Submitted:
22 August 2024
Posted:
23 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
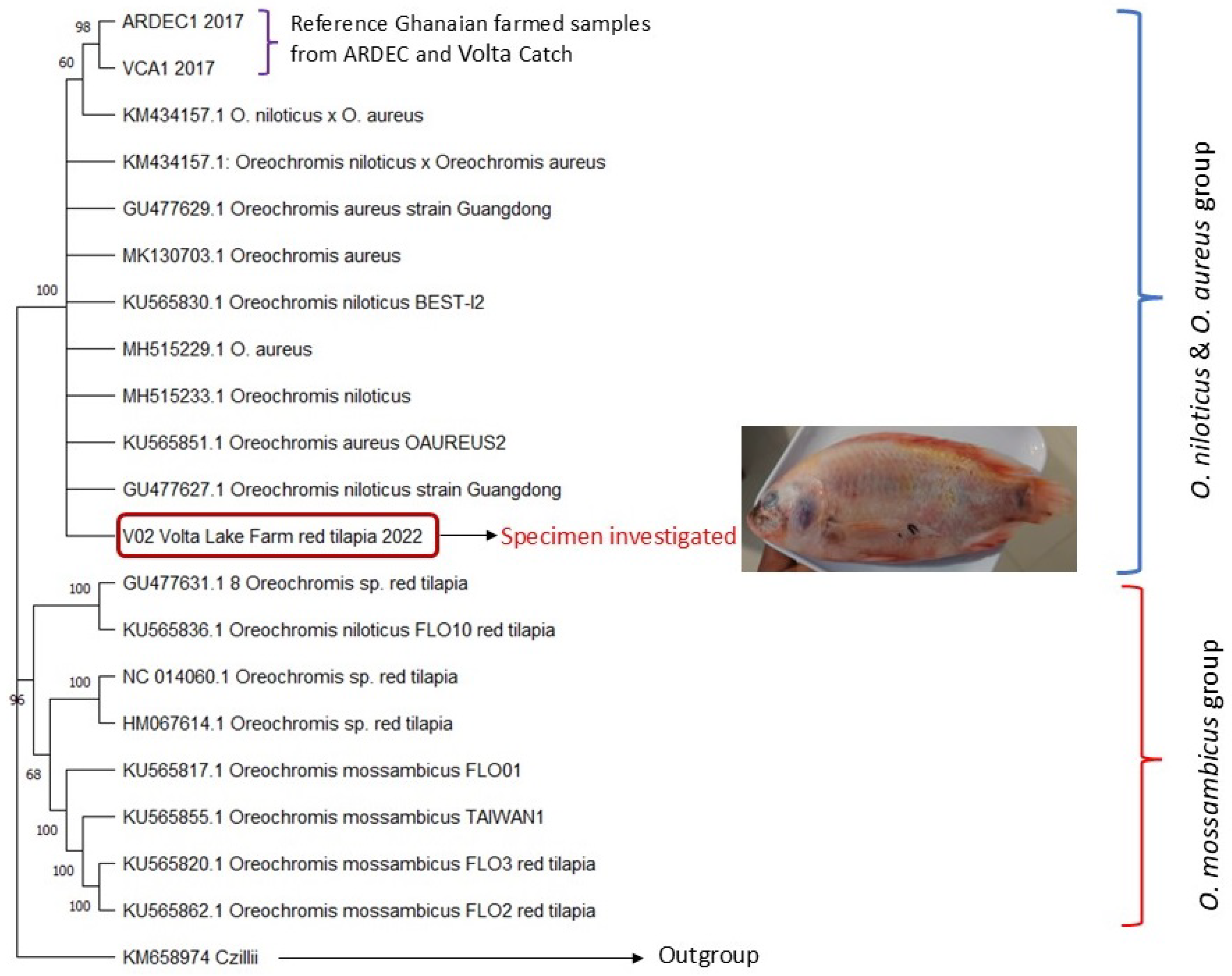
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- MOFAD. Ministry of Fisheries and Aquaculture Development -MOFAD, Annual Report Summary of Important Performance Indicators of the Fisheries Commission for 2017 and 2018. 2019. Available online: https://www.mofep.gov.gh/sites/default/files/pbb-estimates/2018/2018-PBB-MOFAD.pdf (accessed on Aug. 11, 2024).
- Ragasa, C.; Kufoalor, D.; Andam, K.S.; Amewu, S. A blue revolution in sub-Saharan Africa? Evidence from Ghana’s tilapia value chain. 2018. Available online: https://www.researchgate.net/publication/326070066.
- MOFAD. Republic of Ghana, Ministry of Fisheries and Aquaculture Development, Budget Estimates. 2021. Available online: https://mofep.gov.gh/sites/default/files/pbb-estimates/2021/2021-PBB-MOFAD.pdf (accessed on Aug. 11, 2024).
- Trinh, T.Q.; Agyakwah, S.K., Khaw, H.L.; Benzie, J.A.H.; Attipoe, F.K.Y. Performance evaluation of Nile tilapia (Oreochromis niloticus) improved strains in Ghana. Aquaculture 2021, 530. [CrossRef]
- Bentsen, H.B.; Gjerde, B.; Eknath, A.E.; de Vera, M.S.P.; Velasco, R.R.; Danting, J.C. et al. Genetic improvement of farmed tilapias: Response to five generations of selection for increased body weight at harvest in Oreochromis niloticus and the further impact of the project. Aquaculture 2017, 468, 206–217. [CrossRef]
- Eknath, A.E.; Tayamenb, M.M.; Palada-De Vera, M.S.; Dantingb, J.C.; Reyesb, R.A.; Dionisiob, E.E. et al. Genetic improvement of farmed tilapias: the growth performance of eight strains of Oreochromis niloticus tested in different farm environments. Journal 1993, 111, 171–188. [CrossRef]
- Moses, M.; Chauka, L.J.; de Koning, D.J.; Palaiokostas, C.; Mtolera, M.S.P. Growth performance of five different strains of Nile tilapia (Oreochromis niloticus) introduced to Tanzania reared in fresh and brackish waters. Sci. Rep. 2021, 11, 11147. [CrossRef]
- FAO. Food and Agriculture Organization of the United Nations, Country Report Supporting the Preparation of the First Report on The State of the World’s Aquatic Genetic Resources for Food and Agriculture. 2015. Available online: https://openknowledge.fao.org/server/api/core/bitstreams/ (accessed on Jul. 15, 2024).
- Anane-Taabeah, G.; Frimpong, E.; Hallerman, E. Aquaculture-mediated invasion of the Genetically Improved Farmed Tilapia (GIFT) into the Lower Volta Basin of Ghana. Diversity (Basel) 2019, 11, 188. [CrossRef]
- Figueiredo, H.C.P. et al. First report of infectious spleen and kidney necrosis virus in Nile tilapia in Brazil,” Transbound Emerg Dis 2022, 69, 3008–3015. [CrossRef]
- Subramaniam, K.; Shariff, M.; Omar, A.R.; Ong, B.L. Detection and molecular characterization of infectious spleen and kidney necrosis virus from major ornamental fish breeding states in Peninsular Malaysia. J. Fish. Dis. 2014, 37, 609–618. [CrossRef]
- Ayiku, A.N.A.; Adelani, A.A.; Appenteng, P.; Nkansah, M.; Morang, C.M.; Djabeng, F. et al. Molecular epidemiology and current management of Infectious Spleen and Kidney Necrosis Virus (ISKNV) infection in Ghanaian cultured tilapia. Aquaculture 2023, 581, 740330. [CrossRef]
- Zornu, J.; Tavornpanich, S.; Brun, E.; van Zwieten, P.A.M.; van de Leemput, I.; Appenteng, P. et al. Understanding tilapia mortalities and fish health management in Lake Volta: a systematic approach. Front. Sustain. Food Syst. 2023, 7, 1249898. [CrossRef]
- Verner-Jeffreys DW, Wallis TJ, Cano Cejas, I.; Ryder, D.; Haydon, D.J.; Domazoro, J.F. et al. Streptococcus agalactiae Multilocus sequence type 261 is associated with mortalities in the emerging Ghanaian tilapia industry. J. Fish. Dis. 2018, 41, 175–179. [CrossRef]
- Alathari, S.; Chaput, D.L.; Bolaños, L.M.; Joseph, A.; Jackson, V.L.N.; Verner-Jeffreys, D. et al. A Multiplexed, Tiled PCR Method for Rapid Whole-Genome Sequencing of Infectious Spleen and Kidney Necrosis Virus (ISKNV) in Tilapia. Viruses 2023, 15, 965. [CrossRef]
- Hebert, P.D.N.; Cywinska, A.; Ball, S.L.; DeWaard, J.R.Biological identifications through DNA barcodes. Proc. R. Soc. B: Biol. Sci. 2003, 270, 313–321. [CrossRef]
- Tamura, K.; Stecher, G., Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol Biol Evol 2021,38, 3022–3027. [CrossRef]
- Ordoñez, J.F.F.; Ventolero, M.F.H.; Santos, M.D. Maternal mismatches in farmed tilapia strains (Oreochromis spp.) in the Philippines as revealed by mitochondrial COI gene. Taylor & Francis, 2017, 28, 526–535. [CrossRef]
- Romana-Eguia, M.R.R.; Ikeda, M.; Basiao, Z.U.; Taniguchi, N. Genetic diversity in farmed Asian Nile and red hybrid tilapia stocks evaluated from microsatellite and mitochondrial DNA analysis. Aquaculture 2004, 236, 131–150. [CrossRef]
- Amenyogbe, E.; Chen, G.; Wang, Z.; Lin, M.; Lu, X.; Atujona, D.; et al. A Review of Ghana’s Aquaculture Industry. J. Aquac. Res. Dev. 2018, 9. [CrossRef]
- Cai, J., Quagrainie, K.K.; Hishamunda, N. Social and economic performance of tilapia farming in Africa. FAO Fish. Aquac. Circ. 2017, 1130. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




