1. Introduction
Electrofreezing, the process where an applied electric field induces or enhances the nucleation of ice from supercooled water, has garnered significant interest due to its potential applications in cryopreservation, food processing, and climate science [
1,
2,
3,
4,
5]. Traditional studies have largely focused on the effects of electric field strength, electrode materials, and water purity on the freezing process [
1,
2,
4]. However, the role of electrode surface characteristics such as the roughness remains underexplored, despite its potential to significantly influence the nucleation and growth dynamics of ice crystals.
Previous research has highlighted the importance of electrode material in the electrofreezing process. For instance, Hozumi et al. demonstrated that the degree of supercooling required for ice nucleation varies with different electrode materials. Aluminum electrodes, for example, induce freezing at lower supercooling levels compared to carbon electrodes, likely due to their distinct electrochemical properties [
4]. Similarly, Braslavsky and Lipson found that high-voltage pulses could effectively induce ice nucleation in supercooled water, emphasizing the critical role of the electrode properties in determining the efficiency of the electrofreezing process [
1].
Surface features of the electrode material such as average roughness, crystalline phases and microstructure are hypothesized to play a crucial role in the electrofreezing process by providing additional nucleation sites. Rough surfaces with micro-cavities and increased surface area can potentially enhance nucleation of deionized water by offering more sites for ice crystal formation. However, this hypothesis has not been thoroughly investigated. The interplay between the surface microstructural features of the electrodes and nucleation dynamics remains a key area of interest that could offer insights into optimizing electrode design for more effective electrofreezing. For instance Q. Deng et al. [
6] investigated the effect of the microstructure of poly(dymethulsiloxane) (PDMS) and found that the energy consumption during freezing was reduced by combining an electric field and by modifying the applied substrate.
According to previously reported studies [
7], one of the ways to induce changes in the roughness of a metal surface is through LSP treatment due to the plastic deformation it induces on the surface of the treated material [
8]. Depending on the laser parameters, different modifications can be induced on the surface by laser impact [
9].
Studies on the impact of electric fields on freezing have shown that the application of such fields can reduce the size of ice crystals formed during freezing. For instance, Xanthakis et al. (2013) [
10] demonstrated that applying a static electric field during the freezing of pork meat significantly reduced the size of ice crystals, leading to less damage to the tissue microstructure of the meat. This effect is likely due to the influence of the electric field on the molecular dynamics of water, which promotes the formation of a greater number of smaller ice crystals. Similarly, Acharya et al. (2017) [
11] explored the fundamental interfacial mechanisms underlying electrofreezing and found that electric fields can enhance nucleation by aligning water molecules and reducing the energy barrier for ice formation. In addition, several other studies have analyzed the crystallization assisted by static electric fields [
12,
13,
14,
15,
16].
On the other hand, considering the microscopic effects of static electric fields on the crystallization of water, molecular dynamics studies have been carried out, with which critical electric field magnitudes have been identified to polarize the crystal with a structure similar to that of cubic ice [
17,
18].
Electrofreezing also has potential applications in atmospheric sciences, where the freezing of supercooled raindrops in strong electric fields has been studied. Smith et al. (1971) [
3] found that supercooled water drops could be nucleated more efficiently when disrupted in the presence of an electric field, highlighting the relevance of electrofreezing in natural processes such as cloud formation and precipitation .
This study aims to systematically investigate the impact of the Al-electrode surface roughness induced by the Laser Shock Processing process (LSP) on the electrofreezing of deionized water. By fabricating Al-electrodes with varying degrees of roughness and applying a consistent electric field, we will examine how surface texture influences the nucleation rate, the degree of supercooling, and the growth patterns of ice crystals. This research seeks to bridge the gap in existing knowledge by providing a comprehensive understanding of how surface characteristics affect the electrofreezing process. The findings could have significant implications for the design and application of electrofreezing technologies in various industrial and scientific fields, including enhanced cryopreservation techniques, improved food processing methods, and more accurate climate models. This study aims to contribute to the fundamental knowledge of electrofreezing and to provide practical guidelines for improving/optimizing the electrode design to achieve better control over ice formation processes. By exploring the effects of the electrode surface roughness induced by the Laser Shock Processing (LSP) method, we hope to open new avenues for research and application in this area of study. The understanding of the mechanisms behind the electrofreezing and the involved factors that influence the process is essential for advanced applications.
2. Materials and Methods
2.1. Electrofreezing Experiment
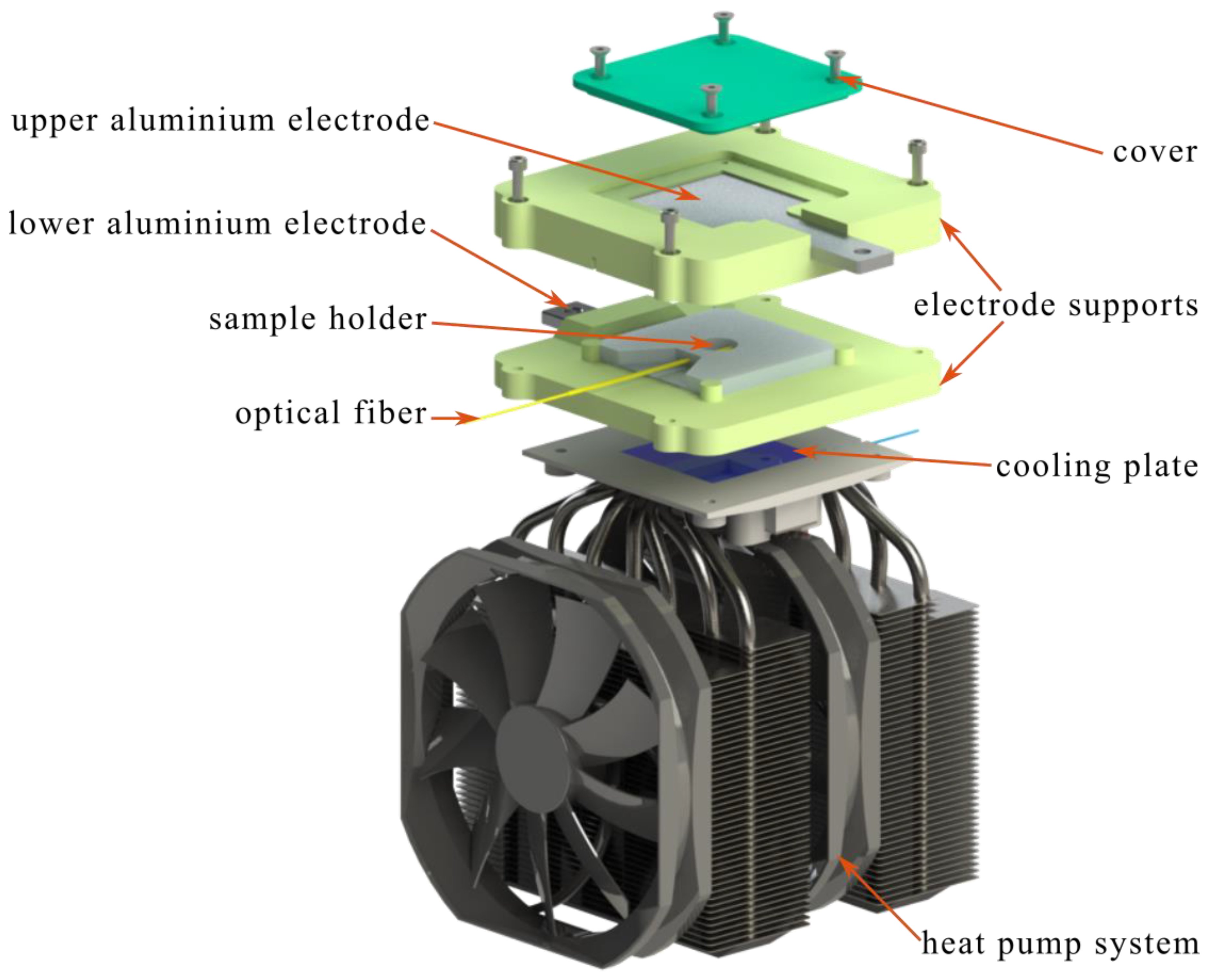
The electro-freezing equipment consists of two main systems, the first system is a heat pump that is made up of a 120 W TEC112715 Peltier effect cell measuring 50×50×3.7 mm (length × width × thickness) which is mounted on a GamerStorm Assassin III heat sink that removes the heat generated by the Peltier effect cell using two fans. The second system is coupled to the first system as can be seen in
Figure 1, it consists of two Al6061 T6 aluminum electrodes with a dimension of 46×46×5 mm (length × width × thickness), these electrodes are coupled to two support elements that keep them separated by a constant distance (parallel), an electric field was generated by the application of voltage ranging from 0 kV to 12 kV with a current of 1 mA is induced through the electrodes, which was supplied by a high voltage source GW INSTEK SPS-1820. The system contains a cavity machined on an insulating material with a diameter of 15 mm and a height of 6 mm. Deionized water with a volume of 1 ml was placed in this cavity, which served as the object of study. A fiber optic temperature sensor OMEGA FOS-LT (accuracy ± 0.8 °C) was passed through the cavity, which crossed exactly through the middle part of the water sample. The sensor monitored the real-time temperature change of the sample at a frequency of 1 Hz, an OMEGA FOM-Series interface was used to save the sensor temperature data, through a serial port, the data were transferred to a PC and graphed in real time with the LabView software.
All experimental tests carried out began with the precooling of the sample to 5 °C.This was achieved using a domestic refrigerator Whirlpool WS6601D and a Peltier effect cooling cell. The main objective of the refrigerator was to cool the heat sinks that are attached to the cooling cell and the heat from the fans, while the cooling cell decreases the temperature of the sample, due to this the electrofreezing system was installed in the inside the refrigerator to have a controlled environment. When the water sample reaches a temperature of 5 °C within the controlled environment, DC voltage was applied to the sample through the electrodes, different voltage values were applied during the experiments (0 kV to 12 kV). When the sample reached the nucleation point, the experiment continued to the crystallization zone and subsequently the sample temperature was allowed to stabilize. An experiment was also performed without application of voltage as a reference for comparison with measurements under electric fields.
2.2. The Electrode Treatments
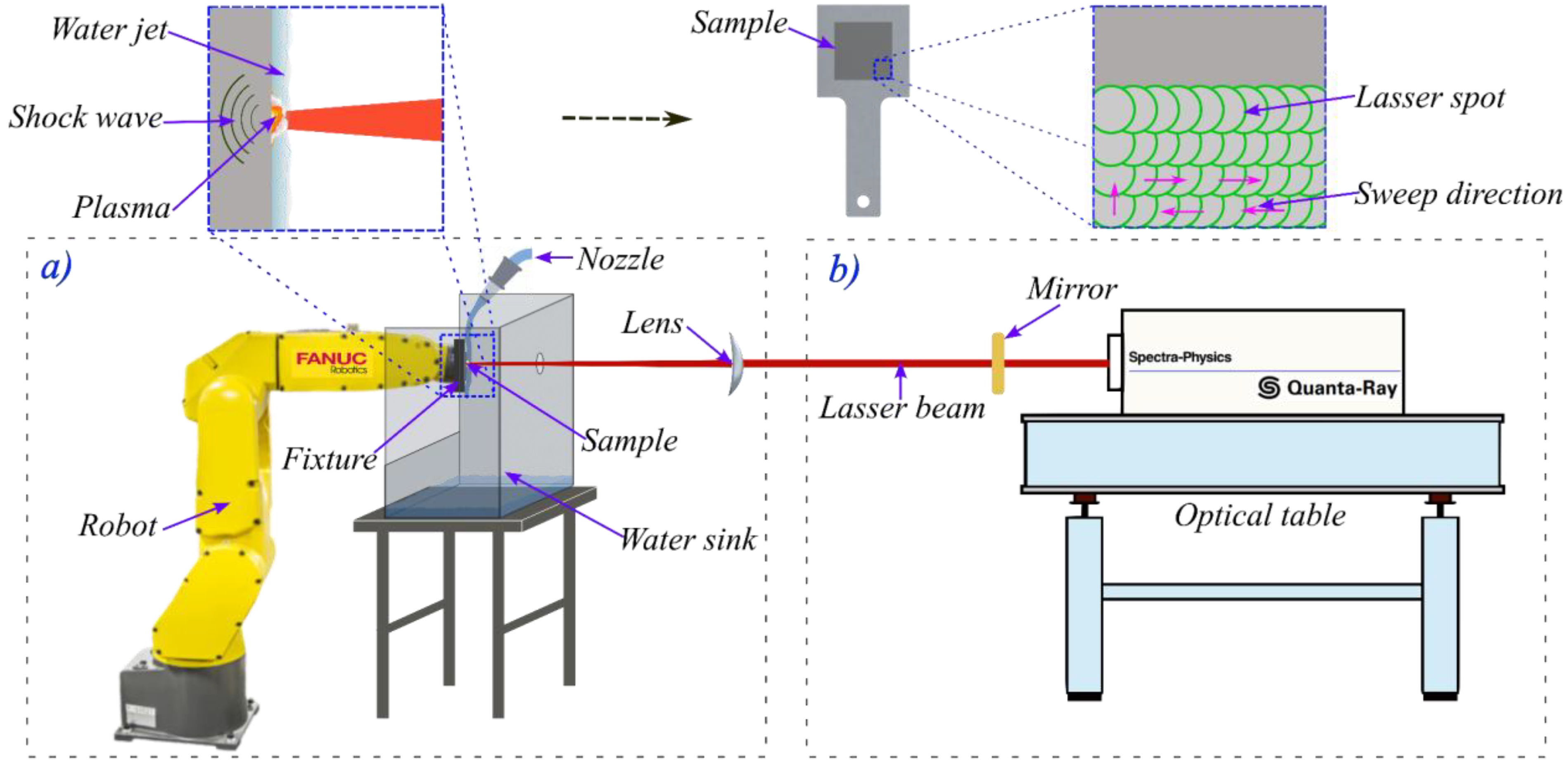
In the present investigation the commercially available Al6061 T6 alloy was used as a substrate electrode. First the Al6061 electrode was fixed in a steel plate and oriented at 90 ° related to the laser beam,
Figure 2. The Laser Shock Processing or Laser Shock Peening (LSP) process was applied using a
converging lens producing 1 J (7.5 GW/cm2) energy, 10 ns FWHM laser pulses by a Q-switched Nd:YAG laser, operating at 10 Hz with infrared (1064 nm) radiation. The pulses are focused to a diameter of 1.3 mm. The pulse density is adjusted by the trajectory and speed of a FANUC robot model LR Mate 200 iB with 6 degrees of freedom. The trajectory of the robot was maintained at a constant linear speed in order to cover the required treatment area of the electrode for the selected pulse density. The applied pulse densities were 900, 1600 & 2500 pulses/cm2 over an area of 625 mm2. The confining medium used for the laser treatment was a 1 mm thin water layer, supplied by a continuous water jet, see Figure 2a.
2.3. Microstructure and Crystalline State
The microstructure and chemical composition of the Al6061 T6 alloys before and after the LSP process were evaluated in a JEOL JSM 7200F Field Emission Scanning Electron Microscope (FE-SEM) equipped with the Ultim Max 100 EDS detector from Oxford Instruments. The crystalline state of the structured electrodes were analyzed in a SmartLab diffractometer from Rigaku applying Cu-kα radiation (40 kV, 44 Amp, λ = 1.5418 Å). The XRD patterns were acquired in a grazing incidence configuration, angle ω = 3.5°, 2θ from 25° to 85° and step size = 0.02.
2.4. Roughness Measurements
The roughness mapping was measured in a Bruker DektakXT contact profilometer, using a 12.5 µm diameter conical Stylus and a 3 mg force. For profile mapping a 400 × 400 µm area was selected with a 1 µm spacing between each profile at 30 sec thus resulting in a resolution = 0.022 µm/pt applying the hills and valleys strategy.
The roughness measurements were carried out following the guidelines of the ISO 4287 standard, a calibrated Mitutoyo SV-C3100H4 contact profilometer with a probe with a tip radius of 2 μm and an inclination of 60° was used. Four electrodes were inspected to obtain the roughness value (Ra), one of them without LSP treatment and other three with LSP treatment with densities of 900, 1600 and 2500 pulses/cm2. Ten measurements were made on each of the electrodes with a sampling length of 12.5 mm to obtain an average roughness value using 200 μm/s speed test.
3. The Effect of Pulse Density on the Surface Structuring
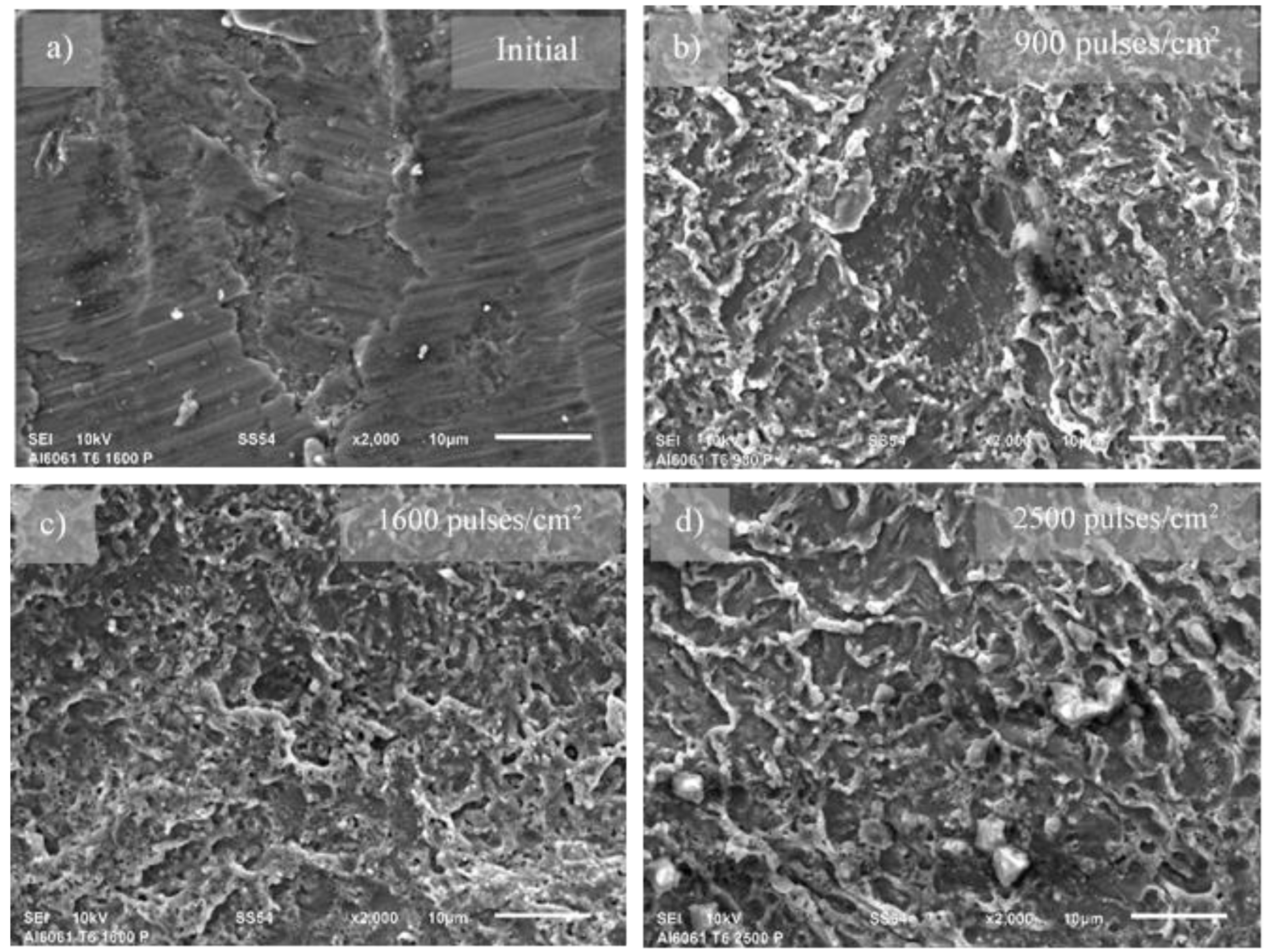
Figure 3 illustrates the impact of pulse density during the LSP process on the surface of the Al6061 T6 alloy. The SEM micrographs show the initial machined microstructure of the alloy (
Figure 3a), which clearly transforms into a wavy-like microstructure following LSP treatment (
Figure 3b to 3d). At first glance, a clear trend is observed in the microstructure as a function of pulse density, ranging from 900 to 2500 pulses/cm². Notably, at the highest pulse density, larger wave patterns emerge. This observation correlates well with the increase in average surface roughness of the LSP-treated alloys.
4. Results and Discussion
4.1. The Effect of Pulse Density on Electrode Roughness
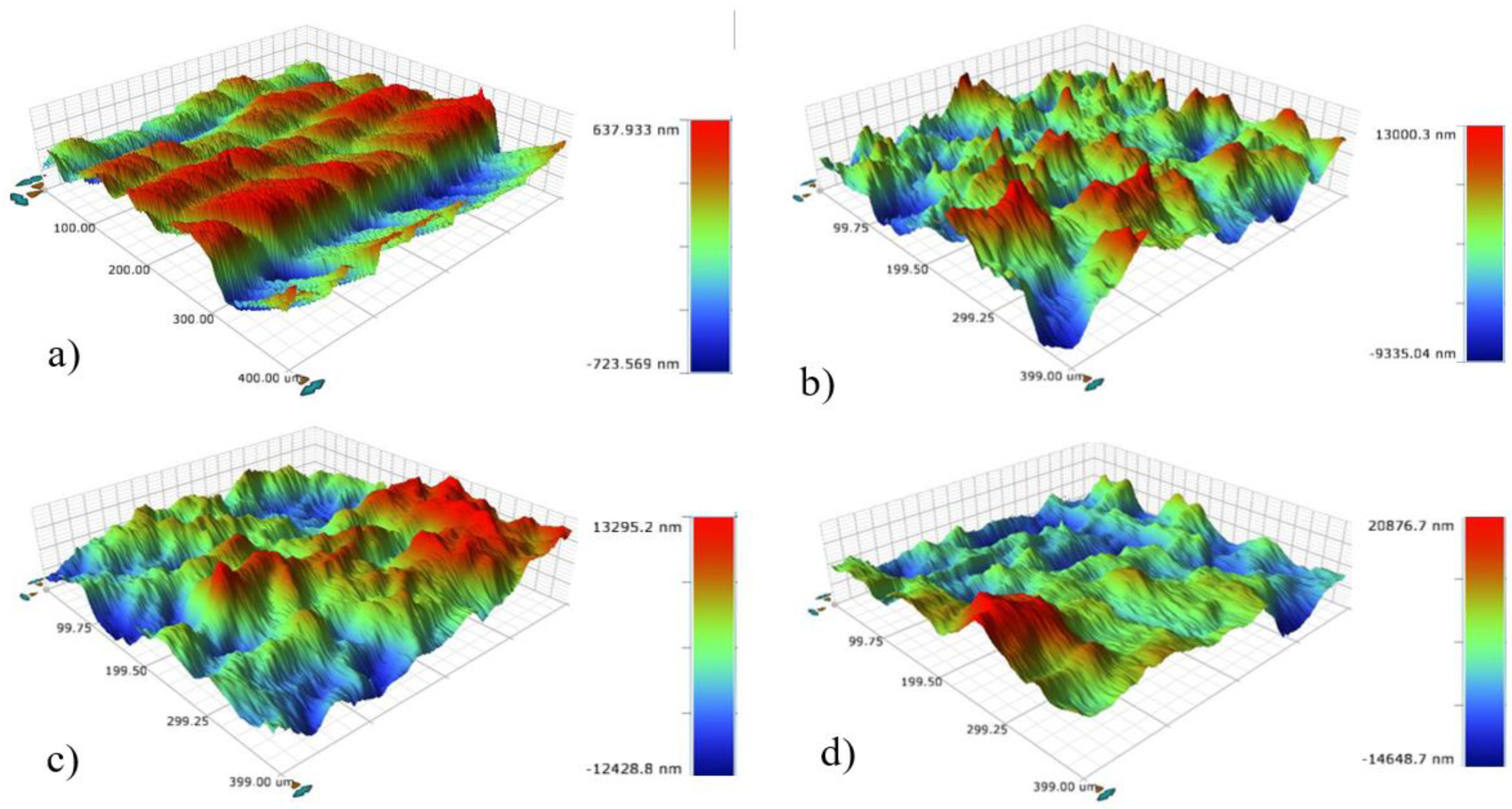
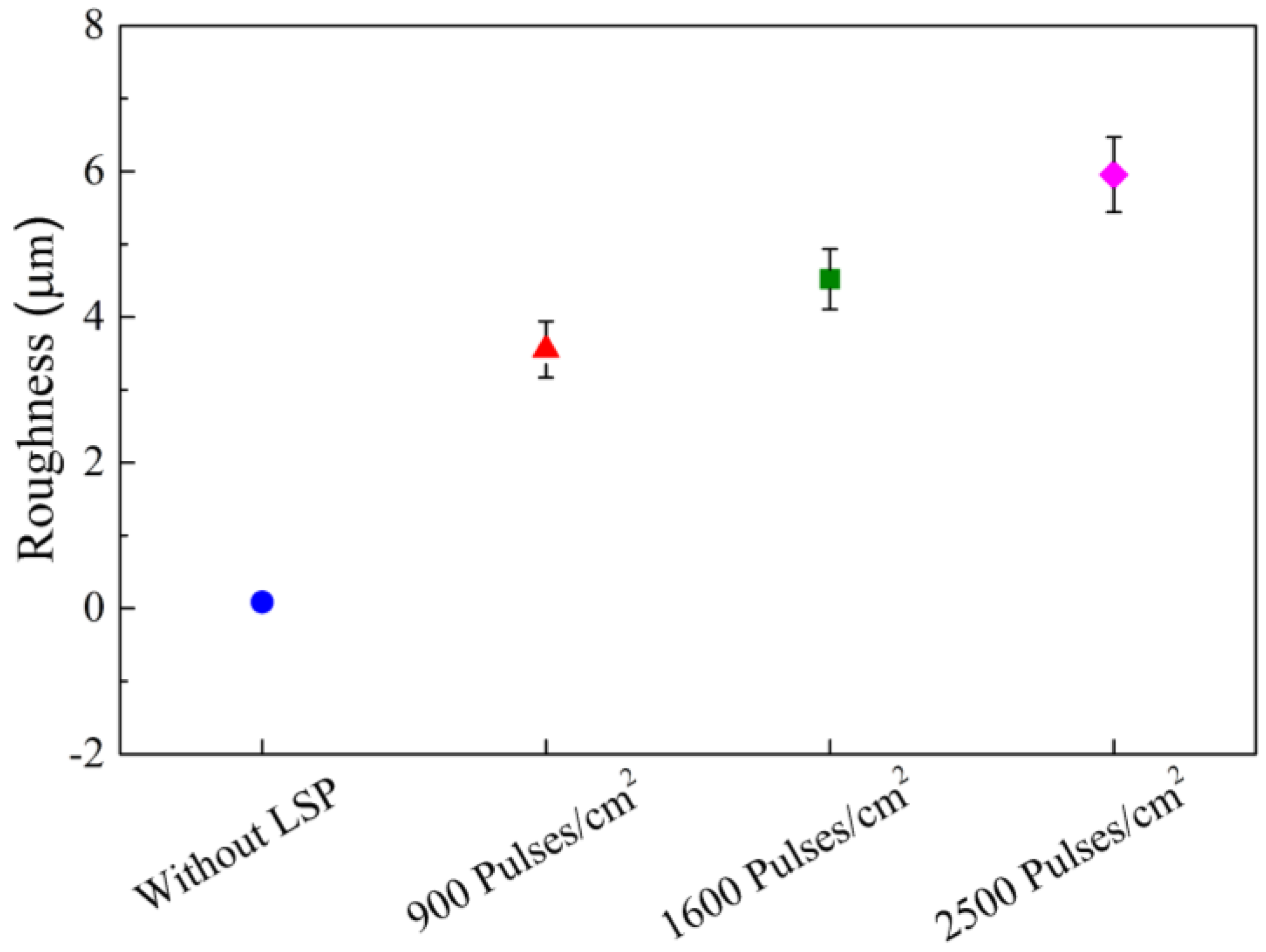
The profile mapping of the 400 × 400 µm surface area reveals a consistent increase in roughness across all electrodes following the LSP treatment. Similar to the microstructural surface evaluation, a significant increase in roughness was observed among the treatments with 900 pulses/cm², 1600 pulses/cm², and 2500 pulses/cm², as shown in
Figure 4b, 4c, and 4d, respectively. The roughness increase is further illustrated in
Figure 5, where it becomes particularly evident after the laser treatment, with the maximum valley-peak distance reaching approximately ~35.5 µm for the electrode treated with 2500 pulses/cm². The increasing trend in Ra as a function of pulse density is detectable over relatively long profile distances, starting at 400 µm. It is clear that the Ra of the Al6061 alloy increases linearly with higher pulse densities during the LSP process. This linear relationship aligns with previously reported correlations between pulse density and surface roughness in Al6061 T6 alloy. [
19].
4.2. The Effect of the LSP Process on the Crystalline State of the Electrodes
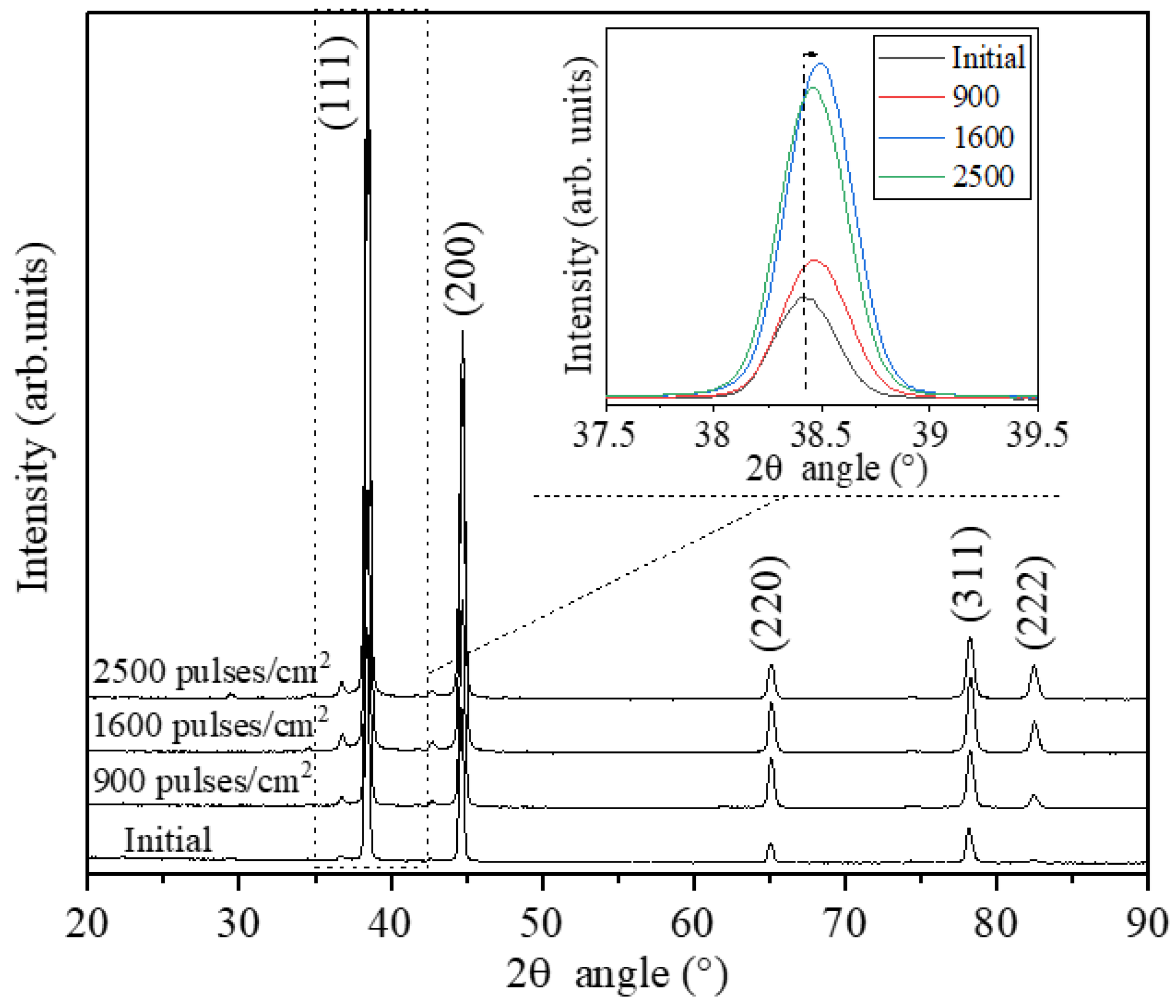
Figure 6 displays the effects of the LSP process and pulsed density on the crystalline state of the treated surface and sub-surface Al6061 T6 electrodes. There is an increasing intensity of the diffraction peaks, which is depicted by the increased intensity of the (111) direction upon the rise of the pulsed density applied during the micro-structuring process, see inset diffraction peak shown in
Figure 6. This is due to the following events induced by the laser energy and the related cooling cycles; a) grain refinement related to the severe plastic deformation processes that induce microstructural defects and high amount of dislocations, and b) the residual stress redistribution caused by the lattice decrease. There is a small shift of the 2θ angles of the diffraction peak of the (111) direction to higher 2θ values that points out to the lattice decrease, see inset image in
Figure 6. Compressive residual stress achieved by work hardening and grain refinement revealed by the micro-hardness increase up to several hundreds of micrometers which is caused by the wave impact during the LSP process has been extensively investigated and reported in the literature [
19,
20,
21]. Those investigations have been carried out mainly to improve the mechanical properties of the treated alloys.
4.3. The Effect of the LSP Treatment with Different Pulses Densities on Nucleation Temperature as a Function of High DC Voltage
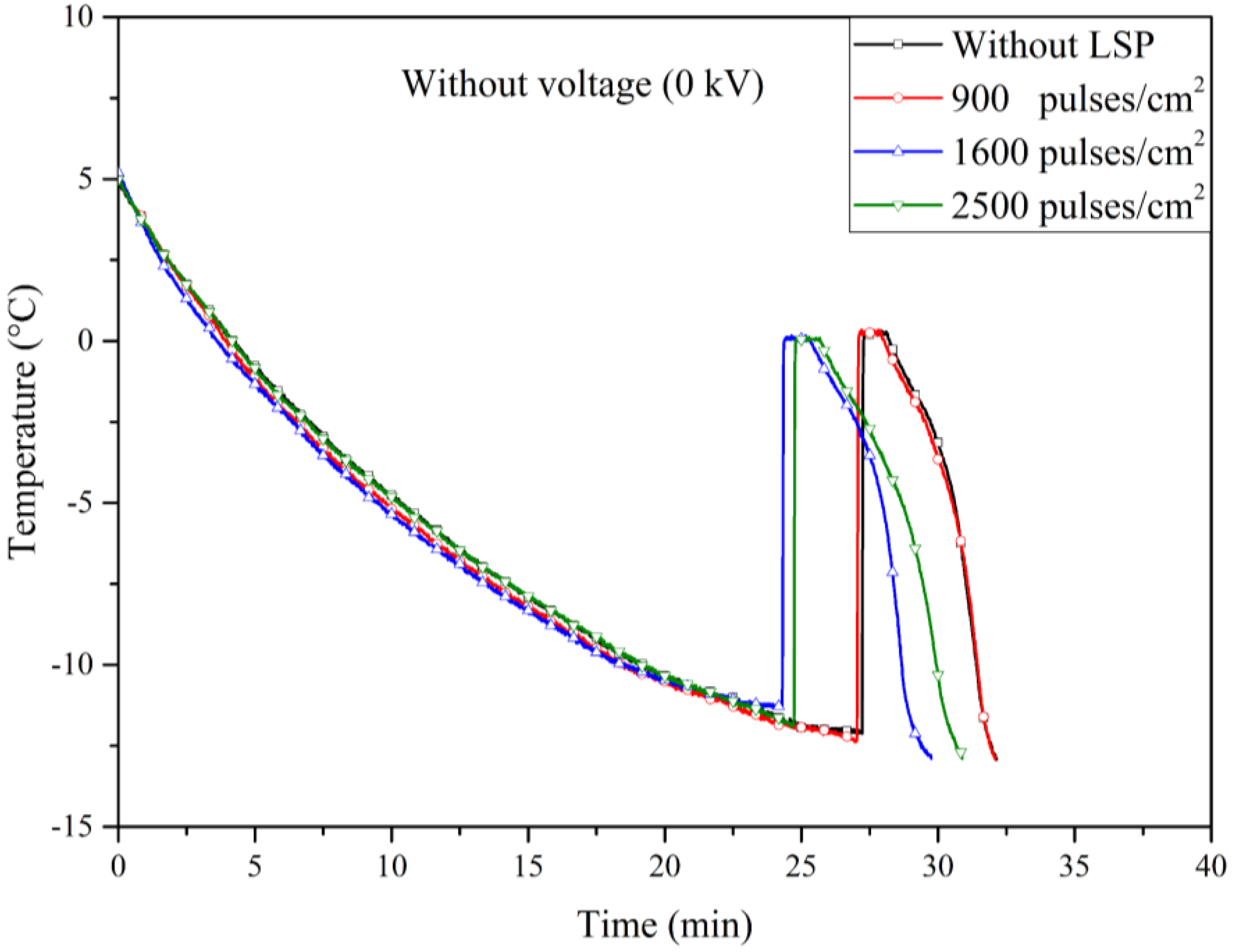
Figure 7 shows four typical temperature-time curves that were obtained during the cooling experiments of 1 ml of deionized water, the curves correspond to experiments performed before the application of voltage using an electrode without LSP treatment (black line), an electrode treated with LSP with 900 pulses/cm
2 (red line), an electrode treated with LSP with 1600 pulses/cm
2 (blue line) and an electrode treated with LSP with 2500 pulses/cm
2 (green line). All curves start their cooling at 5 °C, then they present a very similar cooling slope between them without any apparent effect due to the different pulse densities of the LSP treatment applied on the electrodes. Subsequently, the curve of the experiment with the electrode without LSP presents a nucleation temperature of -12.04 °C, while the curve of the experiment with the electrode with LSP with a density of 900 pulses/cm
2 presents a nucleation temperature of -12.34 °C. On the other hand, the experiment with the electrode with a pulse density of 1600 pulses/cm
2 showed a nucleation temperature of -11.25 °C and finally the nucleation temperature of the experiment with the electrode with LSP treatment with a pulse density of 2500 pulses/cm
2 was -11.88 °C. The freezing temperature of the deionized water during the four experiments was close to 0°.
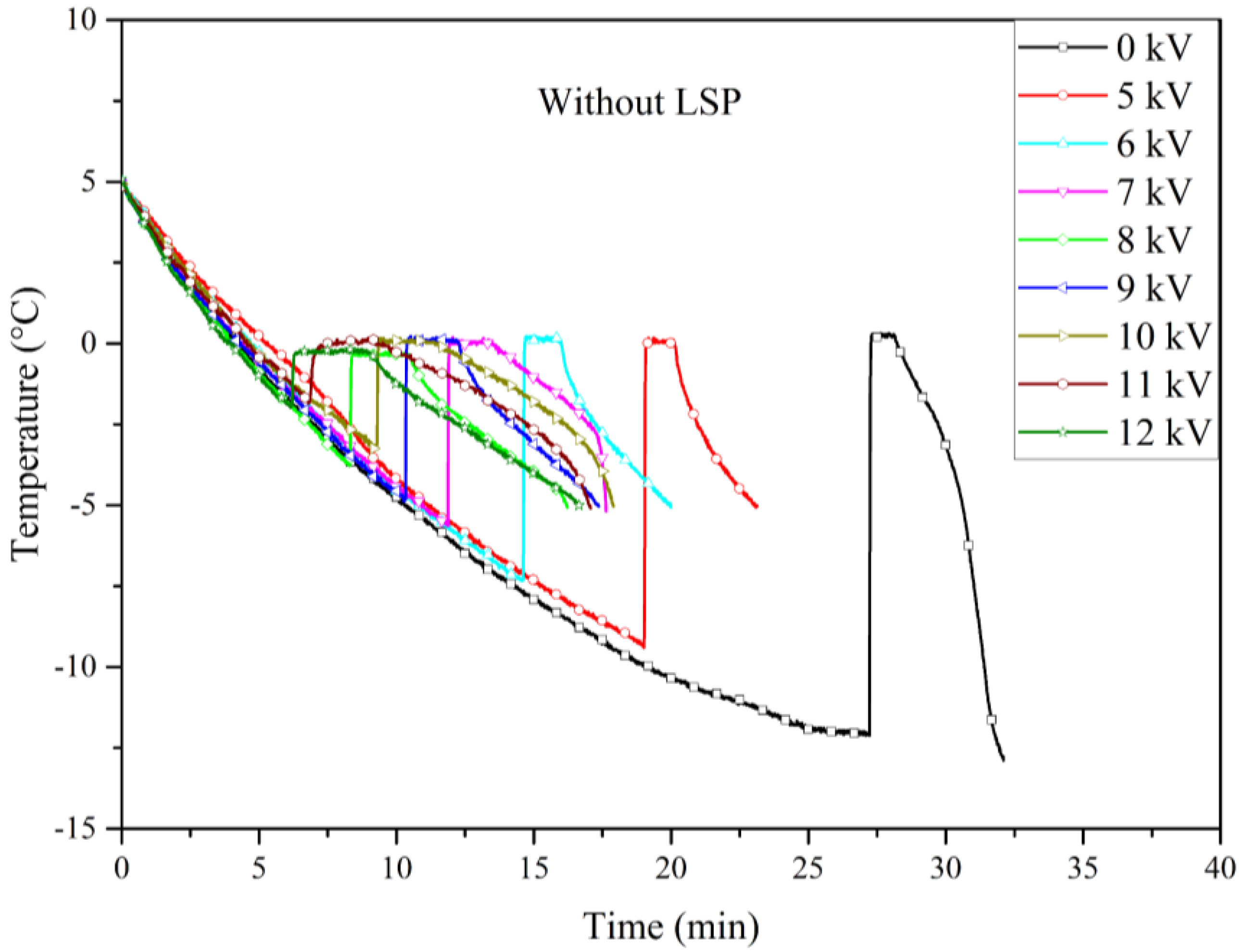
Figure 8 presents the cooling curves from the electrofreezing experiments conducted with electrodes that did not undergo LSP treatment. It was observed that the nucleation temperature increased with the applied voltage, exhibiting a generally linear trend across the range of voltages tested.
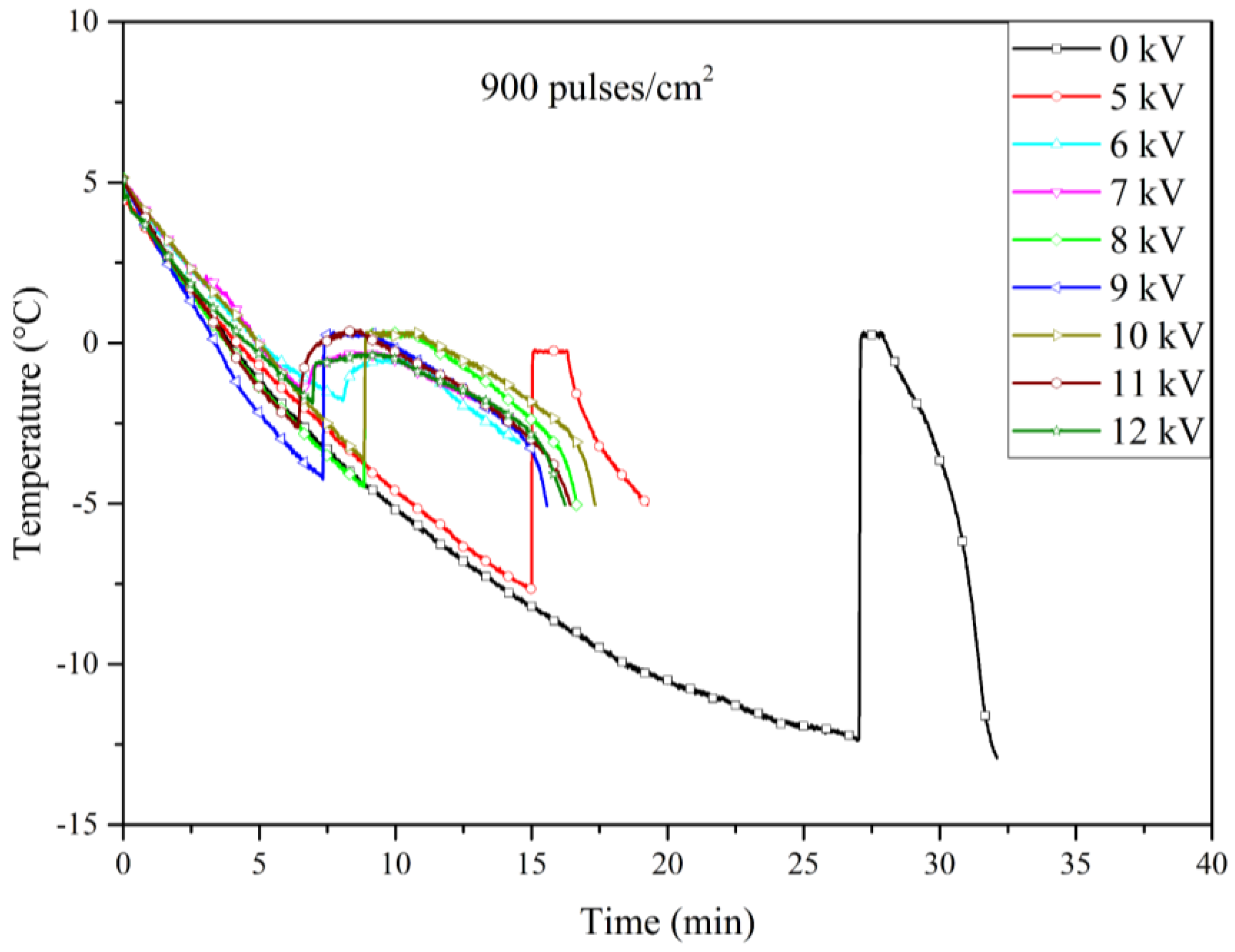
Figure 9 shows the cooling curves for the experiments using electrodes treated with 900 pulses/cm². In this case, the nucleation temperatures exhibited a significant shift to higher values compared to the untreated electrodes, including the temperature at 5 kV, which reached -7.623°C—slightly higher than that of the untreated electrode.
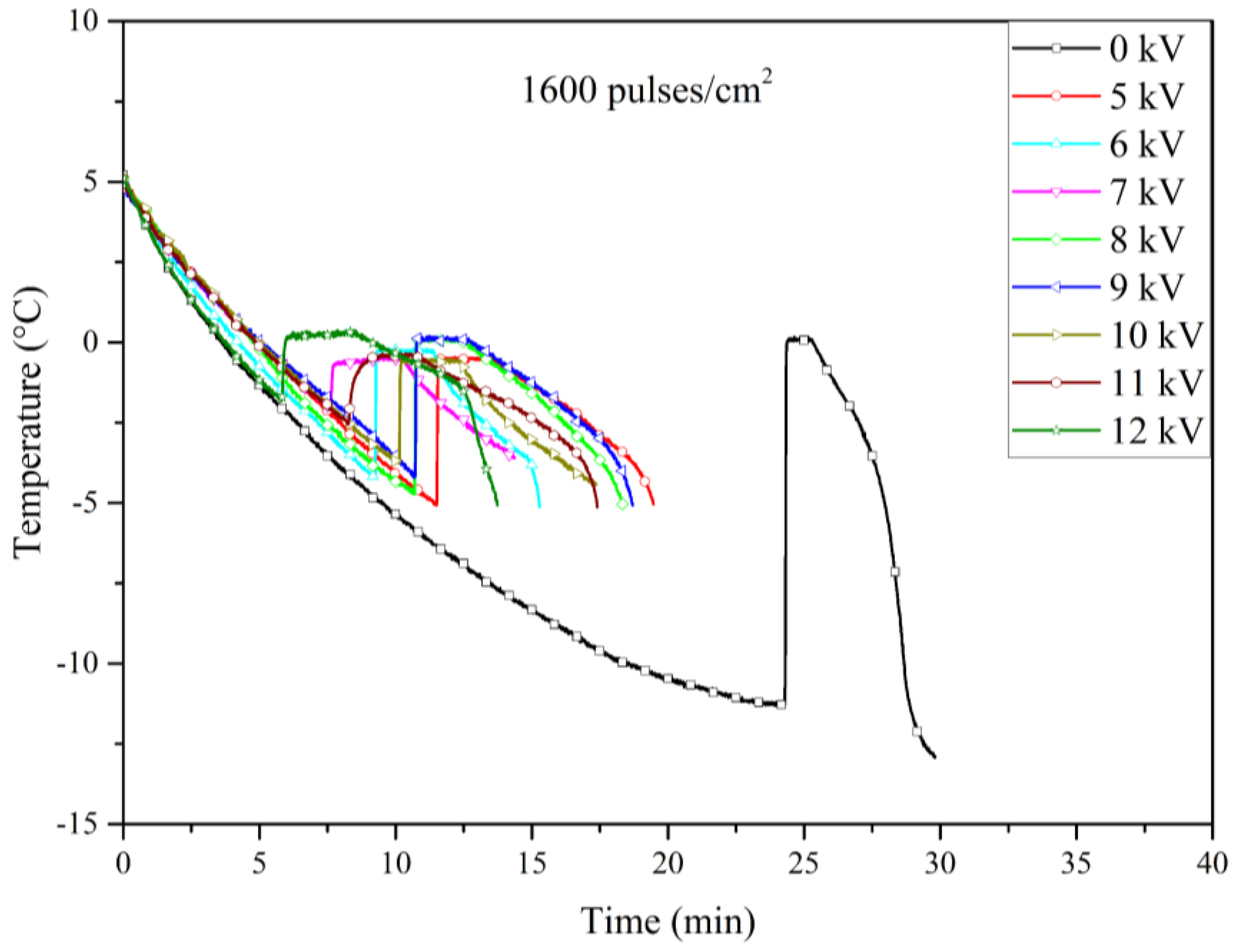
Figure 10 displays the cooling curves for the electrofreezing experiments with electrodes treated with 1600 pulses/cm². In this case, the shift towards higher nucleation temperatures was significant compared to the untreated electrodes. This shift was as pronounced as the shift observed with the 900 pulses/cm² treatment.
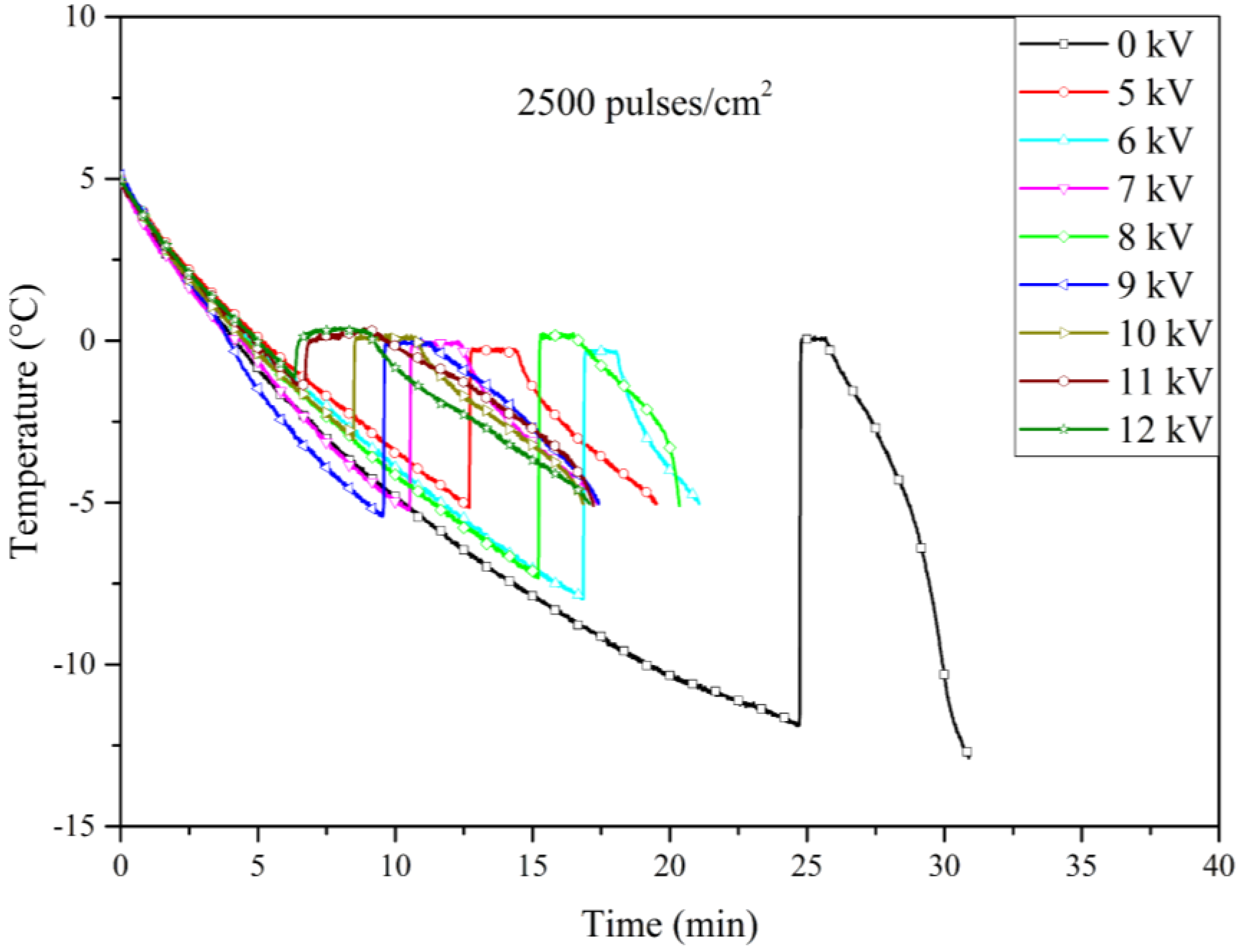
Figure 11 presents the cooling curves for the experiments using electrodes treated with 2500 pulses/cm² under increasing voltage. The shift towards higher nucleation temperatures was less pronounced than those observed with the 900 and 1600 pulses/cm² treatments, but still greater than in the untreated electrodes. The approximately linear increase in nucleation temperature with applied voltage in the untreated electrodes can be attributed to the uniform electric field distribution across their smooth surfaces, which likely does not significantly disturb the molecular structure of the water. In contrast, the LSP-treated electrodes introduce microstructural features that disrupt this uniformity, potentially creating localized electric field enhancements that may either facilitate or inhibit nucleation, depending on their configuration and interaction with water molecules.
Our results indicate that certain levels of roughness are more effective than others; in particular, the electrodes treated with 900 and 1600 pulses/cm² demonstrated superior performance compared to those treated with 2500 pulses/cm². This difference could be explained by the fact that higher pulse densities may result in surfaces that resemble untreated ones. While the increased pulse density creates more pronounced peaks and valleys, it may also reduce the overall number of these features, leading to larger, relatively flat areas. As a result, the surface might lose some of the microstructural advantages that enhance nucleation, making the 900 and 1600 pulses/cm² treatments more effective. Finally,
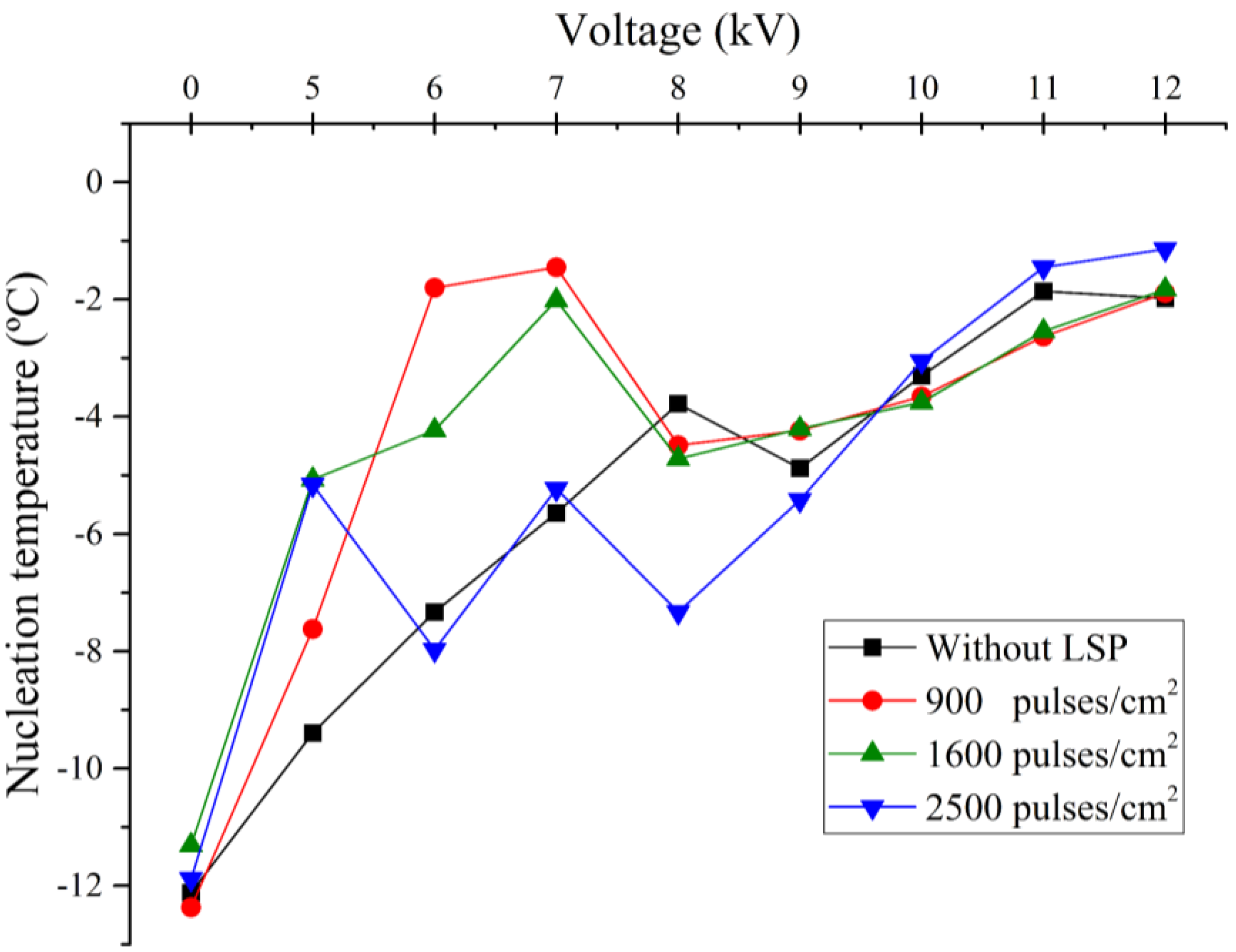
Figure 12 illustrates the relationship between nucleation temperature and the applied voltage across Al6061 T6 electrodes treated with varying pulse densities during the LSP process. While the overall trend of increasing nucleation temperature with higher pulse densities is evident, some variability was noted in the data, particularly at lower voltage levels with 2500 pulses/cm² electrode. This variability could be due to slight inconsistencies in the LSP process.
To the best of our knowledge, no prior analysis has specifically addressed the impact of surface roughness on electrofreezing. Although Hozumi (2003)[
4] conducted a study on the influence of various electrodes on electrofreezing, his investigation was limited to a single electrode tip configuration.
Table 1 presents a detailed comparison of the nucleation temperatures corresponding to each applied pulse density (900, 1600, and 2500 pulses/cm²), as well as for the untreated electrodes. The table also highlights the effects of different applied voltage levels, providing a comprehensive analysis of the relationship between surface microstructure and electrofreezing efficiency under electric field conditions.
5. Conclusions
This study demonstrates that the surface roughness of Al6061 T6 electrodes, induced by Laser Shock Processing (LSP), significantly influences the electrofreezing process of deionized water. The results indicate that increased surface roughness, achieved through varying pulse densities during LSP, plays a crucial role in ice nucleation. Specifically, electrodes with rougher surfaces exhibited higher nucleation temperatures, suggesting enhanced ice nucleation activity. This phenomenon is likely due to the concentration of the electric field at the asperities of the rough surface, which facilitates the alignment of water molecules and the formation of critical ice nuclei.
Moreover, the application of a constant electric field markedly affects the freezing dynamics, with different surface roughness levels of the electrodes leading to varied behaviors under applied voltages. This underscores the importance of precise electrode surface design in controlled electrofreezing applications. The observed correlation between pulse density during LSP and the resulting surface roughness suggests that it is possible to optimize the LSP treatment to achieve specific roughness levels that maximize electrofreezing efficiency. Notably, in our study, electrodes treated with 900 and 1600 pulses/cm² demonstrated greater efficiency in ice nucleation compared to those treated with 2500 pulses/cm². This finding implies that a controlled surface microstructure can be tuned to enhance performance in applications such as cryopreservation and food processing.
The insights gained from this study have significant implications for the design and manufacturing of electrodes in both industrial and scientific applications. By deepening our understanding of how surface roughness affects ice nucleation, we can develop more efficient electrofreezing technologies with potential applications in areas such as biological tissue preservation, the food industry, and climate modeling. This work not only advances our knowledge of the underlying mechanisms of electrofreezing but also highlights the critical role of surface treatment in optimizing the nucleation of ice. The results pave the way for future research and development in electrofreezing, promising substantial advancements in various technological applications.
Acknowledgments
D.P Luis & G.C. Mondragón-Rodríguez acknowledge the program Dirección de Investigadores e Investigadoras por México from CONAHCyT for the financial support granted during the elaboration of this manuscript. Also the authors thank to the LANITEF CONAHCyT (National Laboratory of Cooling Technologies) 322615 project for financial support and providing access to the facilities. Thanks to M.Sc Antonio Banderas for the access to the LSP equipment, and to M.Sc. Santiago Flores García & Ing. Aldair Zambrano for technical assistance during laser structuring and the surface analysis of the Al6061 T6 electrodes, and to José Antonio Sotres López for technical assistance during the cooling measurements.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- I. Braslavsky and S. G. Lipson, “Electrofreezing effect and nucleation of ice crystals in free growth experiments,” Appl. Phys. Lett., vol. 72, no. 2, pp. 264–266, Jan. 1998. [CrossRef]
- P. D. Sanz, C. de Elvira, M. Martino, N. Zaritzky, L. Otero, and J. A. Carrasco, “Freezing rate simulation as an aid to reducing crystallization damage in foods,” Meat Sci., vol. 52, no. 3, pp. 275–278, Jul. 1999. [CrossRef]
- M. H. Smith, R. F. Griffiths, and J. Latham, “The freezing of raindrops falling through strong electric fields,” Quart. J. Roy. Meteor. Soc., vol. 97, no. 414, pp. 495–505, Oct. 1971.
- T. Hozumi, A. Saito, S. Okawa, and K. Watanabe, “Effects of electrode materials on freezing of supercooled water in electric freeze control,” Int. J. Refrig, vol. 26, no. 5, pp. 537–542, Aug. 2003. [CrossRef]
- “Freezing rate simulation as an aid to reducing crystallization damage in foods,” Meat Sci., vol. 52, no. 3, pp. 275–278, Jul. 1999.
- Q. Deng et al.., “Microstructure Enhances the Local Electric Field and Promotes Water Freezing,” Ind. Eng. Chem. Res., Aug. 2022. [CrossRef]
- K. Dyer, S. Ghadar, S. Zulić, D. Rostohar, E. Asadi, and R. Molaei, “The Effect of Laser Shock Peening (LSP) on the Surface Roughness and Fatigue Behavior of Additively Manufactured Ti-6Al-4V Alloy,” Coat. World, vol. 14, no. 1, p. 110, Jan. 2024. [CrossRef]
- “Laser Shock Processing: Process Physics, Parameters, and Applications,” Materials Today: Proceedings, vol. 4, no. 8, pp. 7921–7930, Jan. 2017.
- R. Fabbro, P. Peyre, L. Berthe, A. Sollier, and E. Bartnicki, “Physics and applications of laser shock processing of materials,” in High-Power Lasers in Manufacturing, SPIE, Feb. 2000, pp. 155–164.
- E. Xanthakis, M. Havet, S. Chevallier, J. Abadie, and A. Le-Bail, “Effect of static electric field on ice crystal size reduction during freezing of pork meat,” Innov. Food Sci. Emerg. Technol., vol. 20, pp. 115–120, Oct. 2013. [CrossRef]
- P. V. Acharya and V. Bahadur, “Fundamental interfacial mechanisms underlying electrofreezing,” Adv. Colloid Interface Sci., vol. 251, pp. 26–43, Jan. 2018. [CrossRef]
- “Controlled ice nucleation under high voltage DC electrostatic field conditions,” Food Res. Int., vol. 42, no. 7, pp. 879–884, Aug. 2009.
- “Website.” [Online]. Available: . [CrossRef]
- R. W. Salt, “Effect of Electrostatic Field on Freezing of Supercooled Water and Insects,” Science, vol. 133, no. 3451, pp. 458–459, Feb. 1961. [CrossRef]
- “Website.” [Online]. Available: . [CrossRef]
- “Effect of electric currents on the nucleation of ice crystals in the melt,” J. Cryst. Growth, vol. 54, no. 2, pp. 207–210, Aug. 1981.
- “Electric field induced transitions in water clusters,” Journal of Molecular Structure: THEOCHEM, vol. 593, no. 1–3, pp. 19–32, Sep. 2002.
- “The effect of an external electric field on the structure of liquid water using molecular dynamics simulations,” Chem. Phys., vol. 244, no. 2–3, pp. 331–337, Jun. 1999.
- U. Sánchez-Santana et al.., “Wear and friction of 6061-T6 aluminum alloy treated by laser shock processing,” Wear, vol. 260, no. 7–8, pp. 847–854, Apr. 2006. [CrossRef]
- “Effect of laser shock peening on mechanical and microstructural aspects of 6061-T6 aluminum alloy,” J. Mater. Process. Technol., vol. 282, p. 116640, Aug. 2020.
- “Effect of Laser Shock Peening on surface properties and residual stress of Al6061-T6,” Opt. Lasers Eng., vol. 77, pp. 112–117, Feb. 2016.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).