, yak, and cattle using UPLC–MS/MS. J. Food Compos. Anal. 2021, 103. [Google Scholar] [CrossRef]
- Dunne, P.G.; Monahan, F.J.; O’Mara, F.P.; Moloney, A.P. Colour of bovine subcutaneous adipose tissue: A review of contributory factors, associations with carcass and meat quality and its potential utility in authentication of dietary history. Meat Sci. 2008, 81, 28–45. [Google Scholar] [CrossRef]
- Ahmad, A.A.; Zhang, J.; Liang, Z.; Du, M.; Yang, Y.; Zheng, J.; Yan, P.; Long, R.; Tong, B.; Han, J.; et al. Age-dependent variations in rumen bacterial community of Mongolian cattle from weaning to adulthood. BMC Microbiol. 2022, 22, 213–213. [Google Scholar] [CrossRef]
- Aricha, H.; Simujide, H.; Wang, C.; Zhang, J.; Lv, W.; Jimisi, X.; Liu, B.; Chen, H.; Zhang, C.; He, L.; et al. Comparative Analysis of Fecal Microbiota of Grazing Mongolian Cattle from Different Regions in Inner Mongolia, China. Animals. 2021, 11, 1938–1938. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Wang, Z.; Zhao, M.; Wang, Y.; Zhang, J.; Li, Y.; Gegen, T.; Jia, Y. Study on quality characteristics of natural pastures in different steppe types. Feed. 2022, 45, 75–78. [Google Scholar]
- Wei, S.; Zhang, M.; Zheng, Y.; Yan, P. ZBTB16 Overexpression Enhances White Adipogenesis and Induces Brown-Like Adipocyte Formation of Bovine White Intramuscular Preadipocytes. Cell. Physiol. Biochem. 2018, 48, 2528–2538. [Google Scholar] [CrossRef]
- Shemeis, A.R.; Liboriussen, T.; Andersen, B.B.; Abdallah, O.Y. Changes in carcass and meat quality traits of Danish friesian cull cows with the increase of their age and body condition. Meat Sci. 1994, 37, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Vahmani, P.; Ponnampalam, E.N.; Kraft, J.; Mapiye, C.; Bermingham, E.N.; Watkins, P.J.; Proctor, S.D.; Dugan, M.E.R. Bioactivity and health effects of ruminant meat lipids. Invited Review. Meat Sci. 2020, 165. [Google Scholar] [CrossRef] [PubMed]
- Kaaman, M.; Sparks, L.M.; van Harmelen, V.; Smith, S.R.; Sjölin, E.; Dahlman, I.; Arner, P. Strong association between mitochondrial DNA copy number and lipogenesis in human white adipose tissue. Diabetologia. 2007, 50, 2526–2533. [Google Scholar] [CrossRef]
- Pohjoismäki, J.L.O.; Goffart, S.; Taylor, R.W.; Turnbull, D.M.; Suomalainen, A.; Jacobs, H.T.; Karhunen, P.J. Developmental and Pathological Changes in the Human Cardiac Muscle Mitochondrial DNA Organization, Replication and Copy Number. PLoS ONE. 2010, 5, 1–9. [Google Scholar] [CrossRef]
- Barazzoni, R.; Short, K.R.; Nair, K.S. Effects of aging on mitochondrial DNA copy number and cytochromec oxidase gene expression in rat skeletal muscle, liver, and heart. J. Biol. Chem. 2000, 275, 3343–3347. [Google Scholar] [CrossRef]
- Hou, L.; Xie, M.; Cao, L.; Shi, J.; Xu, G.; Hu, C.; Wang, C. Browning of Pig White Preadipocytes by Co-Overexpressing Pig PGC-1alpha and Mice UCP1. Cell Physiol Biochem. 2018, 48, 556–568. [Google Scholar] [CrossRef]
- Xu, L.; Zhou, L.; Li, P. CIDE Proteins and Lipid Metabolism. Arterioscler Thromb Vasc Biol. 2012, 32, 1094–1098. [Google Scholar] [CrossRef] [PubMed]
- Seale, P.; Conroe, H.M.; Estall, J.; Kajimura, S.; Frontini, A.; Ishibashi, J.; Cohen, P.; Cinti, S.; Spiegelman, B.M. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J Clin Invest. 2011, 121, 96–105. [Google Scholar] [CrossRef]
- Wu, Z.; Puigserver, P.; Andersson, U.; Zhang, C.; Adelmant, G.; Mootha, V.; Troy, A.; Cinti, S.; Lowell, B.; Scarpulla, R.C.; et al. Mechanisms Controlling Mitochondrial Biogenesis and Respiration through the Thermogenic Coactivator PGC-1. Cell. 1999, 98, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.; Prolla, T. PGC-1α in aging and anti-aging interventions. BBA - Gen Subjects. 2009, 1790, 1059–1066. [Google Scholar] [CrossRef]
- Harms, M.; Seale, P. Brown and beige fat: development, function and therapeutic potential. Nat. Med. 2013, 19, 1252–1263. [Google Scholar] [CrossRef] [PubMed]
- Shin, W.; Okamatsu-Ogura, Y.; Machida, K.; Tsubota, A.; Nio-Kobayashi, J.; Kimura, K. Impaired adrenergic agonist-dependent beige adipocyte induction in aged mice. Obesity. 2017, 25, 417–423. [Google Scholar] [CrossRef]
- Silva, G.d.N.; Amato, A.A. Thermogenic adipose tissue aging: Mechanisms and implications. Front. Cell Dev. Biol. 2022, 10, 955612. [Google Scholar] [CrossRef]
- Félix-Soriano, E.; Sáinz, N.; Gil-Iturbe, E.; Castilla-Madrigal, R.; Celay, J.; Fernández-Galilea, M.; Pejenaute, Á.; Lostao, M.P.; Martínez-Climent, J.A.; Moreno-Aliaga, M.J. Differential remodeling of subcutaneous white and interscapular brown adipose tissue by long-term exercise training in aged obese female mice. J. Physiol. Biochem. 2023, 79, 451–465. [Google Scholar] [CrossRef] [PubMed]
- Sano, T.; Sanada, T.; Sotomaru, Y.; Shinjo, T.; Iwashita, M.; Yamashita, A.; Fukuda, T.; Sanui, T.; Asano, T.; Kanematsu, T. Ccr7 null mice are protected against diet-induced obesity via Ucp1 upregulation and enhanced energy expenditure. Nutr. Metab. 2019, 16, 43. [Google Scholar] [CrossRef]
- Shigematsu, M.; Yamada, T.; Wong, Y.Y.; Kanamori, Y.; Murakami, M.; Fujimoto, Y.; Suzuki, M.; Kida, R.; Qiao, Y.; Tomonaga, S. Dietary regulation of Ucp2 and Ucp3 expressions in white adipose tissues of beef cattle. Can. J. Anim. Sci. 2016, 457–460. [Google Scholar] [CrossRef]
- Pousinis, P.; Gowler, P.R.W.; Burston, J.J.; Ortori, C.A.; Chapman, V.; Barrett, D.A. Lipidomic identification of plasma lipids associated with pain behaviour and pathology in a mouse model of osteoarthritis. Metabolomics. 2020, 16, 32. [Google Scholar] [CrossRef]
- Li, J.; Yang, Y.; Zhan, T.; Zhao, Q.; Zhang, J.; Ao, X.; He, J.; Zhou, J.; Tang, C. Effect of slaughter weight on carcass characteristics, meat quality, and lipidomics profiling in longissimus thoracis of finishing pigs. Lwt. 2021, 140. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, C.; Xiao, S.; Zhan, H.; Du, M.; Wu, C.; Ma, C. Lipids deposition, composition and oxidative stability of subcutaneous adipose tissue and Longissimus dorsi muscle in Guizhou mini-pig at different developmental stages. Meat Sci. 2010, 84, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.D.; Enser, M.; Fisher, A.V.; Nute, G.R.; Sheard, P.R.; Richardson, R.I.; Hughes, S.I.; Whittington, F.M. Fat deposition, fatty acid composition and meat quality: A review. Meat Sci. 2008, 78, 343–358. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Annu Rev. Food Sci Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.H.; Vidal, N.P.; Manful, C.F.; Fillier, T.A.; Pumphrey, R.P.; Doody, K.M.; Thomas, R.H. Moose and Caribou as Novel Sources of Functional Lipids: Fatty Acid Esters of Hydroxy Fatty Acids, Diglycerides and Monoacetyldiglycerides. Molecules. 2019, 24. [Google Scholar] [CrossRef]
- Vanni, S.; Riccardi, L.; Palermo, G.; De Vivo, M. Structure and Dynamics of the Acyl Chains in the Membrane Trafficking and Enzymatic Processing of Lipids. Acc. Chem. Res. 2019, 52, 3087–3096. [Google Scholar] [CrossRef]
- Cao, Z.; Xu, M.; Qi, S.; Xu, X.; Liu, W.; Liu, L.; Bao, Q.; Zhang, Y.; Xu, Q.; Zhao, W.; et al. Lipidomics reveals lipid changes in the intramuscular fat of geese at different growth stages. Poultry Sci. 2023, 103, 103172–103172. [Google Scholar] [CrossRef] [PubMed]
- Marcher, A.-B.; Loft, A.; Nielsen, R.; Vihervaara, T.; Madsen, J.G.S.; Sysi-Aho, M.; Ekroos, K.; Mandrup, S. RNA-Seq and Mass-Spectrometry-Based Lipidomics Reveal Extensive Changes of Glycerolipid Pathways in Brown Adipose Tissue in Response to Cold. Cell Rep. 2015, 13, 2000–2013. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, T.; Liu, R.; Chang, M.; Wei, W.; Jin, Q.; Wang, X. Reviews of medium- and long-chain triglyceride with respect to nutritional benefits and digestion and absorption behavior. Food Res. Int. 2022, 15. [Google Scholar] [CrossRef]
- Zhang, R.; Zhu, Z.; Jia, W. Molecular mechanism associated with the use of magnetic fermentation in modulating the dietary lipid composition and nutritional quality of goat milk. Food Chem. 2022, 366, 130554–130554. [Google Scholar] [CrossRef]
- Yan, H.; Jiao, L.; Fang, C.; Benjakul, S.; Zhang, B. Chemical and LC–MS-based lipidomics analyses revealed changes in lipid profiles in hairtail (Trichiurus haumela) muscle during chilled storage. Food Res. Int. 2022, 159. [Google Scholar] [CrossRef] [PubMed]
- Sohlenkamp, C.; López-Lara, I.M.; Geiger, O. Biosynthesis of phosphatidylcholine in bacteria. Prog. Lipid Res. 2003, 42, 115–162. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.; Schweizer, S.; Liebisch, G.; Oeckl, J.; Hoering, M.; Seeliger, C.; Schiebel, C.; Klingenspor, M.; Ecker, J. The lipidome of primary murine white, brite, and brown adipocytes—Impact of beta-adrenergic stimulation. PLOS Biol. 2019, 17. [Google Scholar]
- Guo, X.; Shi, D.; Liu, C.; Huang, Y.; Wang, Q.; Wang, J.; Pei, L.; Lu, S. UPLC-MS-MS-based lipidomics for the evaluation of changes in lipids during dry-cured mutton ham processing. Food Chem. 2022, 377. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




