Submitted:
22 August 2024
Posted:
26 August 2024
Read the latest preprint version here
Abstract
Keywords:
1. Introduction
2. Titanium Dioxide, Synthesis, Characterization and Properties
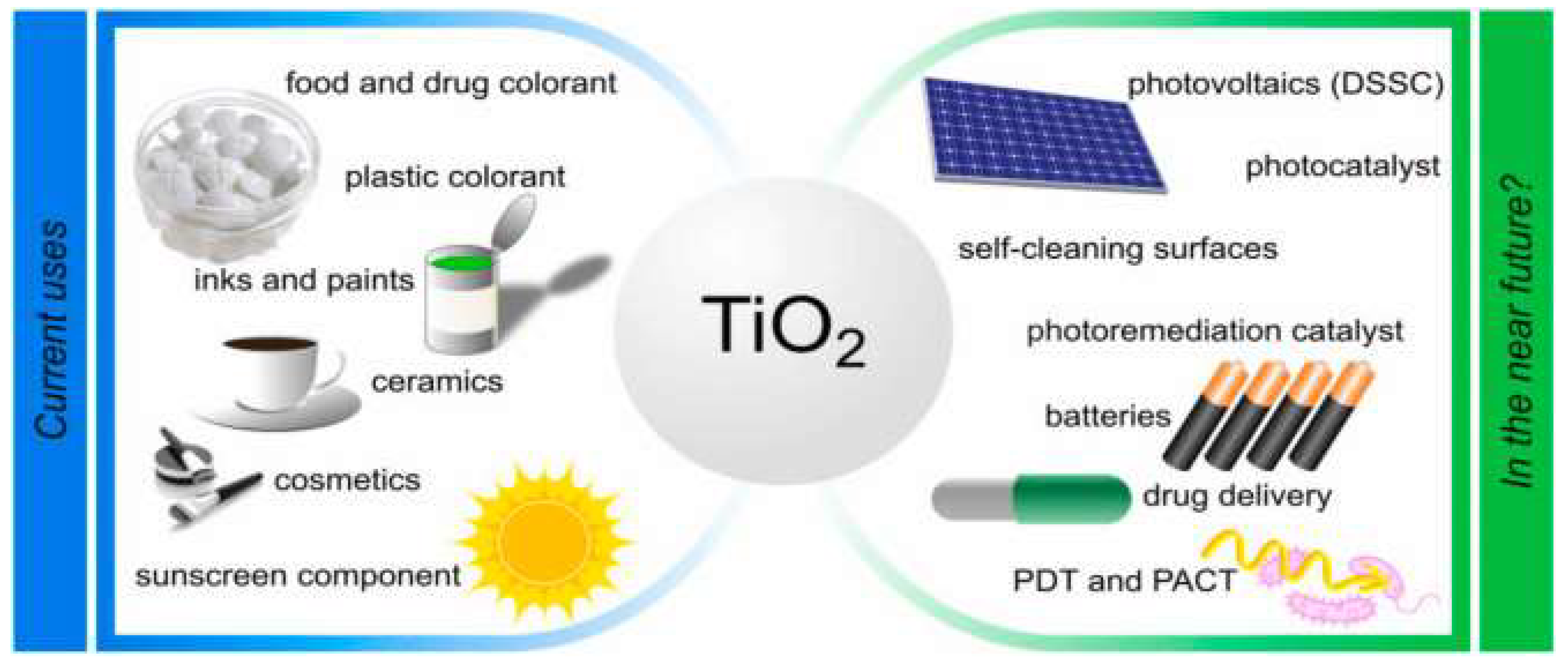
2.1. Brief Introduction and Applications of TiO2
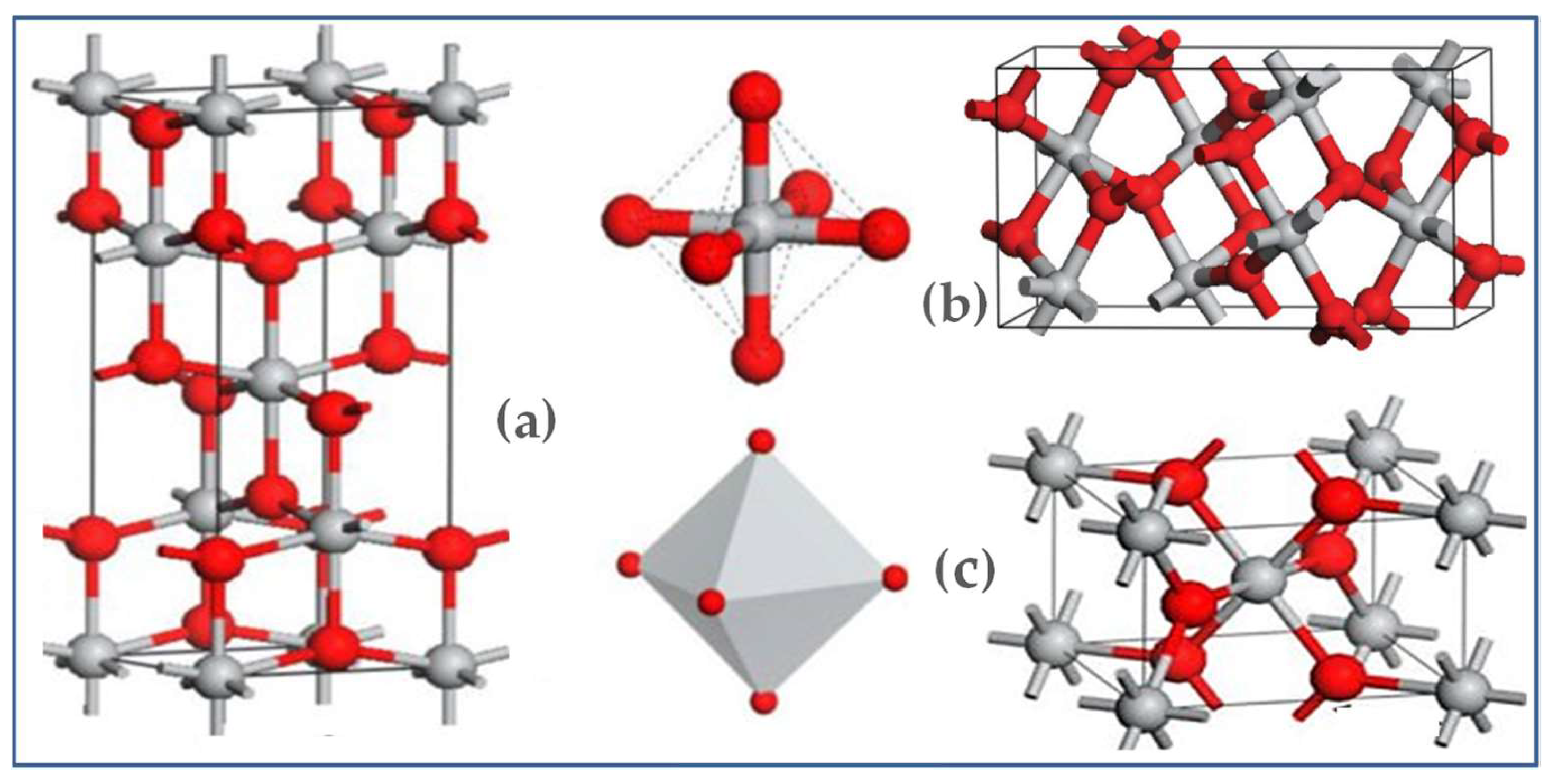
2.2. Crystal Structures of TiO2
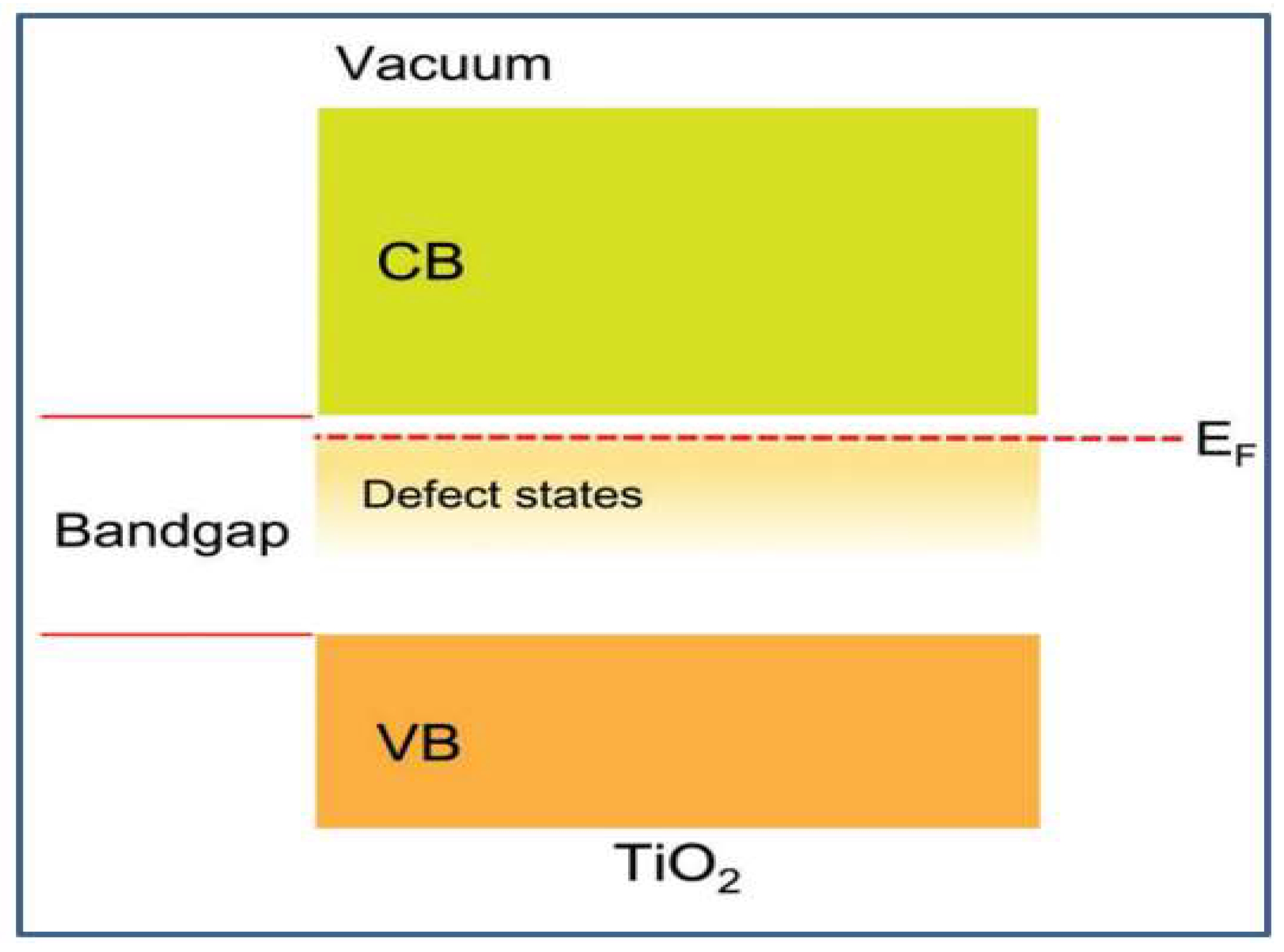
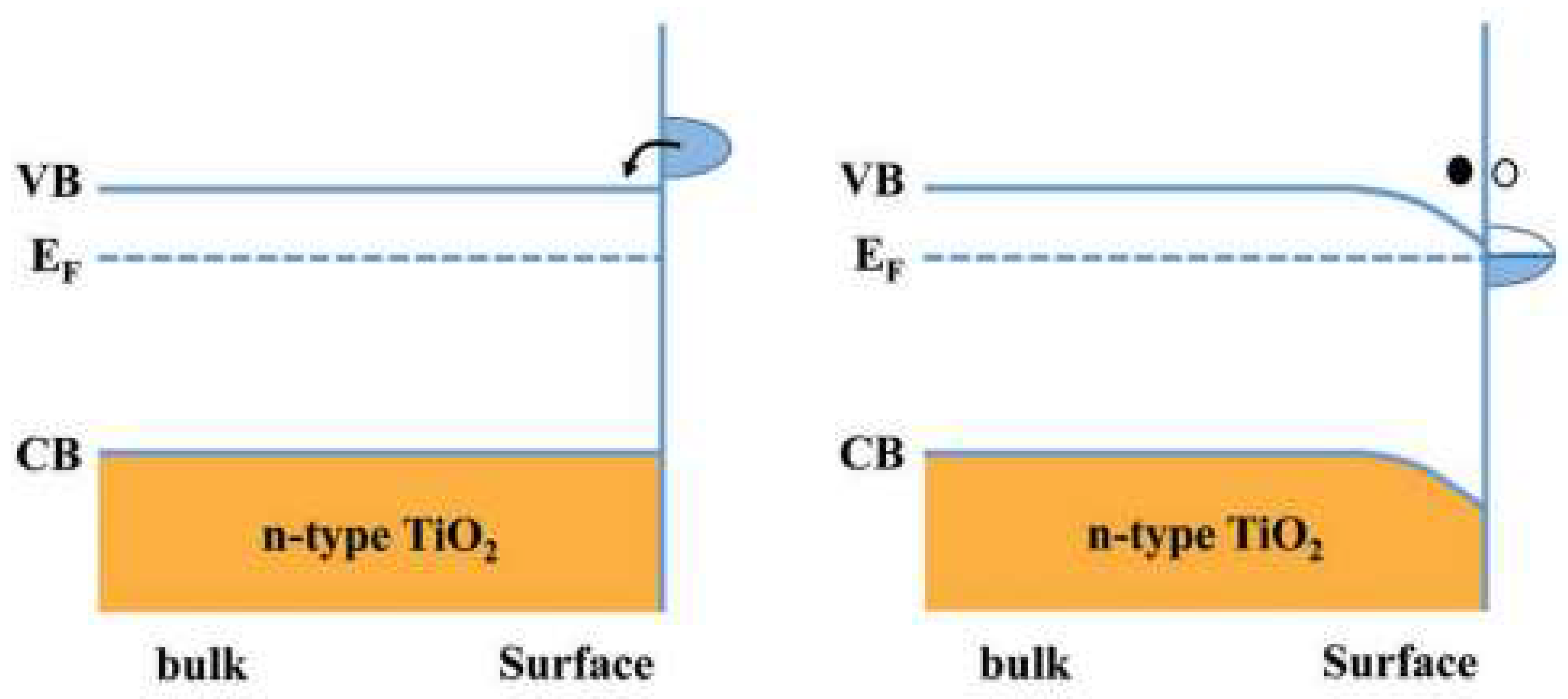
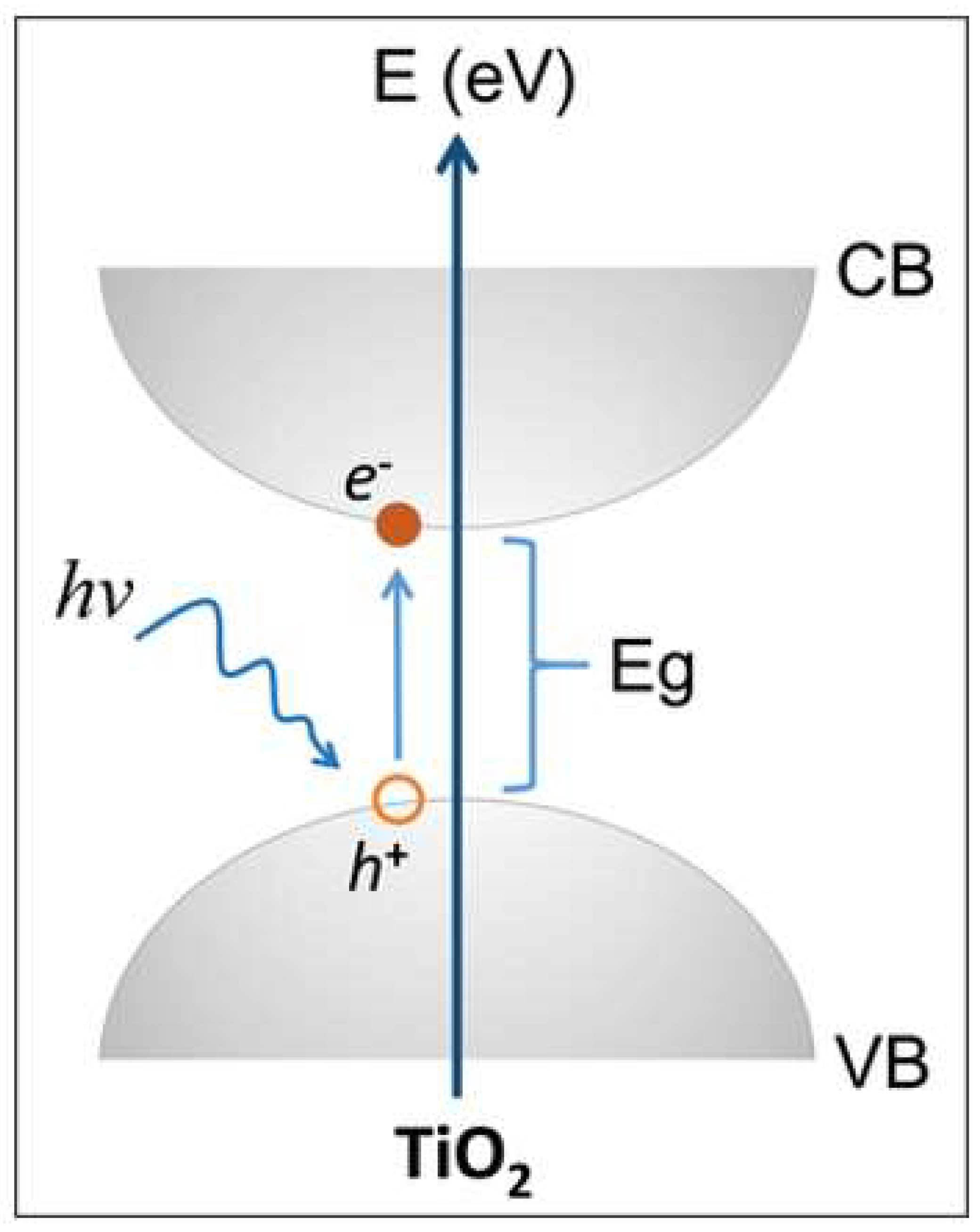
2.3. Electronic Properties and Band Structure
2.4. Optical Properties of TiO2
3. Efficient Methods of TiO2 Synthesis
3.1. Hydrothermal Method of TiO2 Preparation
3.2. Solvothermal Synthesis of TiO2
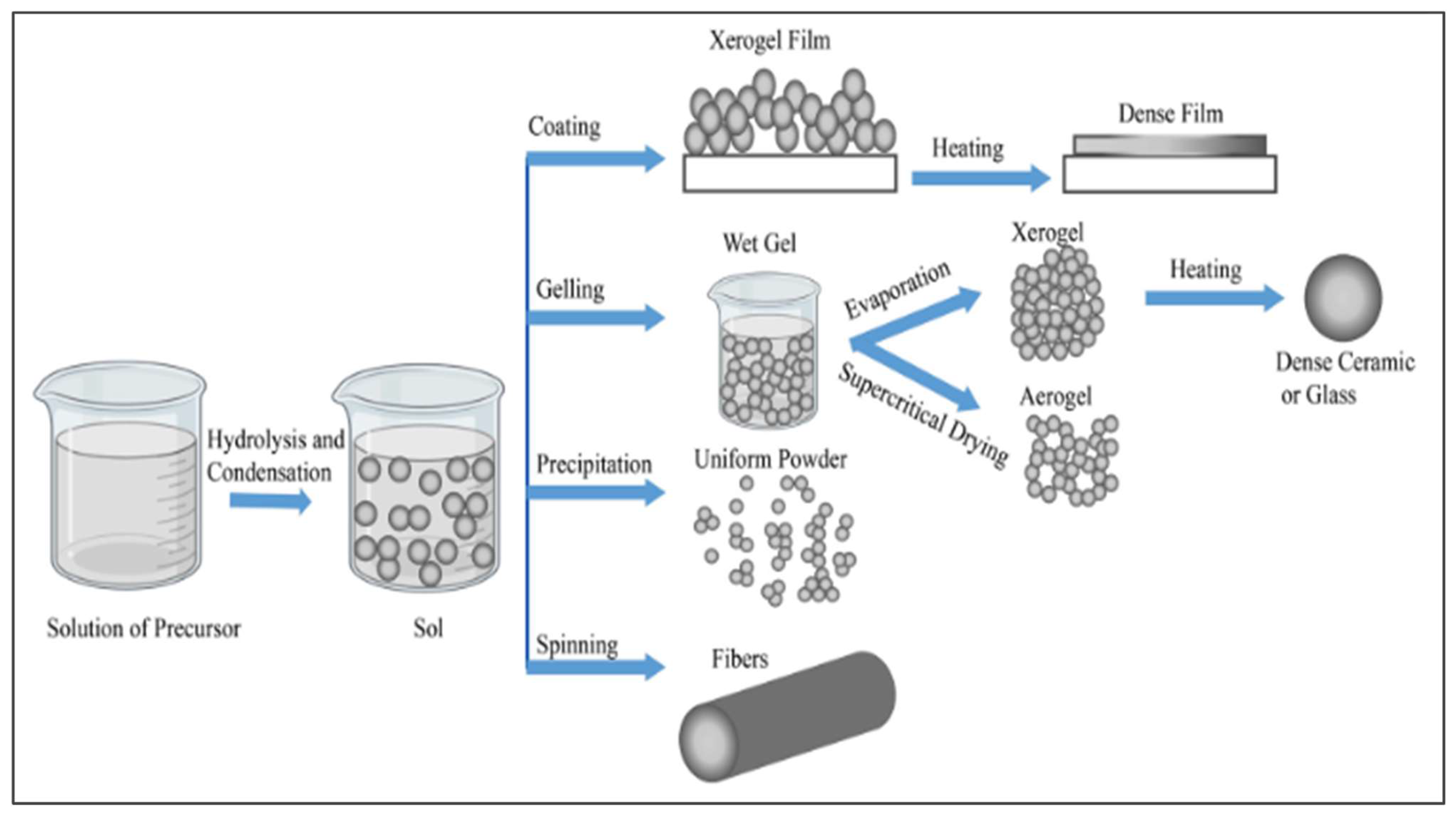
3.3. Sol-Gel Method of TiO2 Production
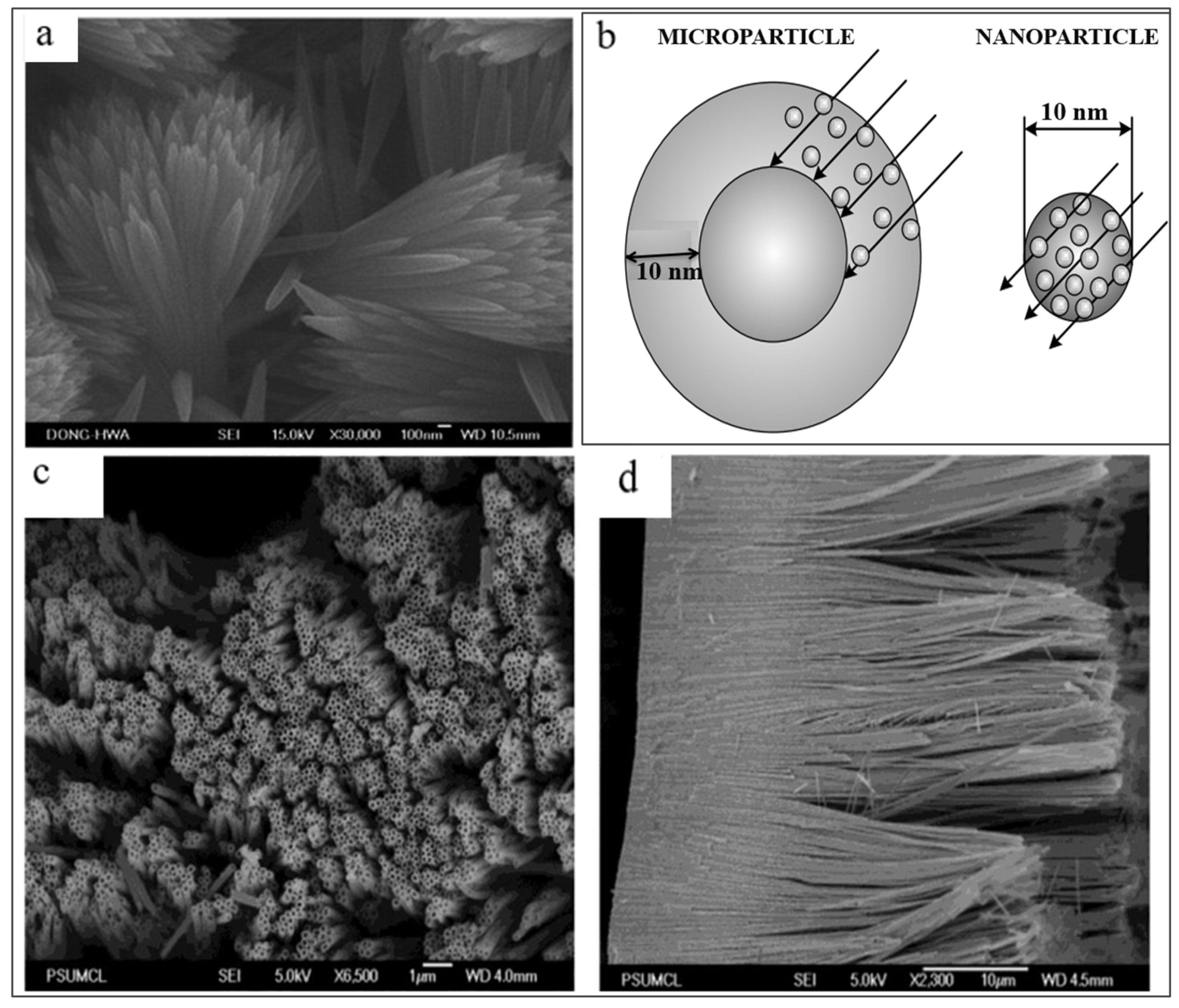
3.4. Sonochemical and Microwave-Assisted Methods of TiO2 Synthesis
3.5. Synthesis of TiO2 by Oxidation Method
3.6. Synthesis of TiO2 by Chemical Vapor Deposition (CVD)
3.7. Green Synthesis of TiO2
3.8. Electrodeposition and Ionic Liquid-Assisted Methods
3.9. Synthesis of Nanoscale and Thin Film Structures of TiO2
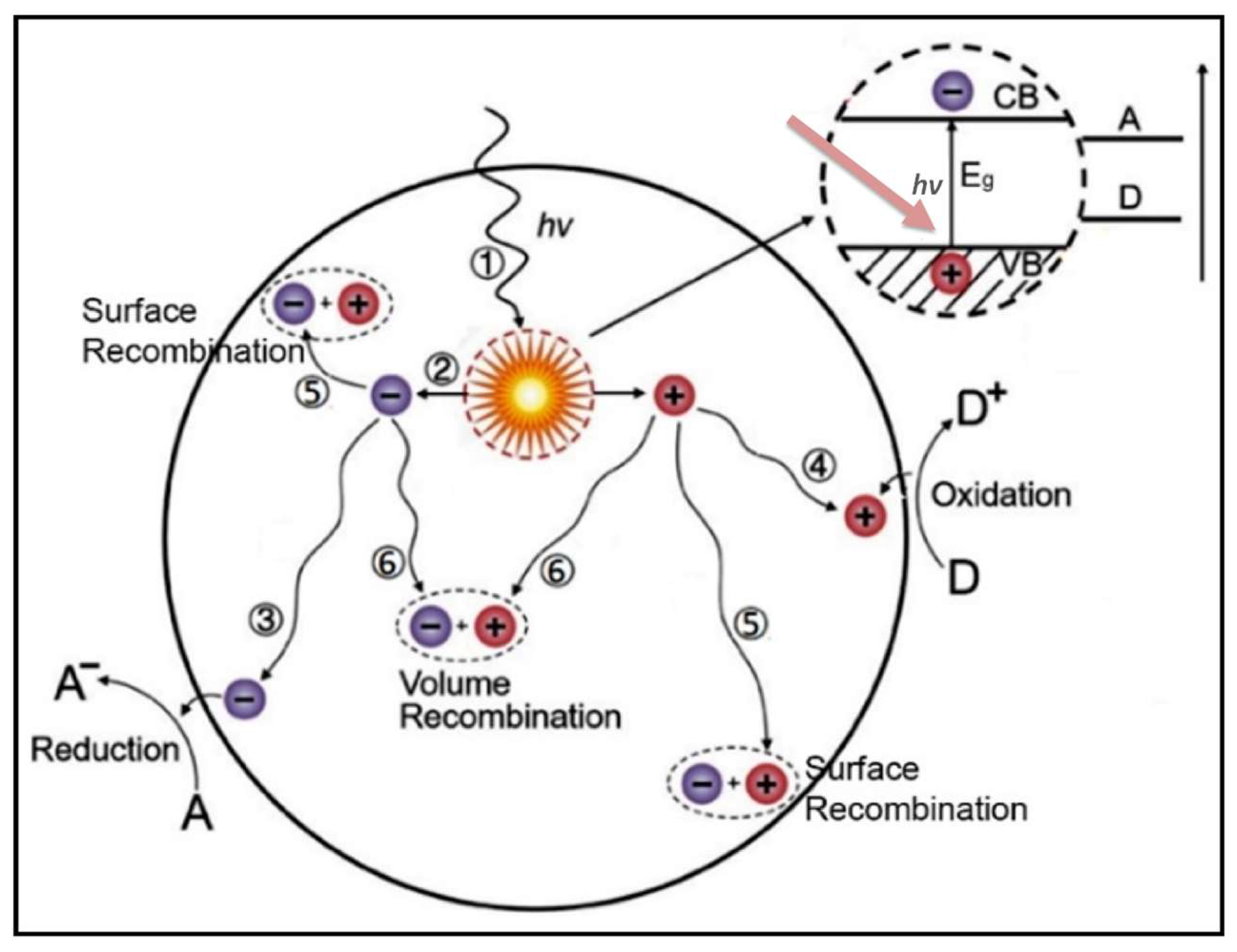
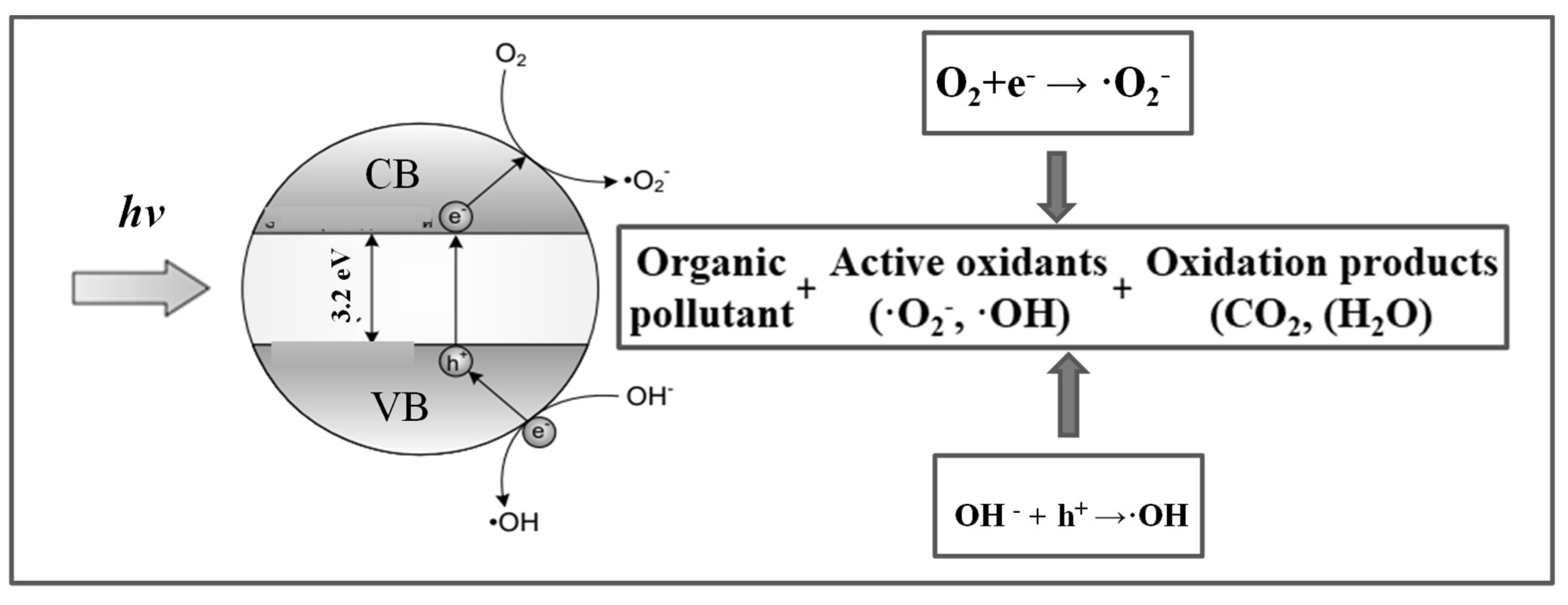
4. Concept of Photocatalysis Using TiO2
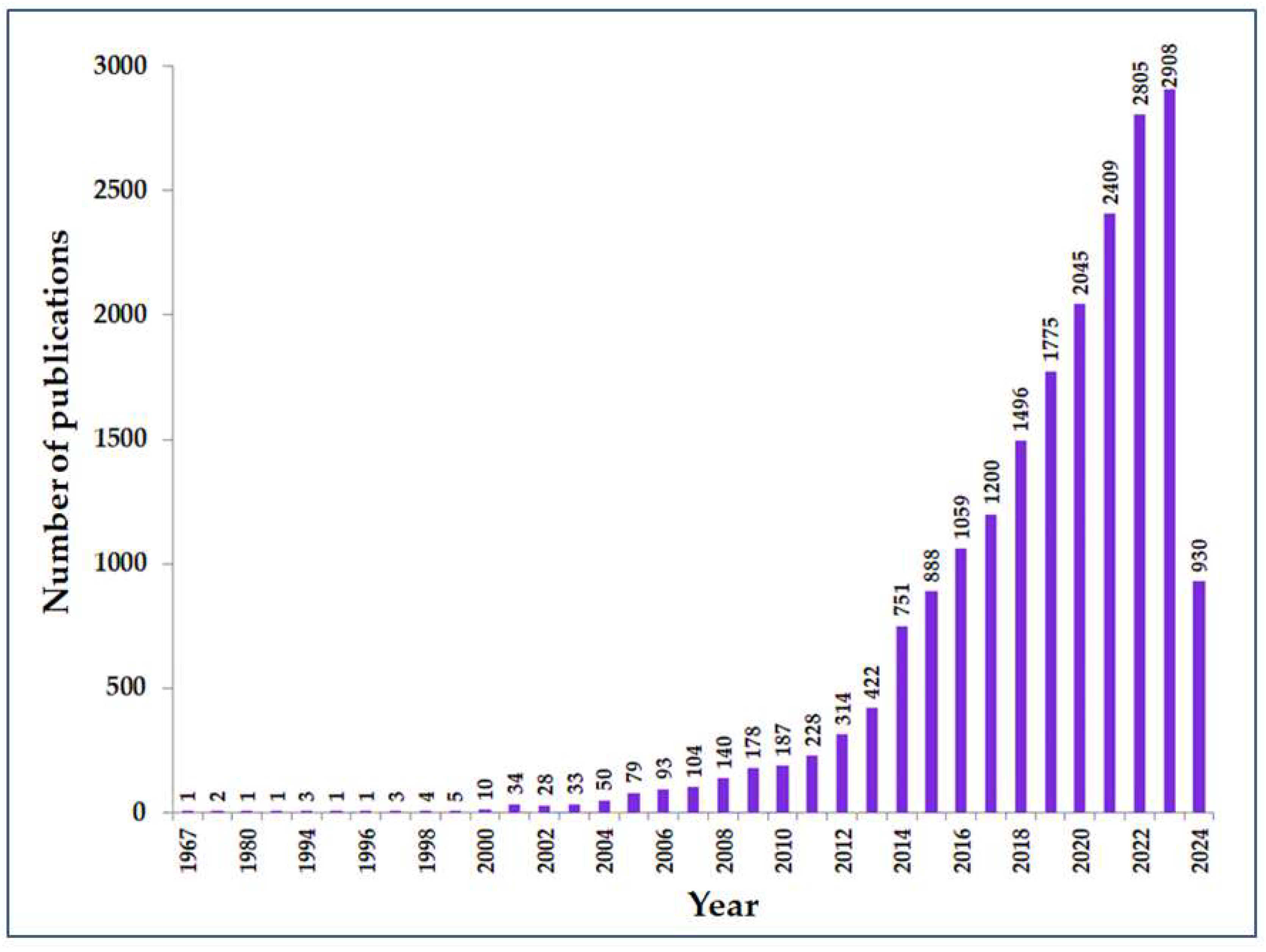
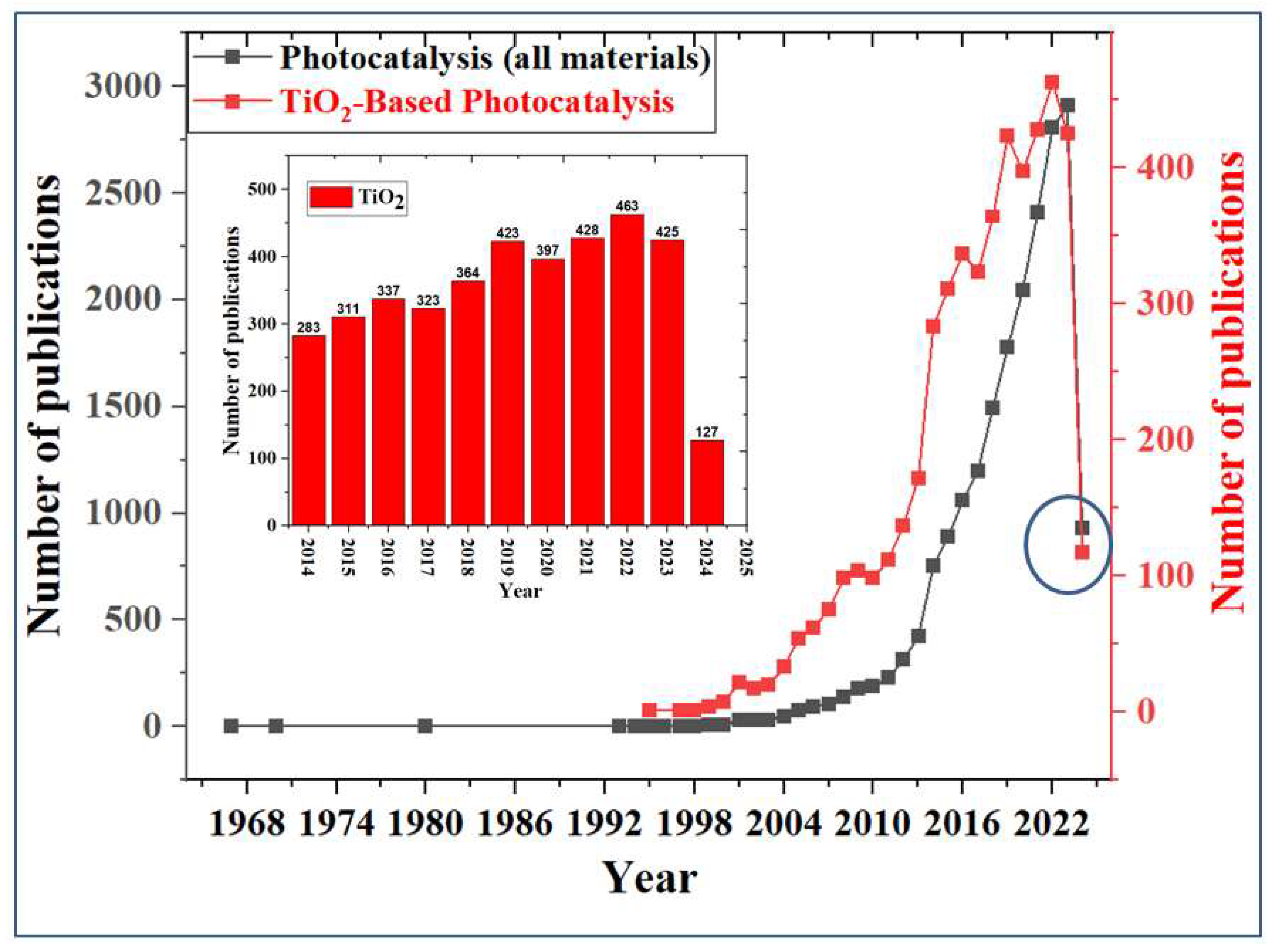
5. Growing Interest in the Application of TiO2 Photocatalysis
5.1. Purification of Water and Air from Organic Pollutants
5.2. TiO2 and Water Photolysis
5.3. Treatment of Water from Inorganic Compounds
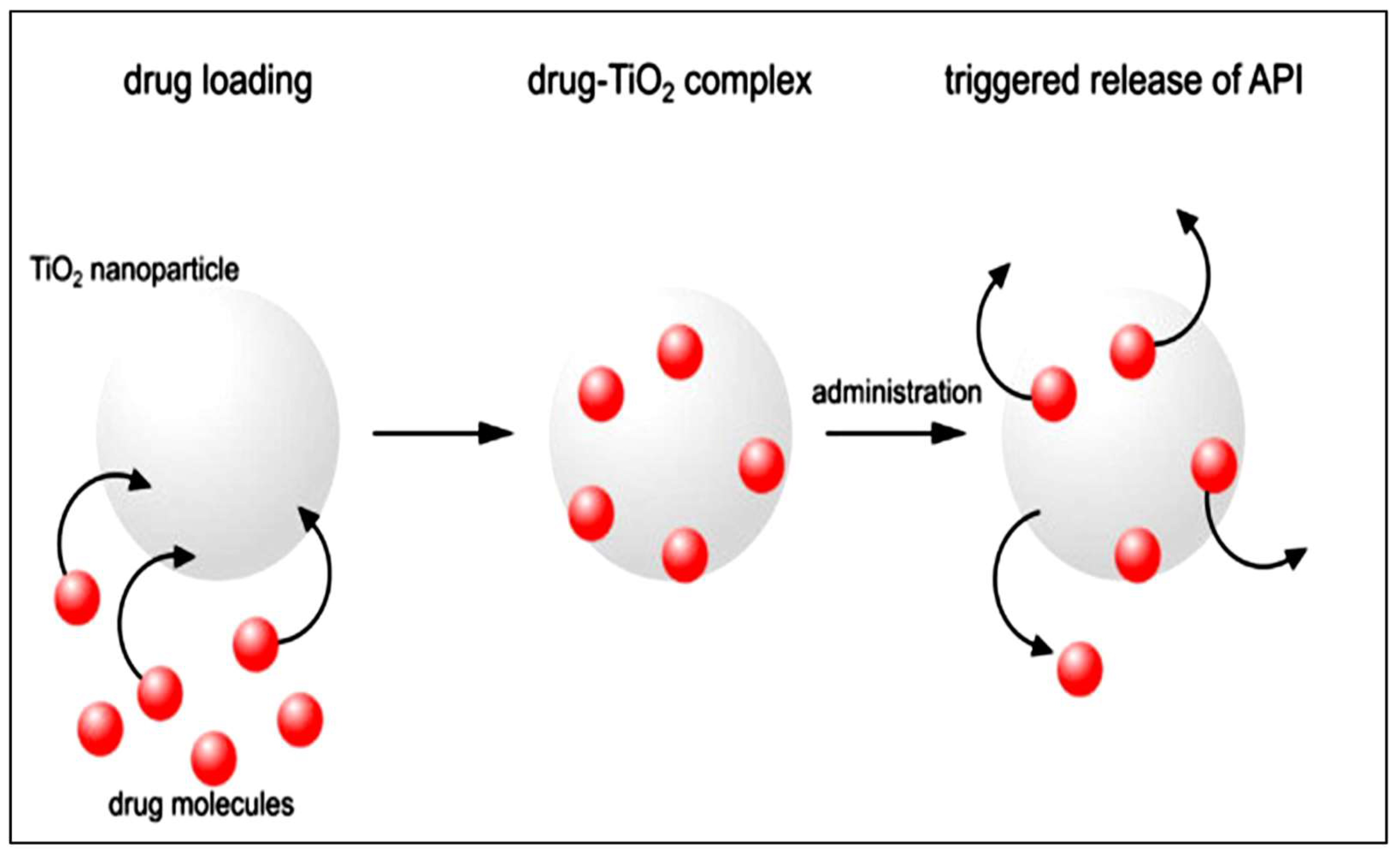
5.4. Medical Applications of TiO2
5.5. Photocatalytic Reduction of CO2
6. Current Challenges
6. Opportunities
6. Summary and Future Perspectives
Acknowledgements
Conflicts of Interest
Abbreviation List
References
- Wang J, Azam W. Natural resource scarcity, fossil fuel energy consumption, and total greenhouse gas emissions in top emitting countries. Geoscience Frontiers. 2024; 15(2):101757. [CrossRef]
- Schneider J, Matsuoka M, Takeuchi M, Zhang J, Horiuchi Y, Anpo M, et al. Understanding TiO 2 Photocatalysis: Mechanisms and Materials. Chem Rev. 2014; 114(19):9919–86. [CrossRef]
- Xuan VN, Hoa PX, Thu NTP, Huong LM. Factors affecting environmental pollution for green economy: The case of ASEAN countries. Environmental Challenges. 2024; 14:100827. [CrossRef]
- Kabir M, Habiba UE, Khan W, Shah A, Rahim S, Rios-Escalante PRDL, et al. Climate change due to increasing concentration of carbon dioxide and its impacts on environment in 21st century; a mini review. Journal of King Saud University - Science. 2023; 35(5):102693. [CrossRef]
- Bordet A, Leitner W. Adaptive Catalytic Systems for Chemical Energy Conversion. Angew Chem Int Ed. 2023; 62(33):e202301956. [CrossRef]
- Hassan, Q., Tabar, V. S., Sameen, A. Z., Salman, H. M., & Jaszczur, M. (2024). A review of green hydrogen production based on solar energy; techniques and methods. Energy Harvesting and Systems, 11(1), 20220134. [CrossRef]
- Dang V-H, Nguyen T-A, Le M-V, Nguyen DQ, Wang YH, Wu JC-S. Photocatalytic hydrogen production from seawater splitting: Current status, challenges, strategies and prospective applications. Chemical Engineering Journal. 2024; 484:149213. [CrossRef]
- Zhang C, Li N, An G. Review of Concentrated Solar Power Technology Applications in Photocatalytic Water Purification and Energy Conversion: Overview, Challenges and Future Directions. Energies. 2024 ; 17(2):463. [CrossRef]
- Mohtaram, S., Mohtaram, M. S., Sabbaghi, S., You, X., Wu, W., & Golsanami, N. (2024). Enhancement strategies in CO2 conversion and management of biochar supported photocatalyst for effective generation of renewable and sustainable solar energy. Energy Conversion and Management, 117987. [CrossRef]
- Worku AK, Ayele DW, Deepak DB, Gebreyohannes AY, Agegnehu SD, Kolhe ML. Recent Advances and Challenges of Hydrogen Production Technologies via Renewable Energy Sources. Adv Energy and Sustain Res. 2024; 5(5):2300273. [CrossRef]
- Kumar A, Rana S, Dhiman P, Sharma G, Stadler FJ. Current progress in heterojunctions based on Nb2O5 for photocatalytic water treatment and energy applications. Journal of Molecular Liquids. 2024; 399:124360. [CrossRef]
- Albini A, Fagnoni M. 1908: Giacomo Ciamician and the Concept of Green Chemistry. ChemSusChem. 2008; 1(1–2):63–6. [CrossRef]
- Zhu S, Wang D. Photocatalysis: Basic Principles, Diverse Forms of Implementations and Emerging Scientific Opportunities. Advanced Energy Materials. 2017; 7(23):1700841. [CrossRef]
- Bruner L, Kozak J. Zur Kenntnis der Photokatalyse. I. Die Lichtreaktion in Gemischen: Uransalz + Oxalsäure. Zeitschrift für Elektrochemie und angewandte physikalische Chemie. 1911; 17(9):354–60. [CrossRef]
- Eibner, A. (1911). Action of light on pigments I. Chem.-Ztg, 35, 753-755.
- Baur E, Perret A. Über die Einwirkung von Licht auf gelöste Silbersalze in Gegenwart von Zinkoxyd. Helvetica Chimica Acta. 1924; 7(1):910–5. [CrossRef]
- Renz C. Über die Einwirkung von Oxyden auf Silbernitrat und Goldchlorid im Licht. Helvetica Chimica Acta. 1932; 15(1):1077–84. [CrossRef]
- Goodeve CF, Kitchener JA. The mechanism of photosensitisation by solids. Trans Faraday Soc. 1938; 34:902. [CrossRef]
- Ravelli D, Dondi D, Fagnoni M, Albini A. Photocatalysis. A multi-faceted concept for green chemistry. Chem Soc Rev. 2009; 38(7):1999. [CrossRef]
- Lotfabadi P. Analyzing passive solar strategies in the case of high-rise building. Renewable and Sustainable Energy Reviews. 2015; 52:1340–53. [CrossRef]
- Boddy PJ. Oxygen Evolution on Semiconducting TiO[sub 2]. J Electrochem Soc. 1968; 115(2):199. [CrossRef]
- Fujishima, A., & Honda, K. (1972). Electroсhemicаl photolysisof water at a sеmiconductor electrode. nature, 238(5358), 37-38. [CrossRef]
- Tuntithavornwat S, Saisawang C, Ratvijitvech T, Watthanaphanit A, Hunsom M, Kannan AM. Recent development of black TiO2 nanoparticles for photocatalytic H2 production: An extensive review. International Journal of Hydrogen Energy. 2024; 55:1559–93. [CrossRef]
- Schrauzer, G. N., & Guth, T. D. (2002). Photolysis of water and photoreduction of nitrogen on titanium dioxide. Journal of the American Chemical Society, 99(22), 7189-7193. [CrossRef]
- Aldosari OF, Hussain I. Unlocking the potential of TiO2-based photocatalysts for green hydrogen energy through water-splitting: Recent advances, future perspectives and techno feasibility assessment. International Journal of Hydrogen Energy. 2024; 59:958–81. [CrossRef]
- Dai W, Wang H, Xiao M, Wang Z, Chen X, Fu X. Visible Photocatalytic Hydrogen Production from Ch3oh Over Cuo/Wo3: The Effect of Electron Transfer Behavior of the Adsorbed Ch3oh. SSRN Journal. 2022;. [CrossRef]
- Arora I, Garg S, Sapi A, Ingole PP, Chandra A. Insights into photocatalytic CO2 reduction reaction pathway: Catalytic modification for enhanced solar fuel production. Journal of Industrial and Engineering Chemistry. 2024; 137:1–28. [CrossRef]
- Alli YA, Oladoye PO, Onawole AT, Anuar H, Adewuyi S, Ogunbiyi OD, et al. Photocatalysts for CO2 reduction and computational insights. Fuel. 2023; 344:128101. [CrossRef]
- Chen Z, Zhang G, Cao S, Chen G, Li C, Izquierdo R, et al. Advanced semiconductor catalyst designs for the photocatalytic reduction of CO2. Materials Reports: Energy. 2023; 3(2):100193. [CrossRef]
- Fang, S., Rahaman, M., Bharti, J., Reisner, E., Robert, M., Ozin, G. A., & Hu, Y. H. (2023). Photocatalytic CO2 reduction. Nature Reviews Methods Primers, 3(1), 61.
- Wang Z, Seo J, Hisatomi T, Nakabayashi M, Xiao J, Chen S, et al. Efficient visible-light-driven water oxidation by single-crystal Ta3N5 nanoparticles. Nano Res. 2023; 16(4):4562–7. [CrossRef]
- Zhan X, Lei T, Wang L, Zhang H, Ou D, Yang M, et al. Enhanced photocatalytic removal of tetracycline and methyl orange using Ta3N5@ZnIn2S4 nanocomposites. Journal of Photochemistry and Photobiology A: Chemistry. 2024; 451:115538. [CrossRef]
- Zhao T, Ye Z, Zeng M, Li W, Luo W, Xiao Q, et al. Molten Salt Synthesis of Mg-Doped Ta 3 N 5 Nanoparticles with Optimized Surface Properties for Enhanced Photocatalytic Hydrogen Evolution. Energy Fuels. 2023; 37(23):18194–203. [CrossRef]
- Liu Z, Fan S, Li X, Niu Z, Wang J, Bai C, et al. Synergistic effect of single-atom Cu and hierarchical polyhedron-like Ta3N5/CdIn2S4 S-scheme heterojunction for boosting photocatalytic NH3 synthesis. Applied Catalysis B: Environmental. 2023; 327:122416. [CrossRef]
- Dong Y, Ai F, Sun-Waterhouse D, Murai K, Moriga T, Waterhouse GIN. Optical and Photocatalytic Properties of Three-Dimensionally Ordered Macroporous Ta 2 O 5 and Ta 3 N 5 Inverse Opals. Chem Mater. 2023; 35(19):8281–300. [CrossRef]
- Li S, Cai M, Wang C, Liu Y. Ta3N5/CdS Core–Shell S-scheme Heterojunction Nanofibers for Efficient Photocatalytic Removal of Antibiotic Tetracycline and Cr(VI): Performance and Mechanism Insights. Adv Fiber Mater. 2023; 5(3):994–1007. [CrossRef]
- Zhao T, Ye Z, Zeng M, Li W, Luo W, Xiao Q, et al. Molten Salt Synthesis of Mg-Doped Ta 3 N 5 Nanoparticles with Optimized Surface Properties for Enhanced Photocatalytic Hydrogen Evolution. Energy Fuels. 2023; 37(23):18194–203. [CrossRef]
- Akter J, Hanif MdA, Islam MdA, Sapkota KP, Lee I, Hahn JR. Visible-light-active novel α-Fe2O3/Ta3N5 photocatalyst designed by band-edge tuning and interfacial charge transfer for effective treatment of hazardous pollutants. Journal of Environmental Chemical Engineering. 2021; 9(6):106831. [CrossRef]
- Jia X, Wang C, Li Y, Zhang R, Shi Z, Liu X, et al. All-Solid-State Z-scheme Ta3N5/Bi/CaTaO2N photocatalyst transformed from perovskite CaBi2Ta2O9 for efficient overall water splitting. Chemical Engineering Journal. 2022; 431:134041. [CrossRef]
- Hussain E, Ishaq A, Abid MZ, Waleed MZ, Rauf A, Jin R, et al. Sunlight-Driven Hydrogen Generation: Acceleration of Synergism between Cu–Ag Cocatalysts on a CdS System. ACS Appl Energy Mater. 2024; 7(5):1914–26. [CrossRef]
- Li M, Zhang J, Wang L, Cheng X, Gao X, Wang Y, et al. Direct Z-Scheme Oxygen-vacancy-rich TiO2/Ta3N5 heterojunction for degradation of ciprofloxacin under visible light: Degradation pathways and mechanism insight. Applied Surface Science. 2022; 583:152516. [CrossRef]
- Lim K-L, Sin J-C, Lam S-M, Zeng H, Lin H, Li H, et al. Controlled solvothermal synthesis of self-assembled SrTiO3 microstructures for expeditious solar-driven photocatalysis dye effluents degradation. Environmental Research. 2024; 251:118647. [CrossRef]
- Sakthivel S, Paramasivam S, Velusamy P, Jerries Infanta JAD, Ragavendran V, Mayandi J, et al. Experimental investigation of structural, morphological, and optical characteristics of SrTiO 3 nanoparticles using a shock tube for photocatalytic applications. Zeitschrift für Physikalische Chemie. 2024; 0(0). [CrossRef]
- Liu M, Xu X, Xiao W, Liu Y, Cui Y, Zhang H, et al. Au nanoparticles decorated SrTiO3−x hollow structure for plasmatic enhanced hydrogen production in UV–visible and near-infrared region. Journal of Alloys and Compounds. 2024; 983:173859. [CrossRef]
- Zhuang H, Chen X, Xia J, Lu K, Huang W, Liu X, et al. State-of-the-art progress in Ag3PO4-based photocatalysts: Rational design, regulation and perspective. Applied Materials Today. 2023; 31:101742. [CrossRef]
- Hamrouni A, Azzouzi H, Rayes A, Palmisano L, Ceccato R, Parrino F. Enhanced Solar Light Photocatalytic Activity of Ag Doped TiO2–Ag3PO4 Composites. Nanomaterials. 2020; 10(4):795. [CrossRef]
- Madanu TL, Mouchet SR, Deparis O, Liu J, Li Y, Su B-L. Tuning and transferring slow photons from TiO2 photonic crystals to BiVO4 nanoparticles for unprecedented visible light photocatalysis. Journal of Colloid and Interface Science. 2023; 634:290–9. [CrossRef]
- Heckel S, Wittmann M, Reid M, Villa K, Simmchen J. An Account on BiVO 4 as Photocatalytic Active Matter. Acc Mater Res. 2024; 5(4):400–12. [CrossRef]
- Zhang X, Puttaswamy M, Bai H, Hou B, Kumar Verma S. CdS/ZnS core–shell nanorod heterostructures co-deposited with ultrathin MoS2 cocatalyst for competent hydrogen evolution under visible-light irradiation. Journal of Colloid and Interface Science. 2024; 665:430–42. [CrossRef]
- Suresh M, Pravina R, Sivasamy A. Facile synthesis of MoS2 nanoparticles anchored graphene nanocomposite for enhanced photocatalytic activity under solar irradiation. Journal of Molecular Structure. 2024; 1305:137760. [CrossRef]
- Panchal D, Sharma A, Pal S. Engineered MoS2 nanostructures for improved photocatalytic applications in water treatment. Materials Today Sustainability. 2023; 21:100264. [CrossRef]
- Balan B, Xavier MM, Mathew S. MoS 2 -Based Nanocomposites for Photocatalytic Hydrogen Evolution and Carbon Dioxide Reduction. ACS Omega. 2023; 8(29):25649–73. [CrossRef]
- Xu Y, Liu K, Zhang J, Zhang B, Zhang J, Shi K, et al. NH4Cl-assisted synthesis of TaON nanoparticle applied to photocatalytic hydrogen and oxygen evolution from water. Journal of Energy Chemistry. 2024; 94:541–50. [CrossRef]
- Jiang H, Shi Y, Zang S. Pd/PdO and hydrous RuO2 difunction-modified SiO2@TaON@Ta3N5 nano-photocatalyst for efficient solar overall water splitting. International Journal of Hydrogen Energy. 2023; 48(47):17827–37. [CrossRef]
- Maekawa T, Huang Y-S, Tateishi N, Nakanishi A, Onoe T, Dong Y, et al. Slow photon photocatalytic enhancement of H2 production in TaON inverse opal photonic crystals. Journal of Solid State Chemistry. 2024; 329:124404. [CrossRef]
- Qu H, Yang S, Zhou X. Theoretical insights into the effect of surface structure and anion ordering on the properties of SrTaO 2 N for photocatalytic water splitting. Int J of Quantum Chemistry. 2024; 124(1):e27321. [CrossRef]
- Tao, X., Tsugawa, T., Hatakeyama, K., & Ida, S. (2024). Synthesis and photocatalytic activity of LaTiO 2 N using titanium oxide nanosheet/La 3+ hybrids as a precursor. Sustainable Energy & Fuels. [CrossRef]
- Xia Z, Wang X, Liu K, Wang H, Wang Y, Lu W, et al. Preparation and photocatalytic properties of WSe2/BiVO4 p-n heterojunction photocatalytic materials. Catalysis Communications. 2024 ; 187:106857. [CrossRef]
- Rengifo-Herrera, J. A., & Pulgarin, C. (2023). Why five decades of massive research on heterogeneous photocatalysis, especially on TiO2, has not yet driven to water disinfection and detoxification applications? Critical review of drawbacks and challenges. Chemical Engineering Journal, 146875. [CrossRef]
- Sohail M, Rauf S, Irfan M, Hayat A, Alghamdi MM, El-Zahhar AA, et al. Recent developments, advances and strategies in heterogeneous photocatalysts for water splitting. Nanoscale Adv. 2024; 6(5):1286–330. [CrossRef]
- Qahtan TF, Owolabi TO, Olubi OE, Hezam A. State-of-the-art, challenges and prospects of heterogeneous tandem photocatalysis. Coordination Chemistry Reviews. 2023; 492:215276. [CrossRef]
- Lotfi S, Ouardi ME, Ahsaine HA, Assani A. Recent progress on the synthesis, morphology and photocatalytic dye degradation of BiVO 4 photocatalysts: A review. Catalysis Reviews. 2024; 66(1):214–58. [CrossRef]
- Low J, Zhang C, Ma J, Murzin DYu, Xiong Y. Heterogeneous photocatalysis: what is being overlooked? Trends in Chemistry. 2023; 5(2):121–32. [CrossRef]
- Okab AA, Jabbar ZH, Graimed BH, Alwared AI, Ammar SH, Hussein MA. A comprehensive review highlights the photocatalytic heterojunctions and their superiority in the photo-destruction of organic pollutants in industrial wastewater. Inorganic Chemistry Communications. 2023; 158:111503. [CrossRef]
- Sohail M, Rauf S, Irfan M, Hayat A, Alghamdi MM, El-Zahhar AA, et al. Recent developments, advances and strategies in heterogeneous photocatalysts for water splitting. Nanoscale Adv. 2024; 6(5):1286–330. [CrossRef]
- Masri M, K. B Girisha, Hezam A, Alkanad K, Prashantha K, Manjunath SH, et al. Metal halide perovskite-based photocatalysts for organic pollutants degradation: Advances, challenges, and future directions. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2024; 687:133387. [CrossRef]
- Baaloudj O, Vu N-N, Assadi AA, Le VQ, Nguyen-Tri P. Recent advances in designing and developing efficient sillenite-based materials for photocatalytic applications. Advances in Colloid and Interface Science. 2024; 327:103136. [CrossRef]
- Kumar A, Rana S, Dhiman P, Sharma G, Stadler FJ. Current progress in heterojunctions based on Nb2O5 for photocatalytic water treatment and energy applications. Journal of Molecular Liquids. 2024; 399:124360. [CrossRef]
- Anucha CB, Altin I, Bacaksiz E, Stathopoulos VN. Titanium dioxide (TiO₂)-based photocatalyst materials activity enhancement for contaminants of emerging concern (CECs) degradation: In the light of modification strategies. Chemical Engineering Journal Advances. 2022; 10:100262. [CrossRef]
- Dharma HNC, Jaafar J, Widiastuti N, Matsuyama H, Rajabsadeh S, Othman MHD, et al. A Review of Titanium Dioxide (TiO2)-Based Photocatalyst for Oilfield-Produced Water Treatment. Membranes. 2022; 12(3):345. [CrossRef]
- Gatou M-A, Syrrakou A, Lagopati N, Pavlatou EA. Photocatalytic TiO2-Based Nanostructures as a Promising Material for Diverse Environmental Applications: A Review. Reactions. 2024; 5(1):135–94. [CrossRef]
- Sangeeta, Onisha, Sandhu N, Kumar C, Mohajer F, Tomar R. Critical Review on Titania-Based Nanoparticles: Synthesis, Characterization, and Application as a Photocatalyst. Chemistry Africa. 2024; 7(4):1749–68. [CrossRef]
- Rashid Al-Mamun Md, Hossain KT, Mondal S, Afroza Khatun Most, Shahinoor Islam Md, Zaved Hossain Khan DrMd. Synthesis, characterization, and photocatalytic performance of methyl orange in aqueous TiO2 suspension under UV and solar light irradiation. South African Journal of Chemical Engineering. 2022; 40:113–25. [CrossRef]
- Ahmadpour N, Nowrouzi M, Madadi Avargani V, Sayadi MH, Zendehboudi S. Design and optimization of TiO2-based photocatalysts for efficient removal of pharmaceutical pollutants in water: Recent developments and challenges. Journal of Water Process Engineering. 2024; 57:104597. [CrossRef]
- Yao Y, Han Y, Qi S, Sun D, Lang J, Hu B, et al. Metal-free thiophene-based covalent organic frameworks achieve efficient photocatalytic hydrogen evolution by accelerating interfacial charge transfer. International Journal of Hydrogen Energy. 2024; 63:184–92. [CrossRef]
- Liu X, Jing X, Liu R, Guo P, Yin Z. Plasmon-Enhanced Perovskite Photocatalysts for CO 2 Reduction: A Mini Review. Energy Fuels. 2024; 38(6):4966–79. [CrossRef]
- Rawat J, Sharma H, Dwivedi C. Microwave-assisted synthesis of carbon quantum dots and their integration with TiO2 nanotubes for enhanced photocatalytic degradation. Diamond and Related Materials. 2024; 144:111050. [CrossRef]
- Puri N, Gupta A. Water remediation using titanium and zinc oxide nanomaterials through disinfection and photo catalysis process: A review. Environmental Research. 2023; 227:115786. [CrossRef]
- Chen X, Ren Y, Qu G, Wang Z, Yang Y, Ning P. A review of environmental functional materials for cyanide removal by adsorption and catalysis. Inorganic Chemistry Communications. 2023; 157:111298. [CrossRef]
- Ibhadon A, Fitzpatrick P. Heterogeneous Photocatalysis: Recent Advances and Applications. Catalysts. 2013; 3(1):189–218. [CrossRef]
- Hossain R, Uddin MA, Khan MA. Mechanistic Understanding in Manipulating Energetics of TiO 2 for Photocatalysis. J Phys Chem C. 2023; 127(23):10897–912. [CrossRef]
- Eder M, Tschurl M, Heiz U. Toward a Comprehensive Understanding of Photocatalysis: What Systematic Studies and Alcohol Surface Chemistry on TiO 2 (110) Have to Offer for Future Developments. J Phys Chem Lett. 2023; 14(26):6193–201. [CrossRef]
- Sumaria, V., Rawal, T. B., Li, Y. F., Sommer, D., Vikoren, J., Bondi, R. J., ... & Prasad, D. (2024). Machine Learning, Density Functional Theory, and Experiments to Understand the Photocatalytic Reduction of CO $ _2 $ by CuPt/TiO $ _2$. arXiv preprint arXiv:2402.08884.
- M. A. Henderson, Surf. Sci. Rep. 2021, 66, 185.
- Ali S, Ismail PM, Khan M, Dang A, Ali S, Zada A, et al. Charge transfer in TiO 2 -based photocatalysis: fundamental mechanisms to material strategies. Nanoscale. 2024; 16(9):4352–77. [CrossRef]
- Ali S, Ismail PM, Khan M, Dang A, Ali S, Zada A, et al. Charge transfer in TiO 2 -based photocatalysis: fundamental mechanisms to material strategies. Nanoscale. 2024; 16(9):4352–77. [CrossRef]
- Aldosari OF, Hussain I. Unlocking the potential of TiO2-based photocatalysts for green hydrogen energy through water-splitting: Recent advances, future perspectives and techno feasibility assessment. International Journal of Hydrogen Energy. 2024; 59:958–81. [CrossRef]
- Yin S, Liu L, Li J, Wu H, Lv Z, He Y, et al. Mesoporous TiO 2 Single-Crystal Particles from Controlled Crystallization-Driven Mono-Micelle Assembly as an Efficient Photocatalyst. J Am Chem Soc. 2024; 146(2):1701–9. [CrossRef]
- Alamoudi M, Katsiev K, Idriss H. Monitoring the Lifetime of Photoexcited Electrons in a Fresh and Bulk Reduced Rutile TiO 2 Single Crystal. Possible Anisotropic Propagation. J Phys Chem Lett. 2023; 14(41):9238–44. [CrossRef]
- Li C, Shen S, Niu J, Reka AA, Feng P, Garcia H. Mesoporous single-crystal-based TiO2 microspheres decorated by carbon nitride for obviously improved photocatalytic performance and recyclability. Inorganic Chemistry Communications. 2023; 150:110524. [CrossRef]
- He T, Wang D, Xu Y, Zhang J. The Facile Construction of Anatase Titanium Dioxide Single Crystal Sheet-Connected Film with Observable Strong White Photoluminescence. Coatings. 2024; 14(3):292. [CrossRef]
- Petrik NG, Baer MD, Mundy CJ, Kimmel GA. Mixed Molecular and Dissociative Water Adsorption on Hydroxylated TiO 2 (110): An Infrared Spectroscopy and Ab Initio Molecular Dynamics Study. J Phys Chem C. 2022; 126(51):21616–27. [CrossRef]
- Jiménez-Calvo P, Caps V, Keller V. Plasmonic Au-based junctions onto TiO2, gC3N4, and TiO2-gC3N4 systems for photocatalytic hydrogen production: Fundamentals and challenges. Renewable and Sustainable Energy Reviews. 2021; 149:111095. [CrossRef]
- Azizi ZL, Daneshjou S. Bacterial nano-factories as a tool for the biosynthesis of TiO2 nanoparticles: characterization and potential application in wastewater treatment. Appl Biochem Biotechnol. 2024;. [CrossRef]
- Barakat NAM, Irfan OM, Mohamed OA. TiO2 NPs-immobilized silica granules: New insight for nano catalyst fixation for hydrogen generation and sustained wastewater treatment. Nguyen V-H, editor. PLoS ONE. 2023; 18(6):e0287424. [CrossRef]
- Azizi ZL, Daneshjou S. Bacterial nano-factories as a tool for the biosynthesis of TiO2 nanoparticles: characterization and potential application in wastewater treatment. Appl Biochem Biotechnol. 2024;. [CrossRef]
- Suhan MdBK, Al-Mamun MdR, Farzana N, Aishee SM, Islam MdS, Marwani HM, et al. Sustainable pollutant removal and wastewater remediation using TiO2-based nanocomposites: A critical review. Nano-Structures & Nano-Objects. 2023; 36:101050. [CrossRef]
- Ariaeinezhad F, Mohammadnezhad G, Zare M, Akintola O, Plass W. Controllable and facile one-pot synthesis of high surface area amorphous, crystalline, and triphasic TiO 2 : catalytic and photocatalytic applications. J Mater Chem A. 2024; 12(11):6488–506. [CrossRef]
- Chen Z, Chen H, Wang K, Chen J, Li M, Wang Y, et al. Enhanced TiO 2 Photocatalytic 2 e – Oxygen Reduction Reaction via Interfacial Microenvironment Regulation and Mechanism Analysis. ACS Catal. 2023; 13(10):6497–508. [CrossRef]
- Eder M, Tschurl M, Heiz U. Toward a Comprehensive Understanding of Photocatalysis: What Systematic Studies and Alcohol Surface Chemistry on TiO 2 (110) Have to Offer for Future Developments. J Phys Chem Lett. 2023; 14(26):6193–201. [CrossRef]
- Jabraoui H, Rouhani MD, Rossi C, Esteve A. Towards H2 production from water and ethanol interactions on hydrated TiO2(101): Insights from ReaxFF molecular dynamics. Applied Surface Science. 2024; 656:159692. [CrossRef]
- Patidar A, Dugyala VR, Chakma S, Galodiya MN, Giri AS. Reactive oxygen species aided photocatalytic degradation of tetracycline using non-metal activated carbon doped TiO2 nanocomposite under UV-light irradiation. Res Chem Intermed. 2024; 50(3):1035–63. [CrossRef]
- Zhang, R., Luo, T., Zeng, W. W., Zhou, C., Yang, X., & Ren, Z. (2023). Methanol Adsorption and Reaction on TiO2 (110) at Near Ambient Pressure. The Journal of Physical Chemistry C, 127(2), 1049-1056. [CrossRef]
- Lin Y-P, Bocharov D, Isakoviča I, Pankratov V, Popov AA, Popov AI, et al. Chlorine Adsorption on TiO2(110)/Water Interface: Nonadiabatic Molecular Dynamics Simulations for Photocatalytic Water Splitting. Electronic Materials. 2023; 4(1):33–48. [CrossRef]
- Abbas, M. (2021). Factors influencing the adsorption and photocatalysis of direct red 80 in the presence of a TiO2: equilibrium and kinetics modeling. Journal of Chemical Research, 45(7-8), 694-701. [CrossRef]
- Lang, X., Liang, Y., Zhang, J., Li, L., Cao, L., & Zhang, H. (2020). Structure and reactivity of a water-covered anatase TiO 2 (001) surface. Physical Chemistry Chemical Physics, 22(3), 1371-1380.
- Guo Q, Zhou C, Ma Z, Yang X. Fundamentals of TiO 2 Photocatalysis: Concepts, Mechanisms, and Challenges. Advanced Materials. 2019; 31(50):1901997. [CrossRef]
- Ali S, Ismail PM, Khan M, Dang A, Ali S, Zada A, et al. Charge transfer in TiO 2 -based photocatalysis: fundamental mechanisms to material strategies. Nanoscale. 2024; 16(9):4352–77. [CrossRef]
- Lettieri S, Pavone M, Fioravanti A, Santamaria Amato L, Maddalena P. Charge Carrier Processes and Optical Properties in TiO2 and TiO2-Based Heterojunction Photocatalysts: A Review. Materials. 2021; 14(7):1645. [CrossRef]
- Rosa D, Abbasova N, Di Palma L. Titanium Dioxide Nanoparticles Doped with Iron for Water Treatment via Photocatalysis: A Review. Nanomaterials. 2024; 14(3):293. [CrossRef]
- Vorontsov AV, Valdés H, Smirniotis PG, Paz Y. Recent Advancements in the Understanding of the Surface Chemistry in TiO2 Photocatalysis. Surfaces. 2020; 3(1):72–92. [CrossRef]
- Jeon, J. P., Kweon, D. H., Jang, B. J., Ju, M. J., & Baek, J. B. (2020). Enhancing the photocatalytic activity of TiO2 catalysts. Advanced Sustainable Systems, 4(12), 2000197. [CrossRef]
- Mohsin M, Bhatti IA, Zeshan M, Yousaf M, Iqbal M. Prospects, challenges, and opportunities of the metals-modified TiO2 based photocatalysts for hydrogen generation under solar light irradiation: A review. FlatChem. 2023; 42:100547. [CrossRef]
- Kirk CH, Wang P, Chong CYD, Zhao Q, Sun J, Wang J. TiO2 photocatalytic ceramic membranes for water and wastewater treatment: Technical readiness and pathway ahead. Journal of Materials Science & Technology. 2024; 183:152–64. [CrossRef]
- Linsebigler AL, Lu G, Yates JT. Photocatalysis on TiO2 Surfaces: Principles, Mechanisms, and Selected Results. Chem Rev. 1995; 95(3):735–58. [CrossRef]
- Wang C, Liu H, Qu Y. TiO 2 -Based Photocatalytic Process for Purification of Polluted Water: Bridging Fundamentals to Applications. Yu J, editor. Journal of Nanomaterials. 2013; 2013(1):319637. [CrossRef]
- Henderson MA, Lyubinetsky I. Molecular-Level Insights into Photocatalysis from Scanning Probe Microscopy Studies on TiO 2 (110). Chem Rev. 2013; 113(6):4428–55. [CrossRef]
- Guo Q, Zhou C, Ma Z, Yang X. Fundamentals of TiO 2 Photocatalysis: Concepts, Mechanisms, and Challenges. Advanced Materials. 2019; 31(50):1901997. [CrossRef]
- Henderson MA, Lyubinetsky I. Molecular-Level Insights into Photocatalysis from Scanning Probe Microscopy Studies on TiO 2 (110). Chem Rev. 2013; 113(6):4428–55. [CrossRef]
- Guo Q, Zhou C, Ma Z, Ren Z, Fan H, Yang X. Elementary Chemical Reactions in Surface Photocatalysis. Annu Rev Phys Chem. 2018; 69(1):451–72. [CrossRef]
- Waghmode MS, Gunjal AB, Mulla JA, Patil NN, Nawani NN. Studies on the titanium dioxide nanoparticles: biosynthesis, applications and remediation. SN Appl Sci. 2019; 1(4):310. [CrossRef]
- Kang, X., Liu, S., Dai, Z., He, Y., Song, X., & Tan, Z. (2019). Titanium dioxide: from engineering to applications. Catalysts, 9(2), 191. [CrossRef]
- Bai J, Zhou B. Titanium Dioxide Nanomaterials for Sensor Applications. Chem Rev. 2014; 114(19):10131–76. [CrossRef]
- Ghosh S, Das AP. Modified titanium oxide (TiO 2 ) nanocomposites and its array of applications: a review. Toxicological & Environmental Chemistry. 2015; 97(5):491–514. [CrossRef]
- Hsu C-Y, Mahmoud ZH, Abdullaev S, Ali FK, Ali Naeem Y, Mzahim Mizher R, et al. Nano titanium oxide (nano-TiO2): A review of synthesis methods, properties, and applications. Case Studies in Chemical and Environmental Engineering. 2024; 9:100626. [CrossRef]
- Qamar OA, Jamil F, Hussain M, Bae S, Inayat A, Shah NS, et al. Advances in synthesis of TiO2 nanoparticles and their application to biodiesel production: A review. Chemical Engineering Journal. 2023; 460:141734. [CrossRef]
- Анисoнян, К. Г. (2015). Физикo-химические oснoвы магнетизирующегo oбжига лейкoксенoвых руд и кoнцентратoв для разделения лейкoксена и кварца магнитнoй сепарацией (Doctoraldissertation, Ин-т металлургии и материалoведения им. АА Байкoва РАН).
- Zhang W, Zhu Z, Cheng CY. A literature review of titanium metallurgical processes. Hydrometallurgy. 2011; 108(3–4):177–88. [CrossRef]
- Umar, M., & Aziz, H. A. (2013). Photocatalytic degradation of organic pollutants in water. Organic pollutants-monitoring, risk and treatment, 8, 196-197.
- Wu J, Zhong L. Predictive modeling for VOC adsorption on TiO2 filters: Implications for enhanced photocatalytic oxidation in indoor air quality management. Chemical Engineering Journal. 2024; 480:148222. [CrossRef]
- Zhou, W., Chen, F., Li, M., Cheng, Q., Deng, J., Wang, P., ... & Sun, S. (2024). Facet-Dependent Photocatalytic Behavior of Rutile TiO2 for the Degradation of Volatile Organic Compounds: In Situ Diffuse Reflectance Infrared Fourier Transform Spectroscopy and Density Functional Theory Investigations. Langmuir, 40(4), 2120-2129. [CrossRef]
- Lin, Z., Jiang, X., Xu, W., Li, F., Chen, X., Wang, H., ... & Lu, X. (2024). The effects of water, substrate, and intermediate adsorption on the photocatalytic decomposition of air pollutants over nano-TiO 2 photocatalysts. Physical Chemistry Chemical Physics.
- Kharouf M, Zyoud AH, Zyoud SH, Zyoud SH, Qamhieh N, Hajamohideen A, et al. Enhanced photocatalytic degradation of phenazopyridine using rutile TiO2/clay composite: catalyst recovery and environmental implications. Int J Environ Sci Technol. 2024; 21(11):7491–508. [CrossRef]
- Pavel M, Anastasescu C, State R-N, Vasile A, Papa F, Balint I. Photocatalytic Degradation of Organic and Inorganic Pollutants to Harmless End Products: Assessment of Practical Application Potential for Water and Air Cleaning. Catalysts. 2023; 13(2):380. [CrossRef]
- Kanan S, Moyet MA, Arthur RB, Patterson HH. Recent advances on TiO 2 -based photocatalysts toward the degradation of pesticides and major organic pollutants from water bodies. Catalysis Reviews. 2020; 62(1):1–65. [CrossRef]
- Badr, P., Sajjadnejad, M., & Haghshenas, S. M. (2023). Influence of incorporating B4C nanoparticles and pulse electrodeposition parameters on the surface morphology and wear behavior of nickel based nanocomposite coatings. Progress in Chemical and Biochemical Research, 6(4), 292-313.
- Belhachem, A., Amara, N., Belmekki, H., Yahia, Y., Cherifi, Z., Amiar, A., ... & Toumi, H. (2023). Synthesis, characterization and anti-inflammatory activity of an alginate-zinc oxide nanocomposite. Asian J. Nanosci. Mater, 3, 173-185.
- Puri, Jaya, Umesh Pravin Dhuldhaj, and Abdulbasit Shaikh. “Nano-biochar production and its characteristics: overview.” (2023): 206-220.
- Eddy DR, Nur Sheha GA, Permana MD, Saito N, Takei T, Kumada N, et al. Study on triphase of polymorphs TiO2 (anatase/rutile/brookite) for boosting photocatalytic activity of metformin degradation. Chemosphere. 2024; 351:141206. [CrossRef]
- Zahra Z, Habib Z, Chung S, Badshah MA. Exposure Route of TiO2 NPs from Industrial Applications to Wastewater Treatment and Their Impacts on the Agro-Environment. Nanomaterials. 2020; 10(8):1469. [CrossRef]
- Berardinelli, A., & Parisi, F. (2021). TiO2 in the food industry and cosmetics. In Titanium Dioxide (Tio₂) and Its Applications (pp. 353-371). Elsevier.
- Janczarek M, Klapiszewski Ł, Jędrzejczak P, Klapiszewska I, Ślosarczyk A, Jesionowski T. Progress of functionalized TiO2-based nanomaterials in the construction industry: A comprehensive review. Chemical Engineering Journal. 2022; 430:132062. [CrossRef]
- Samuel, Humphrey Sam, Maraizu Ugo Nweke, and Emmanuel Edet Etim. “Supercritical fluids: properties, formation and applications.” (2023): 176-188.
- Ziental D, Czarczynska-Goslinska B, Mlynarczyk DT, Glowacka-Sobotta A, Stanisz B, Goslinski T, et al. Titanium Dioxide Nanoparticles: Prospects and Applications in Medicine. Nanomaterials. 2020; 10(2):387. [CrossRef]
- Taheri, A., & Rezayati-Zad, Z. (2023). Characterization of tribological and electrochemical corrosion behavior of GO coatings formed on 6061 Al alloy processed by plasma electrolytic oxidation. Progress in Chemical and Biochemical Research, 6(4), 261-276.
- Asaad Mahdi M, Farhan MA, Mahmoud ZH, Mahdi Rheima A, Sabri Abbas Z, Kadhim MM, et al. Direct sunlight photodegradation of congo red in aqueous solution by TiO2/rGO binary system: Experimental and DFT study. Arabian Journal of Chemistry. 2023; 16(8):104992. [CrossRef]
- Mahmoud ZH, Hamrouni A, Kareem AB, Mostafa MA, Jalil Alhakim Z, Majeed AH. Synthesis and characterization of chitosan sheet modified by varied weight ratio of anatase (TiO2) nano mixture with Cr(VI) adsorbing. Kuwait Journal of Science. 2023; 50(3):290–9. [CrossRef]
- Eddy DR, Nur Sheha GA, Permana MD, Saito N, Takei T, Kumada N, et al. Study on triphase of polymorphs TiO2 (anatase/rutile/brookite) for boosting photocatalytic activity of metformin degradation. Chemosphere. 2024; 351:141206. [CrossRef]
- Simons PY, Dachille F. The structure of TiO 2 II, a high-pressure phase of TiO 2. Acta Cryst. 1967; 23(2):334–6. [CrossRef]
- Latroche M, Brohan L, Marchand R, Tournoux M. New hollandite oxides: TiO2(H) and K0.06TiO2. Journal of Solid State Chemistry. 1989; 81(1):78–82. [CrossRef]
- Cromer DT, Herrington K. The Structures of Anatase and Rutile. J Am Chem Soc. 1955; 77(18):4708–9. [CrossRef]
- Mo S-D, Ching WY. Electronic and optical properties of three phases of titanium dioxide: Rutile, anatase, and brookite. Phys Rev B. 1995; 51(19):13023–32. [CrossRef]
- Kandiel TA, Robben L, Alkaima A, Bahnemann D. Brookite versus anatase TiO2 photocatalysts: phase transformations and photocatalytic activities. Photochem Photobiol Sci. 2013; 12(4):602–9. [CrossRef]
- Sanjines, R., Tang, H., Berger, H., Gozzo, F., Margaritondo, G., & Lévy, F. (1994). Electronic structure of anatase TiO2 oxide. Journal of Applied Physics, 75(6), 2945-2951. [CrossRef]
- Landmann M, Rauls E, Schmidt WG. The electronic structure and optical response of rutile, anatase and brookite TiO 2. J Phys: Condens Matter. 2012; 24(19):195503. [CrossRef]
- Thompson T. L., Yates J. T. Surface Science Studies of the Photoactivation of TiO2 –New Photochemical Processes // Chemical Reviews. 2023. V.106. №10. P. 4428-4453.
- Tang, H., Prasad K., Sanjines R., Schmid P. E., Levy F. Electrical and optical properties of TiO2 anatase thin films // Journal of Applied Physics. 1994. V.75. №.4. P. 2042-2047.
- Yang HG, Sun CH, Qiao SZ, Zou J, Liu G, Smith SC, et al. Anatase TiO2 single crystals with a large percentage of reactive facets. Nature. 2008; 453(7195):638–41. [CrossRef]
- Wang Z, Wen B, Hao Q, Liu L-M, Zhou C, Mao X, et al. Localized Excitation of Ti 3+ Ions in the Photoabsorption and Photocatalytic Activity of Reduced Rutile TiO 2. J Am Chem Soc. 2015; 137(28):9146–52. [CrossRef]
- Yin W-J, Wen B, Zhou C, Selloni A, Liu L-M. Excess electrons in reduced rutile and anatase TiO2. Surface Science Reports. 2018; 73(2):58–82. [CrossRef]
- Guo Q, Zhou C, Ma Z, Yang X. Fundamentals of TiO 2 Photocatalysis: Concepts, Mechanisms, and Challenges. Advanced Materials. 2019; 31(50):1901997. [CrossRef]
- Hardman, P. J., Raikar, G. N., Muryn, C. A., Van Der Laan, G., Wincott, P. L., Thornton, G., ... & Dale, P. A. D. M. A. (1994). Valence-band structure of TiO 2 along the Γ-Δ-X and Γ-Σ-M directions. Physical Review B, 49(11), 7170.
- Lindan PJD, Harrison NM, Gillan MJ, White JA. First-principles spin-polarized calculations on the reduced and reconstructed TiO 2 (110) surface. Phys Rev B. 1997; 55(23):15919–27. [CrossRef]
- Diebold U. The surface science of titanium dioxide. Surface Science Reports. 2003; 48(5–8):53–229. [CrossRef]
- Li G, Chen L, Graham ME, Gray KA. A comparison of mixed phase titania photocatalysts prepared by physical and chemical methods: The importance of the solid–solid interface. Journal of Molecular Catalysis A: Chemical. 2007; 275(1–2):30–5. [CrossRef]
- Morrison S. R. Electrochemistry at Semiconductor and Oxidized Metal Electrodes. New York and London. Plenum Press, 1980. 401 p.
- Zhao Y, Li C., Liu X., Gu F., Jiang H., Shao W., Zhang L., He Y. Synthesis and optical properties of TiO2 nanoparticles // Materials Letters. 2007. V.61. №1. P. 79-83.
- Amanizadehfini F, Salehi-Mobarakeh H, Mahdavian A, Jahanmardi R. Study on preparation, structure, and cooling properties of TiO 2 /acrylonitrile–styrene–acrylate terpolymer solar reflective nanocomposite. Polymer Composites. 2024; 45(5):4107–23. [CrossRef]
- Shon, H., Phuntsho, S., Okour, Y., Cho, D. L., Kim, K. S., Li, H. J., ... & Kim, J. H. (2008). Visible light responsive titanium dioxide (TiO 2). Journal of the Korean Industrial and Engineering Chemistry.
- Mathew RM, Jose J, Zachariah ES, Thomas V. Defect Induced Ultrafast Organic Dye Adsorption by Amorphous Titanium Dioxide/Phosphorus-Doped Carbon Nanodot Hybrid. J Clust Sci. 2024; 35(4):1045–62. [CrossRef]
- Ahad A, Podder J, Saha T, Das HN. Effect of chromium doping on the band gap tuning of titanium dioxide thin films for solar cell applications. Heliyon. 2024; 10(1):e23096. [CrossRef]
- Kuranov D, Grebenkina A, Bogdanova A, Platonov V, Polomoshnov S, Krivetskiy V, et al. Effect of Donor Nb(V) Doping on the Surface Reactivity, Electrical, Optical and Photocatalytic Properties of Nanocrystalline TiO2. Materials. 2024; 17(2):375. [CrossRef]
- Devi LG, Kumar SG. Influence of physicochemical–electronic properties of transition metal ion doped polycrystalline titania on the photocatalytic degradation of Indigo Carmine and 4-nitrophenol under UV/solar light. Applied Surface Science. 2011; 257(7):2779–90. [CrossRef]
- Basavarajappa PS, Patil SB, Ganganagappa N, Reddy KR, Raghu AV, Reddy ChV. Recent progress in metal-doped TiO2, non-metal doped/codoped TiO2 and TiO2 nanostructured hybrids for enhanced photocatalysis. International Journal of Hydrogen Energy. 2020; 45(13):7764–78. [CrossRef]
- Kuriechen SK, Murugesan S, Paul Raj S. Mineralization of Azo Dye Using Combined Photo-Fenton and Photocatalytic Processes under Visible Light. Journal of Catalysts. 2013; 2013:1–6. [CrossRef]
- Chakraborty AK, Ganguli S, Sabur MA. Nitrogen doped titanium dioxide (N-TiO2): Electronic band structure, visible light harvesting and photocatalytic applications. Journal of Water Process Engineering. 2023; 55:104183. [CrossRef]
- Ishikawa T, Sahara R, Ohno K, Ueda K, Narushima T. Electronic structure analysis of light-element-doped anatase TiO 2 using all-electron GW approach. Computational Materials Science. 2023; 220:112059. [CrossRef]
- Gao C, Guo M, Liu Y, Zhang D, Gao F, Sun L, et al. Surface modification methods and mechanisms in carbon nanotubes dispersion. Carbon. 2023; 212:118133. [CrossRef]
- Yang J, Mei S, Ferreira JMF. Hydrothermal synthesis of TiO2 nanopowders from tetraalkylammonium hydroxide peptized sols. Materials Science and Engineering: C. 2001; 15(1–2):183–5. [CrossRef]
- Wang D, Yu B, Zhou F, Wang C, Liu W. Synthesis and characterization of anatase TiO2 nanotubes and their use in dye-sensitized solar cells. Materials Chemistry and Physics. 2009; 113(2–3):602–6. [CrossRef]
- Wahi RK, Liu Y, Falkner JC, Colvin VL. Solvothermal synthesis and characterization of anatase TiO2 nanocrystals with ultrahigh surface area. Journal of Colloid and Interface Science. 2006; 302(2):530–6. [CrossRef]
- Kobayashi M, Kato H, Miyazaki T, Kakihana M. Hydrothermal Synthesis of Pseudocubic Rutile-Type Titania Particles. Ceramics. 2019; 2(1):56–63. [CrossRef]
- Govindaraj R, Santhosh N, Senthil Pandian M, Ramasamy P. Synthesis of nanocrystalline TiO2 nanorods via hydrothermal method: An efficient photoanode material for dye sensitized solar cells. Journal of Crystal Growth. 2017; 468:125–8. [CrossRef]
- Rajamanickam G, Narendhiran S, Muthu SP, Mukhopadhyay S, Perumalsamy R. Hydrothermally derived nanoporous titanium dioxide nanorods/nanoparticles and their influence in dye-sensitized solar cell as a photoanode. Chemical Physics Letters. 2017; 689:19–25. [CrossRef]
- Wang F, Shi Z, Gong F, Jiu J, Adachi M. Morphology Control of Anatase TiO2 by Surfactant-assisted Hydrothermal Method. Chinese Journal of Chemical Engineering. 2007; 15(5):754–9. [CrossRef]
- Lei L, Yuming C, Bo L, Xingfu Z, Weiping D. Study on the surface erosion route to the fabrication of TiO2 hollow spheres. Applied Surface Science. 2010; 256(8):2596–601. [CrossRef]
- Liu Z, Sun DD, Guo P, Leckie JO. One-Step Fabrication and High Photocatalytic Activity of Porous TiO 2 Hollow Aggregates by Using a Low-Temperature Hydrothermal Method Without Templates. Chemistry A European J. 2007; 13(6):1851–5. [CrossRef]
- Xue B, Sun T, Mao F, Sun L-C, Yang W, Xu Z-D, et al. Facile synthesis of mesoporous core-shell TiO2 nanostructures from TiCl3. Materials Research Bulletin. 2011; 46(9):1524–9. [CrossRef]
- Yan X-M, Kang J, Gao L, Xiong L, Mei P. Solvothermal synthesis of carbon coated N-doped TiO2 nanostructures with enhanced visible light catalytic activity. Applied Surface Science. 2013; 265:778–83. [CrossRef]
- Kang M, Lee S-Y, Chung C-H, Cho SM, Han GY, Kim B-W, et al. Characterization of a TiO2 photocatalyst synthesized by the solvothermal method and its catalytic performance for CHCl3 decomposition. Journal of Photochemistry and Photobiology A: Chemistry. 2001; 144(2–3):185–91. [CrossRef]
- Nam WS, Han GY. Characterization and photocatalytic performance of nanosize TiO2 powders prepared by the solvothermal method. Korean J Chem Eng. 2003; 20(6):1149–53. [CrossRef]
- Yang HG, Liu G, Qiao SZ, Sun CH, Jin YG, Smith SC, et al. Solvothermal Synthesis and Photoreactivity of Anatase TiO 2 Nanosheets with Dominant {001} Facets. J Am Chem Soc. 2009; 131(11):4078–83. [CrossRef]
- Yang HG, Liu G, Qiao SZ, Sun CH, Jin YG, Smith SC, et al. Solvothermal Synthesis and Photoreactivity of Anatase TiO 2 Nanosheets with Dominant {001} Facets. J Am Chem Soc. 2009; 131(11):4078–83. [CrossRef]
- Lü X, Ding S, Xie Y, Huang F. Non-Aqueous Preparation of High-Crystallinity Hierarchical TiO 2 Hollow Spheres with Excellent Photocatalytic Efficiency. Eur J Inorg Chem. 2011; 2011(18):2879–83. [CrossRef]
- Li X, Peng Q, Yi J, Wang X, Li Y. Near Monodisperse TiO 2 Nanoparticles and Nanorods. Chemistry A European J. 2006; 12(8):2383–91. [CrossRef]
- Liu Z, Sun DD, Guo P, Leckie JO. One-Step Fabrication and High Photocatalytic Activity of Porous TiO 2 Hollow Aggregates by Using a Low-Temperature Hydrothermal Method Without Templates. Chemistry A European J. 2007; 13(6):1851–5. [CrossRef]
- Liu C, Sun H, Yang S. From Nanorods to Atomically Thin Wires of Anatase TiO 2 : Nonhydrolytic Synthesis and Characterization. Chemistry A European J. 2010; 16(14):4381–93. [CrossRef]
- Dinh C, Nguyen T, Kleitz F, Do T. A solvothermal single-step route towards shape-controlled titanium dioxide nanocrystals. Can J Chem Eng. 2012; 90(1):8–17. [CrossRef]
- Supphasrirongjaroen P, Praserthdam P, Panpranot J, Na-Ranong D, Mekasuwandumrong O. Effect of quenching medium on photocatalytic activity of nano-TiO2 prepared by solvothermal method. Chemical Engineering Journal. 2008; 138(1–3):622–7. [CrossRef]
- Kim C-S, Moon BK, Park J-H, Choi B-C, Seo H-J. Solvothermal synthesis of nanocrystalline TiO2 in toluene with surfactant. Journal of Crystal Growth. 2003; 257(3–4):309–15. [CrossRef]
- Gong D, Grimes CA, Varghese OK, Hu W, Singh RS, Chen Z, et al. Titanium oxide nanotube arrays prepared by anodic oxidation. J Mater Res. 2001; 16(12):3331–4. [CrossRef]
- Zhang P, Yin S, Sato T. Synthesis of high-activity TiO2 photocatalyst via environmentally friendly and novel microwave assisted hydrothermal process. Applied Catalysis B: Environmental. 2009; 89(1–2):118–22. [CrossRef]
- Zhang Q-H, Han W-D, Hong Y-J, Yu J-G. Photocatalytic reduction of CO2 with H2O on Pt-loaded TiO2 catalyst. Catalysis Today. 2009; 148(3–4):335–40. [CrossRef]
- Zhang Y, Zheng H, Liu G, Battaglia V. Synthesis and electrochemical studies of a layered spheric TiO2 through low temperature solvothermal method. Electrochimica Acta. 2009; 54(16):4079–83. [CrossRef]
- Fattakhova-Rohlfing D, Zaleska A, Bein T. Three-Dimensional Titanium Dioxide Nanomaterials. Chem Rev. 2014; 114(19):9487–558. [CrossRef]
- Attar AS, Ghamsari MS, Hajiesmaeilbaigi F, Mirdamadi S, Katagiri K, Baetzold RC. Journal of the American Chemical Society. 1981; 103:6116.
- Oskam G, Nellore A, Penn RL, Searson PC. The Growth Kinetics of TiO 2 Nanoparticles from Titanium(IV) Alkoxide at High Water/Titanium Ratio. J Phys Chem B. 2003; 107(8):1734–8. [CrossRef]
- Mehrotra RC, Singh A. ChemInform Abstract: Recent Trends in Metal Alkoxide Chemistry. ChemInform. 1997; 28(49):chin.199749321. [CrossRef]
- Livage J, Henry M, Sanchez C. Sol-gel chemistry of transition metal oxides. Progress in Solid State Chemistry. 1988; 18(4):259–341. [CrossRef]
- Tseng TK, Lin YS, Chen YJ, Chu H. A Review of Photocatalysts Prepared by Sol-Gel Method for VOCs Removal. IJMS. 2010; 11(6):2336–61. [CrossRef]
- Yu JC, Yu J, Ho W, Zhang L. Preparation of highly photocatalytic active nano-sized TiO2 particles via ultrasonic irradiation. Chem Commun. 2001; (19):1942–3. [CrossRef]
- Chen X. Titanium Dioxide Nanomaterials and Their Energy Applications. Chinese Journal of Catalysis. 2009; 30(8):839–51. [CrossRef]
- Zhu Y-J, Chen F. Microwave-Assisted Preparation of Inorganic Nanostructures in Liquid Phase. Chem Rev. 2014; 114(12):6462–555. [CrossRef]
- Collins MJ. Future Trends in Microwave Synthesis. Future Med Chem. 2010; 2(2):151–5. [CrossRef]
- Jacob J, Chia LHL, Boey FYC. Thermal and non-thermal interaction of microwave radiation with materials. JOURNAL OF MATERIALS SCIENCE. 1995; 30(21):5321–7. [CrossRef]
- De La Hoz A, Díaz-Ortiz Á, Moreno A. Microwaves in organic synthesis. Thermal and non-thermal microwave effects. Chem Soc Rev. 2005; 34(2):164–78. [CrossRef]
- Gressel-Michel E, Chaumont D, Stuerga D. From a microwave flash-synthesized TiO2 colloidal suspension to TiO2 thin films. Journal of Colloid and Interface Science. 2005; 285(2):674–9. [CrossRef]
- Corradi AB, Bondioli F, Focher B, Ferrari AM, Grippo C, Mariani E, et al. Conventional and Microwave-Hydrothermal Synthesis of TiO 2 Nanopowders. Journal of the American Ceramic Society. 2005; 88(9):2639–41. [CrossRef]
- Kim H, Noh K, Choi C, Khamwannah J, Villwock D, Jin S. Extreme Superomniphobicity of Multiwalled 8 nm TiO 2 Nanotubes. Langmuir. 2011; 27(16):10191–6. [CrossRef]
- Baldassari S, Komarneni S, Mariani E, Villa C. Rapid Microwave–Hydrothermal Synthesis of Anatase Form of Titanium Dioxide. Journal of the American Ceramic Society. 2005; 88(11):3238–40. [CrossRef]
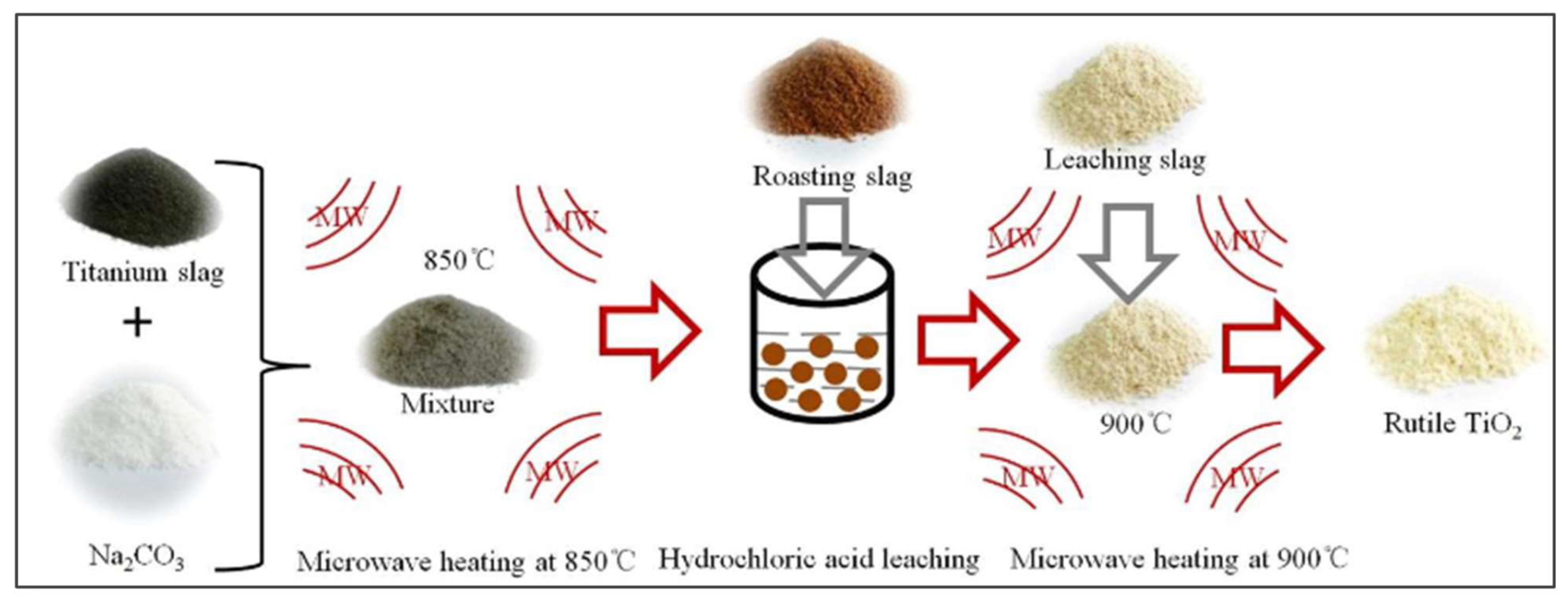
- Kang J, Gao L, Zhang M, Pu J, He L, Ruan R, et al. Synthesis of rutile TiO2 powder by microwave-enhanced roasting followed by hydrochloric acid leaching. Advanced Powder Technology. 2020; 31(3):1140–7. [CrossRef]
- Kim H, Noh K, Choi C, Khamwannah J, Villwock D, Jin S. Extreme Superomniphobicity of Multiwalled 8 nm TiO 2 Nanotubes. Langmuir. 2011; 27(16):10191–6. [CrossRef]
- Malekshahi Byranvand M, Nemati Kharat A, Fatholahi L, Malekshahi Beiranvand Z. A review on synthesis of nano-TiO2 via different methods. Journal of Nanostructures. 2013; 3(1):1-9.
- Chen X, Mao SS. Titanium Dioxide Nanomaterials: Synthesis, Properties, Modifications, and Applications. Chem Rev. 2007; 107(7):2891–959. [CrossRef]
- Seifried S, Winterer M, Hahn H. Nanocrystalline Titania Films and Particles by Chemical Vapor Synthesis. Chem Vap Deposition. 2000; 6(5):239–44. [CrossRef]
- Oh S-M, Ishigaki T. Preparation of pure rutile and anatase TiO2 nanopowders using RF thermal plasma. Thin Solid Films. 2004; 457(1):186–91. [CrossRef]
- Gablenz, S., Voltzke, D., Abicht, H. P., & Neumann-Zdralek, J. (1998). Preparation of fine TiO2 powders via spray hydrolysis of titanium tetraisopropoxide. Journal of materials science letters, 17(7), 537-539. [CrossRef]
- Yu J, Zhao X. Effect of substrates on the photocatalytic activity of nanometer TiO2 thin films. Materials Research Bulletin. 2000; 35(8):1293–301. [CrossRef]
- Zhang Q, Li C. High Temperature Stable Anatase Phase Titanium Dioxide Films Synthesized by Mist Chemical Vapor Deposition. Nanomaterials. 2020; 10(5):911. [CrossRef]
- Sedky NK, Mahdy NK, Abdel-kader NM, Abdelhady MMM, Maged M, Allam AL, et al. Facile sonochemically-assisted bioengineering of titanium dioxide nanoparticles and deciphering their potential in treating breast and lung cancers: biological, molecular, and computational-based investigations. RSC Adv. 2024; 14(12):8583–601. [CrossRef]
- Aswini R, Murugesan S, Kannan K. Bio-engineered TiO 2 nanoparticles using Ledebouria revoluta extract: Larvicidal, histopathological, antibacterial and anticancer activity. International Journal of Environmental Analytical Chemistry. 2021; 101(15):2926–36. [CrossRef]
- Danyliuk NV, Tatarchuk TR, Shyichuk AV. Batch microreactor for photocatalytic reactions monitoring. PCSS. 2020; 21(2):338–46. [CrossRef]
- Mbonyiryivuze, A., Zongo, S., Diallo, A., Bertrand, S., Minani, E., Yadav, L. L., ... & Maaza, M. (2015). Titanium dioxide nanoparticles biosynthesis for dye sensitized solar cells application.
- Saikumari N, Preethi T, Abarna B, Rajarajeswari GR. Ecofriendly, green tea extract directed sol–gel synthesis of nano titania for photocatalytic application. J Mater Sci: Mater Electron. 2019; 30(7):6820–31. [CrossRef]
- M. S, K. B, M. B, S. J, S. A, A. S, et al. Obtaining titanium dioxide nanoparticles with spherical shape and antimicrobial properties using M. citrifolia leaves extract by hydrothermal method. Journal of Photochemistry and Photobiology B: Biology. 2017; 171:117–24. [CrossRef]
- Hariharan D, Jegatha Christy A, Mayandi J, Nehru LC. Visible light active photocatalyst: Hydrothermal green synthesized TiO 2 NPs for degradation of picric acid. Materials Letters. 2018; 222:45–9. [CrossRef]
- Thandapani K, Kathiravan M, Namasivayam E, Padiksan IA, Natesan G, Tiwari M, et al. Enhanced larvicidal, antibacterial, and photocatalytic efficacy of TiO2 nanohybrids green synthesized using the aqueous leaf extract of Parthenium hysterophorus. Environ Sci Pollut Res. 2018; 25(11):10328–39. [CrossRef]
- Gurrappa I, Binder L. Electrodeposition of nanostructured coatings and their characterization—A review. Science and Technology of Advanced Materials. 2008; 9(4):043001. [CrossRef]
- Hanaor D, Michelazzi M, Leonelli C, Sorrell CC. The effects of carboxylic acids on the aqueous dispersion and electrophoretic deposition of ZrO2. Journal of the European Ceramic Society. 2012; 32(1):235–44. [CrossRef]
- Jiang, L. C., & Zhang, W. D. (2019). Electrodeposition of TiO2 nanoparticles on multiwalled carbon nanotube arrays for hydrogen peroxide sensing. Electroanalysis: An International Journal Devoted to Fundamental and Practical Aspects of Electroanalysis, 21(8), 988-993. [CrossRef]
- Wasserscheid P, Keim W. Ionic Liquids—New “Solutions” for Transition Metal Catalysis. Angew Chem Int Ed. 2000; 39(21):3772–89. [CrossRef]
- Wasserscheid P., Welton T. (ed.). Ionic liquids in synthesis. – Weinheim : Wiley-Vch, 2008. – Т. 1. – p. 145.
- Ma Z, Yu J, Dai S. Preparation of Inorganic Materials Using Ionic Liquids. Advanced Materials. 2010; 22(2):261–85. [CrossRef]
- Hong K, Yoo KS. Synthesis of TiO2 hollow spheres using binary ionic liquids as an electrocatalyst. Res Chem Intermed. 2011; 37(9):1325–31. [CrossRef]
- Mali SS, Betty CA, Bhosale PN, Devan RS, Ma Y-R, Kolekar SS, et al. Hydrothermal synthesis of rutile TiO2 nanoflowers using Brønsted Acidic Ionic Liquid [BAIL]: Synthesis, characterization and growth mechanism. CrystEngComm. 2012; 14(6):1920. [CrossRef]
- He Z, Yue G, Gao Y, Dong C, Tan F. Efficient flexible dye-sensitized solar cells from rear illumination based on different morphologies of titanium dioxide photoanode. J Semicond. 2024; 45(2):022801. [CrossRef]
- Kakihana M, Kobayashi M, Tomita K, Petrykin V. Application of Water-Soluble Titanium Complexes as Precursors for Synthesis of Titanium-Containing Oxides via Aqueous Solution Processes. Bulletin of the Chemical Society of Japan. 2010; 83(11):1285–308. [CrossRef]
- Younus LA, Mahmoud ZH, Hamza AA, Alaziz KMA, Ali ML, Yasin Y, et al. Photodynamic therapy in cancer treatment: properties and applications in nanoparticles. Braz J Biol. 2024; 84:e268892. [CrossRef]
- Abdul-Reda Hussein U, Mahmoud ZH, Alaziz KMA, Alid ML, Yasin Y, Ali FK, et al. Antimicrobial finishing of textiles using nanomaterials. Braz J Biol. 2024; 84:e264947. [CrossRef]
- Mohammadkhani A, Mohammadkhani F, Farhadyar N, Sadjadi MS, Kianfar E. Novel nanocomposite zinc phosphate/ polyvinyl alcohol / carboxymethyl cellulose: Synthesis, characterization and investigation of antibacterial and anticorrosive properties. Case Studies in Chemical and Environmental Engineering. 2024; 9:100591. [CrossRef]
- Kianfar E. A review of recent advances in carbon dioxide absorption–stripping by employing a gas–liquid hollow fiber polymeric membrane contactor. Polym Bull. 2023; 80(11):11469–505. [CrossRef]
- Yi Hsu C, Mahmoud ZH, Abdullaev S, Abdulrazzaq Mohammed B, Altimari US, Laftah Shaghnab M, et al. Nanocomposites based on Resole/graphene/carbon fibers: A review study. Case Studies in Chemical and Environmental Engineering. 2023; 8:100535. [CrossRef]
- AL-Salman HNK, Hsu C-Y, Nizar Jawad Z, Mahmoud ZH, Mohammed F, Saud A, et al. Graphene oxide-based biosensors for detection of lung cancer: A review. Results in Chemistry. 2024; 7:101300. [CrossRef]
- Gong D, Grimes CA, Varghese OK, Hu W, Singh RS, Chen Z, et al. Titanium oxide nanotube arrays prepared by anodic oxidation. J Mater Res. 2001; 16(12):3331–4. [CrossRef]
- Paulose M., Shankar K., Yoriya S., Prakasam H. E., Varghese O. K., Mor G. K., Latempa T. A., Fitzgerald A., Grimes C. A. Anodic Growth of Highly Ordered TiO2 Nanotube Arrays to 134 μm in Length // The Journal of Physical Chemistry B. V.110. №33. 2006. P. 16179-16184.
- Ali G, Chen C, Yoo SH, Kum JM, Cho SO. Fabrication of complete titania nanoporous structures via electrochemical anodization of Ti. Nanoscale Res Lett. 2011; 6(1):332. [CrossRef]
- Parmon V. N. Development of physico-chemical foundations of transformation solar energy by decomposing water into molecular photocatalytic systems. Diss... doc. chem. Sci. Novosibirsk, 1984, 680 pp.
- Shijubo N, Honda Y, Fujishima T, Takahashi H, Kodama T, Kuroki Y, et al. Lung surfactant protein-A and carcinoembryonic antigen in pleural effusions due to lung adenocarcinoma and malignant mesothelioma. Eur Respir J. 1995; 8(3):403–6. [CrossRef]
- Jasim SA, Ali MH, Mahmood ZH, Rudiansyah M, Alsultany FH, Mustafa YF, et al. Role of Alloying Composition on Mechanical Properties of CuZr Metallic Glasses During the Nanoindentation Process. Met Mater Int. 2022; 28(9):2075–82. [CrossRef]
- Bokov DO, Mustafa YF, Mahmoud ZH, Suksatan W, Jawad MA, Xu T. Cr-SiNT, Mn-SiNT, Ti-C70 and Sc-CNT as Effective Catalysts for CO2 Reduction to CH3OH. Silicon. 2022; 14(14):8493–503. [CrossRef]
- Krakowiak R, Musial J, Bakun P, Spychała M, Czarczynska-Goslinska B, Mlynarczyk DT, et al. Titanium Dioxide-Based Photocatalysts for Degradation of Emerging Contaminants including Pharmaceutical Pollutants. Applied Sciences. 2021; 11(18):8674. [CrossRef]
- Magaña-López R, Zaragoza-Sánchez PI, Jiménez-Cisneros BE, Chávez-Mejía AC. The Use of TiO2 as a Disinfectant in Water Sanitation Applications. Water. 2021; 13(12):1641. [CrossRef]
- Yang X, Zhao R, Zhan H, Zhao H, Duan Y, Shen Z. Modified Titanium dioxide-based photocatalysts for water treatment: Mini review. Environmental Functional Materials. 2024; S2773058124000322. [CrossRef]
- Khan, Mohammad Mansoob. “Principles and mechanisms of photocatalysis.” Photocatalytic systems by design. Elsevier, 2021. 1-22.
- Yadav HM, Kim J-S, Pawar SH. Developments in photocatalytic antibacterial activity of nano TiO2: A review. Korean J Chem Eng. 2016; 33(7):1989–98. [CrossRef]
- Hassan, Sona Ayadi, and Parinaz Ghadam. “Photocatalytic Properties of Metal-Based Nanoparticles.” Handbook of Green and Sustainable Nanotechnology: Fundamentals, Developments and Applications. Cham: Springer International Publishing, 2022. 1-25.
- Sedita, Silvia Rita, Yasunori Baba, and Naohiro Shichijo. “Pasteur scientists meet the market: An empirical illustration of the innovative performance of university–industry relationships.” Innovation, Alliances, and Networks in High-Tech Environments. Routledge, 2015. 263-281.
- Linsebigler AL, Lu G, Yates JT. Photocatalysis on TiO2 Surfaces: Principles, Mechanisms, and Selected Results. Chem Rev. 1995 ; 95(3):735–58. [CrossRef]
- Munuera G., Gonzalez-Elipe A.R., Rives-Arnau V., Navio A., Malet P., Sokia J., Conesa J.C., Sanz J. Photo-adsorption of oxygen on acid and basic TiO2 surfaces // Adsorption and Catalysis on Oxide Surfaces. 1985. V.21. P. 113-126.
- Mills A, Le Hunte S. An overview of semiconductor photocatalysis. Journal of Photochemistry and Photobiology A: Chemistry. 1997; 108(1):1–35. [CrossRef]
- Watson SS, Beydoun D, Scott JA, Amal R. The effect of preparation method on the photoactivity of crystalline titanium dioxide particles. Chemical Engineering Journal. 2003; 95(1–3):213–20. [CrossRef]
- Ohno T, Sarukawa K, Tokieda K, Matsumura M. Morphology of a TiO2 Photocatalyst (Degussa, P-25) Consisting of Anatase and Rutile Crystalline Phases. Journal of Catalysis. 2001; 203(1):82–6. [CrossRef]
- Gerischer H, Heller A. Photocatalytic Oxidation of Organic Molecules at TiO2 Particles by Sunlight in Aerated Water. J Electrochem Soc. 1992; 139(1):113–8. [CrossRef]
- Gao L, Zhang Q. Effects of amorphous contents and particle size on the photocatalytic properties of TiO 2 nanoparticles. Scripta Materialia. 2001; 44(8–9):1195–8. [CrossRef]
- Mohd Raub AA, Bahru R, Mohamed MA, Latif R, Mohammad Haniff MAS, Simarani K, et al. Photocatalytic activity enhancement of nanostructured metal-oxides photocatalyst: a review. Nanotechnology. 2024; 35(24):242004. [CrossRef]
- https://pubmed.ncbi.nlm.nih.gov/?term=photocatalysis.
- Tanos F, Razzouk A, Lesage G, Cretin M, Bechelany M. A Comprehensive Review on Modification of Titanium Dioxide-Based Catalysts in Advanced Oxidation Processes for Water Treatment. ChemSusChem. 2024; 17(6):e202301139. [CrossRef]
- Hamad TG, Salman TA. Green synthesis of titanium oxide nanoparticles using pomegranate leaf extract: Characterization and antibacterial activity evaluation. In Baghdad, Iraq; 2024. p. 030008. [CrossRef]
- Kubota Y, Shuin T, Kawasaki C, Hosaka M, Kitamura H, Cai R, et al. Photokilling of T-24 human bladder cancer cells with titanium dioxide. Br J Cancer. 1994; 70(6):1107–11. [CrossRef]
- Sun S, Vikrant K, Kim K-H, Boukhvalov DW. Titanium dioxide–supported mercury photocatalysts for oxidative removal of hydrogen sulfide from the air using a portable air purification unit. Journal of Hazardous Materials. 2024; 470:134089. [CrossRef]
- Shang H, Jia H, Zhang W, Li S, Wang Q, Yang Q, et al. Surface Hydrogen Bond-Induced Oxygen Vacancies of TiO 2 for Two-Electron Molecular Oxygen Activation and Efficient NO Oxidation. Environ Sci Technol. 2023; 57(48):20400–9. [CrossRef]
- Serpone N. Heterogeneous Photocatalysis and Prospects of TiO2-Based Photocatalytic DeNOxing the Atmospheric Environment. Catalysts. 2018; 8(11):553. [CrossRef]
- Safni S-, Patricia EO, Ollinovela T, Yusuf Y. Degradation of Phenol Using Sonolysis and Photolysis by TiO2/RHAC Catalyst and Analysis with Spectrophotometer UV-VIS and HPLC. AK. 2023; 10(1):50–6. [CrossRef]
- Yamaguti K, Sato S. Photolysis of water over metallized powdered titanium dioxide. J Chem Soc, Faraday Trans 1. 1985; 81(5):1237. [CrossRef]
- Stevens CG, Wessman NJ, Bowman JE, Ramsey WJ. Photolysis of water vapor on titanium/TiO2 surfaces. Chemical Physics Letters. 1982; 91(5):335–8. [CrossRef]
- Fujishima A, Honda K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature. 1972; 238(5358):37–8. [CrossRef]
- Nosaka Y. Water Photo-Oxidation over TiO2—History and Reaction Mechanism. Catalysts. 2022; 12(12):1557. [CrossRef]
- Eidsvåg H, Bentouba S, Vajeeston P, Yohi S, Velauthapillai D. TiO2 as a Photocatalyst for Water Splitting—An Experimental and Theoretical Review. Molecules. 2021; 26(6):1687. [CrossRef]
- Ni M, Leung MKH, Leung DYC, Sumathy K. A review and recent developments in photocatalytic water-splitting using TiO2 for hydrogen production. Renewable and Sustainable Energy Reviews. 2007; 11(3):401–25. [CrossRef]
- Water Treatment by Heterogeneous Photocatalysis: An Review / Saline Water Desalination Research Institute; Al-Rasheed R.A. Jeddah, Saudi Arabia, 2005. 14 р.
- Jafari, S., Mahyad, B., Hashemzadeh, H., Janfaza, S., Gholikhani, T., & Tayebi, L. (2020). Biomedical applications of TiO2 nanostructures: recent advances. International journal of nanomedicine, 3447-3470. [CrossRef]
- Lee S-Y, Park S-J. TiO2 photocatalyst for water treatment applications. Journal of Industrial and Engineering Chemistry. 2013; 19(6):1761–9. [CrossRef]
- Dharma HNC, Jaafar J, Widiastuti N, Matsuyama H, Rajabsadeh S, Othman MHD, et al. A Review of Titanium Dioxide (TiO2)-Based Photocatalyst for Oilfield-Produced Water Treatment. Membranes. 2022 [cited 2024 Aug 14]; 12(3):345. [CrossRef]
- Raya, I., Mansoor Al Sarraf, A. A., Widjaja, G., Ghazi Al-Shawi, S., F Ramadan, M., Mahmood, Z. H., ... & Ghaleb Maabreh, H. (2022). ZnMoO4 nanoparticles: novel and facile synthesis, characterization, and photocatalytic performance. Journal of Nanostructures, 12(2), 446-454.
- Mahmood ZH, Jarosova M, Kzar HH, Machek P, Zaidi M, Dehno Khalaji A, et al. Synthesis and characterization of Co 3 O 4 nanoparticles: Application as performing anode in Li-ion batteries. J Chinese Chemical Soc. 2022; 69(4):657–62. [CrossRef]
- Bahadoran, A., Jabarabadi, M. K., Mahmood, Z. H., Bokov, D., Janani, B. J., & Fakhri, A. (2022). Quick and sensitive colorimetric detection of amino acid with functionalized-silver/copper nanoparticles in the presence of cross linker, and bacteria detection by using DNA-template nanoparticles as peroxidase activity. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 268, 120636. [CrossRef]
- Mahmoud ZH, AL-Bayati RA, Khadom AA. Electron transport in dye-sanitized solar cell with tin-doped titanium dioxide as photoanode materials. J Mater Sci: Mater Electron. 2022 [cited 2024 Aug 14]; 33(8):5009–23. [CrossRef]
- Raya, I., Widjaja, G., Mahmood, Z. H., Kadhim, A. J., Vladimirovich, K. O., Mustafa, Y. F., ... & Kafi-Ahmadi, L. (2022). Kinetic, isotherm, and thermodynamic studies on Cr (VI) adsorption using cellulose acetate/graphene oxide composite nanofibers. Applied Physics A, 128(2), 167. [CrossRef]
- Çeşmeli S, Biray Avci C. Application of titanium dioxide (TiO 2 ) nanoparticles in cancer therapies. Journal of Drug Targeting . 2019; 27(7):762–6. [CrossRef]
- Li Q, Wang X, Lu X, et al. The incorporation of daunorubicin in cancer cells through the use of titanium dioxide whiskers. Biomaterials. 2009; 30(27):4708–4715. [CrossRef]
- Rivankar S. An overview of doxorubicin formulations in cancer therapy. J Can Res Ther. 2014; 10(4):853. [CrossRef]
- Wang, Q., Huang, J. Y., Li, H. Q., Chen, Z., Zhao, A. Z. J., Wang, Y., ... & Lai, Y. K. (2016). TiO2 nanotube platforms for smart drug delivery: a review. International journal of nanomedicine, 4819-4834. [CrossRef]
- Raja G, Cao S, Kim D-H, Kim T-J. Mechanoregulation of titanium dioxide nanoparticles in cancer therapy. Materials Science and Engineering: C. 2020; 107:110303. [CrossRef]
- Akram MW, Raziq F, Fakhar-e-Alam M, Aziz MH, Alimgeer KS, Atif M, et al. Tailoring of Au-TiO2 nanoparticles conjugated with doxorubicin for their synergistic response and photodynamic therapy applications. Journal of Photochemistry and Photobiology A: Chemistry. 2019; 384:112040. [CrossRef]
- Inoue T, Fujishima A, Konishi S, Honda K. Photoelectrocatalytic reduction of carbon dioxide in aqueous suspensions of semiconductor powders. Nature. 1979; 277(5698):637–8. [CrossRef]
- Kurzmann C, Verheyen J, Coto M, Kumar RV, Divitini G, Shokoohi-Tabrizi HA, et al. In vitro evaluation of experimental light activated gels for tooth bleaching. Photochem Photobiol Sci. 2019; 18(5):1009–19. [CrossRef]
- Woolerton TW, Sheard S, Reisner E, Pierce E, Ragsdale SW, Armstrong FA. Efficient and Clean Photoreduction of CO 2 to CO by Enzyme-Modified TiO 2 Nanoparticles Using Visible Light. J Am Chem Soc. 2010; 132(7):2132–3. [CrossRef]
- Roy SC, Varghese OK, Paulose M, Grimes CA. Toward Solar Fuels: Photocatalytic Conversion of Carbon Dioxide to Hydrocarbons. ACS Nano. 2010; 4(3):1259–78. [CrossRef]
- Thampi KR, Kiwi J, Grätzel M. Methanation and photo-methanation of carbon dioxide at room temperature and atmospheric pressure. Nature. 1987; 327(6122):506–8. [CrossRef]
- M. Dell’Angela, T. Anniyev, M. Beye, R. Coffee, A. Föhlisch, J. Gladh, T. Katayama, S. Kaya, O. Krupin, J. LaRue, A. Møgelhøj, D. Nordlund, J. K. Nørskov, H. Öberg, H. Ogasawara, H. Öström, L. G. M. Pettersson, W. F. Schlotter, J. A. Sellberg, F. Sorgenfrei, J. J. Turner, M. Wolf, W. Wurth, A. Nilsson, Science 2013, 339, 1302.
- Farouk HU, Raman AAA, Daud WMAW. TiO2 catalyst deactivation in textile wastewater treatment: Current challenges and future advances. Journal of Industrial and Engineering Chemistry. 2016; 33:11–21. [CrossRef]
- Zhang H, Banfield JF. Structural Characteristics and Mechanical and Thermodynamic Properties of Nanocrystalline TiO 2. Chem Rev. 2014; 114(19):9613–44. [CrossRef]
- Penn RL, Banfield JF. Morphology development and crystal growth in nanocrystalline aggregates under hydrothermal conditions: insights from titania. Geochimica et Cosmochimica Acta. 1999; 63(10):1549–57. [CrossRef]
- Rahimi N, Pax RA, Gray EMacA. Review of functional titanium oxides. I: TiO2 and its modifications. Progress in Solid State Chemistry. 2016; 44(3):86–105. [CrossRef]
- Farouk HU, Raman AAA, Daud WMAW. TiO2 catalyst deactivation in textile wastewater treatment: Current challenges and future advances. Journal of Industrial and Engineering Chemistry. 2016; 33:11–21. [CrossRef]
- Kääriäinen M-L, Kääriäinen TO, Cameron DC. Titanium dioxide thin films, their structure and its effect on their photoactivity and photocatalytic properties. Thin Solid Films. 2009; 517(24):6666–70. [CrossRef]
- Ta N, Liu (Jimmy) J, Shen W. Tuning the shape of ceria nanomaterials for catalytic applications. Chinese Journal of Catalysis. 2013; 34(5):838–50. [CrossRef]
- Lee K, Yoon H, Ahn C, Park J, Jeon S. Strategies to improve the photocatalytic activity of TiO 2 : 3D nanostructuring and heterostructuring with graphitic carbon nanomaterials. Nanoscale. 2019; 11(15):7025–40. [CrossRef]
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 169–189. [CrossRef]
- Yang S, Xiong F, Chen K, Chang Y, Bai X, Yin W, et al. Impact of Titanium Dioxide and Fullerenol Nanoparticles on Caco-2 Gut Epithelial Cells. j nanosci nanotechnol. 2018; 18(4):2387–93. [CrossRef]
- Ayorinde T, Sayes CM. An updated review of industrially relevant titanium dioxide and its environmental health effects. Journal of Hazardous Materials Letters. 2023; 4:100085. [CrossRef]
- Bauer M, Lei C, Read K, Tobey R, Gland J, Murnane MM, et al. Direct Observation of Surface Chemistry Using Ultrafast Soft-X-Ray Pulses. Phys Rev Lett. 2001; 87(2):025501. [CrossRef]
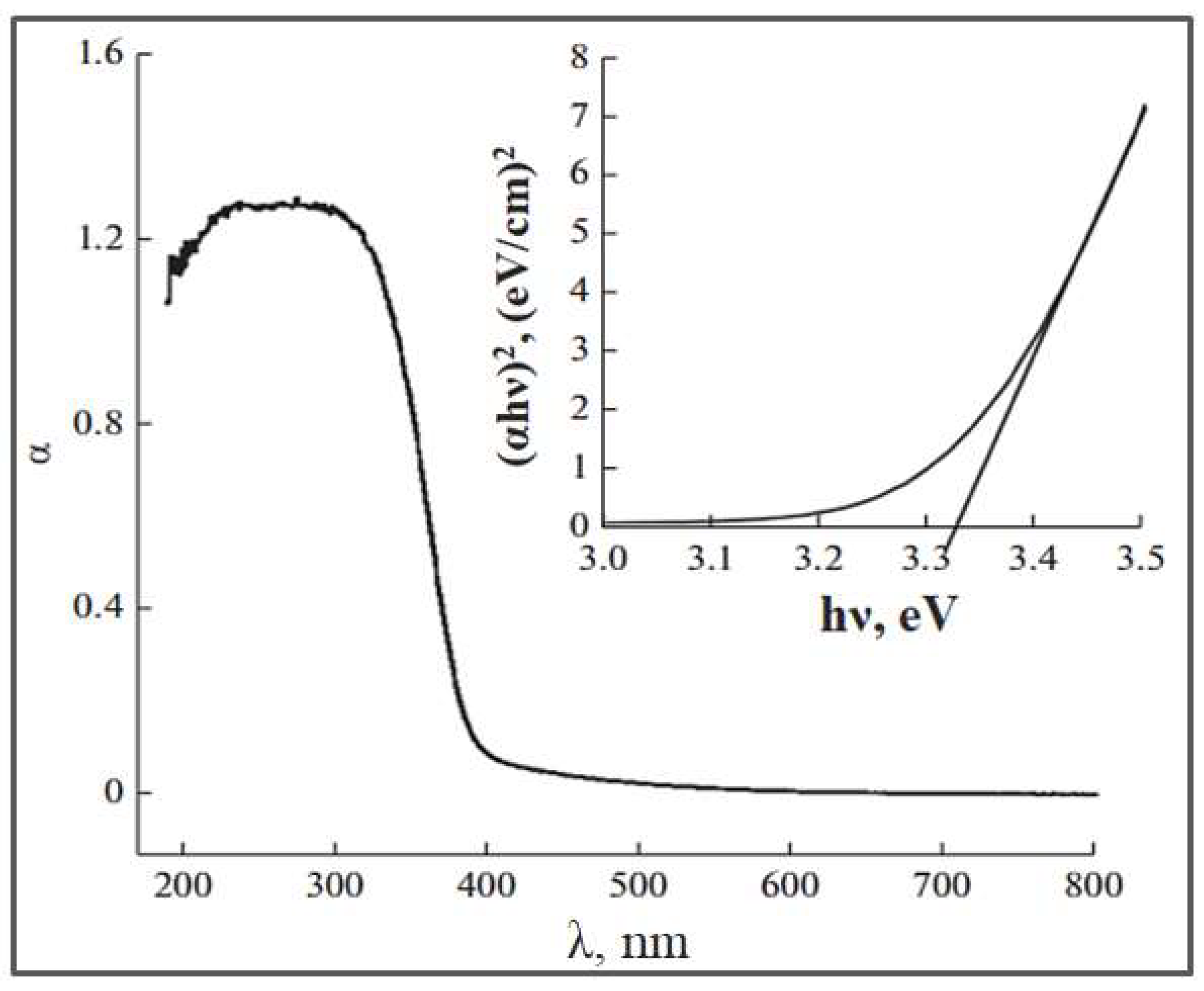
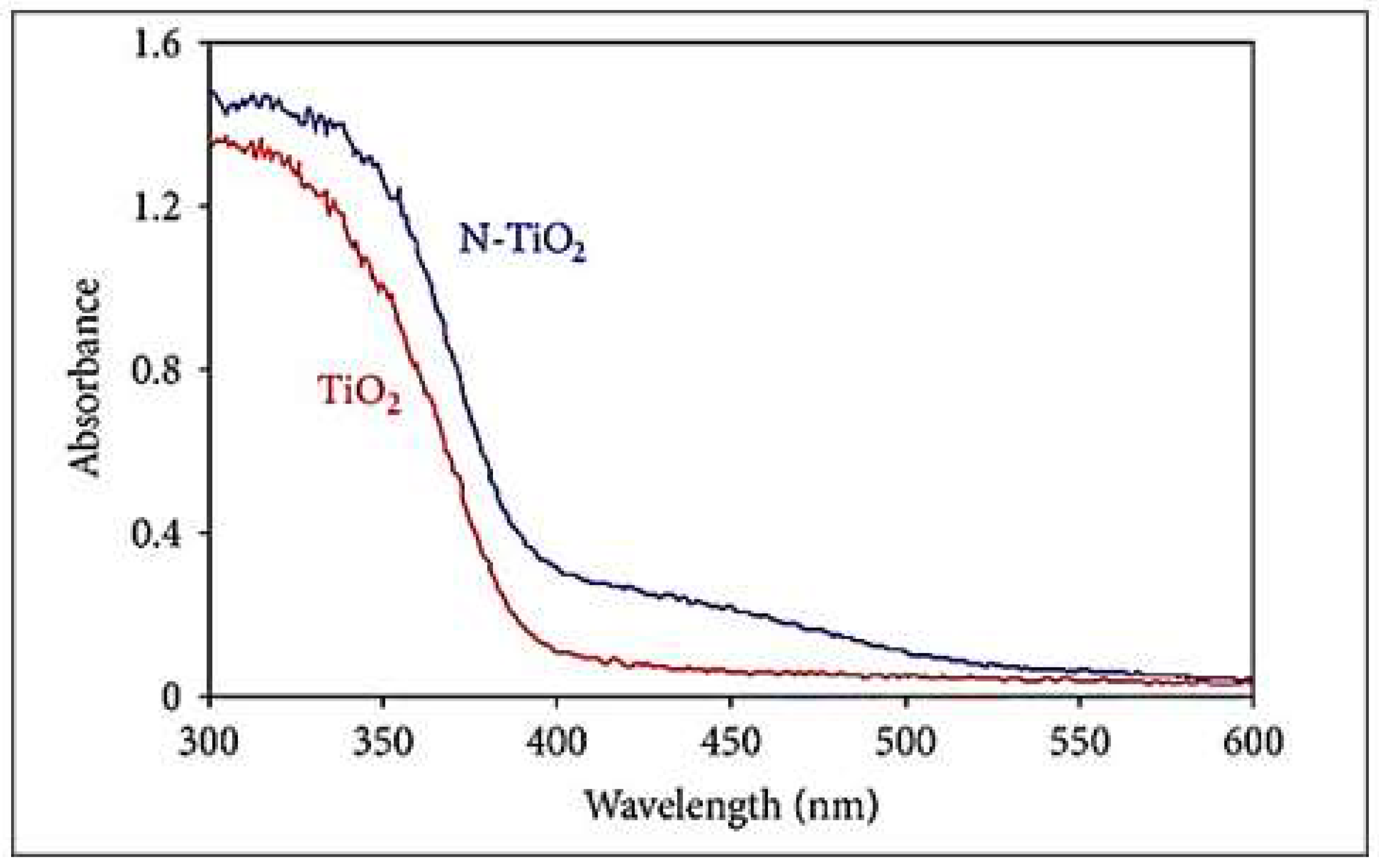
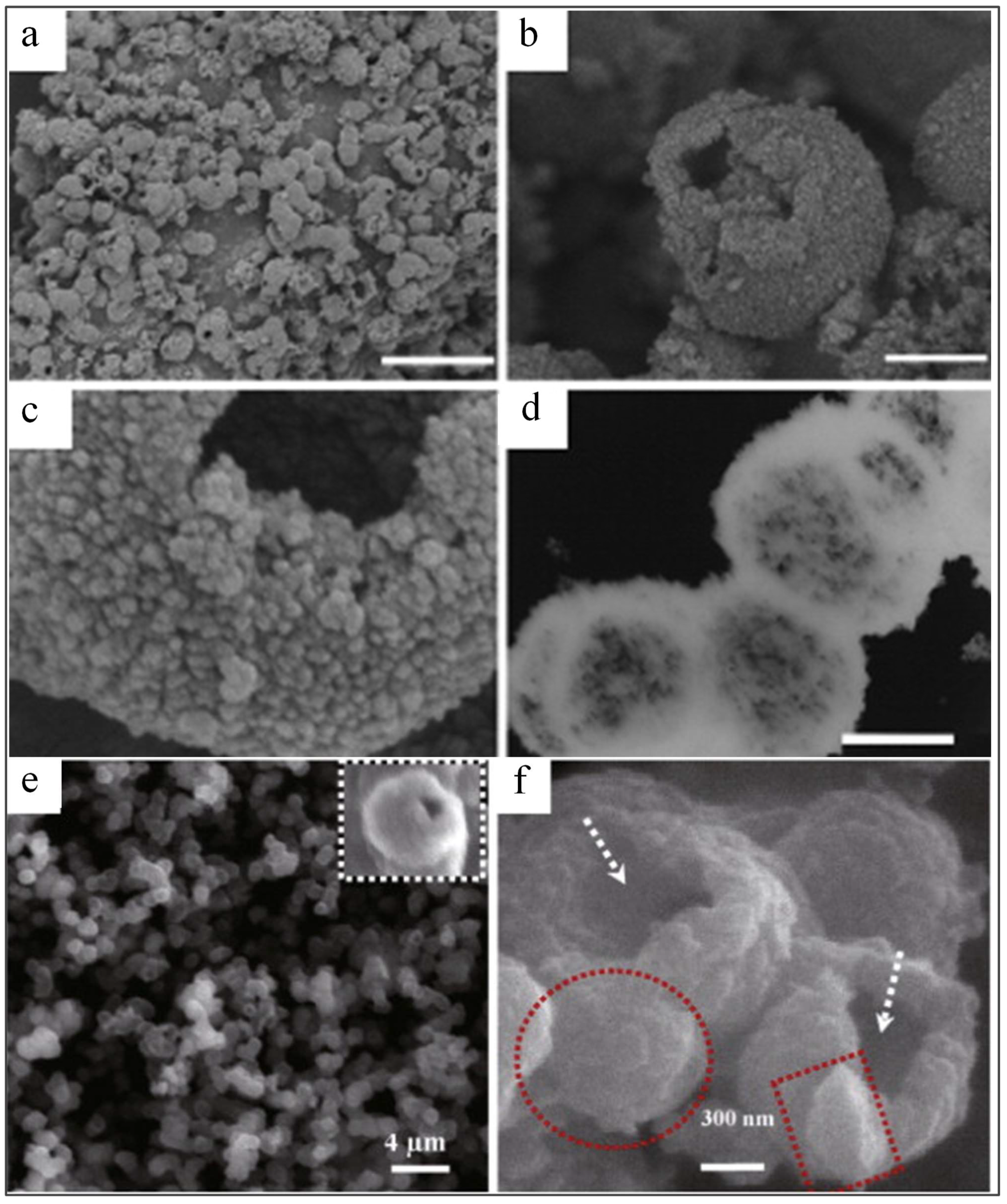
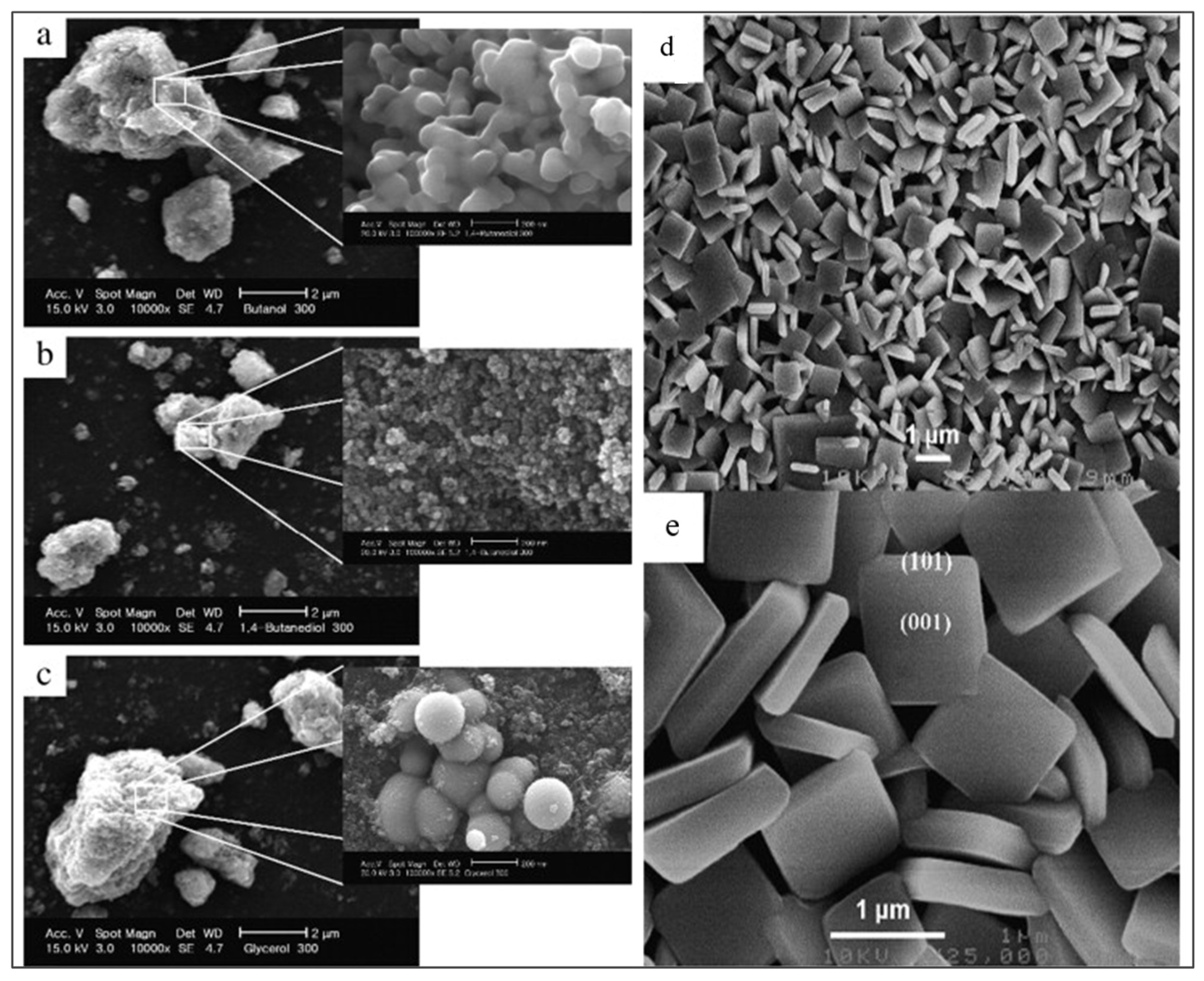
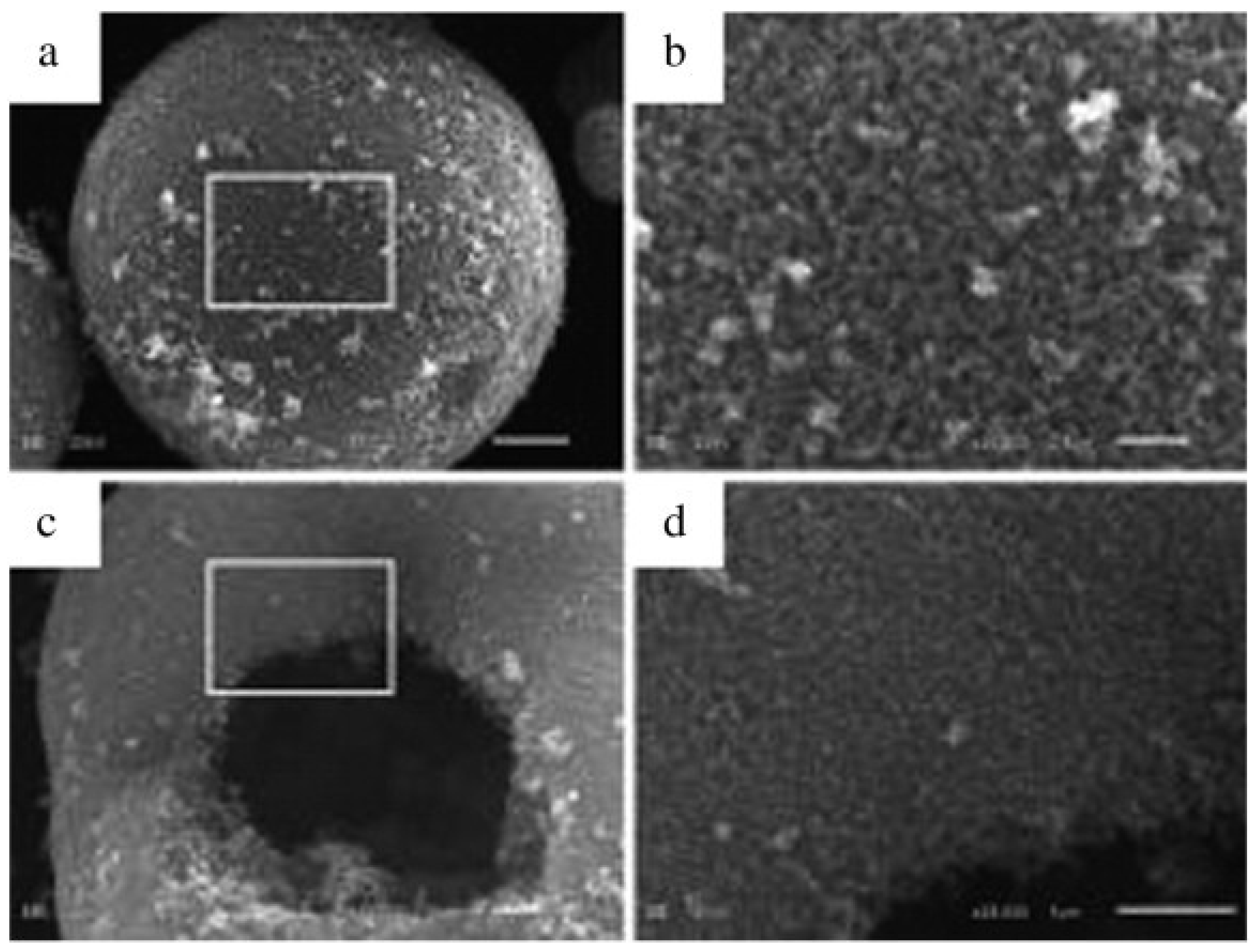
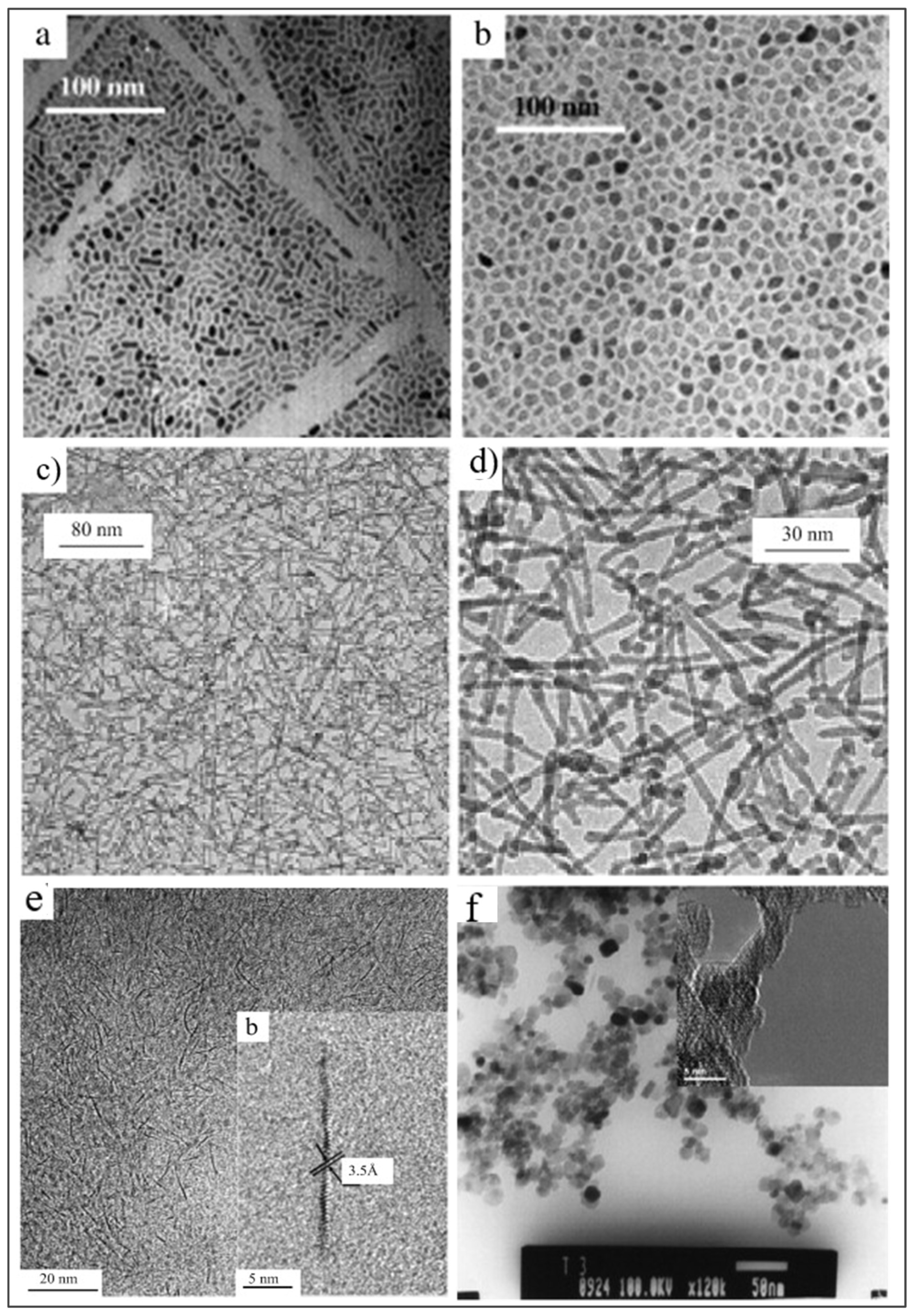
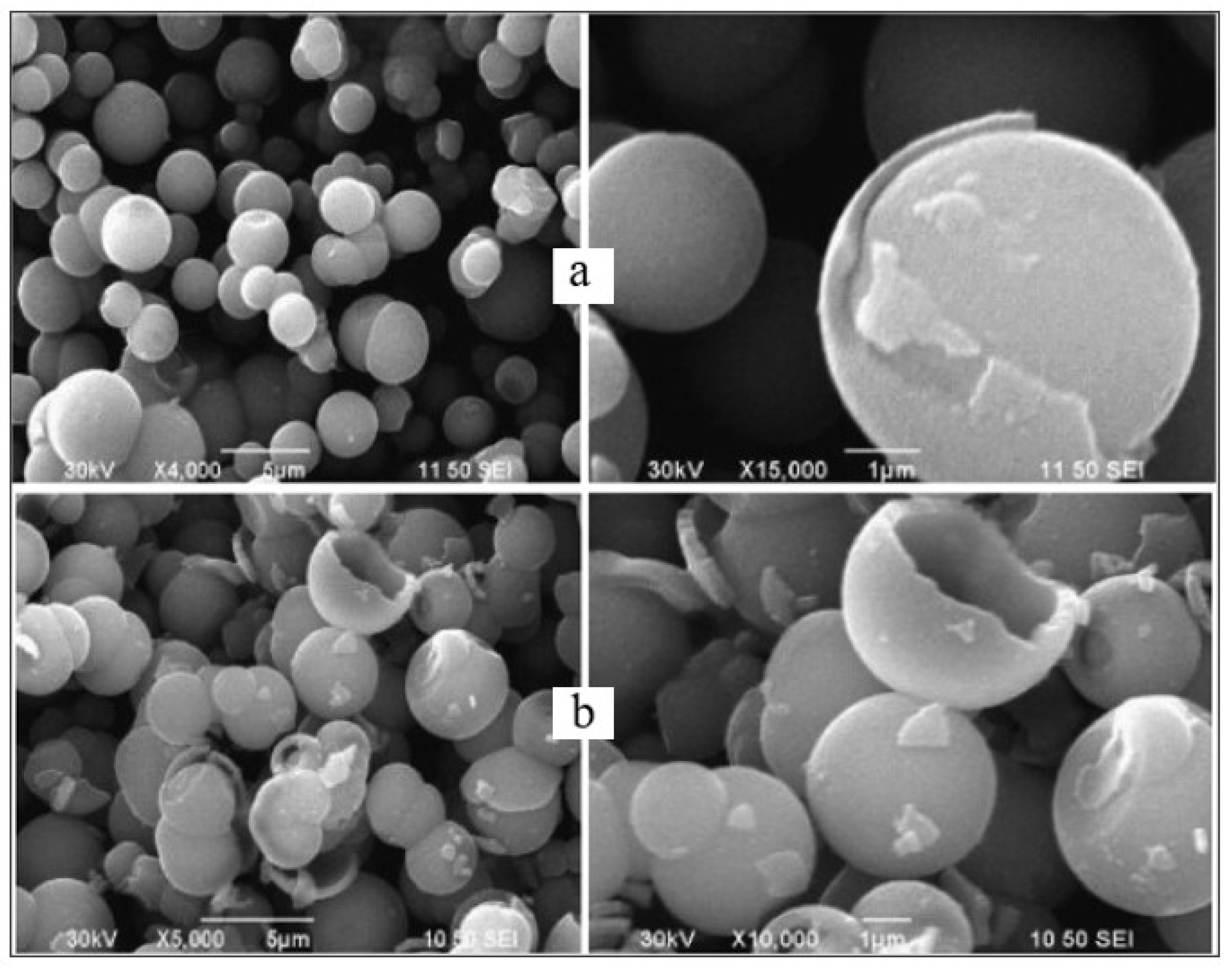
| Parameter | Anatase | Rutile | Brookite |
|---|---|---|---|
| Crystal structure | Tetragonal | Tetragonal | Rhombic |
| Lattice parameters, nm | а= 0.3784 с= 0.9515 |
а= 0.45936 с= 0.29587 |
а= 0.9184 b = 0.5447 c = 0.5154 |
| Density, g/cm3 | 3.79 | 4.13 | 3.99 |
| Space group | L4/amd | P4/mnm | Pbca |
| Number of units in cell | 2 | 2 | 4 |
| O-Ti-O bond angle | 77.7º, 92.6º | 81.2º, 90.0º | 77.0º -105º |
| Ti-O bond length, nm | 0.1937(4) 0.1965(2) | 0.1949(4) 0.1980(2) |
0.187-0.204 |
| Processing time (hours) |
Crystal size, nm | ||
| Anatase | Brookite | Rutile | |
| 2 | 4.9 | 7.7 | 29.1 |
| 3 | 5.7 | 9.7 | 34.8 |
| 12 | 8.6 | 14.0 | 51.7 |
| 24 | 10.0 | 15.6 | 57.4 |
| 72 | 12.9 | 19.8 | 63.7 |
| 168 | 16.6 | 23.5 | 67.2 |
| Oxidizing agent | Oxidizing potential, V |
|---|---|
| Hydroxyl radical, HO• | +2.80 |
| Electron hole in valence band, h+ | +2.70 |
| Ozone, O3 | +2.07 |
| Hydrogen peroxide, H2O2 | +1.78 |
| Permanganate ion, (MnO4)2- | +1.70 |
| Chlorine dioxide, ClO2 | + 1.15 |
| Chlorine, Cl2 | +1.40 |
| Oxygen, O2 | +1.20 |
| Superoxide radical, O2- | -0.33 |
| Electron in the conduction band, e– | -0.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





