1. Introduction
Pain diagnosis is often highly challenging due to several factors. These barriers include but are not limited to the inherently subjective nature of pain, the wide range of pain expressions that are not always clearly distinguishable, and the complexity of identifying a symptom that is intertwined with broader biopsychosocial factors [
1,
2].
In this complex scenario, the potential assistance from technology becomes increasingly relevant. Specifically, artificial intelligence (AI) is emerging as a powerful tool in medicine and healthcare [
3]. It is a multidisciplinary research field which involves the use of mathematic approaches, statistics, and advanced algorithms to simulate human decision-making and problem-solving [
4].
The integration of AI into pain medicine has become a significant area of research [
5]. Early developments centered around data management and basic predictive models, showcasing the potential of computational techniques in diagnosing pain conditions, such as low back pain [
6] and abdominal pain [
7]. With the emergence of big data and more advanced machine learning (ML) techniques in the 2010s, AI's applications expanded to include more complex tasks, like image analysis and sophisticated predictive analytics, marking the beginning of a new era in precision pain medicine across various clinical settings [
8]. Given that ML algorithms and deep learning (DL) can process complex datasets to identify patterns and make predictions, AI has been applied in pain medicine for a range of purposes. In the area of computer-aided diagnosis (CAD), efforts have been made to improve diagnostic accuracy for pain [
9], predict treatment outcomes [
10], and tailor pain management strategies [
11]. Moreover, generative AI, particularly natural language processing (NLP), has been used to extract valuable information from clinical notes and patient reports, contributing to the development of specialized chatbots [
12]. Additionally, automated tools such as smartphone apps and wearable devices have been designed to provide real-time monitoring and analysis of pain-related data [
13]. These technologies enable the integration of various data sources, including self-reported pain levels, facial expressions, and behavioral as well as physiological signals [
13]. This review focuses on computer-aided pain diagnosis, particularly on the role of AI within comprehensive multimodal models.
2. Computer-Aided Diagnosis
AI-driven diagnostic methods hold significant promise, particularly in complex cases where the cause of pain is multifactorial and not easily identifiable [
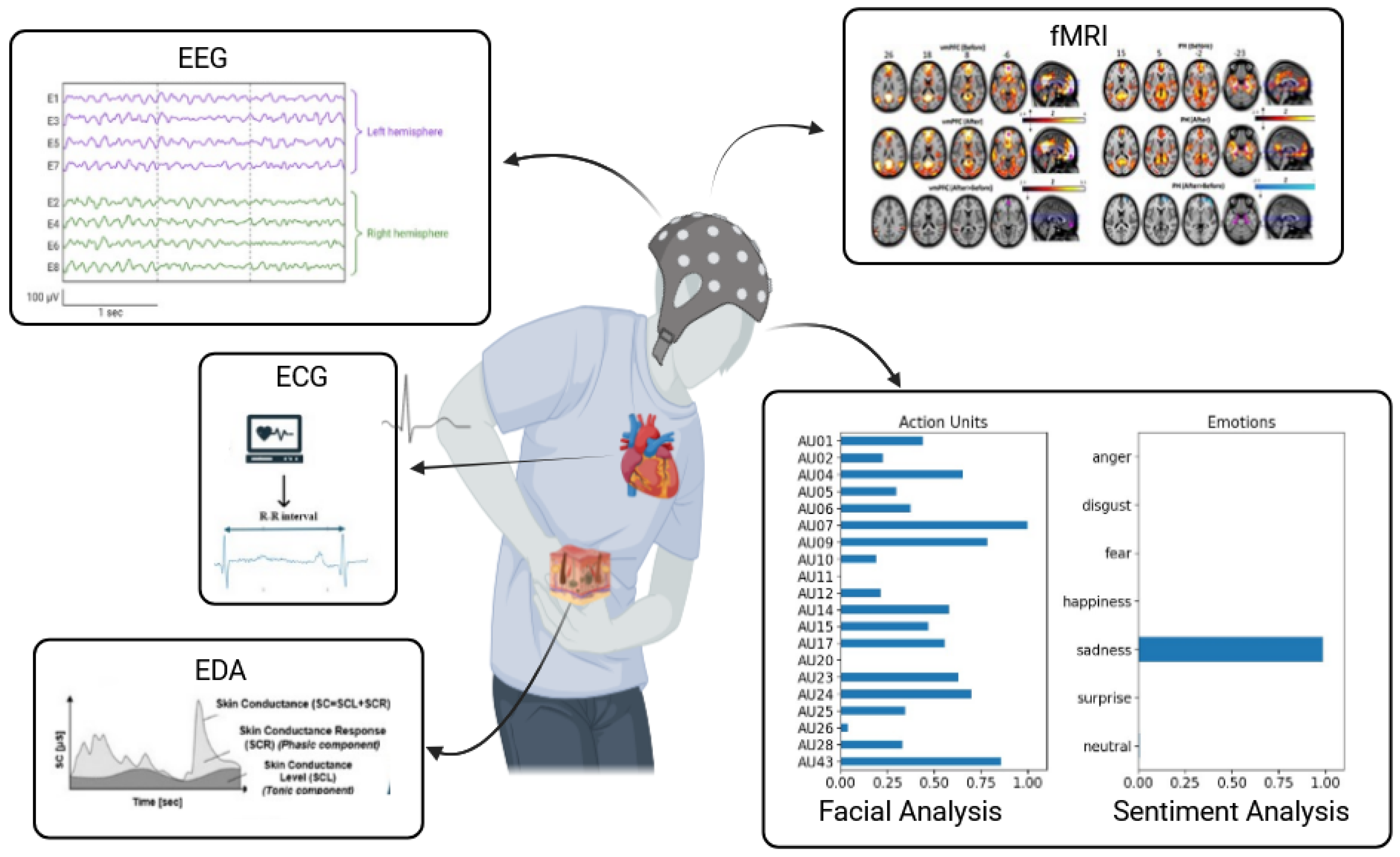
14]. The use of multimodal approaches is considered beneficial for delivering a more precise and comprehensive pain assessment compared to relying solely on subjective self-reports. Therefore, automatic pain assessment (APA) is a set of research, and clinical approaches designed to offer objective and quantifiable measures of pain, aiming to reduce dependence on subjective self-reports. These methodologies generally fall into two primary categories: (1) behavioral-based approaches, which involve analyzing facial expressions, linguistic cues, and nonverbal physical indicators such as body movements, and (2) neurophysiology-based pain detection methods. The latter group includes biosignal strategies such as electrodermal activity (EDA), electroencephalography (EEG), electrocardiography (ECG), electromyography (EMG), and photoplethysmography (PPG) as well as imaging methods such as functional magnetic resonance imaging (fMRI) and other approaches [
13] (
Figure 1).
Various CAD strategies have been developed to support clinicians in achieving more accurate diagnoses. For example, Fontaine et al. [
15] demonstrated that their deep learning model, a ResNet-18 convolutional neural network, which analyzed 2810 facial expressions from 1189 patients before and after surgery, achieved a sensitivity of 89.7% for detecting pain, 77.5% for severe pain, and an accuracy of 53% in estimating pain intensity. In contrast, Bargshady et al. [
16] utilized a three-stream hybrid deep neural network (Ensemble Deep Learning Model) to train on a pain database, reporting an accuracy of 89% and an AUC ROC of 94% for shoulder pain estimation. Similarly, Hosseini et al. [
17] employed a deep learning model with transfer learning, utilizing a pre-trained CNN model with modified upper layers to identify seven pain intensity levels from facial expressions. Additionally, Barua et al. [
18] used the pre-trained DarkNet19 network to achieve a high accuracy of 95% in estimating self-reported shoulder pain. These studies primarily focused on facial expressions for pain recognition.
Other innovative approaches have been explored, particularly for low back pain. Abdollahi et al. [
19] conducted a study using an inertial measurement unit (IMU) to gather kinematic data, developing an ML model to classify patients with nonspecific lower back pain (NSLBP) into high and low-risk categories. Their models achieved 75% accuracy using a support vector machine (SVM) and 60% accuracy with a multi-layer perceptron (MLP). Staartjes et al. [
20] assessed the Five Repetition Sit to Stand Test (5R-STS) combined with ML to classify patients with lumbar disk herniation, lumbar spinal stenosis, or NSLBP, achieving approximately 96% accuracy. Liew et al. [
21] used motion capture and electromyography to evaluate ML models for classifying low back pain subgroups, achieving an AUC of 90.4% for control vs. current pain and 96.7% for chronic vs. current low back pain. On the same topic, a recent evidence-based investigation identified twenty studies utilizing various AI methodologies, including ML and different DL architectures, to diagnose lumbar degenerative disc disease manifestations such as disc degeneration, herniation, and bulging. Interestingly, the AI models consistently surpassed traditional methods in terms of accuracy, sensitivity, and specificity, with performance metrics ranging from 71.5% to 99% across different diagnostic tasks [
22].
In the context of complex pain conditions like fibromyalgia (FM), which involves altered cognitive and emotional processing, AI can offer valuable insights. Sentiment analysis using large language models (LLMs) has been employed to detect subtle nuances in pain expression. Specifically, a study involving 40 FM patients, and 40 chronic pain controls used the LLM Mistral-7B-Instruct-v0.2 to analyze transcribed responses. The prompt-engineered approach showed superior performance with an accuracy of 0.87, precision of 0.92, recall of 0.84, specificity of 0.82, and an AUC ROC of 0.86 for distinguishing FM, compared to the ablated approach with an accuracy of 0.76 [
23]. The statistical significance of these findings suggests that LLM-driven sentiment analysis could enhance FM diagnosis. LLMs are encompassed among the natural language processing methods. It is a field of AI that focuses on the interaction between computers and human language. Therefore, NLP approaches can be used to extract and analyze data from clinical records. Hughes et al. [
24] implemented NLP strategies for building and testing a DL model aimed at intercepting pain in the emergency department.
Furthermore, Lapitov et al. [
25] utilized neuroimaging data, including MRI diffusion tensor and T1-weighted imaging, to identify subtypes of neuropathic facial pain. Using random forest and logistic regression, their ML models achieved up to 95% accuracy in differentiating classical trigeminal neuralgia from healthy controls. Similarly, Peng et al. [
26] explored the application of functional near-infrared spectroscopy (fNIRS) for real-time pain detection under general anesthesia, showcasing innovative approaches to pain management.
3. Integration of AI within a Comprehensive Multimodal Model
AI can significantly augment traditional diagnostic methods by adding deeper layers of analysis and insight. Consequently, research has increasingly focused on integrating AI models to analyze complex physiological and clinical data. For instance, Lee et al. [
27] conducted a study to develop ML models that objectively classify pain levels using neuroimaging and autonomic metrics. Their study, which involved 53 patients with chronic lower back pain, employed support vector machine (SVM) and support vector regression technologies. The models achieved a classification accuracy of 92.45% and an AUC ROC of 0.97 for pain level detection, with the regression model showing a correlation coefficient (r) of 0.63 in predicting pain in new patients.
In another study, researchers created a database encompassing various biosignals, such as EMG, EDA levels, and ECG. They designed an experimental ML system to classify pain levels based on biopotential data from 85 participants exposed to controlled heat stimuli. The model, using SVM, achieved a classification accuracy of 91% for differentiating baseline from pain tolerance thresholds and 79% for baseline versus pain threshold [
28].
The diagnosis of chronic pain must account for the diverse conditions that shape the pain experience. Soin et al. [
29] conducted a pilot study to test ML for diagnosing spinal conditions in chronic pain settings. The study involved 246 patients who provided 85 data points, including demographic and pain-related information, via a Google form on an iPad. A decision tree model processed this data, achieving a 72% accuracy rate compared to practitioner-assigned diagnoses. The study highlighted the potential of AI in enhancing diagnostic accuracy but also emphasized the need for further research to refine these methods and incorporate biopsychosocial factors and data from patient-owned devices.
Cancer pain diagnosis and management often present challenges. A recent study utilized a comprehensive statistical approach, including sensitivity analysis, factorial analysis, and hierarchical clustering on principal components. This analysis integrated demographic, clinical, pain-related variables, and electrodermal activity and ECG signals. The multifactorial analysis revealed links between pain intensity, pain type, Eastern Cooperative Oncology Group (ECOG) performance status, opioid use, and metastasis presence. Clustering analysis identified three distinct patient groups based on pain characteristics, treatments, and ECOG status. Multivariable regression analysis further highlighted associations between pain intensity, breakthrough cancer pain, and opioid dosages [
30,
31].
4. AI-Powered Assessment in Infants and Cognitively Impaired Populations
The proprieties of AI systems can be implemented to assess pain levels and guide treatment, particularly in special populations like infants and the elderly, where pain is challenging to evaluate accurately. Infants cannot verbally communicate their pain, often leading to under recognition and inadequate treatment. This inaccurate pain management in infants is linked to behavioral issues, heightened vigilance, and potential structural brain changes that impact development and learning [
32]. To address these challenges, AI techniques analyze behavioral responses such as facial expressions [
33], crying sounds [
34], and body movements [
35], as well as physiological signals like pupil dilation [
36], skin conductance [
37], heart rate variability [
38], and cerebral hemodynamics [
39]. Multimodal approaches combine these data sources for more accurate assessments [
40]. For example, the PainChek Infant, a mHealth solution, uses AI to evaluate pain intensity based on facial expressions, demonstrating effectiveness in ease of use and accuracy [
41]. Similarly, Carlini et al. [
42] worked on the UNIFESP [
43] and the Classification of Pain Expression (iCOPE) [
44] repositories and developed a mobile app utilizing a convolutional neural network (CNN)-based architecture to classify neonate facial expressions as indicative of pain or not, with low latency and offline functionality. Another group of researchers evaluated EGG, an AI-powered interactive toy developed to assess individual pain levels in children. This device engages young patients through an immersive experience incorporating visual, tactile, and auditory stimuli [
45]. Additionally, Gholami et al. [
46] used ML for evaluating neonate pain intensity through digital imaging analyses.
For cognitively impaired elderly individuals, AI tools analyze non-verbal cues and physiological signals to provide objective pain assessments [
47]. The PainChek application is also used for dementia patients to evaluate pain through facial expressions. For example, a retrospective study by Atee et al. [
48] examined facial expressions in 3,144 individuals with dementia using the PainChek Face domain. The study identified facial action units (AUs) associated with pain intensity, finding AU7 (eyelid tightening) most prevalent during severe pain. Eye-related AUs were more common at higher pain levels than mouth-related AUs. In another investigation, Babicova et al. [
49] tested the tool in UK aged care residents with advanced dementia Additionally, video recordings of non-communicative patients during routine activities were analyzed to observe pain behaviors, with ratings performed using the PAINAD score [
50]. However, real-world applications of these models have shown mixed results in performance metrics [
51].
The main applications are reported in
Table 1.
Legend: Accuracy: the proportion of true results (both true positives and true negatives) among the total number of cases examined. It indicates how often the AI model correctly identifies or excludes the condition; AUC ROC^: represents the model's ability to distinguish between classes, with values closer to 1 indicating better performance. A higher AUC ROC means the model is better at distinguishing between those with and without the condition; Precision‡ (or positive predictive value): the proportion of true positives among the total number of cases that the model predicted as positive. It reflects the accuracy of the model in predicting positive instances; Recall*(or sensitivity or true positive rate): the proportion of actual positives that are correctly identified by the model. It indicates how well the model can identify positive instances; Specificity§: the proportion of true negatives that are correctly identified by the model. It reflects the model's ability to correctly exclude individuals who do not have the condition.
Abbreviations: CNN, Convolutional Neural Network; AUC, Area Under the Curve; ROC, Receiver Operating Characteristic; SVM, Support Vector Machine; ANN, Artificial Neural Network; NSLBP, Non-Specific Low Back Pain; 5R-STS - Five-Repetition Sit-to-Stand Test; DDD, degenerative disc disease; LLM, Large Language Model; SVR, Support Vector Regression.
5. Limitations and Perspectives
While APA methods hold promise for providing more objective assessments of pain intensity, several limitations must be considered. These mostly include a lack of high-quality validation studies, uncertainty regarding which parameters are most appropriate across different clinical settings, and technical challenges such as the timing of their application. To address these issues, a comprehensive approach to pain assessment should integrate both subjective self-reports and objective measures. Additionally, it is essential to use more advanced computational models that account for the variability of clinical data as current research on objective pain intensity assessment often focuses on point estimation. These point estimates can lead to overconfidence and thus inaccurate predictions, which can be detrimental in clinical settings [
52]. Different strategies such as neural network-based prediction interval methods can be adopted for understanding the level of uncertainty in pain intensity [
53].
Moreover, the integration of AI into clinical workflows requires careful consideration of ethical, legal, and technical factors. For example, more efforts should be directed towards the explainable AI [
54,
55]. Additionally, the development of standardized guidelines and protocols for AI implementation will be essential to ensure consistency and safety in patient care [
56]. This step is mandatory for integrating AI into routine clinical practice.
Finally, there is no doubt that pain medicine may have some significant transformation in this “digital age” [
57]. The successful adoption of AI in pain medicine will depend on a collaborative effort between researchers, clinicians, and policymakers to create a framework that balances innovation with patient safety.
6. Conclusions
The potential applications of AI in pain medicine are numerous and intriguing. However, despite its promise, APA methods face important limitations, such as the need for additional validation studies and the difficulty of selecting the most effective parameters for various clinical environments. Reliable data collection is therefore essential for the development of accurate AI models. To address these challenges, it is important to complement traditional clinical evaluations with patient-reported outcomes and biosignal measurements. Given the subjective nature of pain and its complex biopsychosocial dimensions, a multimodal approach that integrates diverse data types within a comprehensive multiprofessional research framework is necessary to obtain more accurate and meaningful insights.
Author Contributions
Conceptualization, M.C. and M.N.S.; methodology, M.C- and M.L.G.L.; software, M.N.S.; validation and formal analysis, M.C., M.L.G.L. and M.N.S.; investigation, M.C. and G.V.; resources, G.V.; data curation, M.C. and M.L.G.L.; writing—original draft preparation, M.C.; writing—review and editing, all the authors; visualization, supervision, and project administration, M.C. and G.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data collected for the review are available from the corresponding author upon reasonable request.
Acknowledgments
The authors are grateful to the Fondazione Paolo Procacci for the support received during the publishing process.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Rahman S, Kidwai A, Rakhamimova E, Elias M, Caldwell W, Bergese SD. Clinical Diagnosis and Treatment of Chronic Pain. Diagnostics. 2023;13:3689. [CrossRef]
- Cohen SP, Vase L, Hooten WM. Chronic pain: an update on burden, best practices, and new advances. Lancet 2021;397(10289):2082-2097. [CrossRef]
- lowais SA, Alghamdi SS, Alsuhebany N, Alqahtani T, Alshaya AI, Almohareb SN, Aldairem A, Alrashed M, Bin Saleh K, Badreldin HA, Al Yami MS, Al Harbi S, Albekairy AM. Revolutionizing healthcare: the role of artificial intelligence in clinical practice. BMC Med Educ. 2023;23(1):689. [CrossRef]
- Xu Y, Liu X, Cao X, Huang C, Liu E, Qian S, Liu X, Wu Y, Dong F, Qiu CW, Qiu J, Hua K, Su W, Wu J, Xu H, Han Y, Fu C, Yin Z, Liu M, Roepman R, Dietmann S, Virta M, Kengara F, Zhang Z, Zhang L, Zhao T, Dai J, Yang J, Lan L, Luo M, Liu Z, An T, Zhang B, He X, Cong S, Liu X, Zhang W, Lewis JP, Tiedje JM, Wang Q, An Z, Wang F, Zhang L, Huang T, Lu C, Cai Z, Wang F, Zhang J. Artificial intelligence: A powerful paradigm for scientific research. Innovation (Camb). 2021;2(4):100179. [CrossRef]
- El-Tallawy SN, Pergolizzi JV, Vasiliu-Feltes I, Ahmed RS, LeQuang JK, El-Tallawy HN, Varrassi G, Nagiub MS. Incorporation of "Artificial Intelligence" for Objective Pain Assessment: A Comprehensive Review. Pain Ther. 2024;13(3):293-317. [CrossRef]
- Mathew B, Norris D, Hendry D, Waddell G. Artificial intelligence in the diagnosis of low-back pain and sciatica. Spine (Phila Pa 1976). 1988;13(2):168-72. [CrossRef]
- Sturman MF, Perez M. Computer-assisted diagnosis of acute abdominal pain. Compr Ther. 1989;15(2):26-35. [PubMed]
- Abd-Elsayed A, Robinson CL, Marshall Z, Diwan S, Peters T. Applications of Artificial Intelligence in Pain Medicine. Curr Pain Headache Rep. 2004;28(4):229-238. [CrossRef]
- Cascella M, Vitale VN, Mariani F, Iuorio M, Cutugno F. Development of a binary classifier model from extended facial codes toward video-based pain recognition in cancer patients. Scand J Pain. 2023;23(4):638-645. [CrossRef]
- Zhao Z, Zhao M, Yang T, Li J, Qin C, Wang B, Wang L, Li B, Liu J. Identifying significant structural factors associated with knee pain severity in patients with osteoarthritis using machine learning. Sci Rep. 2024;14(1):14705. [CrossRef]
- Cascella M, Scarpati G, Bignami EG, Cuomo A, Vittori A, Di Gennaro P, Crispo A, Coluccia S. Utilizing an artificial intelligence framework (conditional generative adversarial network) to enhance telemedicine strategies for cancer pain management. J Anesth Analg Crit Care 2023;3(1):19. [CrossRef]
- Cascella M, Semeraro F, Montomoli J, Bellini V, Piazza O, Bignami E. The Breakthrough of Large Language Models Release for Medical Applications: 1-Year Timeline and Perspectives. J Med Syst. 2024;48(1):22. [CrossRef]
- Cascella M, Schiavo D, Cuomo A, Ottaiano A, Perri F, Patrone R, Migliarelli S, Bignami EG, Vittori A, Cutugno F. Artificial Intelligence for Automatic Pain Assessment: Research Methods and Perspectives. Pain Res Manag. 2023;6018736. [CrossRef]
- Vallejo-De la Cueva A, Aretxabala-Cortajarena N, Quintano-Rodero A, Rodriguez-Nun˜ez C, Pelegrin-Gaspar PM, Gil-Garcia ZI, Margu¨ello-Fernandez AA, Aparicio-Cilla L, Parraza-Diez N. Pupillary dilation reflex and behavioural pain scale: Study of diagnostic test. Intensive Crit Care Nurs. 2023;74:103332. [CrossRef]
- Fontaine D, Vielzeuf V, Genestier P, Limeux P, Santucci-Sivilotto S, Mory E, Darmon N, Lanteri-Minet M, Mokhtar M, Laine M, Vistoli D; DEFI study group. Artificial intelligence to evaluate postoperative pain based on facial expression recognition. Eur J Pain. 2022;26(6):1282-1291. [CrossRef]
- Bargshady G, Zhou X, Deo RC, Soar J, Whittaker F, Wang H. Ensemble neural network approach detecting pain intensity from facial expressions. Artif Intell Med. 2020;109:101954. [CrossRef]
- Hosseini E, Fang R, Zhang R, Chuah CN, Orooji M, Rafatirad S, Rafatirad S, Homayoun H. Convolution Neural Network for Pain Intensity Assessment from Facial Expression. Annu Int Conf IEEE Eng Med Biol Soc 2022:2697-2702. [CrossRef]
- Barua PD, Baygin N, Dogan S, Baygin M, Arunkumar N, Fujita H, Tuncer T, Tan RS, Palmer E, Azizan MMB, Kadri NA, Acharya UR. Automated detection of pain levels using deep feature extraction from shutter blinds-based dynamic-sized horizontal patches with facial images. Sci Rep. 2022;12(1):17297. [CrossRef]
- Abdollahi M, Ashouri S, Abedi M, Azadeh-Fard N, Parnianpour M, Khalaf K, et al. Using a motion sensor to categorize nonspecific low back pain patients: a machine learning approach. Sensors (Basel). 2020;20(12):3600. [CrossRef]
- Staartjes VE, Quddusi A, Klukowska AM, Schröder ML. Initial classification of low back and leg pain based on objective functional testing: a pilot study of machine learning applied to diagnostics. Eur Spine J. 2020;29(7):1702–8. [CrossRef]
- Liew BXW, Rugamer D, De Nunzio AM, Falla D. Interpretable machine learning models for classifying low back pain status using functional physiological variables. Eur Spine J 2024;29(8):1845–59. [CrossRef]
- Liawrungrueang W, Park JB, Cholamjiak W, Sarasombath P, Riew KD. Artificial Intelligence-Assisted MRI Diagnosis in Lumbar Degenerative Disc Disease: A Systematic Review. Global Spine J. 2024 Aug 15:21925682241274372. [CrossRef]
- Venerito V, Iannone F. Large language model-driven sentiment analysis for facilitating fibromyalgia diagnosis. RMD Open 2024;10(2):e004367. [CrossRef]
- Hughes JA, Wu Y, Jones L, Douglas C, Brown N, Hazelwood S, Lyrstedt AL, Jarugula R, Chu K, Nguyen A. Analyzing pain patterns in the emergency department: Leveraging clinical text deep learning models for real-world insights. Int J Med Inform. 2024;190:105544. [CrossRef]
- Latypov TH, So MC, Hung PS, Tsai P, Walker MR, Tohyama S, Tawfik M, Rudzicz F, Hodaie M. Brain imaging signatures of neuropathic facial pain derived by artificial intelligence. Sci Rep 2023;13(1):10699. [CrossRef]
- Peng K, Karunakaran KD, Green S, Borsook D. Machines, mathematics, and modules: the potential to provide real-time metrics for pain under anesthesia. Neurophotonics 2024;11(1):010701. [CrossRef]
- Lee J, Mawla I, Kim J, Loggia ML, Ortiz A, Jung C, et al. Machine learning-based prediction of clinical pain using multimodal neuroimaging and autonomic metrics. Pain. 2025;160(3):550–60. [CrossRef]
- Gruss S, Treister R, Werner P, Traue HC, Crawcour S, Andrade A, et al. Pain intensity recognition rates via biopotential feature patterns with support vector machines. PLoS ONE 2019;10(10):e0140330. [CrossRef]
- Soin A, Hirschbeck M, Verdon M, Manchikanti L (2022) A Pilot Study Implementing a Machine Learning Algorithm to Use Artificial Intelligence to Diagnose Spinal Conditions. Pain Physician. 2022;25(2):171-178. [PubMed]
- Cuomo A, Cascella M, Forte CA, Bimonte S, Esposito G, De Santis S, Cavanna L, Fusco F, Dauri M, Natoli S, Maltoni M, Morabito A, Mediati RD, Lorusso V, Barni S, Porzio G, Mercadante S, Crispo A. Careful Breakthrough Cancer Pain Treatment through Rapid-Onset Transmucosal Fentanyl Improves the Quality of Life in Cancer Patients: Results from the BEST Multicenter Study. J Clin Med. 2020;9(4):1003. [CrossRef]
- Cascella M, Di Gennaro P, Crispo A, Vittori A, Petrucci E, Sciorio F, Marinangeli F, Ponsiglione AM, Romano M, Ovetta C, Ottaiano A, Sabbatino F, Perri F, Piazza O, Coluccia S (2024) Advancing the integration of biosignal-based automated pain assessment methods into a comprehensive model for addressing cancer pain. BMC Palliat Care. 2024;23(1):198. [CrossRef]
- Page GG. Are there long-term consequences of pain in newborn or very young infants? J. Perinatal Edu. 2004;13,3:10-17. [CrossRef]
- Brahnam S, Chuang C-F, Shih FY, Slack MR. Machine recognition and representation of neonatal facial displays of acute pain. Artif. Intell. Med 2006;36,3:211-222. [CrossRef]
- Pal P, Iyer AN, Yantorno RE. Emotion detection from infant facial expressions and cries. Proc. 2006 IEEE Int. Conf. Acoust. Speech Signal Process., vol. 2, pp. II-II, May 2006. [CrossRef]
- Zamzmi G, Pai C-Y, Goldgof D, Kasturi R, Sun Y, Ashmeade T. Automated pain assessment in neonates. Proc. 20th Scand. Conf. Image Anal., 2017. pp. 350-361. [CrossRef]
- Partala T, Surakka V. Pupil size variation as an indication of affective processing. Int. J. Hum.-Comput. Stud. 2003;59;1:185-198. [CrossRef]
- Gruss S, Treister R, Werner P, Traue HC, Crawcour S, Andrade A, Walter S. Pain intensity recognition rates via biopotential feature patterns with support vector machines. PLoS One. 2015;10(10):e0140330. [CrossRef]
- Walter S, Gruss S, Limbrecht-Ecklundt K, Traue HC, Werner P, Al-Hamadi B, Diniz N, da Silva GM, Andrade AO. Automatic pain quantification using autonomic parameters Psychol. Neurosci. 2014;7:363-380. [CrossRef]
- Ranger M, Glinas C. Innovating in pain assessment of the critically ill: Exploring cerebral near-infrared spectroscopy as a bedside approach. Pain Manage Nursing. 2014;15(2):519-529. [CrossRef]
- Zamzmi G, Kasturi R, Goldgof D, Zhi R, Ashmeade T, Sun Y. A Review of Automated Pain Assessment in Infants: Features, Classification Tasks, and Databases. IEEE Reviews in Biomedical Engineering 2018;11:77-96. [CrossRef]
- Hughes JD, Chivers P, Hoti K. The Clinical Suitability of an Artificial Intelligence-Enabled Pain Assessment Tool for Use in Infants: Feasibility and Usability Evaluation Study. J Med Internet Res 2023;25:e41992. [CrossRef]
- Carlini LP, Ferreira LA, Coutrin GAS, Varoto VV, Heiderich TM, Balda RCX, Barros MCM, Guinsburg R. A Convolutional Neural Network-based Mobile Application to Bedside Neonatal Pain Assessment. 34th SIBGRAPI Conference on Graphics, Patterns and Images (SIBGRAPI), Gramado, Rio Grande do Sul, Brazi 2021. pp. 394-401,. [CrossRef]
- Heiderich TM, Leslie AT, Guinsburg R. Neonatal procedural pain can be assessed by computer software that has good sensitivity and specificity to detect facial movements. Acta Paediatr. 2015; 104(2):e63-9. [CrossRef]
- Brahnam S, Chuang CF, Sexton RS, Shih FY. Machine assessment of neonatal facial expressions of acute pain. Decision Support Systems. 2007;43(4):1242–1254. [CrossRef]
- Li J, Chen K, Yang L, Mutsaers M, Barakova E. EGG: AI-Based Interactive Design Object for Managing Post-operative Pain in Children. In: Ferrández Vicente, J.M., Val Calvo, M., Adeli, H. (eds) Artificial Intelligence for Neuroscience and Emotional Systems. IWINAC 2024. Lecture Notes in Computer Science, vol 14674. Springer, Cham. [CrossRef]
- Gholami B, Haddad WM, Tannenbaum AR. Relevance vector machine learning for neonate pain intensity assessment using digital imaging. IEEE Trans Biomed Eng 2010;57(6):1457-66. [CrossRef]
- Smith E, Storch EA, Vahia I, et al. Affective computing for late-life mood and cognitive disorders. Frontiers in Psychiatry. 2021;12. [CrossRef]
- Atee M, Hoti K, Hughes JD. Psychometric Evaluation of the Electronic Pain Assessment Tool: An Innovative Instrument for Individuals with Moderate-to-Severe Dementia. Dementia and Geriatric Cognitive Disorders. 2018;44(5-6):256-267. [CrossRef]
- Babicova I, Cross A, Forman D, Hughes J, Hoti K. Evaluation of the Psychometric Properties of PainChek R in UK Aged Care Residents with advanced dementia. BMC geriatr. 2021; 21(1):337. [CrossRef]
- Warden V, Hurley AC, Volicer L (2003) Development and psychometric evaluation of the Pain Assessment in Advanced Dementia (PAINTED) scale. J Am Med Dir Assoc. 2003;4:9–15. [CrossRef]
- Gomutbutra P, Kittisares A, Sanguansri A, Choosri N, Sawaddiruk P, Fakfum P, Lerttrakarnnon P, Saralamba S. Classification of elderly pain severity from automated video clip facial action unit analysis: A study from a Thai data repository. Front Artif Intell 2022;5:942248. [CrossRef]
- Ak R, Vitelli V, Zio E. An interval-valued neural network approach for uncertainty quantification in short-term wind speed prediction. IEEE transactions on neural networks and learning systems. 2015;26(11):2787–800. [CrossRef]
- Ozek B, Lu Z, Radhakrishnan S, Kamarthi S. Uncertainty quantification in neural-network based pain intensity estimation. PLoS One. 2024;19(8):e0307970. [CrossRef]
- Zednik C. Solving the black box problem: A normative framework for explainable artificial intelligence. Philos Technol 2021;34:265–88. [CrossRef]
- Marcus E, Teuwen J. Artificial intelligence and explanation: How, why, and when to explain black boxes. Eur J Radiol. 2024;173:111393. [CrossRef]
- Collins GS, Dhiman P, Andaur Navarro CL, et al. Protocol for development of a reporting guideline (TRIPOD-AI) and risk of bias tool (PROBAST-AI) for diagnostic and prognostic prediction model studies based on artificial intelligence. BMJ Open. 2021;11(7):e048008. [CrossRef]
- Kiran N, Sapna F, Kiran F, Kumar D, Raja F, Shiwlani S, Paladini A, Sonam F, Bendari A, Perkash RS, Anjali F, Varrassi G. Digital Pathology: Transforming Diagnosis in the Digital Age. Cureus. 2023;15(9):e44620. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).