1. Introduction
Bioprinting is an excellent tool for the production and study of artificial tissues, a potential tool in skin regeneration [
1]. Compared to typical skin regeneration methods, bioprinting offers better automation and standardization for clinical uses and accuracy in the incorporation of living cells, growth factors, and other biomolecules [
2]. Generally, the main advantage of bioprinting over other techniques is the ability to print not only scaffolds of a specific, personalized shape, but also a properly designed internal network in which all processes necessary for regeneration and tissue or organ formation occur.
However, one of the major challenges remains the development of bioink and the application of the appropriate crosslinking technique, which will allow obtaining an object with specific physicochemical and biological properties. Such a bioink must be printable, i.e., have appropriate rheological parameters, and the obtained scaffolds must be mechanically stable and biocompatible, i.e., provide an appropriate environment in which cells will survive, proliferate, migrate, and differentiate, forming tissues [
3,
4]. These three parameters determine the quality of the bioink, which, as it turns out, are contradictory because the higher the viscosity of the bioink, the better the printability, and conversely, the worse the biocompatibility. Therefore, already at the stage of designing the bioink and determining its application, it is necessary to focus research and find a compromise between these properties [
5].
An example of systems that seem to possess both good physical properties and are biocompatible are hydrogels, which form a three-dimensional network of hydrophilic polymer chains. Additionally, hydrogels can effectively recreate the extracellular matrix and the natural environment of cells. They also have mechanical properties similar to tissues and can provide cells with structural support and hydration. Another desirable property of hydrogels is shear-thinning, which allows the extrusion of bioinks under high shear stress while maintaining the mechanical properties of the system [
6].
In tissue engineering and bioprinting, both natural polymers such as alginate, chitosan (CS), hyaluronic acid, gelatin, and synthetic ones, which include polyacrylamide, polyvinyl alcohol, polyethylene glycol, polylactic acid, are used. The main advantage of natural polymers is their biocompatibility, specific enzymatic degradation, and the presence of functional groups and domains that provide better interaction with cells compared to synthetic ones. These, in turn, are characterized by strong water adsorption and high gel strength. However, despite the introduction of synthetic polymers, which allow researchers precise control of gels’ structure and properties, special attention should be paid to their biocompatibility. This problem concerns potential residues during the production of such polymers, i.e., unreacted monomers and remnants of initiators [
7].
This work is a continuation of a series of studies on the development of a hydrogel polymer bioink based on CS obtained by carbon dioxide saturation method and agarose (AG). The concept of combining CS in bioink with fibroblast and keratinocyte stem cells from the confirmed positive impact of this polymer on the skin tissue regeneration processes, as evidenced by numerous studies. The primary mechanism of action of CS is based on the activation of key components of the immune system, namely inflammatory cells, macrophages, and fibroblasts. This, in turn, contributes to the shortening of the inflammatory phase and accelerates the transition to the proliferation phase, which is essential in the process of rebuilding damaged tissue. Furthermore, CS promotes the formation of granulation tissue and angiogenic processes, facilitating the effective deposition of collagen fibers, which is crucial for the repair of skin damage [
8,
9]. Angiogenesis plays a crucial role in regeneration, as vascularization is needed to supply oxygen, nutrients, and growth factors to the regenerating tissue. Proper angiogenesis can accelerate the wound healing process and prevent necrosis; on the other hand, abnormal angiogenesis contributes to a significant slowdown of this process [
10].
The main issue with current bioprinting formulations lies in their limited versatility. Many commercially available and researched systems rely on three primary polymers: alginate, collagen, and gelatin. These systems have gained widespread adoption due to their simplicity in cross-linking using methods like calcium chloride and UV radiation, especially after modification to methacrylated derivatives. However, these formulations predominantly focus on supporting cell viability post-printing, lacking additional biological effects such as promoting angiogenesis or cellular differentiation. To date, only a few solutions in the literature have introduced alternative bioink compositions. One approach involves carboxymethylchitosan combined with agarose and sodium alginate [
5], while another utilizes N,O-carboxymethylchitosan with agarose [
11]. Modifications to chitosan to enhance solubility aim to eliminate the use of cytotoxic acids but necessitate the use of organic solvents for derivatization. Yet, chemical modifications of polymers pose risks of residual toxic solvents and involve time-consuming processes, complicating their practical application and potentially leading to the release of cytotoxic degradation products over time.
The aim of this study is to demonstrate additional physicochemical and biological properties confirming the practical application and meeting acceptance criteria of a chitosan-agarose bioink, essential for biomaterials in tissue engineering. Our previous research has demonstrated that blending two polymers with diverse physicochemical and biological properties enables the creation of a bioink that fulfills all necessary system requirements: it is printable, biocompatible, and possesses suitable rheological properties for bioprinting applications [
12]. Through rheological studies and extrusion tests, we have identified composites with significant application potential. These blends underwent rigorous biological testing to assess their suitability for wound healing and broader tissue engineering applications. This approach represents a straightforward solution for producing a bioink that, based on just two components, already meets numerous critical criteria for tissue engineering applications from inception.
2. Materials and Methods
2.1. Materials
Chitosan – CS (MMW, DD >75%), agarose – AG (low EEO, 34.5-37.5°C gel point), phosphate buffer saline (PBS) sodium hydroxide, and glycolic acid were purchased from Merck (Germany). The carbon dioxide used to saturate the CS precipitate was derived from Linde Gaz Polska Sp. z o. o. (Gdańsk, Poland). Immortalized human keratinocyte HaCaT cells were purchased from the German Cancer Research Center (DKFZ, Heidelberg, Germany). Human skin fibroblast cell line 46BR.1N was obtained from the European Collection of Authenticated Cell Cultures (ECACC, Sigma Aldrich, St. Louis, MO, USA). The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), DMEM medium, antibiotics, propidium iodide, calcein and supplements necessary for cell culture were obtained from Sigma-Aldrich (St. Louis, MO, USA). Wound healing assay kits were obtained from Ibidi GmbH (Germany). MilliQ water was used to prepare all aqueous solutions. All other reagents were of analytical grade or higher. The experiments on mice were conducted in the Tri-City Academic Laboratory Animal Centre of the Medical University of Gdańsk, where the animals were bred and maintained. 3,5-dinitrosalicylic acid, Glucose, Resazurin sodium salt, Methylene blue, Brilliant green, Acid Fuchsin and Nigrosin were purchased from Merck (Germany).
2.2. Hydrogel Preparation
The composition based on chitosan (CS) and agarose (AG), as well as their individual solutions, were prepared according to the methodology described by Banach-Kopeć et al, 2024. To summarize, a 1% CS solution with an average molecular weight was prepared using an innovative carbon dioxide saturation method. Meanwhile, a 1% AG solution was prepared by dissolving it in distilled water at 80°C while stirring at 200 RPM (RA 2020, Heidolph Instruments GmbH & Co. KG, Kelheim, Germany). The CS/AG-based compositions were prepared by cooling the hot AG solution to 45°C and immediately combining it with the CS solution in various weight ratios, using a mechanical stirrer at a speed of 300 revolutions per minute for 2 minutes (BIOMIXBMX-10, Gdańsk, Poland) [
13]. To evaluate the rheological properties, the temperature of the mixture was maintained at 40°C to prevent gelation.
To standardize the water content of the CS/AG hydrogel membranes, it was necessary to initially reduce the water content and then replenish it through conditioning. This process ensured that the samples were consistent for repeatable testing. The membranes were cast onto 90mm Petri dishes, dried at 25°C for 48 hours, and then incubated in distilled water for 24 hours before measurement, resulting in a thickness of 0.4 mm. Samples for angiogenesis assessment were prepared in the same way, with the difference that they were incubated in a 0.9% saline solution. For the evaluation of wound closure, CS/AG and AG samples were cast onto 90mm Petri dishes, and after gelation, 6 mm diameter discs were cut using a biopsy punch.
To conduct biological safety tests and assess the system's impact on cell migration, samples of CS, AG, and their composites were frozen at -80°C and then freeze-dried (Christ Alpha 12–4 LD Plus, Osterode am Harz, Germany). According to the standards of the MTT test and the Ibidi wound healing assay methodology, it is necessary to prepare extracts of the tested materials. To ensure the most rigorous conditions and achieve the highest possible concentrations of the tested extracts, the samples were freeze-dried rather than preparing extracts from their solutions. This approach is particularly important because, during the bioprinting process, the printed scaffolds are placed in the medium only after the process is complete, which can lead to significant water loss, especially in the first printed layers.
2.3. Physiochemical characterization of chitosan-agarose composition
2.3.1. Rheological Properties
The rheological properties of the CS, AG solutions and their composites were measured using oscillating rheometer Anton Paar series MCR 302e (Anton Paar, Warsaw, Poland). A plate-plate system with a 25 mm diameter and 1 mm gap was used for the measurements. The impact of the shear rate on the deformation of a sample was conducted at the temperature of 37°C and the method of a three-interval thixotropy test was applied (three-interval thixotropy test (3ITT) oscillations—rotations—oscillations). In the middle interval, the sample was deformed at a constant shear rate (1000 1/s). In the initial and final interval, the sample was tested through oscillatory shear at a constant deformation amplitude γ = 1% and angular frequency ω = 5 rad/s in order to establish the properties before and after the rotational deformation process.
2.3.2. Permeability Assessment
A modified Kirby-Bauer method was applied to study permeability in AG and CS hydrogels [
14]. This modification involved the use of dyes with varying molecular weights. Initially, 1% solutions of AG and CS and their compositions were prepared and then spread on Petri dishes and left to gel. For CS solutions, this process took 48 hours to allow the release of carbon dioxide necessary for gelling. After the completion of the gelling process, 100 mM solutions of dyes with molecular weights ranging from 251 to 616 g/mol were prepared. In the next step, holes with a diameter of 6 mm were cut in the gelled samples using a biopsy punch. 0.150 mL of the respective dye solution was applied to each of these holes. The samples were incubated at a temperature of 25°C. The diameters of the dye spots were measured with a calliper at regular time intervals, which allowed for the assessment of the degree of diffusion of the dyes in the hydrogel matrices.
To assess glucose permeability, the method described by Reys and co-workers was used with slight modifications [
15]. A laboratory set-up was configured in which a round-bottomed three-necked flask was used as an acceptor, to which a funnel and a metal filter from a filtration system under vacuum were connected to form the receptor part of the apparatus. The flask was placed in a temperature-controlled, i.e. 37°C water bath. Hydrogel membranes based on CS, AG and their composites with a thickness of 0,4 mm were placed in the receptor part of the apparatus, stabilized with a stainless steel clamp. Before measurements, samples were incubated for 24 hours in distilled water. The round-bottom flask was then filled with distilled water and the donor space with a 10% glucose solution. At fixed time points, 2 mL samples were taken from the acceptor chamber, each time topped up with an equal amount of fresh water. Experiments were carried out with continuous stirring of the contents of the round-bottom flask using a magnetic rod to eliminate boundary layer effects that would interfere with the measurement. A colourimetric method for the determination of reducing sugars by reaction with 3,5-dinitrosalicylic acid (DNS method) was used to analyse the concentration of glucose that permeated the membrane into the round-bottom chamber. The measurement was performed at 540 nm using a Mettler UV5 spectrophotometer.
2.4. Biological Properties of Chitosan-Agarose Composition
2.4.1. Cell Culture Conditions
Immortalized human HaCaT keratinocytes (DKFZ, Heidelberg, Germany) [
16,
17] and the human dermal fibroblast cell line 46BR.1N (ECACC, Sigma Aldrich, St. Louis, MO, USA) were used in the study. Cells were routinely grown in culture flasks (growth surface area 25 cm2) in Dulbecco’s modified Eagle medium (DMEM, Sigma Aldrich, St. Louis, MO, USA), with 4500 mg/L of glucose, 584 mg/L of L-glutamine, sodium pyruvate, and sodium bicarbonate, supplemented with 10% FBS and 100 units/mL of penicillin and 100 μg/mL of streptomycin (Sigma Aldrich, St. Louis, MO, USA), under a humidified atmosphere with 5% CO
2 at 37 °C. The medium was changed every 2–3 days.
2.4.2. MTT
AG, CS and CS/AG composites were prepared in accordance with ISO 10993-5:2009 standard. An appropriate amount of medium dedicated to 46BR.1N and HaCaT cells was added to a given composite weight (25 mg per 1 ml culture medium). Then, the prepared composites were placed in a CO2 incubator for 24 hours. After this time, the cells of the 46BR.1N and HaCaT lines seeded in a 96-well plate were replaced with the medium corresponding to a given composite for stimulation - in two concentrations: 100% - adding the full volume of undiluted supernatant from previously prepared composites and 50% - half the volume of the supernatant from a given composite diluted with DMEM HG medium. The cells were incubated for 24 hours. After this time, the MTT test was performed in accordance with the manufacturer's instructions. MTT solution (5mg/ml) was added to each well. After 3 hours, the medium was removed, and isopropanol (6 mM) was added to the cells. Then, the plates with cells were shaken for 30 minutes, and a spectrophotometric reading was performed (Synergy LX, Biotek) at a wavelength of 575 nm.
2.4.5. Cell Viability
To investigate the effectiveness of a newly developed bioink in maintaining cellular viability, an experiment was conducted using a mixture of CS (1% w/v) of medium molecular weight and AG (1% w/v). These components were combined in equal proportions and incubated at 37°C to prevent the composition from gelling. Cells, at a concentration of 1 × 10^6 cells/mL, were integrated with the prepared bioink and then applied to 24-well culture plates using a syringe, forming 3D structures. After the scaffolding gelled below 37°C, the wells were filled with the appropriate medium and placed in an incubator (37°C, 5% CO2).
To assess cellular viability in the produced structures, selective staining was performed to color live cells green and dead cells red. After removing the medium, the scaffolds were cleansed with phosphate-buffered saline (PBS). Then, 3 mL of PBS solution with the addition of Calcein-AM (8 µM) and propidium iodide (2 µg/ml) was applied into each well. The scaffolds were incubated with the dyes in darkness at a constant temperature of 37°C in a 5% CO2 environment for 45 minutes. After the incubation, the staining solution was removed, and fluorescence microscopy observations were made (Nikon Eclipse TE300). The use of CellProfiler software enabled detailed analysis of the obtained images and quantification of live and dead cells.
2.4.6. Wound Healing Ibidi
The study on the impact of CS in combination with AG on the migratory capacity of cells after 24 hours was conducted using Ibidi culture inserts with a predefined cell-free area, crucial for research in wound healing and cell migration. For the experiment, cells were seeded in the inserts at a concentration of 20,000 per well, utilizing DMEM medium enriched with 10% FBS. After 24 hours, the medium was replaced with serum-free DMEM and cellular growth was inhibited by adding mitomycin C (5 μg/ml) for 2 hours. Following this procedure, the medium was changed, and the cells were subjected to the extracts of the tested CS, AG and CS/AG compositions. After another 24 hours, the cells were fixed using 3.7% paraformaldehyde and then stained with a 0.05% solution of crystal violet. The results were evaluated using a Leica DMIL LED inverted microscope and GRAPHAX software.
2.4.7. Angiogenesis
Fertilized chicken eggs were obtained from a local provider (EKO ELITA, Poland) and incubated horizontally with intermittent rotation at 37°C and 65% humidity for 3 days. Subsequently, the eggshells were cracked open, and the embryos were transferred into sterile containers for further incubation at 37°C with 0.5% CO2 for 5 days. On day 8, approximately 5-mm square pieces of various materials (CS, AG, CS/AG, or Whatman filter paper) were placed on the chorioallantoic membrane (CAM). Prior to placement, all materials were UV-sterilized and rinsed with 0.9% NaCl solution. The materials were incubated on the CAM for up to 72 hours. Integration of the materials with the CAM was photographed every 24 hours using a stereomicroscope attached to a digital camera (Olympus Stemi 2000-C). Angiogenesis around the materials was quantified using the AngioTool software on the IKOSA platform. On embryonic development day (EDD) 12, the embryos were humanely sacrificed. In the European Union, the CAM assay is not legally classified as an animal experiment and, therefore, does not require ethical approval (EU Directive 2010/63/EU and European Commission 2021/2784(RSP)).
2.4.8. Wound Healing Model in Mice
A full-thickness excisional skin injury model in mice was used to investigate the effects of hydrogel compositions on wound healing. The mice, 8-10-week-old females of BALB/c strain, were purchased from and maintained in the Tri-City Academic Laboratory Animal Centre of the Medical University of Gdańsk where they were maintained. All animal experiments were performed in compliance with the ARRIVE guidelines and the U.K. Animals (Scientific Procedures) Act, 1986 and associated guidelines. The experiment protocol received approval from the Local Ethical Committee in Bydgoszcz (approval number 51/2020).
Mice were anesthetized with isoflurane inhalations at 2-5% concentration. Before starting the experiment, the animals' backs were shaved and disinfected with Octanisept. Then, the skin on the back was folded and lifted towards the head and tail along the spinal line, and biopsied using 6.0 mm diameter punches to create two symmetrical wounds. Before the application of the tested samples, mice were randomly divided into therapeutic and control groups, each consisting of six animals. Two mm-thick discs of 6 mm diameter prepared from CS/AG, AG hydrogels were placed onto the wounds. The wounds in control mice were treated with 10 µL of carbon dioxide-saturated water. The wounds were then covered with transparent Tegaderm
TM dressing (25×25 mm) and fastened with a self-adhesive bandage wrapped around the animal torso. After 7 days, the dressings and test preparations were replaced to avoid damaging the newly formed skin layer. After 14 days, the dressings were removed. To measure the wound area, a ruler was placed beside the wound, and photographs were taken weekly. Wound sizes were calculated using ImageJ software [
18].
2.4.9. Tissue Isolation for Histological Analyses
After collection, the ears were preserved in 4% formalin buffered with PBS. The tissues were then transferred to a 15% sucrose in PBS for 24 hours and then to a 30% sucrose solution in PBS for another 24 hours. Then, the tissues were sectioned to 10-μm thickness on the cryostat Leica CM1520 and stained with Masson's trichrome. Image acquisition was performed with a Leica DM IL microscope at 40×magnification.
2.5. Statistical Analysis
The Prism 10 V10.0.3 (GraphPad software, Boston, MA, USA) was used for analyzing the results. The statistical significance was determined at p < 0.05. All data reported were based on the means of three replicates (n = 3) or four (n = 12) replicates in case of wound healing tests. Experimental results were expressed as mean ± standard deviation (SD). The differences were considered to be statistically significant at p < 0.05. Statistical analysis of biocompatibility cell culture date was performer with one-way ANOVA (p < 0.05).
3. Results
3.1. Physiochemical Properties of Chitosan-Agarose Composition
3.1.1. Rheological Properties
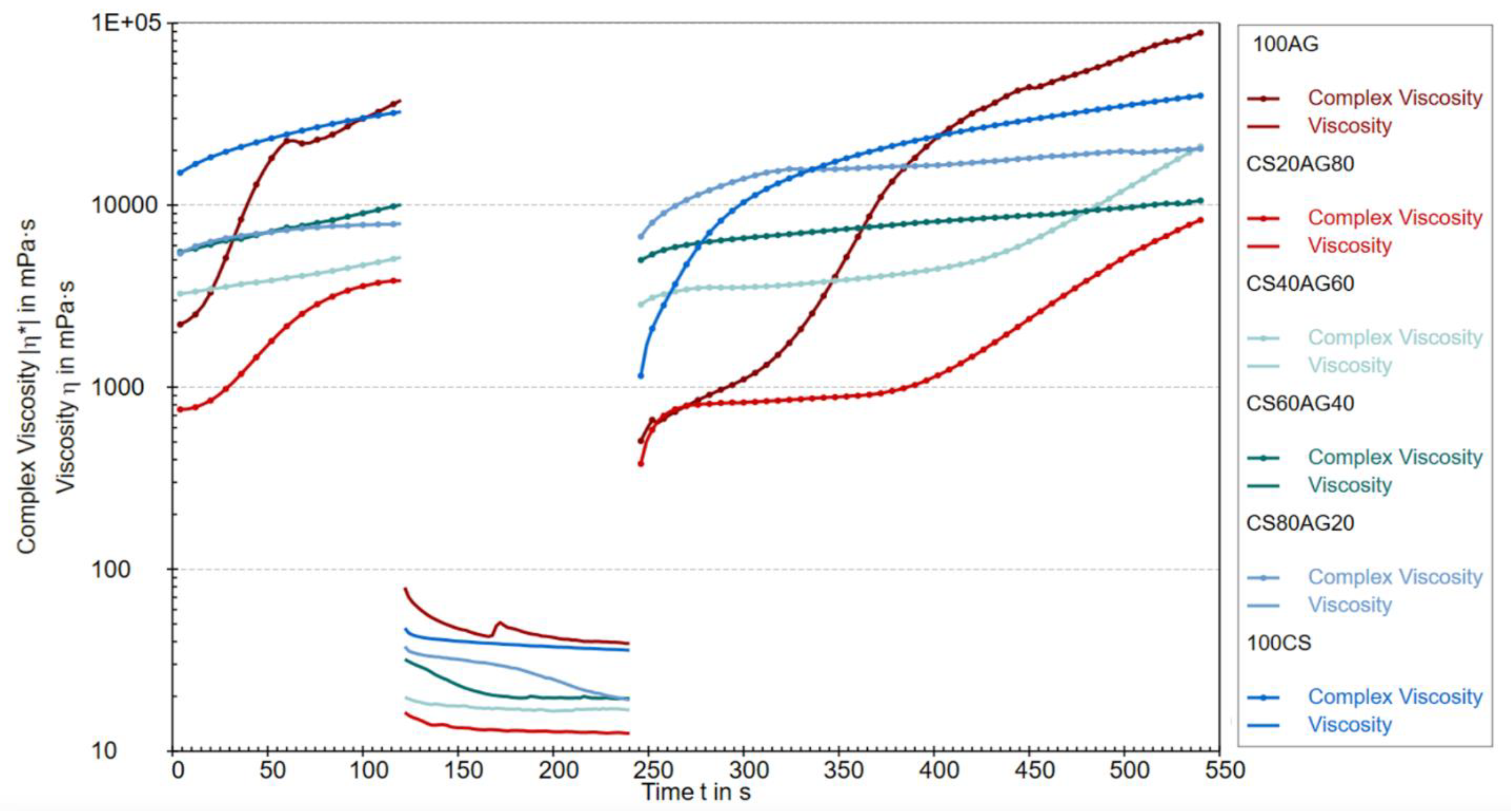
The rheological properties of CS agarose solutions and their composites were evaluated using the three-interval thixotropy test (3ITT), conducted in the oscillatory-rotational-oscillatory (ORO) variant, which applies high rotational stress immediately after the low-strain oscillatory stage within the LVE region. This approach effectively simulates the unidirectional extrusion of bioink from the printer head and assesses its behavior post-stress relaxation under conditions similar to physiological ones optimal for cells in the prepared bioink for printing (37°C) (19).
Figure 1 shows the change in complex viscosity (oscillatory part) and dynamic viscosity (rotational part) over time for 1% solutions of CS and AG, and their mixtures. The viscosity changes over time suggest processes related to the degradation and regeneration of the studied fluids' structure. Complex viscosity describes a material's resistance to deformation under stress, encompassing both viscous (loss) and elastic components, making it particularly useful for analyzing thixotropic materials with time-dependent structure. The viscosity levels in the first test cycle indicate that chitosan and agarose as single-component solutions exhibit a more developed structure, resistant to deformation under dynamic loads. In other words, the internal molecular connections in these single-component materials are more robust compared to their composite materials under oscillatory conditions in the LVE region. Introducing rapid rotation in the second region caused the destruction of the structure of all tested samples. Viscosity values in this range confirm the trend observed in the first cycle, where a chitosan content of 20% and 40% in the composite shows the greatest susceptibility to deformation induced by stresses mimicking unidirectional shear. The third region of the graph indicates the ability to rebuild the structure of the studied polymer sols but with different characteristics. Based on this part of the graph, it can be concluded that the reconstruction of the structure of pure agarose takes the longest, but at the same time, the most durable network is created. The values of complex viscosity of composite materials at the end of the first oscillation and at the beginning of the third are similar, which indicates that the three-dimensional structure of the material will be quickly recovered after unidirectional squeezing from the syringe, which is an advantage of using the composite.
3.1.2. Permeability
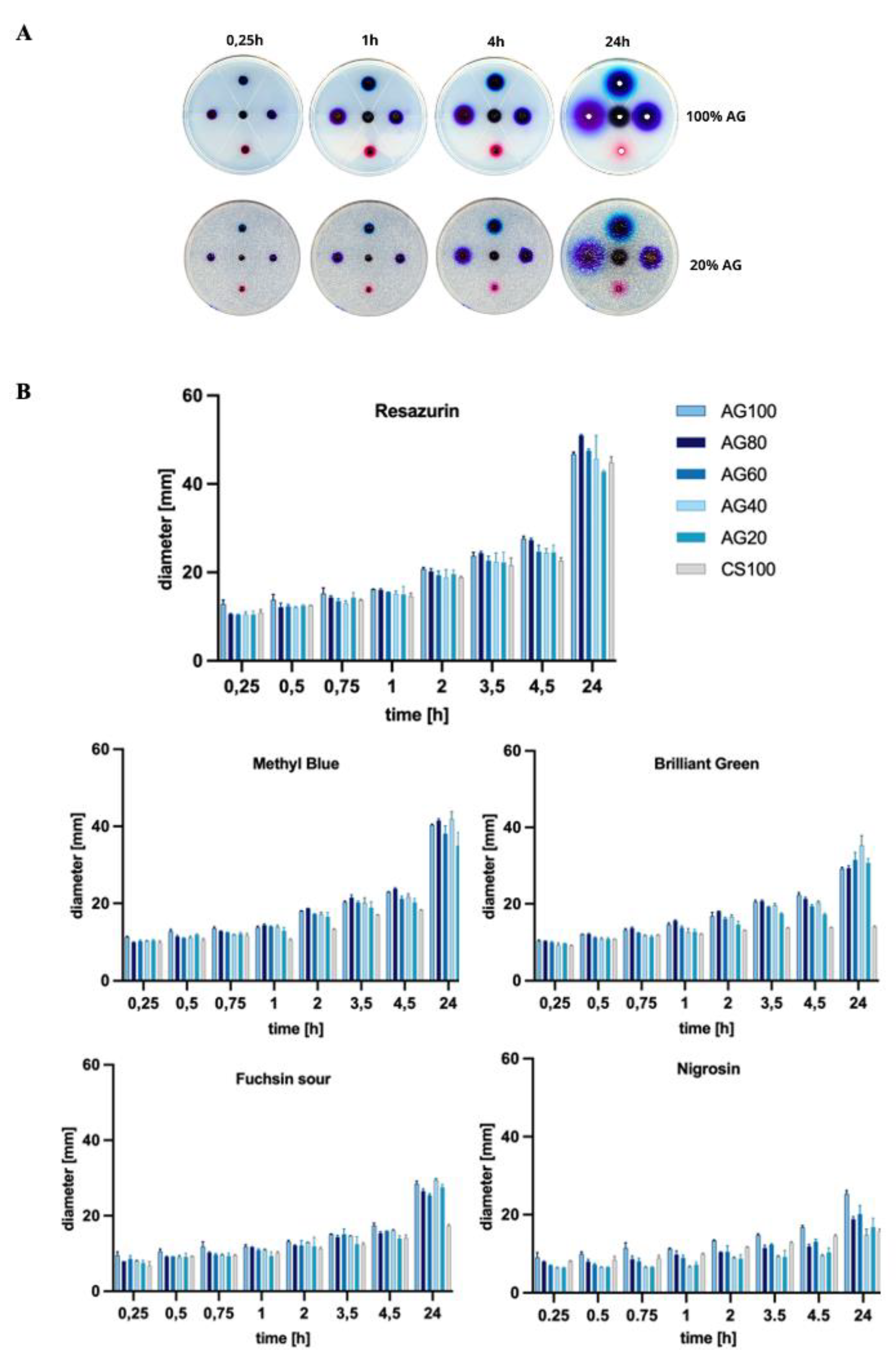
The permeability of scaffolds to nutrients, gases and metabolites is a key feature that provides the scaffold-embedded cells with the correct conditions for proliferation, differentiation and migration. For this purpose, a series of measurements were carried out to determine how changing the composition of the scaffold, i.e. the ratio of AG and CS, affects the migration of components of the standard glucose and compounds differing in molecular weight.
The study of the effect of the molecular weight of dyes on their permeability through membranes showed that the diffusion rate for compounds with the lowest molecular mass is the highest (
Figure 1). Furthermore, for the dye with the lowest molecular weight, resazurin (251.17 g/mol), the largest spot diameters were observed, indicating its highest permeability through hydrogel membranes, more than double compared to Nigrosin (616.49 g/mol). For example, increasing the molecular weight of a compound from approximately 251 to 482 significantly reduced permeability, which after 24 hours of incubation for Resazurin was about 50 mm, and for Brilliant Green, it was below 40 mm in dye spot diameter. Comparing Nigrosin and Resazurin dye diffusion, for the 100%AG system, the average dye stain after 24 hours was approximately 1.6 times smaller.
Based on the conducted studies, it can be concluded that both the composition of CS/AG composites, i.e., the proportion of each polymer, and the molecular weight of diffusing substances have a significant impact on their permeability through hydrogel membranes. It has been shown that 100% AG membranes have greater permeability compared to 100% CS membranes. This phenomenon is consistent with results available in scientific literature, which explain it by the lower crosslinking density and higher porosity of AG membranes. The looser structure of AG facilitates the diffusion of particles. On the other hand, adding CS to the composite reduces the diffusion of dyes through the membrane, which is particularly observed as the molecular weight of the tested substance increases. This phenomenon can be attributed to stronger intermolecular bonds of CS, which in turn affects its lower permeability. Consequently, by mixing CS with AG, it is possible to regulate the porosity and cross-linking density of membranes, allowing for the modification of their permeability.
Also, Reys et al. deduced that pure AG shows better permeability towards methylene blue than its flucoidan-agarose-based hydrogel. As a result of the stronger interactions between the polymers, a more cohesive and compact system is formed, resulting in slower diffusion of the dye. Furthermore, the same study showed that permeability decreases with increasing agarose concentration, resulting in a denser hydrogel matrix [
15]. Based on these results, it can be assumed that compounds with lower molecular weight, such as glucose (180.17 g/mol), will effectively diffuse through hydrogel membranes, regardless of the CS/AG composition. Such properties of membranes open up possibilities for their application in many fields, from biotechnology to regenerative medicine, where the control of substance diffusion through membranes is crucial.
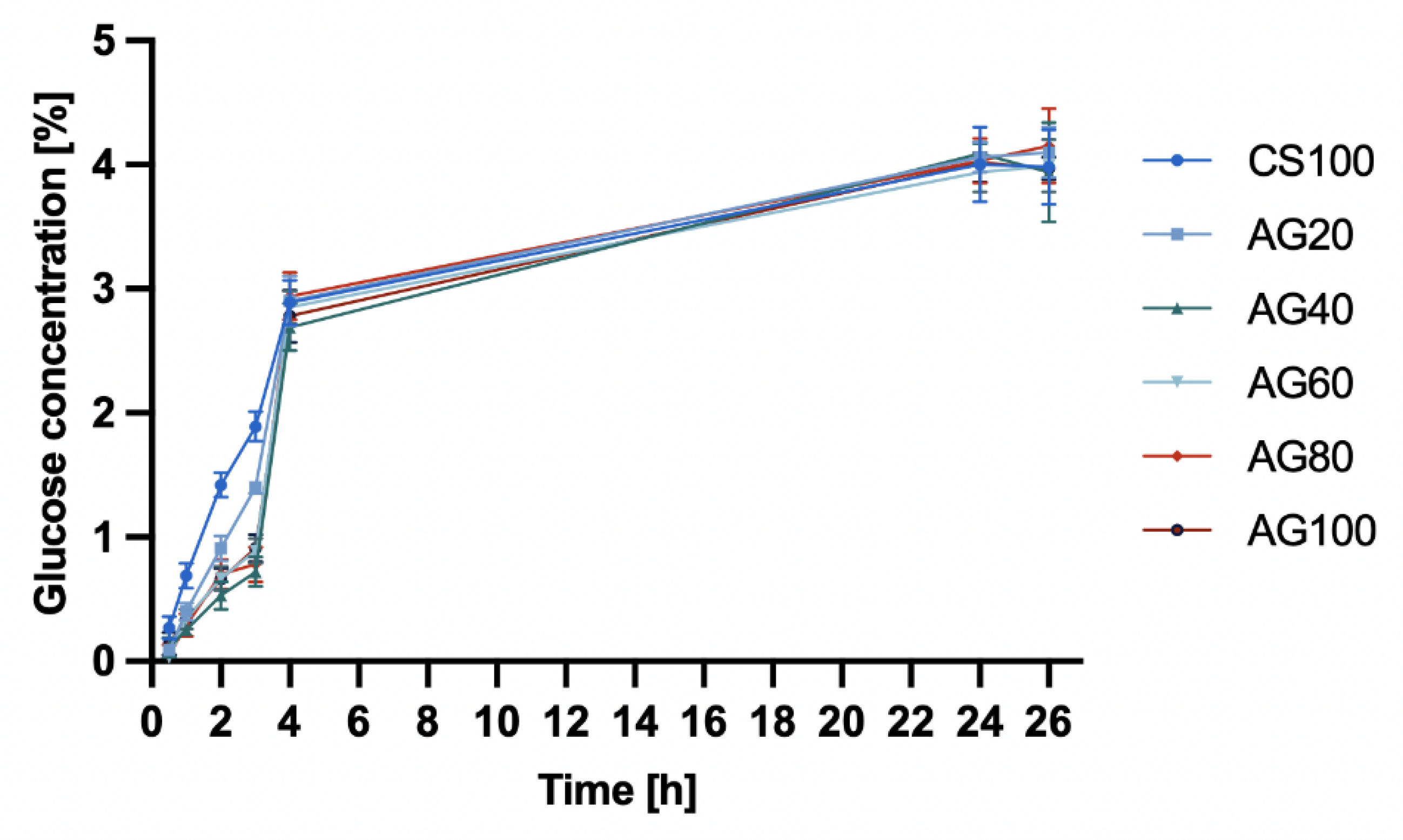
Therefore, in the study of glucose diffusion through hydrogel membranes (
Figure 2) based on CS, CS/AG, and AG, no significant differences were observed in the change of glucose concentration in the acceptor chamber over time. Initially, the CS membrane demonstrated a greater ability to diffuse glucose molecules. However, after 4 hours, the glucose concentration diffusing through the hydrogel membranes became similar. After 24 hours, the glucose concentrations in both the acceptor and donor chambers equalized, with the glucose concentration after 24 hours being 3.97 ± 0.2.
3.2. Biological Properties of Chitosan-Agarose Composition
3.2.1. MTT Test
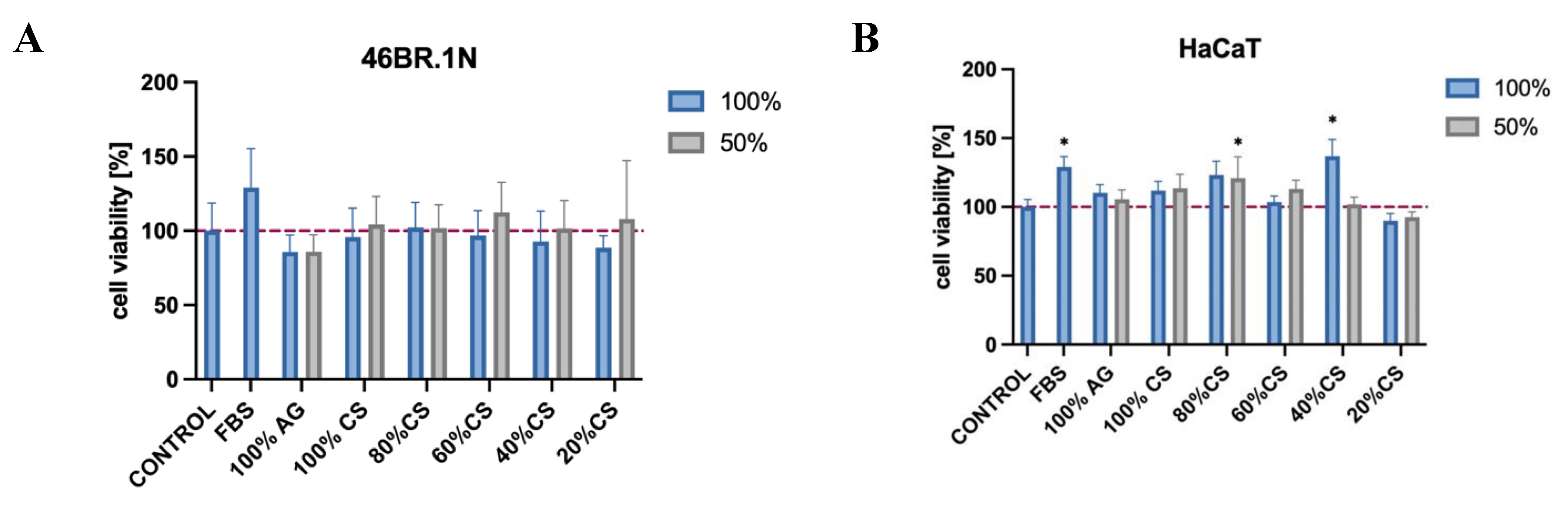
The MTT assay was conducted on skin cell lines, namely fibroblasts 46BR.1N and keratinocytes HaCaT, which are to constitute one of the fundamental components of the developed bioinks for future application uses. The results have provided further evidence confirming the biological safety of CS, AG, and their composites and are consistent with our previous studies, which, according to ISO standard 10993-5, demonstrated that both AG and CS of various degrees of DD and MW are not cytotoxic towards the standardized mouse fibroblast L929 cell line [
13]. In the current study, viability was assessed after a 24-hour contact of cells with extracts from the tested samples to investigate potential cytotoxicity towards skin cells.
According to ISO standard 10993-5, materials for which cell viability exceeds 70% are considered biocompatible. The tested samples showed high cell viability, with values ranging from approximately 85 to 112% (
Figure 3A) for the 46BR.1N cell line and from approximately 90 to 137% (
Figure 3B) for the HaCaT line, suggesting a favorable biological safety of these materials.
3.2.2. Migration Test
An essential aspect of cellular scaffold design is the selection of biomaterials capable of stimulating cell migration processes. This applies to both cells contained in the bioink and those naturally present at the site of the scaffold application. Currently, two approaches are used in bioprinting: the first involves placing cells in the bioink, within which they are supposed to differentiate and migrate; the second promotes the migration of cells from the body into the scaffold.
The use of extracts in the Ibidi wound healing assay model was due to the need to assess the impact of biomaterials under conditions close to real applications, where, in addition to placing cells inside the scaffolds, which are porous, there is also a necessity for cell migration into the pores where they proliferate and differentiate. The fact that extracts were used also stemmed from the fact that AG as well as CS/AG systems gel at 37°C, as do CS solutions under the influence of carbon dioxide release, especially at elevated temperatures. Consequently, examining cell migration using the above set directly in solutions would have been impossible. Furthermore, CS, AG, and CS/AG solutions have different viscosities before and after gelation, which significantly disturbs the measurement. The migratory effect as well as the non-cytotoxic impact of the systems on individual cells was examined during the assessment of viability after extruding materials in the form of scaffolds.
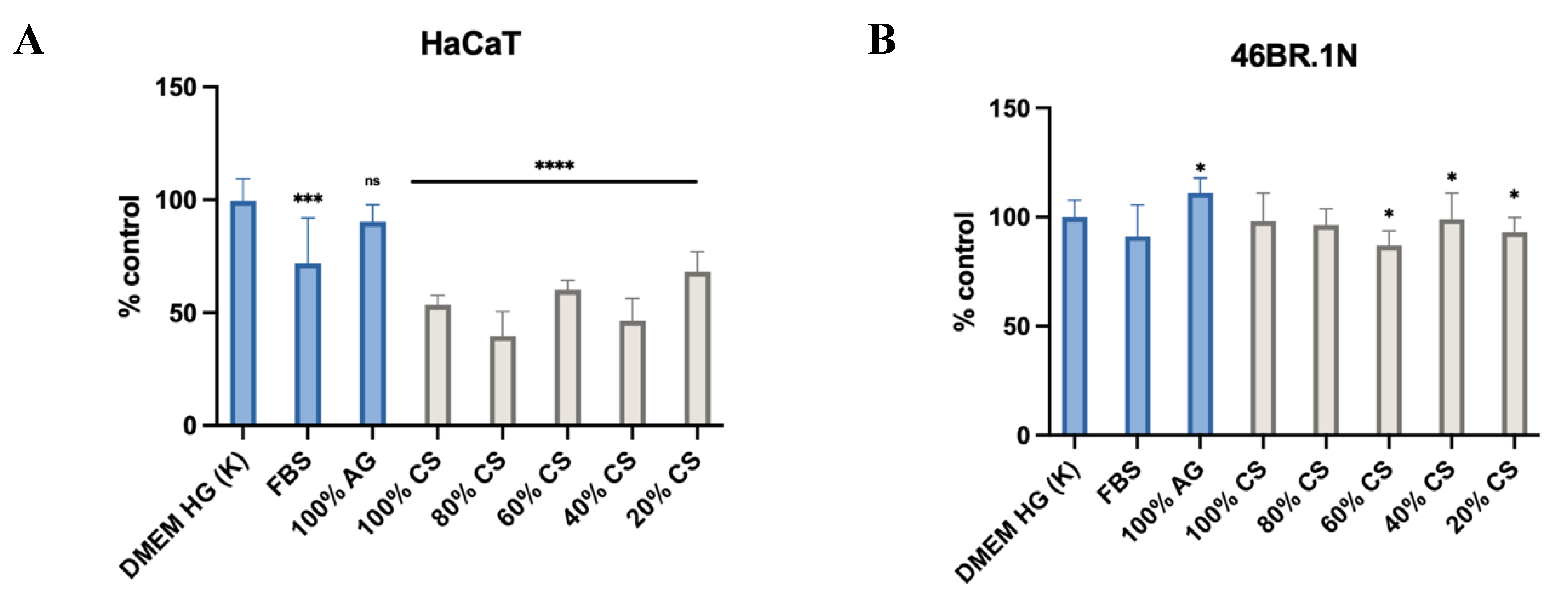
The analysis of the results showed (
Figure 5) that in the case of HaCaT cells, all CS extracts significantly support cell migration, achieving better results than the negative (cells stimulated with DMEM HG medium) and positive (cells stimulated with DMEM HG medium containing 10% FBS). In the case of AG, no statistically significant differences were observed compared to the control. For the 46BR.1N cells, despite statistically significant differences compared to the control, the pro-migratory effect was moderate, and the lack of stimulation by FBS suggests specific requirements of these cells. These results indicate that AG extracts do not support cell migration, while CS show promising pro-migratory properties for HaCaT cells, which is not observed in cultures of 46BR.1N fibroblasts.
3.2.3. Cell Viability
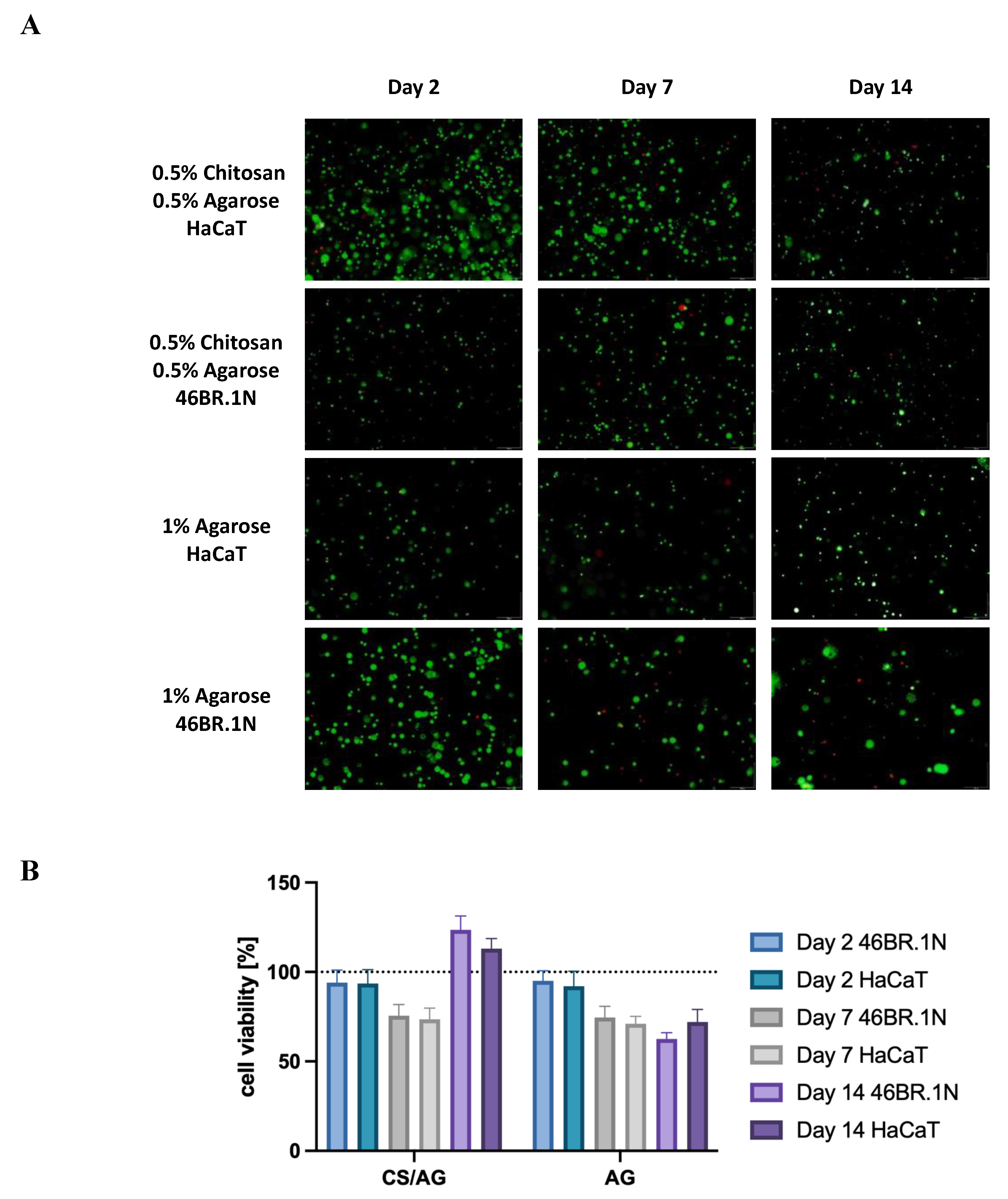
As part of the further analysis of the biological properties of the tested bioinks, cell viability of the HaCaT line and 46BR.1N fibroblasts in contact with scaffolds containing 1% AG and the CS/AG composite in a 1:1 ratio was examined. The viability assessment was conducted at three time points: 2, 7, and 14 days post-scaffold extrusion, allowing for the observation of the long-term effects of the tested biomaterials on the cells. The time points allowed for the investigation of both the biological safety of the system, i.e., the cytotoxic effect, as well as the impact on cell proliferation and migration.
According to the results presented in
Figure 6, after two days of incubation, a similar viability was observed for both cell lines across all tested systems, oscillating around 100%. These results indicate a lack of cytotoxicity, which was previously demonstrated in MTT assays for extracts of the tested biomaterials. Seven days after scaffold extrusion, a slight decrease in the viability of both cell lines and in both types of scaffolds was observed; however, these values remained at an acceptable level, ranging between 70% and 78%. This decrease in living cells may be attributed to the adaptation process to the new environment. Nevertheless, a positive impact on the survival and proliferation of cells was noted for the CS/AG composite after 14 days, both for 46BR.1N and HaCaT cells, where viability reached 117% and 129%, respectively. In systems based solely on AG, cells tended to cluster outside the scaffold area, resulting in a slight decrease in cell viability within the scaffold. Moreover, this observation aligns with the literature data indicating that AG scaffolds do not promote cell adhesion.
In summary, both materials tested demonstrated compatibility with HaCaT and 46BR.1N cells, showing no cytotoxic action over up to 14 days of incubation. Furthermore, the data confirm that the CS/AG composite is not only compatible with the cells but also promotes their proliferation, providing favorable rheological parameters while maintaining high cell viability.
The bioprinting process significantly impacts cell survival, especially during extrusion, influenced by factors like bioink viscosity, printing speed, pressure, nozzle geometry, build platform temperature, needle diameter, and construct geometry [
20]. Insufficient initial cell density can lead to slower cell and tissue growth rates and inadequate scaffold integration in vivo [
21]. The bioink's composition, construct geometry (porosity, permeability, degradability), growth factors, peptide sequences, and culture conditions (temperature, nutrient composition) further affect cell survival [
12]. Comparing cell survival rates over different time intervals is challenging due to numerous variables in bioprinting parameters. For instance, human keratinocytes and fibroblast cells in a genipin-crosslinked CS-PEG system showed up to 85% survival after 7 days [
22]. In a self-crosslinkable CS-gallic acid system, NIH 3T3 cell survival was 92% after 7 days [
23]. Over a longer period, NIH 3T3 cells in an aldehyde hyaluronic acid-N-carboxymethyl chitosan-gelatin-alginate system maintained approximately 91% viability after 29 days [
24].Fluorescent staining of fibroblast and keratinocyte cells in CS/AG and AG systems revealed nearly 100% survival, indicating that the optimal extrusion temperature did not induce cell death. However, survival declined to 70% by day 7, potentially due to new environmental stresses like nutrient deficiency, metabolite buildup, or mechanical stress from the printing process. Beyond this adaptation phase, survival increased to 110% by day 14, suggesting cells adapted and began proliferating, potentially facilitated by biocompatible materials like CS supporting tissue regeneration and creating favorable conditions for cell expansion.
3.2.4. Angiogenesis
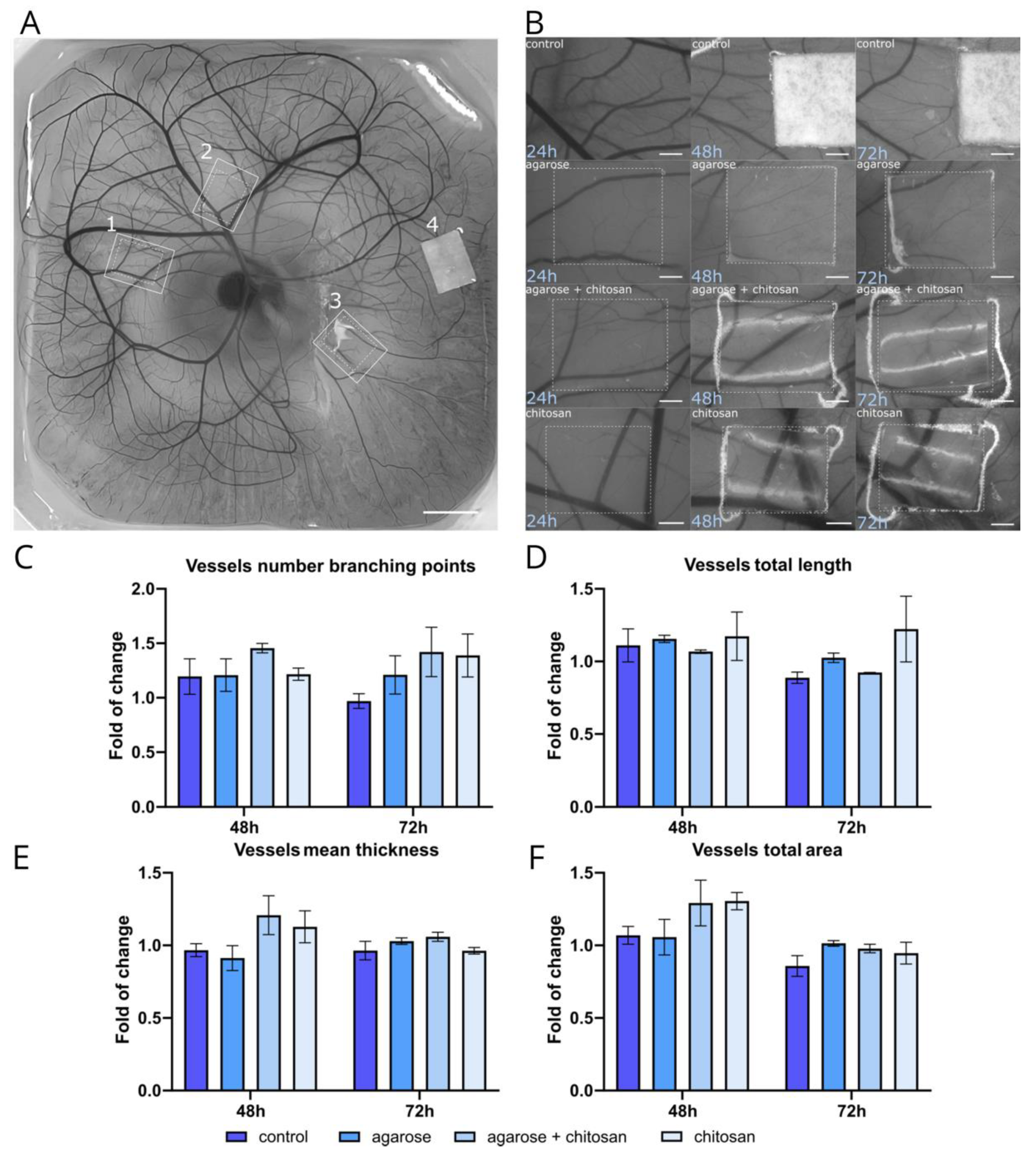
The proangiogenic potential of CS and AG-based materials was evaluated using CAM assay on 8-day-old embryos. Materials, including CS, AG, and a combination of both, were tested against a Whatman filter paper control (
Figure 7A and B). The analysis focused on several parameters of the vascular network, including the number of branching points, vessel network length, total vascular area, and mean vessel thickness. Branching points are crucial for evaluating angiogenesis, as new vascular branches arise through mechanisms like sprouting or intussusception from existing vessels. A notable increase in branching points was observed with CS and the CS/AG combination, particularly at 72 hours, while AG alone showed a slight increase compared to the control (
Figure 7C). Another important phase in angiogenesis is the extension of blood vessels. At 48 hours, the total vessel length was similar across the control, AG and CS/AG combination treatments. However, CS alone exhibited a consistent increase in vessel length at both 48 and 72 hours (
Figure 7D). The total vascular area reflecting the surface area of all vessels in an image increased as the vascular network expanded. As shown in
Figure 7E, the total vascular area displayed a slight increase across all materials over time, and it is more pronounced after 48 hours, whereas after the next 24 hours, it remains almost constant. The thickness of the blood vessels also influences the total vascular area. Since new vascular sprouts that form during angiogenesis are thin, they may not significantly affect the total vascular area compared to other parameters. Alterations in the average thickness of the vasculature were observed, as newly formed thin sprouts contributed to a reduction in the overall diameter of the vascular network. The mean thickness increased only with the combination of CS/AG and CS alone at 48 hours, whereas at 72 hours, the mean vessel thickness remained relatively consistent across all treatments with slight variations (
Figure 7F).
In summary, CS and CS/AG combinations generally promoted slight increases in various vascular parameters compared to the control, while AG alone had minimal effects and showed varied results depending on the specific parameter and time point. CS alone demonstrated the most consistent proangiogenic effects, enhancing both vessel length and the number of branching points. These findings suggest the potential application of CS and CS/AG materials in therapeutic angiogenesis, warranting further investigation into their mechanisms and long-term effects on vascular development.
3.2.5. Wound Healing
An animal model is an essential element in biomedical research. Due to their genetic, physiological, and pathological similarities to humans, mice are helpful in preclinical studies. Experiments conducted on small rodents are essential for evaluating developed biologics, as they provide preliminary information about interactions with living organisms, specifically regarding biocompatibility and therapeutic efficacy. Furthermore, studies on animal models complement earlier biological research on proliferation, cytotoxicity, or cell migration. Moreover, the results from studies conducted on animal models constitute the first step that allows the conduct of clinical trials, which are decisive for confirming the utility of scaffolds in medical applications.
In this experiment, the effect of the CS/AG composition and its individual components, namely CS and AG applied alone, on wound healing was evaluated in a model of excisional dorsal skin injury in mice. CO
2-saturated water (Water CO
2) was applied to wounds in control mice. According to the literature, CS accelerates wound healing by stimulating immune cells like macrophages and fibroblasts, which shortens the inflammatory phase and promotes tissue proliferation. Additionally, CS enhances granulation tissue formation and angiogenesis, aiding in collagen deposition for skin repair [
8,
9]. Therefore, this study aimed to determine whether the CS/AG composition accelerates wound healing, specifically if the addition of agarose affects the efficacy of this process.
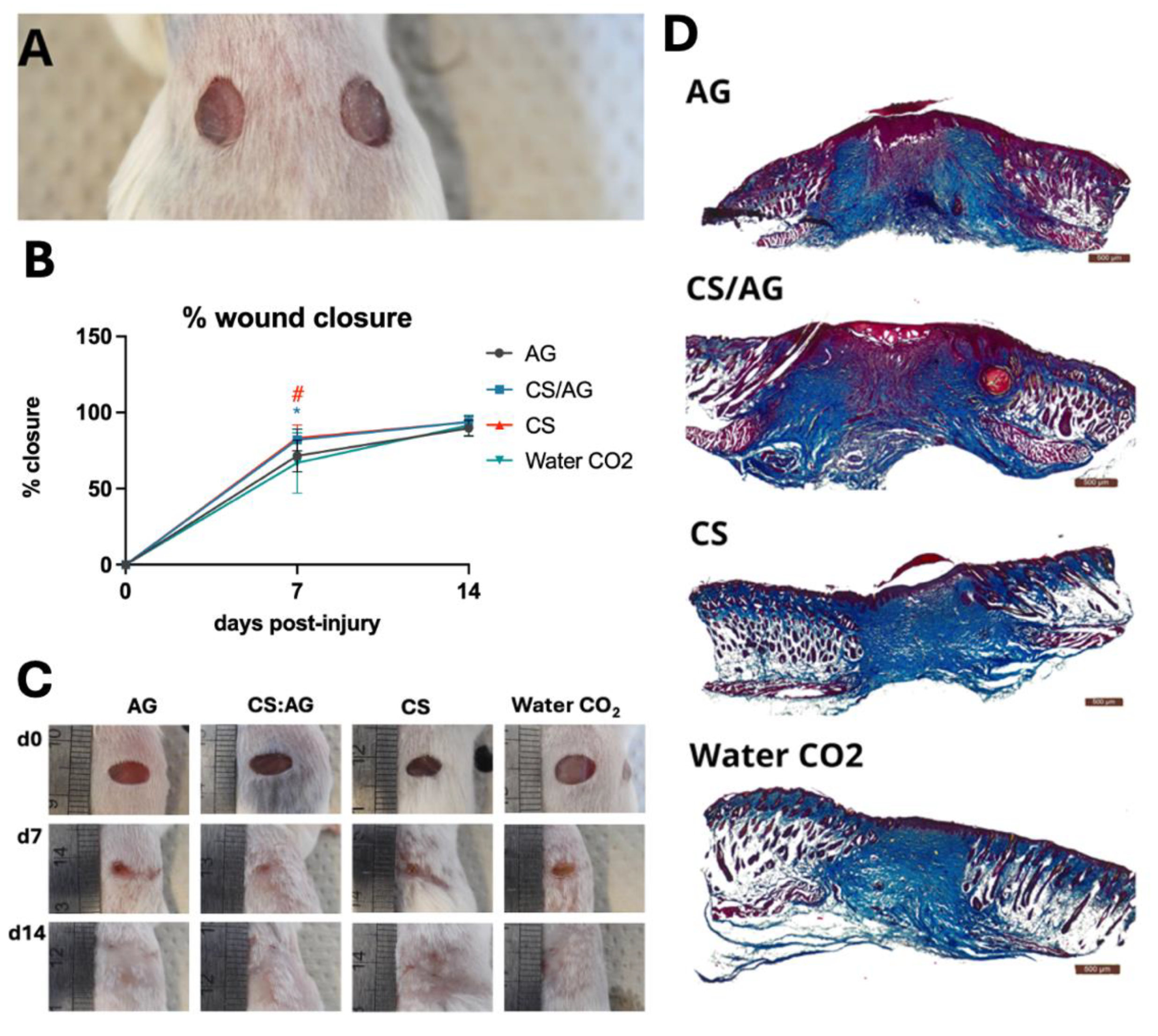
The samples are indicated as follows: CS/AG - chitosan-agarose composition, CS - chitosan alone; AG - agarose alone, Water CO2 –carbon dioxide-saturated water.
CS/AG and CS dressings applied as 6-mm hydrogel disc onto wounds (
Figure 8A) significantly accelerated the wound closure, resulting in 82.0 and 83.2% closure, respectively, on day 7 post-injury compared to the control (66.9%) (
Figure 8B, C). On day 14 post-injury wound closure in mice treated with all three types of hydrogel dressings and in the controls reach approximately 90%. In the case of AG the pace of wound closure was similar the control group (Water CO
2). On day 14 post-injury wound closure equalled in all tested groups, including the controls, achieving approximately 90%. No complications in wounds were observed, thus confirming the biocompatibility of the applied dressings. Histological examination (
Figure 8D) indicated typical wound healing in all groups (
Figure 8D).
It is crucial not only to concentrate on CS but also to apply it to the skin. CS hydrogel rapidly releases CO2 and dries quickly. AG in CS/AG allows for maintaining a moist environment in contact with the wound, providing adequate moisture and protection against drying. This may be because AG creates a more stable and durable scaffold that better mimics the natural tissue environment. As a result, CS/AG dressing can combine chitosan's biological activities with agarose's physicochemical properties, promoting the maintenance of a moist microenvironment.
4. Summary and Conclusion
This research continues the investigative path into an innovative, thermosensitive bioink consisting of a medium molecular weight chitosan hydrogel obtained by CO2 saturation and an agarose hydrogel responsible for the gelling of the system. Demonstrating the feasibility of using this composition in bioprinting by meeting the acceptance criteria for bioinks, we conducted further characterization to determine more detailed parameters that assess the flow characteristics of the bioink under conditions simulating its extrusion from a syringe during 3D printing. The ORO test showed that the three-dimensional structure of the material after unidirectional squeezing from the syringe will be rapidly recovered in the case of the CS/AG composite, which is not the case with single components. Studies on the diffusion rates of dyes through CS/AG hydrogel membranes showed that both the polymer proportions and the molecular weight of the diffusing substances significantly affect diffusion. The results of glucose permeability studies, as a low molecular weight substance serving as a model compound mimicking the diffusion of nutrients, indicate that it effectively diffuses through various CS/AG membrane compositions, highlighting its potential for further applications in regenerative medicine.
In further studies evaluating the biological safety and activity of the developed materials in cell models, additional MTT tests were conducted on selected skin cell lines such as 46BR.1N fibroblasts and HaCaT keratinocytes, which are intended to be key components of the bioink for future applications. These results confirmed the biological safety of CS, AG, and their composites, consistent with previous studies according to ISO 10993-5. The tested samples showed high cell viability, reaching values from about 85 to 112% for the 46BR.1N line and from 90 to 137% for the HaCaT line, suggesting a favorable biological safety profile of these materials. Besides the lack of cytotoxic effect, it is important that cells, both those included in the scaffold and naturally occurring at the site of application, effectively migrate both within and to the scaffold. It was demonstrated that the CS/AG composition favors the migration of HaCaT cells, which is a promising discovery for regenerative applications. However, for 46BR.1N cells, despite statistically significant differences compared to the control, only a moderate pro-migratory effect was observed, suggesting specific requirements of these cells and underscoring the need to further tailor the composition of composites to various cell types.
In addition to cytotoxicity tests conducted on extracts during a 24-hour incubation, it was also crucial to monitor the long-term survival of cells in the CS/AG composition. Cell survival of HaCaT lines and fibroblasts 46BR.1N, at all studied time points, i.e., 2, 7, and 14 days after scaffold extrusion, showed that biomaterials are not only biocompatible but also support cell proliferation and survival. High cell survival rates, especially on day 14, confirm the potential use of the CS/AG composition as key components of the scaffold, indicating the important role of chitosan in enhancing cell survival over time. Based on the results of studies using L929, HaCaT, and 46BR.1N cell lines, and preliminary verification of printability and functionality of CS/AG biomaterials, a detailed analysis of their proangiogenic potential was conducted. Both CS and its CS/AG combinations exhibited better proangiogenic properties compared to pure AG and the control. The absence of angiogenesis inhibition further highlights the unique properties of the studied composition for future applications in tissue engineering. Confirmed biological safety and favorable properties of the CS/AG composition have allowed for the transition to studies in an animal model, which is a crucial step in assessing the effectiveness of biological therapeutic systems. Studies conducted on mice showed that CS/AG and CS dressings accelerated skin wound closure.
In our most recent studies, we were able to confirm the positive impact of the CS/AG composition on many important biological parameters, opening up new possibilities for its use in tissue engineering. The next step will be to investigate the impact of the addition of time-releasing bioactive peptides on migratory effects, the wound healing process, and the possibility of creating a vascularized scaffold. Additionally, future research stages will include designing the geometry of the scaffold, optimizing the arrangement of layers and cells, and determining the porosity of the scaffold to assess its permeability. Such a comprehensive approach will allow for further customization of bioink properties, which is crucial for its future clinical applications.
CRediT authorship contribution statement
Adrianna Banach-Kopeć: Writing – review & editing, Writing – original draft, Visualization, Validation, Conceptualization, Software, Resources, Methodology, Investigation, Formal analysis, Data curation,. Szymon Mania: Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Resources, Software, Supervision, Validation, Writing – original draft, Writing – review & editing. Natalia Maciejewska: Methodology, Investigation, Data curation, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. Katarzyna Czerwiec: Methodology, Investigation, Data curation, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. Paulina Słonimska: Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization. Milena Deptuła: Data curation, Investigation, Methodology, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. Jakub Baczyński-Keller: Data curation, Formal analysis, Investigation, Methodology, Software, Visualization, Validation. Michał Pikuła: Data curation, Investigation, Methodology, Software, Supervision, Validation, Writing – original draft, Writing – review & editing. Paweł Sachadyn: Conceptualization, Supervision, Resources, Data curation, Formal analysis, Funding acquisition, Writing – review & editing. Robert Tylingo: Project administration, Resources, Writing – review & editing, Writing – original draft, Conceptualization, Validation, Supervision, Software, Methodology, Investigation, Formal analysis, Data curation, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Funding
Financial support of these studies from Gdańsk University of Technology by the DEC-10/2021/IDUB/I.3.3 grant under the Argentum Triggering Research Grants - ‘Excellence Initiative - Research University’ program is gratefully acknowledged. Financial support for this research by the Gdansk University of Technology under the minigrant 2022 (036073) - Research University’ program is gratefully acknowledged.
References
- Fornetti E, De Paolis F, Fuoco C, Bernardini S, Giannitelli SM, Rainer A, Seliktar D, Magdinier F, Baldi J, Biagini R, et al. A Novel Extrusion-Based 3D Bioprinting System for Skeletal Muscle Tissue Engineering. Biofabrication. 2023; 15:025009. [CrossRef]
- Antezana PE, Municoy S, Álvarez-Echazú MI, Santo-Orihuela PL, Catalano PN, Al-Tel TH, Kadumudi FB, Dolatshahi-Pirouz A, Orive G, Desimone MF. The 3D Bioprinted Scaffolds for Wound Healing. Pharmaceutics. 2022; 14:464. [CrossRef]
- DeSimone E, Schacht K, Jungst T, Groll J, Scheibel T. Biofabrication of 3D Constructs: Fabrication Technologies and Spider Silk Proteins as Bioinks. Pure Appl Chem. 2015; 87:737-749. [CrossRef]
- Deptuła M, Karpowicz P, Wardowska A, Sass P, Sosnowski P, Mieczkowska A, Filipowicz N, Dzierżyńska M, Sawicka J, Nowicka E, et al. Development of a Peptide Derived from Platelet-Derived Growth Factor (PDGF-BB) into a Potential Drug Candidate for the Treatment of Wounds. Adv Wound Care. 2020; 9:657-675. [CrossRef]
- Gu Z, Fu J, Lin H, He Y. Development of 3D Bioprinting: From Printing Methods to Biomedical Applications. Asian J Pharm Sci. 2020; 15:529-557. [CrossRef]
- Dell AC, Wagner G, Own J, Geibel JP. 3D Bioprinting Using Hydrogels: Cell Inks and Tissue Engineering Applications. Pharmaceutics. 2022; 14:2596. [CrossRef]
- Nie L, Wang C, Deng Y, Shavandi A. Bio-Inspired Hydrogels via 3D Bioprinting. In: Biomimetics. IntechOpen. 2020; 10.
- Feng P, Luo Y, Ke C, Qiu H, Wang W, Zhu Y, Hou R, Xu L, Wu S. Chitosan-Based Functional Materials for Skin Wound Repair: Mechanisms and Applications. Front Bioeng Biotechnol. 2021; 9:650598. [CrossRef]
- Matica MA, Aachmann FL, Tøndervik A, Sletta H, Ostafe V. Chitosan as a wound dressing starting material: Antimicrobial properties and mode of action. Int J Mol Sci. 2019; 20(23):5889. [CrossRef]
- Guo Y., Huang J., Fang Y., Huang H., & Wu J. 1D, 2D, and 3D scaffolds promoting angiogenesis for enhanced wound healing. Chemical Engineering Journal. 2022; 437, 134690.
- Butler H. M., Naseri E., MacDonald D. S., Tasker R. A., & Ahmadi A. Investigation of rheology, printability, and biocompatibility of N, O-carboxymethyl chitosan and agarose bioinks for 3D bioprinting of neuron cells. Materialia. 2021; 18, 101169.
- Banach-Kopeć B, Mania S, Tylingo R. Marine polymers in tissue bioprinting: Current achievements and challenges. Reviews on Advanced Materials Science. 2024; 63(1):20230180.
- Banach-Kopeć A, Mania S, Tylingo R, Wawrzynowicz A, Pawłowska M, Czerwiec K, Pikuła M. Thermosensitive composite based on agarose and chitosan saturated with carbon dioxide. Preliminary study of requirements for production of new CSAG bioink. Carbohydr Polym. 2024; 336:122120. [CrossRef]
- Jan, H. Kirby-Bauer Disk Diffusion Susceptibility Test Protocol. American Society For Microbiology. 2009.
- Reys, L.L., Silva, S.S., Soares da Costa, D., Rodrigues, L.C., Reis, R.L., Silva, T.H. Building Fucoidan/Agarose-Based Hydrogels as a Platform for the Development of Therapeutic Approaches against Diabetes. Molecules. 2023; 28(11):4523. [CrossRef]
- Boukamp, P.; Popp, S.; Altmeyer, S.; Hülsen, A.; Fasching, C.; Cremer, T.; Fusenig, N.E. Sustained nontumorigenic phenotype correlates with a largely stable chromosome content during long-term culture of the human keratinocyte line HaCaT. Genes Chromosomes Cancer. 1997; 19, 201–214.
- Boukamp, P.; et al. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol.1998; 106, 761–771.
- Schneider, C. A., Rasband, W. S., & Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nature methods. 2012; 9(7), 671-675.
- Owczarz P, Ziółkowski P, Modrzejewska Z, Kuberski S, Dziubiński M. Rheo-Kinetic Study of Sol-Gel Phase Transition of Chitosan Colloidal Systems. Polymers. 2018; 10:47. [CrossRef]
- Xu H, Liu JC, Zhang ZY, Xu CX. A review on cell damage, viability, and functionality during 3D bioprinting. Military Medical Research. 2022; 9(1):70.
- Ozbolat IT, Hospodiuk M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials. 2016; 76:321-343. [CrossRef]
- Hafezi F, Shorter S, Tabriz AG, Hurt A, Elmes V, Boateng J, Douroumis D. Bioprinting and Preliminary Testing of Highly Reproducible Novel Bioink for Potential Skin Regeneration. Pharmaceutics. 2020; 12(6):550. [CrossRef]
- Gwak MA, Lee SJ, Lee D, Park SA, Park WH. Highly gallol-substituted, rapidly self-crosslinkable, and robust chitosan hydrogel for 3D bioprinting. Int J Biol Macromol. 2023; 227:493–504.
- Chen H, Fei F, Li X, Nie Z, Zhou D, Liu L, Zhang J, Zhang H, Fei Z, Xu T. A facile, versatile hydrogel bioink for 3D bioprinting benefits long-term subaqueous fidelity, cell viability and proliferation. Regen Biomater. 2021; 8(2). [CrossRef]
Figure 1.
Comparison of complex viscosity and dynamic viscosity during the ORO test.
Figure 1.
Comparison of complex viscosity and dynamic viscosity during the ORO test.
Figure 2.
(A) Images of dye-applied hydrogel samples during 24 hours of incubation. (B) Sizes of dye stain diameters at specific time points from 0.25 to 24 hours. Data represent mean ± standard deviation; p < 0.05.
Figure 2.
(A) Images of dye-applied hydrogel samples during 24 hours of incubation. (B) Sizes of dye stain diameters at specific time points from 0.25 to 24 hours. Data represent mean ± standard deviation; p < 0.05.
Figure 3.
Glucose diffusion into the acceptor chamber through chitosan CS, agarose AG and chitosan/agarose CS/AG membranes at 25°C.
Figure 3.
Glucose diffusion into the acceptor chamber through chitosan CS, agarose AG and chitosan/agarose CS/AG membranes at 25°C.
Figure 4.
The cytotoxicity of chitosan (CS), agarose (AG) and their composites CS/AG following 24 hours incubation in two concentrations against (A) fibroblast 46BR.1N and (B) keratinocytes HaCaT. Data are expressed as the mean ± standard deviation of three independent experiments. The results were analyzed by one-way ANOVA with comparisons vs. control: ns (not statistically significant, p > 0.05), *p < 0.05.
Figure 4.
The cytotoxicity of chitosan (CS), agarose (AG) and their composites CS/AG following 24 hours incubation in two concentrations against (A) fibroblast 46BR.1N and (B) keratinocytes HaCaT. Data are expressed as the mean ± standard deviation of three independent experiments. The results were analyzed by one-way ANOVA with comparisons vs. control: ns (not statistically significant, p > 0.05), *p < 0.05.
Figure 5.
Effects of MMW chitosan (CS), agarose (AG) and their composites on the migration (A) fibroblast 46BR.1N and (B) keratinocytes HaCaT . Graphs show the results of at least three experiments, which are presented as the mean ± SEM. The results were analyzed by one-way ANOVA with comparison to control DMEM HG ns (not statistically significant, p > 0.05), *p < 0.05, **** p<0.0001).
Figure 5.
Effects of MMW chitosan (CS), agarose (AG) and their composites on the migration (A) fibroblast 46BR.1N and (B) keratinocytes HaCaT . Graphs show the results of at least three experiments, which are presented as the mean ± SEM. The results were analyzed by one-way ANOVA with comparison to control DMEM HG ns (not statistically significant, p > 0.05), *p < 0.05, **** p<0.0001).
Figure 6.
(A) Viability of 46BR.1N fibroblasts and HaCaT keratinocytes embedded in chitosan-agarose CS/AG composite and in agarose AG alone. This figure presents fluorescent microscope images showing live-dead staining of cells in the scaffolds on days 2, 7, and 14 of cultivation. Viable cells are stained green with calcein, while dead cells are stained red with propidium iodide; the scale bar 250 µm. (B) Graph of changes in survival rates of both cell lines over time. The results were evaluated using a Leica DMIL LED inverted microscope and GRAPHAX software.
Figure 6.
(A) Viability of 46BR.1N fibroblasts and HaCaT keratinocytes embedded in chitosan-agarose CS/AG composite and in agarose AG alone. This figure presents fluorescent microscope images showing live-dead staining of cells in the scaffolds on days 2, 7, and 14 of cultivation. Viable cells are stained green with calcein, while dead cells are stained red with propidium iodide; the scale bar 250 µm. (B) Graph of changes in survival rates of both cell lines over time. The results were evaluated using a Leica DMIL LED inverted microscope and GRAPHAX software.
Figure 7.
The ex-ovo CAM angiogenesis assay. (A) Representative image of an ex-ovo-cultivated chicken embryo with different tested materials on embryonic development day (EDD) 9. The materials are indicated as follows: 1 - agarose + chitosan, 2 - chitosan, 3 - agarose, 4 - Whatman filter paper. Scale bar = 5 mm. (B) Representative microscopic images showing material-induced angiogenesis after 24, 48, and 72 hours of incubation with the materials. Scale bar = 1 mm. Analysis of changes in the vascular network, including the number of vessel branching points (C), total vessel length (D), mean vessel thickness (E), and total vascular area (F), presented as fold changes relative to the 24-hour incubation. Data represents mean values ± s.d. from at least two independent experiments.
Figure 7.
The ex-ovo CAM angiogenesis assay. (A) Representative image of an ex-ovo-cultivated chicken embryo with different tested materials on embryonic development day (EDD) 9. The materials are indicated as follows: 1 - agarose + chitosan, 2 - chitosan, 3 - agarose, 4 - Whatman filter paper. Scale bar = 5 mm. (B) Representative microscopic images showing material-induced angiogenesis after 24, 48, and 72 hours of incubation with the materials. Scale bar = 1 mm. Analysis of changes in the vascular network, including the number of vessel branching points (C), total vessel length (D), mean vessel thickness (E), and total vascular area (F), presented as fold changes relative to the 24-hour incubation. Data represents mean values ± s.d. from at least two independent experiments.
Figure 8.
Effects of tested hydrogels on skin wound healing in mice. (A) example photo of hydrogel discs applied on dorsal skin wounds; (B) mean percentage of wound closure; error bars represent SD, n =12 - a number of wounds representing 6 mice in each group; statistical significance relative Water CO2 control to was determined using the two-tailed Mann-Whitney test and denoted with an asterisk “*” for CS/AG and a hash “#” for CS alone; (C) representative images of wounds at 0, d7 and d14; (D); representative skin sections were collected on day 14 post-injury and stained with Masson trichrome to visualize tissue architecture: red – muscles and keratin; blue- collagen; pink - cytoplasm; brown - nuclei.
Figure 8.
Effects of tested hydrogels on skin wound healing in mice. (A) example photo of hydrogel discs applied on dorsal skin wounds; (B) mean percentage of wound closure; error bars represent SD, n =12 - a number of wounds representing 6 mice in each group; statistical significance relative Water CO2 control to was determined using the two-tailed Mann-Whitney test and denoted with an asterisk “*” for CS/AG and a hash “#” for CS alone; (C) representative images of wounds at 0, d7 and d14; (D); representative skin sections were collected on day 14 post-injury and stained with Masson trichrome to visualize tissue architecture: red – muscles and keratin; blue- collagen; pink - cytoplasm; brown - nuclei.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).