Submitted:
22 August 2024
Posted:
25 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Extraction of P. gingivalis Outer Membrane Vesicle and Authentication
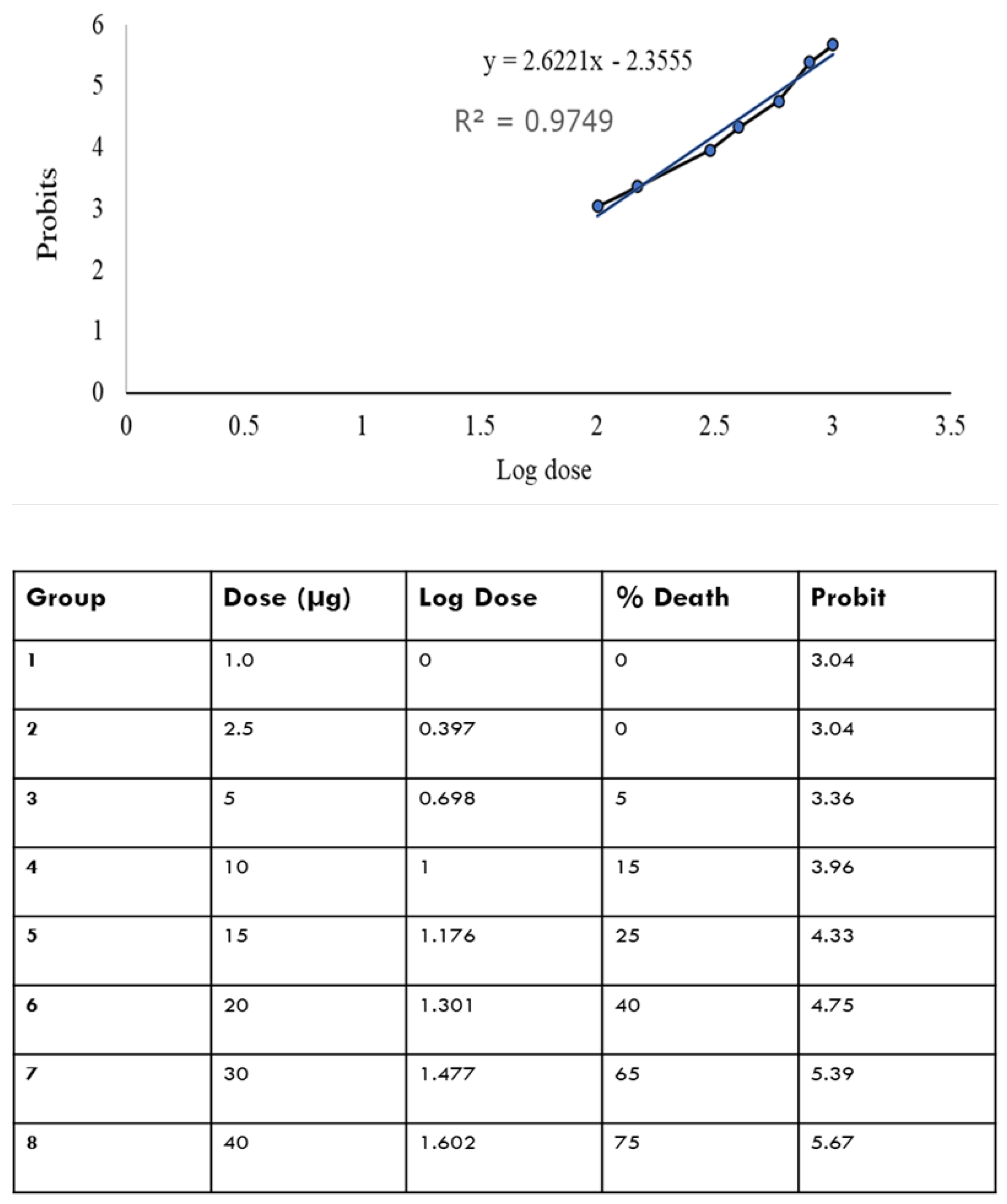
2.2. LD50 of OMV in Adult Zebrafish
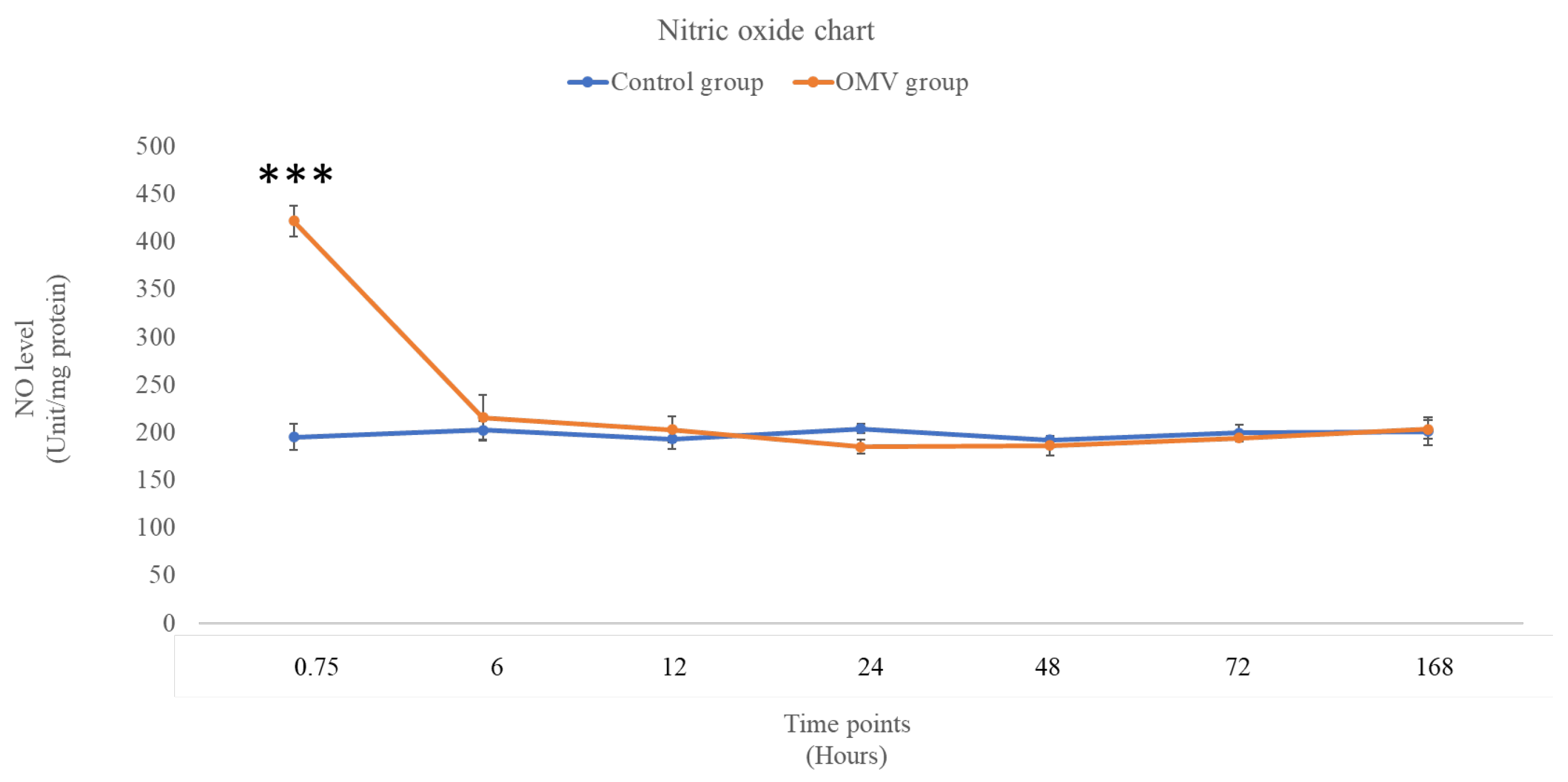
2.3. OMV Triggers Increased NO Level in Zebrafish
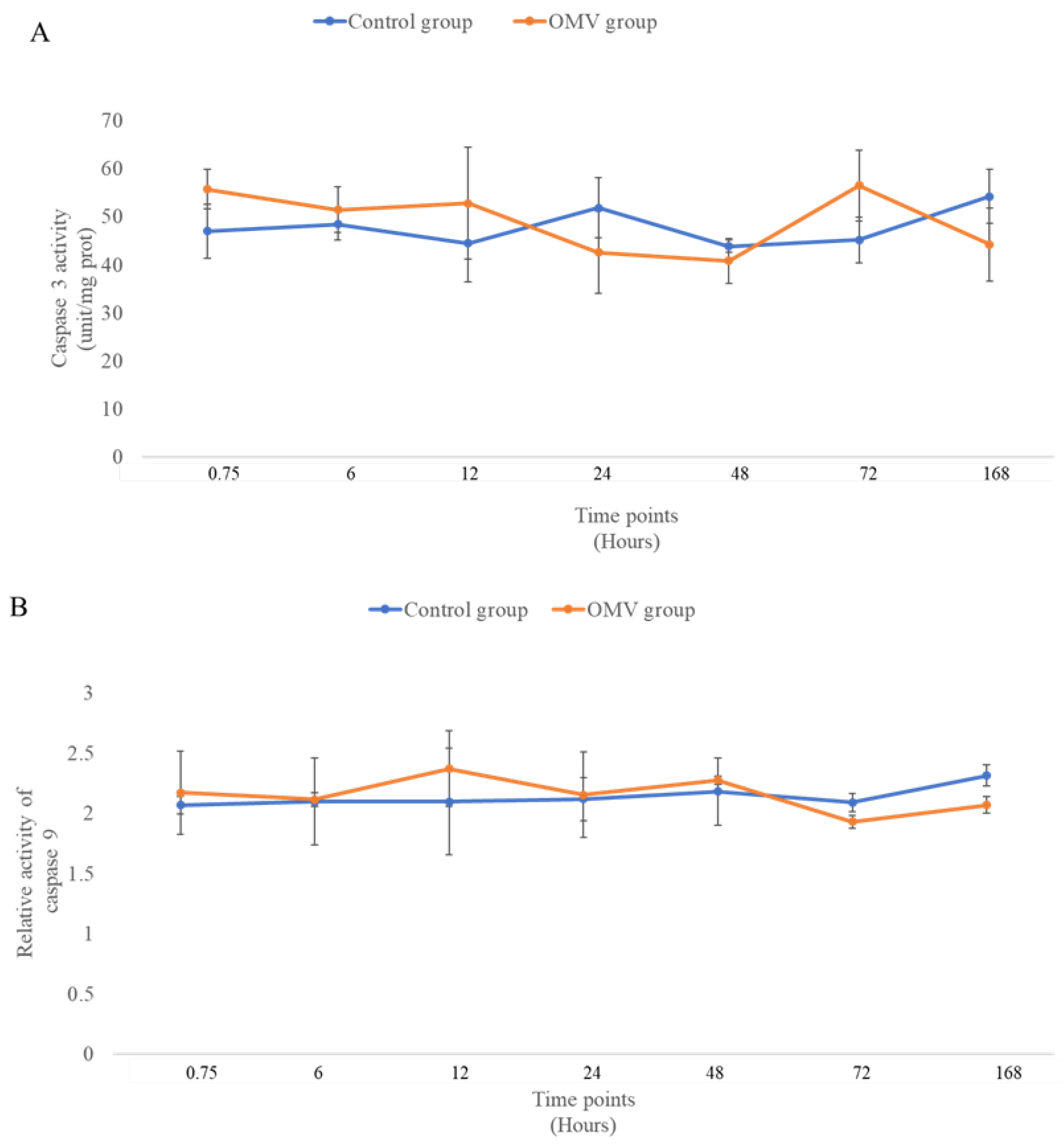
2.4. OMV Has No Effect on Caspase 3 and 9 Activity
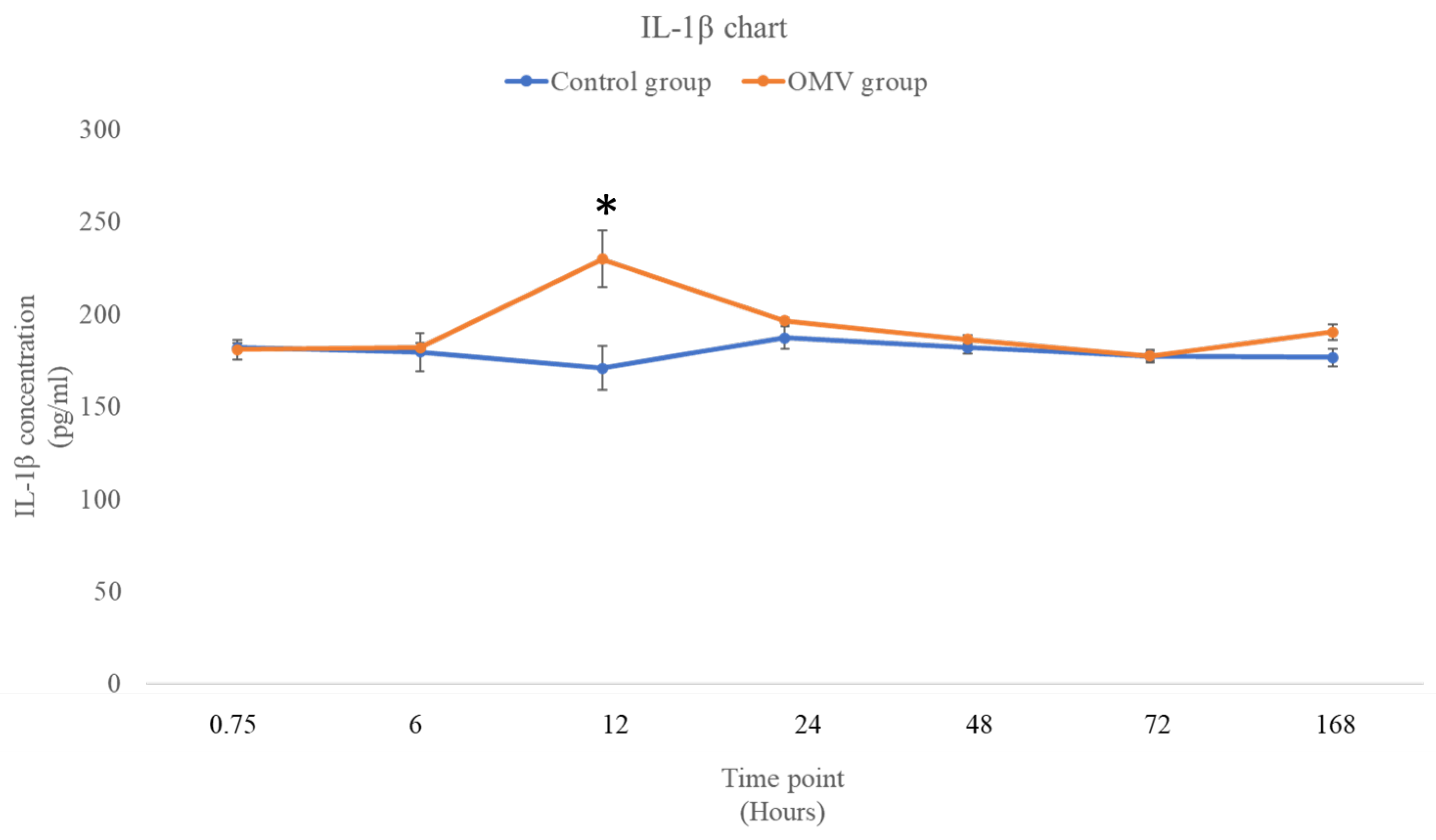
2.5. P. gingivalis OMV Stimulates Production of IL-1β in Zebrafish
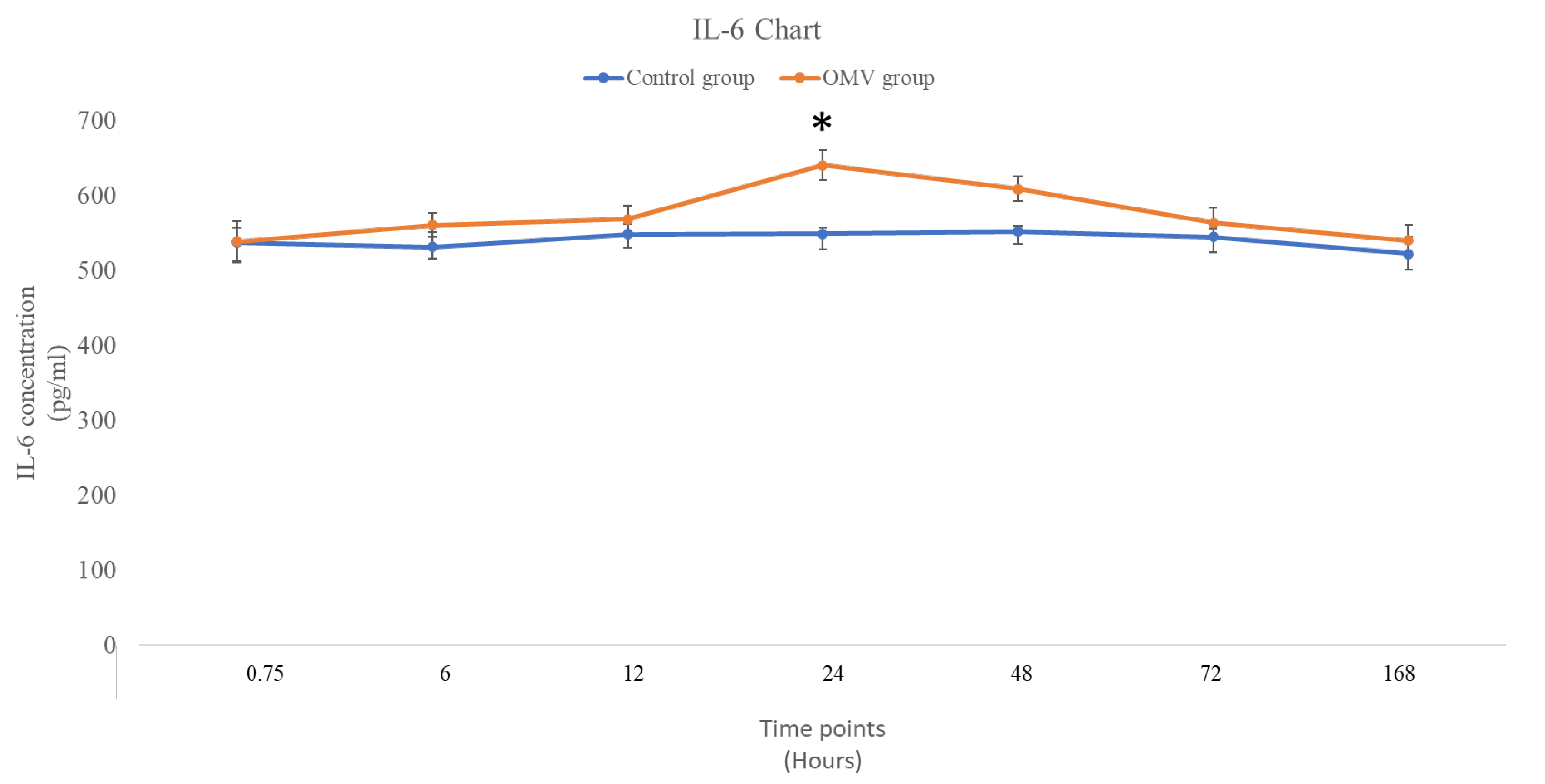
2.6. P. gingivalis OMV Activates Release of IL-6 in Zebrafish
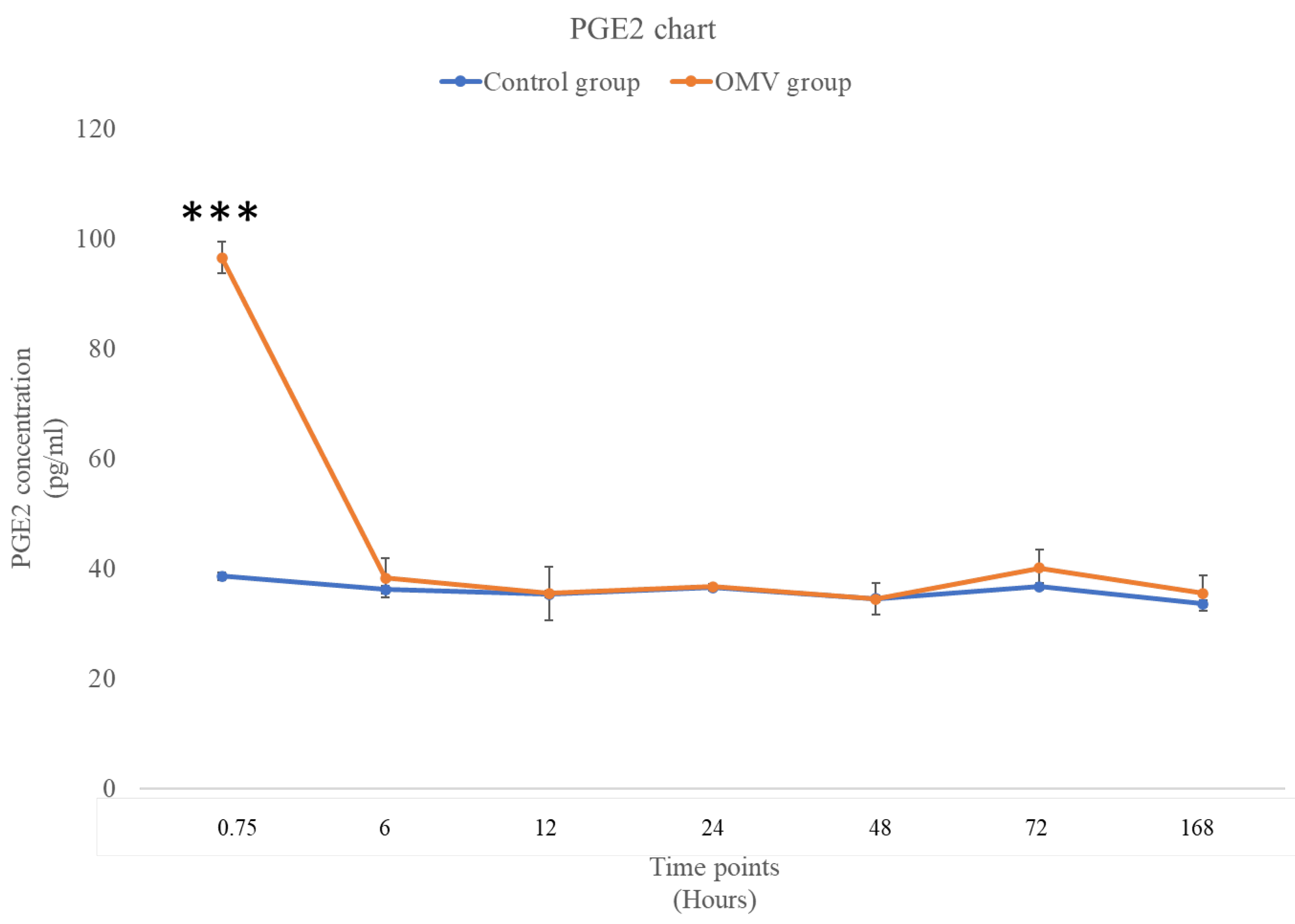
2.7. P. gingivalis OMV Triggers High Concentration of PGE2 in Zebrafish
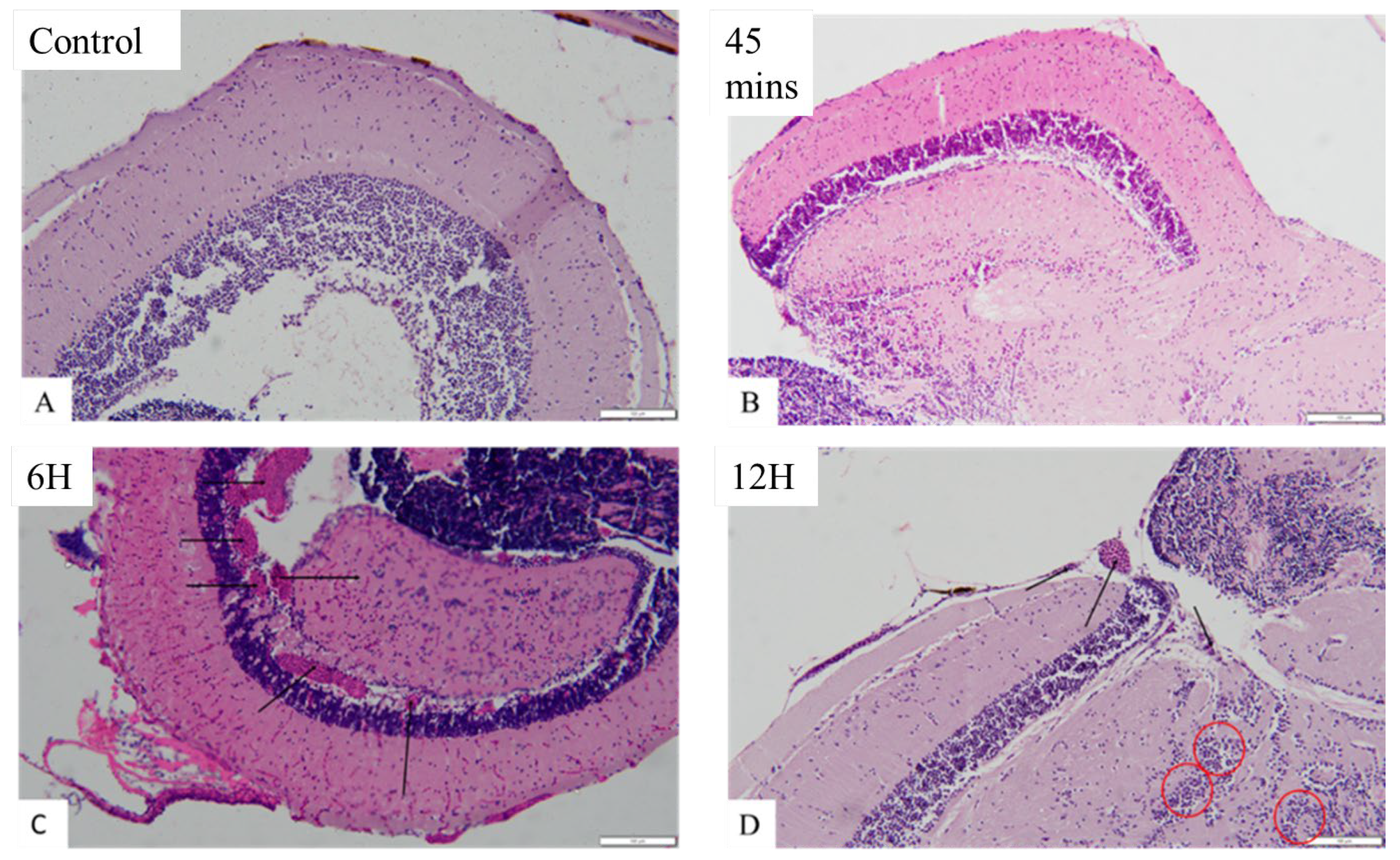
2.8. OMV Induced Histopathological Changes in the Brain of Zebrafish
3. Discussion
4. Materials and Methods
4.1 Porphyromonas Gingivalis Derived Outer Membrane Vesicles (P. gingivalis OMV) Preparation
4.2. Fish Husbandry and Care
4.3. Intraperitoneal Injection of P. gingivalis Derived Outer Membrane Vesicles (OMV)
4.4. Median Lethal Dose Determination
4.5. Determination of Levels of Nitric Oxide (NO), Caspase 3 and 9 Using Colorimetric Assay
4.6. Determination of Concentrations of Interleukin 6 (IL-6), Interleukin 1 Beta (IL-1β) and Prostaglandin E2 (PGE2) Using ELISA
4.7. Evaluation of Zebrafish Brain Histology
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alvarenga MOP, Frazão DR, Matos IG de, et al. Is There Any Association Between Neurodegenerative Diseases and Periodontitis? A Systematic Review. Frontiers in Aging Neuroscience; 13. Epub ahead of print 24 May 2021. [CrossRef]
- Sirisereephap K, Maekawa T, Tamura H, et al. Osteoimmunology in Periodontitis: Local Proteins and Compounds to Alleviate Periodontitis. International Journal of Molecular Sciences; 23. Epub ahead of print 1 May 2022. [CrossRef]
- Teixeira FB, Saito MT, Matheus FC, et al. Periodontitis and alzheimer’s disease: A possible comorbidity between oral chronic inflammatory condition and neuroinflammation. Front Aging Neurosci; 9. Epub ahead of print 10 October 2017. [CrossRef]
- Webers A, Heneka MT, Gleeson PA. The role of innate immune responses and neuroinflammation in amyloid accumulation and progression of Alzheimer’s disease. Immunology and Cell Biology 2020; 98: 28–41.
- Gare J, Kanoute A, Meda N, et al. Zperiodontal conditions and pathogens associated with pre-eclampsia: A scoping review. International Journal of Environmental Research and Public Health; 18. Epub ahead of print 1 July 2021. [CrossRef]
- Jia Y, Guo B, Yang W, et al. Rho kinase mediates Porphyromonas gingivalis outer membrane vesicle-induced suppression of endothelial nitric oxide synthase through ERK1/2 and p38 MAPK. Arch Oral Biol 2015; 60: 488–495.
- Nakao R, Hasegawa H, Ochiai K, et al. Outer membrane vesicles of porphyromonas gingivalis elicit a mucosal immune response. PLoS One; 6. Epub ahead of print 2011. [CrossRef]
- Avila-Calderón ED, Ruiz-Palma M del S, Aguilera-Arreola MG, et al. Outer Membrane Vesicles of Gram-Negative Bacteria: An Outlook on Biogenesis. Frontiers in Microbiology; 12. Epub ahead of print 4 March 2021. [CrossRef]
- Gong T, Chen Q, Mao H, et al. Outer membrane vesicles of Porphyromonas gingivalis trigger NLRP3 inflammasome and induce neuroinflammation, tau phosphorylation, and memory dysfunction in mice. Front Cell Infect Microbiol; 12. Epub ahead of print 9 August 2022. [CrossRef]
- Omar NA, Kumar J, Teoh SL. Neuroprotective effects of Neurotrophin-3 in MPTP-induced zebrafish Parkinson’s disease model. Front Pharmacol; 14. Epub ahead of print 28 November 2023. [CrossRef]
- Gao X, Zhang P, Chen J, et al. Necrostatin-1 Relieves Learning and Memory Deficits in a Zebrafish Model of Alzheimer’s Disease Induced by Aluminum. Neurotoxicity Research 2022 40:1 2022; 40: 198–214.
- Koehler D, Shah ZA, Hensley K, et al. Lanthionine ketimine-5-ethyl ester provides neuroprotection in a zebrafish model of okadaic acid-induced Alzheimer’s disease. Neurochem Int 2018; 115: 61–68.
- Farías-Cea A, Leal C, Hödar-Salazar M, et al. Behavioral Study of 3- and 5-Halocytisine Derivatives in Zebrafish Using the Novel Tank Diving Test (NTT). Int J Mol Sci; 24. Epub ahead of print 1 July 2023. [CrossRef]
- Fontana BD, Alnassar N, Parker MO. The zebrafish (Danio rerio) anxiety test battery: comparison of behavioral responses in the novel tank diving and light–dark tasks following exposure to anxiogenic and anxiolytic compounds. Psychopharmacology (Berl) 2022; 239: 287–296.
- Aparna S, Patri M. Benzo[a]pyrene exposure and overcrowding stress impacts anxiety-like behavior and impairs learning and memory in adult zebrafish, Danio rerio. Environ Toxicol 2021; 36: 352–361.
- Abu Bakar N, Mohd Sata NSA, Ramlan NF, et al. Evaluation of the neurotoxic effects of chronic embryonic exposure with inorganic mercury on motor and anxiety-like responses in zebrafish (Danio rerio) larvae. Neurotoxicol Teratol 2017; 59: 53–61.
- Singh S, Sahu K, Kapil L, et al. Quercetin ameliorates lipopolysaccharide-induced neuroinflammation and oxidative stress in adult zebrafish. Molecular Biology Reports 2022 49:4 2022; 49: 3247–3258.
- Singh S, Sahu K, Kapil L, et al. Administration of Quercetin Ameliorates Lipopolysaccharide Induced Neuroinammation and Oxidative Stress in Adult Zebrash. Epub ahead of print 2021. [CrossRef]
- Valcarce DG, Martínez-Vázquez JM, Riesco MF, et al. Probiotics reduce anxiety-related behavior in zebrafish. Heliyon; 6. Epub ahead of print 1 May 2020. [CrossRef]
- Kaur K, Narang RK, Singh S. AlCl3 induced learning and memory deficit in zebrafish. Neurotoxicology 2022; 92: 67–76.
- Zanandrea R, Abreu MS, Piato A, et al. Lithium prevents scopolamine-induced memory impairment in zebrafish. Neurosci Lett 2018; 664: 34–37.
- Singsai K, Ladpala N, Dangja N, et al. Effect of Streblus asper Leaf Extract on Scopolamine-Induced Memory Deficits in Zebrafish: The Model of Alzheimer’s Disease. Adv Pharmacol Pharm Sci; 2021. Epub ahead of print 2021. [CrossRef]
- Suganya K, Koo BS. Gut–brain axis: Role of gut microbiota on neurological disorders and how probiotics/prebiotics beneficially modulate microbial and immune pathways to improve brain functions. International Journal of Molecular Sciences 2020; 21: 1–29.
- Mottaz H, Schönenberger R, Fischer S, et al. Dose-dependent effects of morphine on lipopolysaccharide (LPS)-induced inflammation, and involvement of multixenobiotic resistance (MXR) transporters in LPS efflux in teleost fish. Environmental Pollution 2017; 221: 105–115.
- Zhang Q, Kopp M, Babiak I, et al. Low incubation temperature during early development negatively affects survival and related innate immune processes in zebrafish larvae exposed to lipopolysaccharide. Sci Rep; 8. Epub ahead of print 1 December 2018. [CrossRef]
- Widziolek M, Prajsnar TK, Tazzyman S, et al. Zebrafish as a new model to study effects of periodontal pathogens on cardiovascular diseases. Sci Rep; 6. Epub ahead of print 25 October 2016. [CrossRef]
- Farrugia C, Stafford GP, Potempa J, et al. Mechanisms of vascular damage by systemic dissemination of the oral pathogen Porphyromonas gingivalis. FEBS Journal 2021; 288: 1479–1495.
- Farrugia C, Stafford GP, Murdoch C. Porphyromonas gingivalis Outer Membrane Vesicles Increase Vascular Permeability. J Dent Res 2020; 99: 1494–1501.
- Guo J, Lin K, Wang S, et al. Effects and mechanisms of Porphyromonas gingivalis outer membrane vesicles induced cardiovascular injury. BMC Oral Health; 24. Epub ahead of print 1 December 2024. [CrossRef]
- Perry VH, Holmes C. Microglial priming in neurodegenerative disease. Nature Reviews Neurology 2014; 10: 217–224.
- Hampel H, Hardy J, Blennow K, et al. The Amyloid-β Pathway in Alzheimer’s Disease. Molecular Psychiatry 2021; 26: 5481–5503.
- Chen Z, Jalabi W, Shpargel KB, et al. Lipopolysaccharide-induced microglial activation and neuroprotection against experimental brain injury is independent of hematogenous TLR4. Journal of Neuroscience 2012; 32: 11706–11715.
- Hai J, Wan JF, Lin Q, et al. Cognitive dysfunction induced by chronic cerebral hypoperfusion in a rat model associated with arteriovenous malformations. Brain Res 2009; 1301: 80–88.
- Zhang Y, Fang Z, Li R, et al. Design of outer membrane vesicles as cancer vaccines: A new toolkit for cancer therapy. Cancers; 11. Epub ahead of print 1 September 2019. [CrossRef]
- Adamski MG, Sternak M, Mohaissen T, et al. Vascular cognitive impairment linked to brain endothelium inflammation in early stages of heart failure in mice. J Am Heart Assoc; 7. Epub ahead of print 1 April 2018. [CrossRef]
- Fulop GA, Ahire C, Csipo T, et al. Cerebral venous congestion promotes blood-brain barrier disruption and neuroinflammation, impairing cognitive function in mice. Geroscience 2019; 41: 575–589.
- Zhang W, Xiao D, Mao Q, et al. Role of neuroinflammation in neurodegeneration development. Signal Transduction and Targeted Therapy; 8. Epub ahead of print 1 December 2023. [CrossRef]
- Toyofuku M, Nomura N, Eberl L. Types and origins of bacterial membrane vesicles. Nature Reviews Microbiology 2019; 17: 13–24.
- Yang WW, Guo B, Jia WY, et al. Porphyromonas gingivalis-derived outer membrane vesicles promote calcification of vascular smooth muscle cells through ERK1/2-RUNX2. FEBS Open Bio 2016; 6: 1310–1319.
- Wei S, Peng W, Mai Y, et al. Outer membrane vesicles enhance tau phosphorylation and contribute to cognitive impairment. J Cell Physiol 2020; 235: 4843–4855.
- Mantri CK, Chen CH, Dong X, et al. Fimbriae-mediated outer membrane vesicle production and invasion of Porphyromonas gingivalis. Microbiologyopen 2015; 4: 53–65.
- Xu W, Zhou W, Wang H, et al. Roles of Porphyromonas gingivalis and its virulence factors in periodontitis. Adv Protein Chem Struct Biol 2020; 120: 45–84.
- Fleetwood AJ, Lee MKS, Singleton W, et al. Metabolic remodeling, inflammasome activation, and pyroptosis in macrophages stimulated by Porphyromonas gingivalis and its outer membrane vesicles. Front Cell Infect Microbiol; 7. Epub ahead of print 4 August 2017. [CrossRef]
- Johnson L, Atanasova KR, Bui PQ, et al. Porphyromonas gingivalis attenuates ATP-mediated inflammasome activation and HMGB1 release through expression of a nucleoside-diphosphate kinase. Microbes Infect 2015; 17: 369–377.
- Vanaja SK, Russo AJ, Behl B, et al. Bacterial Outer Membrane Vesicles Mediate Cytosolic Localization of LPS and Caspase-11 Activation. Cell 2016; 165: 1106–1119.
- Lu F, Lan Z, Xin Z, et al. Emerging insights into molecular mechanisms underlying pyroptosis and functions of inflammasomes in diseases. Journal of Cellular Physiology 2020; 235: 3207–3221.
- Ma L, Liu H, Wang X, et al. CXXC5 orchestrates Stat3/Erk/Akt signaling networks to modulate P. gingivalis-elicited autophagy in cementoblasts. Biochim Biophys Acta Mol Cell Res; 1868. Epub ahead of print 1 March 2021. [CrossRef]
- Noiri Y, Li L, Yoshimura F, et al. Localization of Porphyromonas gingivalis-carrying fimbriae in situ in human periodontal pockets. J Dent Res 2004; 83: 941–945.
- Chopra A, Radhakrishnan R, Sharma M. Porphyromonas gingivalis and adverse pregnancy outcomes: a review on its intricate pathogenic mechanisms. Critical Reviews in Microbiology 2020; 46: 213–236.
- Lamont RJ, Fitzsimonds ZR, Wang H, et al. Role of Porphyromonas gingivalis in oral and orodigestive squamous cell carcinoma. Periodontology 2000 2022; 89: 154–165.
- Yao L, Jermanus C, Barbetta B, et al. Porphyromonas gingivalis infection sequesters pro-apoptotic Bad through Akt n primary gingival epithelial cells. Mol Oral Microbiol 2010; 25: 89–101.
- Hegazy RR, Mansour DF, Salama AA, et al. Regulation of PKB/Akt-pathway in the chemopreventive effect of lactoferrin against diethylnitrosamine-induced hepatocarcinogenesis in rats. Pharmacological Reports 2019; 71: 879–891.
- Ho MH, Chen CH, Goodwin JS, et al. Functional advantages of Porphyromonas gingivalis vesicles. PLoS One; 10. Epub ahead of print 21 April 2015. [CrossRef]
- Omar NA, Kumar J, Teoh SL. Parkinson’s disease model in zebrafish using intraperitoneal MPTP injection. Front Neurosci; 17. Epub ahead of print 2023. [CrossRef]
- Kinkel MD, Eames SC, Philipson LH, et al. Intraperitoneal injection into adult zebrafish. Journal of Visualized Experiments. Epub ahead of print 2010. [CrossRef]
- Chandra M, Raj J, Das Dogra T, et al. Determination of median lethal dose of triazophos with DMSO in wistar rats. Article in Asian Journal of Pharmaceutical and Clinical Research; 6. https://www.researchgate.net/publication/265378795 (2014).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




