Submitted:
23 August 2024
Posted:
26 August 2024
You are already at the latest version
Abstract
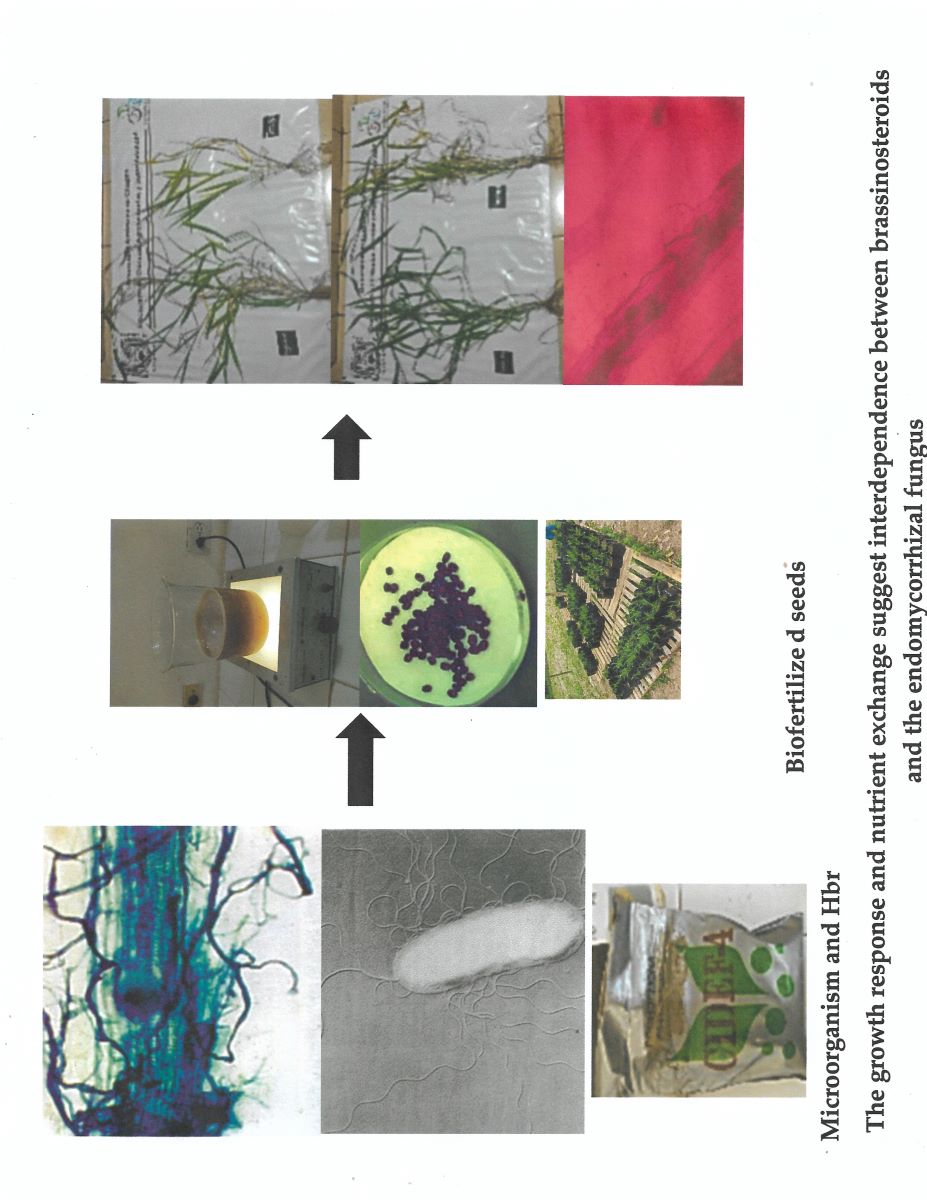
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Area Location
2.2. Edaphoclimatic Conditions of the Study Area
2.3. Biofertilizers and Homobrassinolide
2.4. Experiment Setup and Application of Microorganisms and Homobrassinolide
2.5. Variables Evaluated
2.6. Statistical Analysis
3. Results
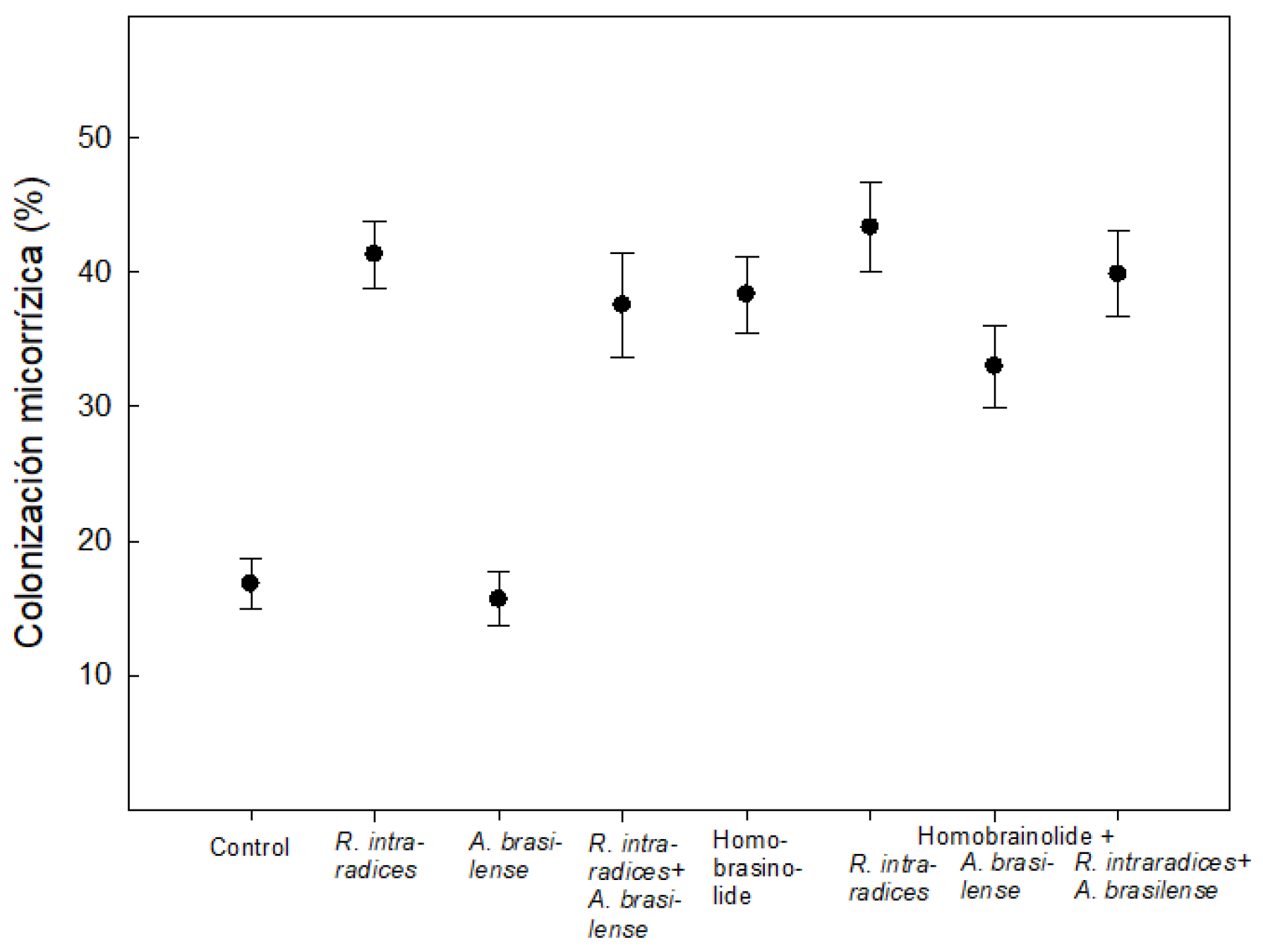
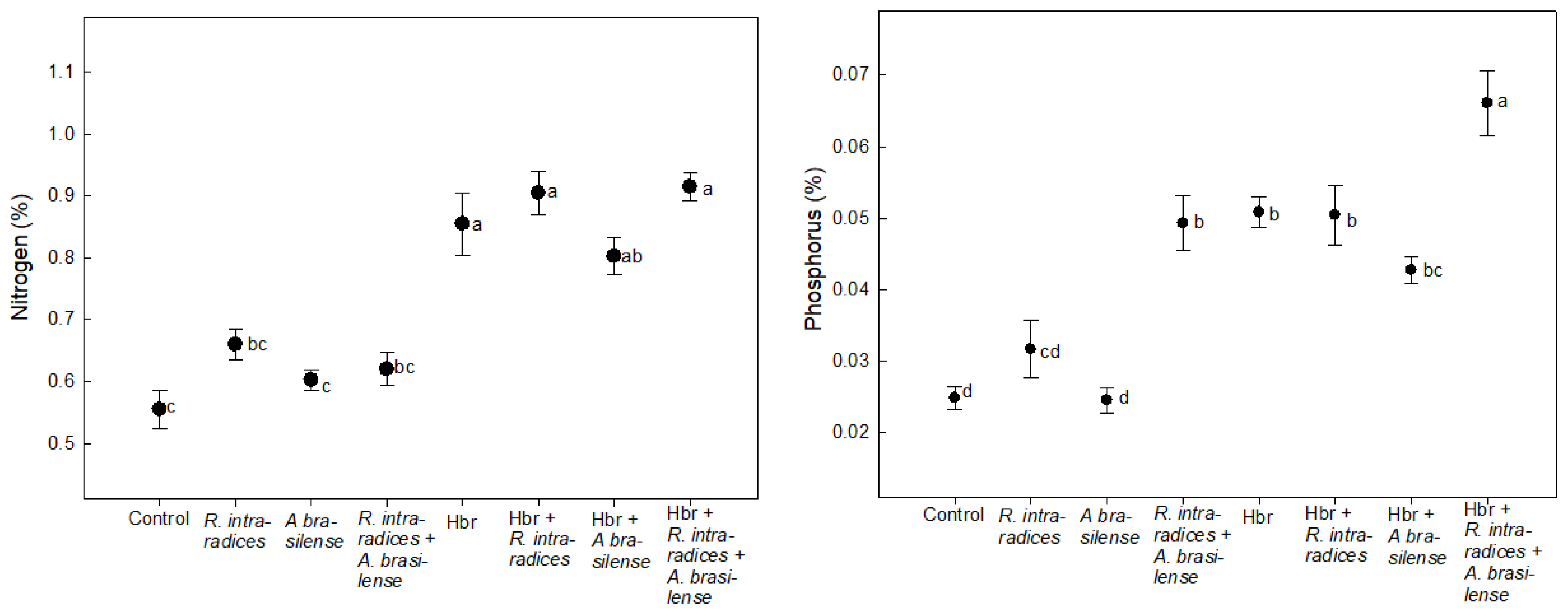
3.1. Morphological and Physiological Yield Components
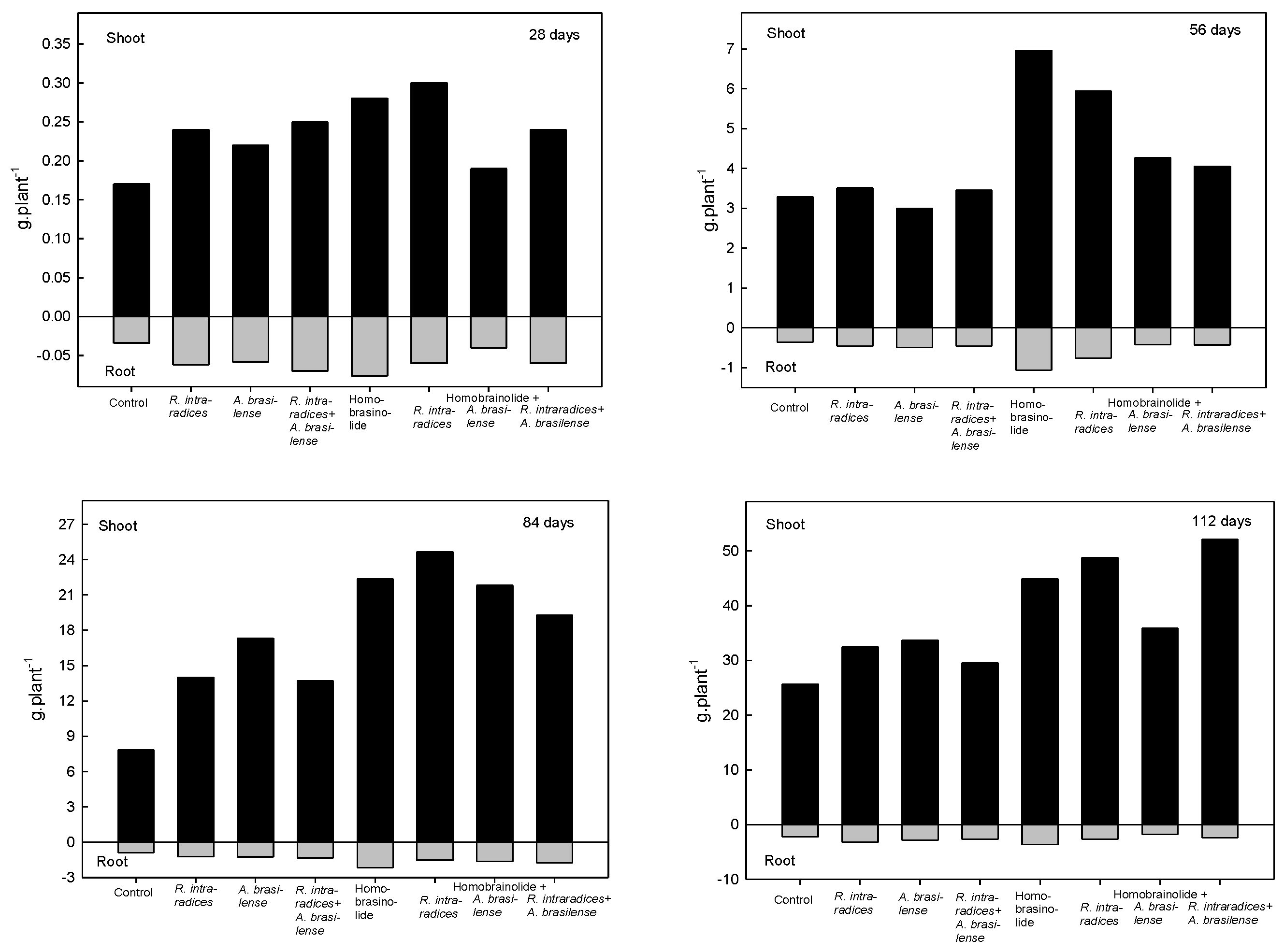
3.2. Biomass Allocation in Shoot and Root
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramírez, R.O.; Hernández-Garay, A.; Da Silva, S.C.; Pérez, P.J.; Enríquez, Q.J.F.; Quero, C.A.R.; et al. Acumulación de forraje, cre-cimiento y características estructurales del pasto Mombaza (Panicum maximum Jacq. Jacq.) cosechado a diferentes intervalos de corte. Téc Pecu Méx. 2009, 47(2), pp. 203-213.
- Redecker, D.; Kodner, R.; Graham, L.E. Glomalean fungi from the Ordovician. Science. 2000, 289 (5486):1920-1. [CrossRef] [PubMed]
- Strack, D.; Fester, T.; Hause, B.; Schliemann, W.; Walter, M.H. Arbuscular mycorrhiza: biological, chemical and molecular aspects, J. Chem. Ecol., 2003, 29, pp. 1955-1979. [CrossRef]
- Aroca, R.; Porcel, R.; Ruiz-Lozano, J.M. Plant drought tolerance enhancement by arbuscular mycorrhizal symbiosis. In Mycorrhizal Fungi, Fulton, S.M. Ed.; Nova Science Publishers lnc, New York, U.S.A. 2011; pp 229–240.
- Dohroo, A. Azospirillum- a potent Biofertilizer in Agriculture. Global Scientific and Academic Research Journal of Multidisciplinary Studies. 2024, 3(2), pp. 8-11. https://gsarpublishers.com/wp-content/uploads/2024/02/GSARJMS192024-Gelary-script.pdf.
- Aguirre-Medina, J.F.; Cadena-Iñiguez, J.; Aguirre-Cadena, J.F. Influence of Endomycorrhizal Fungi on the Growth of Tropical Plant Species. In Mycorrhizal Fungi-Utilization in Agriculture and Industry. R. Radhakrishnan (Ed) IntechOpen. London, England, 2020. [CrossRef]
- Prieto Benavides, O.; Belezaca Pinargote, C.; Mora Silva, W.; Vallejo Zambrano, E.; Gutiérrez Lara1, V.; Pinargote, Mendoza, E. Inoculación de Brachiaria decumbens con hongos formadores de micorriza arbuscular nativos del trópico húmedo ecuatoriano. Ciencia y Tecnología 2011, 4(2), pp. 9-18. file:///C:/Users/franc/Downloads/Dialnet-InoculacionDeBrachiariaDecumbensConHongosFormadore-4149411.pdf.
- Lozano-Contreras, M.G.; Rivas-Pantoja, F.; Castillo-Huchim, J. E. Crecimiento de plántulas de Brachiaria brizantha en respuesta a la aplicación de hongos micorrizógenos y bacterias diazotróficas Pastos y Forrajes, 2013, 36(2), 227-232. http://scielo.sld.cu/scielo.php?script=sci_abstract&pid=S0864-03942013000200007.
- Fariduddin, Q.; Yusuf, M.; Ahmad, I.; Ahmad, A. Brassinosteroids and their role in response of plants to abiotic stresses. Biol. Plant. 2014, 58 (1), pp. 9-17. [CrossRef]
- Zhu, F., Yun, Z., Ma, Q., Gong, Q., Zeng, Y., Xu, J., Cheng, Y., Deng, X. Effects of exogenous 24-epibrassinolide treatment on posthar-vest quality and resistance of Satsuma mandarin (Citrus unshiu), Postharvest Biol. Technol, 2015, 100, pp. 8-15. [CrossRef]
- Zebosi, B.; Vollbrecht, E.; Bes, N.B. Conservation and diversification of genes regulating brassinosteroid biosynthesis and signal-ing. bioRxiv 2024 . [CrossRef]
- Hause, B.; Mrosk, C.; Isayenkov, S.; Strack, D. Jasmonates in arbuscular mycorrhizal interactions. Phytochemistry 2007, 68, pp.101–110. [CrossRef] [PubMed]
- Hansch, F.; Jaspar, H.; von Sivers, L.; Bitterlich, M.; Franken, P.; Kühn, C. Brassinosteroids and sucrose transport in mycorrhizal tomato plants, Plant Signal. Behav 2020, 15:2, 1714292. [CrossRef]
- García, A.E. Modificaciones al sistema de clasificación climática de Köppen (para adaptarlo a las condiciones de la República Mexicana). 5th Ed.; Instituto de Geografía and Universidad Nacional Autónoma de México, México. 2004; 90 p. http://www.publicaciones.igg.unam.mx/index.php/ig/catalog/book/83.
- Phillips, J.M.; Hayman, D.S. Improved Procedures for Clearing Roots and Staining Parasitic Vesicular-Arbuscular Mycorrhizal Fungi for Rapid Assessment of Infection. Trans. Br. Mycol. Soc., 1970, 55, pp. 158-161. [CrossRef]
- Milthorpe, F.L.; Moorby, J. Introducción a la fisiología de los cultivos. Ed. Hemisferio Sur, Buenos Aires, Argentina; 1982. 259 p.
- SAS (Statistical Analysis System) (1999-2000) SAS/STAT User’S Guide: Ver 8.1. SAS Institute Inc., Cary NC.SAS 1999-2000.
- Jäderlund, L.; Arthurson, V.; Granhall, U.; Jansson, J.K. Specific interactions between arbuscular mycorrhizal fungi and plant growth-promoting bacteria: as revealed by different combinations. FEMS Microbiol. Lett. 2008, 287 (2), pp. 174–180. [CrossRef]
- Wright, S.F. Management of Arbuscular Mycorrhizal Fungi. In Roots and Soil Management: Interactions between Roots and the Soil, Zobel, R.W.; Wright, S.F. Eds., American Society of Agronomy, Crop Science Society of America, Soil Science Society of Ameri-ca, Madison, USA, 2005; pp. 183-197.
- von Sivers, L.; Jaspar, H.; Johst, B.; Roese, M.; Bitterlich, M.; Franken, P.; Kühn, C. Brassinosteroids Affect the Symbiosis Between the AM Fungus Rhizoglomus irregularis and Solanaceous Host Plants. Front. Plant Sci. 2019 10:571. [CrossRef]
- Tofighi, C.; Khavari-Nejad, R.A.; Najafi, F.; Razavi, K.; Rejali, F. Responses of wheat plants to interactions of 24-epibrassinolide and Glomus mosseae in saline condition. Physiol. Mol. Biol. Plants 2017, 23, pp. 557–564. [CrossRef] [PubMed]
- Herrera Aguilar, J.; Aguirre Medina, J. F.; Gálvez López, A. L.; Ley de Coss, A.; Martínez Solís, M. Efecto de reguladores de creci-miento en la reproducción in vitro de Musa spp cv gran enano. Agroproductividad 2017, 10 (9), pp. 20-25. http://www.colpos.mx/wb/index.php/agroproductividad#.WnoBa1TibIU.
- González-Olmedo, J.L.; Córdova, A.; Aragón, C. E.; Pina, D.; Rivas, M.; Rodríguez, R. Efecto de un análogo de brasinoesteroides sobre plántulas de FHIA-18 expuestas a un estrés térmico. InfoMusa. 2005, 14 (1), pp. 18-20. https://www.researchgate.net/publication/311736418_Efectos_de_un_analogo_de_brasinoesteroides_sobre_plantulas_de_FHIA-18_expuestas_a_un_estres_termico_InfoMusa_14_1_18-21_2005.
- Zamora Olivo, M.A.; Aguirre Medina, J.F.; Cano García. M.A.; Martínez-Tinajero, J.J. Productividad de Brachiaria brizantha (Hochst. ex A. Rich) y Clitoria ternatea L. con biofertilizantes. Agroproductividad 2013, 6(6), pp. 23-29. http://www.colpos.mx/wb/index.php/agroproductividad#.VB9GcpR5N8E.
- Artursson, V.; Finlay, R.D.; Jansson, J.K. Interactions between arbuscular mycorrhizal fungi and bacteria and their potential for stimulating plant growth. Environ. Microbiol. 2006, 8(1), pp. 1–10. [CrossRef]
- Leigh, J.; Hodge, A.; Fitter, A. H. Arbuscular mycorrhizal fungi can transfer substantial amounts of nitrogen to their host plant from organic material. New Phytol 2009, 181, pp. 199–207. [CrossRef] [PubMed]
- Posta, K.; Duc, H.N. Benefits of arbuscular mycorrhizal fungi application to crop production under water scarcity. In drought - detection and solutions. G. Ondrasek (Ed.) IntechOpen. London, England, 2020. [CrossRef]
- González, P.J.; Plana, R.; Rivera, R.; Fernández, F.; Arbola, J. Efectos de la inoculación de hongos micorrízicos arbusculares en pastos del género Brachiaria, cultivados en suelo Pardo Mullido. Rev. cuba. cienc. agríc, 2008, 42(1), pp. 101-106. https://www.redalyc.org/pdf/1930/193015413016.pdf.
- Howeler, R.H.; Sieverding, E.; Saif, S.R. Practical aspects of mycorrhizal technology in some tropical crops and pastures. Plant and Soil 1987, 100, pp. 249-283. https://www.jstor.org/stable/42939116.
- Ibarra-Puón, J.C.; Aguirre-Medina, J.F.; Ley-De Coss, A.; Cadena-Iñiguez, J.; Zavala-Mata A. Inoculación de Coffea canephora (Pierre) ex Froehner con Rhizophagus intraradices (Schenck et Sm.) Walker et Schuessler y Azospirillum brasilense Tarrand, Krieg et Döbereiner en vivero. Rev Chapingo Ser Hortic, 2014, 20(2), pp. 201-213. [CrossRef]
- Aguirre-Medina, J.F.; Mendoza-López, A.; Cadena-Iñiguez, J.; Avendaño-Arrazate, C.H. La Biofertilización del cacao (Theobroma cacao L.) en vivero con Azospirillum brasilense Tarrand, Krieg et Döbereiner y Glomus intraradices Schenk et Smith. Interciencia 2007, 32 (8), pp. 541-546. http://ve.scielo.org/scielo.php?pid=S0378-18442007000800010&script=sci_abstract&tlng=es.
- Izquierdo, H.; Núñez, M.; González, M.; Proenza, R. Efectos de la aplicación de un análogo espirostánico de brasinoesteroides en vitroplantas de banano (Musa spp.) durante la fase de aclimatización. Cult. trop. 2012, 33, pp. 71-76. https://www.medigraphic.com/cgi-bin/new/resumen.cgi?IDARTICULO=73722.
- Terry, A.E.; Ruiz, P.J.; Tejeda, P.T.; Reynaldo, E.I.; Díaz, M.M. Respuesta del cultivo de la lechuga (Lactuca sativa l.) a la aplicación de diferentes productos bioactivos. Cult. trop, 2011, 32(1), pp. 77-82. https://www.redalyc.org/pdf/1932/193222352010.pdf.
- Aguirre-Medina, J. F.; Gálvez-López, A. L.; Ibarra-Puón, J. C. Growth of Leucaena leucocephala (Lam.) de Wit biofertilized with arbuscular mycorrhizal fungi in the nursery. Rev. Chapingo, Ser. Cienc. For. y del Ambient, 2018, 24(1), pp. 49-58. [CrossRef]
- Doubková P, Vlasáková E, Sudová R. Arbuscular mycorrhizal symbiosis alleviates drought stress imposed on Knautia arvensis plants in serpentine soil. Plant Soil 2013, 370, pp. 149–161. [CrossRef]
- Cruz, R, de Sousa,; Vieira Araújo, F.H.; Cabral França, A.; Tadin Sardinha, L.; Miranda Machado, C.M. Physiological responses of Coffea arabica cultivars in association with arbuscular mycorrhizal fungi. Coffee Sci. 2020. 15, e151641. [CrossRef]
- Jiménez Terry, F.A.; Ramírez Aguilar, D.; Agramonte Peñalver, D. Empleo del BIOBRAS-6 en la micropropagación del cultivar de plátano FHIA21 (AAAB). Biotecnol. veg., 2002, 2 (3), pp. 131-136. https://revista.ibp.co.cu/index.php/BV/rt/printerFriendly/171/html.
- Izquierdo Oviedo, H.; González Cepero, M.C.; Núñez Vázquez, M. de la C.; Proenza Llerena, R.; Cabrera Pino, J.C. Biological activity of biobras and Pectimorf-6 in each of the phases of micropropagation of banana (Musa sp.). J. Research Biol. 2017, 7(3), pp. 2231-2247. file:///C:/Users/franc/Downloads/Biological_activity_of_biobras_and_Pecti%20(1).pdf.
- Neetu, N.; Ashok, A.; Anju, T.; Alpa, A. Influence of arbuscular mycorrhizal fungi and Pseudomonas fluorescens at different su-perphosphate levels on linseed (Linum usitatissimum L.) growth response. Chil. J. Agric. Res. 2012, 72 (2), pp. 237-243.
- Singh, R.; Pandey, D. K.; Kumar, A.; Singh, M. PGPR isolates from the rhizosphere of vegetable crop Momordica charantia: char-acterization and application as biofertilizer. Int J Curr Microbiol Appl Sci, 2017, 6(3), pp. 1789-1802. [CrossRef]
- Desmet, S.; Saeys, Y.; Verstaen, K.; Dauwe, R.; Kim, H.; Niculaes, C.; Fukushima, A.; Goeminne, G.; Vanholme, R.; Ralph, J.; Boer-jan, W.; Morreel, K. Maize specialized metabolome networks reveal organ-preferential mixed glycosides. Comput Struct Biotechnol J. 2021, 19, pp. 1127–1144. [CrossRef]
- Larose, G.; Chênevert, R.; Moutoglis, P.; Gagné, S.; Piché, Y.; Vierheilig, H. Flavonoid levels in roots of Medicago sativa are mod-ulated by the developmental stage of the symbiosis and the root colonizing arbuscular mycorrhizal fungus. J. Plant Physiol. 2002, 159, pp. 1329–1339. [CrossRef]
- Ruiz-Lozano, J.M.; Perálvarez, M.C.; Aroca, R,; Azcón, R. The application of a treated sugar beet waste residue to soil modifies the responses of mycorrhizal and non mycorrhizal lettuce plants to drought stress. Plant Soil 2012, 346, pp. 153–166. [CrossRef]
- Lou, S.; Jiang, H.; Li, J.; Tian, L.; Du, M.; Ma, T.; Zhang, L.; Zhang, P. Effects of Exogenous Brassinosteroid and Reduced Leaf Source on Source–Sink Relationships and Boll Setting in Xinjiang Cotton. Agronomy 2024, 14, 1168. [CrossRef]
- Zhang, X.; Sun, S.; Nie, X.; Boutté, Y.; Grison, M.; Li, P.; Kuang, S.; Men, S. Sterol Methyl Oxidases Affect Embryo Development via Auxin-Associated Mechanisms. Plant Physiol. 2016, 171, pp. 468-482. [CrossRef]
- Liu, C.Y.; Zhang, F.; Zhang, D.J.; Srivastava, A. K.; Wu, Q-S.; Zou, Y-N. Mycorrhiza stimulates root-hair growth and IAA synthesis and transport in trifoliate orange under drought stress. Sci Rep 2018, 8, 1978. [CrossRef] [PubMed]
- Dhiman, M.; Sharma, L.; Kaushik, P.; Singh, A.; Sharma, M.M. Mycorrhiza: An Ecofriendly Bio-Tool for Better Survival of Plants in Nature. Sustainability 2022, 14, 10220. [CrossRef]
- Liao, D.; Wang, S.; Cui, M.; Liu, J.; Chen, A.; Xu, G. Phytohormones regulate the development of Arbuscular mycorrhizal symbio-sis. Int. J. Mol. Sci. 2018, 19, 3146. [CrossRef]
- Rao, A.V.; Tak, R. Growth of different tree species and their nutrient uptake in limestone mine spoil as influenced by Arbuscular mycorrhizal (AM)-fungi in Indian arid zone. J. Arid Environ. 2002, 51, pp. 113–119. [CrossRef]
- He J.D.; Chi G.G.; Zou Y.N.; Shu B.; Wu Q.S. Srivastava A.K., Kuča K. (2020): Contribution of glomalin-related soil proteins to soil organic carbon in trifoliate orange. Appl. Soil Ecol., 2020, 154: 103592. [CrossRef]
- Powell, J.R.; Rillig, M.C. Biodiversity of arbuscular mycorrhizal fungi and ecosystem function. New Phytol. 2018, 220, pp. 1059–1075. [CrossRef]
- Altomare, C.; Tringovska, I. Beneficial Soil Microorganisms, an Ecological Alternative for Soil Fertility Management. In: Licht-fouse E. (eds). Genetics, Biofuels and Local Farming Systems. Sustainable Agriculture Reviews, Springer, Dordrecht, 2011, 7. pp. 161–214. [CrossRef]
- Kanno, T.; Saito, M.; Ando, Y.; Macedo, M. C. M.; Nakamura, T.; Miranda, C. H. B. Importance of indigenous arbuscular mycor-rhiza for growth and phosphorus uptake in tropical forage grasses growing on an acid, infertile soil from the Brazilian savannas. Trop. grassl, 2006, 40, pp. 94–101. Retrieved from http://www.tropicalgrasslands.info/public/journals/4/Historic/Tropical%20Grasslands%20Journal%20archive/PDFs/Vol_40_2006/Vol_40_02_20.
- Mathur, S.; Sharma, M.P.; Jajoo, A. Improved photosynthetic efficacy of maize (Zea mays) plants with Arbuscular mycorrhizal fungi (AMF) under high temperature stress. J. Photochem. Photobiol. Biol. 2018, 180, pp. 149–154. [CrossRef] [PubMed]
- Otie, V.; Ibrahim, A.; Udo, I.; Kashiwagi, J.; Matsuura, A.; Shao, Y.; Itam, M.; An, P.; Eneji, A.E. Foliarly applied 2,4- Epibrassino-steroid modulates the electrical conductivity of the saturated rhizospheric soil extracts of soybean under salinity stress. Plants 2022, 11, 2330. [CrossRef]
- Aguirre-Medina, J.F. 2006. Biofertilizantes microbianos: su aplicación en la agricultura. Folleto Técnico Núm. 1. Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias. Centro de Investigación Regional Pacifico Sur. Campo Experimental Rosario Izapa. Chiapas, México. 1, 24 p.
- Devi, S. H.; Bhupenchandra, I.; Sinyorita, S.; Chongtham, S.; Devi, E. L. Mycorrhizal Fungi and Sustainable Agriculture. In Nitro-gen in Agriculture -Physiological, Agricultural and Ecological Aspects. T. Ohyama, & K. Inubushi (Eds.). IntechOpen, London, England. 2021. [CrossRef]
- França, A. C.; de Freitas A.N.; dos Santos, A. E.; Grazziotti, P.H.; de Andrade Júnio, V. C. Mycorrhizal fungi increase coffee plants competitiveness against Bidens pilosa interference. Pesqui. Agropecu. Trop, 2016, 46(2), pp.132-139. [CrossRef]
- Aguirre Medina, J.F.; Culebro Cifuentes, F.; Cadena Iñiguez, H.; Aguirre Cadena, J.F. Crecimiento de Tabebuia Donnell-Smithii (Rose) Inoculada con Hongos Micorrizicos y Azospirillum brasilense. Agrociencia. 2014, 48 (3), pp. 331-345. http://www.colpos.mx/agrocien/agrociencia.htm.
- Jacott, C.N.; Murray, J.D.; Ridout, C.J. Trade-offs in arbuscular mycorrhizal symbiosis: Disease resistance, growth responses and perspectives for crop breeding. Agronomy 2017, 7(4), 75. [CrossRef]
- Steenhoudt, O.; Vanderleyden, J. Azospirillum, a free living nitrogen-fixing bacterium closely associated with grasses: genetic, biochemical and ecological aspects. FEMS Microbiol. Rev. 2000, 24 (4), pp. 487–506. [CrossRef] [PubMed]
- Jefwa, J.M.; Sinclair, R.W.; Maghembe, J.A. Diversity of Glomale mycorrhizal fungi in Maize/Sesbania intercrops and maize monocrop systems in Southern Malawi. Agrofor. Syst. 2004, 67, pp. 107-114. [CrossRef]
- Perreta, M.G.; Vegetti, A.C. Patrones estructurales en las plantas vasculares: Una Revisión. Gayana Bot. 2005, 62 (1), pp. 9-19. [CrossRef]
- Adolfsson, L.; Solymosi, K.; Andersson, M. X.; Keresztes, Á.; Uddling, J.; Schoefs, B.; Spetea, C. (2015) Mycorrhiza symbiosis in-creases the surface for sunlight capture in Medicago truncatula for better photosynthetic production. PLoS ONE, 10:e0115314. [CrossRef]
- Baum, C.; Toljander, Y.K.; Eckhardt, K.U.; Weih, M. The significance of host-fungus combinations in ectomycorrhizal symbioses for the chemical quality of willow foliage. Plant Soil 2009, 323, pp. 213–224. [CrossRef]
- Tang, H.; Hassan, M.U.; Feng, L.; Nawaz, M.; Shah, A.N.; Qari, S.H.; Liu, Y.; Miao, J. The critical role of arbuscular mycorrhizal fungi to improve drought tolerance and nitrogen use efficiency in crops. Front. Plant Sci. 2022, 13, 919166. [CrossRef] [PubMed]

| Time (days) | Treatment | Height (cm.plant-1) |
Leaves (Number.plant-1) |
Stems (Number.plant-1) |
Stem diameter*** (mm.plant-1) |
| 28 | Control | 14.2 c** | 3.8 c | 2.4 b | 2.0 ab |
| R. intraradices | 15.8 bc | 4.6 abc | 3.2 ab | 2.0 ab | |
| A. brasilense | 15.0 bc | 4.8 ab | 2.8 b | 1.9 ab | |
| R. intraradices+A. brasilense | 16.8 ab | 4.4 bc | 3.0 b | 2.0 ab | |
| Hbr* | 15.8 bc | 5.4 a | 3.8 a | 2.2 a | |
| Hbr + R. intraradices | 18.4 a | 5.4 a | 3.0 ab | 2.3 a | |
| Hbr + A. brasilense | 18.2 a | 4.8 ab | 2.8 b | 1.7 b | |
| Hbr + R. intraradices + A. brasilense | 15.2 bc | 5.0 ab | 2.6 b | 2.0 ab | |
| CV (%) | 5.5 | 9.9 | 14.1 | 8.7 | |
| 56 | Control | 36.6 e | 14.4 f | 3.8 bc | 2.4 a |
| R. intraradices | 47.2 d | 21.0 d | 3.6 c | 2.5 a | |
| A. brasilense | 49.6 bcd | 16.2 ef | 3.8 bc | 2.4 a | |
| R. intraradices+A. brasilense | 46.2 d | 18.0 e | 4.0 bc | 2.5 a | |
| Hbr | 47.6 cd | 32.2 a | 5.0 a | 2.6 a | |
| Hbr + R. intraradices | 58.4 a | 29.2 b | 4.6 ab | 2.5 a | |
| Hbr + A. brasilense | 55.2 abc | 23.8 c | 4.2 abc | 2.6 a | |
| Hbr + R. intraradices + A. brasilense | 56.4 ab | 24.8 c | 3.8 c | 2.6 a | |
| CV (%) | 7.7 | 4.5 | 11.8 | 7.0 | |
| 84 | Control | 61.6 c | 29.6 d | 3.8 d | 2.5 b |
| R. intraradices | 66.8 b | 47.0 c | 4.6 cd | 2.6 b | |
| A. brasilense | 68.8 b | 55.2 bc | 4.6 cd | 2.5 b | |
| R. intraradices+ A. brasilense | 67.6 b | 53.6 bc | 5.4 bc | 2.5 b | |
| Hbr | 72.6 a | 70.2 a | 6.8 a | 2.6 b | |
| Hbr + R. intraradices | 69.4 b | 68.8 a | 5.6 bc | 2.9 a | |
| Hbr + A. brasilense | 69.4 b | 57.6 b | 6.4 ab | 2.7 b | |
| Hbr + R. intraradices + A. brasilense | 72.6 a | 55.0 cb | 6.0 ab | 2.9 a | |
| CV (%) | 2.2 | 7.9 | 10.1 | 2.9 | |
| 112 | Control | 65.4 d | 82.0 c | 5.0 d | 2.6 b |
| R. intraradices | 71.8 c | 98.4 b | 5.8 cd | 2.6 ab | |
| A. brasilense | 73.6 c | 99.2 b | 5.6 cd | 2.7 ab | |
| R. intraradices+ A. brasilense | 76.2 bc | 94.0 b | 6.0 bcd | 2.6 b | |
| Hbr | 82.4 a | 114.8 a | 9.6 a | 2.8 ab | |
| Hbr + R. intraradices | 81.6 a | 124.4 a | 8.8 a | 2.9 a | |
| Hbr + A. brasilense | 82.6 a | 95.4 b | 6.6 bc | 2.7 ab | |
| Hbr + R. intraradices + A. brasilense | 80.8 ab | 122.0a.6 | 7.2 b | 2.8 ab | |
| CV (%) | 3.4 | 5.2 | 8.9 | 5.2 |
| dry weight (g.plant-1) | Leaf area (cm2.plant-1) | |||||
| Time (days) | Treatment | Root | Leaves | Stem | ||
| 28 | Control | 0.034 c** | 0.092 d | 0.044 e | 27.2 c | |
| R. intraradices | 0.062 b | 0.122 b | 0.068 abc | 38.6 b | ||
| A. brasilense | 0.064 b | 0.104 cd | 0.054 de | 41.3 b | ||
| R. intraradices+A. brasilense | 0.070 ab | 0.120 cd | 0.066 bc | 41.6 b | ||
| Hbr* | 0.076 a | 0.128 b | 0.078 a | 53.8 a | ||
| Hbr + R. intraradices | 0.060 b | 0.154 a | 0.076 ab | 61.3 a | ||
| Hbr + A. brasilense | 0.040 c | 0.090 d | 0.064 cd | 35.3 b | ||
| Hbr + R. intraradices + A. brasilense | 0.060 b | 0.112 bc | 0.068 abc | 27.2 c | ||
| CV (%) | 9.7 | 7.1 | 8.8 | 8.9 | ||
| 56 | Control | 0.35 d | 1.92 de | 1.37 d | 285.2 d | |
| R. intraradices | 0.40 cd | 2.00 cde | 1.50 cd | 301.8 d | ||
| A. brasilense | 0.49 c | 1.65 e | 1.34 d | 537.4 a | ||
| R. intraradices+A. brasilense | 0.46 c | 2.00 cde | 1.45 cd | 581.0 a | ||
| Hbr | 1.06 a | 3.91 a | 3.02 a | 469.9 b | ||
| Hbr + R. intraradices | 0.77 b | 3.12 b | 2.61 b | 445.2 bc | ||
| Hbr + A. brasilense | 0.42 cd | 2.53 c | 1.73 c | 456.7 bc | ||
| Hbr + R. intraradices + A. brasilense | 0.42 cd | 2.38 cd | 1.67 cd | 399.1 c | ||
| CV (%) | 8.1 | 11.1 | 9.8 | 6.9 | ||
| 84 | Control | 0.88 e | 4.18 e | 3.65 d | 537.38 f | |
| R. intraradices | 1.23 d | 7.07 d | 6.93 c | 621.13 ef | ||
| A. brasilense | 1.24 d | 8.69 c | 8.63 b | 928.17 d | ||
| R. intraradices+ A. brasilense | 1.32 cd | 7.06 e | 6.64 c | 719.11 e | ||
| Hbr | 2.15 a | 10.00 b | 11.55 a | 1055.43 c | ||
| Hbr + R. intraradices | 1.52 bcd | 12.14 a | 12.52 a | 1427.05 a | ||
| Hbr + A. brasilense | 1.63 bc | 10.51 b | 11.29 a | 1254.99 b | ||
| Hbr + R. intraradices + A. brasilense | 1.75 b | 10.01 b | 9.28 b | 1093.19 c | ||
| CV (%) | 11.3 | 6.0 | 9.2 | 5.7 | ||
| 112 | Control | 2.20 e | 10.73 c | 14.94 ed | 151.18 c | |
| R. intraradices | 3.21 b | 12.92 b | 19.47 d | 174.73 c | ||
| A. brasilense | 2.86 c | 13.41 b | 20.26 cd | 169.85 c | ||
| R. intraradices+ A. brasilense | 2.61 cd | 12.71 bc | 16.79 e | 171.86 c | ||
| Hbr | 3.64 a | 18.26 a | 26.63 b | 288.16 a | ||
| Hbr + R. intraradices | 2.69 cd | 19.85 a | 28.92 b | 274.60 a | ||
| Hbr + A. brasilense | 1.80 f | 13.58 b | 22.26 c | 206.41 c | ||
| Hbr + R. intraradices + A. brasilense | 2.44 de | 19.32 a | 32.79 a | 2812.44 a | ||
| CV (%) | 6.0 | 6.4 | 4.9 | 6.4 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




