1. Introduction
Rabies is a contagious zoonotic disease caused by the rabies virus, with a mortality rate of approximately 100% [
1]. There are currently no effective treatments for this condition. Unlike many other human infectious diseases, the timely administration of a rabies vaccine can prevent the development of rabies, even after exposure to the virus. Therefore, post-exposure vaccination is considered an effective method for preventing rabies [
2].
Due to the higher immunogenicity of cell-culture vaccines, Europe pioneered a six-dose vaccination program with injections on days 0, 3, 7, 14, 28, and 90 [
3]. In 1984, the Zagreb Institute of Public Health in former Yugoslavia developed a four-dose immunization regimen (2-1-1) [
4]. The United States Advisory Committee on Immunization Practices recommended a simplified four-dose immunization program, reducing the final dose from the original five-dose regimen in 2009 [
5]. The 2010 edition of "Rabies vaccines: WHO position paper" recommended two muscle-based post-exposure vaccination programs, the "five-dose Essen regimen" and "four-dose Zagreb regimen" [
6]. Previous studies have shown that both regimens perform well in safety and immunogenicity [7-9].
The short-term immune effects of both vaccination procedures have been well-studied and confirmed [
10], but reports on immune persistence are scarce. Studies have shown a decrease in the conversion rate of positive anti-rabies virus-neutralizing antibodies one year after rabies vaccination [
2,
7]. In 2011, The World Health Organization (WHO) ranked China’s vaccine regulatory system at a functional level of maturity according to the WHO’s global classification system for national medical product regulatory authorities. The immunogenicity and safety of the improved vaccine production process have been evaluated in Phase III clinical trials. Therefore, this study aimed to analyze vaccine quality under the positive trend in China's vaccine market and to optimize vaccine production processes. Given that studies and evidence on vaccine immune persistence are still limited, the aim of this study was to evaluate the immune persistence of a purified Vero cell-based rabies vaccine produced by Shandong Yeedu Biotechnology Co., 12 months after full vaccination in a five-dose Essen regimen and a four-dose Zagreb regimen. We hypothesized that the vaccine would maintain a high seroconversion rate after a follow-up in December.
2. Materials and Methods
2.1. Patients
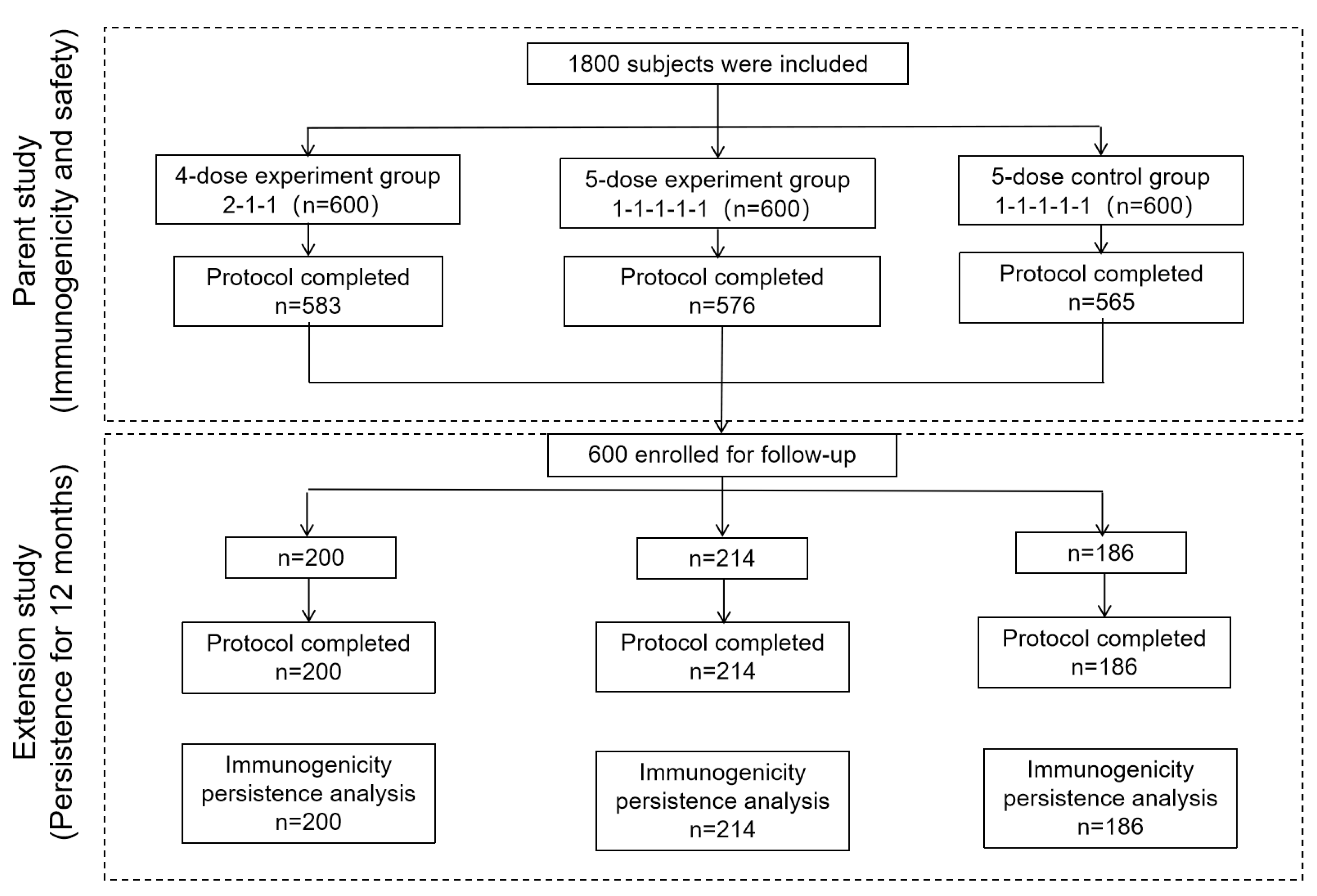
The trial included Guangxi Province residents aged 10–60 years who had not been vaccinated against rabies, had not received any other vaccines, antisera, human immunoglobulins, or similar products within the past month, and excluded those with a history of severe allergy to vaccines, as well as patients with severe cardiovascular diseases, liver or kidney diseases, and mental illnesses. A total of 1800 participants were enrolled in the Phase III clinical trial and randomized into three groups in a 1:1:1 ratio, with 600 participants in each group. Recruitment of participants began in March 2018 based on informed consent and voluntary participation principles. Blood sample collection lasted until May 2019, covering the entire immunization process and 12 months post-immunization. This study subsequently enrolled patients sequentially into the four-dose experimental, five-dose experimental, and five-dose control groups.
2.2. Intervention
The experimental vaccine was Vero cells produced by Shandong Yeedo Biotechnology Co., Ltd. (Dongying, China) (0.5 mL per vial after reconstitution, with a potency of rabies vaccine not less than 2.5 IU), batch number T20170901. The control vaccine was Vero cells produced by Liaoning Chengda Biotechnology Co., Ltd. (0.5 mL per vial), batch number 201704110. Liaoning Chengda was selected as the control vaccine, considering the same cell matrix and virulence for production and close production process [
11,
12]. All vaccines were transported and stored at 2–8°C. Randomization codes were generated using the SAS 9.4 software for the allocation and numbering of experimental and control vaccines. The five-dose experimental group and five-dose control group received five doses of the experimental or control vaccine at 0, 3, 7, 14, and 28 days, while the four-dose experimental group received four doses of the experimental vaccine at 0, 7, and 21 days (two doses injected on day 0 in both arms).
2.3. Sample Testing
Blood samples were collected from all patients before the first immunization, 7 days after the first immunization, 14 days after the first immunization, and on the 14th day after completion of the full immunization for antibody detection. Blood was collected 12 months after the completion of full immunization (window period: +2 months) to assess immune persistence. Approximately 3.0 mL of venous blood was collected, and the serum was separated and stored at -20°C or below. The rapid fluorescent focus inhibition test was used to detect rabies vaccine virus neutralizing antibodies in serum, with antibody concentration ≥0.5IU/mL considered positive. The serum antibody positivity rate and geometric mean concentration (GMC) were calculated for the three groups 12 months after the completion of full immunization.
2.4. Evaluation of Immune Persistence
The antibody positivity rate and antibody GMC at 12 months after the completion of full immunization were used for evaluation. Antibody positivity was defined as participant pre-immunization antibody concentrations of <0.5 IU/mL and post-immunization antibody concentrations ≥0.5 IU/mL. The positivity rate is defined as the proportion of seropositive cases.
2.5. Statistical Analysis
According to the Chinese Guidelines for Clinical Research on Human Rabies Vaccines [
13], 600 participants from the Phase III clinical trial were selected, with 200 in the four-dose experimental group and 400 in the combined five-dose experimental and five-dose control groups. The Phase III clinical trial was still blinded, consecutive number ranges were used to ensure similar numbers of participants in the five-dose experimental and five-dose control groups. All statistical analyses were performed using the SAS 9.4 software. The analysis set at 12 months after the completion of full immunization (IPS-12) included all participants who entered the immune persistence evaluation, completed blood collection 12 months after full immunization, and had valid antibody values. Descriptive statistics were used for continuous data, including means and standard deviations, and categorical data, including frequencies and percentages. The Clopper–Pearson method was used to calculate the 95% confidence interval for the serum antibody positivity rate 12 months after full immunization for each group, and the chi-square test or Fisher's exact probability test was used to statistically analyze the differences between groups. Geometric means and 95% confidence intervals were used to describe the antibody GMC at 12 months after full immunization for each group and the fold increase compared to that at 14 days after full immunization. Statistical analysis of differences between groups was performed using analysis of variance with log-transformed data. Reverse distribution plots of antibody concentration at pre-immunization, 7 days after the first immunization, 14 days after the first immunization, 14 days after full immunization, and 12 months after full immunization as well as antibody concentration-time semi-log plots before and after immunization were generated for each group. Subgroup analysis was conducted based on pre-immunization-positive and pre-immunization-negative participants.
2.6. Ethical Review
Prior to the start of this study, it was approved by the Ethics Review Committee of the Guangxi Zhuang Autonomous Region Center for Disease Control and Prevention. All participants or legal guardians were fully informed about the relevant matters of the clinical trial and provided voluntary informed consent.
3. Results
3.1. Baseline Characteristics
A total of 600 patients were enrolled: 200 in the four-dose experimental group, 214 in the five-dose experimental group, and 186 in the five-dose control group. All the patients were included in the IPS-12 analysis set (
Table 1 and
Figure 1). There were no statistically significant differences in age or sex among the patients (P>0.05).
3.2. Immunological Characteristics Analysis
A comparison of pre-immune antibody positivity rates and GMC among the groups is shown in
Table 2. The antibody positivity rates 7 days after the first dose were 79.00%, 46.07%, and 59.46% in the four-dose experimental, five-dose experimental, and five-dose control groups, respectively, which were statistically different among the three groups (P<0.001). The antibody positivity rate 14 days after the first dose immunization and 14 days post-vaccination was 100% in all three groups, but the difference was not statistically significant (P>0.05).
3.3. Analysis of Immunogenic Persistence
Twelve months post-immunization, the overall antibody positivity rates were 97.00%, 93.55%, and 94.86% in the four-dose experimental, five-dose control, and five-dose experimental groups, respectively, with no statistically significant differences among the three groups (P=0.277). The overall antibody GMCs at 12 months post-immunization were 2.50 IU/mL, 2.05 IU/mL, and 2.04 IU/mL in the four-dose experimental, five-dose control, and five-dose experimental groups, respectively, with no statistically significant differences among the groups (P=0.184). The fold increases in overall antibody GMCs at 12 months post-immunization compared with 14 days post-immunization were 0.05, 0.05, and 0.04 in the four-dose experimental, five-dose control, and five-dose experimental groups, respectively, with no statistically significant differences among the three groups (P=0.421) (
Table 3).
3.4. Distribution of Antibody Levels at 12 Months Post-Immunization
The reverse distribution table of overall antibody concentrations at 12 months post-immunization indicates that all groups were able to effectively produce neutralizing antibodies in participants positive for antibodies (neutralizing antibody concentration ≥0.5 IU/mL), with no significant differences among groups, except neutralizing antibody concentration ≥ 4 (
Table 4).
3.5. Subgroup Analysis
Among pre-immune positive participants (antibody concentration ≥0.5 IU/mL), the GMCs remained at relatively high levels at 12 months post-immunization, with GMCs of 5.71 IU/mL, 8.24 IU/mL, and 6.15 IU/mL in the four-dose experimental, five-dose control, and five-dose experimental groups, respectively, with no statistically significant differences among the three groups (P=0.771). In pre-immunity-negative participants, the positivity rates at 12 months post-immunization were above 85% (88.0%, 87.6%, and 86.9%, respectively), with no statistically significant differences among the groups (P=0.308) (
Table 5).
4. Discussion
This study compared and analyzed the immunogenicity of four-dose experimental, five-dose experimental, and five-dose control groups as an extension of a phase III clinical trial. The results showed that, after full immunization for 12 months, the overall antibody positivity rates of the four-dose experimental, five-dose control, and five-dose experimental groups, were slightly lower than those 14 days after full immunization, but remained at a relatively high level (97.00% for the four-dose experimental, 93.55% for the five-dose control, and 94.86% for the five-dose experimental groups).
Currently, the main immunization programs in China are the five-dose Essen scheme and the four-dose Zagreb scheme, both of which have good immunogenicity and safety [
2,
4]. The study results indicate that over time, the protective level of neutralizing antibodies gradually decreases, but remains at a relatively high level after reaching its peak 14 days after the first immunization. Notably, all three groups were able to maintain a positivity rate of over 90% after 12 months of vaccination, which is much higher than the antibody positivity rate of participants in previous studies after one year [
2,
14] and similar to the antibody positivity rate after one year reported by Cramer et al. [
15]. The present study also showed that in the subgroup analysis, antibody levels in seronegative individuals before vaccination remained high, with a positivity rate of over 85%. This trend suggests that the vaccine generated an effective immune response after initial immunization, providing recipients with good protection and long-lasting immunity. This sustained immune effect may be related to the specificity of vaccine design and immunization programs.
Although no statistically significant differences were observed in GMC levels among the three groups during the 12-month follow-up period after immunization the GMC levels in all groups remained above 0.5 IU/mL, indicating antibody positivity. This suggests that all vaccination regimens can maintain sufficiently high levels of antibodies, ensuring effective protection against the rabies virus, even in the absence of statistical differences. Notably, the GMC antibody in the four-dose trial group was significantly higher than those in the five-dose trial group. This may indicate that with this specific vaccine regimen, lower doses can generate a stronger and more durable immune response, possibly related to factors, such as immunological memory and response intensity [
16]. However, in Zhang et al.'s research [
17], the seroconversion rate of Liaoning Chengda vaccine gradually declined from 90.5% at one year to 34.0% at five years, suggesting that the durability of antibodies in this study's experimental vaccine warrants investigation through a longer follow-up.
In addition, the antibody positivity rate of all three groups did not reach 100% after one year, which is consistent with the results of previous studies [
1], suggesting that WHO-recommended immunization procedures should be followed [
6] to achieve protection. Booster vaccinations were administered six months after full immunization, if necessary.
The strengths of this study include improvements to the vaccine production process, such as using advanced microcarrier bioreactors combined with microcarrier perfusion culture technology to ensure vaccine quality and safety and using single-use sterile bags for aseptic transfer from the bioreactor to the lyophilized formulation. After process optimization, the seroconversion rate after 12 months remains higher than in previous studies [
2,
7]. Following the enactment of the National Law on Vaccine Management [
18], which provides a legal framework for vaccine clinical trials, this study offers new evidence for vaccine research in China.
This study has some limitations; the follow-up lasted only one year and longer follow-up periods are required. The study selected a population aged 10–60 years without age stratification. Compliance was not analyzed in this study. According to a previous report [
19], compliance with the four-dose Zagreb regimen was significantly higher than that with the five-dose Essen regimen, possibly due to patients forgetting vaccination times or inability to adhere to the specified vaccination schedule due to work or study commitments. Future research should include long-term compliance analyses for both vaccination regimens.
5. Conclusions
Both four-dose and five-dose vaccination regimens can induce sustained immune memory and have good immunological durability within 12 months, effectively immunizing the body against the rabies virus. The results of this study provide a basis for the selection and refinement of various immunization programs. In future, we will focus on long-term follow-up studies and post-exposure booster immunization research. We will conduct stratified analyses for different age groups to optimize vaccination strategies. In addition, we will evaluate the cost-effectiveness of different vaccination regimens to guide public health policy formulation.
Supplementary Materials
Not Applicable.
Author Contributions
Conceptualization, Z.W. (Zhiang Wu), Z.M. (Zhaojun Mo) and S.C. (Shouchun Cao); methodology, J.L. (Jia Li), S.C., L.S. (Leitai Shi), D.Z. (Danhua Zhao) and X.W. (Xiaohong Wu); software, S.C., L.S., D.Z., X.W. and J.L.; validation, H.L. (Haiyan Liang), Z.M., D.Z., X.W. and J.L.; formal analysis, S.C., L.S., D.Z., X.W. and J.L.; investigation, Z.M. and Y.W. (Yunpeng Wang); resources, Z.W., H.L., Z.M., Y.W. and J.L.; data curation, H.L., Y.W., L.S. and D.Z.; writing—original draft preparation, Y.W., S.C., J.L., Z.M. and H.L.; writing—review and editing, Y.W., Z.M., J.L., S.C., L.S., D.Z., Z.W., H.L. and X.W.; visualization, Z.W., Z.M. and S.C.; supervision, Z.W., Z.M., S.C. and X.W.; project administration, H.L., Z.M.,Y.W. and L.S.; funding acquisition, Z.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Shandong Yeedo Biotechnology Co., Ltd.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of the Guangxi Zhuang Autonomous Region Center for Disease Control and Prevention (Protocol code: GXIBR2017-0013-4, approval date: 25 July 2017).
Informed Consent Statement
Informed consent was obtained from all participants involved in the study.
Data Availability Statement
The data that support the findings of this study are available from Shandong Yeedo Biotechnology Co., Ltd. but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are, however, available from the authors upon reasonable request and with permission of Shandong Yeedo Biotechnology Co., Ltd.
Acknowledgments
Not Applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wang, L. Y.; Sun, M. P.; Zhang, X. C.; Suo, L. D.; Xu, R. H.; Zou, Y. J.; Zuo, L. B.; Qi, H. Safety and Immunogenicity of Two Freeze-Dried Vero Cell Rabies Vaccines for Human Use in Post-Exposure Prophylaxis. Vaccine 2011, 24, 2679–2681. [Google Scholar] [CrossRef] [PubMed]
- Shi, N.; Zhang, Y.; Zheng, H.; Zhu, Z.; Wang, D.; Li, S.; Li, Y.; Yang, L.; Zhang, J.; Bai, Y.; et al. Immunogenicity, Safety and Antibody Persistence of a Purified Vero Cell Cultured Rabies Vaccine (Speeda) Administered by the Zagreb Regimen or Essen Regimen in Post-Exposure Subjects. Hum Vaccin Immunother 2017, 13, 1–8. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Expert Consultation on Rabies. Second report. Technical report series no. 931. Available online: https://www.who.int/publications/i/item/who-trs-931 (accessed on 24 July 2024).
- Vodopija, I.; Sureau, P.; Smerdel, S.; Lafon, M.; Baklaić, Z.; Ljubicić, M.; Svjetlicić, M. Interaction of Rabies Vaccine with Human Rabies Immunoglobulin and Reliability of a 2-1-1 Schedule Application for Postexposure Treatment. Vaccine 1988, 6, 283–286. [Google Scholar] [CrossRef]
- Rupprecht, C. E.; Briggs, D.; Brown, C. M.; Franka, R.; Katz, S. L.; Kerr, H. D.; Lett, S. M.; Levis, R.; Meltzer, M. I.; Schaffner, W.; et al. Use of a Reduced (4-Dose) Vaccine Schedule for Postexposure Prophylaxis to Prevent Human Rabies: Recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep 2010, 59, 1–9. [Google Scholar] [PubMed]
- World Health Organization. Rabies vaccines: WHO position paper–recommendations. Vaccine, 2010; 28, 7140–7142. [CrossRef]
- Ma, J.; Wang, H.; Li, J.; Chang, L.; Xie, Y.; Liu, Z.; Zhao, Y.; Malerczyk, C. A Randomized Open-Labeled Study to Demonstrate the Non-Inferiority of Purified Chick-Embryo Cell Rabies Vaccine Administered in the Zagreb Regimen (2-1-1) Compared with the Essen Regimen in Chinese Adults. Hum Vaccin Immunother 2014, 10, 2805–2812. [Google Scholar] [CrossRef] [PubMed]
- Mahendra, B. J.; Narayana, D. A.; Agarkhedkar, S.; Ravish, H. S.; Harish, B. R.; Agarkhedkar, S.; Madhusudana, S. N.; Belludi, A.; Ahmed, K.; Jonnalagedda, R.; et al. Comparative Study on the Immunogenicity and Safety of a Purified Chick Embryo Cell Rabies Vaccine (PCECV) Administered According to Two Different Simulated Post Exposure Intramuscular Regimens (Zagreb versus Essen). Hum Vaccin Immunother 2015, 11, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Li, Y.; Wen, S.; Wen, H.; Nong, Y.; Mo, Z.; Xie, F.; Pellegrini, M. Immunogenicity and Safety of Purified Chick-Embryo Cell Rabies Vaccine Under Zagreb 2-1-1 or 5-Dose Essen Regimen in Chinese Children 6 to 17 Years Old and Adults Over 50 Years: A Randomized Open-Label Study. Hum Vaccin Immunother 2015, 11, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, J.; Meng, S.; Ge, L.; You, Y.; Xu, Q.; Wang, H.; Yang, J.; Wang, S.; Wu, H. Safety and Immunogenicity of Human Rabies Vaccine for the Chinese Population After PEP: A Systematic Review and Meta-Analysis. Vaccine 2022, 40, 4371–4379. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Li, J.; Zhou, L.; Chen, J.; Jin, Z.; Meng, Q.; Chai, J.; Gao, H.; Wang, Y.; Zhao, D.; et al. A Randomized, Double-Blind, Controlled Phase III Clinical Trial to Evaluate the Immunogenicity and Safety of a Lyophilized Human Rabies Vaccine (Vero Cells) in Healthy Participants Aged 10-60 Years Following Essen and Zagreb Vaccination Procedures. Vaccines (Basel) 2023, 11, 1311. [Google Scholar] [CrossRef]
- Wang, L. Y.; Sun, M. P.; Zhang, X. C.; Suo, L. D.; Xu, R. H.; Zou, Y. J.; Zuo, L. B.; Qi, H. Safety and Immunogenicity of Two Freeze-Dried Vero Cell Rabies Vaccines for Human Use in Post-Exposure Prophylaxis. Vaccine 2011, 29, 2679–2681. [Google Scholar] [CrossRef] [PubMed]
- Center For Drug Evaluation, China. Guidelines for Clinical Research on Human Rabies Vaccines. Available online: https://www.cde.org.cn/main/news/viewInfoCommon/c670820fd316d8977dc72c4fa7aaa749 (accessed on 12 July 2024).
- Lim, P. L.; Barkham, T. M. Serologic Response to Rabies Pre-Exposure Vaccination in Persons with Potential Occupational Exposure in Singapore. Int J Infect Dis 2010, 14, e511–e513. [Google Scholar] [CrossRef] [PubMed]
- Cramer, J. P.; Jelinek, T.; Paulke-Korinek, M.; Reisinger, E. C.; Dieckmann, S.; Alberer, M.; Bühler, S.; Bosse, D.; Meyer, S.; Fragapane, E.; et al. One-year Immunogenicity Kinetics and Safety of a Purified Chick Embryo Cell Rabies Vaccine and an Inactivated Vero Cell-Derived Japanese Encephalitis Vaccine Administered Concomitantly According to a New, 1-Week, Accelerated Primary Series. J Travel Med 2016, 23, taw011. [Google Scholar] [CrossRef] [PubMed]
- Overduin, L. A.; van Dongen, J. J. M.; Visser, L. G. The Cellular Immune Response to Rabies Vaccination: A Systematic Review. Vaccines (Basel) 2019, 7, 110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhu, Z.; Wang, C. Persistence of Rabies Antibody 5 Years After Postexposure Prophylaxis with Vero Cell Antirabies Vaccine and Antibody Response to a Single Booster Dose. Clin Vaccine Immunol 2011, 18, 1477–1479. [Google Scholar] [CrossRef] [PubMed]
- National People’s Congress of, P.R.C. The China vaccine administration law. Available online: http://www.npc.gov.cn/npc/c30834/201907/11447c85e05840b9b12c62b5b645fe9d.shtml (accessed on 12 July 2024).
- Yan, X. Comparison of Compliance and Economic Cost of Two Different Immunization Procedures for Rabies Vaccine. Contemporary Medicine 2020, 26, 44–46. (In Chinese) [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).