1. Introduction
As fat is an important factor in determining the taste and profile of meat, adding fat is essential to manufacturing cultured meat with characteristics similar to traditional meat [
1,
2]. To enhance the quality of cultured meat in terms of taste, flavor, and texture, researchers are undertaking numerous endeavors [
3,
4,
5,
6]. Among various studies, one research has suggested that taste of cultured tissue can vary depending on amino acid composition and umami substances and that the flavor of cultured meat can differ based on fat composition [
7]. Therefore, the provision of fat is indispensable in the production of cultured meat. Various methods of adding fat have been performed to manufacture numerous types of cultured meat containing fat [
8,
9,
10]. Among them, adding cultured fat has recently captured interest. Cultured fat can be supplied by culturing adipocytes, which can be obtained by isolating fat tissues or inducing trans-differentiation from other types of cells [
11,
12]. Trans-differentiation is a transition in which differentiated cells are irreversibly converted to another cell type without going through the progenitor cell [
13]. It can be induced by changing the microenvironment of a cell or providing specific signalling molecules [
14]. Several cell types originated from mesenchymal stem cells (MSCs), such as fibroblasts and osteoblasts, can be transformed into mature adipocytes through trans-differentiation [
15,
16]. However, it is necessary to make conditions or treatments different, since transcription factors related to trans-differentiation are different depending on the animal species.
Fat can be efficiently added to cultured meat by trans-differentiation even when primary cells are used. To utilize primary cells for culture meat production, an essential process of sorting, which involves isolating targeted cells, is required. Ensuring the purity of cells destined to differentiate into muscle through cell sorting is also crucial for creating cultured tissues [
17]. Several methods for sorting cells based on cellular characteristic have been studied [
18,
19,
20,
21]. Among them, pre-plating can isolate satellite cells based on difference in adherent time of cells [
22]. Various cell species can be attached to the flask during the pre-plating step to increase the purity of satellite cells [
23]. Pre-plating has an advantage in that cells that can rapidly attach to the flask can be trans-differentiated to adipocytes without any additional cell isolation. Most studies utilizing trans-differentiation to produce cultured fat have focused on the conversion of fibroblasts into adipocytes [
11,
24,
25]. In addition, research has indicated that various cells such as myocytes and osteoblasts can trans-differentiate into adipocytes [
11,
15,
26,
27].
Fibroblasts are present throughout the animal body. They can be easily observed in the primary culture of satellite cells. They are suitable for adipogenic trans-differentiation during the production of cultured meat [
28,
29]. Fibroblasts can maintain their stemness for a long time in vitro. Human fibroblasts are known to be able to maintain potency for up to 30 passages [
30]. Due to their high cell plasticity, fibroblasts are suitable for trans-differentiation, enabling them to transform into other cell types [
14]. Based on these characteristics of fibroblasts, it is believed that cell yield will significantly increase, contributing to cultured meat production when fat is produced through trans-differentiation. Hence, this study aimed to explore how many passages chicken embryonic fibroblasts (CEF) could maintain their characteristics in vitro and to determine differences in trans-differentiation of fibroblasts into adipocytes according to passages.
2. Materials and Methods
2.1. Animal Care and Research Ethics Statements
Treatment and use of experimental animals were approved by the Institutional Animal Care and Use Committee (IACUC) at Gyeongsang National University (approval no. GNU-231127-C0215). All experimental steps complied with the IACUC standard procedures.
2.2. Isolation of Chicken Embryo Fibroblasts (CEF)
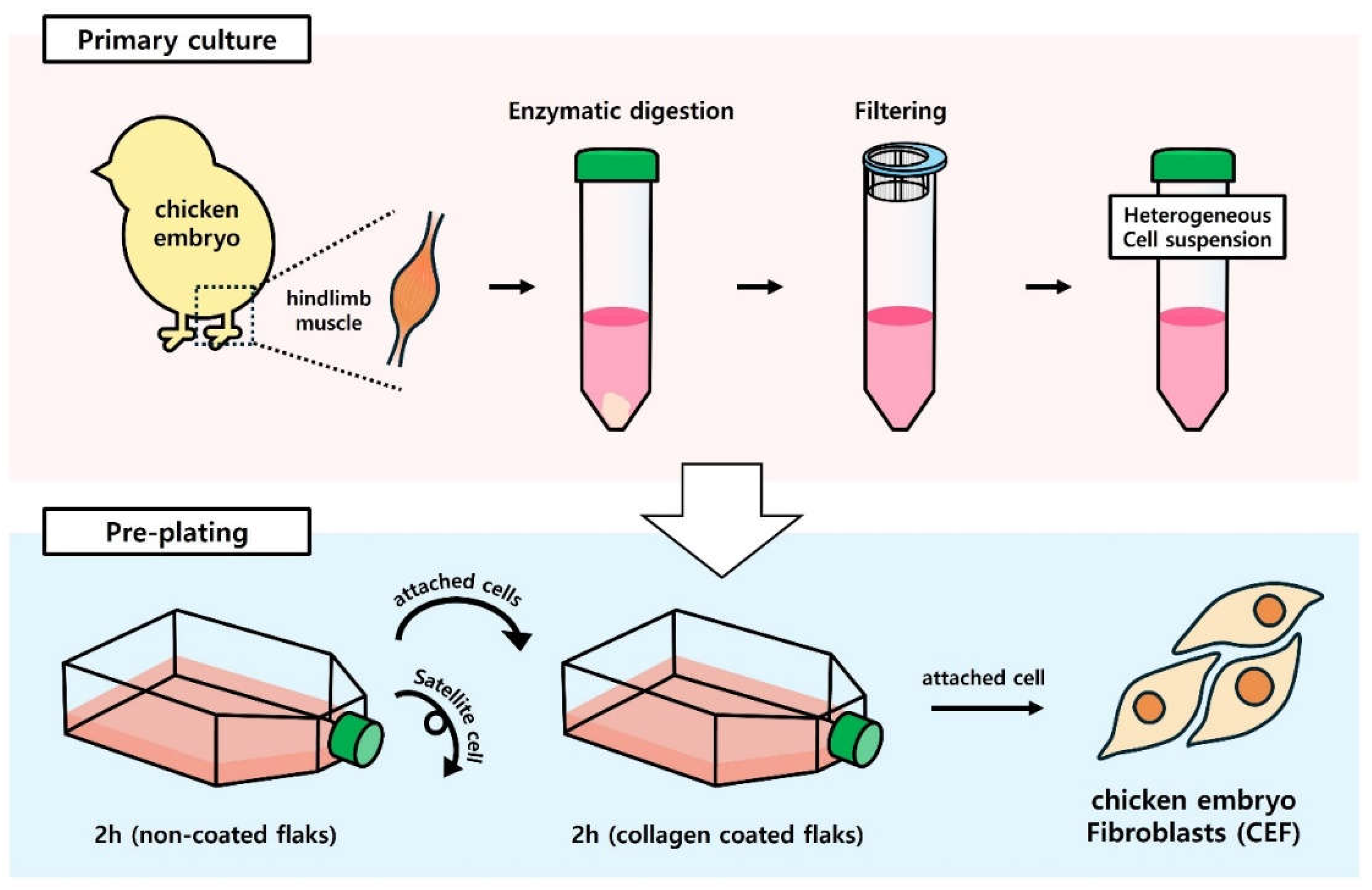
Hindlimb muscle tissues were isolated from 18-day-old chicken embryos, removing bone and cartilage as much as possible. These tissues were then washed and immersed in Hanks balanced salt solution (HBSS; Welgene, Korea) containing 1% antibiotic-antimycotic (anti-anti) (Thermo Fisher, USA) to disinfect and eliminate extrinsic debris including hair and blood. The sample was minced with scissors until it became a thick liquid. The tissue sample was digested with Type I collagenase (0.1%; Worthington Biochemical Corporation, LS004194) and incubated at 37 °C for 1 h. During enzymatic digestion with collagenase, suspension was aspirated and discharged from a syringe with an 18-gauge needle every 15 min. Digested tissue sample was centrifuged at 4 °C, 800 x g for 5 min and the supernatant was removed. To obtain single cell suspension, 0.25% trypsin-EDTA (Gibco™, 25200056) was added to the cell pellet. The cell suspension added with trypsin was incubated at 37 °C for 20 min. After digestion, fetal bovine serum (FBS) was added to the single cell suspension to inhibit the action of trypsin. After Dulbecco's phosphate-buffered saline (DPBS) (Welgene, LB001-02) was added to the cell suspension, the cell suspension was mixed gently in a circular pattern. When viscous lump started to rise, amorphous and flouting liquid was removed by aspirating with a syringe with an 18-gauge needle. During removal, loss of cell suspension had to be minimized. The cell suspension was filtered using cell strainers with pore sizes in the order of 100 μm and 40 μm. It was then centrifuged at 4 °C at 800 x g for 5 min. Red blood cell lysis buffer (InvitrogenTM, 00-4333-57) was added to the pellet after removing supernatant. Detailed experimental procedures followed the manufacturer's guidelines.
2.3. Purification of Chicken Myofibroblast
The cell suspension isolated through primary culture was purified using the pre-plating method. Cells obtained from primary culture were incubated in un-coated flasks for 2 hours. After this incubation, adherent cells were transferred to collagen-coated flasks and incubated for an additional 2 hours. Following this incubation, adherent cells were identified as CEF. The growth medium was subsequently replaced. The supernatant was transferred to another culture flask to start the main culture.
2.4. Culture Condition of Chicken Myofibroblast
Cells were seeded at 2500 cells/cm2 for both proliferation and differentiation. Growth media (GM; 20 % FBS, 1% Glutamax supplement (Thermo Fisher, 35050061) and 1% anti-anti in DMEM) were used for proliferation of CEF. Cells were incubated at 41 °C with 5% CO2 for proliferation. Myofibroblast was cultured in trans-differentiation media (DM) supplemented with 10 % FBS, 1 % Glutamax supplement, 1 % anti-anti in DMEM, 400 µM oleic acid (Santa Cruz), and 5 µM or 10 µM rosiglitazone (Med Chem Express, HY-17386) to induce adipogenic trans-differentiation. GM was replaced with DM after 2 days of proliferation. Cells were incubated at 41 °C with 5% CO2. Adipogenic trans-differentiation was progressed for 14 days.
2.5. Immunofluorescence Staining
Cells were fixed with 4% paraformaldehyde (PFA) for 15 min at room temperature (RT) and washed with DPBS for 5 min at least twice. Depending on the substance to be stained, permeabilization was conducted with 0.5% Triton X-100 for 15 min following by washing with DPBS for 5 min at least twice. After permeabilization, blocking of cell was executed with 3% bovine serum albumin (BSA; Sigma Aldrich, A9647) in DPBS. Cells were incubated with primary antibodies including mouse anti-Pax7 (Paired-box protein 7) conjugated with Alexa fluor 488 (1:200, Santa Cruz Biotechnology, Cat #sc-81648 AF488) and mouse anti-MHC (1:40, DSHB, MF20) at 4 °C overnight. After washing with DPBS for 5 min twice after incubation, cells were incubated with secondary antibodies, goat anti-mouse IgG2b cross-adsorbed secondary antibody conjugated with Alexa Fluor 488 (1:500, Thermo Scientific) (diluted in 3% BSA in DPBS), for 2 h at RT in a dark room. Nuclei were counterstained with Hoechst 33342 (InvitrogenTM, H3570). Immunofluorescence images were captured using Olympus fluorescence microscope CKX 53 (Olympus, Japan) and processed with ImageJ software (NIH, USA). For statistical analysis, mean image data derived from five randomly selected images were used.
2.6. Proliferation Analysis
2.6.1. Cumulative Cell Numbers
Cumulative cell number indicates cell growth rate by calculating the number of cells that have proliferated at each passage. Cumulative cell number of cells from passage 1 (P1) to passage 9 (P9) was calculated based on the following equation:
where S was the number of cells seeded, N was the final number of cells proliferated, and N(0) was the initial number of cells.
2.6.2. Population Doubling Time
Population doubling time (PDT) was calculated based on the following equation referenced from the method described previously [
31]:
where N0 was the number of cells seeded, N1 was the final number of cells proliferated, and dT was the duration of culture. In the present study, the duration of culture (dT) was calculated by seeding 5,000 cells/cm2 from passage 0 (P0) to passage 15 (P15).
2.6.3. EdU Assay
EdU (5-Ethynyl-2'-deoxyuridine) assay was conducted using Click-iT Plus EdU Imaging Kits (Invitrogen™, C10640). Cells were incubated overnight after seeding. After incubation, 10 µM of EdU was added to live cells. Imaging was conducted after 4 h of EdU treatment. Detailed experimental procedures were conducted according to the manufacturer's instructions. Mean image data derived from five randomly selected EdU assay images are presented for statistical analysis.
2.6.4. Proliferation Capacity Assay Using Cell Counting Kit-8
The proliferation capacity of live cells was analysed using Cell counting Kit-8 (CCK-8) (Dojindo, CK04). The assay was conducted with eight replicates by culturing cells in a 96-well plate. After 24 h and 72 h of seeding cells, CCK-8 reagent was added to live cells. After incubating for 3 h, optical density (O.D) was measured at 450 nm with a microplate reader (HiPo MPP-96, bioSan). Specific experimental procedures were performed according to the manufacturer's instructions.
2.7. Adipogenic Trans-Differentiation Analysis
2.7.1. Neutral Lipid Staining
Neutral lipid in adipocyte was stained with HCS LipidTOX™ (Thermo Fisher, H34475). Cells were fixed with 4 % formaldehyde solution in DPBS for 20 min at RT. After fixation, cells were rinsed with DPBS two times to remove remaining formaldehyde solution. LipidTOX diluted in DPBS at 1:500 was added to cells. Staining was conducted at RT for 45 h. Detailed experimental procedures were performed according to the manufacturer's instructions.
2.7.2. Flow Cytometry for Adipogenesis Analysis
Trans-differentiated fibroblasts were stained with HCS LipidTOX and Hoechst 33342 according to the manufacturer’s instruction. Cells were rinsed with DPBS twice and resuspended in FACS buffer. Flow cytometry was performed using a FACS Analyzer (Becton Dickinson, USA).
2.8. Statistical Analysis
All statistical analyses were performed using SAS 9.4 (SAS, USA) and GraphPad Prism 10.3.2 (GraphPad, USA). Statistical analysis was performed via one-way Analysis of variance (ANOVA) with multiple comparisons. Tukey’s honestly significant difference (HSD) post-hoc test was performed to compare means. All error bars in figures indicate SD. P-value ≤ 0.05 indicated statistical significance.
3. Results and Discussion
3.1. Isolation of Chicken Embryo Fibroblasts by Pre-Plating
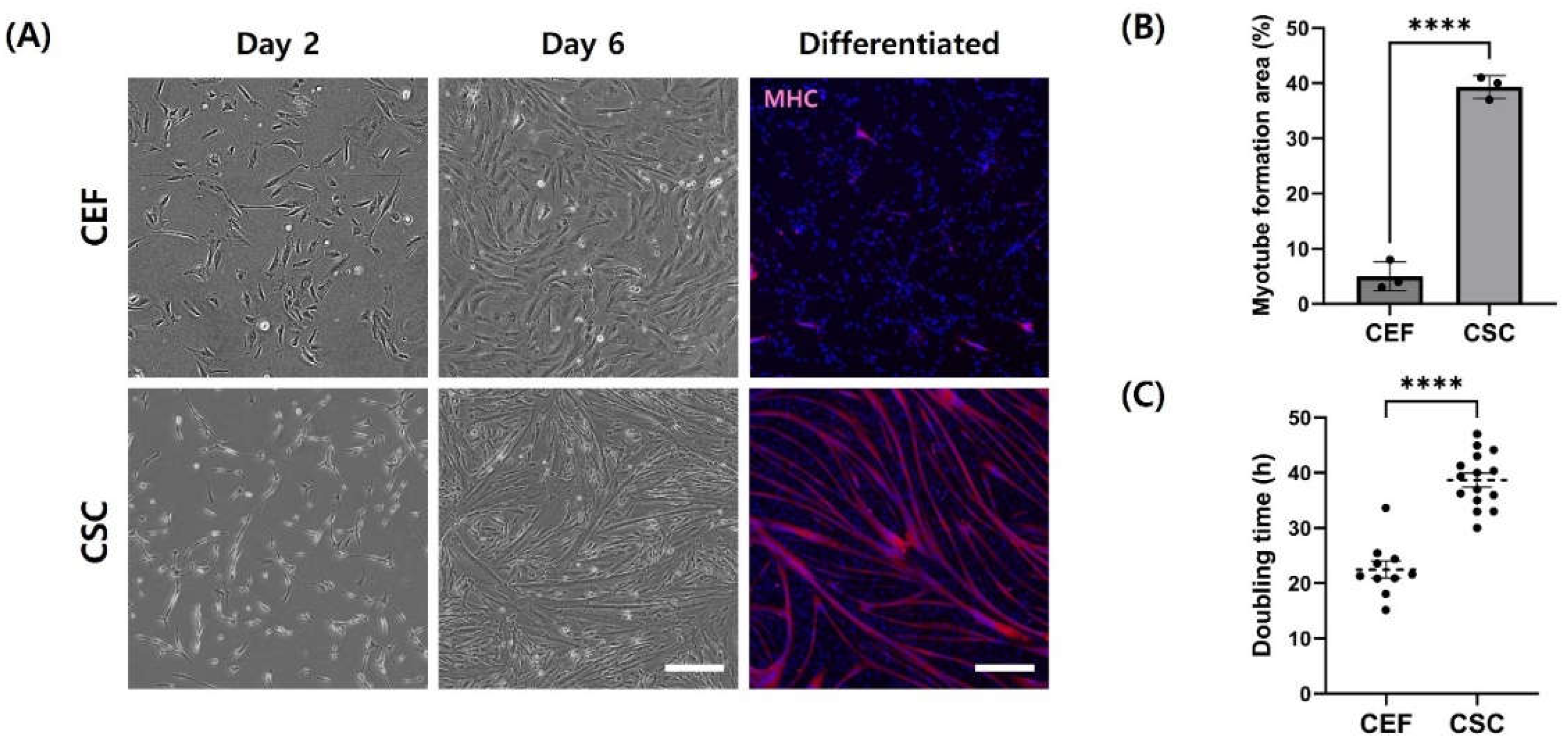
A heterogeneous cell suspension obtained by filtering hindlimb muscle solution isolated from chicken embryo was enzymatic digested and sorted through pre-plating (
Figure 1). Cells that adhered quickly to the flask within 2 hours during pre-plating were classified as fibroblasts, while those sorted in subsequent pre-plating steps were categorized as CSC. After cell purification, attachment and morphological characteristics of CEF were analyzed and compared to isolated CSC. Both CEF and CSC were observed to have a similar morphology under the microscope when they were on 2 days of culture (
Figure 2A). However, when differentiation was induced for cells after starvation on day 6 of culture, CSC formed myotubes whereas fibroblasts exhibited a distinct fibroblastic pattern, failing to form myotubes. This observation was consistent with the result of MHC expression, which was the lowest in fibroblasts (
Figure 2B) (p < 0.05). Additionally, the doubling time of CEF was 1.6 times shorter than that of CSC (
Figure 3C) (p < 0.05).
In the process of isolating satellite cells for cultured meat production, fibroblasts are the main contaminating cell type. Fibroblasts could adhere to the flask and proliferate more quickly than satellite cells. Pre-plating is one of the processes used to isolate cells based on this difference in adhesion time of cells [
22]. In this study, fibroblasts were isolated and cultured by removing slowly adhering cells with pre-plating. Cells attached to the flask during pre-plating were utilized as CEF. Primary CEF and CSC isolated by 2 hours of pre-plating were morphologically indistinguishable until the second day of culture. However, fibroblasts were observed to adhere by spreading on the bottom surface of the flask. After induction of differentiation, distinct morphologies between these cells became evident. Especially, CSC formed myotubes shaped long tunnel through differentiation. Also, totally different doubling time support cells attached after 2 hours of pre-plating is CSC, a majority cell of muscle. In other word, it indicates that CEF were isolated well from heterogeneous cell containing CSC.
3.2. Identification of Chicken Embryo Fibroblasts
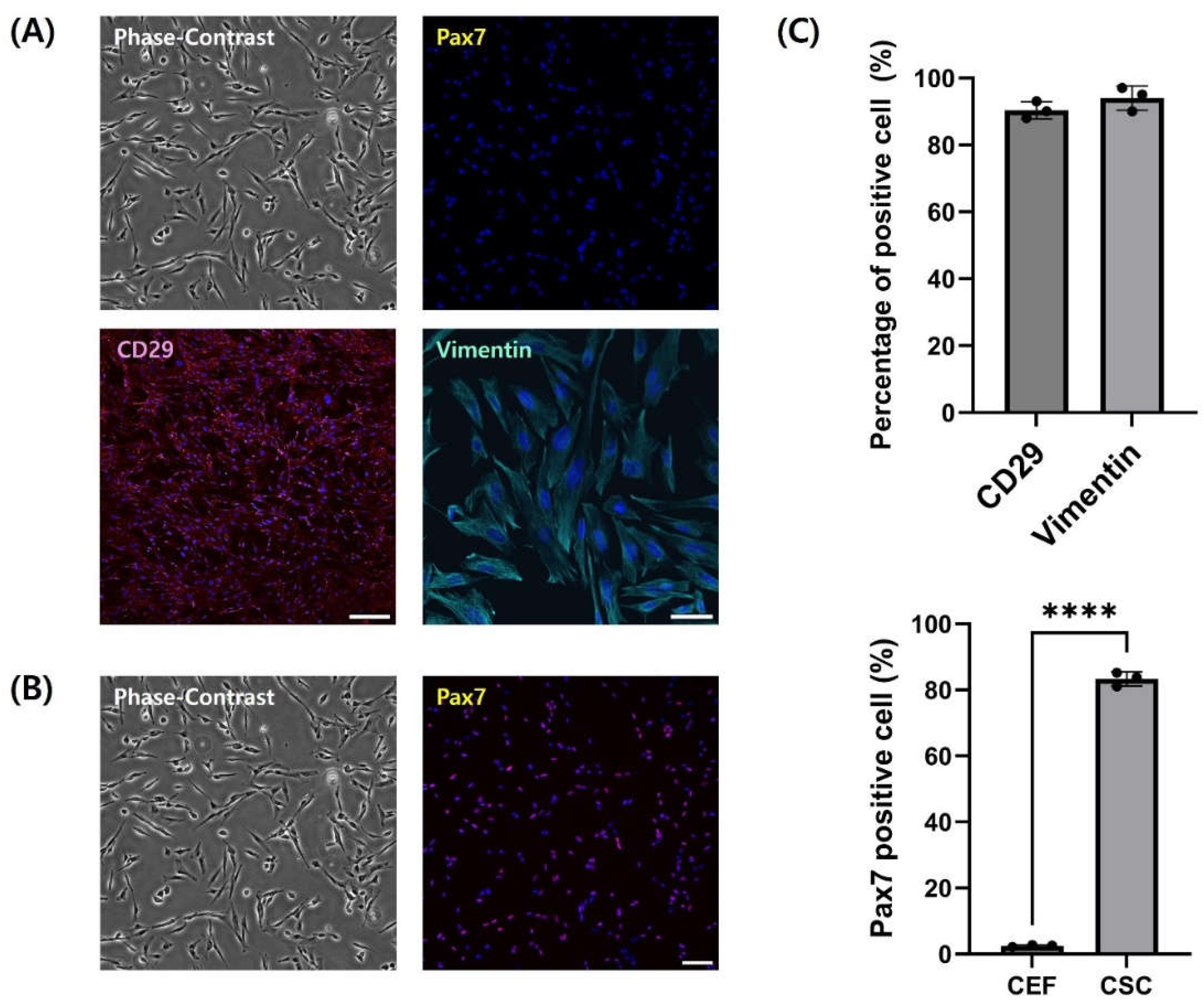
Cells were stained with several specific markers to identify fibroblasts isolated by pre-plating. Isolated CEF and CSC were immunofluorescence-stained with Pax7 (
Figure 3). CEF showed 2% positivity for Pax7, whereas 90% of cells purified through subsequent pre-plating steps expressed Pax7 (
Figure 3C) (p < 0.05). This is consistent with the results of previous morphological differences and doubling time variations among the cells (
Figure 2). For additional identification, fibroblasts were stained with fibroblast markers CD29 and vimentin (
Figure 3A). Stained images revealed that over 90% of fibroblasts expressed CD29 and vimentin (
Figure 3C).
To identify isolated fibroblasts CEF and slow attached cells were immunofluorescence-stained with Pax7. Pax7 is a distinctive transcription factor prevalent in satellite cells [
32,
33]. It is expressed during their quiescent phase and then gradually decreases [
34]. CEF attached to the flask within 2 hours exhibited significantly lower expression rates of pax7 compared to CSC, which took longer to attach (p < 0.05). A 40-fold difference in Pax7 expression rates indicated that CEF could be effectively isolated from muscle tissue which CSC is abundant through pre-coating. The results of staining cell with fibroblast marker showed over 90% of rapidly adhered cells within 2 hours were the fibroblasts. CD29, also known as integrin beta1, and Vimentin are cell proteins that can serve as markers for chicken fibroblasts. This demonstrates that CEF can adhere to flasks coated with collagen within 2 hours.
Figure 3.
Identification of CEF using immunofluorescence-staining. (A) Representative images of CEF stained with Pax7 (yellow), CD29 (red) and Vimentin (cyan). (B) Phase-contrast and staining image of CSC. CSC was stained with Pax7 (red). (C) Percentage of cell expressing CD29, Vimentin and Pax7. Scale bar = 100 μm. Data are presented as means plus standard deviation from three independent experiments.**** indicates statistically significant difference at p < 0.0001.
Figure 3.
Identification of CEF using immunofluorescence-staining. (A) Representative images of CEF stained with Pax7 (yellow), CD29 (red) and Vimentin (cyan). (B) Phase-contrast and staining image of CSC. CSC was stained with Pax7 (red). (C) Percentage of cell expressing CD29, Vimentin and Pax7. Scale bar = 100 μm. Data are presented as means plus standard deviation from three independent experiments.**** indicates statistically significant difference at p < 0.0001.
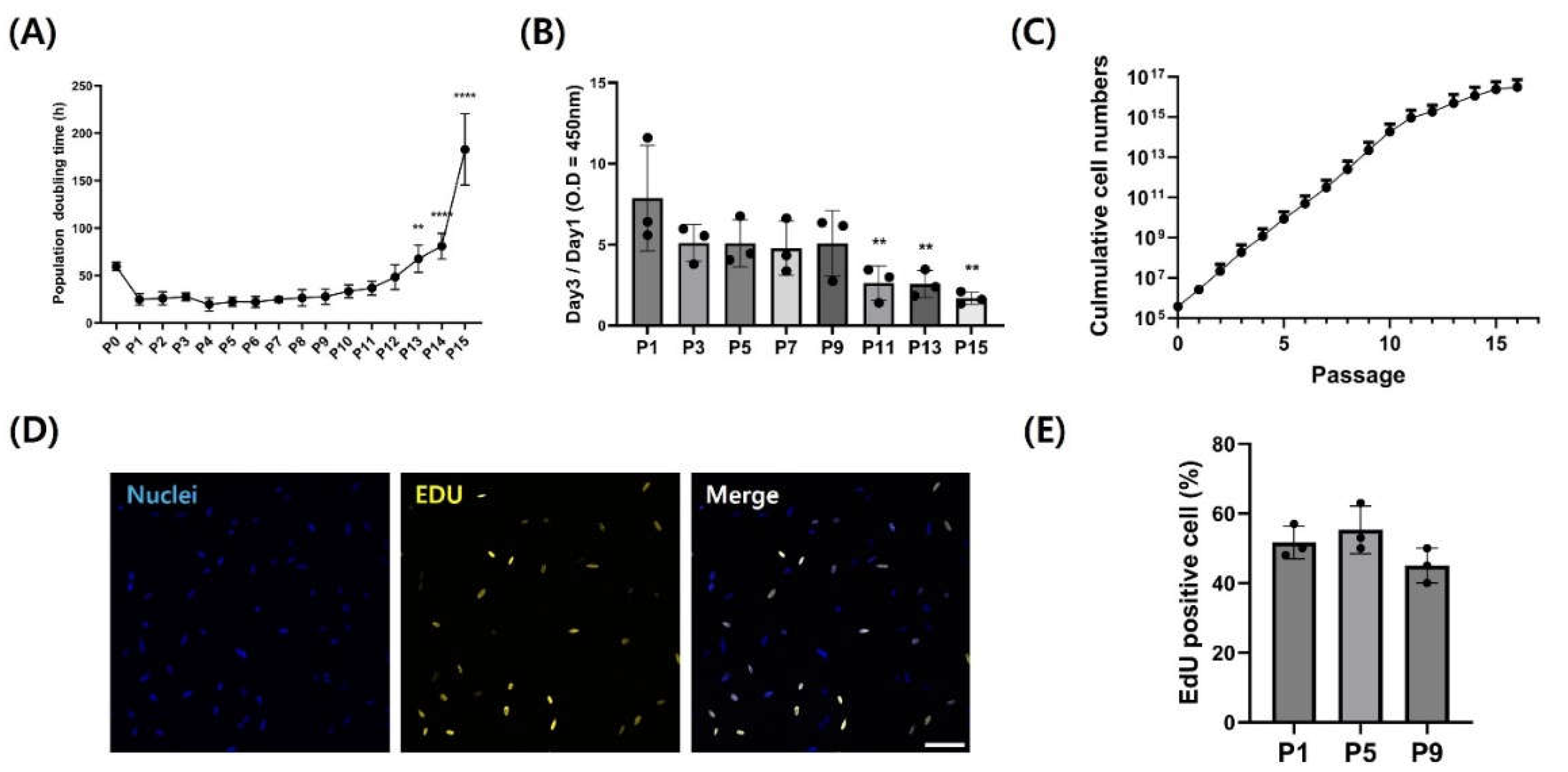
3.3. Analysis of Proliferation Capacity of in Chicken Embryo Fibroblasts
The proliferative capacity of fibroblasts was evaluated through passages 15 to assess their suitability as materials for cultured meat production. Doubling times showed consistent and stable proliferation capacity across passages (
Figure 4A). Except for cells at passage 0 immediately following primary culture, fibroblasts could maintain significant constant proliferate ability up to passage 12 (p < 0.05). The growth rate assessed by CCK-8 assay showed no significant difference up to passage 9, indicating sustained proliferation and survival even in higher passages (
Figure 4B) (p < 0.05). Moreover, cumulative cell numbers measured based on the proliferation of cells continuously increased up to passage 11 (
Figure 4C). Additionally, cells at passages 1, 7, and 11 were stained with EdU to observe actively proliferating cells (
Figure 4D and
Figure 4E). The proportion of cells expressing EdU did not show significant differences among passages (
Figure 4E). However, from passage 11, doubling time and CCK-8 assay revealed proliferation ability of fibroblasts was significantly decreased (p < 0.05).
Fibroblasts have an advantage over satellite cells primarily utilized in cultured meat production by providing a more stable cell supply due to their rapid proliferation capacity. With the aim of leveraging benefits of fibroblasts for cultured meat production, the proliferative potential of CEF was analyzed. Doubling time, which is required for the number of cultured cells to double, signifies how many passages isolated primary fibroblasts can sustain their proliferation capacity and how much time fibroblasts take to double in vitro. Initially, due to impairment of various enzymes, attachment proteins, and substances involved in cell interaction, the doubling time was significantly high in passage 0. However, as cultivation commenced, the cells stabilized their proliferation and showed consistent growth capability. Based on the analysis of population doubling time and CCK absorbance results, it was observed that cell growth began to decelerate, and viability started to decline with continued cultivation from at least the passage 11 onward. Furthermore, this aligned with results of EdU assays, which did not show significant differences in proliferation capacity up to passage 9. In the EdU analysis under the same conditions, fibroblasts from passage 11 showed scarce cells expressing EdU. These comprehensive results of proliferation analysis indicate CEF proliferated very slowly or ceased proliferation from passage 11 onward. Namely, CEF can consistently survive and proliferate up to passage 9 in vitro. This was supposed to the loss of stemness due to senescence in primary cells [
35]. Loss of stemness in primary fibroblasts can significantly impact cultured meat yield. The decline in cellular productivity is directly related to the cost of mass production, which is a critical issue in the cultured meat industry [
36]. Our comprehensive proliferation analysis demonstrated stable proliferation of CEF and 46 x 1012 of cumulative cell number up to passage 9. The observation that primary CEF achieved stable proliferation up to the 9th passage with only 20% FBS, without additional aid of other growth factors, indicates a high level of productivity from a food production perspective. These outcomes suggest the potential application of CEF to utilized for cultured meat components.
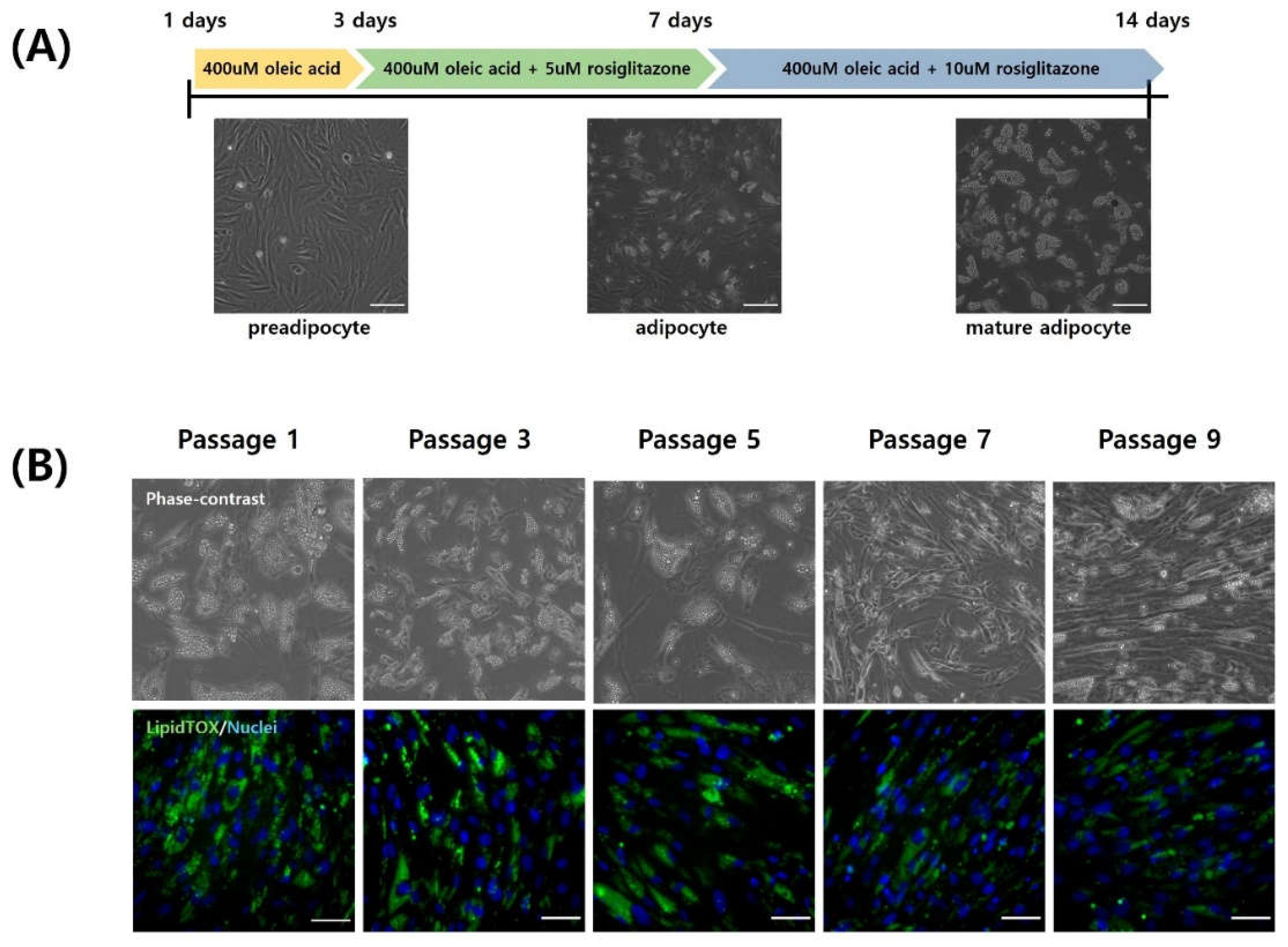
3.4. Adipogenic Trans-Differentiation of Chicken Embryo Fibroblasts
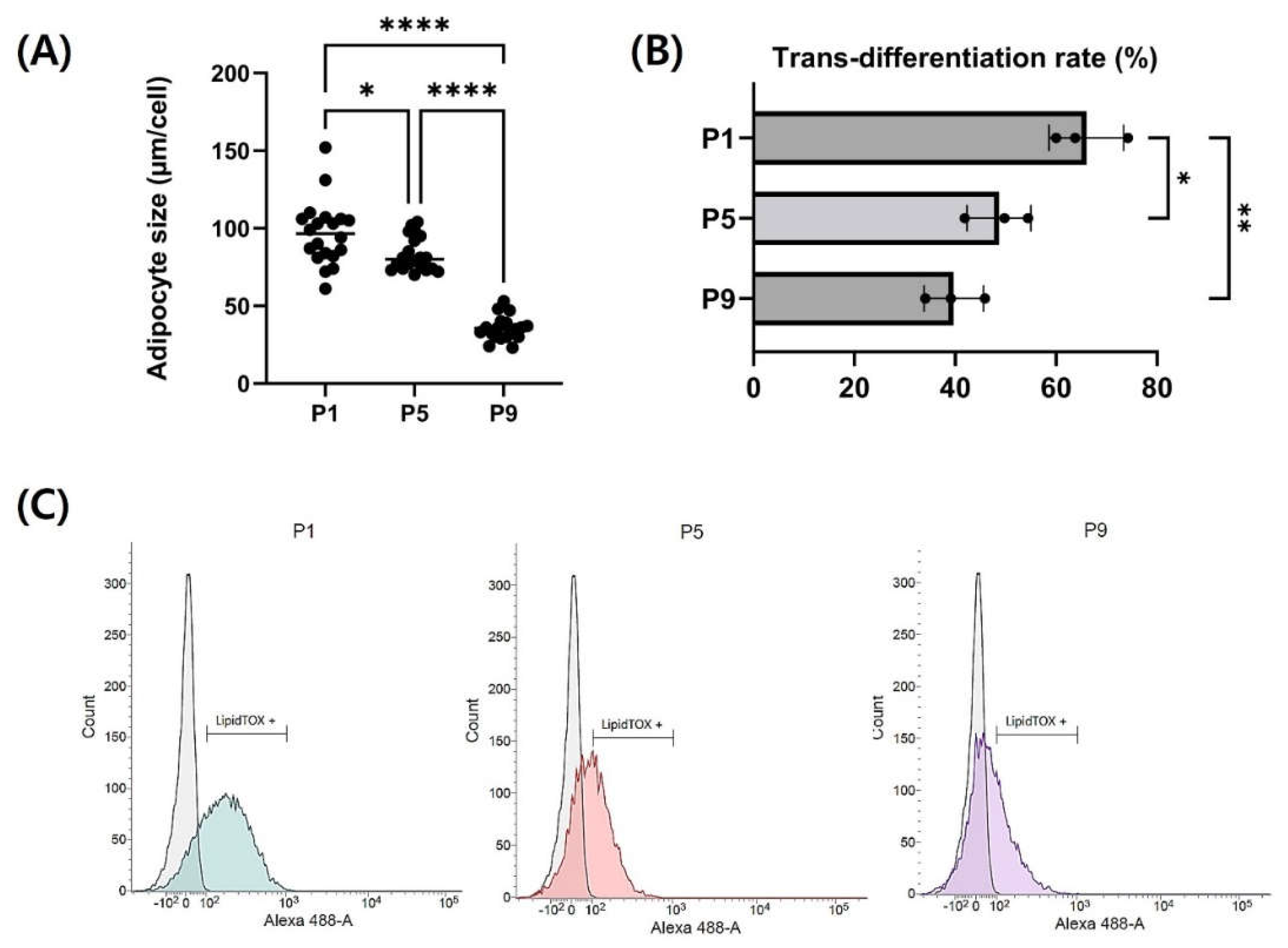
Each passage of CEF was induced for adipogenic trans-differentiation for a total of 14 days in DM (
Figure 5A). For the initial 3 days post-differentiation, a DM containing 400 µM of oleic acid was utilized, followed by differentiation induction with additional 5 µM and 10 µM rosiglitazone in order. Differentiated fibroblasts from each passage were stained with LipidTOX to assess the level of differentiation. Stained neutral lipids are presented in green (
Figure 5B). Particularly in passages 7 and 9, fewer lipid droplets were accumulated. The expression of LipidTOX was notably higher in passage 1, whereas its expression was observed at a lower rate in passage 9. The lipid droplet size of adipocytes from each three passages were measured and compared (
Figure 6A). Lipid droplet was also significantly the highest at Passage 1 (p < 0.05). These findings were consistent with those obtained through flow cytometry analysis (
Figure 6B and
Figure 6C). As passages increased, there was a significant decrease in neutral lipid accumulation within adipocytes (P < 0.05).
Oleic acid is a fatty acid capable of inducing differentiation of chicken preadipocytes into adipocytes [
26]. It has been reported that oleic acid can elevate lipid droplet accumulation and regulate the activity of glycerol 3-phosphate dehydrogenase (GPDH) involved in lipid synthesis [
37,
38]. It can also upregulate fatty acid-binding protein (FABP), a lipid mediator gene. Particularly, oleic acid or oleate is predominantly utilized in the adipogenic differentiation of mouse and chicken cells via trans-differentiation [
26]. During the initial three days of differentiation, 400 µM oleic acid was added to the DM. The addition of oleic acid induced morphological changes in fibroblasts, transitioning them into preadipocytes and starting lipid droplet formation. Large size of lipid droplet is specific morphologies of mature adipocytes [
39]. However, as fibroblasts maintained their fusiform shape and failed to accumulate sufficient lipid droplets, the addition of only oleic acid was insufficient to induce complete trans-differentiation into mature adipocytes. There was also previous study that the addition of oleic acid alone did not fully induce adipogenic trans-differentiation in chicken fibroblasts [
11]. There was a report that chicken preadipocytes can be induced to mature adipocytes with addition of rosiglitazone to DM [
40]. Rosiglitazone, a member of the thiazolidinedione family, functions as a peroxisome proliferator-activated receptor-gamma (PPARγ)-specific agonist capable of robustly inducing differentiation into adipocytes [
16,
24]. PPARγ acts as a master regulator of adipogenesis, playing a decisive role in adipocyte differentiation [
41,
42]. It has been reported that culturing chicken fibroblasts in differentiation media supplemented with rosiglitazone can significantly increase the activity of factors related to peroxisome proliferator response [
37,
40]. Hence, rosiglitazone was supplemented in later stages of differentiation to facilitate the differentiation of adipocytes into mature adipocytes in conjunction with oleate activity.
Figure 6.
Analysis of Adipogenic trans-differentiaion level of CEF at P1, P5, P9 (A) Differnece in adipocyte size by passage (B) and (C) Trans-differentiation level by passage with flow cytometry during long-term culture. The zone within the gate-bar means LipidTOX-positive cells. Data are presented as means plus standard deviation from three independent experiments. * and ** indicate significant differences at p < 0.05 and p < 0.01, respectively.
Figure 6.
Analysis of Adipogenic trans-differentiaion level of CEF at P1, P5, P9 (A) Differnece in adipocyte size by passage (B) and (C) Trans-differentiation level by passage with flow cytometry during long-term culture. The zone within the gate-bar means LipidTOX-positive cells. Data are presented as means plus standard deviation from three independent experiments. * and ** indicate significant differences at p < 0.05 and p < 0.01, respectively.
By the seventh day of differentiation, cells transformed from fusiform to round-shaped mature adipocytes laden with lipid droplets. Adipocytes from passage 1 exhibited relatively rounder and larger morphology compared to those from passage 9, attributing to an increase in lipid droplet size. Adipocyte from passage 9 exhibited a spindle shape like fibroblasts. This is attributed that fibroblast in higher passage possessing lower adipogenic differentiation capability compared to fibroblasts in lower passage, resulting in slower differentiation. Particularly when DM was applied to the cells, cell death occurred frequently in higher passages like passages 9, 11. This is believed to stem from the adipogenic induction substances and the DMSO used as their solvent in DM. It was supposed that cells at higher passages exhibited heightened sensitivity to these differentiation factors and have lower viability. The lower cells viability due to differentiation-inducing substances in the culture medium, along with the progressed passage, is likely a natural consequence of the senescence of primary cells. These results imply that decreased lipid droplet accumulation due to cell senescence in high passages can prevent complete differentiation. Similarly, decreases of adipogenesis were reported in a previous research study about human adipocytes due to activin A secreted from senescent cells [
43]. In this manner, it is essential to further investigate the causes of reduced adipogenesis due to cellular senescence in chickens. This will enhance our understanding of the mechanisms involved and potentially identify targets for mitigating the impact of senescence on adipose tissue function. The results of study also imply growth factor supplementation or use of specific cell lines needs to be considered to sustain the viability of primary fibroblasts during adipogenesis for cultured meat production.
5. Conclusions
In this study, CEF could be isolated by 2 hours of pre-plating. Primary CEF have decreased proliferation capacity from passage 11, while they could only survive up to passage 15. Stable proliferation and stemness of CEF were sustained up to passages 9 due to senescence of CEF. Cell senescence prevented complete adipogenic trans-differentiation of CEF. This means that as passage progresses, adipogenesis was decreased by senescence of primary cell. Also, to prevent decline of cultured fat yield derived from primary cell, growth factor supplementation or the usage of cell lines must be considered for cultured meat production.
Author Contributions
Conceptualization, So-Hee Kim.; methodology, So-Hee Kim.; validation, Young-Hwa Hwang.; formal analysis, So-Hee Kim.; investigation, So-Hee Kim.; resources, Young-Hwa Hwang.; data curation, Chan-Jin Kim, Eun-Yeong Lee.; writing—original draft preparation, So-Hee Kim.; writing—review and editing, Chan-Jin Kim, Seon-Tea Joo.; visualization, Chan-Jin Kim.; supervision, Seon-Tea Joo.; project administration, Seon-Tea Joo.; funding acquisition, Young-Hwa Hwang, Seon-Tea Joo.
Funding
This work was funded by the National Research Foundation of Korea (NRF), Korea government (MSIT) [No. 2020R1I1A2069379] and Korean Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through the Agri-Bioindustry Technology Development Program, funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA) [No. 321028–5].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available on request.
Conflicts of Interest
The authors do not have any conflict of interest in connection with the work submitted.
References
- Hossain, Md.J.; Alam, A.N.; Lee, E.-Y.; Hwang, Y.-H.; Joo, S.-T. Umami Characteristics and Taste Improvement Mechanism of Meat. Food Science of Animal Resources 2024, 44, 515–532. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.-H.; Lee, E.-Y.; Lim, H.-T.; Joo, S.-T. Multi-Omics Approaches to Improve Meat Quality and Taste Characteristics. Food Science of Animal Resources 2023, 43, 1067–1086. [Google Scholar] [CrossRef]
- Alam, A.M.M.; Kim, C.-J.; Kim, S.-H.; Kumari, S.; Lee, E.-Y.; Hwang, Y.-H.; Joo, S.-T. Scaffolding fundamentals and recent advances in sustainable scaffolding techniques for cultured meat development. Food Research International 2024, 189, 114549. [Google Scholar] [CrossRef] [PubMed]
- Bomkamp, C.; Skaalure, S.C.; Fernando, G.F.; Ben-Arye, T.; Swartz, E.W.; Specht, E.A. Scaffolding Biomaterials for 3D Cultivated Meat: Prospects and Challenges. Advanced Science 2022, 9, 2102908. [Google Scholar] [CrossRef]
- Kim, C.-J.; Kim, S.-H.; Lee, E.-Y.; Hwang, Y.-H.; Joo, S.-T. Effect of Chicken Age on the Proliferation and Differentiation Abilities of Muscle Stem Cells and Nutritional Characteristics of Cultured Meat Tissue. Food Science of Animal Resources 2024, in press. [Google Scholar] [CrossRef]
- Yun, S.H.; Lee, D.Y.; Lee, J.; Mariano, E.; Choi, Y.; Park, J.; Han, D.; Kim, J.S.; Hur, S.J. Current Research, Industrialization Status, and Future Perspective of Cultured Meat. Food Science of Animal Resources 2024, 44, 326–355. [Google Scholar] [CrossRef]
- Joo, S.-T.; Choi, J.-S.; Hur, S.-J.; Kim, G.-D.; Kim, C.-J.; Lee, E.-Y.; Bakhsh, A.; Hwang, Y.-H. A Comparative Study on the Taste Characteristics of Satellite Cell Cultured Meat Derived from Chicken and Cattle Muscles. Food Science of Animal Resources 2022, 42, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.-M.; Lee, D.B.; Kim, H.-Y. Industrial Research and Development on the Production Process and Quality of Cultured Meat Hold Significant Value: A Review. Food Science of Animal Resources 2024, 44, 499–514. [Google Scholar] [CrossRef] [PubMed]
- Yuen, J.S.K., Jr.; Saad, M.K.; Xiang, N.; Barrick, B.M.; DiCindio, H.; Li, C.; Zhang, S.W.; Rittenberg, M.; Lew, E.T.; Zhang, K.L.; Leung, G.; Pietropinto, J.A.; Kaplan, D.L. Aggregating in vitro-grown adipocytes to produce macroscale cell-cultured fat tissue with tunable lipid compositions for food applications. eLife 2023, 12, e82120. [Google Scholar] [CrossRef]
- Yuen, J.S.K., Jr.; Stout, A.J.; Kawecki, N.S.; Letcher, S.M.; Theodossiou, S.K.; Cohen, J.M.; Barrick, B.M.; Saad, M.K.; Rubio, N.R.; Pietropinto, J.A.; DiCindio, H.; Zhang, S.W.; Rowat, A.C.; Kaplan, D.L. Perspectives on scaling production of adipose tissue for food applications. Biomaterials 2022, 280, 121273. [Google Scholar] [CrossRef] [PubMed]
- Pasitka, L.; Cohen, M.; Ehrlich, A.; Gildor, B.; Reuveni, E.; Ayyash, M.; Wissotsky, G.; Herscovici, A.; Kaminker, R.; Niv, A.; Bitcover, R.; Dadia, O.; Rudik, A.; Voloschin, A.; Shimoni, M.; Cinnamon, Y.; Nahmias, Y. Spontaneous immortalization of chicken fibroblasts generates stable, high-yield cell lines for serum-free production of cultured meat. Nature Food 2022, 4, 35–50. [Google Scholar] [CrossRef]
- Zagury, Y.; Ianovici, I.; Landau, S.; Lavon, N.; Levenberg, S. Engineered marble-like bovine fat tissue for cultured meat. Communications Biology 2022, 5, 927. [Google Scholar] [CrossRef] [PubMed]
- Cieślar-Pobuda, A.; Knoflach, V.; Ringh, M.V.; Stark, J.; Likus, W.; Siemianowicz, K.; Ghavami, S.; Hudecki, A.; Green, J.L.; Łos, M.J. Transdifferentiation and reprogramming: Overview of the processes, their similarities and differences. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research 2017, 1864, 1359–1369. [Google Scholar] [CrossRef]
- Merrell, A.J.; Stanger, B.Z. Adult cell plasticity in vivo: De-differentiation and transdifferentiation are back in style. Nature Reviews Molecular Cell Biology 2016, 17, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Clabaut, A.; Grare, C.; Rolland-Valognes, G.; Letarouilly, J.-G.; Bourrier, C.; Andersen, T.L.; Sikjær, T.; Rejnmark, L.; Ejersted, C.; Pastoureau, P.; Hardouin, P.; Sabatini, M.; Broux, O. Adipocyte-induced transdifferentiation of osteoblasts and its potential role in age-related bone loss. PLOS ONE 2021, 16, e0245014. [Google Scholar] [CrossRef]
- Hu, E.; Tontonoz, P.; Spiegelman, B.M. Transdifferentiation of myoblasts by the adipogenic transcription factors PPARy and C/EBPa! Proc. Natl. Acad. Sci. USA 1995. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Wang, F.; Liu, Y.; Li, S.; Zhou, G.; Hu, P. Characterization and isolation of highly purified porcine satellite cells. Cell Death Discovery 2017, 3, 17003. [Google Scholar] [CrossRef] [PubMed]
- Baquero-Perez, B.; Kuchipudi, S.V.; Nelli, R.K.; Chang, K.-C. A simplified but robust method for the isolation of avian and mammalijan muscle satellite cells. BMC Cell Biology 2012, 13, 16. [Google Scholar] [CrossRef]
- Choi, K.-H.; Kim, M.; Yoon, J.W.; Jeong, J.; Ryu, M.; Jo, C.; Lee, C.-K. Purification of Pig Muscle Stem Cells Using Magnetic-Activated Cell Sorting (MACS) Based on the Expression of Cluster of Differentiation 29 (CD29). Food Science of Animal Resources 2020, 40, 852–859. [Google Scholar] [CrossRef]
- Matsuyoshi, Y.; Akahoshi, M.; Nakamura, M.; Tatsumi, R.; Mizunoya, W. Isolation and Purification of Satellite Cells from Young Rats by Percoll Density Gradient Centrifugation. In Myogenesis; Rønning, S.B., Ed.; Springer: New York, 2019; Vol. 1889, pp. 81–93. [Google Scholar] [CrossRef]
- Yoshioka, K.; Kitajima, Y.; Okazaki, N.; Chiba, K.; Yonekura, A.; Ono, Y. A Modified Pre-plating Method for High-Yield and High-Purity Muscle Stem Cell Isolation From Human/Mouse Skeletal Muscle Tissues. Frontiers in Cell and Developmental Biology 2020, 8, 793. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Kim, C.-J.; Lee, E.-Y.; Son, Y.-M.; Hwang, Y.-H.; Joo, S.-T. Optimal Pre-Plating Method of Chicken Satellite Cells for Cultured Meat Production. Food Science of Animal Resources 2022, 42, 942–952. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, A.; Cera, G.; De Meo, D.; Villani, C.; Bouche, M.; Lozanoska-Ochser, B. A novel approach for the isolation and long-term expansion of pure satellite cells based on ice-cold treatment. Skeletal Muscle 2021, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Song, W.; Bassey, A.P.; Tang, C.; Li, H.; Ding, S.; Zhou, G. Preparation and Quality Evaluation of Cultured Fat. Journal of Agricultural and Food Chemistry 2023, 71, 4113–4122. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, Y.; Wang, L.; Wang, N.; Li, Y.; Li, H. Transdifferentiation of fibroblasts into adipocyte-like cells by chicken adipogenic transcription factors. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 2010, 156, 502–508. [Google Scholar] [CrossRef]
- Liu, S.; Wang, L.; Wang, N.; Wang, Y.; Shi, H.; Li, H. Oleate induces transdifferentiation of chicken fibroblasts into adipocyte-like cells. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 2009, 154, 135–141. [Google Scholar] [CrossRef]
- Sugathan, S.; Lee, S.-J.; Shiwani, S.; Singh, N.K. Transdifferentiation of bovine epithelial cells towards adipocytes in the presence of myoepithelium. Asian-Australasian Journal of Animal Sciences 2020, 33, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, I.R.; Russo, F.B.; Pignatari, G.C.; Evangelinellis, M.M.; Tavolari, S.; Muotri, A.R.; Beltrão-Braga, P.C.B. Fibroblast sources: Where can we get them? Cytotechnology 2016, 68, 223–228. [Google Scholar] [CrossRef]
- Plikus, M.V.; Wang, X.; Sinha, S.; Forte, E.; Thompson, S.M.; Herzog, E.L.; Driskell, R.R.; Rosenthal, N.; Biernaskie, J.; Horsley, V. Fibroblasts: Origins, definitions, and functions in health and disease. Cell 2021, 184, 3852–3872. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S.; Hanwate, M.; Deb, K.; Sharma, V.; Totey, S. FGF2 secreting human fibroblast feeder cells: A novel culture system for human embryonic stem cells. Molecular Reproduction and Development 2008, 75, 1523–1532. [Google Scholar] [CrossRef]
- Kim, C.-J.; Kim, S.-H.; Lee, E.-Y.; Son, Y.-M.; Bakhsh, A.; Hwang, Y.-H.; Joo, S.-T. Optimal temperature for culturing chicken satellite cells to enhance production yield and umami intensity of cultured meat. Food Chemistry Advances 2023, 2, 100307. [Google Scholar] [CrossRef]
- Allouh, M.Z.; Yablonka-Reuveni, Z.; Rosser, B.W.C. Pax7 Reveals a Greater Frequency and Concentration of Satellite Cells at the Ends of Growing Skeletal Muscle Fibers. Journal of Histochemistry & Cytochemistry 2008, 56, 77–87. [Google Scholar] [CrossRef]
- Zammit, P.S.; Relaix, F.; Nagata, Y.; Ruiz, A.P.; Collins, C.A.; Partridge, T.A.; Beauchamp, J.R. Pax7 and myogenic progression in skeletal muscle satellite cells. Journal of Cell Science 2006, 119, 1824–1832. [Google Scholar] [CrossRef]
- Schmidt, M.; Schüler, S.C.; Hüttner, S.S.; von Eyss, B.; von Maltzahn, J. Adult stem cells at work: Regenerating skeletal muscle. Cellular and Molecular Life Sciences 2019, 76, 2559–2570. [Google Scholar] [CrossRef]
- Zhao, R.; Jin, J.; Sun, X.; Jin, K.; Wang, M.; Ahmed, M.F.; Zuo, Q.; Zhang, Y.; Zhao, Z.; Chen, G.; Li, B. The establishment of clonally derived chicken embryonic fibroblast cell line (CSC) with high transfection efficiency and ability as a feeder cell. Journal of Cellular Biochemistry 2018, 119, 8841–8850. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Lee, D.Y.; Mariano, E.J.; Yun, S.H.; Lee, J.; Park, J.; Choi, Y.; Han, D.; Kim, J.S.; Joo, S.-T.; Hur, S.J. Study on the current research trends and future agenda in animal products: An Asian perspective. Journal of Animal Science and Technology 2023, 65, 1124–1150. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, Y.; Sato, K.; Ishii, H.; Akiba, Y. Changes in mRNA expression of regulatory factors involved in adipocyte differentiation during fatty acid induced adipogenesis in chicken. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 2005, 141, 108–115. [Google Scholar] [CrossRef]
- Shi, H.; Zhang, Q.; Wang, Y.; Yang, P.; Wang, Q.; Li, H. Chicken adipocyte fatty acid-binding protein knockdown affects expression of peroxisome proliferator-activated receptor γ gene during oleate-induced adipocyte differentiation. Poultry Science 2011, 90, 1037–1044. [Google Scholar] [CrossRef]
- Rizzatti, V.; Boschi, F.; Pedrotti, M.; Zoico, E.; Sbarbati, A.; Zamboni, M. Lipid droplets characterization in adipocyte differentiated 3T3-L1 cells: Size and optical density distribution. European Journal of Histochemistry 2013, 57, 24. [Google Scholar] [CrossRef]
- Cheng, B.; Wu, M.; Xu, S.; Zhang, X.; Wang, Y.; Wang, N.; Leng, L.; Li, H. Cocktail supplement with rosiglitazone: A novel inducer for chicken preadipocyte differentiation in vitro. Bioscience Reports 2016, 36, e00401. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, S.; Sharma, S.; Gupta, P.; Saini, A.; Kaushal, C. The peroxisome proliferator-activated receptor: A family of nuclear receptors role in various diseases. Journal of Advanced Pharmaceutical Technology & Research 2011, 2, 236. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, C.; Sheng, X.; Gong, Z.; Zang, Y.Q. Lecithin promotes adipocyte differentiation and hepatic lipid accumulation. International Journal of Molecular Medicine 2009, 23. [Google Scholar] [CrossRef]
- Xu, M.; Palmer, A.K.; Ding, H.; Weivoda, M.M.; Pirtskhalava, T.; White, T.A.; Sepe, A.; Johnson, K.O.; Stout, M.B.; Giorgadze, N.; Jensen, M.D. Targeting senescent cells enhances adipogenesis and metabolic function in old age. eLife 2015, 4, e12997. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).