1. Introduction
Modern agriculture has been intensified to increase crop production and become highly dependent on chemical fertilizers by which excessive or imbalanced use can decrease soil productivity and cause environmental problems. Improper use of these fertilizers can cause soil acidification, decrease in soil organic carbon and beneficial soil microorganisms, stunted plant growth and yield, and even leading to the emission of greenhouse gases [
1,
2]. Utilizing organic wastes, on the other hand, as soil amendment can enhance the physicochemical and biological properties of the soil. However, as a substitute for chemical fertilizers, large quantities of organic material would be required to satisfy the nutritional needs of crops [
3]. Farmers also struggle to achieve high yields with this kind of fertilization compared to the conventional practice. Therefore, efforts must be made to develop an efficient crop production strategy while reducing the negative impacts of agrochemical inputs on soil health and environment.
An approach called integrated nutrient management (INM) aims to address constraints on soil and crop productivity by optimizing the benefits derived from chemical, organic, and biological sources in an integrated manner. A comprehensive literature review revealed that compared with conventional practices, INM increased crop yields and income of farmers while improving soil health [
4].
Several studies have also reported the influence of microbial interactions in the sustainable management of agricultural soils. Roles of arbuscular mycorrhizal fungi (AMF) in plant nutrition have been documented to enhance plant growth, increase nutrient uptake and yield of crops, and improve tolerance to several biotic and abiotic stresses as they colonize plant root systems [
5,
6,
7]. Plant growth-promoting bacteria (PGPB) including nitrogen-fixing bacteria (NFB) are another promising soil microorganisms for crop production as they interact with plant roots. Among these is the genus
Azospirillum which has been studied for its interaction with plants and their beneficial impacts on plant growth [
8].
Azospirillum bacteria were determined to improve plant growth through the biological fixation of nitrogen and production of several plant hormones [
9,
10,
11]. These mechanisms make the utilization of these beneficial soil microorganisms promising for optimizing nutrient management and increasing crop productivity.
Tomato (
Solanum lycopersicum L.) is one of the most widely grown and consumed crops in the world as it is rich in vitamins, minerals and antioxidants [
12]. Nutrient management plays an important role in the productivity of tomato as nutrients affect its morphological growth, photosynthetic processes and accumulation of yield [
13]. Several studies revealed that the integrated use of chemical and organic sources of nutrients can increase the yield of tomato [
14,
15]. The significant effect of beneficial soil microorganisms on tomato yield was documented under various levels of chemical fertilization [
16,
17]. Still, their synergy with the optimal combination of nutrient sources in the actual tomato production field is a new investigation. The present work aimed to optimize nutrient management to increase tomato production through the development of INM strategies using integrated nutrient sources and beneficial soil microorganisms.
2. Materials and Methods
2.1. Experimental Design and Treatments
An open-field experiment was conducted in a tomato farm in Cabuyao, Laguna, Philippines (N 14°11’55’’, E 121°2’1’’) from February to May 2022 following a Randomized Complete Block Design with four replicates. The treatments were detailed in
Table 1.
2.2. Soil Properties of the Experimental Site
Soil samples from the experimental site were collected at 0-15 cm depth and subjected to laboratory analyses. The soils have a dark brown color and clay loam texture with a pH of 5.0 in H2O (1:1). It also contains 4.28% organic matter, 0.23% total N, 7.79 ppm of available phosphorus, high exchangeable potassium at 2.06 cmol/kg, and 37.29 cmolc/kg of cation exchange capacity.
2.3. Characterization of Vermicompost
The study used vermicompost derived from a mixture of poultry manure and guinea grass (Panicum maximum) substrates and produced at the DA-BSWM – National Soil and Water Resources Research and Development Center for Hillyland Pedo-ecological Zone, Tanay, Rizal, Philippines. It consists of 0.77% total nitrogen, 3.45% total phosphorus (P2O5), 0.66% total potassium (K2O), 3.42% calcium oxide, 0.60% magnesium oxide, 543 ppm of copper, 656 ppm of zinc, 906 ppm of manganese and 26.28% organic matter at 35% moisture.
2.4. Application of Microbial Inoculants
The AMF inoculant was prepared as described by Aggangan and Moon (2013) [
18]. The soil-based mycorrhizal inoculant contained 12 species belonging to the genera
Glomus, Gigaspora, Entrophospora, and
Acaulospora. The NFB inoculant consists of
Azospirillum spp. originally isolated from the roots of
talahib (Saccharum spontaneum L.). These inoculants were developed and commercially produced at the National Institute of Molecular Biology and Biotechnology, University of the Philippines Los Banos (BIOTECH UPLB), Laguna, Philippines.
For INM 2, the seedlings were inoculated with AMF inoculant at the rate of 5 g per seedling placed in a 2-3 inches depth of hole beneath and in contact with the roots, and for INM 3, the seedlings were inoculated with 5 g of 1:1 mixture of AMF and Azospirillum inoculants.
2.5. Nutrient Management
The recommended rates of chemical fertilizers were based on the results of soil analyses. The recommended rate of vermicompost based on nutrient content was applied during basal fertilization. Half dose of inorganic N fertilizer and full dose of P2O5 fertilizer were applied during transplanting, and the remaining dose of N fertilizer was applied one month after transplanting.
2.6. Agronomic Parameters
Aboveground parts of tomato were measured for plant height, collected, washed, air-dried, and oven-dried at 60°C until a constant weight for the measurement of dry weight. Samples were ground and analyzed for nitrogen concentration using Kjeldahl titration, phosphorus concentration using colorimetry, and potassium, calcium, magnesium and micronutrient concentrations using atomic absorption spectrometry. Nutrient uptake was calculated by multiplying the nutrient concentrations with the dry weight of aboveground biomass. Tomato fruits were harvested from the sample plants in the central rows of each plot. At each harvest, the weight of marketable and unmarketable fruits in each plot was recorded.
2.7. Assessment of Mycorrhizal Root Colonization and Number of Spores
Fine (<0.02 mm diameter) roots were also collected, cleared, and stained following the method of Phillips and Hayman (1970) [
19] and observed under a stereomicroscope. All roots that crossed the grid lines were counted, and roots with vesicles, hyphae, or other AMF propagules were also considered as mycorrhiza-infected roots. The percentage of mycorrhizal root colonization was calculated based on a formula [
20].
AMF spores were separated from the rhizosphere soil following the wet sieving and centrifugation technique [
21], and the estimated number of spores was counted using light microscopy [
22].
2.8. Statistical Analysis
All data were subjected to One-Way ANOVA in RCBD using a statistical software (SAS). Treatment means were compared using LSD if ANOVA showed a significant difference at p<0.05. Correlation (Pearson) analyses were done to determine the relationship between the observation variables.
3. Results
3.1. Plant Growth and Yield
No significant difference was observed among treatments in terms of growth parameters, but higher values were observed on treatments with integrated nutrient management particularly INM 1 for plant height (77.59 cm), INM 3 for dry weight (112.10 g plant
-1) and number of marketable fruits per plant, and INM 2 for fruit weight (40.42 g fruit
-1) (
Table 2). Treatment with combined chemical fertilizers and vermicompost recorded significantly higher yield than RRC at 38.92 t ha
-1. AMF inoculation with combined nutrient sources further increased tomato yield by 13.40% relative to those applied with RRC. Dual inoculation with AMF and
Azospirillum obtained the highest yield among the integrated nutrient management strategies 40.96 t ha
-1, however it is not statistically different with sole AMF inoculation.
3.2. Nutrient Uptake
Plant uptake of various macro- and micronutrients was significantly affected by nutrient management strategies and microbial inoculation. Treatment of dual inoculation with AMF and
Azospirillum recorded the highest nitrogen uptake at 3.53 g plant
-1 and is not significantly different with RRC even with a 50% reduction of inorganic nitrogen fertilizer rate. AMF inoculation in synergy with combined chemical and organic nutrient sources significantly increased phosphorus, potassium, calcium, and magnesium uptake of tomato over the RRC by 33.0%, 8.4%, 20.8%, and 10.7% respectively (
Table 3). Integrating chemical fertilizers and vermicompost resulted in a significant increase in copper uptake even without the use of microbial inoculants while no significant effect was observed on zinc, iron and manganese uptake among the treatments (
Table 4).
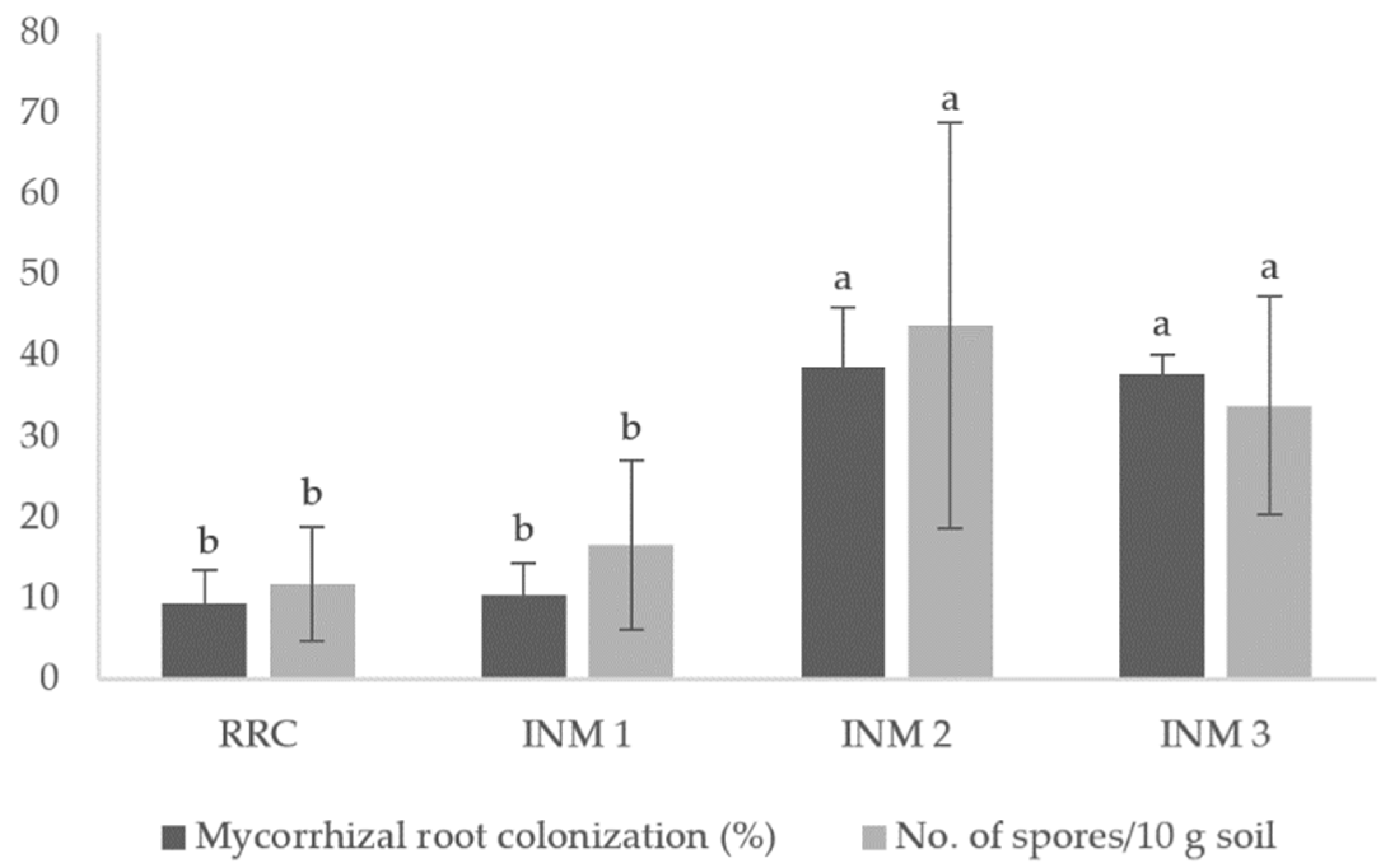
3.3. Mycorrhizal Root Colonization and Spore Count
Mycorrhizal inoculation of tomato plants resulted to significantly higher root colonization (38-39%) and estimated number of spores in the tomato rhizosphere than non-mycorrhizal treatments (
Figure 1).
3.4. Correlation Analyses
Correlation analyses show that fruit yield is positively correlated with AMF root colonization and nutrient uptake particularly on calcium (0.64), copper (0.66), and manganese (0.65) (
Table 5). AMF root colonization is significantly correlated with plant uptake of phosphorus (0.62), potassium (0.66), calcium (0.69), magnesium (0.77), and copper (0.58).
4. Discussion
In this study, fertilization strategies integrating chemical and organic nutrient sources, and beneficial soil microorganisms such as AMF and Azospirillum spp. were assessed in an actual tomato farm. This approach gives direct agronomic information under field conditions on the potential synergy of nutrient sources and microbial inoculants to optimize soil nutrient management for tomato.
Plant growth and development primarily depend on the availability of essential nutrients in the soil. The use of organic amendments containing various essential elements and plant-microbial interactions are other factors that can influence the nutrient uptake of crops. In this study, statistically higher values of nutrient uptake were recorded in the mycorrhizal treatments particularly on P, K, Ca, and Mg. AMF root colonization is also significantly correlated with the uptake of these nutrients as well as copper. Findings on the ability of AMF to increase crop uptake of various nutrients were also documented by various studies on cacao (
Theobroma cacao L.) [
23], on
Eunymus japonica [
24] and on
Antirrhinum majus [
25]. When AMF colonizes the root, their hyphae extend and establish a mycelial network or extraradical mycelium (ERM) which transfers these nutrients to the intraradical mycelium (IRM) where nutrients are exchanged from the host plant for carbon [
26]. These mechanisms of mycorrhizal symbiosis can lead to improved soil rhizosphere and increased nutrient uptake. The highest nitrogen uptake of tomato plants inoculated with
Azospirillum spp. under 50% reduction of nitrogen fertilizer rate demonstrated similar findings with Fernandez et al. [
27] where potato (
Solanum tuberosum L.) and cassava (
Manihot esculenta Crantz) inoculated with
Azospirillum recorded higher nitrogen concentrations in leaves at lower rates of nitrogen fertilization. The nutrient contents and humic acid derived from vermicompost may have also influenced the increased uptake of these nutrients [
28].
Increased crop production is of high economic interest. Soil nutrient management plays an important role in achieving higher productivity of crops by managing nutrient sources, and their amount and timing of application. Results of the study indicate that 4 t ha
-1 of vermicompost used in the trial can reduce the rate of chemical fertilizers by 50% without compromising the yield. This can be attributed to the nutrient contents of vermicompost and the increase in uptake of several nutrients. These results agreed with the findings of Islam et al. [
14], Mengistu et al. [
15], and Qasim et al. [
29] where combination of chemical fertilizers and organic amendments significantly increased the yield of tomato. Higher fruit yield was also observed by Zhang et al. [
30] on treatments with application of organic fertilizer even under reduced nitrogen fertilization. Slow release of nutrients from organic amendments like vermicompost can optimize the uptake of nutrients. Its micronutrient contents which are not usually common in chemical fertilizers can further increase the yield of crops. Vermicompost also encompasses different enzymes which are necessary in the decomposition of soil organic matter and the release of various nutrients, making them available for plant uptake [
31]. Vermicompost application was also documented by Liu et al. [
32] to enhance photosynthesis and chlorophyll fluorescence traits which are essential in the productivity of tomato.
The use of efficient microbial inoculants can also enhance overall plant growth and productivity even with the minimized use of agrochemicals. The results of a study conducted by Bona et al. [
14] revealed that the use of AMF and PGPB allowed to spare 30% of the recommended rate of chemical fertilizer without significant yield reduction. Increased yield of mycorrhizal treatments in the present work can be attributed to increased nutrient uptake of tomato and its significant correlation with mycorrhizal root colonization. Under low phosphorus soil conditions, the yield of tomato was significantly increased by AMF inoculation regardless of the varying rates of chemical NPK fertilizers [
15].
No significant effect was observed on additional
Azospirillum inoculation which might be due to high nitrogen fertilizer rate used. Similar findings were observed by Andrade-Sifuentes et al. [
33] where inoculation with
Azospirillum spp. had no significant effect on the yield of tomato on treatments with higher fertilizer rates in contrast with Aseri et al. [
34] where dual inoculation of AMF and N-fixing bacteria increased the fruit yield of pomegranate under field conditions.
Higher mycorrhizal root colonization and estimated number of spores were observed in treatments with AMF inoculation. The values of AMF root colonization in tomato roots are higher than those recorded by Bona et al. [
16]. Results also agree with the study of Aggangan et al. [
23] on the effects of AMF inoculation using the same AMF inoculant root colonization and AMF spores in a cacao rhizosphere. Similar findings with those previously obtained by Bona et al. [
16] where no effect on mycorrhizal root colonization by dual inoculation with plant growth-promoting bacteria (PGPB) was documented in this study incontrast with the other studies where colonization was further increased by PGPB in cucumber (
Cucumis sativus) [
35] and eggplant (
Solanum melongena L.) [
36]. These studies indicate that the combined application of different microorganisms like AMF and
Azospirillum spp. may have different effects on various plants.
5. Conclusions
The present study documented a significant improvement in nutrient uptake and fruit yield of tomato upon integration of chemical and organic nutrient sources and beneficial soil microorganisms, particularly AMF. The findings reveal how this strategy can optimize nutrient management and reduce the dosage of chemical fertilizers while maintaining and even increasing tomato fruit yield. This nutrient management strategy for tomato farming systems can potentially lead to economic, soil health and environmental benefits in relation to reduced input costs and addressing the negative impacts of conventional fertilization on soil health and environment.
Author Contributions
L.C.B.: methodology, investigation, writing – original draft, visualization; J.P.E.: project administration; J.A.A.: formal analysis; N.S.A.: writing – review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
These findings are from the project entitled “Development of Integrated Nutrient Management Strategy for Improving Soil and Tomato Productivity” which was funded by the Department of Agriculture-Bureau of Agricultural Research (DA-BAR), Philippines.
Data Availability Statement
All data included in this published article are generated from the abovementioned project.
Acknowledgments
Appreciation is due to Dr. Gina P. Nilo, DA-BSWM Director, Ms. Denise A. Solano, DA-BSWM Assistant Director, and the project implementation team of the National Soil and Water Resources Research and Development Center for Hillyland Pedo-ecological Zone (NSWRRDC HILLPEZ) of DA-BSWM for their support and contributions in the realization of the project.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Savci, S. Investigation of Effect of Chemical Fertilizers on Environment. APCBEE Procedia 2012, 1, 287–292. [Google Scholar] [CrossRef]
- Pahalvi, H.N.; Rafiya, L.; Rashid, S.; Nisar, B.; Kamili, A.N. Chemical Fertilizers and Their Impact on Soil Health. In Microbiota and Biofertilizers; Dar, G.H., Bhat, R.A., Mehmood, M.A., Hakeem, K.R., Eds.; Springer: Cham, Switzerland, 2021; Volume 2, pp. 1–20. [Google Scholar] [CrossRef]
- Hernandez, T.; Chocano, C.; Moreno, J.L.; Garcia, C. Towards a more sustainable fertilization: Combined use of compost and inorganic fertilization for tomato cultivation. Agriculture, Ecosystems and Environment 2014, 196, 178–184. [Google Scholar] [CrossRef]
- Wu, W.; Ma, B. Integrated nutrient management (INM) for sustaining crop productivity and reducing environmental impact: a review. Science of the Total Environment 2015, 512, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Balliu, A.; Sallaku, G.; Rewald, B. AMF inoculation enhances growth and improves the nutrient uptake of transplanted, salt-stressed tomato seedlings. Sustainability 2015, 7, 15967–15981. [Google Scholar] [CrossRef]
- Berruti, A.; Lumini, E.; Balestrini, R.; Bianciotto, V. (2016). Arbuscular mycorrhizal fungi as natural biofertilizers: let’s benefit from past successes. Front. Microbiol. 2016, 6, 1559. [Google Scholar] [CrossRef]
- Kim, S.J.; Eo, J.K.; Lee, E.H.; Park, H.; Eom, A.H. Effects of arbuscular mycorrhizal fungi and soil conditions on crop plant growth. Microbiology 2017, 45(1), 20–24. [Google Scholar] [CrossRef]
- Cassán, F.; Coniglio, A.; López, G.; Molina, R.; Nievas, S.; de Carlan, C.L.; Donadio, F.; Torres, D.; Rosas, S.; Pedrosa, F.O. Everything you must know about Azospirillum and its impact on agriculture and beyond. Biol. Fertil. Soils 2020, 56, 461–479. [Google Scholar] [CrossRef]
- Contreras-Liza, S.; Villadeza, C.Y.; Rodriguez-Grados, P.M.; Palomares, E.G.; Arbizu, C.I. Yield and Agronomic Performance of Sweet Corn in Response to Inoculation with Azospirillum sp. under Arid Land Conditions. Int. J. Plant Biol. 2024, 15, 683–691. [Google Scholar] [CrossRef]
- Pankievicz, V.C.S.; do Amaral, F.P.; Santos, K.F.D.N.; Agtuca, B. , Xu; Y., Schueller, M.J. Robust biological nitrogen fixation in a model grass-bacterial association. The Plant Journal 2015, 81, 907–919. [Google Scholar] [CrossRef]
- Fibach-Paldi, S.; Burdman, S.; Okon, Y. Key physiological properties contributing to rhizosphere adaptation and plant growth promotion abilities of Azospirillum brasilense. FEMS Microbiol. Lett. 2012, 326, 99–108. [Google Scholar] [CrossRef]
- Dumas, Y.; Dadomo, M.; Di Lucca, G.; Grolier, P. Effects of environmental factors and agricultural techniques on antioxidant content of tomatoes. J. Sci. Food Agric. 2003, 83, 369–382. [Google Scholar] [CrossRef]
- Guo, L.; Yu, H.; Kharbach, M.; Wang, J. The Response of Nutrient Uptake, Photosynthesis and Yield of Tomato to Biochar Addition under Reduced Nitrogen Application. Agronomy 2021, 11, 1598. [Google Scholar] [CrossRef]
- Islam, M.A.; Islam, S.; Akter, A.; Rahman, M.H.; Nandwani, D. Effect of organic and inorganic fertilizers on soil properties and the growth, yield and quality of tomato in Mymensingh, Bangladesh. Agriculture. 2017, 7, 18. [Google Scholar] [CrossRef]
- Mengistu, T.; Gebrekidan, H.; Kibret, K.; Woldetsadik, K.; Shimelis, B.; Yadav, H. The integrated use of excreta-based vermicompost and inorganic NP fertilizer on tomato (Solanum lycopersicum L.) fruit yield, quality and soil fertility. Int J Recycl Org Waste Agricult. 2017, 6, 63–77. [Google Scholar] [CrossRef]
- Bona, E.; Todeschini, V.; Cantamessa, S.; Cesaro, P.; Copetta, A.; Lingua, G.; Gamalero, E.; Berta, G.; Massa, N. Combined bacterial and mycorrhizal inocula improve tomato quality at reduced fertilization. Scientia Horticulturae 2018, 234, 160–165. [Google Scholar] [CrossRef]
- Ziane, H.; Hamza, N.; Meddad-Hamza, A. Arbuscular mycorrhizal fungi and fertilization rates optimize tomato (Solanum lycopersicum L.) growth and yield in a Mediteranean agroecosystem. Journal of the Saudi Society of Agricultural Sciences 2021, 20 (7), 454-458. [CrossRef]
- Aggangan, N.S.; Moon, H.K. The effects of soil sterilization, mycorrhizal inoculation and rates of phosphorus on growth and survival of Kalopanax septemlobus microplants during the acclimization period. Plant Biotechnol. Rep. 2013, 7(1), 71–82. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Ishii, T.; Kadoya, K. Effects of charcoal as a soil conditioner on citrus growth and vesicular-arbuscular mycorrhizal development. J. Jpn. Soc. Hortic. Sci. 1994, 63, 529–535. [Google Scholar] [CrossRef]
- Brundrett, M.; Bougher, N.; Grove, T.; Malajczuk, N. Working with Mycorrhizas in Forestry and Agriculture. ACIAR Monograph 32. Australian Centre for International Agricultural Research, Canberra, Australia, 1996, p. 34. [CrossRef]
- Moreira, S.D.; Baretta, D.; Tsai, S.M.; Cardoso, E.J.B.N. Spore density and root colonization by arbuscular mycorrhizal fungi in preserved or disturbed Araucariaa angustifolia (Bert.) O. Ktze. Ecosystems. Sci. Agric. 2006, 63, 380–385. [Google Scholar] [CrossRef]
- Aggangan, N.S.; Cortes, A.D.; Reaño, C.E. Growth response of cacao (Theobroma cacao L.) plant as affected by bamboo biochar and arbuscular mycorrhizal fungi in sterilized and unsterilized soil. Biocatalysis and Agricultural Biotechnology 2019, 22, 1–11. [Google Scholar] [CrossRef]
- Gomez-Bellot, M.J.; Ortuño, M.F.; Nortes, P.A.; Vicente-Sanchez, J.; Bañon, S.; Sanchez Blanco, M.J. Mycorrhizal euonymus plants and reclaimed water: biomass, water status and nutritional responses. Sci. Hort. 2015, 186, 61–69. [Google Scholar] [CrossRef]
- Asrar, A.; Abdel-Fattah, G.M.; Elhindi, K.M. Improving growth, flower yield and water relations of snapdragon Antirhinum majus L. plants grown under well-watered and water-stress conditions using arbuscular mycorrhizal fungi. Photosynthetica. 2012, 50, 306-316. [CrossRef]
- Fellbaum, C.R.; Mensah, J.A.; Cloos, A.J.; Strahan, G.E.; Pfeffer, P.E.; Kiers, E.T.; Bucking, H. Fungal nutrient allocation in common mycorrhizal networks is regulated by the carbon source strength of individual host plants. New Phytol. 2014, 203(2), 646–656. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.M.; da Silva, J.A.; Eburneo, J.A.M.; Leonel, M.; Garreto, F.G.d.S.; Nunes, J.G.d.S. Growth and Nitrogen Uptake by Potato and Cassava Crops Can Be Improved by Azospirillum brasilense Inoculation and Nitrogen Fertilization. Horticulturae 2023, 9, 301. [Google Scholar] [CrossRef]
- Rehman, S.u.; De Castro, F.; Aprile, A.; Benedetti, M.; Fanizzi, F.P. Vermicompost: Enhancing Plant Growth and Combating Abiotic and Biotic Stress. Agronomy 2023, 13, 1134. [Google Scholar] [CrossRef]
- Qasim, M.; Ju, J.; Zhao, H.; Bhatti, S.M.; Saleem, G.; Memon, S.P.; Ali, S.; Younas, M.U.; Rajput, N.; Jamali, Z.H. Morphological and Physiological Response of Tomato to Sole and Combined Application of Vermicompost and Chemical Fertilizers. Agronomy 2023, 13, 1508. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, Y.; Liang, Y.; Dai, Z.; Zhao, Y.; Shi, Y.; Gao, J.; Hou, L.; Zhang, Y.; Ahammed, G.J. Improving the Yield and Quality of Tomato by Using Organic Fertilizer and Silicon Compared to Reducing Chemical Nitrogen Fertilization. Agronomy 2024, 14, 966. [Google Scholar] [CrossRef]
- Aslam, Z.; Ahmad, A.; Bellitürk, K.; Kanwal, H.; Asif, M.; Ullah, E. Integrated Use of Simple Compost, Vermicompost, Vermi-Tea and Chemical Fertilizers NP on the Morpho-Physiological, Yield and Yield Related Traits of Tomato (Solanum lycopersicum L.). J. Innov. Sci. 2023, 9, 1–12. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, J.; Wang, Q.; Chang, T.; Shaghaleh, H.; Hamoud, Y. A. Improvement of Photosynthesis by Biochar and Vermicompost to Enhance Tomato (Solanum lycopersicum L.) Yield under Greenhouse Conditions. Plants 2022, 11, 3214. [Google Scholar] [CrossRef]
- Andrade-Sifuentes, A.; Fortis-Hernandez, M.; Preciado-Rangel, P.; Orozco-Vidal, J.A.; Yescas-Coronado, P.; Rueda-Puente, O. Azospirillum brasilense and Solarized Manure on the Production and Phytochemical Quality of Tomato Fruits (Solanum lycopersicum L.). Agronomy. 2020, 10. [Google Scholar] [CrossRef]
- Aseri, G.K.; Jain, N.; Panwar, J.; Rao, A.V.; Meghwal, P.R. Biofertilizers improve plant growth, fruit yield, nutrition, metabolism and rhizosphere enzyme activities of pomegranate (Punica granatum L.) in Indian Thar Desert. Sci Hortic. 2008; 117, 130–135. [Google Scholar] [CrossRef]
- Gamalero, E.; Berta, G.; Massa, N.; Glick, B.R.; Lingua, G. Synergistic interactions between the ACC deaminase-producing bacterium Pseudomonas putida UW4 and the AM fungus Gigaspora rosea positively affect cucumber plant growth. FEMS Microbiol. 2008, 37 (Suppl. 1), 55. [Google Scholar] [CrossRef]
- Sharma, M.; Delta, A.K.; Brar, N.S.; Yadav, A.; Dhanda, P.S.; Baslam, M.; Kaushik, P. Rhizophagus irregularis and Azotobacter chroococcum Uphold Eggplant Production and Quality under Low Fertilization. Int. J. Plant Biol. 2022, 13, 601–612. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).