1. Introduction
Researchers in recent years show that the prospect of using magnetically controlled nanoparticles is becoming more and more actual. The nanocomposites created on their basis are equipped with the functions of medical nanobots [
1], such as: the ability to distinguish microbiological objects in the biological area [
2], targeted transportation of medicinal drugs to the target organ [
3] diagnosis and therapy of diseases at the cellular level [
4] adsorption and removal of toxins through a magnetic field. The results of physical, chemical, and biological experiments confirm the potential of multifunctional magnetic nanoparticles as medicinal drug delivery systems [
2]. However, at the same time, aspects of the interaction of magnetic nanoparticles with living organisms and cells still relevant [
5]. The consequences of damaging effects of nanomaterials on the cellular structure, biodegradation processes of nanoparticles in the biological medium, dependence of toxicity on nanosystems concentration, etc., have not been fully studied [
6].
The main goal of the present research was the synthesis of medical multifunctional iron oxide nanoparticles and the study of the effectiveness of the synthesized samples in an experimental tumor model in vitro. Different configurations of magnetic nanoparticles loaded with doxorubicin (DOX), an antitumor chemotherapy agent was evaluated, using a variety of experimental methods and used in an in vivo tumor model, in order to obtain a new prototype of an effective antitumor therapeutic nanosystem with fewer side effects. To achieve this goal, it was necessary to create an optimal tumor model in experimental mice, for which preliminary in vitro studies were conducted on different tumor cell lines. As a result, it was possible to evaluate the antitumor efficiency of different concentrations of DOX-loaded nanoparticles and to select the least resistant culture for further studies.
Recent studies have shown that magnetic field-controlled nanoparticles hold significant promise in the treatment of neurodegenerative diseases such as Alzheimer's and Parkinson's [
7]. For instance, nanoparticles functionalized with specific ligands can cross the blood-brain barrier and target amyloid plaques, a hallmark of Alzheimer's disease [
8], these nanoparticles can be engineered to deliver therapeutic agents directly to the brain, potentially reducing the progression of neurodegenerative symptoms [
9]. In Parkinson's disease, it was demonstrated that magnetic nanoparticles could facilitate the delivery of neuroprotective drugs to dopaminergic neurons, offering a targeted approach to mitigate neuronal loss and improve motor function [
10].
Moreover, infectious diseases are another critical area where magnetically controlled nanoparticles are making an impact. Recent advancements have explored the use of these nanoparticles in the targeted treatment of bacterial infections, particularly those caused by antibiotic-resistant strains [
11]. For example, one of the recent studies developed magnetically guided nanoparticles that can deliver antibiotics directly to the site of infection, enhancing the drug's efficacy while minimizing systemic side effects [
12]. In addition, it has been shown that these nanoparticles can be designed to disrupt bacterial biofilms, which are often resistant to conventional treatments, thereby providing a potent tool against persistent infections [
13].
Prostate cancer is another area where magnetically controlled nanoparticles have shown substantial potential. Prostate cancer remains one of the most common cancers among men, and traditional treatments such as surgery, radiation, and chemotherapy often come with significant side effects [
14]. One of the promising recent research projects has demonstrated that iron oxide nanoparticles can be used to deliver chemotherapeutic agents specifically to prostate cancer cells, reducing the impact on surrounding healthy tissues [
15]. These nanoparticles can be guided with magnetic field to the tumor site, ensuring that a higher concentration of the drug is delivered directly to the cancer cells, thereby improving treatment efficacy and minimizing systemic toxicity.
Chronic lymphocytic leukemia (CLL), a type of cancer that affects the blood and bone marrow, has also been a focus of nanoparticle research. Traditional treatments for CLL, such as immunotherapy and chemotherapy, can cause severe side effects and may not always be effective in advanced stages [
16]. Recent studies, have explored the use of magnetic nanoparticles to deliver targeted therapies directly to leukemic cells [
17]. These nanoparticles can be engineered to carry therapeutic agents that specifically bind to cancerous B-cells, which are characteristic of CLL. This targeted approach not only enhances the effectiveness of the treatment but also significantly reduces adverse effects, offering new hope for patients with CLL.
By integrating these recent findings, we can appreciate the broad potential of magnetically controlled nanoparticles in addressing some of the most challenging medical conditions of our time. Their ability to deliver targeted therapy with precision not only enhances treatment efficacy but also reduces side effects, paving the way for more effective and safer medical interventions.
Magnetically controlled nanoparticles are emerging as a promising tool in medicine, offering targeted drug delivery, diagnosis, and even toxin removal. Recent research has highlighted their potential in treating various diseases, including cancer. One of our recent findings demonstrated the effectiveness of doxorubicin (DOX)-loaded citric-acid-modified magnetic nanoparticles in inhibiting the growth and proliferation of triple-negative breast cancer cells [
18]. This study highlights the potential of these nanoparticles for targeted drug delivery, minimizing side effects, and enhancing the cytotoxic effect of DOX on cancer cells.
This research aims to further explore the potential of magnetic nanoparticles in cancer therapy by synthesizing and evaluating the effectiveness of the same above -mentioned nanoparticles on different types of tumors on one hand and adding different configurations of these nanoparticles in a Leukemia and prostate cancer experimental tumor model. Our goal is to develop a new prototype of an effective antitumor therapeutic nanosystem with fewer side effects.
2. Materials and Methods
Synthesis of Nanoparticles. The synthesis of magnetic nanofluid containing citric acid coated (or folium acid conjugated) and DOX-loaded Iron Oxid Nanoparticles was carried out by modifying the standard chemical co-precipitation procedure in an automated continuous technology line. The dissolution processes were carried out in a reactor under vacuum (-0.98 MPa), and the reaction processes in an inert gas (N2) atmosphere using a Schlenk line connected to the automated continuous technology line, this automatization makes the synthesis process more efficient and allow obtaining highly dispersive, multifunctionalized, reproducible, nanofluids with narrow size distribution of nanoparticles - such method was used to prevent possible unwanted oxidation of divalent iron ions (Fe2+). Furthermore, some samples were additionally subjected to an electrohydraulic discharge (EHD) treatment at one stage of the synthesis.
The process of nanoparticle synthesis can be divided into the following stages:
Stage I – synthesis of ferromagnetic nanofluid containing iron oxide nanoparticles: First, FeCl3 · 6H2O (9 g) + 333 mL of deionized water (DW) (0.1 M solution) was prepared in the jacketed reactor with mechanical stirring (temperature 45°C, mixing duration 20 min. At the same time, 6.86 g (0.024 mol) of FeSO4 · 7H2O (stirring time 30 min) was dissolved in deionized water in a chemical reactor, under mechanical stirring (500 rpm) at 45°C with inert gas system (Schlenk line). Also separately, 26 ml of 25% ammonium hydroxide (NH4OH) was dissolved in 66 ml of distilled water under magnetic stirring (500 rpm) at 40°C. A separately dissolved 0.2 M iron (III) chloride aqueous solution was then added to the chemical reactor (in which 0.2 M iron (II) sulphide aqueous solution was previously prepared) via a peristaltic pump. In the vacuum medium, at 45°C, the said iron salts were stirred with a mechanical stirrer (750 rpm) for 10 minutes, after which 0.2 molar ammonium hydroxide was added through a peristaltic pump (at a speed of 3 ml/min). After complete delivery of ammonium hydroxide, mechanical stirring (750 rpm, 45°C) of the resulting colloidal solution in the chemical reactor was continued for 40 minutes.
Stage II: leaching of synthesized ferromagnetic nanofluid, ultrasonic treatment and coating with citric acid.
The magnetic colloid containing iron oxide nanoparticles was washed with distilled water by decantation on a permanent magnet to remove unwanted reaction products (ammonium chloride and sulphate) formed as a result of the synthesis until the pH of the suspension became equal to 6.5.
Finally, the washed sample was added to 200 ml of deionized water and treated with an ultrasonic disperser at 80% power for 5 min.
After that, the coating with citric acid or conjugated with folic acid was carried out: by adding a pre-prepared aqueous solution of citric acid /folic acid (0.585 g CA /0.585 g FA) drop by drop to the colloid with a peristaltic pump, after which the ultrasonic treatment of the surfaced liquid continued for 20 min. Finally, the encapsulated ferromagnetic nanofluid was centrifuged at 4000 rpm for 10 min.
In stage III, the loading of the synthesized magnetic nanoparticles with an antitumor drug (doxorubicin). Citric acid/folic acid-stabilized magnetic nanofluid (100 µg) was added to 2.5 mL of doxorubicin solution (10 mg/mL) and mixed together with the doxorubicin at room temperature under continues shaking conditions.
Cell cultivation. The research involved the use of important methods for cell cultivation. All cell lines were cultured in RPMI-1640 supplemented with 10% fetal calf serum (FCS) and 1% Penicillin Streptomycin (all from Sigma-Aldrich). Cell culture maintenance, including multiplication, seeding, and separation, as well as freezing methods, were also employed.
Cell cultures used: RM1 is a murine prostate cancer cell line that was induced in male C57BL/6 strain mice. Urogenital sinus cells of 17-day-old C57BL/6 mouse embryos were infected with Zipras/myc9 retrovirus and transplanted under the renal capsule of isogenic adult male mice. The cell line is metastatic, therefore the tumor developed and it was from here that the cell line was isolated. The RM1 tumor line is often used to create an experimental tumor model.
The MEC1 cell line consists of transformed B lymphocytes obtained from a 58-year-old man diagnosed with stage 2 chronic lymphocytic leukemia (CLL) [
19]. The cell line was established from a patient with unmutated IGHV genes who exhibited progression towards prolymphocytic leukemia. The patient was Epstein-Barr virus (EBV)-positive, consistent with most CLL patients, who also have the EBV receptor CD21 on their lymphocytes. The primary aim of establishing the MEC1 B cell line was to create an
in vitro model that closely mimics CLL, facilitating the study of the biological processes involved in the disease.
Characterization of nanoparticles. The determination of the hydrodynamic diameters and zeta potential was conducted using dynamic light scattering (DLS) and electrophoretic light scattering (ELS) techniques with a Litesizer 500 particle analyzer from Anton Paar, Graz, Austria. The pH range studied was from 2 to 12, with a semiconductor laser as the light source (40 MW, 658 nm). For crystal structure and phase analysis, X-ray diffraction (XRD) was employed on dried aqueous suspensions of bare SPIONs, CA-SPIONs, FA-SPIONs, DOX-FA-SPIONs and DOX-CA-SPIONs using a DRON 3M X-ray diffractometer. Transmission electron microscopy (TEM) was carried out on samples prepared by drop-drying suspensions on holey carbon-foil-coated copper grids using a JEOL 3010 transmission electron microscope. Magnetization curves of magnetic nanofluids were measured with a vibrating sample magnetometer (VSM) (7300 Series VSM System, Lake Shore Cryotronics, Inc., Westerville, OH, USA), and FTIR spectroscopic studies were performed using a Thermo Scientific™ Nicolet™ iS™ 5 instrument with a diamond ATR crystal. UV-visible absorption measurements of doxorubicin solutions and nanoparticles were recorded on an AVAspec HS 2048XL spectrometer in the wavelength range of 200–1100 nm at room temperature.
Determination of optical density of cells in solutions. The optical density (OD562) of the cell suspension was determined by analyzing filled 96-cell plates using a scanning multiscan spectrophotometer at a wavelength of 562 nm. The number of cells was determined at 0, 24 and 48 hours. 100 µl of the cell suspension was transferred to each well. After shaking the cassette three times (20 s), the absorbance at 562 nm was immediately measured. Thus, a linear relationship between OD values and cell concentrations is determined.
MTT method. Cytotoxicity of the samples were assessed via MTT (2-3-[4,5-dimethyltetrazolium-2-yl]-2, 5 dimethyl tetrazolium bromide) test. MTT cell proliferation assay, which is based on the ability of the enzyme mitochondrial dehydrogenase in viable cells to disperse the bright yellow color of tetrazolium MTT and convert it to a dark blue formazan crystal. The number of surviving cells is directly proportional to the number of formazan crystals, which is measured by spectrophotometry at a wavelength of 570 nm (ELISA counter).
Production of cytopreps. The cell concentration was brought to 2x106/mL, after fixing 100μL of cells, the suspension was transferred to the cuvettes of a cytocentrifuge (Shandon Cytospin 2). The cuvette consisted of a slide, a metal holder, a funnel and a paper filter. The suspension was added to the cuvette funnel. Centrifugation was performed for 10 minutes at 600 (RPM). Then the samples were observed with a fluorescence microscope (Zeiss).
3. Results
3.1. Characterization of Nanoparticles
3.1.1. ELS and DLS Results
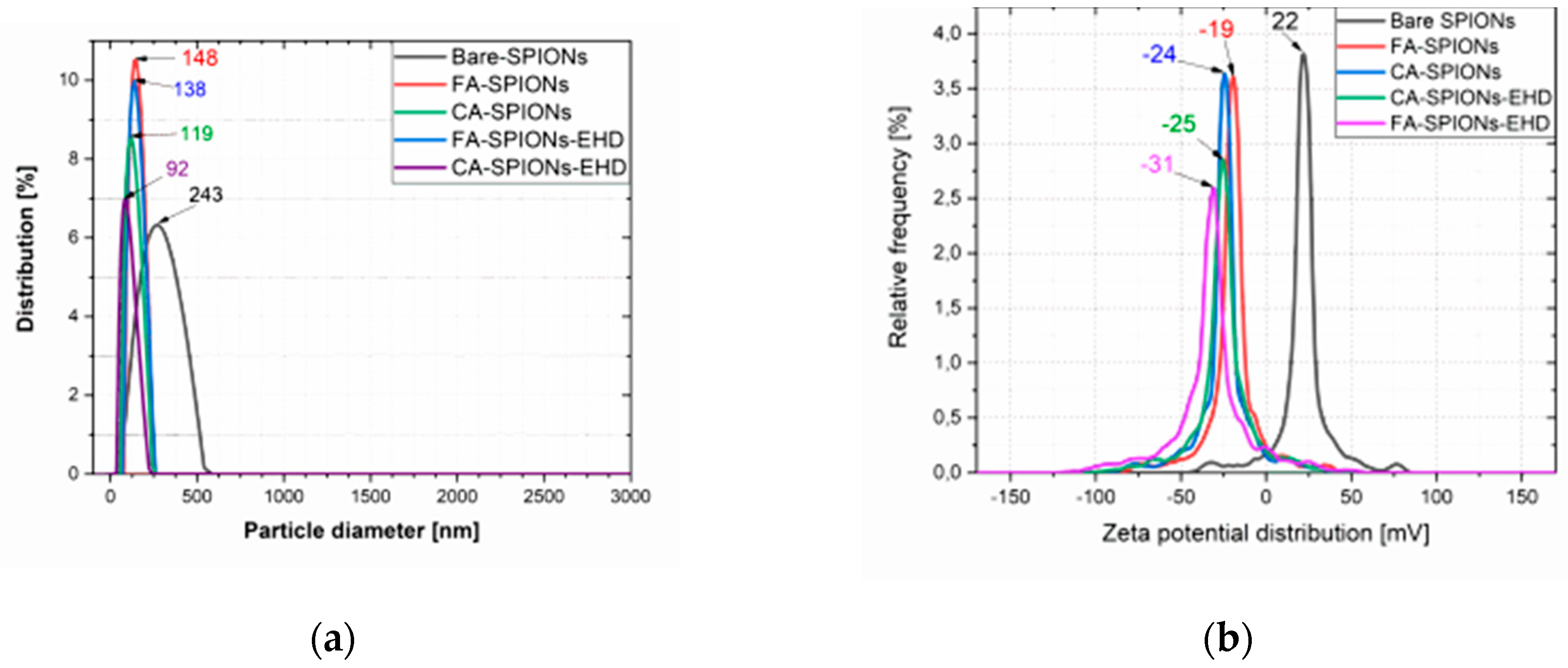
The zeta potential is a measure of the electrostatic repulsion between particles in a solution. A higher absolute value of zeta potential indicates greater stability. Both the CA-modified and FA-modified iron oxide nanoparticles and the electrohydraulic discharge-processed CA-SPIONs had negative zeta potentials from -19 mV to -31 mV, at neutral pH (6.5). This suggests that these nanoparticles are well-dispersed in solution and less likely to aggregate. The Bare SPIONs had a positive zeta potential of 22 mV, indicating a change in surface charge due to the CA coating or FA conjugation.
Figure 1.
Particle size and ζ potential of Bare SPIONs; CA-SPIONs, FA-SPIONs, CA-SPIONs-EHD and FA-SPIONs-EHD (a) Particle size (b) ζ potential.
Figure 1.
Particle size and ζ potential of Bare SPIONs; CA-SPIONs, FA-SPIONs, CA-SPIONs-EHD and FA-SPIONs-EHD (a) Particle size (b) ζ potential.
The hydrodynamic diameter refers to the size of a particle in solution, including any surrounding hydration layer. The CA-modified iron oxide nanoparticles and the electrohydraulic discharge-processed CA-SPIONs had smaller hydrodynamic diameters of 119 nm and 92 nm, respectively, compared to FA-modified iron oxide nanoparticles and the electrohydraulic discharge-processed FA-SPIONs had a larger hydrodynamic diameter of 148 nm and 138 nm, respectively. However, all the samples were relatively smaller than Bare-SPIONs, with 243 nm particle diameter, likely due to the stabilization by the CA Molecules on the nanoparticle surface.
3.1.2. XRD Results
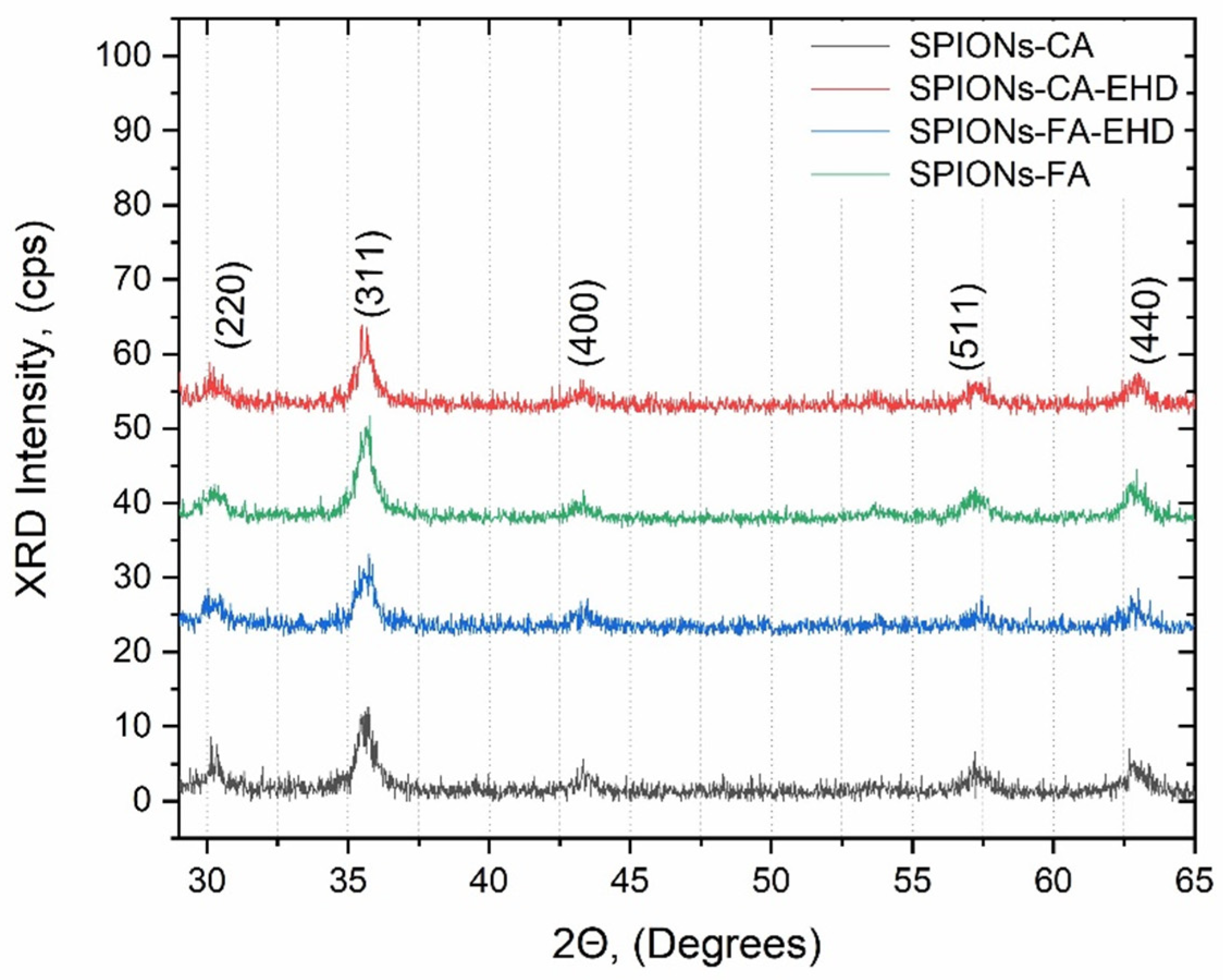
The X-ray diffraction (XRD) is a technique used to identify the crystal structure of a material. The XRD patterns of all synthesized samples (CA-SPIONs, FA-SPIONs, CA-SPIONs-EHD and FA-SPIONs-EHD) showed the same characteristic peaks corresponding to the cubic inverse spinel structure of magnetite (Fe3O4). This indicates that the modification process with citric acid and the conjugation with folic acid did not alter the fundamental crystal structure of the iron oxide nanoparticles.
Figure 2.
Diffraction patterns of the synthesized samples: CA-SPIONs, FA-SPIONs, CA-SPIONs-EHD and FA-SPIONs-EHD.
Figure 2.
Diffraction patterns of the synthesized samples: CA-SPIONs, FA-SPIONs, CA-SPIONs-EHD and FA-SPIONs-EHD.
3.1.3. TEM Results
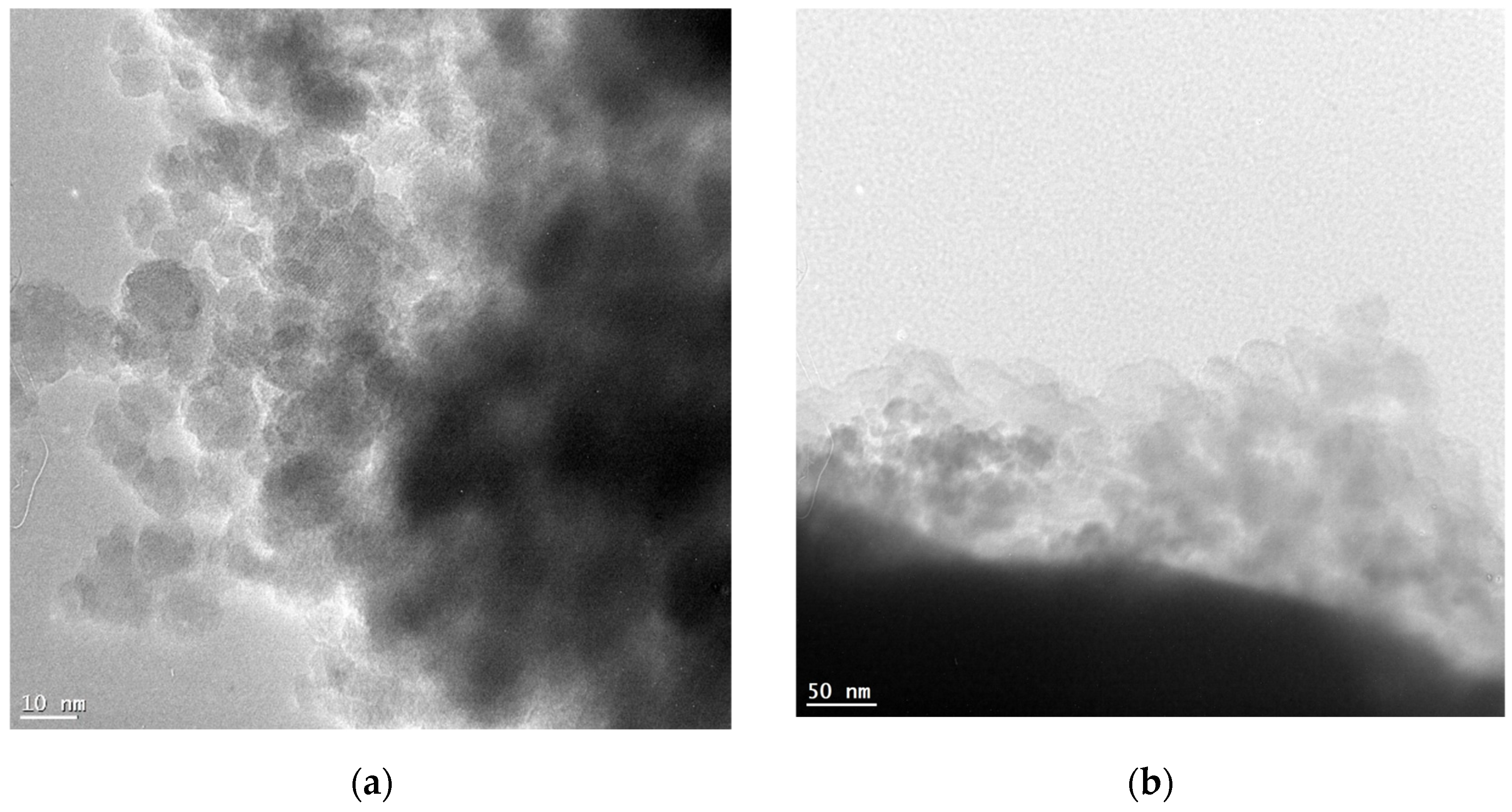
Transmission electron microscopy (TEM) is a technique used to visualize the morphology and size of nanoparticles. The TEM images revealed that the synthesized nanoparticles had a spherical morphology with a narrow size distribution. The CA-modified iron oxide nanoparticles (CA-SPIONs) appeared slightly sparse compared to the FA-modified nanoparticles (FA-SPIONs) which is consistent with the DLS results.
Figure 3.
TEM images of (a) CA-modified and (b) FA-modified iron oxide nanoparticles.
Figure 3.
TEM images of (a) CA-modified and (b) FA-modified iron oxide nanoparticles.
3.1.4. VSM Results
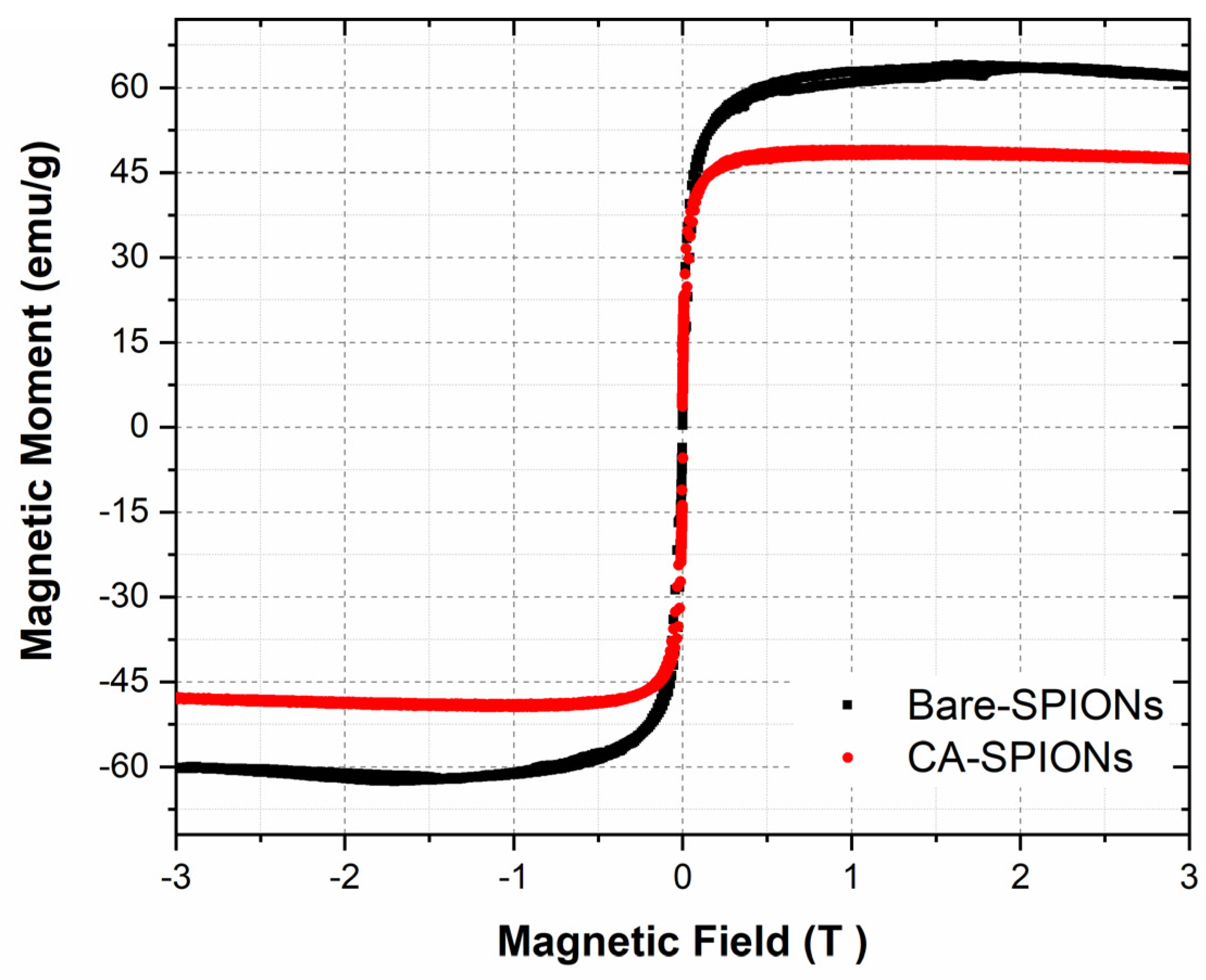
Vibrating sample magnetometry (VSM) is a technique used to measure the magnetic properties of materials. The magnetization curves of the synthesized magnetic nanofluids showed that both bare and citric-acid-modified iron oxide nanoparticles exhibited superparamagnetic behavior. This means that they are magnetic only when an external magnetic field is applied and lose their magnetism when the field is removed. This property is important for potential applications in targeted drug delivery, as the nanoparticles can be guided to the tumor site using an external magnetic field.
Figure 4.
Magnetization curves of magnetic fluids with bare SPIONs and Citric acid coated magnetite nanoparticles were measured on a Vibrating Sample Magnetometer at room temperature under an applied field up to 3 Tesla.
Figure 4.
Magnetization curves of magnetic fluids with bare SPIONs and Citric acid coated magnetite nanoparticles were measured on a Vibrating Sample Magnetometer at room temperature under an applied field up to 3 Tesla.
3.1.5. UV-Vis Optical Studies
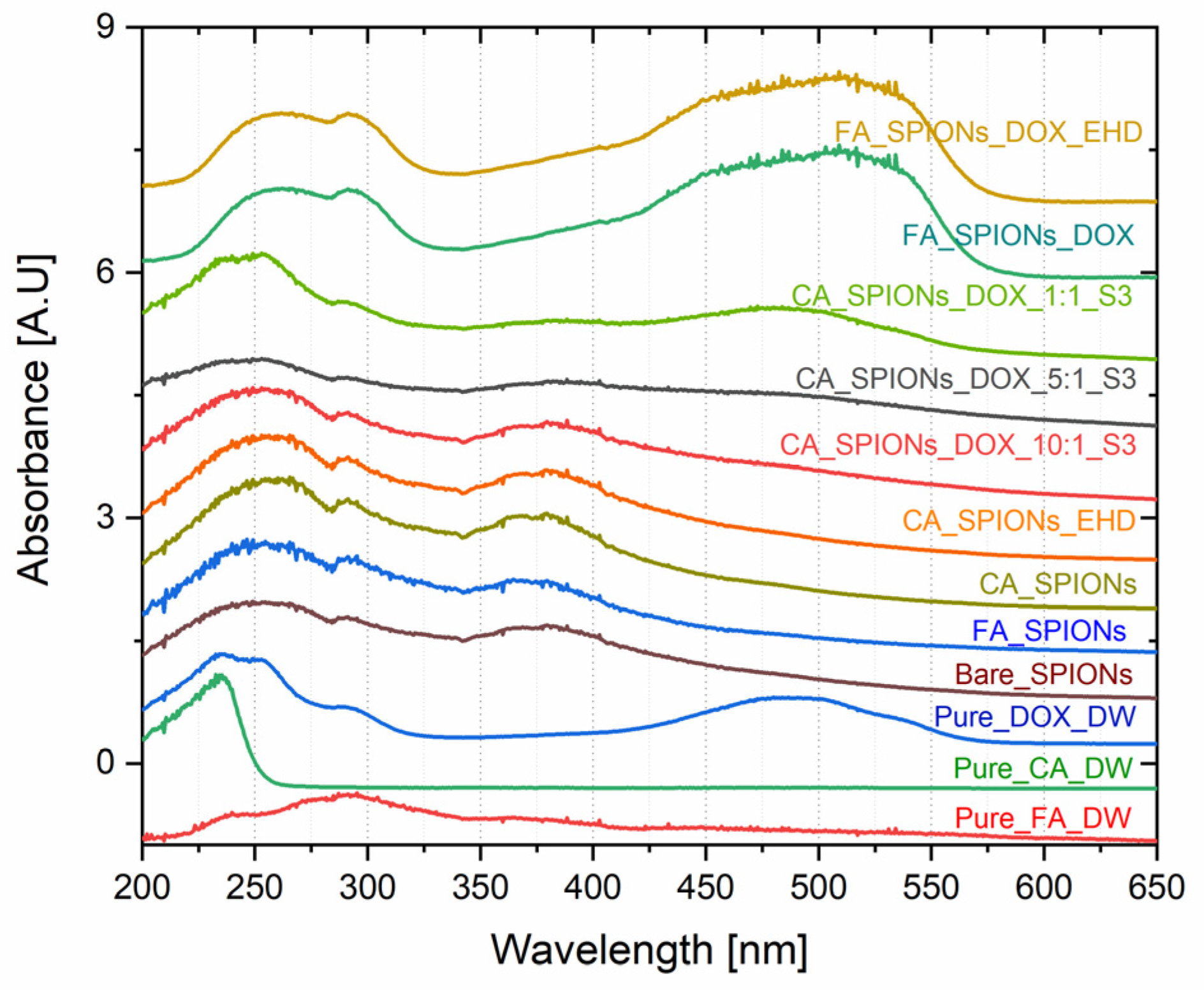
UV-Vis spectroscopy is a technique used to identify and quantify the presence of specific molecules based on their absorption of light at different wavelengths. The UV-Vis absorption spectra of the DOX-loaded samples showed a characteristic absorption peak at around 480 nm, which is the absorption peak of doxorubicin. The intensity of this peak increased with increasing doxorubicin loading concentration, confirming the successful loading of doxorubicin onto the CA-SPIONs. This is important for the development of a drug delivery system, as it demonstrates that the nanoparticles can effectively encapsulate and carry the anticancer drug.
Figure 5.
UV-VIS spectra of magnetic nanoparticles containing Bare SPIONs, citric acid coated, Folic acid conjugated, electro-hydraulically treated and DOX loaded NPs (with different ratio of DOX to magnetite in nanofluid (parity - 1: 1; 1: 5; 1:10).
Figure 5.
UV-VIS spectra of magnetic nanoparticles containing Bare SPIONs, citric acid coated, Folic acid conjugated, electro-hydraulically treated and DOX loaded NPs (with different ratio of DOX to magnetite in nanofluid (parity - 1: 1; 1: 5; 1:10).
3.1.6. FTIR Analysis
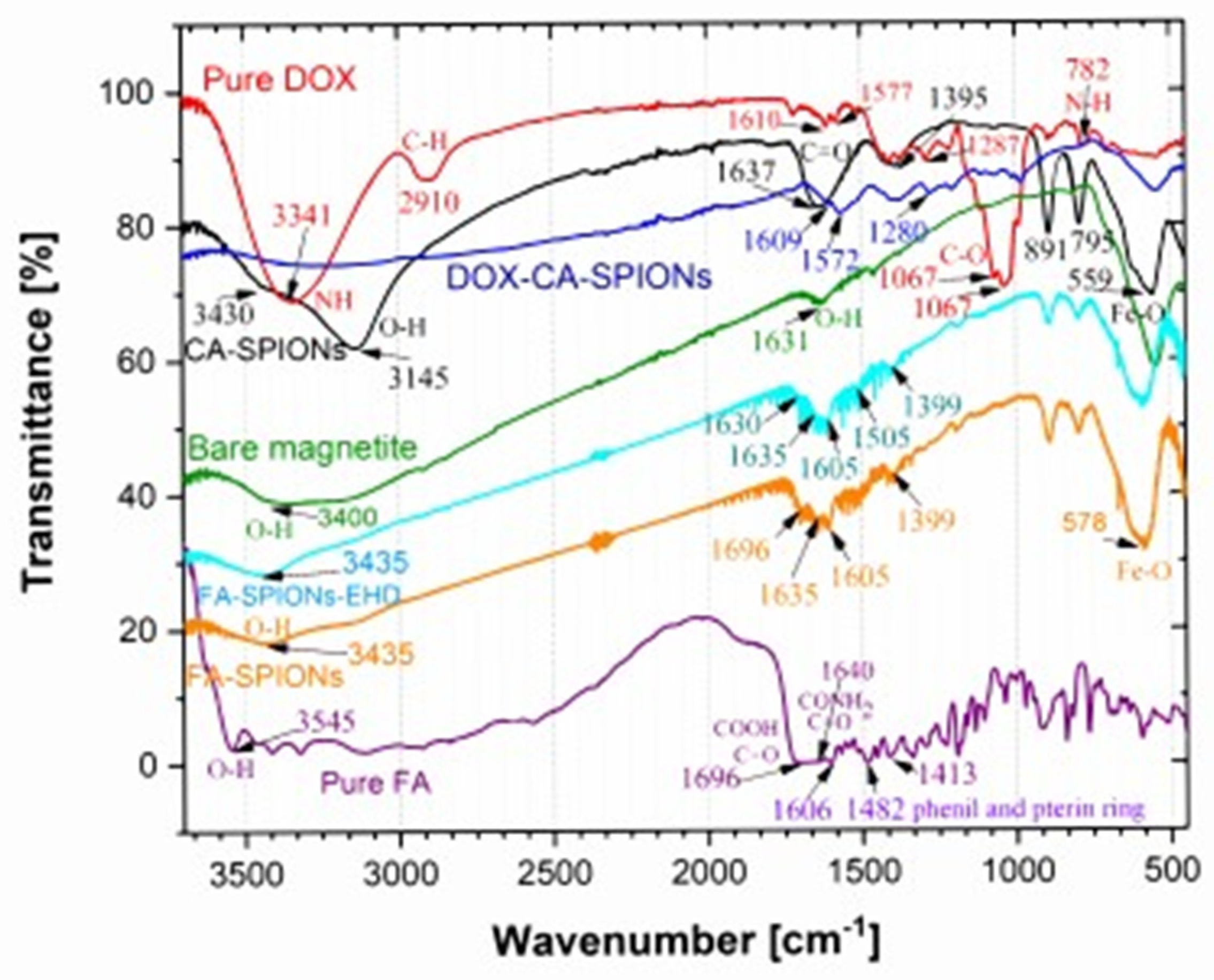
The FTIR analysis is crucial for confirming the successful synthesis and characterization of the nanoparticles. It provides evidence for the presence of the desired coating, such as citric acid (CA) or conjugation, as folic acid (FA), on the iron oxide nanoparticles. The distinct peaks observed in the spectra of CA-SPIONS and FA-SPIONS, compared to the bare SPIONs, confirm the successful attachment of these coatings. Additionally, the presence of doxorubicin peaks in the DOX-CA-SPIONS spectrum indicates successful drug loading onto the nanoparticles. The FTIR results provide valuable information for optimizing the synthesis process and ensuring the desired properties of the nanoparticles for their intended therapeutic applications.
Figure 6.
FTIR spectra of pure citric acid (CA), pure folic acid (FA), bare SPIONs (); CA-SPIONs, pure DOX, DOX-FA-SPION and DOX-CA-SPION.
Figure 6.
FTIR spectra of pure citric acid (CA), pure folic acid (FA), bare SPIONs (); CA-SPIONs, pure DOX, DOX-FA-SPION and DOX-CA-SPION.
3.2. Results of in vitro experiments
following steps were performed:
Cultivation of cell lines;
Obtaining the required concentration for cell research (10,000 cells/mL);
Making different combinations of nanoparticle-drugs systems (table 1);
Incubation of cells and nanofluid in an environment as close as possible to the organism (37°C, 5% CO2);
Cytotoxicity assessment of samples at 0, 24 and 48 hours using MTT-method.
Incubation of cells is carried out at 0 hours, 24 hours and 48 hours. Then the test substances were added: - S0-uncoated nanoparticles at 1.00 mg/mL, 0.50 mg/mL, and 0.20 mg/mL;
- S1-(SPIONs-CA) – citric acid coated nanoparticles at 1.00 mg/mL, 0.50 mg/mL, and 0.20 mg/mL;
- S2-(SPIONs–HYD-CA) – electrohydraulically treated + citric acid coated nanoparticles at 1.00 mg/mL, 0.50 mg/mL, and 0.20 mg/mL;
- DOX-doxorubicin at 1.84 mM, 0.92 mM, and 0.37 mM;
- S3-(SPIONs-CA-Dox) - nanoparticles coated with citric acid and functionalized with Doxorubicin at 0.50 mg/mL, 0.25 mg/mL, and 0.10 mg/mL (1:1 ratio), 0.83 mg/mL, 0.42 mg/mL, and 0.17 mg/mL (1:5 ratio), and 0.91 mg/mL, 0.45 mg/mL, and 0.18 mg/mL (1:10 ratio);
- S4 - (CA-SPIONs-HYD-Dox) - nanoparticles processed electrohydraulically, coated with citric acid, and loaded with Doxorubicin at 0.50 mg/mL, 0.25 mg/mL, and 0.10 mg/mL (1:1 ratio), 0.83 mg/mL, 0.42 mg/mL, and 0.17 mg/mL (1:5 ratio), and 0.91 mg/mL, 0.45 mg/mL, and 0.18 mg/mL (1:10 ratio).
- SPIONs-FA – Folic acid conjugated nanoparticles at 0.45 mg/mL and 0.18 mg/mL
- SPIONs–HYD-FA - Electrohydraulically treated + Folic acid conjugated nanoparticles at 0.50 mg/mL and 0.20 mg/mL
- SPIONs-FA-Dox - Folic acid conjugated nanoparticles functionalized with Doxorubicin at 0.45 mg/mL and 0.18 mg/mL
- SPIONs-HYD-FA-Dox - Electrohydraulically treated + Folic acid conjugated nanoparticles functionalized with Doxorubicin at 0.45 mg/mL and 0.18 mg/mL
Table 1.
column 1 - Designations Corresponding to Different Concentrations (w/v) and column 2 - Concentration (mg/mL).
Table 1.
column 1 - Designations Corresponding to Different Concentrations (w/v) and column 2 - Concentration (mg/mL).
| Designations (w/v) |
Concentrations (mg/mL) |
| DOX–0.1% |
1.84 mM |
| DOX–0.05% |
0.92 mM |
| DOX–0.02% |
0.37 mM |
| S0–0.1% |
1.00 mg/mL |
| S0–0.05% |
0.50 mg/mL |
| S0–0.02% |
0.20 mg/mL |
| S1–0.1% |
1.00 mg/mL |
| S1–0.05% |
0.50 mg/mL |
| S1–0.02% |
0.20 mg/mL |
| S2–0.1% |
1.00 mg/mL |
| S2–0.05% |
0.50 mg/mL |
| S2–0.02% |
0.20 mg/mL |
| S3–1:1–0.1% |
0.50 mg/mL |
| S3–1:1–0.05% |
0.25 mg/mL |
| S3–1:1–0.02% |
0.10 mg/mL |
| S3–1:5–0.10% |
0.83 mg/mL |
| S3–1:5–0.05% |
0.42 mg/mL |
| S3–1:5–0.02% |
0.17 mg/mL |
| S3–1:10–0.10% |
0.91 mg/mL |
| S3–1:10–0.05% |
0.45 mg/mL |
| S3–1:10–0.02% |
0.18 mg/mL |
| S4–1:1–0.1% |
0.50 mg/mL |
| S4–1:1–0.05% |
0.25 mg/mL |
| S4–1:1–0.02% |
0.10 mg/mL |
| S4–1:5–0.1% |
0.83 mg/mL |
| S4–1:5–0.05% |
0.42 mg/mL |
| S4–1:5–0.02% |
0.17 mg/mL |
| S4–1:10–0.1% |
0.91 mg/mL |
| S4–1:10–0.05% |
0.45 mg/mL |
| S4–1:10–0.02% |
0.18 mg/mL |
Test substances were added at the specified concentrations, and after incubation, 10μL of MTT solution was added and incubation was carried out for 3-4 hours at 37°C, finally 100μL MTT solvent was added and gentle pipetting was performed. The optical density was measured with an ELISA counter at a wavelength of 570 nm.
3.2.1. Effects of Nanoparticles on RM1 Cell Lines
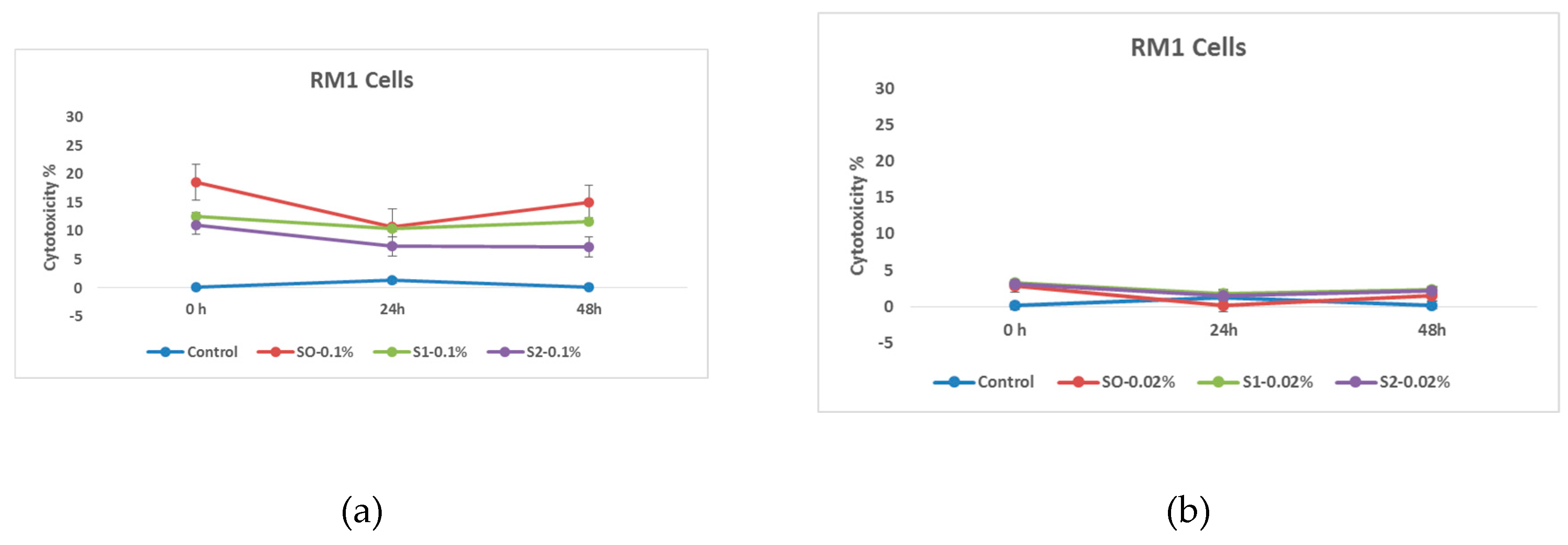
At the initial stage of the research, the cytotoxic effect of citric acid coated nanoparticles was studied. Based on the obtained data, it can be concluded at a glance that the RM1 cell line is a much more stable line, and the effect of doxorubicin on them is expressed only by a small variation in the indicators of cell survival, although it is worth noting that the efficiency of both citric acid-coated and doxorubicin-loaded nanoparticles is similar to the indicators observed as a result of previous studies.
Figure 7.
Effects of nanoparticles on RM1 cell line, (MTT assay - cytotoxicity assessed at 570 nm) where control - represents untreated tumor cells; (a) S0-0.1%– cells treated with 1.00 mg/mL concentrations of superparamagnetic iron oxide nanoparticles; S1-0.1% - cells treated with 1.00 mg/mL concentration of superparamagnetic iron oxide nanoparticles coated with citric acid; and, S2-0.2%- cells treated with 1.00 mg/mL concentration of electrohydraulic processed superparamagnetic iron oxide nanoparticles coated with citric acid. (b) S0-0.02%– cells treated with 0.20 mg/mL concentrations of superparamagnetic iron oxide nanoparticles; S1-0.02% - cells treated with 0.20 mg/mL concentration of superparamagnetic iron oxide nanoparticles coated with citric acid; and, S2-0.02% - cells treated with 0.20 mg/mL concentration of electrohydraulic processed superparamagnetic iron oxide nanoparticles coated with citric acid.
Figure 7.
Effects of nanoparticles on RM1 cell line, (MTT assay - cytotoxicity assessed at 570 nm) where control - represents untreated tumor cells; (a) S0-0.1%– cells treated with 1.00 mg/mL concentrations of superparamagnetic iron oxide nanoparticles; S1-0.1% - cells treated with 1.00 mg/mL concentration of superparamagnetic iron oxide nanoparticles coated with citric acid; and, S2-0.2%- cells treated with 1.00 mg/mL concentration of electrohydraulic processed superparamagnetic iron oxide nanoparticles coated with citric acid. (b) S0-0.02%– cells treated with 0.20 mg/mL concentrations of superparamagnetic iron oxide nanoparticles; S1-0.02% - cells treated with 0.20 mg/mL concentration of superparamagnetic iron oxide nanoparticles coated with citric acid; and, S2-0.02% - cells treated with 0.20 mg/mL concentration of electrohydraulic processed superparamagnetic iron oxide nanoparticles coated with citric acid.
In this case, two different concentrations of nanoparticles were compared. As expected, nanoparticles coated with citric acid and loaded with chemotherapy drug are more effective. This pattern is also well expressed in time and as expected the cytotoxic effect of the samples on RM1 line tumor cells is much higher at the concentration of 1.00 mg/mL
Figure 8.
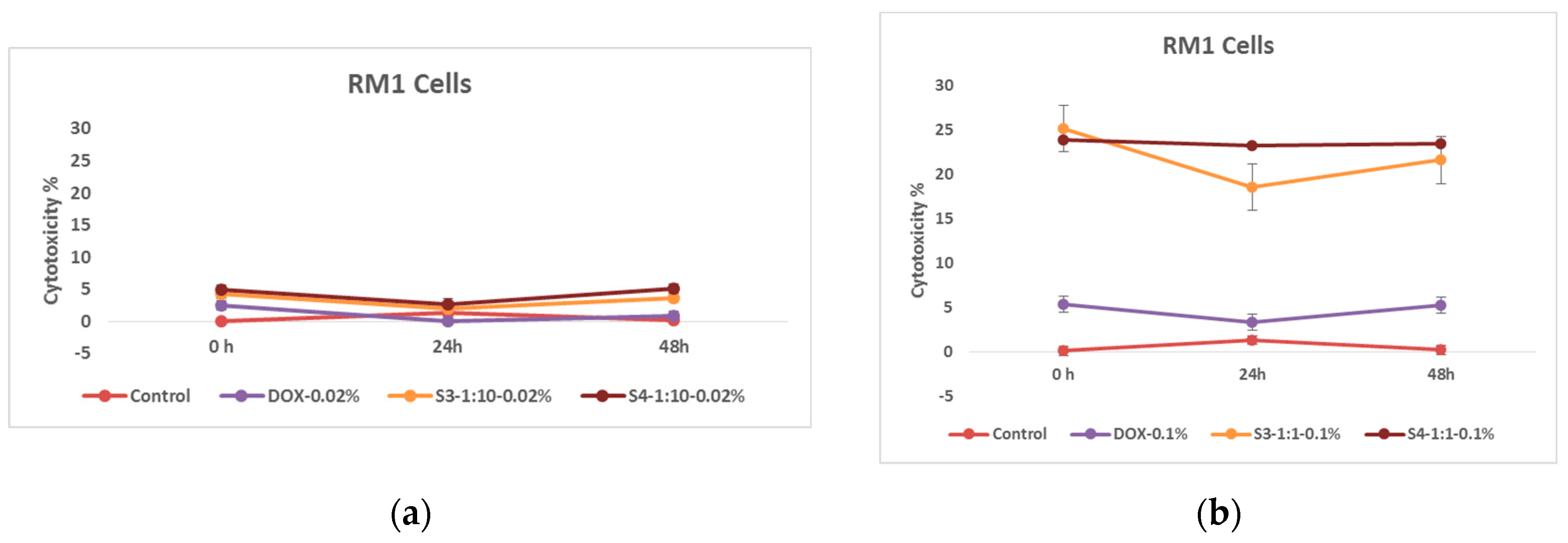
Effects of nanoparticles on RM 1 cell line, (MTT assay - optical density assessed at 570 nm) where control represents untreated tumor cells; (a) DOX-0.02% - cells treated with doxorubicin at 0.37 mM concentrations; S3-1:10-0.02%- cells treated with 0.18 mg/mL concentrations of superparamagnetic iron oxide nanoparticles functionalized with doxorubicin and coated with citric acid; And, S4 -1:10-0.02%- cells treated with 0.18 mg/mL concentrations of electrohydraulic processed superparamagnetic iron oxide nanoparticles functionalized with doxorubicin and coated with citric acid. (b) DOX-0.1%- cells treated with doxorubicin at 1.84 mM, S3-1:1-0.1%- cells treated with 0.50 mg/mL concentrations of superparamagnetic iron oxide nanoparticles functionalized with doxorubicin and coated with citric acid; And, S4-1:1--0.1%- cells treated with 0.50 mg/mL concentrations of electrohydraulic processed superparamagnetic iron oxide nanoparticles functionalized with doxorubicin and coated with citric acid.
Figure 8.
Effects of nanoparticles on RM 1 cell line, (MTT assay - optical density assessed at 570 nm) where control represents untreated tumor cells; (a) DOX-0.02% - cells treated with doxorubicin at 0.37 mM concentrations; S3-1:10-0.02%- cells treated with 0.18 mg/mL concentrations of superparamagnetic iron oxide nanoparticles functionalized with doxorubicin and coated with citric acid; And, S4 -1:10-0.02%- cells treated with 0.18 mg/mL concentrations of electrohydraulic processed superparamagnetic iron oxide nanoparticles functionalized with doxorubicin and coated with citric acid. (b) DOX-0.1%- cells treated with doxorubicin at 1.84 mM, S3-1:1-0.1%- cells treated with 0.50 mg/mL concentrations of superparamagnetic iron oxide nanoparticles functionalized with doxorubicin and coated with citric acid; And, S4-1:1--0.1%- cells treated with 0.50 mg/mL concentrations of electrohydraulic processed superparamagnetic iron oxide nanoparticles functionalized with doxorubicin and coated with citric acid.
3.2.2. Effects of Functionalized Nanoparticles on MEC1 Cell Lines
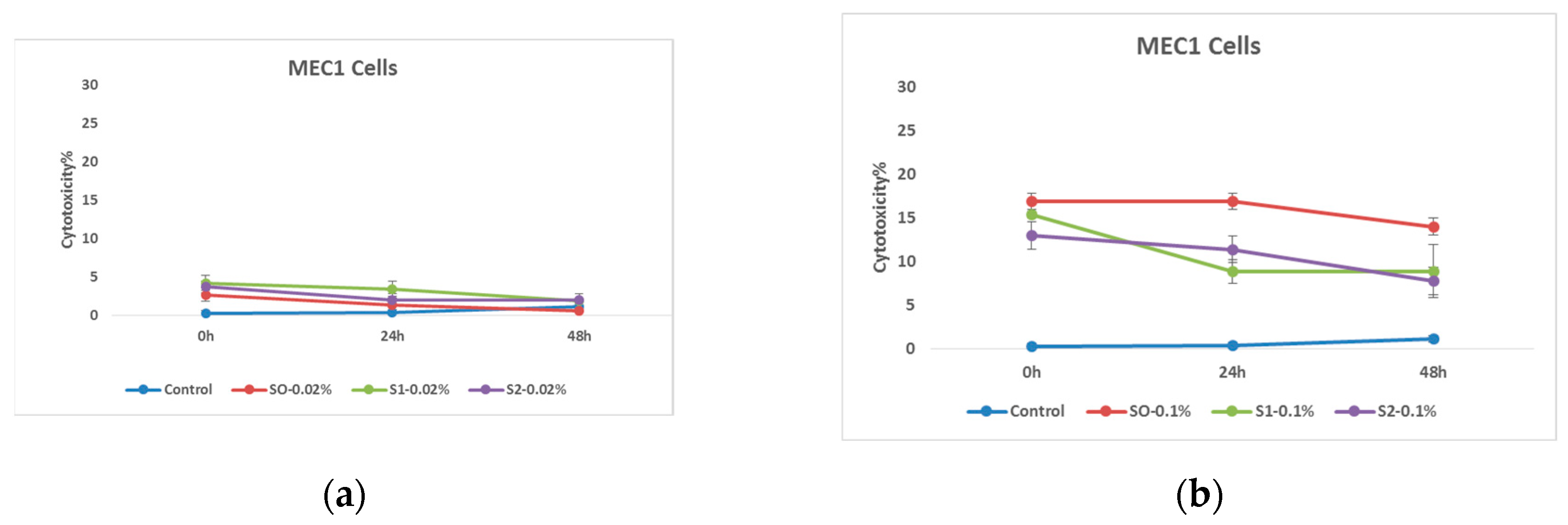
In the first stage of the research, the cytotoxic effect of nanoparticles coated with citric acid was determined. The obtained results emphasize the fact that the nanoparticles coated with citric acid have a certain cytotoxic effect on the MEC 1 cell line, and if they are added in the form where they are also loaded with chemotherapy drug, it is assumed that this nanosystem will significantly increase the effectiveness of the test substance. The effect is also well timed and, in most cases, only fades slightly as time goes on. It is also worth noting the effectiveness of the drug coated with citric acid, which is detected immediately after treatment, but then the initial effect is overcome by tumor cells. The presented graph shows the cytotoxic effect of the nanosystems on tumor cells after the exposure at relatively high 1.00 mg/mL and relatively low 0.20 mg/mL concentrations.
Figure 9.
Effects of nanoparticles on MEC 1 cell line, (MTT assay - cytotoxicity assessed at 570nm) where control represents untreated tumor cells; (a) S0-0.02% – cells treated with 0.20 mg/mL concentrations of superparamagnetic iron oxide nanoparticles; S1-0.02% - cells treated with 0.20 mg/mL concentrations of superparamagnetic iron oxide nanoparticles coated with citric acid; And, S2-0.02% - cells treated with 0.20 mg/mL concentrations of electrohydraulic processed superparamagnetic iron oxide nanoparticles coated with citric acid. (b) S0-0.1%– cells treated with 1.00 mg/mL concentrations of superparamagnetic iron oxide nanoparticles; S1-0.1%- cells treated with the 1.00 mg/mL concentrations of superparamagnetic iron oxide nanoparticles coated with citric acid; And, S2-0.1%- cells treated with - 1.00 mg/mL concentrations of electrohydraulic processed superparamagnetic iron oxide nanoparticles coated with citric acid.
Figure 9.
Effects of nanoparticles on MEC 1 cell line, (MTT assay - cytotoxicity assessed at 570nm) where control represents untreated tumor cells; (a) S0-0.02% – cells treated with 0.20 mg/mL concentrations of superparamagnetic iron oxide nanoparticles; S1-0.02% - cells treated with 0.20 mg/mL concentrations of superparamagnetic iron oxide nanoparticles coated with citric acid; And, S2-0.02% - cells treated with 0.20 mg/mL concentrations of electrohydraulic processed superparamagnetic iron oxide nanoparticles coated with citric acid. (b) S0-0.1%– cells treated with 1.00 mg/mL concentrations of superparamagnetic iron oxide nanoparticles; S1-0.1%- cells treated with the 1.00 mg/mL concentrations of superparamagnetic iron oxide nanoparticles coated with citric acid; And, S2-0.1%- cells treated with - 1.00 mg/mL concentrations of electrohydraulic processed superparamagnetic iron oxide nanoparticles coated with citric acid.
As for the cytotoxic effect of nanoparticles loaded with doxorubicin, two different concentrations were compared for conspicuous results. As expected, nanoparticles coated with citric acid are more effective after being loaded with chemotherapeutic drugs. The effect is also well expressed in time, and as expected, the cytotoxic effect of the nanosystems on tumor cells after the exposure is relatively high at the 1.00 mg/mL concentration.
Figure 10.
Effects of nanoparticles on MEC 1 cell line, (MTT assay - optical density assessed at 570 nm) where control - represents untreated tumor cells; (a) DOX-0.02% - cells treated with doxorubicin 0.37 mg/mL concentration; S3-1:5-0.02% - cells treated with 0.17 mg/mL concentration of doxorubicin functionalized citric acid coated superparamagnetic iron oxide nanoparticles; And, S4-1:5-0.02% - cells treated with 0.17 mg/mL concentration of electrohydraulic processed superparamagnetic iron oxide nanoparticles coated with citric acid and loaded with doxorubicin (b) DOX-0.1% - cells treated with doxorubicin 1.84 mg/mL concentration; S3-1:1-0.1% - cells treated with 0.83 mg/mL concentration of doxorubicin functionalized citric acid coated superparamagnetic iron oxide nanoparticles; And, S4-1:1-0.1% - cells treated with 0.83 mg/mL concentration of electrohydraulic processed superparamagnetic iron oxide nanoparticles coated with citric acid and loaded with doxorubicin .
Figure 10.
Effects of nanoparticles on MEC 1 cell line, (MTT assay - optical density assessed at 570 nm) where control - represents untreated tumor cells; (a) DOX-0.02% - cells treated with doxorubicin 0.37 mg/mL concentration; S3-1:5-0.02% - cells treated with 0.17 mg/mL concentration of doxorubicin functionalized citric acid coated superparamagnetic iron oxide nanoparticles; And, S4-1:5-0.02% - cells treated with 0.17 mg/mL concentration of electrohydraulic processed superparamagnetic iron oxide nanoparticles coated with citric acid and loaded with doxorubicin (b) DOX-0.1% - cells treated with doxorubicin 1.84 mg/mL concentration; S3-1:1-0.1% - cells treated with 0.83 mg/mL concentration of doxorubicin functionalized citric acid coated superparamagnetic iron oxide nanoparticles; And, S4-1:1-0.1% - cells treated with 0.83 mg/mL concentration of electrohydraulic processed superparamagnetic iron oxide nanoparticles coated with citric acid and loaded with doxorubicin .
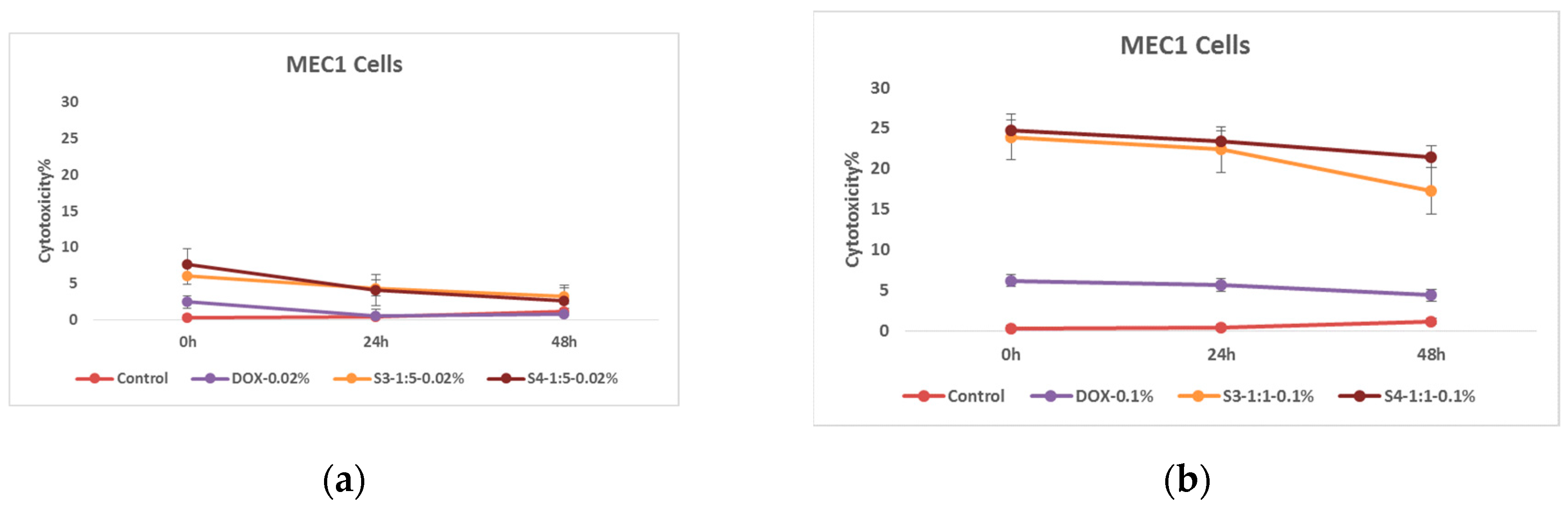
The interesting data was obtained as a result of the research emphasized the effectiveness of nanoparticles. However, in order to conduct a complete study, it was desirable to evaluate the efficiency of nanosystems obtained in a different way. For this very reason, after confirming the cytotoxic efficiency of nanoparticles coated with citric acid, it became interesting to evaluate the effect of nanoparticles conjugated with folic acid. In particular, the nanosystem were loaded with doxorubicin and the cytotoxic effect on tumor cells was evaluated with the help of the same MTT immunofluorescence method. The cytotoxic effect of nanoparticles surrounded by folic acid and with number of modifications was determined. In this case, it is notable that we have got a slightly different picture.
Figure 11.
Effect of nanoparticles on tumor cells of MEC1 line, (MTT analysis - estimated optical density at 570 nm), where Control - represents untreated cells; (a) Bare – cells treated with solutions of 0.50 mg/mL and 0.20 mg/mL concentration of bare superparamagnetic iron oxide nanoparticles; (b) SPIONs-FA- cells treated with 1:10 concentration of 0.45 mg/mL and 0.18 mg/mL solutions of superparamagnetic iron oxide nanoparticles conjugated with folic acid; and, (c) SPIONs-HYD-FA- cells treated with 0.50 mg/mL and 0.20 mg/mL concentration of electrohydraulic processed superparamagnetic iron oxide nanoparticles solutions conjugated with folic acid at 1:10 concentration.
Figure 11.
Effect of nanoparticles on tumor cells of MEC1 line, (MTT analysis - estimated optical density at 570 nm), where Control - represents untreated cells; (a) Bare – cells treated with solutions of 0.50 mg/mL and 0.20 mg/mL concentration of bare superparamagnetic iron oxide nanoparticles; (b) SPIONs-FA- cells treated with 1:10 concentration of 0.45 mg/mL and 0.18 mg/mL solutions of superparamagnetic iron oxide nanoparticles conjugated with folic acid; and, (c) SPIONs-HYD-FA- cells treated with 0.50 mg/mL and 0.20 mg/mL concentration of electrohydraulic processed superparamagnetic iron oxide nanoparticles solutions conjugated with folic acid at 1:10 concentration.
In the graphs above, different effects are clearly expressed when nanoparticles are used in different concentrations. What is particularly noteworthy is the almost total disappearance of the antitumor effect of nanoparticles conjugated with folic acid after 48 hours of incubation, a similar effect was not observed in the case of samples coated with citric acid.
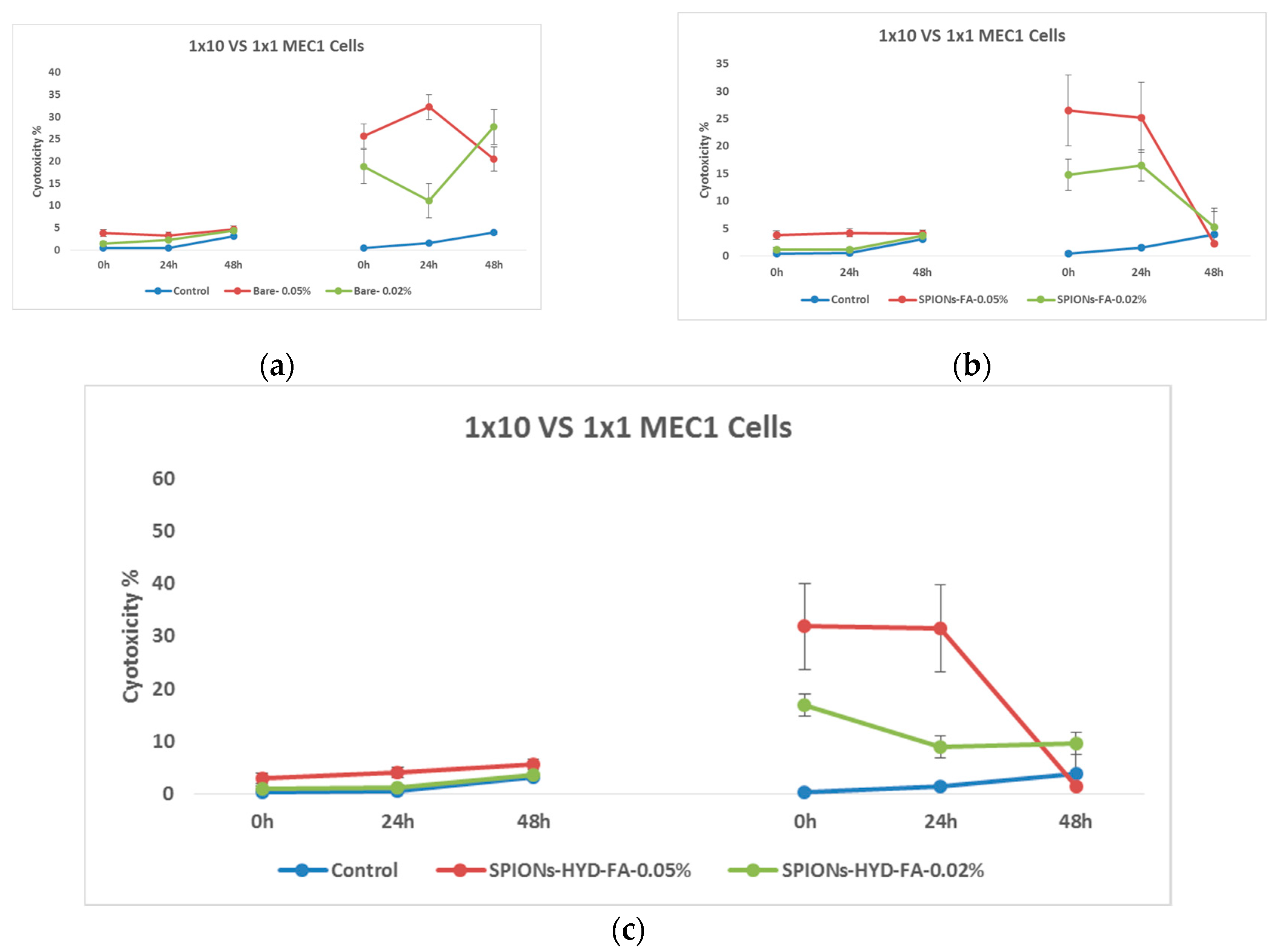
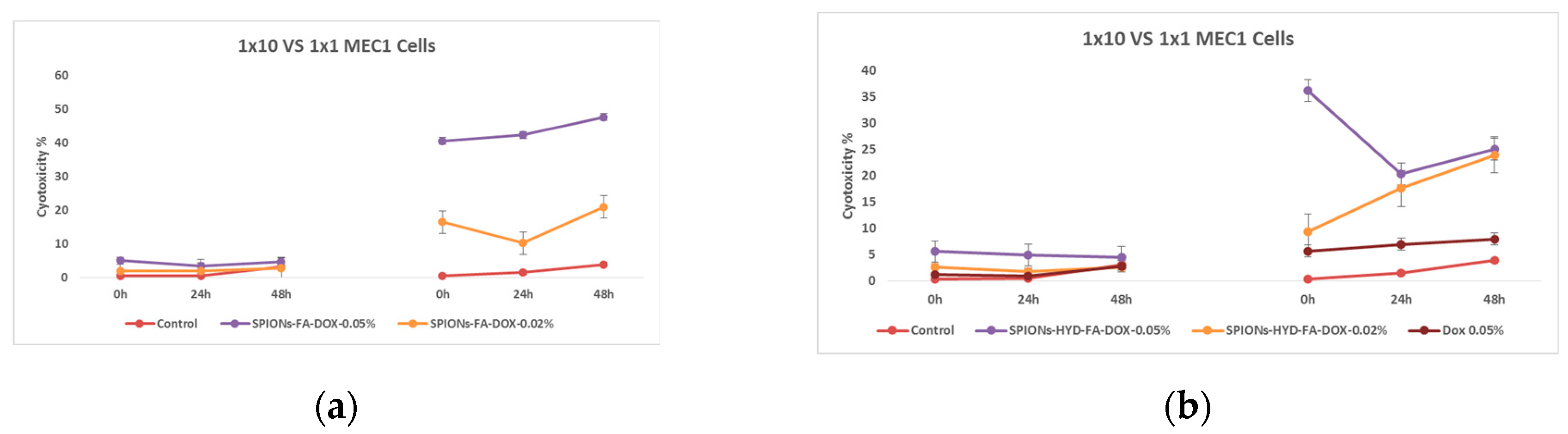
Figure 12.
Effect of nanoparticles on tumor cells of MEC1 line, (MTT analysis - estimated optical density at 570 nm), where Control - represents untreated cells; SPIONs-FA-DOX - cells treated with 1:10 concentration of 0.45 mg/mL and 0.18 mg/mL solutions of superparamagnetic iron oxide nanoparticles loaded with doxorubicin and conjugated with folic acid; and SPIONs-HYD-FA-DOX- cells treated with 1:10 concentration of 0.45 mg/mL and 0.18 mg/mL solutions of electrohydraulic processed, folic acid conjugated and doxorubicin loaded superparamagnetic iron oxide nanoparticles; DOX - cells treated with 1:10 concentration of 0.45 mg/mL solution of doxorubicin.
Figure 12.
Effect of nanoparticles on tumor cells of MEC1 line, (MTT analysis - estimated optical density at 570 nm), where Control - represents untreated cells; SPIONs-FA-DOX - cells treated with 1:10 concentration of 0.45 mg/mL and 0.18 mg/mL solutions of superparamagnetic iron oxide nanoparticles loaded with doxorubicin and conjugated with folic acid; and SPIONs-HYD-FA-DOX- cells treated with 1:10 concentration of 0.45 mg/mL and 0.18 mg/mL solutions of electrohydraulic processed, folic acid conjugated and doxorubicin loaded superparamagnetic iron oxide nanoparticles; DOX - cells treated with 1:10 concentration of 0.45 mg/mL solution of doxorubicin.
It can be clearly seen from the mentioned graphs that the use of the same concentration of doxorubicin that was loaded on the nanoparticles conjugated with folic acid (0.045 mg/mL) does not actually give a cytotoxic effect, both at low and high doses. Which is a remarkable fact. Based on the results of these last studies, it was considered necessary to evaluate the ability of both doxorubicin (because it has the ability to fluoresce) and also the ability of nanoparticles loaded with doxorubicin to penetrate cells using fluorescence microscopy.
3.2.3. Results of Fluorescence Microscopy Performed on MEC1 Cell Culture
In the next stage of the research, it was important to evaluate how efficiently doxorubicin-loaded nanoparticles reached the tumor cells. It was also of interest to see if there was any difference between the nanoparticles coated with citric acid and nanoparticles conjugated with folic acid.
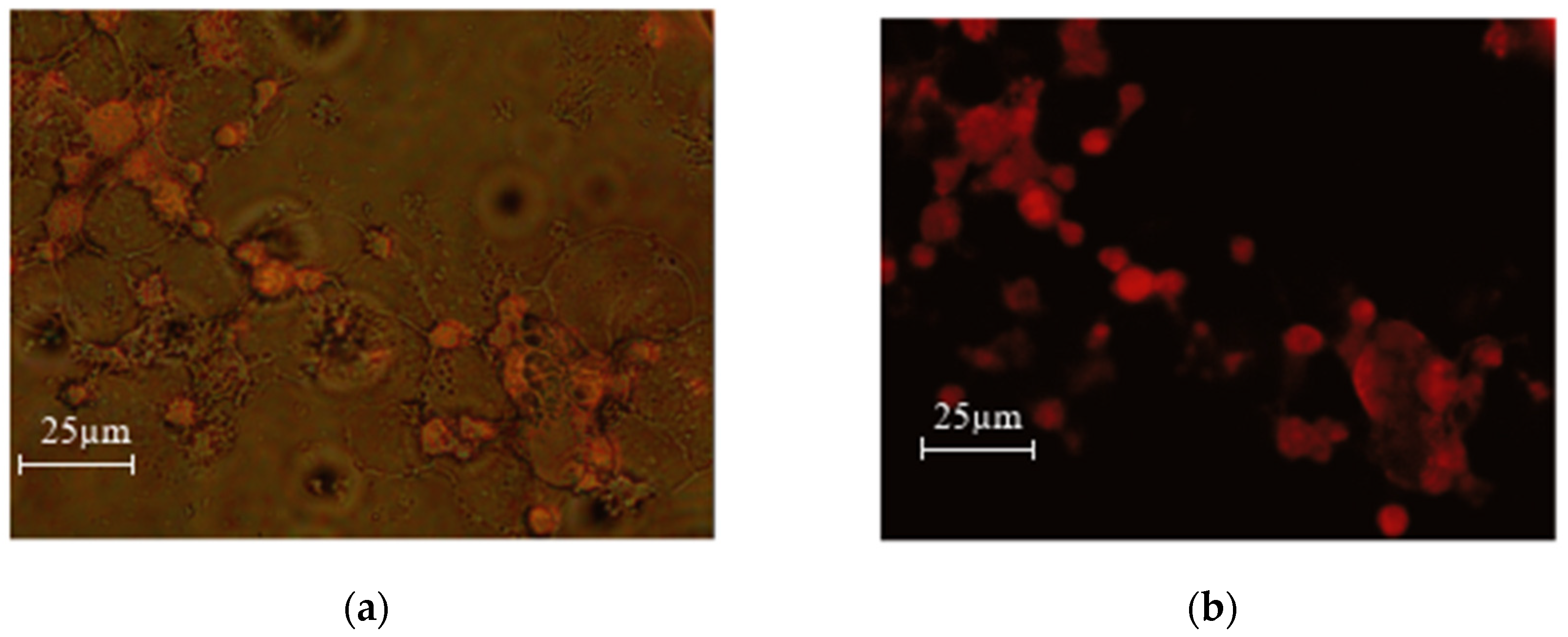
Figure 13.
MEC1 cells +DOX 0.05% concentration (0.045 mg/mL) (a) overlay (b) green laser.
Figure 13.
MEC1 cells +DOX 0.05% concentration (0.045 mg/mL) (a) overlay (b) green laser.
Figure 14.
MEC1 cells +SPIONs-FA-DOX-0.05% (0.045 mg/mL) (a) overlay (b) green laser.
Figure 14.
MEC1 cells +SPIONs-FA-DOX-0.05% (0.045 mg/mL) (a) overlay (b) green laser.
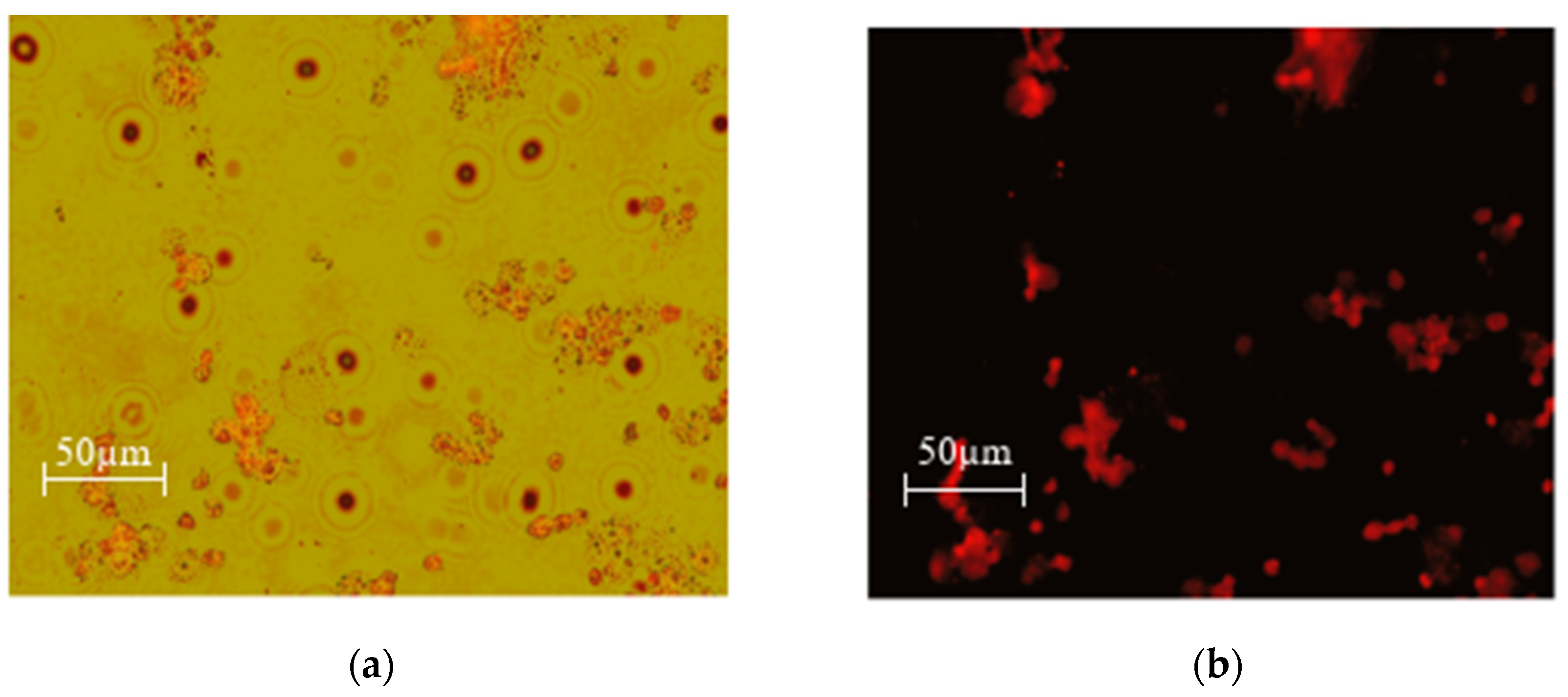
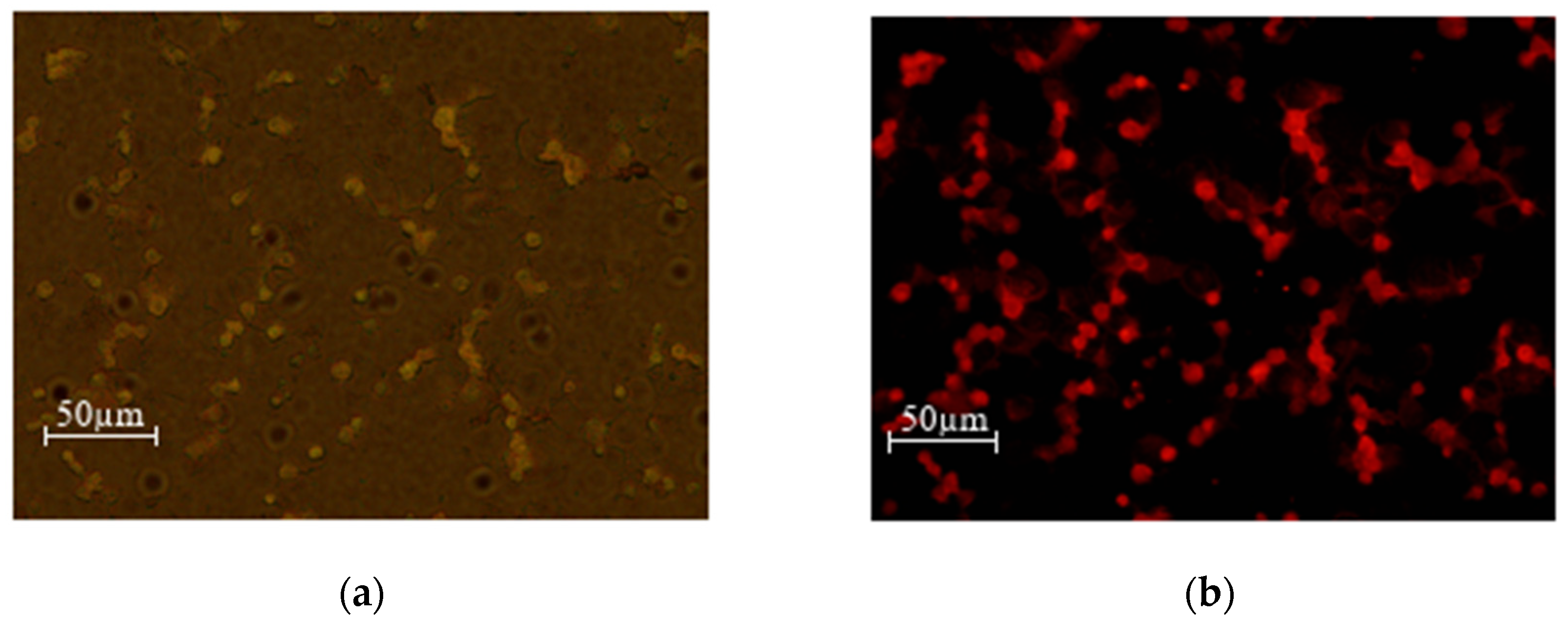
Figure 15.
MEC1 cells +SPIONs-CA-DOX-0.05% (0.025 mg/mL) (a) overlay (b) green laser.
Figure 15.
MEC1 cells +SPIONs-CA-DOX-0.05% (0.025 mg/mL) (a) overlay (b) green laser.
As can be seen from the microscopy photos, when the drug enters the cells and the samples are irradiated with a green laser, doxorubicin fluoresces red, which makes it especially easy to detect the entry of the nanoparticles loaded with the chemotherapy drug into the cells.
4. Discussion
Our study demonstrated that citric acid-coated nanoparticles, especially when loaded with doxorubicin, exhibit significant cytotoxic effects on both RM1 and MEC1 cell lines. This supports their potential as effective therapeutic agents for cancer treatment. Additionally, enhanced cytotoxicity observed with doxorubicin-loaded nanoparticles validates their role in targeted drug delivery, minimizing off-target effects compared to conventional chemotherapy.
Our research highlighted a notable difference in efficacy between nanoparticles coated with citric acid and those conjugated with folic acid. Citric acid-coated nanoparticles consistently maintained their cytotoxic effect over time of 48 hours, whereas the effectiveness of folic acid-conjugated nanoparticles diminished. This finding underscores the importance of coating selection in optimizing nanoparticle stability and effectiveness in therapeutic applications. On the one hand we have a more stable Citric acid-coated nanoparticles and on the other hand targeted delivery with the folic acid.
While the study demonstrates the promising potential of citric acid-coated nanoparticles loaded with doxorubicin, it's important to acknowledge certain limitations. The in vitro studies, while valuable, do not fully capture the complex interactions that occur in vivo. Further research is needed to evaluate the biodistribution, pharmacokinetics, and long-term effects of these nanoparticles in animal models. Additionally, the study focused on two specific cancer cell lines. Expanding the investigation to include a wider range of cancer types would provide a more comprehensive understanding of the nanoparticles' efficacy and potential for personalized medicine. Future research should also explore the potential for combining these nanoparticles with other therapeutic modalities, such as radiation therapy or immunotherapy, to enhance treatment outcomes.
The findings of this study highlight the potential of nanotechnology to revolutionize cancer treatment. The ability to target specific cancer cells with minimal off-target effects offers a significant advantage over conventional chemotherapy, which often leads to debilitating side effects. The use of nanoparticles for targeted drug delivery opens up exciting possibilities for personalized medicine, where treatment strategies can be tailored to the individual patient's needs. Further research into the development of nanoparticle-based therapies could lead to more effective, less toxic, and more personalized cancer treatments, ultimately improving the lives of cancer patients.
5. Conclusion
This study demonstrates the promising potential of citric acid-coated superparamagnetic iron oxide nanoparticles (CA-SPIONs) as effective therapeutic agents for cancer treatment, particularly when loaded with doxorubicin. The characterization results confirmed the stability and favorable properties of the nanoparticles, including appropriate zeta potential values and hydrodynamic diameters that suggest a well-dispersed system. Importantly, the cytotoxicity assessments revealed that CA-SPIONs significantly enhance the therapeutic efficacy against both RM1 and MEC1 cancer cell lines compared to non-coated and folic acid-conjugated nanoparticles.
The results indicate a clear advantage of citric acid coatings over folic acid in maintaining therapeutic effects over time. While folic acid serves as a targeted delivery agent, its effectiveness appeared to diminish, highlighting the critical role that surface modifications play in nanoparticle stability and efficacy. Furthermore, the successful drug loading confirmed the potential of these nanoparticles to deliver chemotherapeutic agents directly to tumor sites, thereby minimizing systemic toxicity often associated with conventional treatments.
Despite the positive findings, the study acknowledges limitations inherent to in vitro research and emphasizes the need for further exploration of the long-term effects, biodistribution, and pharmacokinetics of CA-SPIONs and FA-SPIONs in vivo. Future research should also consider the incorporation of these nanoparticles into combination therapies, which may enhance treatment outcomes through synergistic effects.
In conclusion, our findings underscore the transformative potential of nanotechnology in oncology, paving the way for innovative, personalized approaches to cancer treatment. By leveraging the unique properties of nanoparticles for targeted drug delivery, we can envision a new paradigm in cancer therapy that enhances treatment efficacy while minimizing adverse effects, ultimately improving the quality of life for patients. Further investigation and development could lead to more effective, less toxic, and personalized cancer treatments, significantly impacting the future of oncology.
Author Contributions
Conceptualization, Nino Maisuradze, Shalva Kekutia and Nunu Mitskevichi; Data curation, Nino Maisuradze, Jano Markhulia, Tamar Tsertsvadze, Vladimer Mikelashvili, Liana Saneblidze and Niko Chkhaidze; Formal analysis, Nino Maisuradze, Jano Markhulia, Tamar Tsertsvadze, Vladimer Mikelashvili, Liana Saneblidze and Niko Chkhaidze; Funding acquisition, Nino Maisuradze; Investigation, Nino Maisuradze, Jano Markhulia, Tamar Tsertsvadze, Vladimer Mikelashvili, Liana Saneblidze and Zsolt Horváth; Methodology, Nino Maisuradze, Jano Markhulia, Tamar Tsertsvadze and Niko Chkhaidze; Project administration, Shalva Kekutia, Laszlo Almasy and Nunu Mitskevichi; Supervision, Shalva Kekutia, Laszlo Almasy and Nunu Mitskevichi; Visualization, Nino Maisuradze; Writing – original draft, Nino Maisuradze; Writing – review & editing, Shalva Kekutia, Jano Markhulia and Nunu Mitskevichi.
Funding
This research [PHDF-19-2646] has been supported by Shota Rustaveli National Science Foundation of Georgia (SRNSFG)
Acknowledgments
We thank the Faculty of Exact and Natural Sciences of Tbilisi State University and the Georgian Technical University, the administration of the Vladimer Chavchanidze Institute of Cybernetics for facilitating and supporting the project.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Huang, K.; Yao, J. Beam Theory of Thermal–Electro-Mechanical Coupling for Single-Wall Carbon Nanotubes. Nanomaterials 2021, 11, 923. [Google Scholar] [CrossRef] [PubMed]
- Lage, T.; Rodrigues, R.O.; Catarino, S.; Gallo, J.; Bañobre-López, M.; Minas, G. Graphene-Based Magnetic Nanoparticles for Theranostics: An Overview for Their Potential in Clinical Application. Nanomaterials 2021, 11, 1073. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Morales, M.A.; Goldt, A.E.; Kalachikova, P.M.; Ramirez, B.J.A.; Suzuki, M.; Zhigach, A.N.; Ben Salah, A.; Shurygina, L.I.; Shandakov, S.D.; Zatsepin, T.; et al. lbumin Stabilized Fe@C Core–Shell Nanoparticles as Candidates for Magnetic Hyperthermia Therapy. Nanomaterials 2022, 12, 2869. [Google Scholar] [CrossRef] [PubMed]
- Kargol, A.; Malkinski, L.; Caruntu, G. Biomedical Applications of Multiferroic Nanoparticles. In Advanced Magnetic Materials; Malkinski, L., Ed.; InTech, 2012 ISBN 978-953-51-0637-1.
- Rodrigues, R.O.; Sousa, P.C.; Gaspar, J.; Bañobre-López, M.; Lima, R.; Minas, G. Organ-on-a-Chip: A Preclinical Microfluidic Platform for the Progress of Nanomedicine. Small 2020, 16, 2003517. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, Q.; Han, N.; Bai, L.; Li, J.; Liu, J.; Che, E.; Hu, L.; Zhang, Q.; Jiang, T.; et al. Mesoporous Silica Nanoparticles in Drug Delivery and Biomedical Applications. Nanomedicine Nanotechnol. Biol. Med. 2015, 11, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Jose, S.; Sowmya, S.; Cinu, T.A.; Aleykutty, N.A.; Thomas, S.; Souto, E.B. Surface Modified PLGA Nanoparticles for Brain Targeting of Bacoside-A. Eur. J. Pharm. Sci. 2014, 63, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Chaparro, C.I.P.; Simões, B.T.; Borges, J.P.; Castanho, M.A.R.B.; Soares, P.I.P.; Neves, V. A Promising Approach: Magnetic Nanosystems for Alzheimer’s Disease Theranostics. Pharmaceutics 2023, 15, 2316. [Google Scholar] [CrossRef] [PubMed]
- Poudel, P.; Park, S. Recent Advances in the Treatment of Alzheimer’s Disease Using Nanoparticle-Based Drug Delivery Systems. Pharmaceutics 2022, 14, 835. [Google Scholar] [CrossRef] [PubMed]
- Obaidat, I.M.; Narayanaswamy, V.; Alaabed, S.; Sambasivam, S.; Muralee Gopi, C.V.V. Principles of Magnetic Hyperthermia: A Focus on Using Multifunctional Hybrid Magnetic Nanoparticles. Magnetochemistry 2019, 5, 67. [Google Scholar] [CrossRef]
- Ioannou, P.; Baliou, S.; Samonis, G. Nanotechnology in the Diagnosis and Treatment of Antibiotic-Resistant Infections. Antibiotics 2024, 13, 121. [Google Scholar] [CrossRef] [PubMed]
- Palzer, J.; Eckstein, L.; Slabu, I.; Reisen, O.; Neumann, U.P.; Roeth, A.A. Iron Oxide Nanoparticle-Based Hyperthermia as a Treatment Option in Various Gastrointestinal Malignancies. Nanomaterials 2021, 11, 3013. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Li, J.; Yan, S.; Zhang, H.; Zhang, W.; Zhang, F.; Jiang, J. siRNA Delivery with Stem Cell Membrane-Coated Magnetic Nanoparticles for Imaging-Guided Photothermal Therapy and Gene Therapy. ACS Biomater. Sci. Eng. 2018, 4, 3895–3905. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, P.; Roberts, A.M.; Khan, S.; Hafeez, B.B.; Chauhan, S.C.; Jaggi, M.; Yallapu, M.M. Magnetic Nanoformulations for Prostate Cancer. Drug Discov. Today 2017, 22, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Sherwood, J.A.; Sun, Z. Magnetic Iron Oxide Nanoparticles as T 1 Contrast Agents for Magnetic Resonance Imaging. J. Mater. Chem. C 2018, 6, 1280–1290. [Google Scholar] [CrossRef]
- Hillmen, P.; Pitchford, A.; Bloor, A.; Broom, A.; Young, M.; Kennedy, B.; Walewska, R.; Furtado, M.; Preston, G.; Neilson, J.R.; et al. Ibrutinib and Rituximab versus Fludarabine, Cyclophosphamide, and Rituximab for Patients with Previously Untreated Chronic Lymphocytic Leukaemia (FLAIR): Interim Analysis of a Multicentre, Open-Label, Randomised, Phase 3 Trial. Lancet Oncol. 2023, 24, 535–552. [Google Scholar] [CrossRef] [PubMed]
- Russell, E.; Dunne, V.; Russell, B.; Mohamud, H.; Ghita, M.; McMahon, S.J.; Butterworth, K.T.; Schettino, G.; McGarry, C.K.; Prise, K.M. Impact of Superparamagnetic Iron Oxide Nanoparticles on in Vitro and in Vivo Radiosensitisation of Cancer Cells. Radiat. Oncol. 2021, 16, 104. [Google Scholar] [CrossRef] [PubMed]
- Markhulia, J.; Kekutia, S.; Mikelashvili, V.; Saneblidze, L.; Tsertsvadze, T.; Maisuradze, N.; Leladze, N.; Czigány, Z.; Almásy, L. Synthesis, Characterization, and In Vitro Cytotoxicity Evaluation of Doxorubicin-Loaded Magnetite Nanoparticles on Triple-Negative Breast Cancer Cell Lines. Pharmaceutics 2023, 15, 1758. [Google Scholar] [CrossRef] [PubMed]
- Stacchini, A.; Aragno, M.; Vallario, A.; Alfarano, A.; Circosta, P.; Gottardi, D.; Faldella, A.; Rege-Cambrin, G.; Thunberg, U.; Nilsson, K.; et al. MEC1 and MEC2: Two New Cell Lines Derived from B-Chronic Lymphocytic Leukaemia in Prolymphocytoid Transformation. Leuk. Res. 1999, 23, 127–136. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).