1. Introduction
Polyphenolic compounds used as food additives or prebiotics are known to have positive effects on metabolic health [
1,
2]. Hypoglycemic, hypolipidemic, anti-inflammatory, antioxidant, and other beneficial effects were demonstrated for synthetic [
3] and natural [
2] polyphenols such as resveratrol [
4], sylimarin [
5], flavonoids [
6], alkylresorcinols [
7], etc.
5-Pentadecylresorcinol (C15) is a natural alkylresorcinol that has been shown to protect against complications caused by a high-fat diet (HFD), such as hyperglycemia, and induce changes in microbial communities in both the large and small intestines of mice [
8]. In our previous research, we have shown that supplementation with C15 with HFD significantly increased the representation of the probiotic bacteria
Akkermansia muciniphila and
Bifidobacterium pseudolongum in the mouse gut, as well as the increased alpha diversity of microbial communities in the large, but not small, intestines. Although the exact mechanisms of beneficial activity of alkylresorcinol are not known, we assume that the protective effects of C15 and other resorcinol homologues on metabolic health are mediated at least partially by their modulatory influence on intestinal microbiota composition and functional activity.
Imbalanced nutrition, such as HFD, can lead to multiple fast and delayed disturbances, including obesity, nonalcoholic liver steatosis, insulin resistance, type 2 diabetes mellitus, dyslipidaemia, atherosclerosis, etc. Furthermore, both short-term and prolonged HFD are associated with the development of gut microbiota dysbiosis [
9,
10], which has been shown to contribute significantly to the dysmetabolic state of the host. Previously, we have shown that the obesogenic microbiota of leptin receptor-deficient mice (db/db) has a much lower predicted representation of metabolic pathways and enzymes for vitamin B12 biosynthesis compared to C57Bl6 mice that receive a normal or high-fat diet [
11]. We have also found that C57Bl6 mice that receive an HFD have a lower representation of the metabolic pathways for vitamin B12 synthesis compared to C57Bl6 mice that receive a normal diet. Furthermore, in HFD-fed mice, the representation of several bacterial species was substantially related to the representation of enzymes involved in cobalamin production, whereas in
db/db mice, there were almost no correlations. Therefore, the decrease in the representation of the vitamin B12 synthesis pathways in the gut may be related to the specific obesogenic gut microbiota. Our findings are confirmed by several other works, although there are few studies investigating the microbial synthesis of vitamin B12 by the gut microbiota based on environmental factors such as diet.
In the study by Degnan et al., it is discussed that vitamin B12 may have an unrecognised role in influencing the composition and function of human gut microbial communities [
12]. Less than 25% of sequenced human gut bacteria possess the genetic ability to generate corrinoids, despite the fact that more than 80% of these bacteria consume them [
12]. Because corrinoids, unlike many other vitamins, are made only by bacteria and archaea and because various bacteria require different groups of corrinoids, the authors postulated that microbial communities can be manipulated by changing the levels of specific corrinoids [
12].
This statement was confirmed in a recent study by Sun et al. in which silymarin (a mixture of flavonolignans and some other polyphenolic compounds) has been shown to cause an improvement in lipid metabolism through the production of bacterial vitamin B12 that was also associated with changes in the intestinal microbial community [
13]. Thus, cobalamin is not only produced by the gastrointestinal microbiota, but also contributes to the ecology of the gut microbiota.
In the study by Mok et al. [
14] the advantageous human gut bacteria
Akkermansia muciniphila has been shown to be capable of using a wide variety of cobamides due to its ability to modify the structure of cobamides through a process known as cobamide remodelling. The researchers claim that the role of
A. muciniphila as a key stone species of the microbial community [15] is defined not only by its ability to degrade mucin to provide nutrients to the intestinal microbiota [
15], but also by altering the structure of cobamide. Therefore, cobamides are believed to be crucial modulators of mammalian intestinal ecosystems because they participate in multiple metabolic pathways, only a minority of prokaryotes can produce them, and because various microorganisms can access their complex structures differently [
14].
Taking into account the beneficial properties of ARs on metabolic health and their modulatory activity in the gut microbiota, we hypothesised that pentadecylresorcinol may influence the representation of enzymes and pathways for vitamin B12 synthesis in the gut microbiome, providing compositional and functional changes in the microbial community. High-throughput sequencing of the contents of the small and large intestines of C57Bl6 mice fed a regular or high-fat diet with or without C15 supplementation was performed followed by reconstruction of microbiota metabolic activity due to the PICRUSt2 algorithm to clarify the role of C15 in vitamin B12 synthesis by the gut microbiota.
2. Materials and Methods
2.1. Experimental Animals and Study Design
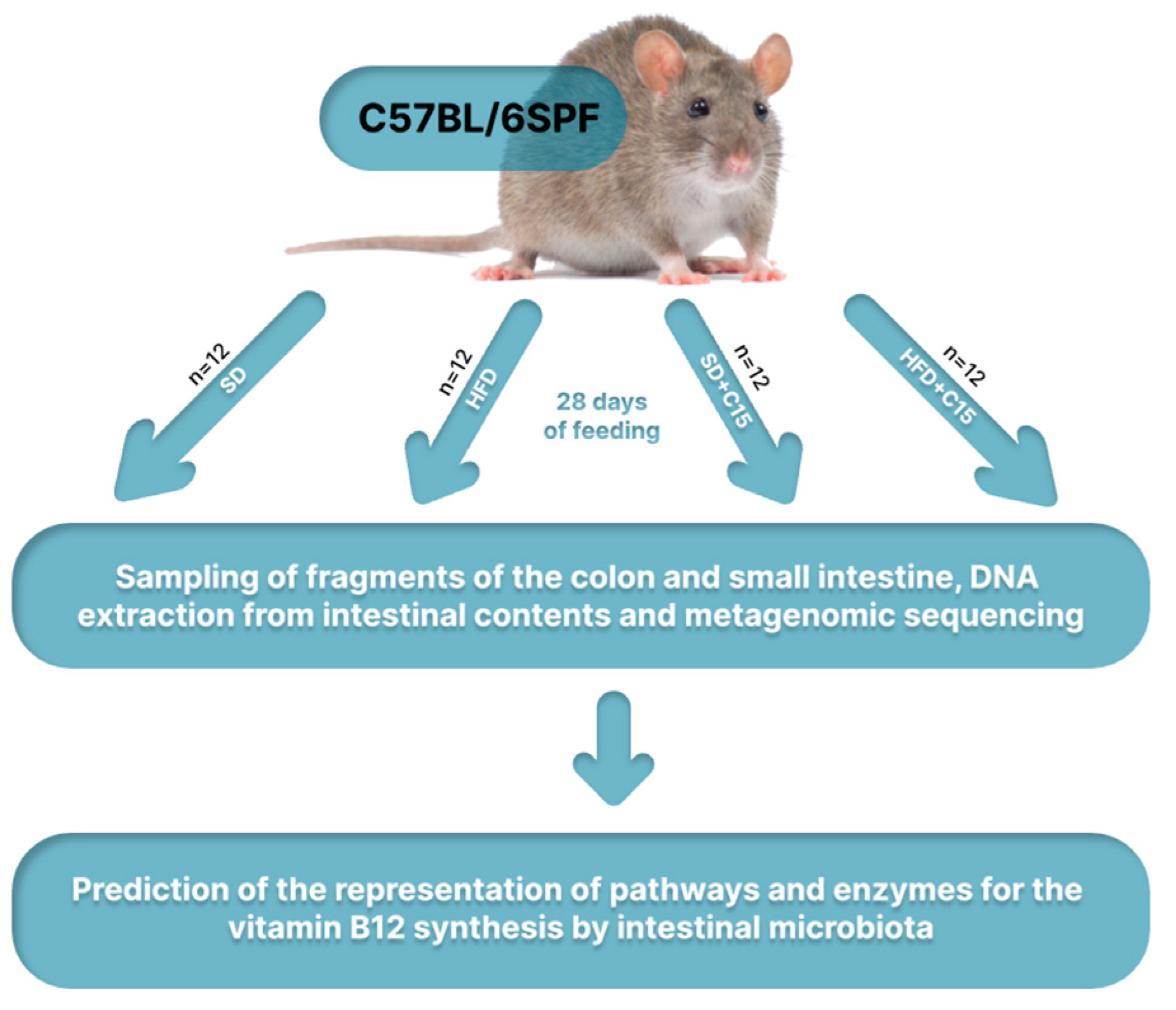
The following conditions were applied to the 48 female C57BL/6SPF mice that were raised at the Laboratory Animal Nursery in Puschino, Russia, and kept in the animal center of the SPF level of Sechenov First Moscow State Medical University (Moscow, Russia): 55% humidity, 22 °C, and a 12-hour light-dark cycle. One week prior to the start of the formal trial, the experimental animals were provided with sterile food (Altromin 1324 FORTI, Lage, Germany) and water
ad libitum. After the adaptation phase, the mice were divided into four groups of 12 animals each, with a maximum of ±10% variation in total weight between the groups. At the beginning of the trial, the mice were 4-5 weeks old and had an average weight of 14.4 ± 0.96 g. By giving laboratory animals a high-fat diet (HFD) (Altromin C 1090-30, Lage, Germany) that was enhanced with triglycerides produced from animals and constituted as much as 30% of their total caloric intake, a high-fat dietary model was created. Throughout the duration of the experiment, the animals in the control group were fed a standard diet (SD) (Altromin 1324 FORTI, Lage, Germany). 5-n-Pentadecylresorcinol (C15) was administered by injection in conjunction with a standard or high-fat diet (Hangzhou ROYAL Import & Export Co., Ltd., Hangzhou, China) (
Figure 1).
All experimental animal procedures were approved by the Ethics Committee for Animal Research, I.M. Sechenov First Moscow State Medical University, Moscow, Russia (protocol number 96 from 2 September 2021). All experimental procedures were performed according to the relevant guidelines and regulations. All methods are reported following the ARRIVE guidelines.
2.2. Sampling of the Large Intestinal and Small Intestinal Microbiota for Metagenome Analysis
Tissue samples from the colon and jejunum were obtained under sterile conditions as previously described [
16]. For metagenomic analysis, the jejunum and its contents were sectioned into 1-cm-long sections. After that, each piece was placed in a different sterile Eppendorf tube, dried, and delivered for high-throughput sequencing analysis. In the same manner, colon samples were collected and preserved. Amplicon concentration was ascertained using the Qubit dsDNA High Sensitivity Assay Kit (Invitrogen, Carlsbad, CA, USA) and the Qubit 2.0 fluorometer (Invitrogen, Carlsbad, CA, USA). The concentration of amplicons was measured using a Qubit 2.0 fluorometer and the Qubit dsDNA High Sensitivity Assay Kit (Invitrogen, Carlsbad, CA, USA). Before sequencing, the components were combined in an equal mole ratio to finish library preparation. The libraries were then sequenced at high throughput (Illumina MiSeq, Illumina, CA, USA) using 2 × 300 bp reads. PICRUSt2 v2.5.2 and QIIME2 v2023.7.0 [
17] were used to process the raw readings.
2.3. High-Throughput Sequencing Analysis and Reconstruction of Intestinal Microbiota Metabolic Activity
The microbiota investigation was carried out by the Scientific Research Laboratory "Multiomics Technologies of Living Systems" in Kazan, Russia. Using the FastDNA TM Spin Kit for Feces (MP Biomedicals, Santa Ana, CA, USA), genomic DNA was extracted from the contents of the mouse intestine. The V3–V4 region of the bacterial 16S rRNA gene was amplified using certain primers (forward: TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGCCTACGGGAGGCAGCAG and reverse: GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGACTACAAGGGTATCTAATCC). Each sample was barcoded using index primers during the second round of PCR amplification following purification of the AMPure XP bead-based PCR product (Beckman Coulter, Brea, CA, USA, CB55766755). The concentration of amplicons was measured using a Qubit 2.0 fluorometer and the Qubit dsDNA High Sensitivity Assay Kit (Invitrogen, Carlsbad, CA, USA). Before sequencing, the components were combined in an equal mole ratio to finish library preparation. The libraries were then sequenced at high throughput (Illumina MiSeq, Illumina, CA, USA) using 2 × 300 bp reads. PICRUSt2 v2.5.2 software
https://huttenhower.sph.harvard.edu/picrust/ (accessed on 12 September 2023) and QIIME2 v2023.7.0 [
17] were used to process the raw readings. The results of the sequencing data of the PICRUSt2 v2.5.2 algorithm were used to examine the microbial metabolic pathways encoded by the discovered bacterial genomes. Multiple t test analysis was used to determine which paths were the most abundant.
2.4. Statistical Data Analysis
Nonparametric statistics approaches were used to process the data using GraphPad Prism 10 v10.0.2 (171), a statistical program. All in vivo experimental data were analyzed using Welch's one-way analysis of variance (ANOVA), t-test or multiple Mann-Whitney tests with the two-stage step-up technique (Benjamini, Krieger, and Yekutieli) (false discovery rate Q = 5%). Statistical significance was defined as p-values less than 0.05. A correlation analysis was performed according to Spearman with an assessment of the statistical significance of the correlation coefficient.
3. Results
3.1. Predicted Representation of Pathways and Enzymes for Vitamin B12 Synthesis in the Small Intestine of Mice
We carried out microbiota metagenome sequencing analysis for both small and large intestinal microbiota samples. The compositional characteristics, as well as the diversity and richness of the microbial communities of mice fed a standard or high-fat diet with or without C15 supplementation, are represented in previous work [
8]. Briefly, we have established that supplementation with C15 with an HFD increased the alpha diversity indices of the microbial community of the large, but not small intestine compared to a standard or an HFD alone. Furthermore, supplementation with C15 with an HFD significantly increased the representation of several probiotic species such as
A. mucicnifila and
B. pseudolongum both in the small and large intestinal microbiota. However, the exact mechanisms of C15 modulatory activity are not known.
To establish whether enzymes and metabolic pathways for B12 synthesis are under the influence of diet type and C15 supplementation, we performed a reconstruction of microbiota metabolic activity using a PICRUSt2 tool based on metagenome sequencing data analysis, which allowed us to estimate the predicted abundance of bacterial genes in microbial communities of the mouse gut.
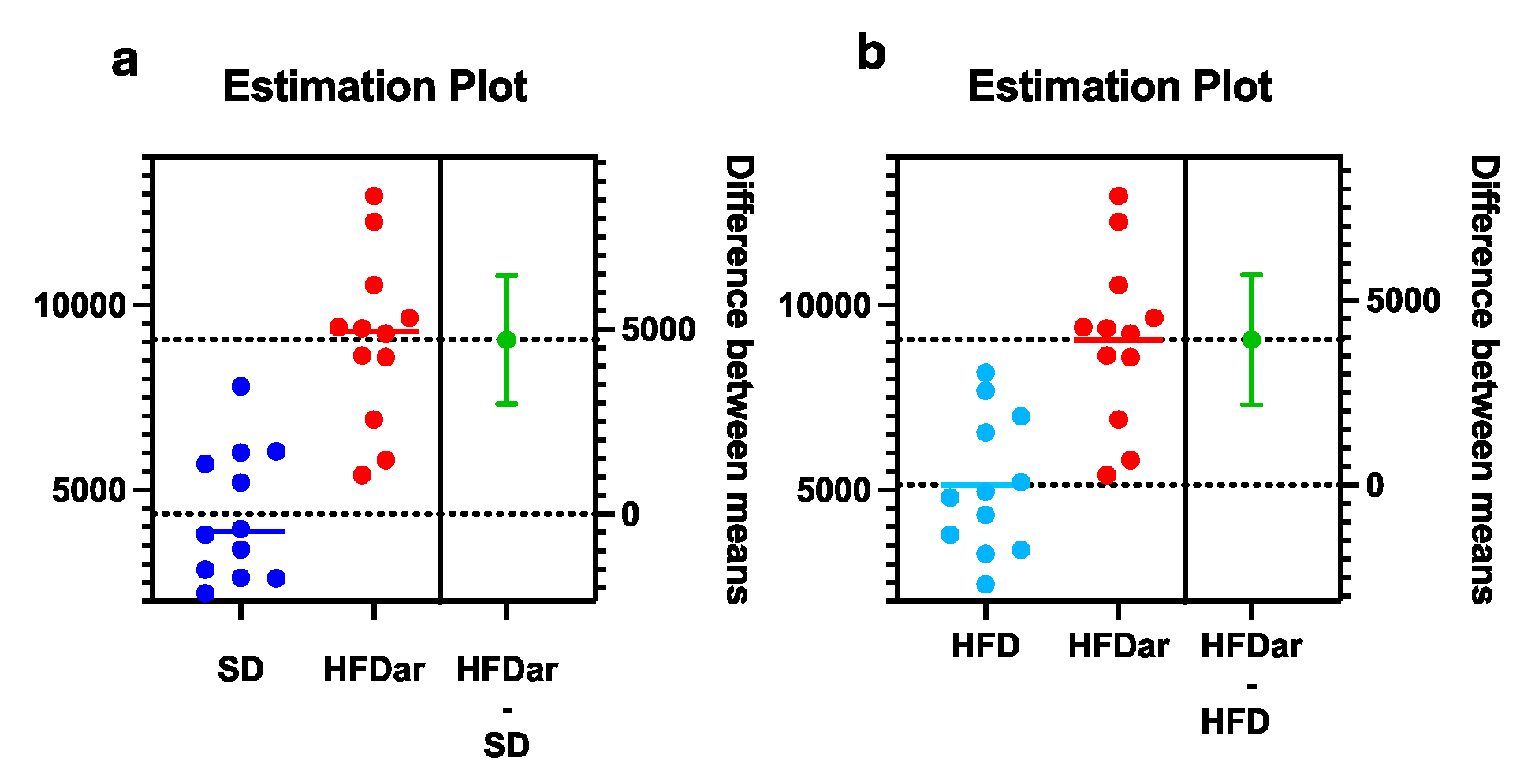
According to the results of PICRUSt2, multiple nonparametric t tests, and Welch's t tests we revealed that among the 438 metabolic pathways analysed, the abundance of only one pathway involved in B12 metabolism - salvage of adenosylcobalamine from cobinamide I in the microbiota of the large intestine was over-represented in the group that received HFD + C15 compared to the groups that received SD or HFD without C15 (
Figure 2), while there were no differences in the representation of pathways for B12 synthesis in the small intestine or between other groups.
Therefore, we have established that the representation of the pathway for B12 synthesis was not dependent on diet type but was dependent on C15 supplementation.
PICRUSt2 analysis has been used to estimate the predicted representation of enzymes involved in B12 synthesis. To this end, we chose 37 enzymes required for B12 synthesis or salvage among more than 8000 enzymes represented in the microbiomes investigated. Among these enzymes in the microbiome of the large intestine, only four enzymes were over-represented in the group of mice that received an HFD+C15 compared to the group that received HFD, and two enzymes were under-represented in this group (
Table 1).
A similar result was obtained in comparison of mice fed an SD compared to mice fed an HFD+C15 (
Table 2).
In particular, all enzymes (EC:2.4.2.21; EC:2.7.1.156; EC:2.7.8.26; EC:6.3.1.10) except two enzymes (EC:2.1.1.107; EC:3.6.3.33) over-represented in the microbiota of mice received an HFD+C15 were also correlated with the salvage of adenosylcobalamine from the cobinamide I pathway.
Therefore, the predicted representation of enzymes for B12 synthesis in the large intestine was not influenced by the HFD diet alone but was dependent on HFD C15 supplementation. On the other hand, C15 did not influence vitamin B12 synthesis in the small intestine and was used as a supplement to an SD.
3.2. Correlation Analysis for Enzyme and Microbial Representation in the Small Intestine of Mice
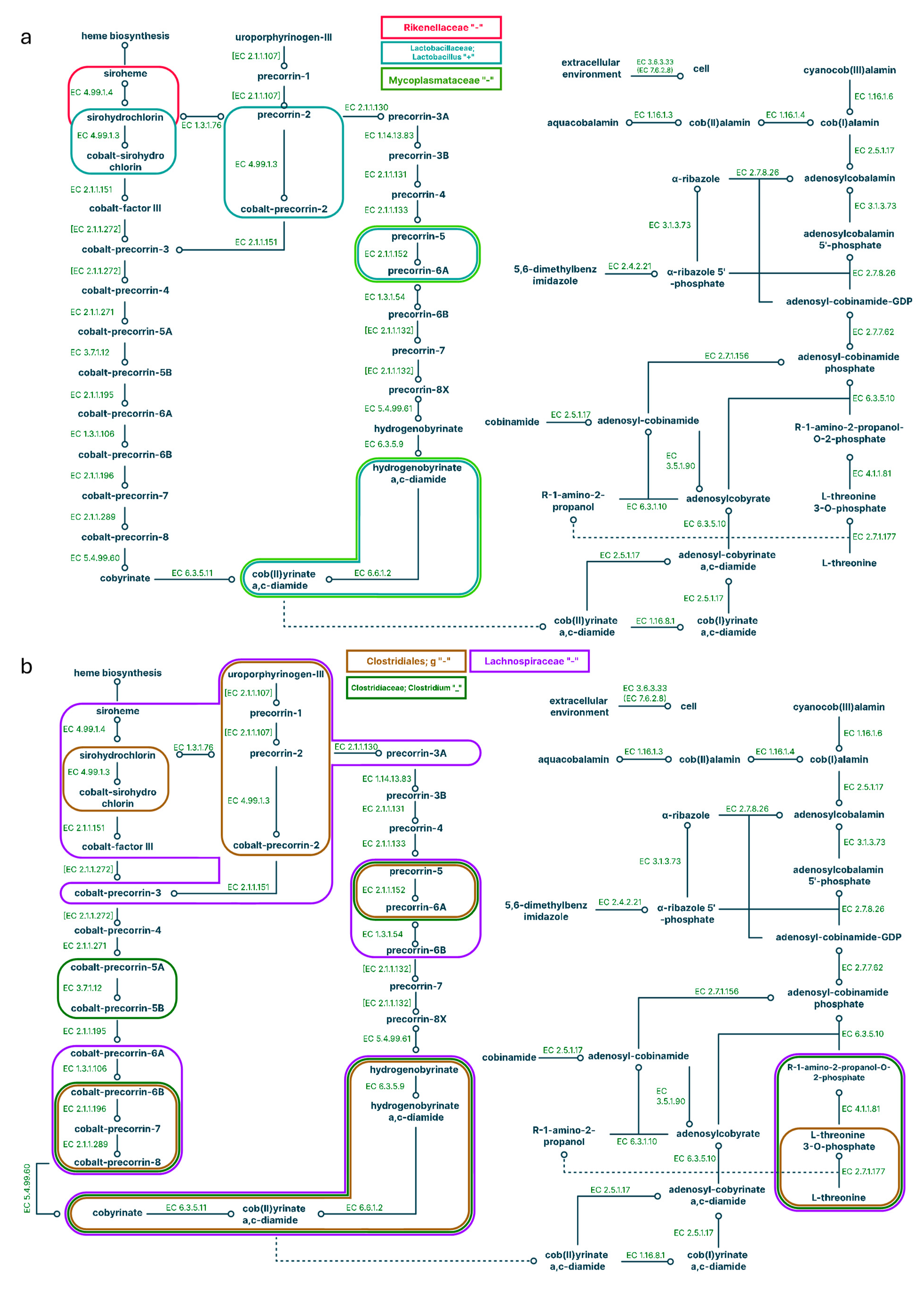
To further investigate the relationship of the representation of the B12 synthesis enzyme and the taxonomy composition of the gut microbiota at the genus level of mice, we performed a correlation analysis. In the small intestine, we have found only few correlations in the SD group that were related to AF12 and unidentified genera of the
Rikenellaceae family (
Figure 3a), while many correlations have emerged in an SD+C15 group (
Figure 3b,c;
Supplementary Tables ST1, ST2).
It is noteworthy that most of the correlations were related to different genera of the
Clostridia class, namely
Clostridium,
Ruminococcus,
Coprococcus,
Oscillaspira and several unidentified genera (
Supplementary Table ST2). Furthermore, all significant correlations were negative in the 'SD + C15' group, in contrast to the ‘SD’ group, where the correlations had a bidirectional character (some were positive and some were negative). However, there were no correlations for genera differentially represented in the ‘SD’ and ‘SD+C15’ groups.
When looking at the correlation in the ‘HFD’ and ‘HFD + C15’ groups (small intestine) we have found that the representation of 10 of 37 enzymes for the synthesis of B12 was positively correlated with the
Akkermansia genus in the HFD group, while all these correlations except one (for EC 2.1.1.152) were lost in the ‘HFD + C15’ group (
Supplementary Tables ST3, ST4). In particular, we had established that the genus
Akkermansia was over-represented in both the small and large intestines of mice fed an HFD + C15 (
Supplementary Tables ST7, ST14, ST16). Furthermore, in the 'HFD + C15' group we observed negative correlations for the unidentified genus of the
Mycoplasmataceae and
Desulfovibrionaceae families, which showed positive correlations in the 'HFD' group and became less represented in the 'HFD + C15' group compared to the 'SD' group (
Supplementary Tables ST3, ST4, ST7). Furthermore, positive correlations for
Suterella and
Prevotella have appeared in the 'HFD + C15' group, and thus the representation of
Suterella has increased in the 'HFD + C15' group compared to the 'HFD' group (
Figure 4;
Supplementary Tables ST4, ST8).
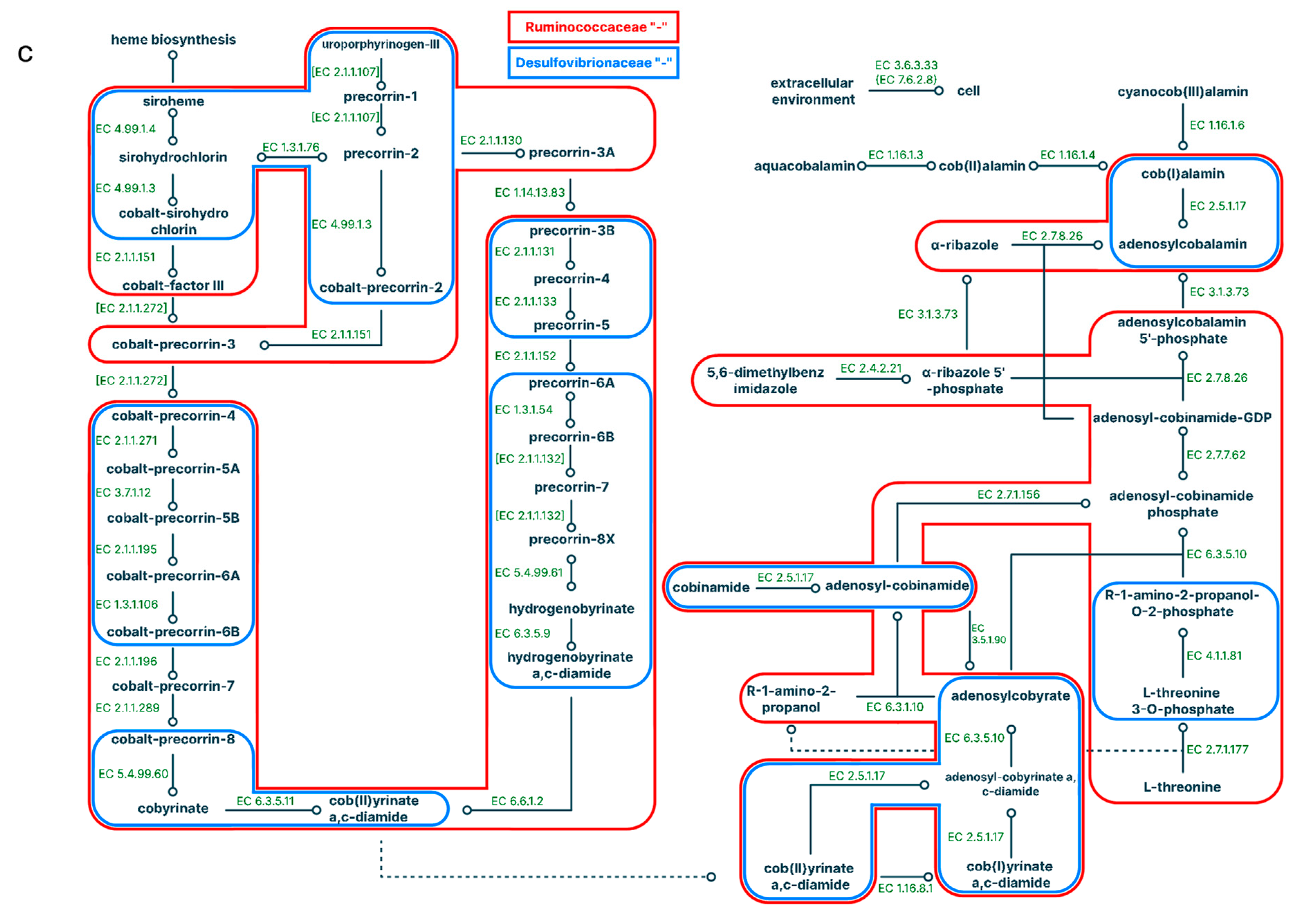
3.3. Correlation Analysis for Enzyme and Microbial Representation in the Large Intestine of Mice
In the large intestines in the 'SD' group again there were only few negative correlations for separate enzymes with the genera
Prevotella,
AF12,
Allobaculum, [
Ruminococcus] and an unidentified genus of the
Clostridiales family, while in the 'SD + C15' group strong negative correlations for the genus
AF12 and positive correlations for the
Akkermansia genus have appeared (
Figure 5;
Supplementary Tables ST9, ST10).
In the 'HFD' group, three genera (
Allobaculum and unidentified genera from
Clostridiaceae and
Peptostreptococcaceae families) showed strong negative associations with the most enzymes for B12 synthesis, and there were almost no correlations for the
Akkermansia genus. In particular, the representation of
Allobaculum and the unidentified genera of
Clostridiaceae and
Peptostreptococcaceae families increased in the 'HFD' group compared to the 'SD' group, while the
Akkermansia genus was decreased in contrast (
Supplementary Tables ST11, ST12).
In the 'HFD + C15' group, we observed a shift of correlations: correlations with genera
AF12,
Clostridium and the unidentified genus of the
Peptostreptococcaceae family became positive, furthermore positive correlations with the genus [
Ruminococcus] have appeared (
Figure 6). However, the representation of genera
AF12,
Clostridium, [
Ruminococcus] and the unidentified genus of the
Peptostreptococcaceae family decreased in ‘HFD+C15’ compared to ‘HFD’ (
Supplementary Table ST16).
Such discrepancies between a decrease in microbial abundance and a simultaneous increase in the number of correlations with enzyme abundance may indicate regulatory functions of the metabolic pathways for B12 synthesis. A shift of negative correlations into positive ones with a simultaneous decrease in microbe representation (as we observed for the genera
AF12,
Clostridium, and [
Ruminococcus] genera) pointed that C15 is more likely to increase the representation of pathways and enzymes for cobalamin salvage by increasing the alpha diversity of the microbe community, thus reaching the representation of bacteria using such pathways. An increase in the number of correlations of enzymes with certain microorganisms may indicate the importance of the pathway as a source of signaling metabolites that determine the representation of key species in the community. On the other hand, changes in microbiota communities may be associated with redistribution of different metabolites in the B12 biosynthetic pathway by decreasing specific bacteria that inhibit the growth of probiotic species. This point is confirmed by observation that in the 'HFD + C15' group the representation of
Clostridium,
AF12, and [
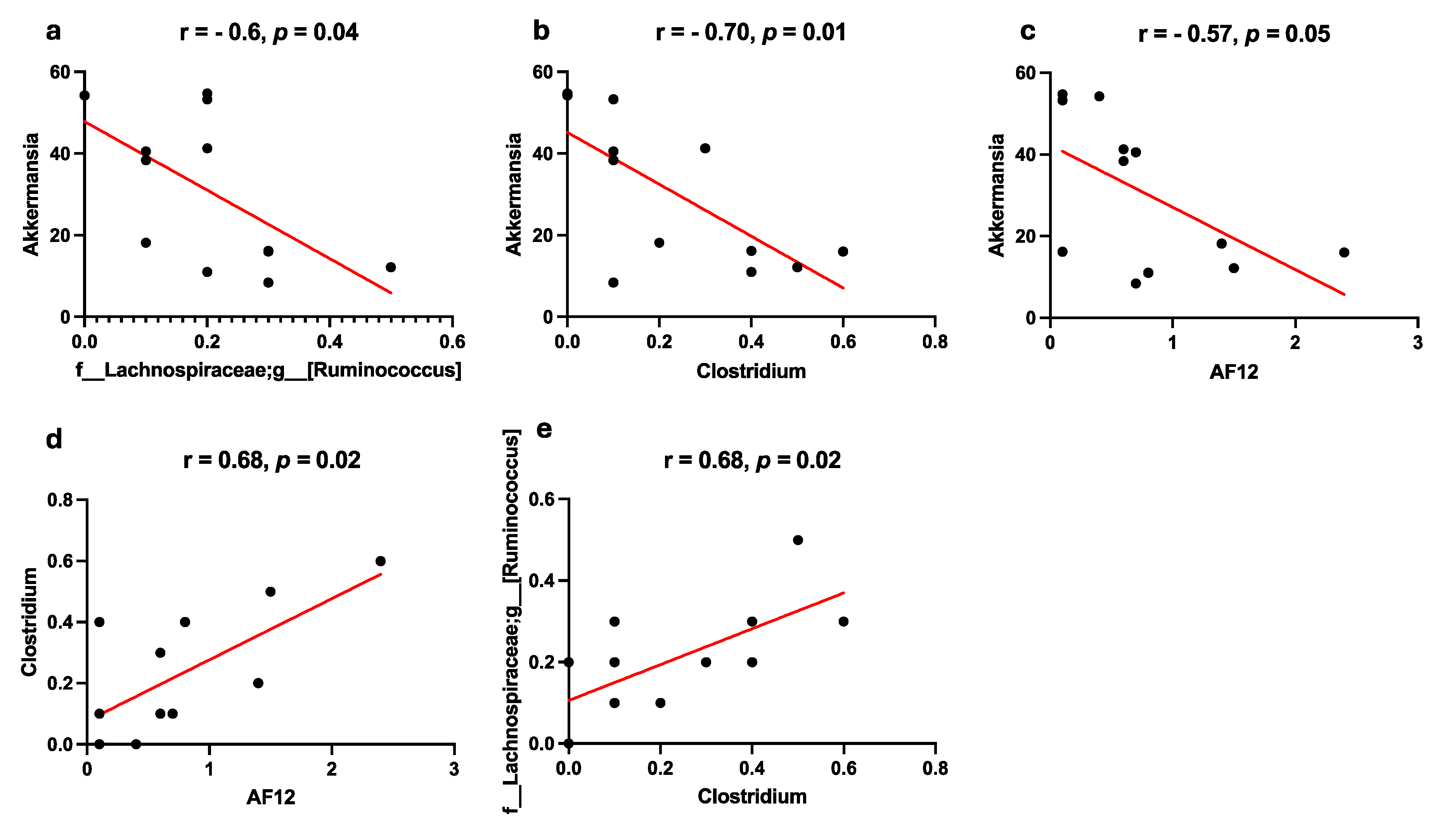
Ruminococcus] genera (that were decreased in this group compared to the HFD and SD groups) was strongly negative associated with the representation of Akkermansia (that was increased after supplementation with C15 (
Figure 1). Furthermore, the representation of the
Clostridium,
AF12, and [
Ruminococcus] genera was strongly positive correlated with each other (
Figure 7).
4. Discussion
Many living organisms require vitamin B12, which is the only vitamin made exclusively by bacteria and archaea [
18]. Vitamin B12 synthesis is an energy investment process that requires more than thirty distinct enzymes [
19]. It has been established that about 37% of prokaryotes have the genetic capacity to
de novo synthesis of vitamin B12, including
Bacillus,
Clostridium,
Mycobacterium,
Salmonella,
Streptococcus, etc., while other microbes rely on salvage pathways, in which bacteria known as auxotrophs - which cannot synthesise certain necessary nutrients - get these nutrients from other organisms in their community [
19]. For example, B12 produced by
Blautia ([
Ruminococcus])
hydrogenotropica,
Marvinbryantia formatexigens, and
Blautia ([
Ruminococcus])
producta has been demonstrated to promote the conversion of succinate to propionate in two prevalent B12-auxotrophic gut bacteria:
Akkermansia muciniphila and
Bacteroides thetaiotaomicron [
20]. Therefore, the representation of keystone species in the community is strongly dependent on the representation of microbes-producers of regulatory molecules or dietary factors. Vitamin B12, in addition to acting as an enzyme cofactor for many bacterial enzymes, serves as an essential signaling molecule and plays a crucial role in determining the functional organisation and spatial arrangement of gut microecology. In the study by Degnan et al., it was shown that 313 gut microbiota genomes contain vitamin B12 riboswitches predicted to regulate 3,868 genes, the majority of which are related to enzymes, transporters, and isoezymes related to vitamin B12, but some of which have not been linked to vitamin B12 before [
12]. These findings imply that vitamin B12 riboswitches may influence the ecology of the gut microbiota due to their strong correlation with a range of relative abundances of vitamin B12-dependent and / or regulated protein expression.
Alkylresorcinols are known to modulate the composition of the intestinal microbial community by increasing the representation of probiotic or keystone species, thus alleviating the dysbiosis state [
8,
21,
22]. We assumed that modulatory effects of alkylresorcinols and particularly pentadecylresorcinol are at least partly associated with changes in the representation of enzymes and the pathway involved in B12 synthesis.
In this study using metagenome sequencing technology followed by reconstruction of functional activity of the gut microbiota, we investigated the influence of a supplement of C15 to a standard or a high-fat diet on small- or large-intestinal microbiota communities in association with the predicted representation of enzymes and pathways for vitamin B12 synthesis.
We have established that the administration of C15 together with HDF significantly increased the representation of enzymes and pathways for the salvage of cobalamin in the microbiome of the large, but not small intestine compared to the HFD or SD-fed groups. This finding cannot be explained only by differences in microbe representation observed after C15 supplementation.
We performed correlation analysis for genera identified in different groups and enzymes involved in B12 synthesis to establish whether C15 administration was associated with an increase in B12 producers. In the small intestine we have found that there were almost no correlations between enzymes and microbes that can be explained by the predominant representation of B12 producers in the colon but not the small intestine [
12]. However, C15 supplementation significantly increases the number of genera and enzymes with strong correlations that were not differentially represented in microbe communities of the investigation groups. Furthermore, as previously demonstrated, C15 did not change the alpha diversity of the small intestinal microbiota.
In the large intestine, we observed the bidirectional character of the correlations depending on the type of diet: for example, negatively correlated in the SD and SD + C15 groups, the AF12 genus became positively correlated in the HFD + C15 group. Furthermore, such positively correlated genera (Clostridium, AF12 and [Ruminococcus]) were decreased in the group 'HFD + C15' compared to the group 'HFD' and were strongly negatively associated with Akkermansia representation, which increased in contrast to the group 'HFD + C15' compared to ‘HFD’.
Therefore, an increase in the representation of enzymes for cobalamin salvage and the number of correlations for enzymes with certain microorganisms in the C15-treated groups may indicate the importance of the pathway as a source of signaling metabolites that determine the representation of keystone species in the community. This preliminary study shows the potential of pentadecylresorcinol as well as other alkylresorcinols to be used as effective prebiotic molecules that change the shape of the intestinal microbiota community through influence on complex intramicrobial interactions. However, direct experiments to confirm the modulatory effects of pentadecylresorcinol, including those affecting B12 synthesis, are needed to further confirm these observations.
5. Conclusions
For the first time, we have investigated the dependence of pathways and enzymes for vitamin B12 synthesis by the microbiota of the small and large intestine of mice on pentadecylresorcinol supplementation. We have established that C15 significantly increases the representation of the cobalamin salvage pathway and enzymes that were not associated with the representation of individual microbes.
C15 had a significant impact on the distribution of the correlation between enzymes and bacteria, by reversing or increasing the number of correlations in the gut microbiota communities.
Supplementation of C15 with an HFD led to a decrease in the representation of Clostridium, AF12 and [Ruminococcus] genera that were negatively associated with Akkermansia representation, which increased with C15 administration. Considering that the Clostridium, AF12 and [Ruminococcus] genera had shown the greatest number of correlations with enzymes for B12 synthesis and were negatively associated with probiotic bacteria, we can assume that the beneficial effect of alkylresorcinol can be achieved in the gut microbiota community by modulating B12 synthesis which in turn serves as one of the key regulators of gut microbiota ecology. However, direct experiments are needed to investigate the role of C15 in vitamin B12 synthesis and its impact on microbe interactions to confirm these observations.
Supplementary Materials
The following supporting information can be downloaded at:
Preprints.org, Figure S1: title; Table S1: title; Video S1: title.
Author Contributions
Conceptualization, A.A.Z. and A.V.S.; methodology, M.D.M. and A.V.F.; software, M.D.M.; validation, A.V.F.; formal analysis, A.A.Z.; investigation, A.A.Z., A.M.G., M.D.M. and A.V.F.; resources, S.A.R.; data curation, A.V.S.; writing—original draft preparation, A.A.Z.; writing—review and editing, A.V.S.; visualization, A.A.Z.; supervision, A.V.S.; project administration, A.V.S.; funding acquisition, A.V.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Review Board (or Ethics Committee) of the Ethics Committee for Animal Research of I.M. Sechenov First Moscow State Medical University, Russia (protocol number 96 from 2 September 2021).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- de Araújo, F.F.; de Paulo Farias, D.; Neri-Numa, I.A.; Pastore, G.M. Polyphenols and Their Applications: An Approach in Food Chemistry and Innovation Potential. Food Chem 2021, 338, 127535. [Google Scholar] [CrossRef]
- Gasmi, A.; Mujawdiya, P.K.; Noor, S.; Lysiuk, R.; Darmohray, R.; Piscopo, S.; Lenchyk, L.; Antonyak, H.; Dehtiarova, K.; Shanaida, M.; et al. Polyphenols in Metabolic Diseases. Molecules 2022, 27, 6280. [Google Scholar] [CrossRef] [PubMed]
- Pérez de Vega, M.J.; Moreno-Fernández, S.; Pontes-Quero, G.M.; González-Amor, M.; Vázquez-Lasa, B.; Sabater-Muñoz, B.; Briones, A.M.; Aguilar, M.R.; Miguel, M.; González-Muñiz, R. Characterization of Novel Synthetic Polyphenols: Validation of Antioxidant and Vasculoprotective Activities. Antioxidants 2020, 9, 787. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Geng, J.; Zhao, H.; Li, X.; Song, G. Effects of Resveratrol on Metabolic Indicators in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis. Int J Clin Pract 2022, 2022, 1–19. [Google Scholar] [CrossRef] [PubMed]
- El-Kot, S.M.; Wanas, W.; Hafez, A.M.; Mahmoud, N.A.; Tolba, A.M.; Younis, A.H.; Sayed, G.E.; Abdelwahab, H.E. Effect of Silymarin on the Relative Gene Expressions of Some Inflammatory Cytokines in the Liver of CCl4-Intoxicated Male Rats. Sci Rep 2023, 13, 15245. [Google Scholar] [CrossRef] [PubMed]
- Zern, T.L.; Fernandez, M.L. Cardioprotective Effects of Dietary Polyphenols. J Nutr 2005, 135, 2291–2294. [Google Scholar] [CrossRef]
- Zabolotneva, A.A.; Shatova, O.P.; Sadova, A.A.; Shestopalov, A. V.; Roumiantsev, S.A. An Overview of Alkylresorcinols Biological Properties and Effects. J Nutr Metab 2022, 2022, 1–12. [Google Scholar] [CrossRef]
- Zabolotneva, A.A.; Vasiliev, I.Yu.; Grigoryeva, T.; Gaponov, A.M.; Chekhonin, V.P.; Roumiantsev, S.A.; Shestopalov, A. V. Supplementation of a High-Fat Diet with Pentadecylresorcinol Increases the Representation of Akkermansia Muciniphila in the Mouse Small and Large Intestines and May Protect against Complications Caused by Imbalanced Nutrition. Int J Mol Sci 2024, 25, 6611. [Google Scholar] [CrossRef]
- Shang, Y.; Khafipour, E.; Derakhshani, H.; Sarna, L.K.; Woo, C.W.; Siow, Y.L.; O, K. Short Term High Fat Diet Induces Obesity-Enhancing Changes in Mouse Gut Microbiota That Are Partially Reversed by Cessation of the High Fat Diet. Lipids 2017, 52, 499–511. [Google Scholar] [CrossRef]
- Machate, D.J.; Figueiredo, P.S.; Marcelino, G.; Guimarães, R. de C.A.; Hiane, P.A.; Bogo, D.; Pinheiro, V.A.Z.; Oliveira, L.C.S. de; Pott, A. Fatty Acid Diets: Regulation of Gut Microbiota Composition and Obesity and Its Related Metabolic Dysbiosis. Int J Mol Sci 2020, 21, 4093. [Google Scholar] [CrossRef]
- Zabolotneva, A.A.; Kolesnikova, I.M.; Vasiliev, I.Yu.; Grigoryeva, T. V.; Roumiantsev, S.A.; Shestopalov, A. V. The Obesogenic Gut Microbiota as a Crucial Factor Defining the Depletion of Predicted Enzyme Abundance for Vitamin B12 Synthesis in the Mouse Intestine. Biomedicines 2024, 12, 1280. [Google Scholar] [CrossRef] [PubMed]
- Degnan, P.H.; Taga, M.E.; Goodman, A.L. Vitamin B 12 as a Modulator of Gut Microbial Ecology. Cell Metab 2014, 20, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.-L.; Hua, S.; Li, X.-Y.; Shen, L.; Wu, H.; Ji, H.-F. Microbially Produced Vitamin B12 Contributes to the Lipid-Lowering Effect of Silymarin. Nat Commun 2023, 14, 477. [Google Scholar] [CrossRef]
- Mok, K.C.; Sokolovskaya, O.M.; Nicolas, A.M.; Hallberg, Z.F.; Deutschbauer, A.; Carlson, H.K.; Taga, M.E. Identification of a Novel Cobamide Remodeling Enzyme in the Beneficial Human Gut Bacterium Akkermansia Muciniphila. mBio 2020, 11. [Google Scholar] [CrossRef]
- Belzer, C.; Chia, L.W.; Aalvink, S.; Chamlagain, B.; Piironen, V.; Knol, J.; de Vos, W.M. Microbial Metabolic Networks at the Mucus Layer Lead to Diet-Independent Butyrate and Vitamin B 12 Production by Intestinal Symbionts. mBio 2017, 8. [Google Scholar] [CrossRef]
- Martinez-Guryn, K.; Hubert, N.; Frazier, K.; Urlass, S.; Musch, M.W.; Ojeda, P.; Pierre, J.F.; Miyoshi, J.; Sontag, T.J.; Cham, C.M.; et al. Small Intestine Microbiota Regulate Host Digestive and Absorptive Adaptive Responses to Dietary Lipids. Cell Host Microbe 2018, 23, 458–469. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat Biotechnol 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Roth, J.; Lawrence, J.; Bobik, T. COBALAMIN (COENZYME B 12 ): Synthesis and Biological Significance. Annu Rev Microbiol 1996, 50, 137–181. [Google Scholar] [CrossRef]
- Shelton, A.N.; Seth, E.C.; Mok, K.C.; Han, A.W.; Jackson, S.N.; Haft, D.R.; Taga, M.E. Uneven Distribution of Cobamide Biosynthesis and Dependence in Bacteria Predicted by Comparative Genomics. ISME J 2019, 13, 789–804. [Google Scholar] [CrossRef]
- Kundra, P.; Greppi, A.; Duppenthaler, M.; Plüss, S.; Pugin, B.; Lacroix, C.; Geirnaert, A. Vitamin B12 Analogues from Gut Microbes and Diet Differentially Impact Commensal Propionate Producers of the Human Gut. Front Nutr 2024, 11. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, D.; Yang, Z.; Hao, Y.; Wang, Z.; Wang, J.; Wang, Z. Wheat Alkylresorcinols Modulate Glucose Homeostasis through Improving GLP-1 Secretion in High-Fat-Diet-Induced Obese Mice. J Agric Food Chem 2023, 71, 16125–16136. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, Y.; Wang, Z.; Hao, Y.; Bai, W.; Wang, Z.; Wang, J. 5-Heptadecylresorcinol, a Biomarker for Whole Grain Rye Consumption, Ameliorates Cognitive Impairments and Neuroinflammation in APP/PS1 Transgenic Mice. Mol Nutr Food Res 2020, 64. [Google Scholar] [CrossRef]
Figure 1.
Design of the experiment.
Figure 1.
Design of the experiment.
Figure 2.
Differences in the representation of the salvage of adenosylcobalamine from the cobinamide I pathway in the microbiome of the large intestine of mice received an SD compared to HFD+C15 (HFDar) (a) or an HFD compared to HFD+C15 (b). Unpaired t test with Welch’s correction was applied, p < 0.001.
Figure 2.
Differences in the representation of the salvage of adenosylcobalamine from the cobinamide I pathway in the microbiome of the large intestine of mice received an SD compared to HFD+C15 (HFDar) (a) or an HFD compared to HFD+C15 (b). Unpaired t test with Welch’s correction was applied, p < 0.001.
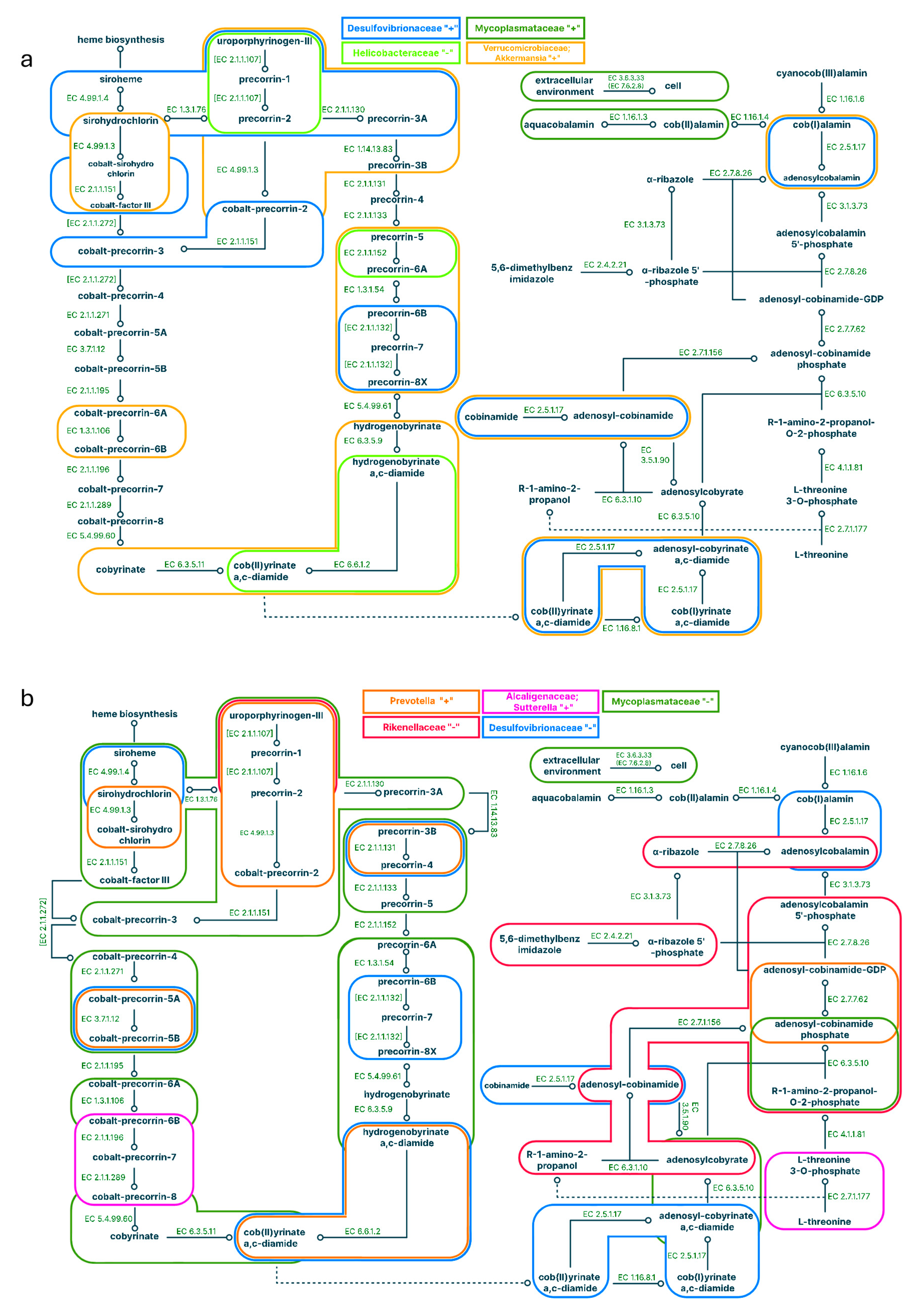
Figure 3.
Pathways correlated with the representation of bacteria in the small intestine for a standard diet (a) and a standard diet supplemented with C15 (b and c). Positive correlations are signed with '+', negative correlations are signed with “-”; different colours correspond to different microbes.
Figure 3.
Pathways correlated with the representation of bacteria in the small intestine for a standard diet (a) and a standard diet supplemented with C15 (b and c). Positive correlations are signed with '+', negative correlations are signed with “-”; different colours correspond to different microbes.
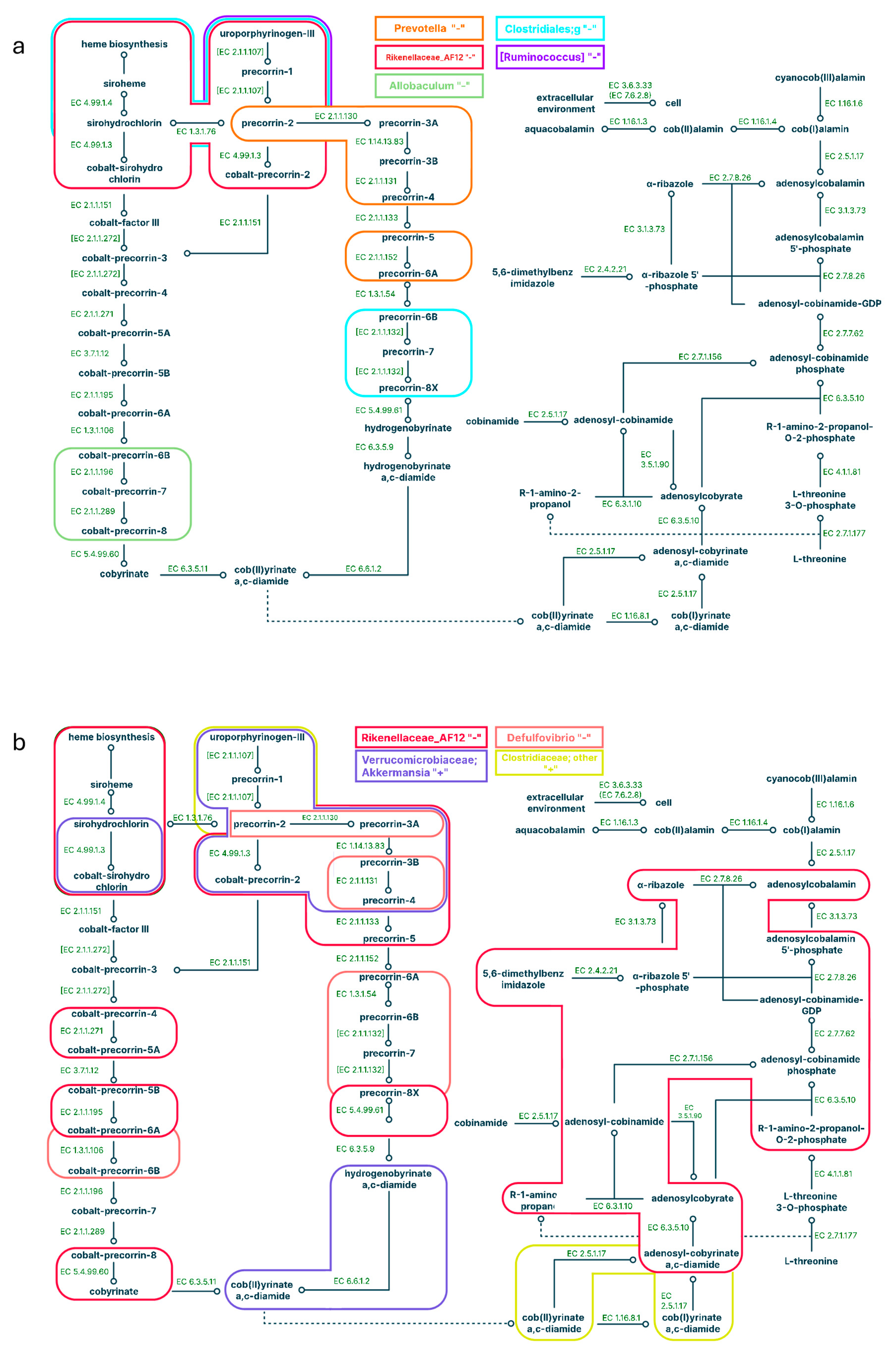
Figure 4.
Pathways correlated with bacteria representation in the small intestine for a high-fat diet (a) and a high-fat diet supplemented with C15 (b). Positive correlations are signed with '+', negative correlations are signed with “-”; different colours correspond to different microbes.
Figure 4.
Pathways correlated with bacteria representation in the small intestine for a high-fat diet (a) and a high-fat diet supplemented with C15 (b). Positive correlations are signed with '+', negative correlations are signed with “-”; different colours correspond to different microbes.
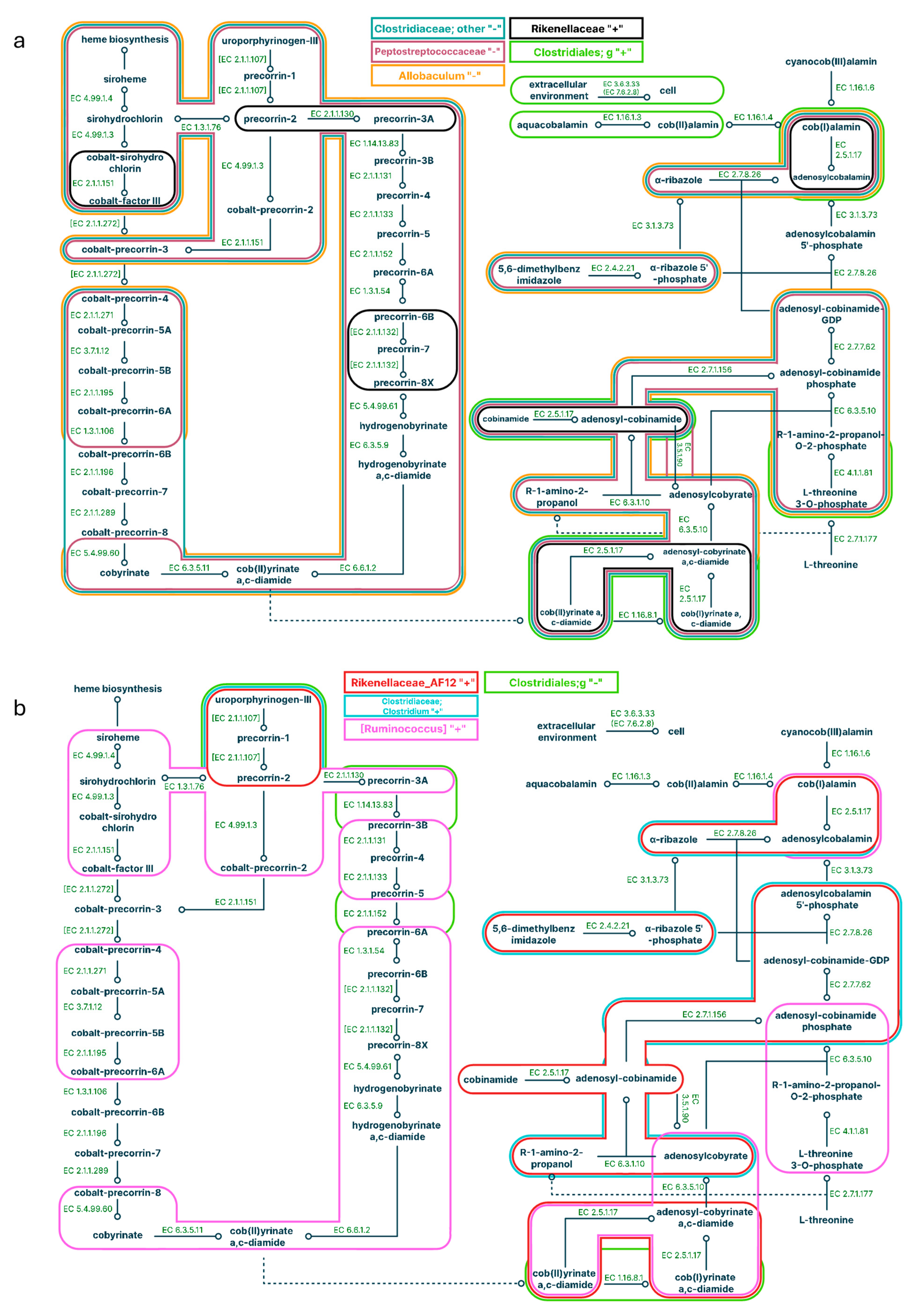
Figure 5.
Pathways correlated with the representation of bacteria in the large intestine for a standard diet (a) and a standard diet supplemented with C15 (b). Positive correlations are signed with '+', negative correlations are signed with “-”; different colours correspond to different microbes.
Figure 5.
Pathways correlated with the representation of bacteria in the large intestine for a standard diet (a) and a standard diet supplemented with C15 (b). Positive correlations are signed with '+', negative correlations are signed with “-”; different colours correspond to different microbes.
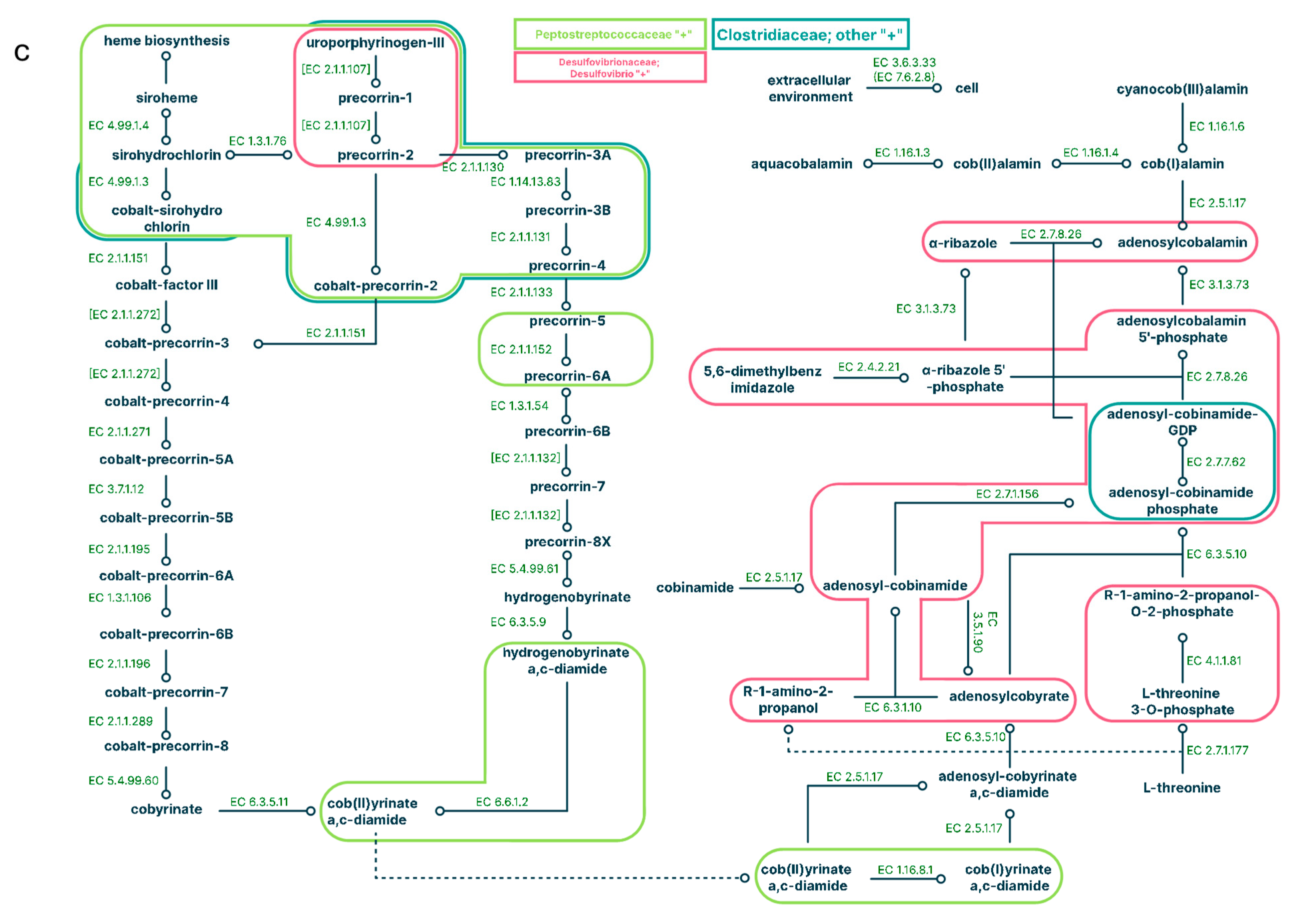
Figure 6.
Pathways correlated with bacteria representation in the large intestine for a high-fat diet (a) and a high-fat diet supplemented with C15 (b and c). Positive correlations are signed with '+', negative correlations are signed with “-”; different colours correspond to different microbes.
Figure 6.
Pathways correlated with bacteria representation in the large intestine for a high-fat diet (a) and a high-fat diet supplemented with C15 (b and c). Positive correlations are signed with '+', negative correlations are signed with “-”; different colours correspond to different microbes.
Figure 7.
Correlation analysis of microbe representation in the large intestine of mice received an HFD+C15. Spearman correlations are shown for: a – Akkermansia and [Ruminococcus], b – Akkermansia and Clostridium, c – Akkermansia and AF12, d – Clostridium and AF12, e – Clostridium and [Ruminococcus] genera.
Figure 7.
Correlation analysis of microbe representation in the large intestine of mice received an HFD+C15. Spearman correlations are shown for: a – Akkermansia and [Ruminococcus], b – Akkermansia and Clostridium, c – Akkermansia and AF12, d – Clostridium and AF12, e – Clostridium and [Ruminococcus] genera.
Table 1.
Differentially represented enzymes involved in vitamin B12 synthesis in HFD and HFD+C15-fed groups of mice according to the results of multiple Mann–Whitney tests with the two-stage step-up method (Benjamini, Krieger and Yekutieli) (false discovery rate Q = 5%). P values less than 0.05 were considered to indicate statistical significance. The mean rank difference below zero pointed to an over-representation of the enzyme in the HFD + C15-fed group compared to the HFD group, and values above zero pointed to an under-representation of the enzyme in the HFD + C15-fed group compared to the HFD group.
Table 1.
Differentially represented enzymes involved in vitamin B12 synthesis in HFD and HFD+C15-fed groups of mice according to the results of multiple Mann–Whitney tests with the two-stage step-up method (Benjamini, Krieger and Yekutieli) (false discovery rate Q = 5%). P values less than 0.05 were considered to indicate statistical significance. The mean rank difference below zero pointed to an over-representation of the enzyme in the HFD + C15-fed group compared to the HFD group, and values above zero pointed to an under-representation of the enzyme in the HFD + C15-fed group compared to the HFD group.
| Enzyme name |
P value |
Mean rank diff. |
q value |
| cobU, cobT; nicotinate-nucleotide--dimethylbenzimidazole phosphoribosyltransferase [EC:2.4.2.21] |
0,000103 |
-10,33 |
0,002678 |
| cobP, cobU; adenosylcobinamide kinase / adenosylcobinamide-phosphate guanylyltransferase [EC:2.7.1.156] |
0,000103 |
-10,33 |
0,002678 |
| cobS, cobV; adenosylcobinamide-GDP ribazoletransferase [;] |
0,000103 |
-10,33 |
0,002678 |
| cbiB, cobD; adenosylcobinamide-phosphate synthase [EC:6.3.1.10] |
0,000201 |
-10 |
0,004033 |
| cbiE; cobalt-precorrin-7 (C5)-methyltransferase [EC:2.1.1.289] |
0,001433 |
8,833 |
0,017084 |
| cbiT; cobalt-precorrin-6B (C15)-methyltransferase [EC:2.1.1.196] |
0,00183 |
8,667 |
0,020269 |
Table 2.
Differentially represented enzymes involved in vitamin B12 synthesis in SD and HFD+C15-fed groups of mice according to the results of multiple Mann–Whitney tests with the two-stage step-up method (Benjamini, Krieger and Yekutieli) (false discovery rate Q = 5%). P values less than 0.05 were considered to indicate statistical significance. The mean rank difference below zero pointed to an over-representation of the enzyme in the HFD + C15-fed group compared to the SD group.
Table 2.
Differentially represented enzymes involved in vitamin B12 synthesis in SD and HFD+C15-fed groups of mice according to the results of multiple Mann–Whitney tests with the two-stage step-up method (Benjamini, Krieger and Yekutieli) (false discovery rate Q = 5%). P values less than 0.05 were considered to indicate statistical significance. The mean rank difference below zero pointed to an over-representation of the enzyme in the HFD + C15-fed group compared to the SD group.
| Enzyme name |
P value |
Mean rank diff. |
q value |
| cobU, cobT; nicotinate-nucleotide--dimethylbenzimidazole phosphoribosyltransferase [EC:2.4.2.21] |
0,00005 |
-10,67 |
0,001295 |
| cobP, cobU; adenosylcobinamide kinase / adenosylcobinamide-phosphate guanylyltransferase [EC:2.7.1.156] |
0,00005 |
-10,67 |
0,001295 |
| cobS, cobV; adenosylcobinamide-GDP ribazoletransferase [EC:2.7.8.26] |
0,00005 |
-10,67 |
0,001295 |
| cbiB, cobD; adenosylcobinamide-phosphate synthase [EC:6.3.1.10] |
0,000072 |
-10,5 |
0,001679 |
| cobA-hemD; uroporphyrinogen III methyltransferase / synthase [EC:2.1.1.107] |
0,000274 |
-9,833 |
0,004186 |
| ABC.VB12.S1, btuF; vitamin B12 transport system substrate-binding protein [EC:3.6.3.33] |
0,000489 |
-9,25 |
0,006336 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).