1. Introduction
Vitamin D (Vit-D) is considered a critical and important micronutrient for overall health [
1,
2], with much attention given to its importance in bone and skeletal muscle health [
3,
4].
The bioactive form of Vit-D acts through specific nuclear receptors (VDR) [
5,
6,
7], which have been identified in most tissues of the human body [
8,
9,
10]. In skeletal muscle, Vit-D exerts both genomic and non-genomic effects [
11,
12]. In human myoblasts, Vit-D has been shown to stimulate protein synthesis [
13]. In older women, Vit-D supplementation increased intramyonuclear VDR concentration and muscle fiber size [
14].
However, recent literature reviews on the effects of Vit-D supplementation on muscle strength, an important indicator of muscle health [
15], have led to conflicting conclusions. Meta-analysis by Tomlinson et al. [
16] showed that Vit-D supplementation increases both upper and lower limb muscle strength, while Han et al. [
17], also based on meta-analysis, concluded that Vit-D supplementation does not improve muscle strength. Meta-analysis by Zhang et al. [
18] revealed no impact of Vit-D supplementation on overall muscle strength outcomes. However, further analysis showed positive effect on lower limb muscle strength, but not upper limb muscle strength or muscle power. The authors [
18] concluded, that different muscle groups and functions may respond differently to Vit-D supplementation. Chiang et al. [
19], conducting a systematic literature review, found that Vit-D2 supplementation was ineffective at impacting muscle strength, whereas Vit-D3 ingestion had positive influence. Meta-analysis by Abshirini et al. [
20] focused on the effect of Vit-D supplementation on hand grip strength. Their pooled findings showed that Vit-D did not influence hand grip strength. However, the subgroup analyses revealed that Vit-D supplementation improved hand grip strength with respect to dosages >1000 IU/day, a treatment duration of 3 months, and subjects with baseline serum 25(OH)D levels <30 ng/mL (75 nmol/L) [
20]. Another meta-analysis [
21] showed positive effect of Vit-D supplementation on lower extremity muscle strength. Of note, majority of the literature reviews cited above [
16,
17,
18,
19] included studies of relatively young athletic populations. Only Abshirini et al. [
20] and Muir & Montero-Odasso [
21] focused on data available for middle-aged and older subjects.
Undoubtedly, the most effective strategy for increasing muscle strength and mass is resistance training (RT) [
22,
23,
24], but it is less clear whether combining Vit-D supplementation with RT improves its outcomes. Recent meta-analysis revealed that Vit-D supplementation had an additive effect to RT in increasing muscle strength of the lower limbs in older adults [
25], but this conclusion was based on the results of only three studies. A more recent study [
26] in elderly persons with or without chronic obstructive pulmonary disease showed the effectiveness of a 13-week RT program in increasing muscle mass and strength but found no additional effect of concomitant vitamin D supplementation on RT-associated changes in muscle mass or function.
Maximal oxygen uptake (VO
2max) is often referred to as cardiorespiratory fitness and is considered an objective measure of health [
27,
28]. The functional state of the lungs, heart, blood vessels and skeletal muscles together with the oxygen transport capacity of the blood are the major determinants of an individual’s VO
2max [
29]. VDR is expressed in all these organs, i.e. lungs, heart, blood vessels, skeletal muscle [
11,
30,
31,
32]. Vit-D can promote erythropoiesis and hemoglobin synthesis [
33], also affect the binding affinity of oxygen to hemoglobin [
34]. Furthermore, Vit-D may be crucial for mitochondrial oxidative phosphorylation capacity [
35]. Therefore, it is not surprising that an independent robust association have been observed between serum Vit-D levels and VO
2max in adults over an age range of 20–73 years [
36,
37].
However, it is not clear whether Vit-D supplementation is effective in increasing VO
2max. A recent literature review [
38] concluded that Vit-D supplementation can significantly improve VO
2max in elite athletes, but this claim was based on the results of only two studies. Research in athletes of varying levels and in recreationally active youth have yielded inconclusive results showing no effect [
39,
40] or a marginal positive influence [
41] of Vit-D supplementation on VO
2max. In some of the studies in young men [
42,
43] that reported a positive effect of vitamin D supplementation on cardiorespiratory fitness, VO
2max was not actually measured but indirectly calculated. In elderly patients with chronic obstructive pulmonary disease participating in a rehabilitation program, Hornikx et al. [
44] observed significantly greater improvements in VO
2max and inspiratory muscle strength as a result of Vit-D supplementation compared to placebo.
Muscle mass and strength [
45] as well as VO
2max [
46] decline with increased age and may lead to decreased quality of life, loss of independence and disability in elderly. Thus, measures that can forestall declines in muscle strength and VO
2max may be important considerations for middle-aged adults that may help maintain better health status and reduce the risk of substantial decline in quality of life in older age. The literature cited above suggests that RT combined with Vit-D supplementation may be an effective intervention in this context, but the available data are limited and inconclusive, particularly for the middle-age population. Therefore, the objective of this study was to assess whether systematic RT combined with Vit-D supplementation is more effective in terms of increasing muscle strength and lean body mass (LBM) than the same RT program without Vit-D supplementation in Vit-D insufficient middle-aged healthy men. The secondary objective was to elucidate whether Vit-D supplementation during participation in RT program affects cardiorespiratory fitness in the same men.
3. Results
Energy and nutrient intake of the participants is shown in
Table 3. As compliance of many participants with requirements of reporting daily energy and nutrients intake was low, only reliable data, that were limited to six men from both groups, are presented. No main effects of group or time or group by time interaction (in all cases
p > 0.05) occurred for any of the dietary parameters measured.
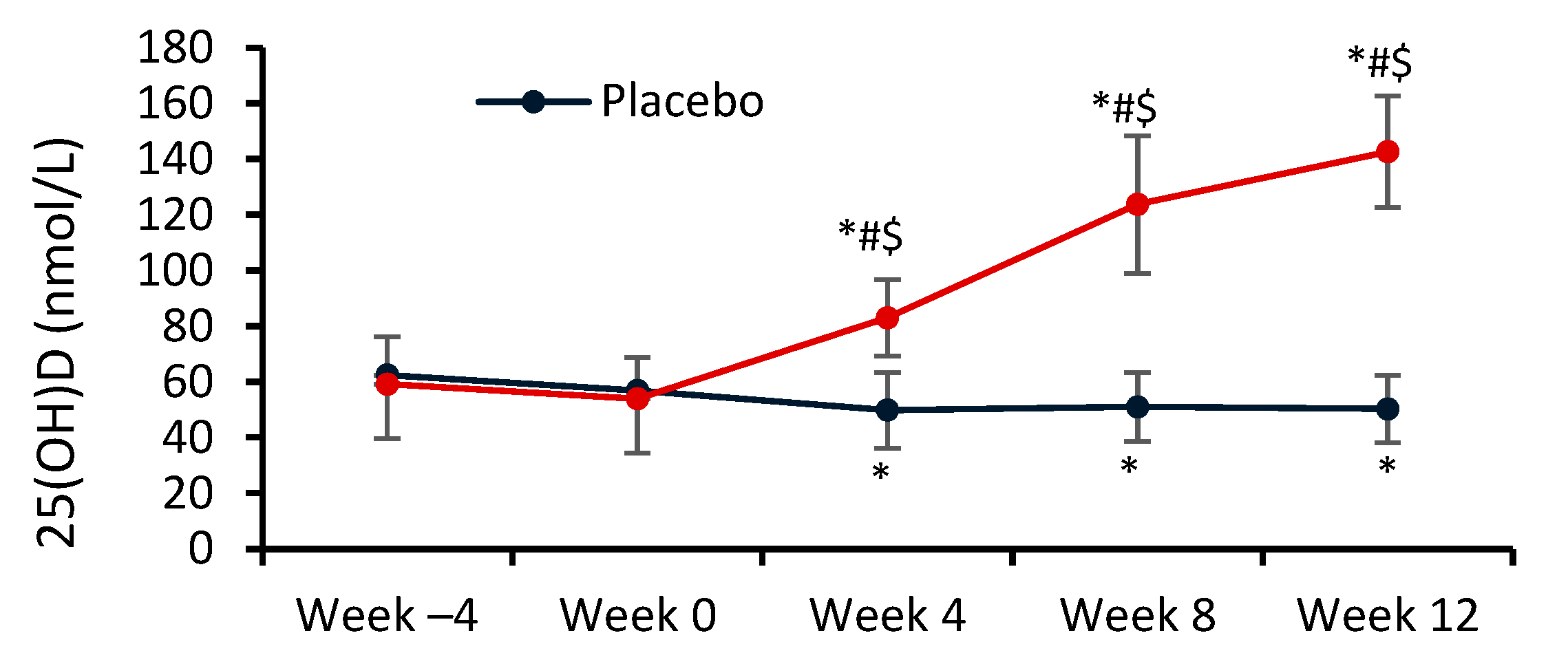
For serum 25(OH)D levels, significant main effects of group (F = 47.91) and time (F = 113.29), and a significant group by time interaction (F = 170.25) were observed (
p < 0.0001 in all cases) (
Figure 2). At weeks ‒4 and 0, serum 25(OH)D concentrations were similar in the two groups. At week 4, i.e. 4 weeks after starting Vit-D and placebo administration, serum 25(OH)D levels were significantly higher in the VD group than in the PLC group (83.0 ± 13.7 nmol/L vs. 49.9 ± 13.6 nmol/L, respectively;
p = 0.0002). In the VD group, serum 25(OH)D continued to rise throughout the rest of the main phase of the study, remaining consistently significantly higher than in the PLC group, reaching a level of 142.7 ± 20.1 nmol/L at week 12. In the PLC group, there was a significant (
p = 0.010) decrease in serum 25(OH)D from week ‒4 to week 4, after which 25(OH)D remained consistently low from 51.0 ± 12.3 to 50.3 ± 12.2 nmol/L until week 12.
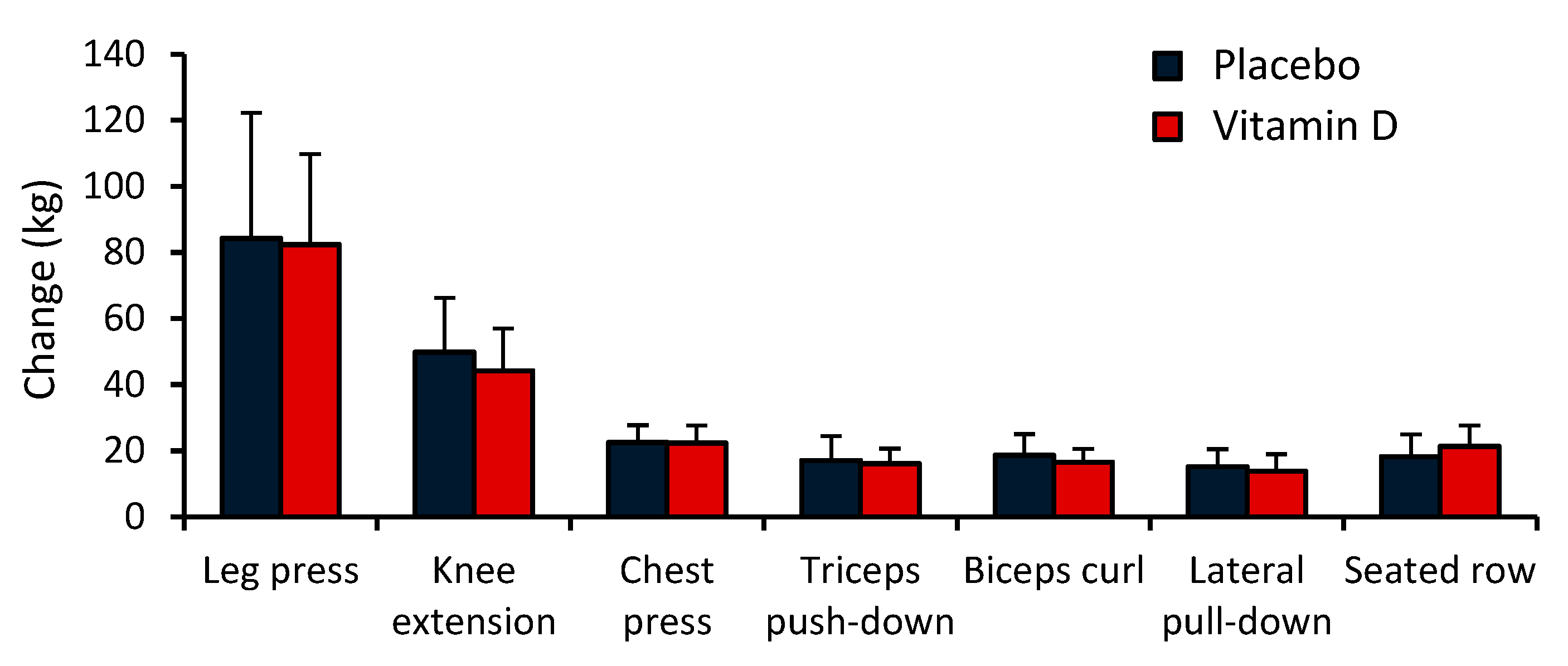
Compliance to the RT protocol was high (91.5% on average) and similar in the two groups (91% in PLC and 92% in VD). A significant main effect of time was observed for 1RM for all seven exercises: leg press (F = 133.03), knee extension (F = 158.90), chest press (F = 351.12), triceps push-down (F = 132.29), biceps curl (F = 209.50), lateral pull-down (F = 143.11), and seated row (F = 176.44) (
p < 0.0001 in all cases) (
Table 4). However, no significant main effect of group or group by time interaction was observed for 1RM for any of the exercise (
p > 0.05 in all cases). In both groups, the greatest increase in 1RM was seen in knee extension (69.3% and 83.6% in VD and PLC, respectively), and the smallest change in triceps push-down (23.3% and 26.4% in VD and PLC, respectively) (
Figure 3).
Significant main effects of time were found for body mass (F = 4.68;
p = 0.005), total fat mass (F = 18.05), total fat percentage (F = 48.88), android fat percentage (F = 29.62), total lean mass (F = 78.34), trunk lean mass (F = 29.19), arms lean mass (F = 33.86), and legs lean mass (F = 28.20) (
p < 0.001 in all cases) (
Table 5). No significant main effect of time was observed for android fat mass (F = 3.059;
p = 0.092). There were no main effects of group or group by time interactions for any of the body composition parameter (
p > 0.05 in all cases). Over the 12-week RT program, both the PLC and VD groups exhibited significant increases in total lean mass, trunk lean mass, arms lean mass, and legs lean mass, as well as decreases in total fat mass, fat percentage and android fat percentage (
p < 0.05 in all cases). However, there were no significant between-group differences in the extent of changes in any body composition parameter (
p > 0.05 in all cases).
A significant main effect of time was found for relative VO
2max (mL/min/kg) levels (F = 5.158;
p = 0.032), with no significant main effect of group (
p = 0.473) or group by time interaction (
p = 0.839) (
Table 6). A small (2.3%) overall decrease in relative VO
2max occurred between weeks 0 and 12 (
p = 0,031). For absolute VO
2max (L/min), there was no significant main effect of group (
p = 0.533) or time (
p = 0.146), nor a significant group by time interaction (
p = 0.551). No significant main effect of group (
p = 0.841) or time (
p = 0.062), nor group by time interaction (
p = 0.445), were observed for the RER. A significant group by time interaction occurred for peak HR (F = 4.979;
p = 0.035) with no significant main effects of time (
p = 0.406) or group (
p = 0.301). No significant main effects of group or time, or group-by-time interactions, were observed for ventilation (VE) and breathing frequency (BF) (
p > 0.05 in all cases) (
Table 6).
A significant group by time interaction occurred for parathormone levels (F = 8.768;
p = 0.0005), with no main effect of time or group (
p > 0.05 in both cases) (
Table 7). In PLC group parathormone levels increased by 22.8% from week 0 to week 12 (
p = 0.008). A significant main effect of time (F = 8.357;
p = 0.0007), but not that of group (
p = 0.916) occurred for cortisol. An overall decrease of 16.1% (
p = 0,002) in cortisol levels was evident from week 0 to week 12, but there was no significant group by time interaction for cortisol (
p = 0.787). No main effects of group or time, nor group by time interactions were observed for IGF-1, testosterone, growth hormone and insulin levels (
p > 0.05 in all cases) (
Table 7).
No significant main effects of group and time, or group-by-time interactions were observed for serum levels of IL-1α, IL-1β, IL-4, IL-6, IL-8, IL-10, TNF-α, and MCP-1, or for the IL-10/TNF-α ratio (
p > 0.05 in all cases;
Table 8).
There were no significant main effects of group or time and no group-by-time interactions for hemoglobin and glucose concentrations (in all cases
p > 0.05;
Table 9). A significant main effect of time (F = 7.595;
p = 0.0013), but not that of group (
p = 0.837) or group-by-time interaction (
p = 0.984) occurred for serum ferritin levels (
Table 9). Overall serum ferritin levels decreased from 194.4 ± 116.3 μg/L at week 0 to 167.6 ± 105.2 μg/L at week 12, i.e. by an average 13.8% (
p = 0.011). There was a significant group-by-time interaction (F = 3.532;
p = 0.036), but no significant main effects of group or time (in both cases
p > 0.05) for HOMA-IR (
Table 9).
Table 8.
Serum cytokine levels during 12-week supplementation and resistance training period.
Table 8.
Serum cytokine levels during 12-week supplementation and resistance training period.
| Variables |
Placebo (n = 14) |
Vitamin D (n = 10) |
| Week 0 |
Week 12 |
Week 0 |
Week 12 |
| IL-1α (pg/mL) |
0.09 ± 0.12 |
0.08 ± 0.13 |
0.05 ± 0.06 |
0.05 ± 0.08 |
| IL-1β (pg/mL) |
1.02 ± 0.71 |
1.06 ± 0.83 |
0.83 ± 0.24 |
0.75 ± 0.19 |
| IL-4 (pg/mL) |
1.08 ± 0.32 |
1.21 ± 0.48 |
1.07 ± 0.35 |
1.02 ± 0.32 |
| IL-6 (pg/mL) |
0.81 ± 0.58 |
0.80 ± 0.44 |
1.07 ± 0.67 |
1.11 ± 0.78 |
| IL-8 (pg/mL) |
10.20 ± 4.77 |
9.92 ± 4.92 |
11.24 ± 4.84 |
10.25 ± 3.90 |
| IL-10 (pg/mL) |
0.60 ± 0.43 |
0.55 ± 0.28 |
0.47 ± 0.18 |
0.42 ± 0.13 |
| TNF-α (pg/mL) |
2.68 ± 0.95 |
2.58 ± 1.11 |
2.44 ± 0.91 |
2.40 ± 0.65 |
| IL-10/TNF-α |
0.21 ± 0.09 |
0.21 ± 0.06 |
0.20 ± 0.08 |
0.18 ± 0.06 |
| MCP-1 (pg/mL) |
219.1 ± 60.7 |
225.3 ± 94.5 |
201.3 ± 93.2 |
196.9 ± 90.5 |
No main effects of group or time or group-by-time interactions were observed for serum ionized calcium and calcium concentrations and creatine kinase activity (in all cases
p > 0.05;
Table 10). A significant main effect of time (F = 3.824;
p = 0.028) and group-by-time interaction (F = 4.704;
p = 0.013) with no significant main effect of group (
p = 0.555) occurred for serum urea levels. In VD group only, a significant increase in serum urea level was observed in week 8 (
p = 0.005) and week 12 (
p = 0.036) compared to week 0 (
Table 10).
4. Discussion
The primary objective of this study was to assess whether systematic RT combined with Vit-D supplementation is more effective in terms of increasing muscle strength and LBM than the same RT program without Vit-D supplementation in Vit-D insufficient healthy middle-aged men. The results show that over the course of 12 weeks of RT, 1RM increased significantly and to a similar extent in all seven exercises in both the VD and PLC groups. During the same period, serum 25(OH)D increased approximately 2.6-fold in the VD group but remained stably low in the PLC group. These data are consistent with previous findings of a positive impact of RT on muscle strength in middle-aged men [
61,
62,
63] but indicate that Vit-D supplementation does not potentiate the effects of RT in this age and sex group.
Four previous studies [
26,
53,
64,
65] have also shown that Vit-D supplementation has no additional effect on RT in increasing muscle strength in middle-aged and elderly individuals. However, in two studies the participants were exclusively [
65] or mostly (90%) [
64] elderly women. It should also be noted that these two studies only measured maximal isometric quadriceps strength [
65] or quadriceps and handgrip strength [
64]. Given that testosterone can influence the RT-induced gains in muscle strength [
66,
67] and that Vit-D supplementation can increase serum testosterone levels [
68,
69], sex differences in the effect of Vit-D supplementation on the efficacy of RT cannot be excluded
a priori. It is also relevant to evaluate the potential effect of combined RT and Vit-D supplementation on strength gains in different muscle groups, as they may respond differently to vitamin D supplementation [
18]. Nevertheless, our data presented here on middle-aged men agree with those obtained in studies of older women [
64,
65] and show that RT improves muscle strength, but Vit-D supplementation does not increase the efficacy of RT. In addition, our muscle strength data from seven different exercises show no differences between muscle groups in terms of response to the Vit-D supplementation in middle-aged men.
Agergaard et al. [
53] investigated the possible effects of Vit-D supplementation during 12 weeks of systematic RT on the quadriceps muscle strength gain in somewhat older healthy men (60–75 years) than our participants (52–66 years) and found no additive effect of Vit-D on either muscle hypertrophy or muscle strength. However, they observed improved muscle quality (increased muscle strength/cross sectional area ratio) in Vit-D supplemented group compared to placebo group. In healthy subjects and patients with chronic obstructive pulmonary disease (age 56–77 years; 46% men), Vit-D supplementation during 13 weeks of RT did not affect maximal muscle strength, assessed as unilateral knee extension and leg press or bilateral chest press [
26]. Our data showing absence of an additive effect of Vit-D supplementation on muscle strength gains during RT are generally consistent with those of these researchers [
26,
53], but extend to much more muscle groups in a slightly younger age group of men.
In our participants, 12 weeks of systematic RT had no effect on body weight but induced significant positive health-related changes in body composition consisting of significant decreases in total fat mass, total fat percentage and android fat percentage. At the same time, significant increases occurred in total lean mass, trunk lean mass and appendicular lean mass.
However, all changes in body composition were of similar extent in the VD and PLC groups, indicating that vitamin D supplementation did not increase the efficacy of RT. Mølmen et al. [
26] reported increases in total lean mass, total fat mass and visceral fat mass, which are in good agreement with our findings, showing efficacy of RT with no additive effect of Vit-D supplementation.
One of our considerations for why vitamin D might improve the efficacy of RT in terms of muscle strength gains in middle-aged men was the vitamin’s potential positive effect on testosterone levels [
68,
69]. However, in our participants, Vit-D supplementation had no effect on serum testosterone or other hormones (insulin, growth hormone, IGF-1, cortisol) that may affect muscle protein metabolism and muscle function. Similar findings were reported by other researchers [
26,
64].
The secondary objective of this study was to elucidate whether systematic RT or Vit-D supplementation during participation in RT program affects cardiorespiratory fitness in Vit-D insufficient healthy middle-aged men. Our results show no effect of 12 weeks of RT with or without concomitant Vit-D supplementation on absolute VO2max. Overall, a small (2.3%) decrease in relative VO2max occurred during 12 weeks of RT.
We considered assessment of the potential effect of interventions applied in this study on VO
2max important by several reasons. First, skeletal muscle oxidative capacity that is among the factors determining VO
2max, is dependent on the mitochondrial function [
70], and systematic RT may lead to positive mitochondrial adaptations [
71,
72,
73]. Second, in middle-aged to elderly subjects, systematic RT may produce significant increases in VO
2max that are as large as in case of aerobic training [
74], or slightly smaller [
75,
76]. Third, VDR is expressed in all human organs, the function of which may influence VO
2max [
11,
30,
31,
32], Vit-D can promote erythropoiesis and hemoglobin synthesis [
33] and affect the binding affinity of oxygen to hemoglobin [
34]. Finally, Vit-D may be crucial for mitochondrial oxidative phosphorylation capacity [
35]. Nevertheless, 12 weeks of RT with or without concomitant Vit-D supplementation did not influence blood hemoglobin levels or absolute VO
2max in our participants. At the same time, there was a small but statistically significant decrease in relative VO
2max, probably due to a tendency to gain weight.
An overall significant 13.8% decline in serum ferritin levels across the 12-week supplementation and RT period indicates a decrease in body’s iron stores [
77] and is consistent with our previous observation in young Vit-D deficient men who completed similar RT program with concomitant Vit-D supplementation [
78]. However, serum ferritin levels <35 μg/L, indicating iron-deficient state [
77], occurred only in two participants at week 0 and in one of these two participants also at weeks 8 and 12. Both men belonged into the VD group. Considering that hemoglobin concentrations were constantly >130 g/L in all participants, i.e. there were no manifestations of anemia, and that iron deficiency without anemia does not affect VO
2max [
77], it seems unlikely that low iron status masked the potential effect of Vit-D supplementation on VO
2max in our participants. Moreover, Shoemaker et al. [
79] demonstrated significant training-induced increases in VO
2max despite 73% decreases in serum ferritin levels in young men. The reasons why training loads can lead to a decrease in ferritin levels are not entirely clear and they may differ in different situations. It has been speculated that the improvement of various aspects of performance against the background of a decrease in serum ferritin may reflect training tolerance of athletes [
80] and physiological adaptation reactions, for example, at the level of intensification of the synthesis of iron-containing enzyme proteins [
81].
Previous studies on middle-aged adults [
72] and elderly men [
73] demonstrated mitochondrial adaptations as a result of 10 and 6 weeks of RT, respectively, but VO
2max was not measured by these researchers. In studies showing a significant positive effect of RT on VO
2max [
74,
75], the duration of systematic RT was 6 months, i.e., twice as long as in our participants. Thus, the RT program in our participants may have been too short-term to induce measurable changes in VO
2max.
In our participants, there were no between-group differences or changes across time in physiological parameters measured during VO
2max tests (respiratory exchange ratio, maximal heart rate, maximal breath frequency, maximal pulmonary ventilation). These findings are consistent with the lack of meaningful changes in VO
2max. Interestingly, Kujach et al. [
39] have observed increases in maximal breath frequency and maximal lung ventilation due to Vit-D supplementation in college aged males.
Bioactive form of Vit-D is known to inhibit and stimulate the production of pro- and anti-inflammatory cytokines, respectively [
7,
82,
83]. In young healthy Vit-D deficient men, 12 weeks of RT with concomitant Vit-D supplementation improved the inflammatory status as reflected by an increase in the serum IL-10/TNF-α ratio [
78]. In the middle-aged men in the present study, RT and Vit-D supplementation did not exhibit this kind of interaction, and no changes were observed in any of the measured cytokine levels. The reasons for the discrepancy in the results of these two studies remain unclear, but it can be assumed that they may be partly related to the fact that our middle-aged men had higher pre-intervention Vit-D status compared to young participants in the previous [
78] study. On the other hand, recent findings of Silva et al. [
84] show that 12 weeks of RT per se, i.e. without Vit-D supplementation, may have an anti-inflammatory effect, including a strong tendency to improved IL-10/TNF-α ratio, in middle-aged and elderly patients with chronic obstructive pulmonary disease (COPD). The difference between our data and that of Silva et al. [
84] regarding the anti-inflammatory effect of RT in middle-aged and elderly people, may be at least partially explained by the fact that COPD is an inflammatory disease [
85], but our subjects were healthy men.
Chronic Vit-D deficiency is associated with insulin resistance [
86]. Six-month Vit-D supplementation in obese Vit-D insufficient adolescents [
87] and 12 weeks of RT in obese middle-aged men [
88] improved insulin resistance which was reflected in a significant decrease in HOMA-IR index. In our normal weight middle-aged men, 12 weeks of RT with or without Vit-D supplementation did not affect serum glucose and insulin levels or HOMA-IR. Our results are consistent with those of three previous studies in overweight and obese young adults with varying Vit-S status [
89], overweight and obese middle-aged older adults with sufficient Vit-D status and type 2 diabetes [
90], and normal weight young Vit-D deficient men [
78] who participated in a similar 12-week RT program with concomitant Vit-D supplementation.
In our VD group participants, the daily dose of supplemental Vit-D was 8000 IU, but the tolerable upper intake level of Vit-D established for adults is 4000 IU per day [
91]. On the other hand, a daily dose of 10000 IU is considered the lowest-observed-adverse-effect-level of Vit-D intake [
91], and the position of The Endocrine Society is that daily intake of this amount may be necessary to treat Vit-D deficiency [
92]. Nevertheless, Vit-D oversupply may lead to serious health problems including formation of kidney stones, calcification of soft tissues and vasculature [
50,
93]. Hypercalcemia is a sensitive marker of the potential harmful effects of Vit-D [
92,
94,
95]. It is widely accepted that hypercalcemia induced by vitamin D intoxication usually only occur at serum 25(OH)D concentrations above 375 nmol/L and is very rare [
92,
95,
96]. In our VD group participants, serum calcium levels remained unchanged throughout the study period. The highest 25(OH)D concentrations in their serum occurred at week 12 and were in the range of 120.7–188.1 nmol/L, i.e. well below the critical level.
In our PLC group participants, serum 25(OH)D levels did not increase during the RT and placebo supplementation period and were in the range of 23.5–63.1 nmol/L at week 12. Vit-D insufficiency may lead to secondary hyperparathyroidism that has negative implications for cardiovascular and bone health [
97,
98]. In PLC group, but not in VD group, serum parathormone levels increased during the 12 weeks of RT and supplementation, but still remained within the physiologically normal range. Thus, the data discussed in the last two sections show that neither high-dose vitamin D supplementation nor placebo posed a risk to the health of our participants.
Our participants donated blood samples always in the morning after two resting days. Under these conditions, fasting serum urea concentrations below 7.5 mmol/L and moderate levels of creatine kinase activity suggest that our participants tolerated training loads well and started each consecutive training week in a well-recovered state [
99].
The main strengths of our study are the supervised RT and dietary supplementation program and high compliance rate of participants with both requirements. On the other hand, the main limitation of the study was the low compliance of the subjects with the guidelines for reporting daily energy and nutrient intake for three 3-day periods of the 12-week intervention. Although all participants verbally confirmed that their usual diet did not change during their participation in the study, and the data of the men who followed the corresponding instructions correctly for at least half the duration of the study confirm that, our data remain incomplete in this regard. Nevertheless, we are not aware of any circumstances that directly or indirectly would indicate that changes in habitual diet could have masked the possible effects of Vit-D supplementation on the outcomes of our study.