1. Introduction
Endotheliopathy is dysfunction of the endothelium and plays a crucial role in various complications associated with hematopoietic stem cell transplantation (HSCT) in pediatric and young adult patients. These complications can include diffuse alveolar hemorrhage (DAH), sinusoidal obstruction syndrome (SOS), and thrombotic microangiopathy (TMA), among others [
1,
2,
3,
4,
5,
6]. The presence of endotheliopathy can lead to organ dysfunction, the requirement for critical care, and even mortality in these patients [
7,
8]. It is important for healthcare providers to remain vigilant for signs and symptoms of endotheliopathy and its associated complications in pediatric and young adult patients undergoing HSCT. Early recognition and intervention may help in managing these serious conditions, and hence has the potential to improve patient outcomes. While research is ongoing in the discovery of biomarkers that could help predict endothelial dysfunction in patients receiving HSCT, a specific marker or set of markers has not yet been shown to be fully predictive of such injury [
3,
5,
6].
It is interesting to note the role of the B-type natriuretic peptides such as NT-proBNP and BNP in the context of HSCT. These markers are easily obtained in most clinical laboratories and are typically thought of as markers associated with left ventricular dysfunction and cardiac failure [
9]. The elevation of these markers following HSCT in adult patients is often attributed to subclinical myocardial toxicity from preparative regimens or prior chemotherapy, even in the absence of clinical or echocardiographic changes [
10,
11,
12,
13,
14,
15,
16,
17,
18]. In pediatric and young adult patients, it is also common practice to monitor NT-proBNP or BNP following HSCT to potentially detect cardiac toxicity. Interestingly, while changes in NT-proBNP levels occur during the peri-transplant and post-transplant time period, studies have not definitively linked NT-proBNP elevation to cardiac outcomes in this specific population [
19,
20,
21,
22]. These findings suggest that while B-type natriuretic peptides can serve as valuable markers for cardiac function assessment in HSCT patients, their relationship to clinical outcomes, especially in the pediatric and young adult population, may be more complex than simply a marker of cardiac dysfunction.
BNP has been shown to be an independent risk factor for ICU mortality in adult patients receiving HSCT [
23]. Elevated levels of BNP have been associated with increased critical care utilization in pediatric HSCT patients and have also been linked to higher mortality rates in pediatric HSCT patients requiring care in the pediatric intensive care unit (PICU) [
24,
25]. These studies highlight the potential utility of B-type natriuretic peptides as prognostic markers in HSCT setting, both in the pediatric and adult populations. The associations between BNP elevation and adverse outcomes underscores the importance of monitoring B-type natriuretic peptides in HSCT patients to identify those at higher risk for complications and potentially intervene earlier to improve outcomes.
The relationship between B-type natriuretic peptides (BNP and NT-proBNP) and inflammation is multifaceted. Although ventricular stress is thought to be the most important factor for BNP or NT-pro-BNP secretion, there is evidence that the B-type natriuretic peptides are also upregulated with inflammation in cardiac myocytes and other cell types [
26,
27,
28]. Additionally, these peptides have been studied as markers of endotheliopathy in peripheral artery disease and coronary artery disease [
28,
29]. Given that clinical cardiac toxicity is rare in children undergoing HSCT despite the observed correlation between BNP elevation and critical care utilization and mortality in pediatric patients, we sought to evaluate NT-proBNP as a potential biomarker of endotheliopathy which is more commonly seen than traditional cardiac toxicity [
22].
Assessment of endotheliopathy through biomarkers such as the B-type natriuretic peptides could provide insight into vascular health and potential cardiovascular risk in HSCT patients, beyond traditional cardiac function assessment. The association between BNP elevation and sinusoidal obstruction syndrome (SOS) in adults has been documented in the literature [
30]. However, there is limited research on this relationship in the pediatric population. To address this gap, we hypothesized that elevation in NT-proBNP was associated with the development of endotheliopathy syndromes such as DAH, SOS, and TMA in the first 100 days following HSCT in children and young adults. We submit our results from a retrospective case-control study at a quaternary pediatric cancer hospital. Our study aimed to provide insight into the utility of NT-proBNP as a biomarker of endothelial dysfunction and development of specific post-transplant complications in children and young adults.
2. Materials and Methods
This was a retrospective single center case-control study conducted at St. Jude Children’s Research Hospital. IRB exempt status was obtained prior to the initiation of the study (IRB number 22-1131). All allogenic or autologous hematopoietic cell transplants at St. Jude Children’s Research Hospital between 2016 and 2020 were initially reviewed (
Figure 1).
Cases were then selected based on the diagnosis of DAH, SOS, or TMA in the first 100 days following HSCT. This was performed using a local database. The database was created for clinical and quality improvement purposes to track transplant complications, including DAH, SOS, or TMA. DAH was diagnosed based on bronchioalveolar lavage findings. SOS and TMA were diagnosed using prior published criteria [
31,
32]. Complications were recorded as per the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE). All adverse events were reviewed by the attending physicians prior to inclusion in the database.
Potential cases were verified by our study team via chart review. Patients that did not receive a diagnosis of TMA, SOS, or DAH during the first 100 days following HSCT were excluded as cases but were available for potential use as controls. Patients that were labeled as only possible, probable, potential, or evolving cases of DAH, SOS, or TMA were excluded for use as cases or controls. Additionally, patients were excluded from selection as cases or controls if baseline and/or near-event NT-proBNP levels were not available.
Cases were matched with controls. Matching factors included transplant protocol, age group (<2 years of age, 2-10 years of age, and >10 years of age), and time-period of transplant (day of transplant between January 1st, 2016, and December 31st, 2018, or day of transplant between January 1st, 2019, and December 31st, 2020). If more than one potential control met matching criteria for any given case, computer randomization was utilized to select the control. If cases did not have a control with adequate matching factors, they were removed from the pool of cases. There were two patients that were noted to have received a diagnosis of SOS that both received tandem transplants. Hence each of these patients were counted as one case each, and the first 100 days following the first HSCT was considered for the purpose of this study. Controls were verified to be true controls by chart review. This resulted in one additional control being excluded for diagnosis of Idiopathic Pneumonia Syndrome discovered during chart review.
Baseline NT-proBNP level, echocardiogram data, and renal function by estimated glomerular filtration rate (eGFR) on the closest day prior to transplant were obtained for cases and controls. Near-event NT-proBNP and echocardiogram data were obtained for cases. Near-event was defined as the NT-proBNP level and echocardiogram on the day closest to but preceding the day of diagnosis of the event (diagnosis of DAH, SOS or TMA). If data points were not available between the time of transplant and the time of the event, then the time point closest to but after the event was used. These data points were obtained by manual chart review. For the controls, data extracts from the day after transplant that correlated to the same day after transplant as the matched case’s day of event (diagnosis of endotheliopathy) was used as the near-event timepoint. In the rare case that there was not an echocardiogram available after the baseline echocardiogram, we searched for the first available echocardiogram after transplant even if it was after 100 days following HSCT. Ejection fraction was measured via M-mode. The status of pulmonary hypertension was assessed by indirect measures of tricuspid regurgitation gradient and qualitative assessment.
Demographic and clinical characteristics were compared between the case (N = 31) and the control (N = 31) groups using the exact Pearson Chi-square test for the categorical variables and the Wilcoxon rank-sum test for the numeric variables. Based on the comparison results and clinical relevance, a series of conditional logistic regression models were constructed to estimate the effect of near-event NT-proBNP level on the odds of developing endotheliopathy within the first 100 days following HSCT, controlling for different subsets of covariates. The primary model used baseline and near-event NT-proBNP level, baseline and near-event ejection fraction and transplant number as covariates. The secondary model further accounted for potential confounding factors including transplant indication, and age difference (difference between age at transplant and the mean age of the corresponding age grouping) in addition to covariates utilized in the primary model. Akaike information criterion (AIC) scores were calculated for the models. The estimated odds ratio (OR) and 95% Wald confidence interval (CI) were reported for the covariates from the models. The statistical significance cut-off was a p-value of 0.05 for all analyses in the study. We used SAS 9.4 to conduct statistical analyses.
3. Results
3.1. Demographic and Baseline Clinical Characteristics
There was a total of 375 hematopoietic stem cell transplants performed between 2016 and 2020 available for review in the database. The study included 31 cases and 31 matched controls after application of inclusion and exclusion criteria. Demographic and baseline clinical characteristics are demonstrated in
Table 1. Most of the included patients (44, 71%) were between 2 and 10 years of age. Forty-two percent of the patients were female. Fifty-two percent of the patients received HSCT between January 1st, 2019, and December 31st, 2020. The included patients underwent haploidentical (24, 39%), autologous (20, 32%), matched unrelated donor (12, 19%), and matched sibling donor (6, 10%) HSCT. For most of the patients included, this was the first HSCT they had received. There was no significant difference between the cases and controls in the proportion who had received one or more transplant in the past (p = 0.27). There was a variety of diagnoses that served as the primary indication for HSCT, though acute leukemias and neuroblastoma were the most prevalent indications for HSCT. Other indications only accounted for 13% of patients. Interestingly, there was a difference in the distribution of transplant indications seen between cases and controls, with ALL being the most prevalent transplant indication for HSCT in the case group (p = 0.02). Radiation was performed as a part of the preparative regimen in the ALL patients with similar frequency between the cases and controls. Most patients had a myeloablative preparative regimen with no significant difference between cases and controls in the preparative regimen type (p = 1.00).
None of the included patients had evidence of pulmonary hypertension based on baseline pre-HSCT echocardiogram. In addition, all the included patients had a baseline ejection fraction (EF) greater than or equal to 50% by echocardiogram. All baseline echocardiograms were performed within 32 days prior to transplant. The baseline eGFR was largely normal (>60 mL/min/1.73 m2) in most included patients (60, 97%) with no significant difference in eGFR found between cases and controls (p = 1.00).
3.2. Endotheliopathy Data
Of the 31 cases that were diagnosed with endotheliopathy in the first 100 days following HSCT, 22 (71%) had SOS, 8 (26%) had TMA, and 1 (3%) had DAH (
Table 2).
The median day of diagnosis of endotheliopathy was 15 days following HSCT (range 6 to 85 days following HSCT). The median near-event ejection fraction by echocardiogram was normal at 70% for the cases and controls (p = 0.57). Only two patients had near-event echocardiograms with an EF of 45% in the case group. The rest of the patients had an EF greater than or equal to 50%. There were two patients in the case group and one patient in the control group with near-event echocardiograms showing pulmonary hypertension.
3.3. NT-proBNP Levels
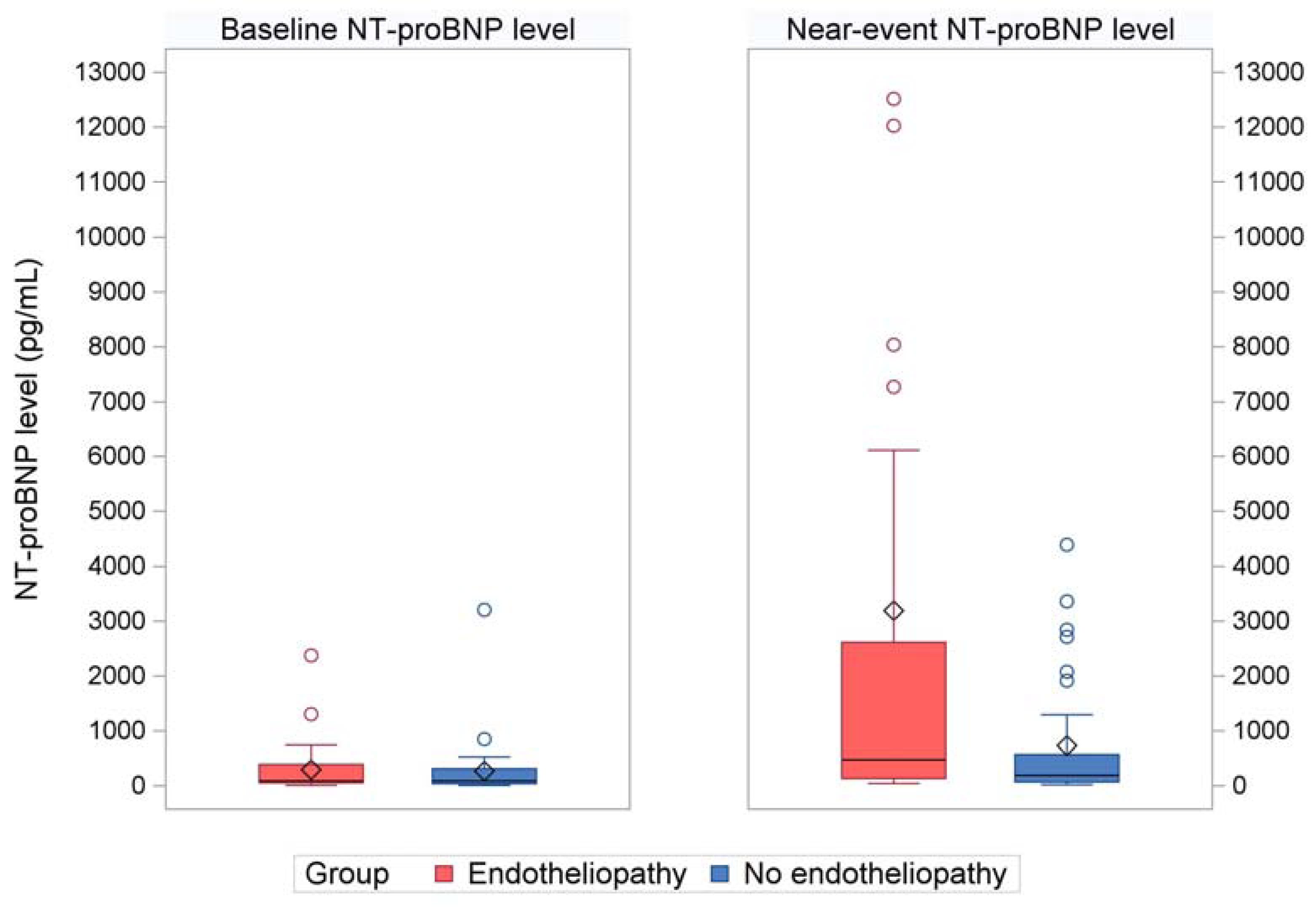
Near-event NT-proBNP levels were significantly elevated in cases compared to controls at the most similar timepoint (Median 473 pg/mL vs. 187 pg/mL, p = 0.03) as displayed in
Table 2 and
Figure 2. In contrast, the baseline NT-proBNP levels were comparable between cases and controls with median in both groups of 86 pg/mL (p = 0.51) as displayed
Table 2 and
Figure 2.
To confirm differences in timing of NT-proBNP levels compared to time of event in the cases were not introducing another variable, we performed an analysis of the timing of the near-event NT-proBNP levels. On average, the difference between the day of diagnosis of endotheliopathy and the day of near-event NT-proBNP level was 2.84 days with a standard deviation of 2.79 days (range -2 to 10). Only 2 patients out of 30 cases had a difference of -2 (two days after diagnosis of endotheliopathy). In other words, on average, the near-event NT-proBNP levels in the case group occurred between 2 and 3 days prior to diagnosis of endotheliopathy.
3.4. Conditional Logistic Regression
Based on the association of elevated near-event NT-proBNP levels and endotheliopathy, we further evaluated the effect of near-event NT-proBNP levels as well as other variables on the odds of developing endotheliopathy using a conditional logistic regression. None of the variables tested in the primary model including near-event NT-proBNP level, baseline NT-proBNP level, near-event EF, baseline EF, and prior transplant(s) had a statistically significant association with the odds of developing endotheliopathy (
Table 3).
Given the unexpected finding of a difference in transplant indication distribution observed between cases and controls we explored the effect of transplant indication on the development of endotheliopathy utilizing the secondary model (
Table 3). In the secondary model, the estimated effect of transplant indication on developing endotheliopathy was significant controlling for other covariates (OR = 20.16 with a 95% CI of 1.90 to 213.56, p = 0.01). In other words, a transplant indication of ALL increased the odds of developing endotheliopathy 20.16 times compared to other indications in our study sample. The effect of transplant number was also noteworthy (OR = 6.36 with a 95% CI of 0.95 to 42.48, p = 0.06). However, the estimated effect of near-event NT-proBNP was not significant (OR = 1.09 with a 95% CI of 0.83 to 1.43, p-value = 0.54) after controlling for the covariates in this model. Nevertheless, as seen in
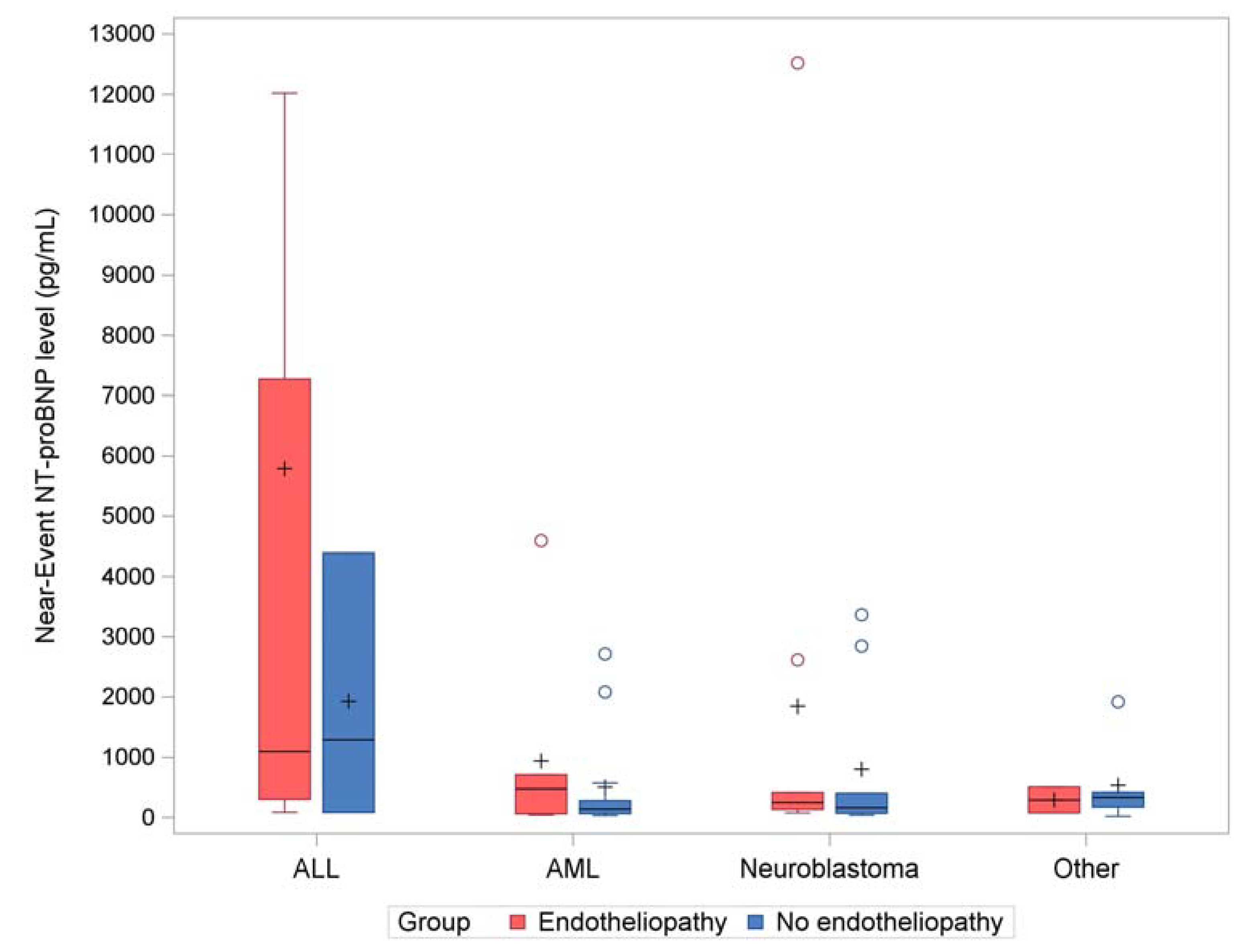
Figure 3, the common trends from the most common transplant indication categories (ALL, AML, and neuroblastoma) suggested an association between elevated near-event NT-proBNP level and endotheliopathy.
4. Discussion
The identification of non-invasive and readily obtainable biomarkers for endotheliopathy in pediatric and young adult HSCT is imperative to facilitate earlier diagnosis and intervention and eventual improved outcomes. We posit that NT-proBNP is a potential biomarker for endotheliopathy in pediatric and young adult patients undergoing HSCT. While baseline NT-proBNP levels were found to be comparable between cases and controls, our study revealed that near-event NT-proBNP levels were elevated in pediatric and young adult patients diagnosed with endotheliopathy within the first 100 days following HSCT when compared to matched controls without endotheliopathy. This novel finding has not been previously reported in pediatric and young adult HSCT patients.
Due to small numbers, our study possibly lacked the statistical power to uncover an association between elevated NT-proBNP levels and development of endotheliopathy following HSCT in a multifactorial logistic regression model. Even with small numbers, having multiple transplants remained an independent risk factor for endotheliopathy in the secondary model.
An interesting and unexpected finding was the difference in distribution of transplant indications seen between the endotheliopathy group and the group without endotheliopathy. Specifically, there was a higher proportion of patients with a primary HSCT indication of ALL in the patients with endotheliopathy compared to those without endotheliopathy. Furthermore, the odds of developing endotheliopathy increased by over 20 times for ALL versus all other diagnoses in the secondary model. A visual inspection of
Figure 3 suggests that the effect of NT-proBNP on developing endotheliopathy might be heightened in ALL patients compared to the other diagnoses.
We speculate that ALL patients likely received intensive chemotherapy before HSCT, rendering them potentially more susceptible to both endothelial damage and elevated NT-proBNP levels. Several prospective studies in adult ALL patients undergoing HSCT have demonstrated persistent elevation in NT-proBNP levels in some patients even in absence of clinically significant cardiac symptoms [
15,
21]. One of these studies also showed persistent NT-proBNP elevation in patients with higher cumulative dose of anthracycline and preparative regimens with total body irradiation or higher dose of cyclophosphamide [
21]. A rising NT-proBNP level, particularly in a patient undergoing HSCT for ALL, may suggest that further screening for endotheliopathy is warranted.
Our study had several limitations. Being retrospective in nature, we were unable to fully control or standardize the timing of NT-proBNP level measurements. This was mitigated as much as possible by obtaining retrospective NT-proBNP levels that were temporally very close to the diagnosis of endotheliopathy. However, reliance on retrospectively identifying the adverse events can also introduce variability in terms of the timing and sensitivity of DAH, SOS, and TMA diagnoses. While all patients undergoing HSCT at our institution are screened for endotheliopathy, more subtle cases may be missed in the absence of a prospective trial deliberately screening for them. As a single-center study, this may limit the generalizability of our findings.
The findings of our study suggest a potential association between elevated NT-proBNP levels and endotheliopathy, particularly in patients undergoing HSCT for acute lymphoblastic leukemia (ALL). To the best of our knowledge, this is the first instance where NT-proBNP has been associated with endotheliopathy syndromes in pediatric and young adult patients undergoing hematopoietic stem cell transplantation (HSCT). NT-proBNP is a laboratory test that is widely available and cost-effective in most clinical settings, aligning with the characteristics of an ideal biomarker [
33]. If NT-proBNP could be utilized in conjunction with other clinical symptoms or laboratory tests for early detection of endotheliopathy, patients stand to benefit from prompt diagnosis and intervention. The use of NT-proBNP as a biomarker for endotheliopathy in pediatric and young adult HSCT patients needs further validation and exploration in future studies, including multi-center and prospective investigations.
Author Contributions
Conceptualization, J.M., K.U., S.G., X.Y.; methodology, D.K., H.P., J.M., K.U., S.G., X.Y.; software, X.Y.; validation, X.Y.; formal analysis, H.P., X.Y.; investigation, D.K., K.T., K.U., R.L.; resources, D.K., H.P., X.Y., Y.A.; data curation, D.K., K.T., K.U., R.L., X.Y., Y.A.; writing—original draft preparation, K.U., X.Y.; writing—review and editing, H.P., J.M., K.U., M.H., S.G., X.Y.; visualization, K.U., X.Y.; supervision, A.Q., H.M., H.P., J.M., K.U., M.H., S.G.; project administration, K.U., S.G., Y.A.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (IRB) of St. Jude Children’s Research Hospital for IRB exempt status (IRB Number 22-1131, 08/19/2022).
Informed Consent Statement
Patient consent was waived due to the IRB determining this research meets the requirements for the Waiver and Alteration of Consent under 45 CFR 46.116(f)(3).
Data Availability Statement
The data presented in this study are available only on request from the corresponding author due to the privacy of participants/patients.
Acknowledgments
The authors would like to acknowledge Dr. A. Nico West, MD, PhD.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ahmad, A.H.; Mahadeo, K.M. Perspective: A Framework to Screen Pediatric and Adolescent Hematopoietic Cellular Therapy Patients for Organ Dysfunction: Time for a Multi-Disciplinary and Longitudinal Approach. Front. Oncol. 2021, 11, 622630. [Google Scholar] [CrossRef] [PubMed]
- Carreras, E.; Diaz-Ricart, M. The Role of the Endothelium in the Short-Term Complications of Hematopoietic SCT. Bone Marrow Transplant. 2011, 46, 1495–1502. [Google Scholar] [CrossRef]
- Pagliuca, S.; Michonneau, D.; Sicre de Fontbrune, F.; Sutra del Galy, A.; Xhaard, A.; Robin, M.; Peffault de Latour, R.; Socie, G. Allogeneic Reactivity–Mediated Endothelial Cell Complications after HSCT: A Plea for Consensual Definitions. Blood Adv. 2019, 3, 2424–2435. [Google Scholar] [CrossRef]
- Cooke, K.R.; Jannin, A.; Ho, V. The Contribution of Endothelial Activation and Injury to End-Organ Toxicity Following Allogeneic Hematopoietic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2008, 14, 23–32. [Google Scholar] [CrossRef]
- Hildebrandt, G.C.; Chao, N. Endothelial Cell Function and Endothelial-related Disorders Following Haematopoietic Cell Transplantation. Br. J. Haematol. 2020, 190, 508–519. [Google Scholar] [CrossRef]
- Lia, G.; Giaccone, L.; Leone, S.; Bruno, B. Biomarkers for Early Complications of Endothelial Origin After Allogeneic Hematopoietic Stem Cell Transplantation: Do They Have a Potential Clinical Role? Front. Immunol. 2021, 12, 641427. [Google Scholar] [CrossRef] [PubMed]
- Zinter, M.S.; Dvorak, C.C.; Spicer, A.; Cowan, M.J.; Sapru, A. New Insights Into Multicenter PICU Mortality Among Pediatric Hematopoietic Stem Cell Transplant Patients*: Crit. Care Med. 2015, 43, 1986–1994. [Google Scholar] [CrossRef]
- Zaidman, I.; Mohamad, H.; Shalom, L.; Ben Arush, M.; Even-Or, E.; Averbuch, D.; Zilkha, A.; Braun, J.; Mandel, A.; Kleid, D.; et al. Survival of Pediatric Patients Requiring Admission in the Intensive Care Unit Post Hematopoietic Stem Cell Transplantation: Prognostic Factors Associated with Mortality. Pediatr. Blood Cancer 2022, 69, e29549. [Google Scholar] [CrossRef] [PubMed]
- Rubattu, S.; Sciarretta, S.; Valenti, V.; Stanzione, R.; Volpe, M. Natriuretic Peptides: An Update on Bioactivity, Potential Therapeutic Use, and Implication in Cardiovascular Diseases. Am. J. Hypertens. 2008, 21, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Chung, T.; Lim, W.-C.; Sy, R.; Cunningham, I.; Trotman, J.; Kritharides, L. Subacute Cardiac Toxicity Following Autologous Haematopoietic Stem Cell Transplantation in Patients with Normal Cardiac Function. Heart 2008, 94, 911–918. [Google Scholar] [CrossRef]
- Masuko, M.; Ito, M.; Kurasaki, T.; Yano, T.; Takizawa, J.; Toba, K.; Aoki, S.; Fuse, I.; Kodama, M.; Furukawa, T.; et al. Plasma Brain Natriuretic Peptide during Myeloablative Stem Cell Transplantation. Intern. Med. 2007, 46, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Roziakova, L.; Bojtarova, E.; Mistrik, M.; Dubrava, J.; Gergel, J.; Lenkova, N.; Mladosievicova, B. Serial Measurements of Cardiac Biomarkers in Patients after Allogeneic Hematopoietic Stem Cell Transplantation. J. Exp. Clin. Cancer Res. 2012, 31, 13. [Google Scholar] [CrossRef]
- Horacek, J.M.; Tichy, M.; Pudil, R.; Jebavy, L.; Zak, P.; Ulrychova, M.; Slovacek, L.; Maly, J. Multimarker Approach to Evaluation of Cardiac Toxicity during Preparative Regimen and Hematopoietic Cell Transplantation.
- Snowden, J.; Hill, G.; Hunt, P.; Carnoutsos, S.; Spearing, R.; Espiner, E.; Hart, D. Assessment of Cardiotoxicity during Haemopoietic Stem Cell Transplantation with Plasma Brain Natriuretic Peptide. Bone Marrow Transplant. 2000, 26, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Roziakova, L.; Bojtarova, E.; Mistrik, M.; Krajcovicova, I.; Mladosievicova, B. Abnormal Cardiomarkers in Leukemia Patients Treated with Allogeneic Hematopoietic Stem Cell Transplantation. Bratisl. Med. J. 2012, 113, 159–162. [Google Scholar] [CrossRef]
- Se, Z.; Zhou, H.; Li, H.; Sun, J.; Zhan, Q.; Zeng, Q.; Liu, Q.; Xu, D. Clinical Characteristics of Patients with Different N-Terminal Probrain Natriuretic Peptide Levels after Hematopoietic Stem Cell Transplantation. Dis. Markers 2020, 2020, 1–7. [Google Scholar] [CrossRef]
- Roziakova, L.; Mistrik, M.; Batorova, A.; Kruzliak, P.; Bojtarova, E.; Dubrava, J.; Gergel, J.; Mladosievicova, B. Can We Predict Clinical Cardiotoxicity with Cardiac Biomarkers in Patients After Haematopoietic Stem Cell Transplantation? Cardiovasc. Toxicol. 2015, 15, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Niwa, N.; Watanabe, E.; Hamaguchi, M.; Kodera, Y.; Miyazaki, H.; Kodama, I.; Ohono, M. Early and Late Elevation of Plasma Atrial and Brain Natriuretic Peptides in Patients after Bone Marrow Transplantation. Ann. Hematol. 2001, 80, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Rotz, S.J.; Ryan, T.D.; Hlavaty, J.; George, S.A.; El-Bietar, J.; Dandoy, C.E. Cardiotoxicity and Cardiomyopathy in Children and Young Adult Survivors of Hematopoietic Stem Cell Transplant. Pediatr. Blood Cancer 2017, 64, e26600. [Google Scholar] [CrossRef]
- Rotz, S.J.; Dandoy, C.E.; Taylor, M.D.; Jodele, S.; Jefferies, J.L.; Lane, A.; El-Bietar, J.A.; Powell, A.W.; Davies, S.M.; Ryan, T.D. Long-Term Systolic Function in Children and Young Adults after Hematopoietic Stem Cell Transplant. Bone Marrow Transplant. 2017, 52, 1443–1447. [Google Scholar] [CrossRef]
- Horacek, J.M.; Pudil, R.; Tichy, M.; Jebavy, L.; Zak, P.; Slovacek, L.; Maly, J. Biochemical Markers and Assessment of Cardiotoxicity During Preparative Regimen and Hematopoietic Cell Transplantation in Acute Leukemia. Exp. Oncol. 2007. [Google Scholar]
- Zaucha-Prażmo, A.; Sadurska, E.; Drabko, K.; Kowalczyk, J.R. Can We Find a Good Biochemical Marker of Early Cardiotoxicity in Children Treated with Haematopoietic Stem Cell Transplantation? Współczesna Onkol. 2016, 3, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Huo, W.; Zhao, H.; Lv, J.; Lv, S.; An, Y. Risk Factors and Predictive Model for Mortality in Patients Undergoing Allogeneic Hematopoietic Stem Cell Transplantation Admitted to the Intensive Care Unit. Exp. Ther. Med. 2024, 27, 168. [Google Scholar] [CrossRef] [PubMed]
- McArthur, J.; Fitzgerald, J.; Bajwa, R.; Margossian, S.; Yates, A.; Duncan, C.; Talano, J.; Spear, D.; Luther, K.; Tamburro, R. 763: Brain Natriuretic Peptide (Bnp) Levels As A Predictor Of Need For Critcal Care Resources In Pediatric Hematopoietic Stem Cell Transplant (HSCT) Patients. Crit. Care Med. 2012, 40, 1–328. [Google Scholar] [CrossRef]
- Kim, D.H.; Ha, E.J.; Park, S.J.; Koh, K.-N.; Kim, H.; Im, H.J.; Jhang, W.K. Prognostic Factors of Pediatric Hematopoietic Stem Cell Transplantation Recipients Admitted to the Pediatric Intensive Care Unit. Acute Crit. Care 2021, 36, 380–387. [Google Scholar] [CrossRef]
- Fu, S.; Ping, P.; Wang, F.; Luo, L. Synthesis, Secretion, Function, Metabolism and Application of Natriuretic Peptides in Heart Failure. J. Biol. Eng. 2018, 12, 2. [Google Scholar] [CrossRef]
- Ma, K.K.; Ogawa, T.; De Bold, A.J. Selective Upregulation of Cardiac Brain Natriuretic Peptide at the Transcriptional and Translational Levels by Pro-Inflammatory Cytokines and by Conditioned Medium Derived from Mixed Lymphocyte Reactions via P38 MAP Kinase. J. Mol. Cell. Cardiol. 2004, 36, 505–513. [Google Scholar] [CrossRef]
- Kuhn, M. Endothelial Actions of Atrial and B-type Natriuretic Peptides. Br. J. Pharmacol. 2012, 166, 522–531. [Google Scholar] [CrossRef]
- Hall, C. NT-ProBNP: The Mechanism Behind the Marker. J. Card. Fail. 2005, 11, S81–S83. [Google Scholar] [CrossRef]
- Kataoka, K.; Nannya, Y.; Iwata, H.; Seo, S.; Kumano, K.; Takahashi, T.; Nagai, R.; Kurokawa, M. Plasma Brain Natriuretic Peptide Is Associated with Hepatic Veno-Occlusive Disease and Early Mortality after Allogeneic Hematopoietic Stem Cell Transplantation. Bone Marrow Transplant. 2010, 45, 1631–1637. [Google Scholar] [CrossRef]
- Corbacioglu, S.; Carreras, E.; Ansari, M.; Balduzzi, A.; Cesaro, S.; Dalle, J.-H.; Dignan, F.; Gibson, B.; Guengoer, T.; Gruhn, B.; et al. Diagnosis and Severity Criteria for Sinusoidal Obstruction Syndrome/Veno-Occlusive Disease in Pediatric Patients: A New Classification from the European Society for Blood and Marrow Transplantation. Bone Marrow Transplant. 2018, 53, 138–145. [Google Scholar] [CrossRef]
- Jodele, S.; Davies, S.M.; Lane, A.; Khoury, J.; Dandoy, C.; Goebel, J.; Myers, K.; Grimley, M.; Bleesing, J.; El-Bietar, J.; et al. Diagnostic and Risk Criteria for HSCT-Associated Thrombotic Microangiopathy: A Study in Children and Young Adults. Blood 2014, 124, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Navanandan, N.; Searns, J.; Ambroggio, L. Method/Ology of Phases of Biomarker Discovery. Hosp. Pediatr. 2023, 13, e181–e185. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).