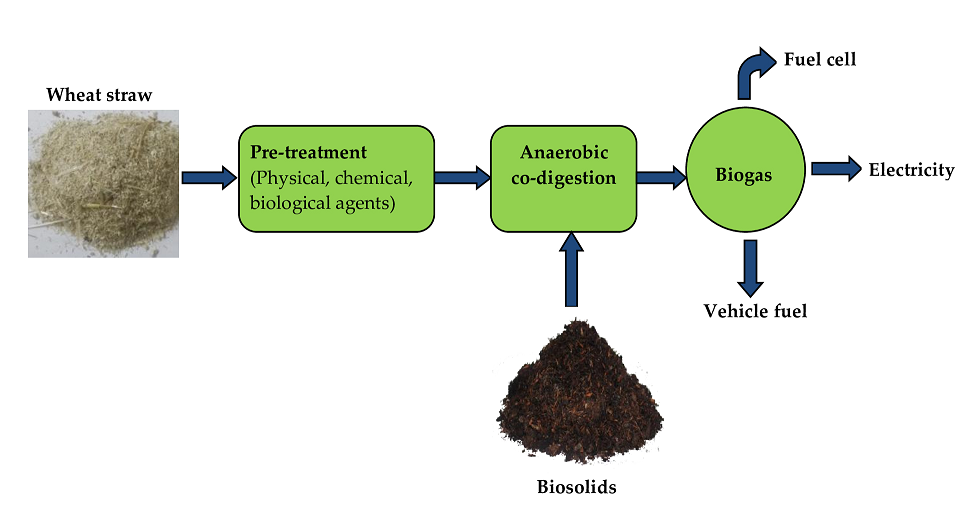
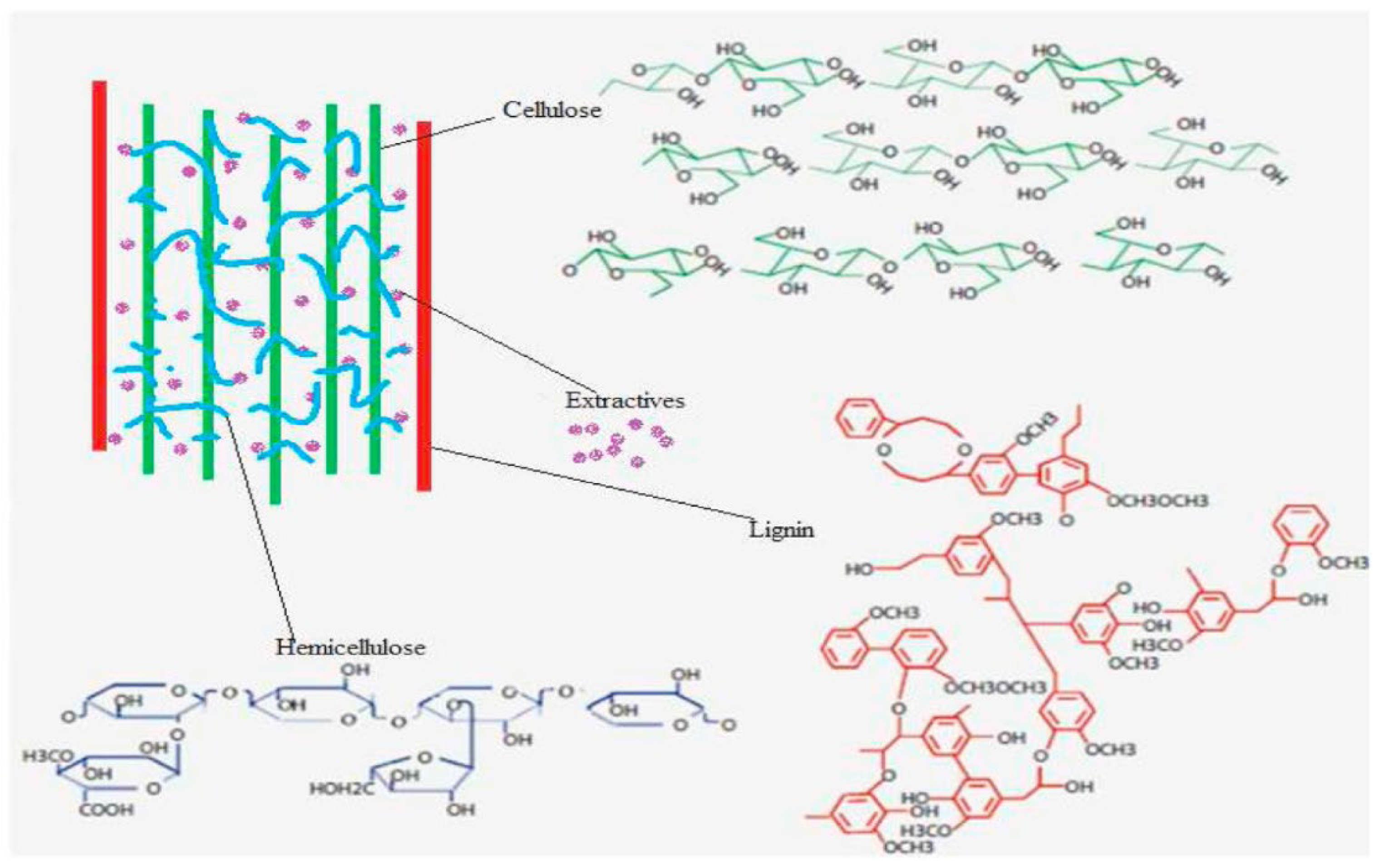
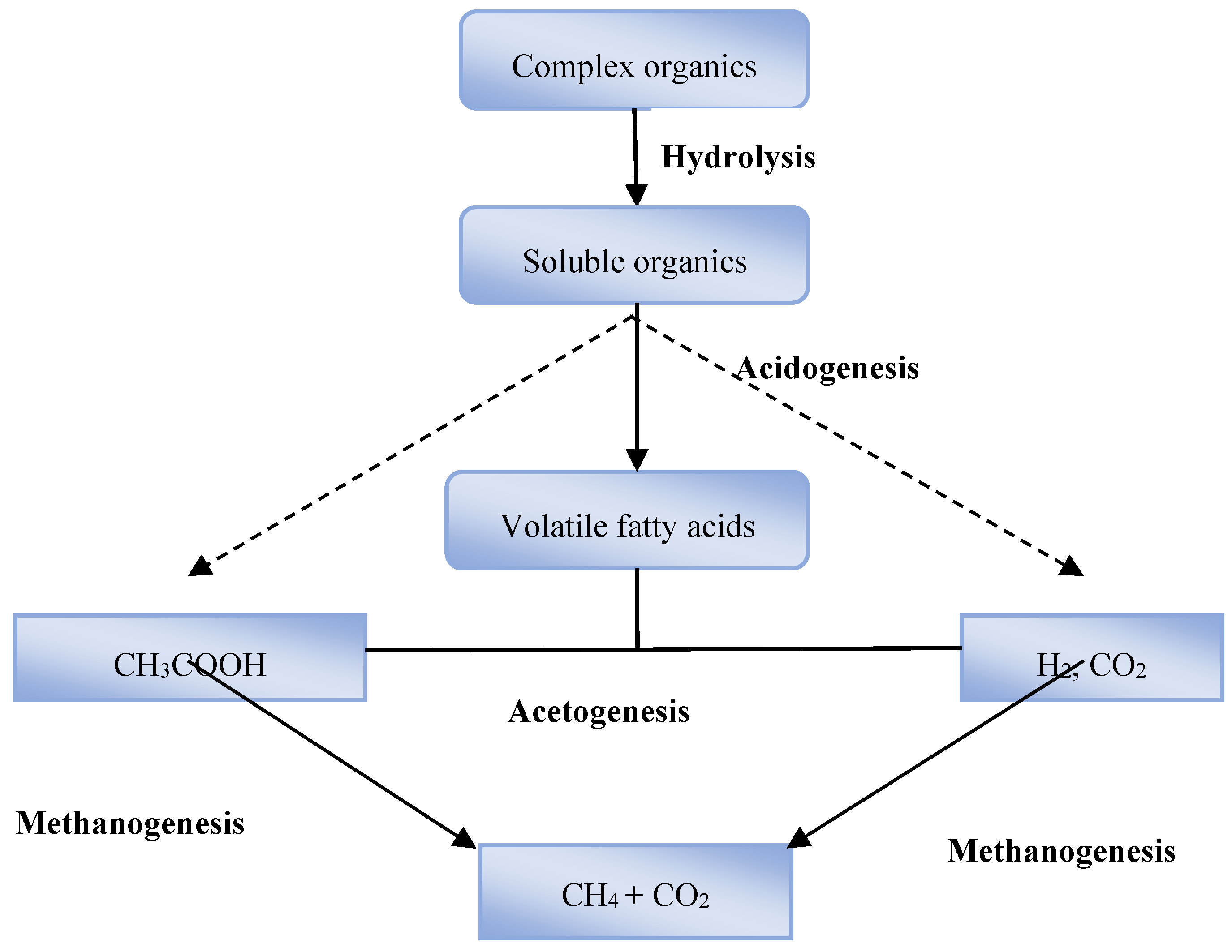
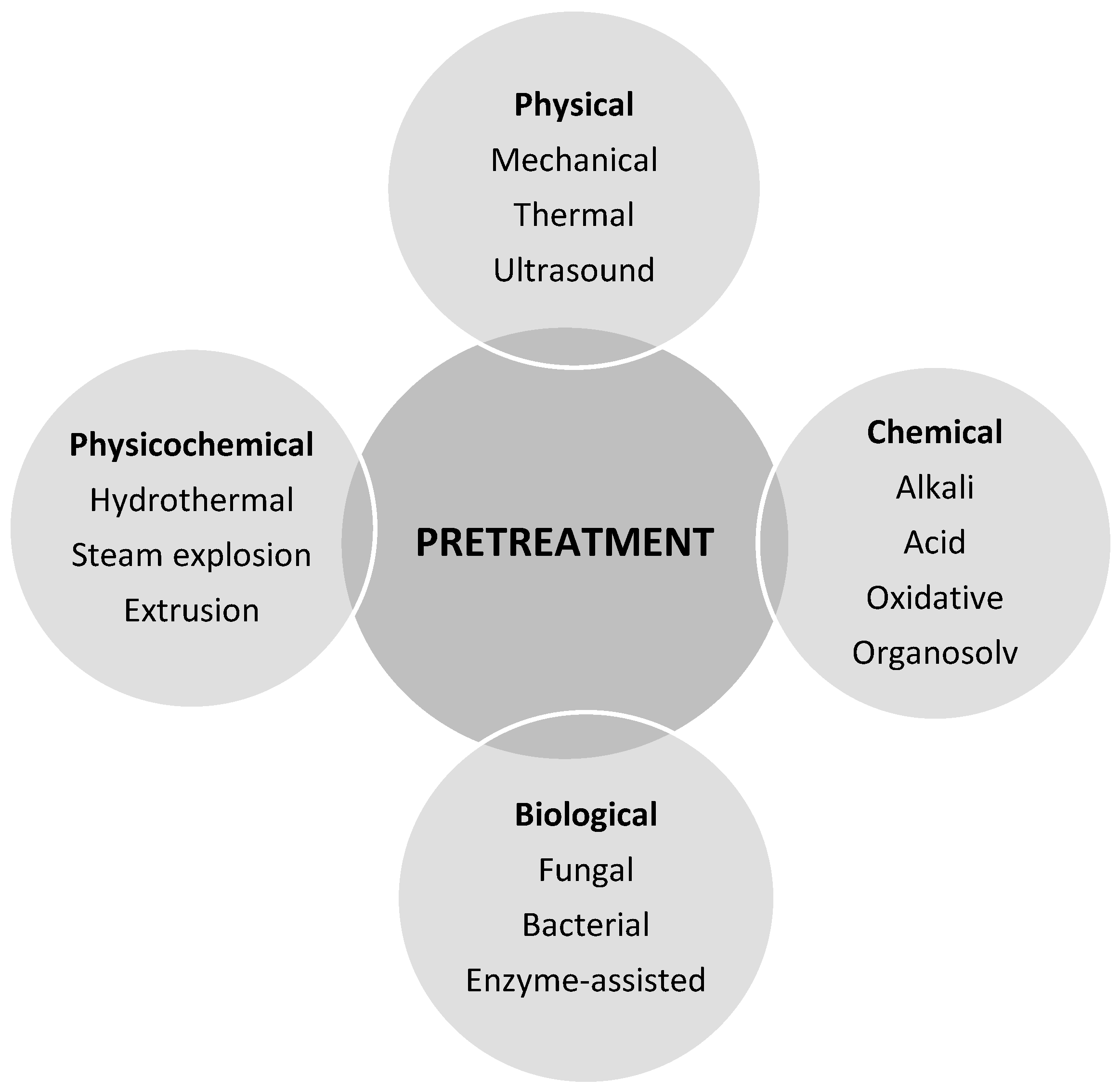
The choice of pre-treatment is critical in developing a viable scheme for biogasification of WS. Pre-treatment enhances the AD hydrolysis and promotes the availability of sugars and other small molecules to microbiota [
17]. There are several pre-treatment methods reported in literature. Pre-treatment methods can be broadly categorized into physical, chemical, biological and physico-chemical processes (
Figure 3) [
47,
48]. Physical and chemical methods are fast and effective, but however, they have limited value at industrial
-scale due to high cost of resources, energy and operation, and formation of toxic compounds. Biological pretreatments are still at their infant stages of development [
27,
49].
5.1.1. Physical Pre-Treatment
Physical pre-treatment is designed to trim the size of particles of a substrate by mechanical comminution or boost the surface area through mechanical refining. The final goal is to enhance the efficiency of hydrolysis and the yield of biogas [
50]. All methods that do not utilize water, chemicals and microorganisms are regarded as physical pre-treatments. These include mechanical, ultrasound and thermal pre-treatment methods [
50,
51].
Table 4 shows studies that have been performed to ascertain the effect of physical pre-treatment methods on biogas production from WS. Physical methods can increase the AD reaction kinetics of WS, but require more energy and capital [
2].
Mechanical pre-treatment includes a gamut of methods, such as grinding, milling, chipping or extrusion. Chipping is believed to be the most suitable mechanical method for treatment of WS. Mechanical pre-treatment reduces the crystallinity of cellulose and its extent of polymerization [
50]. Studies performed on mechanical pre-treatment of WS have shown a strong impact on biogasification. For example, Dell’Omo & Spena [
52] reported 49.1% more cumulative biogas yield from milled WS than control. Similarly, multistage knife milling of WS improved CH
4 production by 49.3% after 28 d of mesophilic digestion [
53]. Size reduction in the range of 0.5-2 mm can enhance heat and mass transfer to achieve adequate levels of biodegradation. Despite, the method is not sustainable because of high energy input [
45,
52,
54].
Thermal pre-treatment can be sub-divided into conventional method and microwave irradiation. Conventional thermal pre-treatment deconstructs cellulosic material by combined use of heat and water at a temperature range of 50-250°C [
9]. Heat and water destroys the crystalline structure of cellulose and other lignocelluloses matter, and converts hemicelluloses into VFAs and simple sugars [
2]. This results in enhanced biomethane production. For example, Bolado-Rodriguez et al. [
55] observed 20% higher CH
4 yield by exposing WS to thermal heat at 121°C for 60 min than the control. Similarly, Abdul-Wahab et al. [
56] generated 20% more CH
4 at 250°C and 1 min upon pre-treating WS at 150-220°C for 1-15 min.
Microwave method utilizes thermal energy from stimulation of vibration of molecules by non-ionizing radiation [
50]. Thermal heat disrupts the crystal structure of cellulose by cutting the β
-1,4
-glucan bonds, consequently enhancing the surface area of WS for AD [
51]. Microwave method could be a cheap alternative to conventional heating in the foreseeable future. With microwave pre-treatment, high temperatures are attained within a short period, thus saving energy [
33]. However, the process is often associated with high processing times, which may lead to sugar degradation [
50]. Furthermore, high capital requirements for installation limit its applicability at industrial scale [
54]. Information on microwave processing of WS to enhance biogas seems to be patchy. As an exemplar, CH
4 yield of WS was raised by 28% via solubilization with microwave irradiation at 150°C [
57].
Ultrasound pre-treatment uses acoustic energy in form of high frequency waves to induce cell lysis. Microbubbles are generated due to cavitation of cells in liquid solutions by high frequency sonic waves. Disintegration of microbubbles ruptures plant cell walls and exposes cellular contents, resulting in improved hydrolysis [
51,
58]. Ultrasound pre-treatment has advantages of short treatment time and low temperature needs, although it integrates the use of chemicals [
50]. Few studies were found in literature on ultrasound pre-treatment of WS for biogas production. For instance, a 63% increase in CH
4 content was recorded from ultrasound pre-treatment of WS at 20 kHz for 36 h [
59].
Table 4.
Physical pre-treatment of wheat straw for biogas production
Table 4.
Physical pre-treatment of wheat straw for biogas production
| Physical agent |
Pre-treatment conditions |
Findings |
Reference |
| Mechanical |
Knife milling, 0.3-1.2 mm particle size |
Methane yield increased by 49.3% |
[53] |
| |
Roll milling |
21% increase in methane yield |
[14] |
| |
Cutting (3-5 cm), milling (<1 mm) |
5-13% more methane for 3-5 cm particles with faster kinetics |
[43] |
| |
Chopping (2 cm), extruder-grinding (0.2 cm) |
Size reduction improved methane yield by 26% |
[60] |
| Conventional thermal |
150-220°C, 1-15 min |
20% increase in methane yield |
[43] |
| 200°C, 5 min |
27% more methane production |
[4] |
| 121°C, 60 min |
20% increase in methane yield |
[55] |
| 150-220°C, 1-15 min |
Methane yield enhanced by 20% |
[56] |
| Microwave |
Power of 400 - 1600 W, 150°C |
28% increase in methane yield |
[57] |
| |
200 -300°C, 15 min |
No increase in methane yield |
[61] |
| Ultrasound |
4% KOH, 20 kHz, ambient temperature, 36 h |
63% higher methane yield |
[59] |
| |
Hydrodynamic cavitation, 2 300-2 700 rpm, 2-6 min |
145% increased methane yield |
[62] |
| |
4% (w/w) H2O2, 36°C, 10 min, 25 kHz |
64% enhanced methane yield |
[63] |
5.1.2. Chemical Pre-Treatment
Chemical pre-treatment is based on substances, including acids, alkalis and ionic liquids to degrade the crystallinity of recalcitrant biomass. It can be classified into acid, alkaline, oxidative and organosolv pre-treatments [
11]. The use of chemicals is the most well-known pre-treatment method. Nevertheless, the method has been extensively reported in cellulosic bioethanol production compared to biogas production [
34].
Table 5 shows studies that have been conducted on chemical pre-treatment of WS for biogas production. Chemical pre-treatment is intended to improve biogas yield through disintegration of holocellulose.
Alkali pre-treatment can be deployed to solubilize lignin and holocellulose, thus rendering cellulosic materials to biological degradation. The method exploits bases, such as sodium hydroxide (NaOH), calcium hydroxide (Ca(OH)
2), potassium hydroxide (KOH) and ammonium hydroxide (NH
3.H
2O) to liquefy and cleave lignin-carbohydrate bonds [
54]. The purpose is to destruct the rigidity and structural complexity of WS, and increase the surface area for microbial attack [
32,
51]. NaOH pre-treatment is the most effective and widely studied alkali method for enhanced AD [
34]. For example, a 112% enhanced CH
4 yield was reported from NaOH-pre-treated WS [
64]. However, NaOH must be treated with caution as it generates sodium ions that can inhibit the AD process [
11].
Acid pre-treatment is yet another chemical method considered to be effective against hemicelluloses and lignin [
50]. This technique leads to ease of access to cellulose by microbial agents [
34]. Nitric acid (HNO
3), sulfuric acid (H
2SO
4), phosphoric acid (H
3PO
4) and hydrochloric acid (HCl) are typical examples of inorganic acids that can be used for biomass pre-treatment [
32,
54]. Dilute acid is believed to be more effective than concentrated acid for pre-treatment of lignocellulose. It is possible to solubilize up to 100% hemicelluloses into its monomeric units using dilute acid. Dilute acid can destroy lignin to a high degree, even though it is considered to be less efficient in dissolving the lignin [
32]. Concentrated acid is very effective against cellulose, but it is highly toxic, corrosive and requires specialized equipment. H
2SO
4 is the most extensively studied acidic pre-treatment method [
34]. There is limited information on acidic pre-treatment of WS for biogas production. Even so, a 16% rise in CH
4 production was obtained from WS pre-treated with H
2SO
4 [
65].
Oxidative pre-treatment is the degradation of lignin and hemicelluloses by oxidants, such as H
2O
2 and ozone gas [
11,
17]. This leads to nucleophilic substitution, destruction of aromatic nuclei, removal of side chains and dislocation of alkyl aryl ether bonds. Hydroxyl radicals (
-OH) and superoxides (O
2-) released from H
2O
2 promote the delignification of organic matter and release more fermentable sugars [
9]. H
2O
2 is very effective in alkaline solutions (pH 11.5) and does not generate toxic compounds [
9,
27]. Oxidative pre-treatment of WS with N-methylmorpholine N-oxide (NMMO) increased CH
4 yield by 11% [
66]. An increase of 27% in CH
4 yield was reported from photo-oxidative pre-treatment of WS using titanium oxide (TiO
2) [
67].
During organosolv pre-treatment, organic solvents are used to destroy internal linkages of lignin and hemicelluloses to ensure pure cellulose in WS is available for AD [
11]. Frequently utilized organic solvents, include methanol, ethanol, tetrahydrofuranol, acetone and ethylene glycol. Organosolv reaction is catalyzed by acids like H
2SO
4 and HCl or bases, such as NaOH, NH
3 and calcium carbonate (CaCO
3) [
17]. Mancini et al. [
66] observed up to 15% enhanced CH
4 yield from pre-treatment of WS with 50% ethanol. Likewise, an improved biogas production (47%) was found from pre-treatment of WS using NMMO [
68]. However, other organic solvents are exempted for WS pre-treatment because they are expensive, flammable, volatile, non-biodegradable and have low lignin removal efficiency [
2].
Table 5.
Chemical pre-treatment of wheat straw for biogas production
Table 5.
Chemical pre-treatment of wheat straw for biogas production
| Chemical agent |
Pre-treatment conditions |
Findings |
Reference |
| Acid |
1% H2SO4, 121°C, 10-120 min |
Increased methane yield by 16% |
[65] |
| |
0.5-5% H2SO4, 90-100°C, 2 h |
Biogas yield increased by 32% using 0.5% H2SO4 while 5% H2SO4 did not improve biogas yield |
[69] |
| |
2% HCl, 90°C, 2 h |
59% more biogas yield |
[70] |
| Alkaline |
1.6% NaOH, 30°C, 24 h |
15% enhanced methane yield |
[66] |
| |
NH3 (2, 4, 6%), 35°C, 7 d |
52% increased methane yield |
[71] |
| |
4% NaOH, 37°C, 5 d |
Biogas yield increased by 87.5% |
[64] |
| |
7 g KOH, 25°C, 24 h |
128% methane yield increment |
[47] |
| |
75 mM NaOH, 16 h |
Methane yield increased by 23% |
[72] |
| |
0.05 M NaOH, 25°C, 22 h |
22% increase in cumulative methane |
[44] |
| |
0.08 M Ca(OH)2, 20°C, 48 h |
Methane yield increased by 315% |
[73] |
| Oxidative |
TiO2-assisted photo-oxidation |
Improved methane yield by 27% |
[67] |
| |
NMMO, 120°C, 3 h |
11% methane yield improvement |
[66] |
| Organosolv |
NMMO, 90°C, 7 h |
47% increase in methane production |
[68] |
| 50% ethanol, 180°C, 1 h |
15% improved methane yield |
[66] |
| NMMO, 120°C, 3 h |
11% enhanced methane yield |
[66] |
5.1.3. Physico-Chemical Pre-Treatment
Physico-chemical pre-treatment amalgamates different methods to depolymerize lignin and hemicelluloses so that more fermentable sugars in WS are released for AD [
33]. The most suitable temperature for physico-chemical pre-treatment varies from 50
-250°C [
11]. Heat is applied to disrupt hydrogen bonds in WS, thereby increasing the surface area for microbes. It is prudent to recycle heat as a strategy to save energy during physico-chemical processing. Avoid extended pre-treatment times to prevent accumulation of inhibitory by-products [
51]. Potent physico-chemical pre-treatments include extrusion, steam explosion and hydrothermal processing [
33].
During extrusion pre-treatment, thermal and mechanical methods are combined in a single unit to modify the physical and chemical properties of plant biomass [
17]. Biomass is subjected to distressful conditions like heating and mixing with rapid fall in pressure [
11]. As biomass is discharged from the extruder, cellulose dissociates from complex polymers by breaking the β
-O-4 linkage in lignin and the plant cell wall structure is destroyed [
17]. Extrusion results in deconstruction of cellulose, hemicelluloses, lignin and proteins [
74]. The most favorable operational conditions for extrusion are temperature and pressure ranges of 160
-250°C and 0.5
-5.0 MPa, respectively [
17]. Chen et al. [
75] evaluated the effect of extrusion at 37ºC on biogas production from WS. In this study, biogas and CH
4 production increased by 23% and 27%, respectively. In a related study, an improved daily CH
4 production of 28% was reported from twin-screw extruded WS [
14]. However, the BMP value of WS was not significantly improved by extrusion. The AD of extruded-WS for 28 and 90 d enhanced CH
4 production by 14-28% and 1-16%, respectively [
76].
Steam explosion is a promising eco-friendly strategy for pre-treatment of WS for AD. In this technology, complex plant polymers are exposed to high pressure (5
-50 bar) and saturated steam at 160
-250°C for short residence times [
77]. Pressure is then rapidly lowered leading to depolymerization of the plant biomass [
11]. The conversion of WS into biofuels and other multiple products via steam explosion pre-treatment has gained interest in the 21
st century. For example, steam explosion pre-treatment of WS was studied by Kaldis [
78] who reported 20% enhanced CH
4 productivity. However, steam-explosion pre-treatment of WS did not provide positive results with regards to improvement of biogas production [
79,
80].
Hydrothermal liquefaction is an excellent method for enhanced energy recovery from WS to biogas. It is realistic to recuperate about 80% of the energy from WS biomass using this technology [
64]. Hydrothermal pre-treatment exploits hot water under high pressure at a reaction temperature of around 200ºC to permeate biomass, solubilize cellulose, and destroy hemicelluloses and a portion of lignin [
11,
64]. Although, there is no use of chemicals and rust-proof equipment [
64], the method releases phenolics and furfurals that may inhibit AD [
11]. Chandra et al. [
64] improved biogas and CH
4 production by 9.2 and 20.0%, respectively, using hydrothermally pre-treated WS at 200°C (1.55 MPa equivalent) for 10 min. Around 34% more biogas yield was achieved from hydrothermal and thermal-alkali pre-treatment of WS compared to control [
81]. In another investigation, exposure of WS to hydrothermal pre-treatment at an optimum temperature of 120°C enhanced CH
4 yield by 32% [
82]. The optimal operating temperature of 180ºC was found to increase CH
4 yield by 53% from hydrothermally-treated WS [
83].
5.1.4. Biological Pre-Treatment
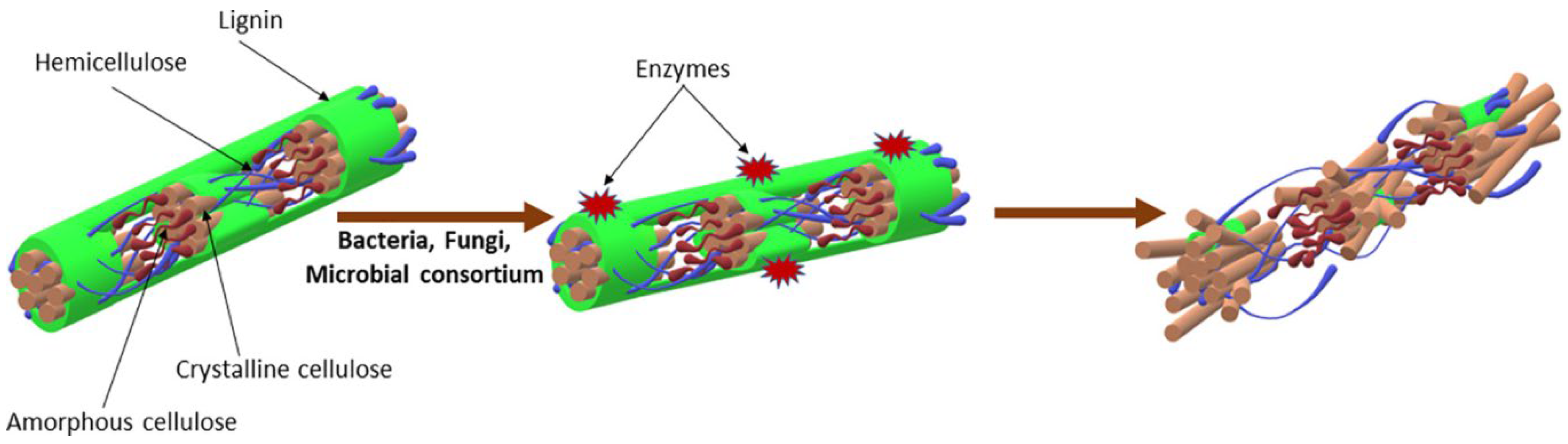
Biological pre-treatment entails the use of microbial metabolism or by-products to pre-digest recalcitrant biomass [
9]. The main effect is delignification to provide more cellulose and hemicelluloses for fermentation [
2]. As shown on
Figure 4, a sole or a consortium of microbes is generally applied to degrade polymeric substances. Biological method is more favorable for pre-treatment of WS than the other pre-treatment methods. It is an eco
-friendly technology that does not pollute the environment, generates little or no toxic by-products and has low energy demands. Contrarily, the method is considered to be slow, often with prolonged incubation times [
2,
9]. Studies that have conducted on biological pre-treatment of WS to improve biogas production are shown on
Table 6. Predominantly used biological agents for pre-treatment of organic matter comprise fungi, bacteria and enzymes.
Fungal pre-treatment utilizes white
-rot fungi, brown
-rot fungi, soft
-rot fungi or other fungi to delignify WS [
24,
33]. Lignin is a potential source of several materials and biochemicals for biorefineries [
84]. White
-rot fungi are considered to be the most effective fungal pre-treatment agents [
33]. Basidiomycetes (
Phanerochaete chrysosporium) are the most widely studied white
-rot fungi for delignification of cellulosic biomass [
17]. Factors such as moisture content, substrate particle size, temperature, pH, oxygen concentration, incubation time and nutrient availability must be optimized for efficient degradation of lignin [
11]. Fungal pre-treatment do not always promote CH
4 production to a larger extent. For example, pre-treatment of WS using fungus
Polyporus brumalis BRFM 985 strain increased CH
4 yield by merely 18% from 215 to 254 mL g VS
-1 [
85]. In addition, a slight rise of 31% in biogas yield was reported upon subjecting WS to
Chaetomium globosporum pre-treatment [
86]. Prominent barriers to fungal pre-treatment are prolonged residence times and consumption of fermentable sugars by fungi. Hence, full-scale adoption of fungal pre-treatment is still scarce [
86,
87].
Bacteria pre-treatment involves the use of enzyme
-secreting bacteria to combat the polymerization of biomass. Many anaerobic bacteria have high capacity to hydrate the structure of WS and improve CH
4 production [
2]. Destruction of WS using bacteria possesses numerous distinct traits over fungal pre-treatment. Bacteria can induce C
α-oxidation and cleave C
β-C
β linkages in lignin [
11]. They possess rapid growth rate with shorter incubation periods and are more cost
-effective than fungi. In addition, the genome of bacteria can be more easily modified than the fungal genome [
88].
Clostridium, Bacillus and Pseudomonas have been found to degrade plant materials through secretion of cellulases, xylanases and other hydrolytic enzymes. These bacteria occupy diverse extreme conditions, including decomposing forestry matter, compost, agricultural waste, organic matter and soil, and hot springs [
88].
Bacillus is one of the most promising genus to decompose WS due to its strong cellulose
-degrading capacity. Further, the bacteria can tolerate high temperatures and diverse pH conditions [
88]. No studies were found in literature on pre-treatment of WS using single strains of bacteria. However, pre-treatment of maize straw using
B. subtilis generated 17.35% higher CH
4 yield than control [
89].
Construction of microbial consortium was proposed as a panacea to limited utility of biological pre-treament at pilot-scale [
32]. The method is believed to be more effective than a single microorganism in enhancing the degradation of cellulosic wastes. A microbial consortium is a group of species with distinct delignification efficiencies and it is functional in diverse ecological conditions. It can deploy discrete delignification mechanisms with improved potential to exploit a substrate compared to indigenous microorganisms [
11]. Microbia consortia are isolated from natural conditions, where decomposing cellulosic waste is the main substrate [
34]. Unlike fungi, which mostly act on lignin, microbial consortium has high affinity for holocellulose [
17,
34]. The advantage of using microbial consortia is that sterilization may not be required. Pre-treatment of WS using microbial consortium improved CH
4 production by 80.34% than unpre-treated counterpart [
90]. A microbial consortium TC
-5 offered a rise in CH
4 yield of 36.6% after 35 d of AD of WS [
91].
Exogenous hydrolytic or oxidative enzymes can promote the degradation of lignocellulosic substrates. The most widely reported classes of enzymes for pre-treatment of biomass are cellulases and hemicellulases [
11,
17]. Enzymes have short reaction periods and can reduce the loss of holocellulose during hydrolysis. Moreover, enzymes have ease of access to a substrate with increased mass transfer rate [
11]. However, enzyme-assisted pre-treatment is limited due to high cost of commercial enzymes [
34]. Operational parameters, such as enzyme activity and specificity, enzyme concentration, inhibitor concentration, digester design, residence time, temperature and pH must be optimized for enhanced enzymatic pre-treatment [
27]. Combining different enzymes is an approach that can improve the efficacy of enzymatic pre-treatment. Screening enzymes with high specific activity and cross specificity can lower the quantity of the enzymes required as well pre-treatment cost [
92]. Literature seems to be scant considering enzyme-assisted pre-treatment of WS for biogas production. Even so, a 14% increase in CH
4 production was observed after enzymatic pre-treatment of WS using a complex mixture of hydrolytic enzymes [
72].
Table 6.
Biological pre-treatment of wheat straw for biogas production
Table 6.
Biological pre-treatment of wheat straw for biogas production
| Biological agent |
Microbes and enzymes |
Pre-treatment conditions |
Findings |
Reference |
| Fungi |
Penicillium aurantiogriseum, Trichoderma reesei, Gilbertella persicaria, Rhizomucor miehei |
100 mL batch reactors, 37ºC, 10 d |
Highest methane yield increase of 48% from P. aurantiogriseum pre-treated wheat straw |
[93] |
| |
Polyporus brumalis |
40 L aerobic reactors, 31ºC, 90% moisture, 13 d |
18% increase in methane yield |
[85] |
| |
Chaetomium globosporum |
Reagent bottles, 36ºC, 81% moisture, 14 d |
31% enhanced methane yield |
[86] |
| |
Ganoderma lobatum, Gloeophyllum trabeum |
250 mL Erlenmeyer flasks, dark, 25ºC, 10-40 d |
43.6 and 26.1 % increase in glucose yield by G. lobatum and G. trabeum, respectively |
[94] |
| |
Ligninolytic fungi |
250 mL Erlenmeyer flasks, 28ºC, 150 rpm, 7 d |
5-fold higher biogas yield |
[95] |
| Microbial consortium |
Microbial consortium TC-5 |
1 L anaerobic bottlles, 50ºC, 3 d |
22.2 and 36.6% increase in methane yield under mesophilic and thermophilic conditions, respectively |
[91] |
| |
Microbial consortium |
Batch, 37ºC, 20 d |
80.34% improved methane yield |
[90] |
| |
Cow rumen-derived microbial consortium |
35ºC, 15 d |
55.5% lignocellulose degradation |
[1] |
| |
Microbial consortium composed of fungi and bacteria |
|
39.24 and 80.34% increase in biogas and methane yield, respectively |
[90] |
| Enzymes |
Cellulase, xylanase, arabinase, pectinase, other carbohydrases, β-glucosidase |
100 mL glass reactors, 50ºC, 16 h |
14% enhanced methane yield |
[72] |
| |
Laccase, peroxidase |
30ºC, 60 rpm, 6 and 24 h |
11% increased methane yield after 6 h pre-treatment and 15% decreased methane yield after 24 h pre-treatment |
[96] |