Submitted:
23 August 2024
Posted:
25 August 2024
You are already at the latest version
Abstract
Keywords:
Introduction
Materials and Methods
Patients
Diagnosis of Lymph Node Metastasis
Statistical Analysis
Results
Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
List of Abbreviations
References
- Lamarca, A.; Edeline, J.; Goyal, L. How I treat biliary tract cancer. ESMO Open 2022, 7, 100378. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, Y.; Nishio, H.; Ebata, T.; Igami, T.; Sugawara, G.; Nagino, M. Value of indocyanine green clearance of the future liver remnant in predicting outcome after resection for biliary cancer. Br. J. Surg. 2010, 97, 1260–1268. [Google Scholar] [CrossRef] [PubMed]
- Kato, A.; Shimizu, H.; Ohtsuka, M.; Yoshitomi, H.; Furukawa, K.; Takayashiki, T.; Nakadai, E.; Kishimoto, T.; Nakatani, Y.; Yoshidome, H.; et al. Downsizing Chemotherapy for Initially Unresectable Locally Advanced Biliary Tract Cancer Patients Treated with Gemcitabine Plus Cisplatin Combination Therapy Followed by Radical Surgery. Ann. Surg. Oncol. 2015, 22, 1093–1099. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, S.; Horiguchi, A.; Miyakawa, S.; Endo, I.; Miyazaki, M.; Takada, T. Biliary tract cancer registry in Japan from 2008 to 2013. J. Hepato-Biliary-Pancreatic Sci. 2015, 23, 149–157. [Google Scholar] [CrossRef]
- Nagino, M.; Ebata, T.; Yokoyama, Y.; Igami, T.; Sugawara, G.; Takahashi, Y.; Nimura, Y. Evolution of Surgical Treatment for Perihilar Cholangiocarcinoma. Ann. Surg. 2013, 258, 129–140. [Google Scholar] [CrossRef]
- Endo, I.; Shimada, H.; Tanabe, M.; Fujii, Y.; Takeda, K.; Morioka, D.; Tanaka, K.; Sekido, H.; Togo, S. Prognostic Significance of the Number of Positive Lymph Nodes in Gallbladder Cancer. J. Gastrointest. Surg. 2006, 10, 999–1007. [Google Scholar] [CrossRef]
- Sierzega, M.; Nowak, K.; Kulig, J.; Matyja, A.; Nowak, W.; Popiela, T. Lymph node involvement in ampullary cancer: The importance of the number, ratio, and location of metastatic nodes. J. Surg. Oncol. 2009, 100, 19–24. [Google Scholar] [CrossRef]
- Ganeshalingam, S.; Koh, D.-M. Nodal staging. Cancer Imaging 2009, 9, 104–11. [Google Scholar] [CrossRef]
- Murakami, Y.; Uemura, K.; Hayashidani, Y.; Sudo, T.; Ohge, H.; Sueda, T. Pancreatoduodenectomy for Distal Cholangiocarcinoma: Prognostic Impact of Lymph Node Metastasis. World J. Surg. 2006, 31, 337–342. [Google Scholar] [CrossRef]
- Sierzega, M.; Nowak, K.; Kulig, J.; Matyja, A.; Nowak, W.; Popiela, T. Lymph node involvement in ampullary cancer: The importance of the number, ratio, and location of metastatic nodes. J. Surg. Oncol. 2009, 100, 19–24. [Google Scholar] [CrossRef]
- Matsuyama, R.; Morioka, D.; Mori, R.; Yabushita, Y.; Hiratani, S.; Ota, Y.; Kumamoto, T.; Endo, I. Our Rationale of Initiating Neoadjuvant Chemotherapy for Hilar Cholangiocarcinoma: A Proposal of Criteria for “Borderline Resectable” in the Field of Surgery for Hilar Cholangiocarcinoma. World J. Surg. 2018, 43, 1094–1104. [Google Scholar] [CrossRef]
- Matsuyama, R.; Mori, R.; Ota, Y.; Homma, Y.; Yabusita, Y.; Hiratani, S.; Murakami, T.; Sawada, Y.; Miyake, K.; Shimizu, Y.; et al. Impact of Gemcitabine Plus S1 Neoadjuvant Chemotherapy on Borderline Resectable Perihilar Cholangiocarcinoma. Ann. Surg. Oncol. 2022, 29, 2393–2405. [Google Scholar] [CrossRef]
- Morimoto, H.; Ajiki, T.; Ueda, T.; Sawa, H.; Fujita, T.; Matsumoto, I.; Yasuda, T.; Fujino, Y.; Kuroda, Y.; Ku, Y. Histological features of lymph node metastasis in patients with biliary tract cancer. J. Surg. Oncol. 2008, 97, 423–427. [Google Scholar] [CrossRef]
- Sugai, K.; Sekine, Y.; Kawamura, T.; Yanagihara, T.; Saeki, Y.; Kitazawa, S.; Kobayashi, N.; Kikuchi, S.; Goto, Y.; Ichimura, H.; et al. Sphericity of lymph nodes using 3D-CT predicts metastasis in lung cancer patients. Cancer Imaging 2023, 23, 1–8. [Google Scholar] [CrossRef]
- Ganeshalingam, S.; Koh, D.-M. Nodal staging. Cancer Imaging 2009, 9, 104–11. [Google Scholar] [CrossRef] [PubMed]
- Noji, T.; Kondo, S.; Hirano, S.; Tanaka, E.; Suzuki, O.; Shichinohe, T. Computed tomography evaluation of regional lymph node metastases in patients with biliary cancer. Br. J. Surg. 2007, 95, 92–96. [Google Scholar] [CrossRef]
- Promsorn, J.; Soontrapa, W.; Somsap, K.; Chamadol, N.; Limpawattana, P.; Harisinghani, M. Evaluation of the diagnostic performance of apparent diffusion coefficient (ADC) values on diffusion-weighted magnetic resonance imaging (DWI) in differentiating between benign and metastatic lymph nodes in cases of cholangiocarcinoma. Abdom. Imaging 2018, 44, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Kurosawa, J.; Tawada, K.; Mikata, R.; Ishihara, T.; Tsuyuguchi, T.; Saito, M.; Shimofusa, R.; Yoshitomi, H.; Ohtsuka, M.; Miyazaki, M.; et al. Prognostic relevance of apparent diffusion coefficient obtained by diffusion-weighted MRI in pancreatic cancer. J. Magn. Reson. Imaging 2015, 42, 1532–1537. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Lee, J.M.; Kim, H.; Yoon, J.H.; Han, J.K.; Choi, B.I. Role of diffusion-weighted magnetic resonance imaging in the diagnosis of gallbladder cancer. J. Magn. Reson. Imaging 2012, 38, 127–137. [Google Scholar] [CrossRef]
- Yu, M.H.; Kim, Y.J.; Park, H.S.; Jung, S.I. Benign gallbladder diseases: Imaging techniques and tips for differentiating with malignant gallbladder diseases. World J. Gastroenterol. 2020, 26, 2967–2986. [Google Scholar] [CrossRef]
- Yoo, R.-E.; Lee, J.M.; Yoon, J.H.; Kim, J.H.; Han, J.K.; Choi, B.I. Differential diagnosis of benign and malignant distal biliary strictures: Value of adding diffusion-weighted imaging to conventional magnetic resonance cholangiopancreatography. J. Magn. Reson. Imaging 2013, 39, 1509–1517. [Google Scholar] [CrossRef]
- Miyazaki, K.; Morine, Y.; Yamada, S.; Saito, Y.; Tokuda, K.; Okikawa, S.; Yamashita, S.; Oya, T.; Ikemoto, T.; Imura, S.; et al. Stromal tumor-infiltrating lymphocytes level as a prognostic factor for resected intrahepatic cholangiocarcinoma and its prediction by apparent diffusion coefficient. Int. J. Clin. Oncol. 2021, 26, 2265–2274. [Google Scholar] [CrossRef] [PubMed]
- Holzapfel, K.; Gaa, J.; Schubert, E.C.; Eiber, M.; Kleeff, J.; Rummeny, E.J.; Loos, M. Value of diffusion-weighted MR imaging in the diagnosis of lymph node metastases in patients with cholangiocarcinoma. Abdom. Imaging 2016, 41, 1937–1941. [Google Scholar] [CrossRef]
- Lee, J.H.; Han, S.-S.; Hong, E.K.; Cho, H.J.; Joo, J.; Park, E.Y.; Woo, S.M.; Kim, T.H.; Lee, W.J.; Park, S.-J. Predicting lymph node metastasis in pancreatobiliary cancer with magnetic resonance imaging: A prospective analysis. Eur. J. Radiol. 2019, 116, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mandriota, S.J.; Jussila, L.; Jeltsch, M.; Compagni, A.; Baetens, D.; Prevo, R.; Banerji, S.; Huarte, J.; Montesano, R.; Jackson, D.G.; et al. Vascular endothelial growth factor-C-mediated lymphangiogenesis promotes tumour metastasis. EMBO J. 2001, 20, 672–682. [Google Scholar] [CrossRef]
- Skobe, M.; Hawighorst, T.; Jackson, D.G.; Prevo, R.; Janes, L.; Velasco, P.; Riccardi, L.; Alitalo, K.; Claffey, K.; Detmar, M. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat. Med. 2001, 7, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Stacker, S.A.; Caesar, C.; Baldwin, M.E.; Thornton, G.E.; Williams, R.A.; Prevo, R.; Jackson, D.G.; Nishikawa, S.-I.; Kubo, H.; Achen, M.G. VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nat. Med. 2001, 7, 186–191. [Google Scholar] [CrossRef]
- Pastushenko, I.; Blanpain, C. EMT Transition States during Tumor Progression and Metastasis. Trends Cell Biol. 2019, 29, 212–226. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.H.; Yang, J. Epithelial–mesenchymal plasticity in carcinoma metastasis. Genes Dev. 2013, 27, 2192–2206. [Google Scholar] [CrossRef]
- Karaman, S.; Detmar, M. Mechanisms of lymphatic metastasis. J. Clin. Investig. 2014, 124, 922–928. [Google Scholar] [CrossRef]
- Nathanson, S.D.; Mahan, M. Sentinel Lymph Node Pressure in Breast Cancer. Ann. Surg. Oncol. 2011, 18, 3791–3796. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.; Pereira, E.R.; Padera, T.P. Growth and Immune Evasion of Lymph Node Metastasis. Front. Oncol. 2018, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Beasley, N.J.P.; Prevo, R.; Banerji, S.; Leek, R.D.; Moore, J.; Van Trappen, P.; Cox, G.; Harris, A.L.; Jackson, D.G. Intratumoral lymphangiogenesis and lymph node metastasis in head and neck cancer. Cancer Res. 2002, 62, 1315–20. [Google Scholar] [PubMed]
- Zhou, H.; Lei, P.-J.; Padera, T.P. Progression of Metastasis through Lymphatic System. Cells 2021, 10, 627. [Google Scholar] [CrossRef]
- Hosokawa, I.; Hayano, K.; Furukawa, K.; Takayashiki, T.; Kuboki, S.; Takano, S.; Matsubara, H.; Miyazaki, M.; Ohtsuka, M. Preoperative Diagnosis of Lymph Node Metastasis of Perihilar Cholangiocarcinoma Using Diffusion-Weighted Magnetic Resonance Imaging. Ann. Surg. Oncol. 2022, 29, 5502–5510. [Google Scholar] [CrossRef]
- Morine, Y.; Shimada, M.; Imura, S.; Ikemoto, T.; Hanaoka, J.; Kanamoto, M.; Ishibashi, H.; Utsunomiya, T. Detection of Lymph Nodes Metastasis in Biliary Carcinomas: Morphological Criteria by MDCT and the Clinical Impact of DWI-MRI. Hepatogastroenterology 2015, 62, 777–81. [Google Scholar]
- Kobayashi, S.; Nagano, H.; Hoshino, H.; Wada, H.; Marubashi, S.; Eguchi, H.; Takeda, Y.; Tanemura, M.; Kim, T.; Shimosegawa, E.; et al. Diagnostic value of FDG-PET for lymph node metastasis and outcome of surgery for biliary cancer. J. Surg. Oncol. 2010, 103, 223–229. [Google Scholar] [CrossRef]
- Shuto, K.; Kono, T.; Shiratori, T.; Akutsu, Y.; Uesato, M.; Mori, M.; Narushima, K.; Imanishi, S.; Nabeya, Y.; Yanagawa, N.; et al. Diagnostic performance of diffusion-weighted magnetic resonance imaging in assessing lymph node metastasis of esophageal cancer compared with PET. Esophagus 2019, 17, 239–249. [Google Scholar] [CrossRef]
- Yoo, J.; Lee, J.M.; Yoon, J.H.; Joo, I.; Lee, D.H. Additional Value of Integrated 18F-FDG PET/MRI for Evaluating Biliary Tract Cancer: Comparison with Contrast-Enhanced CT. Korean J. Radiol. 2021, 22, 714–724. [Google Scholar] [CrossRef]
- Zhan, P.-C.; Yang, T.; Zhang, Y.; Liu, K.-Y.; Li, Z.; Zhang, Y.-Y.; Liu, X.; Liu, N.-N.; Wang, H.-X.; Shang, B.; et al. Radiomics using CT images for preoperative prediction of lymph node metastasis in perihilar cholangiocarcinoma: a multi-centric study. Eur. Radiol. 2023, 34, 1280–1291. [Google Scholar] [CrossRef]
- Ji, G.-W.; Zhu, F.-P.; Liu, X.-S.; Wu, F.-Y.; Wang, K.; Xia, Y.-X.; Zhang, Y.-D.; Jiang, W.-J.; Li, X.-C.; Wang, X.-H. A radiomics approach to predict lymph node metastasis and clinical outcome of intrahepatic cholangiocarcinoma. Eur. Radiol. 2019, 29, 3725–3735. [Google Scholar] [CrossRef] [PubMed]
- Bian, Y.; Zheng, Z.; Fang, X.; Jiang, H.; Zhu, M.; Yu, J.; Zhao, H.; Zhang, L.; Yao, J.; Lu, L.; et al. Artificial Intelligence to Predict Lymph Node Metastasis at CT in Pancreatic Ductal Adenocarcinoma. Radiology 2023, 306, 160–169. [Google Scholar] [CrossRef]
- Bedrikovetski, S.; Dudi-Venkata, N.N.; Kroon, H.M.; Seow, W.; Vather, R.; Carneiro, G.; Moore, J.W.; Sammour, T. Artificial intelligence for pre-operative lymph node staging in colorectal cancer: a systematic review and meta-analysis. BMC Cancer 2021, 21, 1–10. [Google Scholar] [CrossRef]
- Wang, C.; Yu, P.; Zhang, H.; Han, X.; Song, Z.; Zheng, G.; Wang, G.; Zheng, H.; Mao, N.; Song, X. Artificial intelligence–based prediction of cervical lymph node metastasis in papillary thyroid cancer with CT. Eur. Radiol. 2023, 33, 6828–6840. [Google Scholar] [CrossRef]
- Chu, L.C.; Fishman, E.K. Artificial Intelligence Outperforms Radiologists for Pancreatic Cancer Lymph Node Metastasis Prediction at CT. Radiology 2023, 306, 170–171. [Google Scholar] [CrossRef]
- Kasai, S.; Shiomi, A.; Kagawa, H.; Hino, H.; Manabe, S.; Yamaoka, Y.; Chen, K.; Nanishi, K.; Kinugasa, Y. The Effectiveness of Machine Learning in Predicting Lateral Lymph Node Metastasis From Lower Rectal Cancer: A Single Center Development and Validation Study. Ann. Gastroenterol. Surg. 2021, 6, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Wang, L.; Wu, C.; Wei, Y.; Zhang, Y.; Chen, J. Preoperative Prediction of Lymph Node Metastasis of Pancreatic Ductal Adenocarcinoma Based on a Radiomics Nomogram of Dual-Parametric MRI Imaging. Front. Oncol. 2022, 12, 927077. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Pu, H.; Chen, X.-L.; Yuan, Y.; Zhang, F.; Li, H. MRI radiomics signature to predict lymph node metastasis after neoadjuvant chemoradiation therapy in locally advanced rectal cancer. Abdom. Imaging 2023, 48, 2270–2283. [Google Scholar] [CrossRef]
- Yamamoto, H. Micrometastasis in lymph nodes of colorectal cancer. Ann. Gastroenterol. Surg. 2022, 6, 466–473. [Google Scholar] [CrossRef]
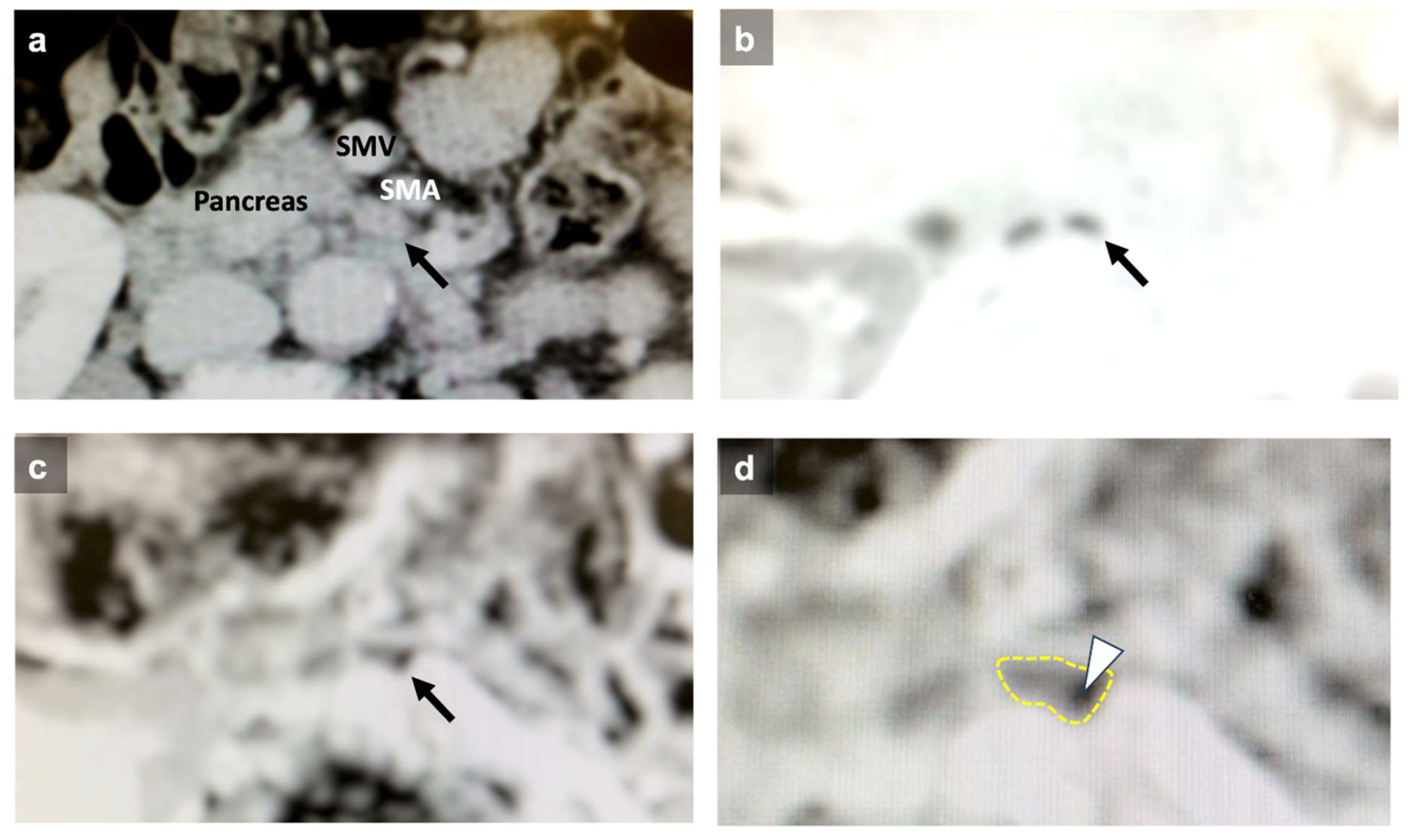
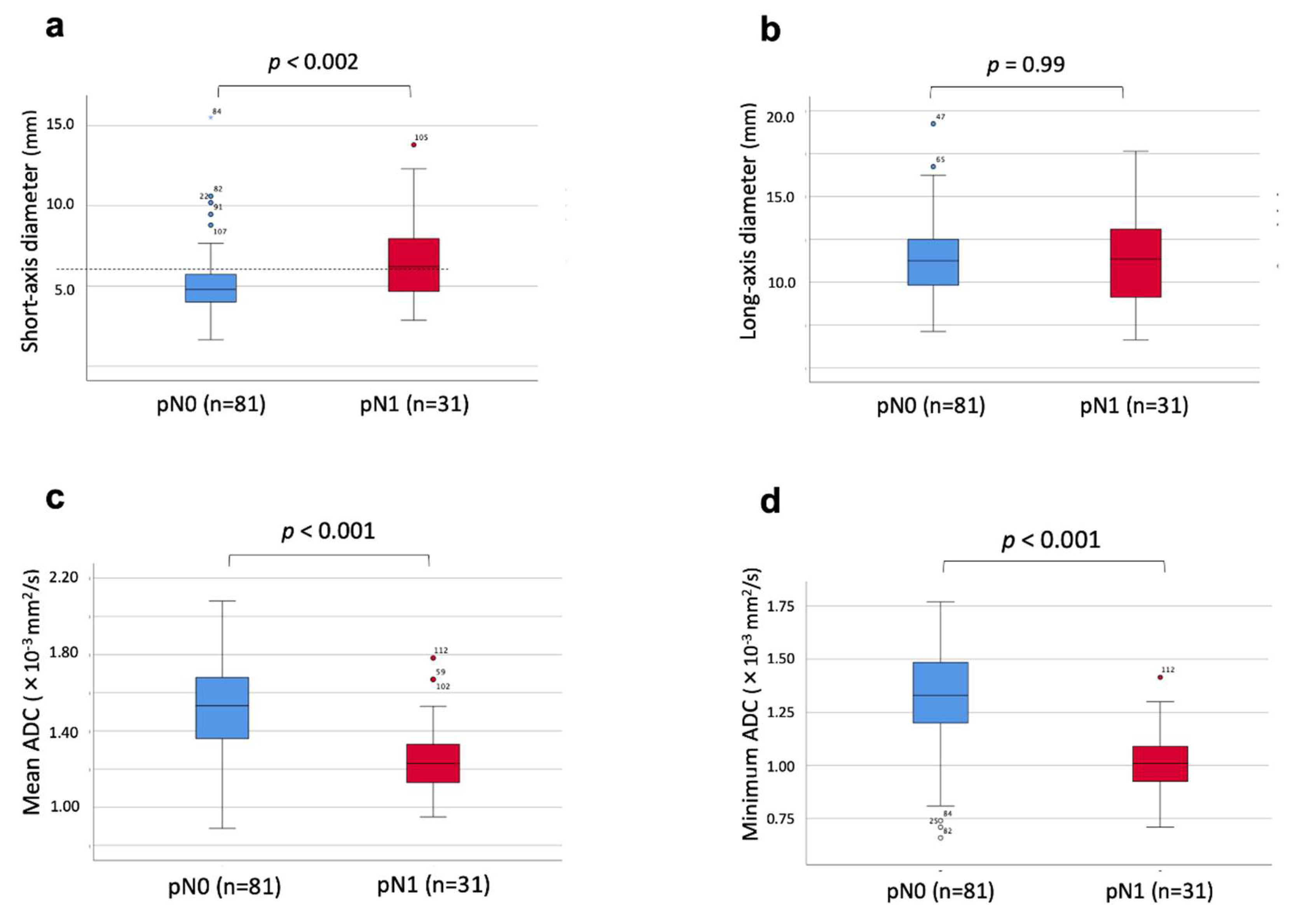
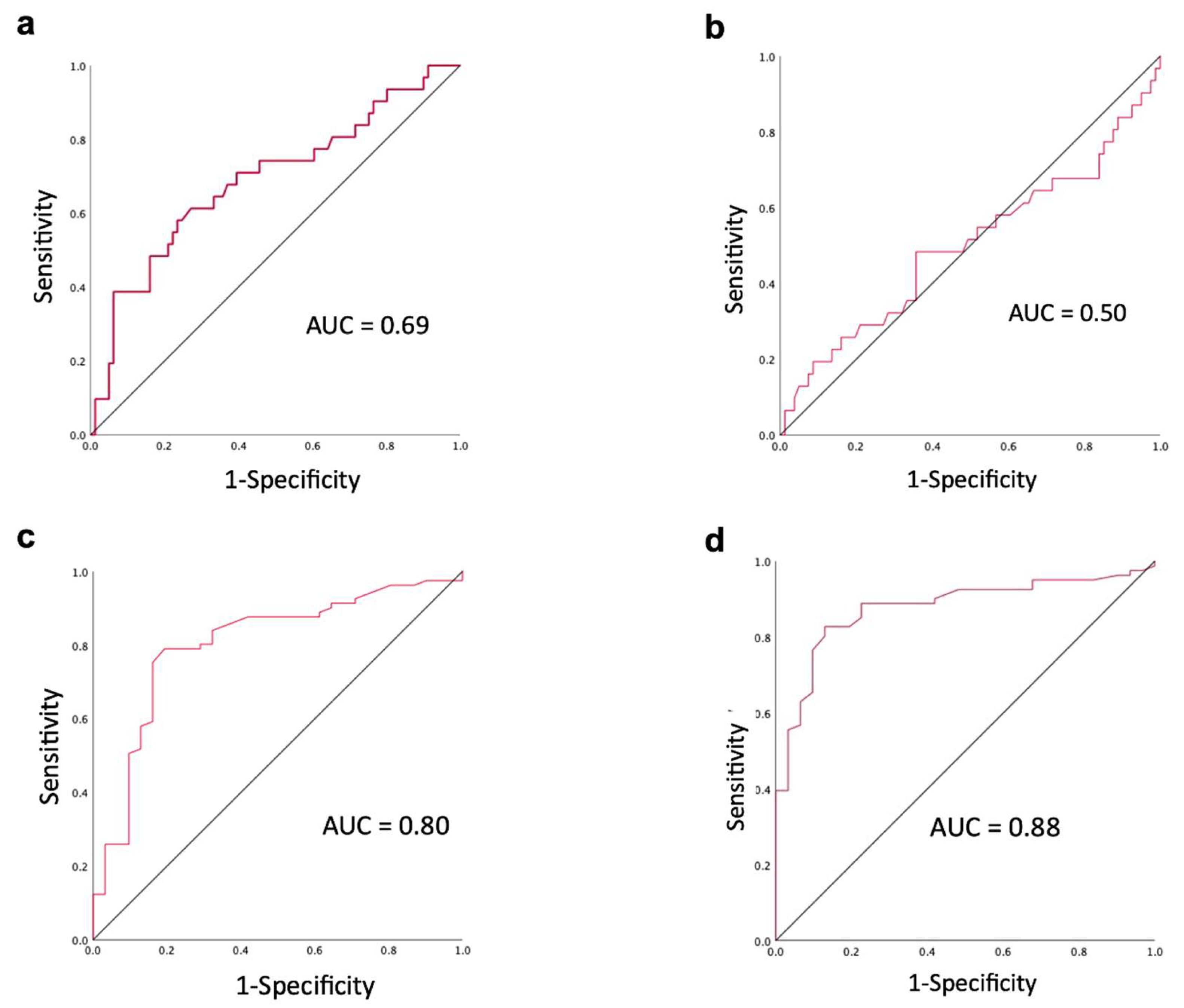
| Factors | |
|---|---|
| Age, years (mean ± SD) | 73 ± 8 |
| Sex Male Female |
21 14 |
| Anatomical tumor location Intrahepatic cholangiocarcinoma Perihilar cholangiocarcinoma Distal bile duct cancer Gallbladder cancer Ampullary carcinoma |
1 10 10 10 4 |
| Operative procedure Right hemihepatectomy + caudate lobectomy + BDR Left hemihepatectomy + caudate lobectomy + BDR Hepatectomy of segment 4a+5 + BDR Hepatic segmentectomy + BDR Pancreatoduodenectomy |
6 6 8 1 14 |
| Preoperative cholangitis Yes No |
11 24 |
| Lymph nodes to be analyzed pN+ pN- |
112 31 81 |
| Locations of lymph nodes | pN+ | pN- | Total |
|---|---|---|---|
| Common hepatic artery | 4 | 19 | 23 |
| Hepatoduodenal ligament | 9 | 49 | 58 |
| Pancreatic head | 12 | 8 | 20 |
| Jejunal mesentery | 2 | 2 | 4 |
| Para-aorta | 3 | 3 | 6 |
| Diaphragm | 1 | 0 | 1 |
| Total | 31 | 81 | 112 |
| Diagnostic criteria for lymph node metastasis | Sensitivity | Specificity | Accuracy |
|---|---|---|---|
| Short-axis diameter > 5.785 mm | 58.1% | 75.3% | 70.5% |
| Mean ADC < 1.345x10-3mm2/s | 80.6% | 79.0% | 79.5% |
| Minimum ADC < 1.14 x10-3mm2/s | 87.1% | 82.7% | 83.9% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





