Submitted:
23 August 2024
Posted:
26 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Vitamin D Analysis
2.3. Statistical Methods
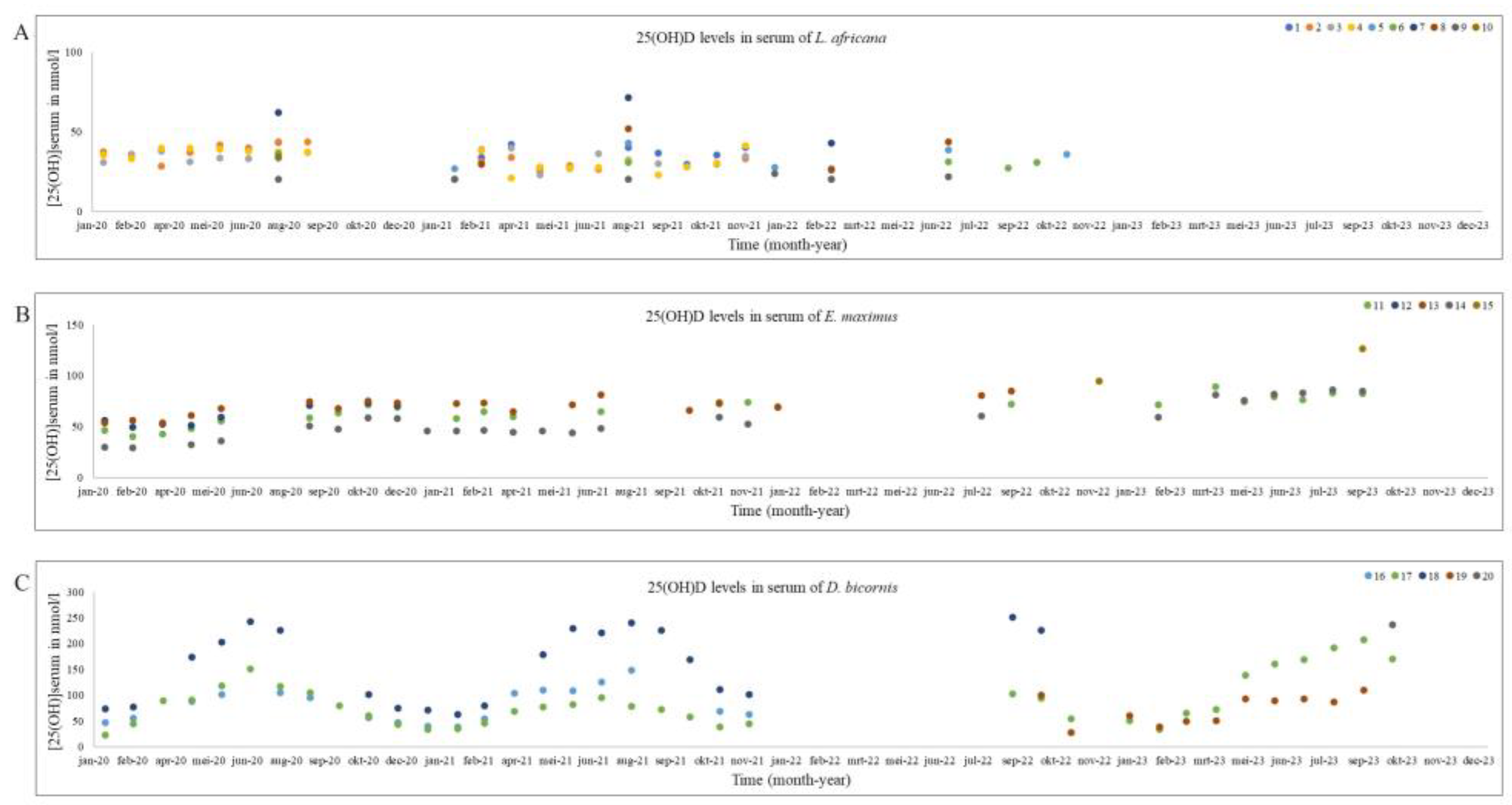
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roach, J.O.; Benyon, S. Nutrition. In Metabolism and nutrition, 2nd ed.; Roach, J.O., Ed.; Elsevier Ltd.: London, NY, USA, 2003; pp. 154–156. [Google Scholar]
- Thitaram, C.; Pongsopawijit, P.; Thongtip, N.; Angkavanich, T.; Chansittivej, S.; Wongkalasin, W. Dystocia following prolonged retention of a dead fetus in an Asian elephant (Elephas maximus). Theriogenology 2006, 66, 1284–1291. [Google Scholar] [CrossRef] [PubMed]
- Mahasawangkul, S.; Angkawanich, T. Elephant health status in Thailand—the role of elephant hospitals and mobile elephant clinics. Eu-Asia link project symposium. In Managing the health and reproduction of elephant populations in Asia; Elephant Hospital, National Elephant Institute, Forest Industry Organization: Lampang, Thailand, 2007; pp. 32–37. [Google Scholar]
- Sanyathitisaeree, P.; Yartbantoong, N.; Thongthipsiridej, S.; Theeraphan, W. Elephant health problems: an accumulative case report from the Kasetsart university veterinary teaching hospital– Kamphaengsaen. Eu-Asia link project symposium. In Managing the Health and Reproduction of Elephant Populations in Asia; Faculty of Veterinary Medicine, Kasetsart University: Bangkok, Thailand, 2007; pp. 41–48. [Google Scholar]
- Hermes, R.; Saragusty, J.; Schaftenaar, W.; Göritz, F.; Schmitt, D.L.; Hildebrandt, T.B. Obstetrics in elephants. Theriogenology 2008, 70, 131–144. [Google Scholar] [CrossRef] [PubMed]
- van der Kolk, J.H.; van Leeuwen, J.P.T.M.; van den Belt, A.J.M.; van Schaik, R.H.N.; Schaftenaar, W. Subclinical hypocalcaemia in captive Asian elephants (Elephas maximus). Veterinary Record 2008, 162, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Van Sonsbeek, G.R.; van der Kolk, J.H.; van Leeuwen, J.P.T.M.; Everts, H.; Marais, J.; Schaftenaar, W. Effect of calcium and cholecalciferol supplementation on several parameters of calcium status in plasma and urine of captive Asian (Elephas maximus) and African elephants (Loxodonta africana). Journal of Zoo and Wildlife Medicine 2013, 44, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Ensley, P.T.; Anderson, M.; Osborn, K.; Bissonnette, S.; Deftos, L. Osteodystrophy in an orphan Asian elephant. In Proceedings of the AAZV, Pittsburgh, Pennsylvania; 1994; pp. 142–143. [Google Scholar]
- Sach, F.; Dierenfeld, E.S.; Langley-Evans, S.C.; Watts, M.J.; Yon, L. African savanna elephants (Loxodonta africana) as an example of a herbivore making movement choices based on nutritional needs. PeerJ 2019, 7, e6260. [Google Scholar] [CrossRef] [PubMed]
- Childs-Sanford, S.E.; Makowski, A.J.; Wakshlag, J.J. The vitamin D status of Asian elephants (Elephas maximus) managed in a northern temperate climate. Journal of Zoo and Wildlife Medicine 2020, 51, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.; Chen, T.C.; Holick, M.F.; Mikota, S.; Dierenfeld, E. Serum concentrations of calcium, phosphorus, and 25-hydroxyvitamin D in captive African elephants (Loxodonta africana). Journal of Zoo and Wildlife Medicine 2009, 40, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Dosi, M.C.M.C.; McGorum, B.C.; Kirton, R.D.; Cillán-García, E.; Mellanby, R.J.; Keen, J.A.; Hurst, E.A.; Morgan, R.A. The effect of season, management and endocrinopathies on vitamin D status in horses. Equine Veterinary Journal 2022, 55, 672–680. [Google Scholar] [CrossRef] [PubMed]
- BioMérieux. Vidas® 25 OH vitamin D total (vitD). 2016, pp. 1–9.
- KNMI/ESA UV index. Available online: https://www.esa.int/Applications/Observing_the_Earth/Thanks_to_ESA_KNMI_offers_a_UV_forecasting_Service (accessed on 22 November 2023).
- Van Sonsbeek, G.R.; van der Kolk, J.H.; van Leeuwen, J.P.T.M.; Schaftenaar, W. Preliminary validation of assays to measure parameters of calcium metabolism in captive Asian and African elephants in Western Europe. Journal of Veterinary Diagnostic Investigation 2011, 23, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Childs-Sanford, S.E.; Makowski, A.J.; Hilliard, R.L.; Wakshlag, J.J. Experimental cholecalciferol supplementation in a herd of managed Asian elephants (Elephas maximus). Journal of Zoo and Wildlife Medicine 2023, 54, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Childs-Sanford, S.E.; Kiso, W.K.; Schmitt, D.L. Serum vitamin D and selected biomarkers of calcium homeostasis in Asian elephants (Elephas maximus) managed at a low latitude. Journal of Zoo and Wildlife Medicine 2024, 55, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Perumbilly, A.J.; Balagangatharathilagar, M.; Kalaivanan, M.; Vishnurahav, R.B.; Begum, M.M. Quantitaive determination of 25-hydroxyvitamin D in adult captive Asian elephants (Elephas maximus) from Southern tropical regions of Tamil Nadu. International Journal of advanced biochemistry research 2024. [Google Scholar] [CrossRef]
- Olds, J.; Oltman, W.; Makowski, M.; Householder, H.; Keeley, L.L. Seasonal variation of serum 25-hydroxy-vitamin D in two captive Eastern black rhinoceros (Diceros bicornis michaeli) housed in a North American zoo. Journal of Zoo and Wildlife Medicine 2018, 49, 943–951. [Google Scholar] [PubMed]
- Clauss, M.; Jessup, D.A.; Norkus, E.B.; Chen, T.C.; Holick, M.F.; Streich, J.; Dierenfeld, E.S. Fat soluble vitamins in blood and tissues of free-ranging and captive rhinoceros. Journal of Wildlife Diseases 2002, 38, 402–413. [Google Scholar] [CrossRef] [PubMed]
- Hurst, E.A.; Homer, N.Z.; Mellanby, J. Vitamin D metabolism and profiling in veterinary species. Metabolites 2020, 10, 371. [Google Scholar] [CrossRef] [PubMed]
- Azarpeykan, S.; Gee, E.K.; Thompson, K.G.; Dittmer, K.E. Undetectable vitamin D3 in equine skin irradiated with ultraviolet light. Journal of Equine Science 2022, 33, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Bapodra, P.; Dierenfeld, E.; Wolfe, B.A. Evaluation of season-related dietary changes on the serum profiles of fat soluble vitamins, mineral, fatty acids, and lipids in the captive greater-one horned rhinoceros (Rhinoceros unicornis). Zoo Biology 2014, 33, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Bruins-van Sonsbeek, L.G.R. Personal observations, 2024.
- Cavaleros, M.; Buffenstein, R.; Ross, F.P.; Pettifor, J.M. Vitamin D metabolism in frugivorous nocturnal mammal, the Egyptian fruit bat (Rousettus aegyptiacus). General and Comparative Endocrinology 2003, 133, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Southworth, L.O.; Holick, M.F.; Chen, T.C.; Kunz, T.H. Effects of sunlight on behaviour and 25-hydroxyvitamin D levels in two species of Old World fruit bats. Dermato-Endocrinology 2013, 5, 192–198. [Google Scholar] [CrossRef] [PubMed]
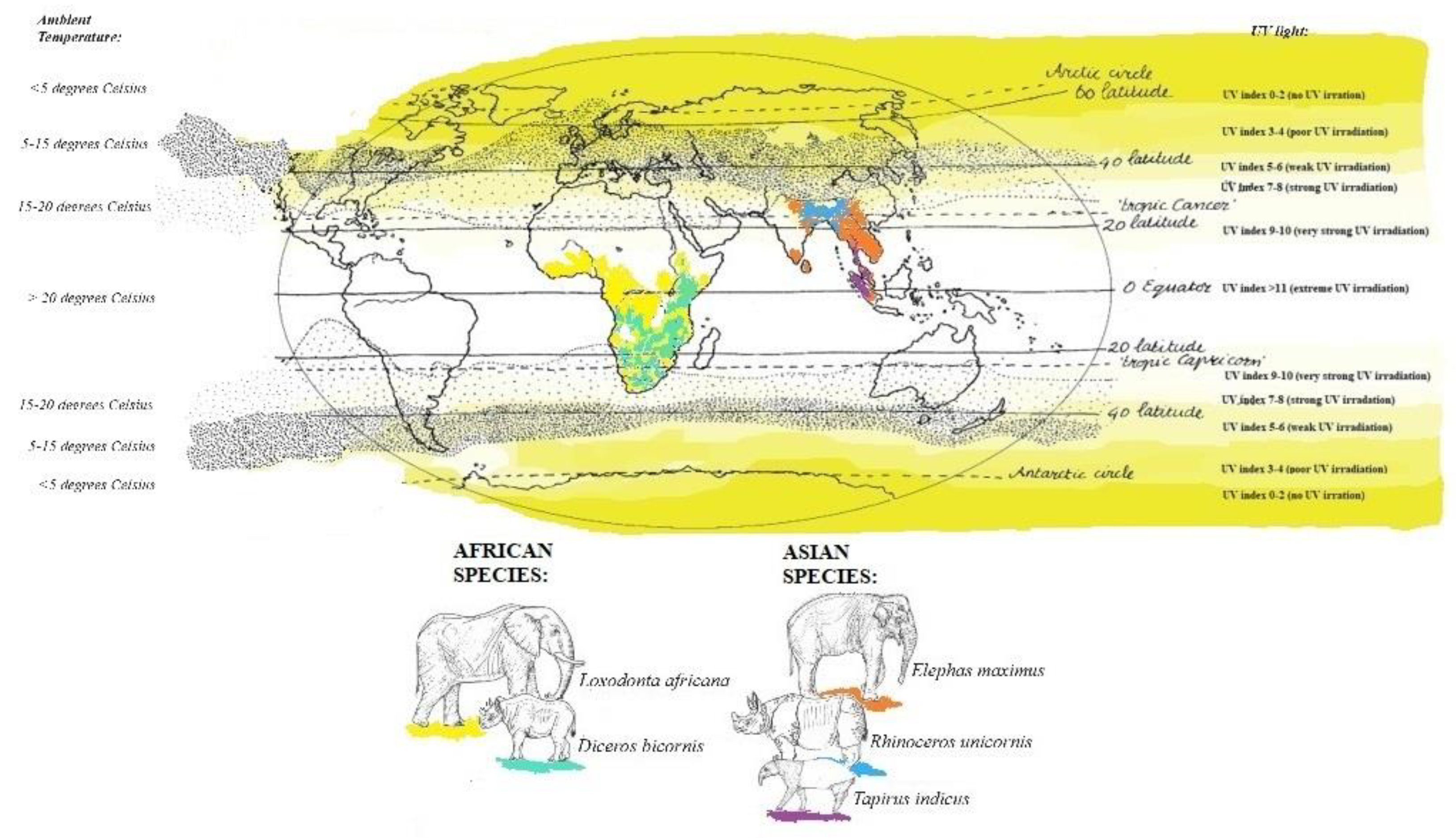

| Zoo* | Individual | Species | Sex | Year of birth (years) |
n | Cholecalciferol intake (IU/kg BW)** |
Recommended vitamin D intake (IU/kg BW) |
| A | 1 | L. Africana | f | 2015 | 9 | 6.5 | 8–121 |
| 2 | L. Africana | f | 1985 | 18 | 6.5 | ||
| 3 | L. Africana | f | 1986 | 18 | 6.5 | ||
| 4 | L. Africana | f | 1992 | 19 | 6.5 | ||
| B | 5 | L. Africana | f | 1993 | 6 | 3.6 | |
| 6 | L. Africana | f | 2007 | 3 | 3.6 | ||
| 7 | L. Africana | f | 2016 | 5 | 3.6 | ||
| 8 | L. Africana | m | 2019 | 6 | 3.6 | ||
| 9 | L. Africana | m | 1993 | 5 | 3.6 | ||
| 10 | L. Africana | f | 1992 | 1 | 3.6 | ||
| C | 11 | E. maximus | f | 1970 | 25 | 5 | 12–151 |
| 12 | E. maximus | f | 2000 | 22 | 5 | ||
| 13 | E. maximus | f | 2003 | 20 | 5 | ||
| 14 | E. maximus | f | 2010 | 25 | 5 | ||
| 15 | E. maximus | m | 2021 | 2 | 5 | ||
| 16 | D. bicornis | m | 2001 | 19 | 2.5-6.6*** | 3-92 | |
| 17 | D. bicornis | f | 2011 | 37 | 2.5-6.6*** | ||
| 18 | D. bicornis | f | 2017 | 22 | 2.5-6.6*** | ||
| 19 | D. bicornis | m | 2020 | 11 | 2.5-6.6*** | ||
| 20 | D. bicornis | m | 2019 | 1 | 2.5-6.6*** | ||
| 21 | R. unicornis | f | 2017 | 4 | 1.5 | - | |
| 22 | T. indicus | f | 2011 | 32 | 10-30**** | 10.53 | |
| 23 | T. indicus | m | 2016 | 8 | 5 |
| Species*** | Mean (±SD) |
Median (range) | Median per season (range) | ||||
| Summer | Winter | ||||||
| Calendar (Apr-Sept) |
UV index >6 (May-July) |
Calendar (Oct-Mar) |
UV index <2 (Nov-Jan) |
||||
|
L. africana* n = 90, 10+ |
34.5 (± 9.0) |
33.2 (21.2-58.8) |
33.5 n = 47, 6 (20.3-43.8) |
31.3 n = 23, 6 (22.0-41.8) |
33.0 n = 30, 6 (20.3-41.3) |
33.3 n = 13, 6 (23.8-41.3) |
|
|
E. maximus n = 94, 5 |
75.3 (± 21.1) |
68.7 (55.5-110.6) |
71.2 n = 48, 4 (32.5-122.0) |
66.5 n = 24, 4 (32.5-112.0) |
60.2 n = 44, 4 (29.0-88.3) |
69.0 n = 21, 4 (46.0-74.8) |
|
|
D. bicornis n = 90, 5 |
126.2 (± 70.2) |
86.3 (72.9-237.0) |
109.0† n = 44, 4 (51.5-251.0) |
118.0† n = 23, 4 (77.5-243.0) |
58.3 n = 45, 4 (23.5-226.0) |
57.9 n = 22, 4 (26.0-111.0) |
|
|
R. unicornis n = 4, 1 |
- | 107.9 (106.3-132.8) |
109.5 (105.3-132.8) |
121.2 (109.5-132.8) |
106.3 |
106.3 | |
|
T. indicus** n = 26, 2 |
- | < 20.3 (< 20.3-33.5) |
< 20.3 (< 20.3-< 20.3) |
< 20.3 (< 20.3-< 20.3) |
20.3 (< 20.3-33.5) |
20.3 (< 20.3-33.5) |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





