Submitted:
24 August 2024
Posted:
26 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
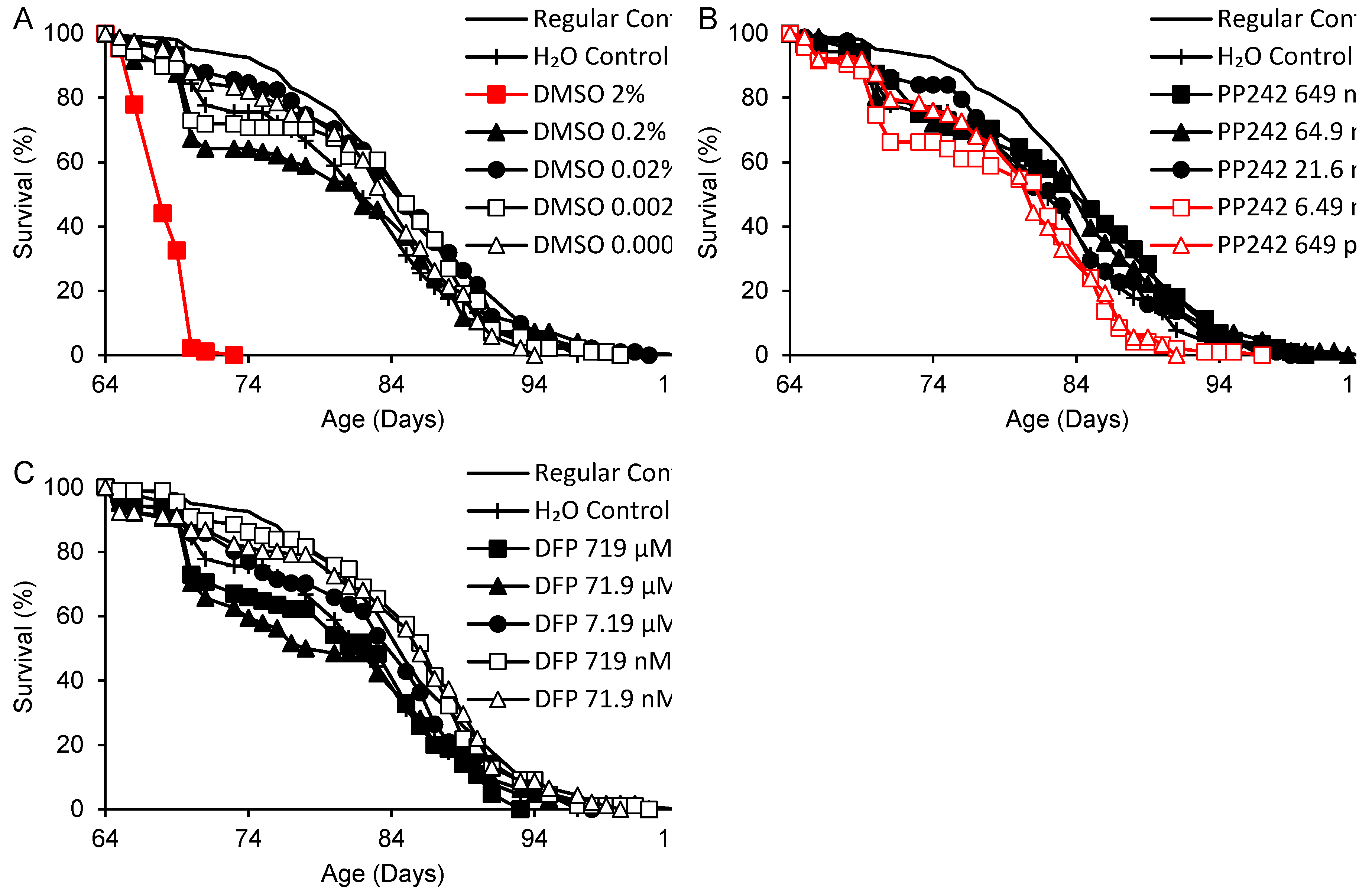
2.1. Longevity Effects of DMSO, PP242 and Deferiprone Supplementation Late in Adult Life
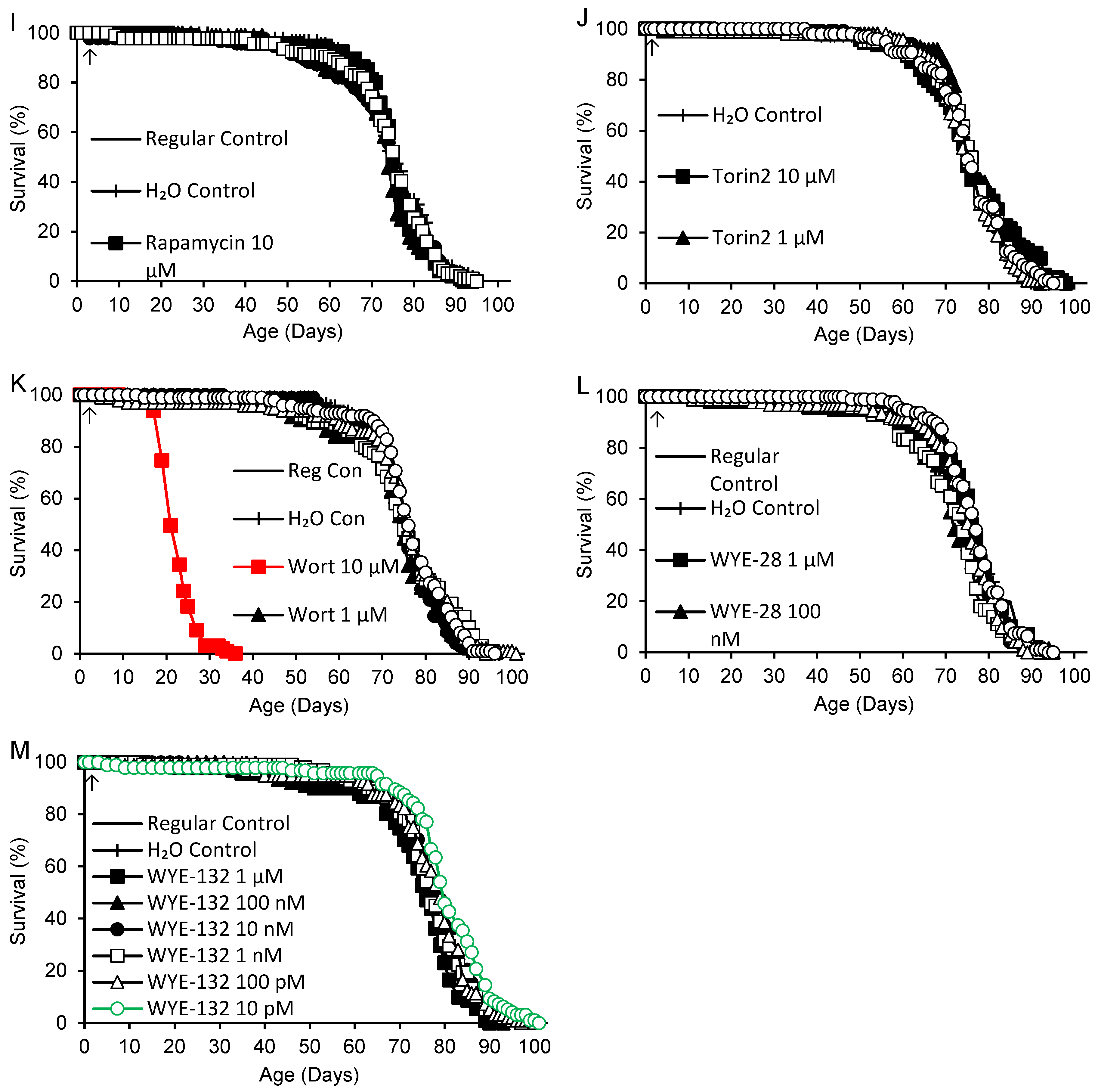
2.2. Longevity Effects of Spermidine and TOR/PI3K Inhibitors – Screening Study
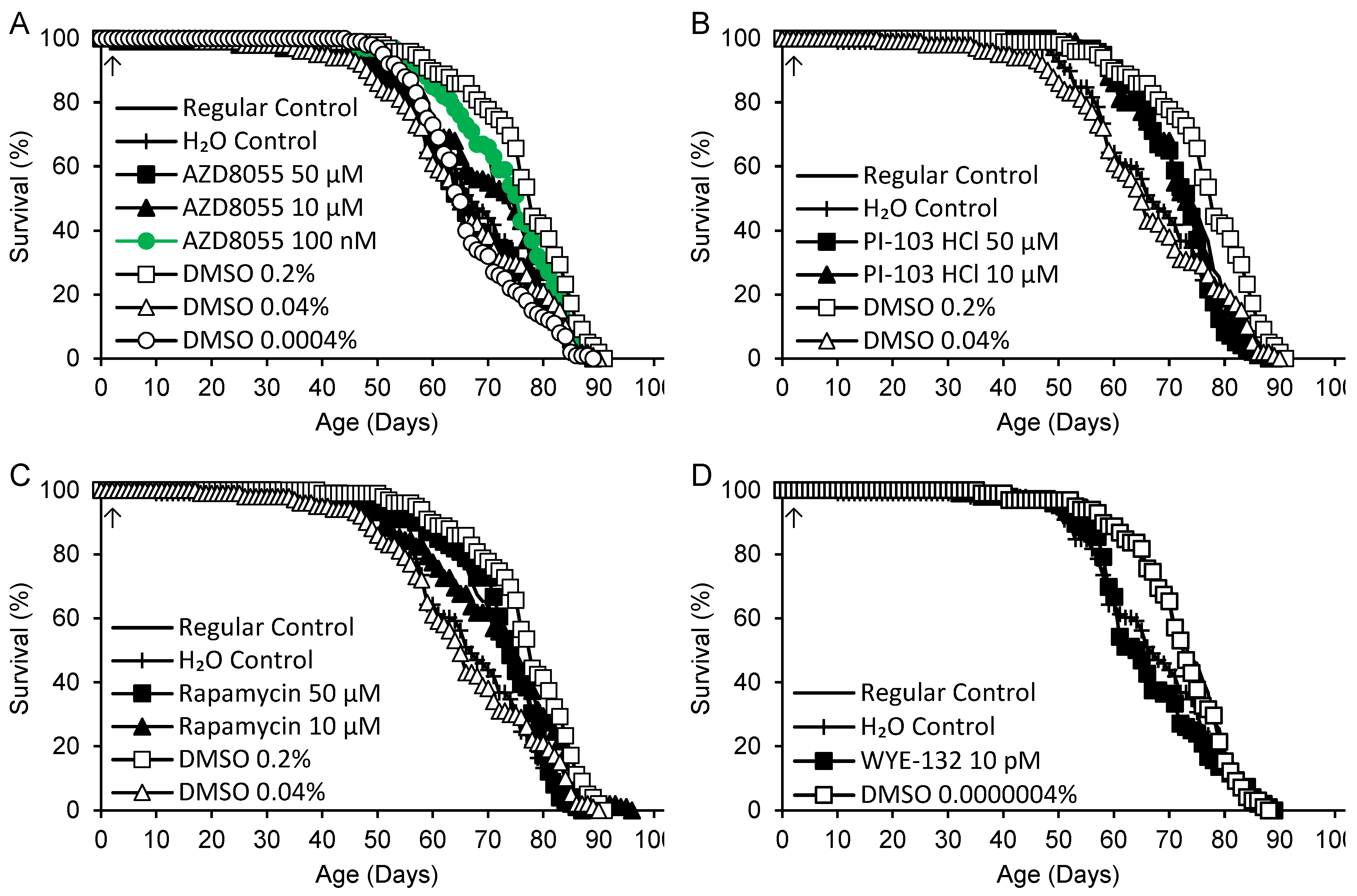
2.3. Longevity effects of TOR/PI3K inhibitors – confirmation study
2.4. Fecundity and Fertility Effects of TOR/PI3K Inhibitors in the Confirmation Study
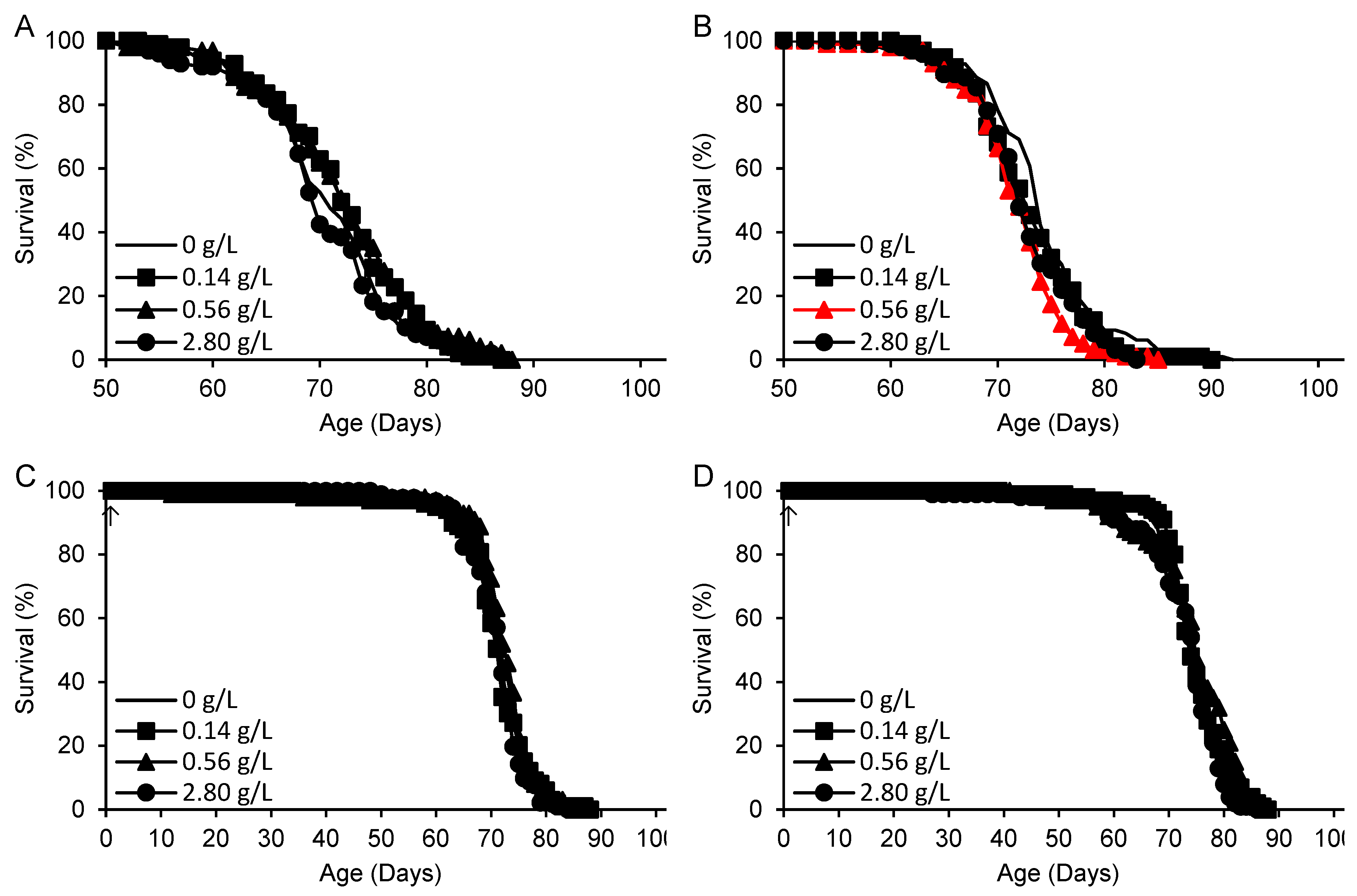
2.5. Longevity Effect of 2-Hydroxypropyl-β-Cyclodextrin (2-HP-β-CD)
3. Discussion
4. Materials and Methods
4.1. Fly Strain and Media
4.2. Supplements
4.3. Life Span
4.4. Fertility
4.5. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barardo, D.; Thornton, D.; Thoppil, H.; Walsh, M.; Sharifi, S.; Ferreira, S.; Anžič, A.; Fernandes, M.; Monteiro, P.; Grum, T.; et al. The DrugAge database of aging-related drugs. Aging Cell 2017, 16, 594–597. [Google Scholar] [CrossRef] [PubMed]
- Bjedov, I.; Toivonen, J.M.; Kerr, F.; Slack, C.; Jacobson, J.; Foley, A.; Partridge, L. Mechanisms of life span extension by rapamycin in the fruit fly Drosophila melanogaster. Cell Metab. 2010, 11, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Juricic, P.; Lu, Y.-X.; Leech, T.; Drews, L.F.; Paulitz, J.; Lu, J.; Nespital, T.; Azami, S.; Regan, J.C.; Funk, E.; et al. Long-lasting geroprotection from brief rapamycin treatment in early adulthood by persistently increased intestinal autophagy. Nat. Aging 2022, 2, 824–836. [Google Scholar] [CrossRef] [PubMed]
- Spindler, S.R.; Li, R.; Dhahbi, J.M.; Yamakawa, A.; Sauer, F. Novel protein kinase signaling systems regulating lifespan identified by small molecule library screening using Drosophila. PLoS ONE 2012, 7, e29782. [Google Scholar] [CrossRef]
- Eisenberg, T.; Knauer, H.; Schauer, A.; Büttner, S.; Ruckenstuhl, C.; Carmona-Gutierrez, D.; Ring, J.; Schroeder, S.; Magnes, C.; Antonacci, L.; et al. Induction of autophagy by spermidine promotes longevity. Nat. Cell. Biol. 2009, 11, 1305–1314. [Google Scholar] [CrossRef]
- Morselli, E.; Galluzzi, L.; Kepp, O.; Criollo, A.; Maiuri, M.C.; Tavernarakis, N.; Madeo, F.; Kroemer, G. Autophagy mediates pharmacological lifespan extension by spermidine and resveratrol. Aging (Albany NY) 2009, 1, 961–970. [Google Scholar] [CrossRef]
- Yuan, T.L.; Cantley, L.C. PI3K pathway alterations in cancer: variations on a theme. Oncogene 2008, 27, 5497–5510. [Google Scholar] [CrossRef]
- Harrison, D.E.; Strong, R.; Sharp, Z.D.; Nelson, J.F.; Astle, C.M.; Flurkey, K.; Nadon, N.L.; Wilkinson, J.E.; Frenkel, K.; Carter, C.S.; et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 2009, 460, 392–395. [Google Scholar] [CrossRef]
- Lamming, D.W. Rapamycin and rapalogs. In Anti-Aging Pharmacology; Koltover, V.K., Ed.; Academic Press: Cambridge, MA, USA, 2023; pp. 89–118. [Google Scholar] [CrossRef]
- Blagosklonny, M.V. Cancer prevention with rapamycin. Oncotarget 2023, 14, 342–350. [Google Scholar] [CrossRef]
- Trelinska, J.; Dachowska, I.; Kotulska, K.; Fendler, W.; Jozwiak, S.; Mlynarski, W. Complications of mammalian target of rapamycin inhibitor anticancer treatment among patients with tuberous sclerosis complex are common and occasionally life-threatening. Anti-Cancer Drugs 2015, 26, 437–442. [Google Scholar] [CrossRef]
- Bissler, J.J.; Kingswood, J.C.; Radzikowska, E.; Zonnenberg, B.A.; Belousova, E.; Frost, M.D.; Sauter, M.; Brakemeier, S.; de Vries, P.J.; Berkowitz, N.; et al. Everolimus long-term use in patients with tuberous sclerosis complex: Four-year update of the EXIST-2 study. PLoS ONE 2017, 12, e0180939. [Google Scholar] [CrossRef]
- Pezzicoli, G.; Filoni, E.; Gernone, A.; Cosmai, L.; Rizzo, M.; Porta, C. Playing the devil’s advocate: Should we give a second chance to mTOR inhibition in renal clear cell carcinoma? - ie strategies to revert resistance to mTOR inhibitors. Cancer Manag. Res. 2021, 13, 7623–7636. [Google Scholar] [CrossRef]
- Kiesewetter, B.; Melhorn, P.; Macheiner, S.; Wolff, L.; Kretschmer-Chott, E.; Haug, A.; Mazal, P.; Raderer, M. Does the dose matter? Antiproliferative efficacy and toxicity of everolimus in patients with neuroendocrine tumors – Experiences from a tertiary referral center. J. Neuroendocrinol. 2023, 35, e13319. [Google Scholar] [CrossRef] [PubMed]
- Karatrasoglou, E.A.; Dimou, M.; Piperidou, A.; Lakiotaki, E.; Korkolopoulou, P.; Vassilakopoulos, T.P. The role of mTOR in B cell lymphoid malignancies: biologic and therapeutic aspects. Int. J. Mol. Sci. 2023, 24, 14110. [Google Scholar] [CrossRef] [PubMed]
- Kaeberlein, T.L.; Green, A.S.; Haddad, G.; Hudson, J.; Isman, A.; Nyquist, A.; Rosen, B.S.; Suh, Y.; Zalzala, S.; Zhang, X.; et al. Evaluation of off-label rapamycin use to promote healthspan in 333 adults. GeroScience 2023, 45, 2757–2768. [Google Scholar] [CrossRef] [PubMed]
- Silkenstedt, E.; Dreyling, M. Mantle cell lymphoma – Update on molecular biology, prognostication and treatment approaches. Hematol. Oncol. 2023, 41(S1), 36–42. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Wang, X.; Proud, C.G. mTOR inhibitors in cancer therapy. F1000Res. 2016, 5, F1000. [Google Scholar] [CrossRef]
- Heitman, J.; Movva, N.R.; Hall, M.N. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science 1991, 253, 905–909. [Google Scholar] [CrossRef]
- Castellano, B.M.; Thelen, A.M.; Moldavski, O.; Feltes, M.; van der Welle, R.E.N.; Mydock-McGrane, L.; Jiang, X.; van Eijkeren, R.J.; Davis, O.B.; Louie, S.M.; et al. Lysosomal cholesterol activates mTORC1 via an SLC38A9-Niemann-Pick C1 signaling complex. Science 2017, 355, 1306–1311. [Google Scholar] [CrossRef]
- Hay, N.; Sonenberg, N. Upstream and downstream of mTOR. Genes Dev. 2004, 18, 1926–1945. [Google Scholar] [CrossRef]
- Minard, A.Y.; Tan, S.-X.; Yang, P.; Fazakerley, D.J.; Domanova, W.; Parker, B.L.; Humphrey, S.J.; Jothi, R.; Stöckli, J.; James, D.E. mTORC1 is a major regulatory node in the FGF21 signaling network in adipocytes. Cell Rep. 2016, 17, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.J.; Beal, P.A.; Keith, C.T.; Chen, J.; Shin, T.B.; Schreiber, S.L. Control of p70 S6 kinase by kinase activity of FRAP in vivo. Nature 1995, 377, 441–446. [Google Scholar] [CrossRef]
- Burnett, P.E.; Barrow, R.K.; Cohen, N.A.; Snyder, S.H.; Sabatini, D.M. RAFT1 phosphorylation of the translational regulators p70 S6 kinase and 4E-BP1. Proc. Natl. Acad. Sci. USA 1998, 95, 1432–1437. [Google Scholar] [CrossRef]
- von Manteuffel, S.R.; Gingras, A.-C.; Ming, X.-F.; Sonenberg, N.; Thomas, G. 4E-BP1 phosphorylation is mediated by the FRAP-p70s6k pathway and is independent of mitogen-activated protein kinase. Proc. Natl. Acad. Sci. USA 1996, 93, 4076–4080. [Google Scholar] [CrossRef]
- Ragupathi, A.; Kim, C.; Jacinto, E. The mTORC2 signaling network: targets and cross-talks. Biochem. J. 2024, 481, 45–91. [Google Scholar] [CrossRef] [PubMed]
- Lamming, D.W.; Sabatini, D.M. A central role for mTOR in lipid homeostasis. Cell Metab. 2013, 18, 465–469. [Google Scholar] [CrossRef]
- Lamming, D.W.; Ye, L.; Katajisto, P.; Goncalves, M.D.; Saitoh, M.; Stevens, D.M.; Davis, J.G.; Salmon, A.B.; Richardson, A.; Ahima, R.S.; et al. Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science 2012, 335, 1638–1643. [Google Scholar] [CrossRef] [PubMed]
- Sarbassov, D.D.; Ali, S.M.; Sengupta, S.; Sheen, J.-H.; Hsu, P.P.; Bagley, A.F.; Markhard, A.L.; Sabatini, D.M. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol. Cell 2006, 22, 159–168. [Google Scholar] [CrossRef]
- Watanabe, R.; Wei, L.; Huang, J. mTOR signaling, function, novel inhibitors, and therapeutic targets. J. Nucl. Med. 2011, 52, 497–500. [Google Scholar] [CrossRef]
- O’Reilly, K.E.; Rojo, F.; She, Q.-B.; Solit, D.; Mills, G.B.; Smith, D.; Lane, H.; Hofmann, F.; Hicklin, D.J.; Ludwig, D.L.; et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006, 66, 1500–1508. [Google Scholar] [CrossRef]
- Julien, L.-A.; Carriere, A.; Moreau, J.; Roux, P.P. mTORC1-activated S6K1 phosphorylates Rictor on threonine 1135 and regulates mTORC2 signaling. Mol. Cell. Biol. 2010, 30, 908–921. [Google Scholar] [CrossRef]
- Thoreen, C.C.; Sabatini, D.M. Rapamycin inhibits mTORC1, but not completely. Autophagy 2009, 5, 725–726. [Google Scholar] [CrossRef] [PubMed]
- Choo, A.Y.; Yoon, S.-O.; Kim, S.G.; Roux, P.P.; Blenis, J. Rapamycin differentially inhibits S6Ks and 4E-BP-1 to mediate cell-type-specific repression of mRNA translation. Proc. Natl. Acad. Sci. USA 2008, 105, 17414–17419. [Google Scholar] [CrossRef] [PubMed]
- Mason, J.S.; Wileman, T.; Chapman, T. Lifespan extension without fertility reduction following dietary addition of the autophagy activator Torin1 in Drosophila melanogaster. PLoS ONE 2018, 13, e0190105. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wang, J.; Kang, S.A.; Thoreen, C.C.; Hur, W.; Ahmed, T.; Sabatini, D.M.; Gray, N.S. Discovery of 9-(6-aminopyridin-3-yl)-1-(3-(trifluoromethyl)-phenyl)benzo[h][1,6]naphthyridin-2(1H)-one (Torin2) as a potent, selective, and orally available mammalian target of rapamycin (mTOR) inhibitor for treatment of cancer. J. Med. Chem. 2011, 54, 1473–1480. [Google Scholar] [CrossRef] [PubMed]
- Waetzig, R.; Matthes, M.; Leister, J.; Penkivech, G.; Heise, T.; Corbacioglu, S.; Sommer, G. Comparing mTOR inhibitor rapamycin with Torin-2 within the RIST molecular-targeted regimen in neuroblastoma cells. Int. J. Med. Sci. 2021, 18, 137–149. [Google Scholar] [CrossRef]
- Vershinina, Y.S.; Krasnov, G.S.; Garbuz, D.G.; Shaposhnikov, M.V.; Fedorova, M.S.; Pudova, E.A.; Katunina, I.V.; Kornev, A.B.; Zemskaya, N.V.; Kudryavtsev, A.A.; et al. Transcriptomic analysis of the effect of Torin-2 on the central nervous system of Drosophila melanogaster. Int. J. Mol. Sci. 2023, 24, 9095. [Google Scholar] [CrossRef] [PubMed]
- Chresta, C.M.; Davies, B.R.; Hickson, I.; Harding, T.; Cosulich, S.; Critchlow, S.E.; Vincent, J.P.; Ellston, R.; Jones, D.; Sini, P.; et al. AZD8055 is a potent, selective, and orally bioavailable ATP-competitive mammalian target of rapamycin kinase inhibitor with in vitro and in vivo antitumor activity. Cancer Res. 2010, 70, 288–298. [Google Scholar] [CrossRef]
- Feldman, M.E.; Apsel, B.; Uotila, A.; Loewith, R.; Knight, Z.A.; Ruggero, D.; Shokat, K.M. Active-site inhibitors of mTOR target rapamycin-resistant outputs of mTORC1 and mTORC2. PLoS Biol. 2009, 7, e1000038. [Google Scholar] [CrossRef]
- Xu, W.; Zhao, T.; Chen, H.; Huang, N.; Gong, H.; Zhang, J.; Yang, Y.; Li, T.; Zhang, G.; Gong, C.; et al. Pan-mTOR inhibitors sensitize the senolytic activity of navitoclax via mTORC2 inhibition-mediated apoptotic signaling. Biochem. Pharmacol. 2022, 200, 115045. [Google Scholar] [CrossRef]
- Chen, T.; Shen, L.; Yu, J.; Wan, H.; Guo, A.; Chen, J.; Long, Y.; Zhao, J.; Pei, G. Rapamycin and other longevity-promoting compounds enhance the generation of mouse induced pluripotent stem cells. Aging Cell 2011, 10, 908–911. [Google Scholar] [CrossRef] [PubMed]
- Vlahos, C.J.; Matter, W.F.; Hui, K.Y.; Brown, R.F. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002). J. Biol. Chem. 1994, 269, 5241–5248. [Google Scholar] [CrossRef] [PubMed]
- Brunn, G.J.; Williams, J.; Sabers, C.; Wiederrecht, G.; Lawrence, J.C.; Abraham, R.T. Direct inhibition of the signaling functions of the mammalian target of rapamycin by the phosphoinositide 3-kinase inhibitors, wortmannin and LY294002. EMBO J. 1996, 15, 5256–5267. [Google Scholar] [CrossRef] [PubMed]
- Gharbi, S.I.; Zvelebil, M.J.; Shuttleworth, S.J.; Hancox, T.; Saghir, N.; Timms, J.F.; Waterfield, M.D. Exploring the specificity of the PI3K family inhibitor LY294002. Biochem. J. 2007, 404, 15–21. [Google Scholar] [CrossRef]
- Moskalev, A.A.; Shaposhnikov, M.V. Pharmacological inhibition of phosphoinositide 3 and TOR kinases improves survival of Drosophila melanogaster. Rejuv. Res. 2010, 13, 246–247. [Google Scholar] [CrossRef]
- Arcaro, A.; Wymann, M.P. Wortmannin is a potent phosphatidylinositol 3-kinase inhibitor: the role of phosphatidylinositol 3,4,5-trisphosphate in neutrophil responses. Biochem. J. 1993, 296, 297–301. [Google Scholar] [CrossRef]
- Powis, G.; Bonjouklian, R.; Berggren, M.M.; Gallegos, A.; Abraham, R.; Ashendel, C.; Zalkow, L.; Matter, W.F.; Dodge, J.; Grindey, G.; et al. Wortmannin, a potent and selective inhibitor of phosphatidylinositol-3-kinase. Cancer Res. 1994, 54, 2419–2423. [Google Scholar]
- Danilov, A.; Shaposhnikov, M.; Plyusnina, E.; Kogan, V.; Fedichev, P.; Moskalev, A. Selective anticancer agents suppress aging in Drosophila. Oncotarget 2013, 4, 1507–1526. [Google Scholar] [CrossRef]
- García-Martínez, J.M.; Moran, J.; Clarke, R.G.; Gray, A.; Cosulich, S.C.; Chresta, C.M.; Alessi, D.R. Ku-0063794 is a specific inhibitor of the mammalian target of rapamycin (mTOR). Biochem. J. 2009, 421, 29–42. [Google Scholar] [CrossRef]
- Zask, A.; Kaplan, J.; Verheijen, J.C.; Richard, D.J.; Curran, K.; Brooijmans, N.; Bennett, E.M.; Toral-Barza, L.; Hollander, I.; Ayral-Kaloustian, S.; et al. Morpholine derivatives greatly enhance the selectivity of mammalian target of rapamycin (mTOR) inhibitors. J. Med. Chem. 2009, 52, 7942–7945. [Google Scholar] [CrossRef]
- Yu, K.; Shi, C.; Toral-Barza, L.; Lucas, J.; Shor, B.; Kim, J.E.; Zhang, W.-G.; Mahoney, R.; Gaydos, C.; Tardio, L.; et al. Beyond rapalog therapy: preclinical pharmacology and antitumor activity of WYE-125132, an ATP-competitive and specific inhibitor of mTORC1 and mTORC2. Cancer Res. 2010, 70, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Wipf, P.; Minion, D.J.; Halter, R.J.; Berggren, M.I.; Ho, C.B.; Chiang, G.G.; Kirkpatrick, L.; Abraham, R.; Powis, G. Synthesis and biological evaluation of synthetic viridins derived from C(20)-heteroalkylation of the steroidal PI-3-kinase inhibitor wortmannin. Org. Biomol. Chem. 2004, 2, 1911–1920. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.S.; Bowles, D.W.; Falchook, G.S.; Messersmith, W.A.; George, G.C.; O’Bryant, C.L.; Vo, A.C.H.; Klucher, K.; Herbst, R.S.; Eckhardt, S.G.; et al. A multicenter phase I trial of PX-866, an oral irreversible phosphatidylinositol 3-kinase inhibitor, in patients with advanced solid tumors. Clin. Cancer Res. 2012, 18, 4173–4182. [Google Scholar] [CrossRef] [PubMed]
- Klucher, K.M.; Vo, A.; Walker, C.; Rosler, R.; Taylor, J.; Millard, J.; Peterson, S. 17-hydroxy-PX-866, the primary metabolite of PX-866, an irreversible, pan-isoform inhibitor of phosphatidylinositol-3 (PI3) kinase, has increased activity in biochemical and cellular assays. In Proceedings of the AACR Special Conference Targeting PI3K/mTOR signaling in cancer, San Francisco, CA, USA, 24-27 February 2011. [Google Scholar]
- Hayakawa, M.; Kaizawa, H.; Moritomo, H.; Koizumi, T.; Ohishi, T.; Yamano, M.; Okada, M.; Ohta, M.; Tsukamoto, S.-i.; Raynaud, F.I.; et al. Synthesis and biological evaluation of pyrido[3’,2’:4,5]furo[3,2-d]pyrimidine derivatives as novel PI3 kinase p110α inhibitors. Bioorg. Med. Chem. Lett. 2007, 17, 2438–2442. [Google Scholar] [CrossRef]
- Knight, Z.A.; Gonzalez, B.; Feldman, M.E.; Zunder, E.R.; Goldenberg, D.D.; Williams, O.; Loewith, R.; Stokoe, D.; Balla, A.; Toth, B.; et al. A pharmacological map of the PI3-K family defines a role for p110α in insulin signaling. Cell 2006, 125, 733–747. [Google Scholar] [CrossRef]
- Toral-Barza, L.; Zhang, W.-G.; Lamison, C.; LaRocque, J.; Gibbons, J.; Yu, K. Characterization of the cloned full-length and a truncated human target of rapamycin: activity, specificity, and enzyme inhibition as studied by a high capacity assay. Biochem. Biophys. Res. Commun. 2005, 332, 304–310. [Google Scholar] [CrossRef]
- Lehman, J.A.; Calvo, V.; Gomez-Cambronero, J. Mechanism of ribosomal p70S6 kinase activation by granulocyte macrophage colony-stimulating factor in neutrophils. J. Biol. Chem. 2003, 278, 28130–28138. [Google Scholar] [CrossRef] [PubMed]
- Hara, Y.; Yanatori, I.; Tanaka, A.; Kishi, F.; Lemasters, J.J.; Nishina, S.; Sasaki, K.; Hino, K. Iron loss triggers mitophagy through induction of mitochondrial ferritin. EMBO Rep. 2020, 21, e50202. [Google Scholar] [CrossRef]
- Soriano, S.; Llorens, J.V.; Blanco-Sobero, L.; Gutiérrez, L.; Calap-Quintana, P.; Morales, M.P.; Moltó, M.D.; Martínez-Sebastián, M.J. Deferiprone and idebenone rescue frataxin depletion phenotypes in a Drosophila model of Friedreich’s ataxia. Gene 2013, 521, 274–281. [Google Scholar] [CrossRef]
- Sun, H.; Zong, H.; Wu, G. 2-Hydroxypropyl-β-cyclodextrin blocks autophagy flux and triggers caspase-8-mediated apoptotic cascades in HepG2 cells. Mol. Med. Rep. 2020, 22, 1901–1909. [Google Scholar] [CrossRef]
- Massie, H.R.; Williams, T.R.; Iodice, A.A. Influence of anti-inflammatory agents on the survival of Drosophila. J. Gerontol. 1985, 40, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, X.; Li, L.; Wang, D. Lifespan extension in Caenorhabditis elegans by DMSO is dependent on sir-2.1 and daf-16. Biochem. Biophys. Res. Commun. 2010, 400, 613–618. [Google Scholar] [CrossRef]
- Harrison, B.; Tran, T.T.; Taylor, D.; Lee, S.-D.; Min, K.-J. Effect of rapamycin on lifespan in Drosophila. Geriatr. Gerontol. Int. 2010, 10, 110–112. [Google Scholar] [CrossRef]
- Carvalho, G.B.; Kapahi, P.; Benzer, S. Compensatory ingestion upon dietary restriction in Drosophila melanogaster. Nat. Methods 2005, 2, 813–815. [Google Scholar] [CrossRef]
- Kraig, E.; Linehan, L.A.; Liang, H.; Romo, T.Q.; Liu, Q.; Wu, Y.; Benavides, A.D.; Curiel, T.J.; Javors, M.A.; Musi, N.; et al. A randomized control trial to establish the feasibility and safety of rapamycin treatment in an older human cohort: Immunological, physical performance, and cognitive effects. Exp. Gerontol. 2018, 105, 53–69. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, J.; Mathieu, J.; Alvarez, P. 2-Hydoxypropyl-beta-cyclodextrin (HPβCD) reduces age-related lipofuscin accumulation through a cholesterol-associated pathway. Sci. Rep. 2017, 7, 2197. [Google Scholar] [CrossRef] [PubMed]
- Brunk, U.T.; Terman, A. The mitochondrial-lysosomal axis theory of aging. Eur. J. Biochem. 2002, 269, 1996–2002. [Google Scholar] [CrossRef]
- Ramirez, C.M.; Liu, B.; Taylor, A.M.; Repa, J.J.; Burns, D.K.; Weinberg, A.G.; Turley, S.D.; Dietschy, J.M. Weekly cyclodextrin administration normalizes cholesterol metabolism in nearly every organ of the Niemann-Pick type C1 mouse and markedly prolongs life. Pediatr. Res. 2010, 68, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Gould, S.; Scott, R.C. 2-Hydroxypropyl-β-cyclodextrin (HP-β-CD): A toxicology review. Food Chem. Toxicol. 2005, 43, 1451–1459. [Google Scholar] [CrossRef]
- Trotta, F.; Zanetti, M.; Camino, G. Thermal degradation of cyclodextrins. Polym. Degrad. Stab. 2000, 69, 373–379. [Google Scholar] [CrossRef]
- Mockett, R.J.; Nobles, A.C. Lack of robustness of life extension associated with several single-gene P element mutations in Drosophila melanogaster. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 1157–1169. [Google Scholar] [CrossRef] [PubMed]
- Orr, W.C.; Sohal, R.S. The effects of catalase gene overexpression on life span and resistance to oxidative stress in transgenic Drosophila melanogaster. Arch. Biochem. Biophys. 1992, 297, 35–41. [Google Scholar] [CrossRef]
- Mockett, R.J.; Bearden, A.A.; Nobles, A.C. Longevity effects of DMSO-solubilized rapamycin and other compounds in y w male Drosophila melanogaster. FASEB J. 2022, 36S1. [Google Scholar] [CrossRef]


| Inhibitor | TORC1 (nM) 2 | TORC2 (nM) 3 | PI3Kinase (nM) 4 | Reference |
|---|---|---|---|---|
| AZD8055 | 0.13 ± 0.05 5 | 3200-18 900 6 | 39 | |
| Ku-0063794 | ~10 | ~10 | >10 000 | 50 |
| LY294002 | 1500 5 ~5000 7 . | 1400 | 43,44,58 | |
| PI-103 hydrochloride 8 | 20 | 83 | 3-250 6 | 56,57 |
| PP242 | 30 9 | 58 | 102-2200 6 | 40 |
| PX-866-17OH | 10 | 14-57 6,10 | 54 | |
| Rapamycin | 2 5,11 | — | — | 58 |
| Torin2 | 0.25 12 | 200 12 | 36 | |
| Wortmannin | 200 5 ~200 7, 300 13 . | 0.3-4 | 44,47,48,53,58 | |
| WYE-28 | 0.22 ± 0.06 5 | 4271 | 51 | |
| WYE-132 | 0.19 ± 0.07 5 | 3 | 1179->10 000 6 | 52 |
| Supplement (Concentration) 1 | p 2 | Survival (Days) | % vs. H2O (p) | % vs. Regular (p) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Control (Regular) | 21.0 | ||||||||
| Control (H2O) | 17.4 | -17.0 | (0.002) | ||||||
| DMSO | <0.0005 | ||||||||
| DMSO (2%) | 4.4 | -74.7 | (< 0.0005) | -79.0 | (< 0.0005) | ||||
| DMSO (0.2%) | 16.1 | -7.4 | (0.92) | -23.1 | |||||
| DMSO (0.02%) | 19.8 | +13.7 | (0.055) | -5.6 | |||||
| DMSO (0.002%) | 18.1 | +3.8 | (0.21) | -13.8 | |||||
| DMSO (0.0002%) | 18.5 | +6.3 | (0.79) | -11.8 | |||||
| PP242 | <0.0005 | ||||||||
| PP242 (649 nM) | 19.1 | +9.7 | (0.048) | -8.9 | (0.60) | ||||
| PP242 (64.9 nM) | 18.5 | +6.0 | (0.15) | -12.0 | |||||
| PP242 (21.6 nM) | 18.2 | +4.3 | (0.63) | -13.4 | |||||
| PP242 (6.49 nM) | 15.0 | -13.7 | (0.030) | -28.3 | (< 0.0005) | ||||
| PP242 (649 pM) | 15.9 | -8.8 | (0.028) | -24.3 | (< 0.0005) | ||||
| DFP | 0.003 | ||||||||
| DFP (719 µM) | 16.2 | -6.9 | (0.45) | -22.7 | |||||
| DFP (71.9 µM) | 15.9 | -8.9 | (0.99) | -24.3 | |||||
| DFP (7.19 µM) | 18.6 | +6.4 | (0.19) | -11.6 | |||||
| DFP (719 nM) | 20.9 | +19.6 | (0.012) | -0.7 | (0.98) | ||||
| DFP (71.9 nM) | 20.2 | +16.0 | (0.006) | -3.7 | (0.82) | ||||
| Supplement (Concentration) 1 | p 2 | Life Span (Days) | % vs. H2O (p) | % vs. Regular (p) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Control (Regular) | 73.9 | ||||||||
| Control (H2O) | 74.1 | 0.2 | |||||||
| Spermidine | <0.0005 | ||||||||
| Spermidine (10 mM) | <0.0005 | 66.2 | -10.6 | (<0.0005) | -10.4 | (<0.0005) | |||
| Spermidine (1 mM) | 0.87 | 73.6 | -0.7 | -0.5 | |||||
| Spermidine (100 µM) | 0.92 | 74.3 | +0.2 | +0.5 | |||||
| Spermidine (10 µM) | 0.93 | 73.5 | -0.8 | -0.6 | |||||
| DMSO | 0.89 | ||||||||
| DMSO (0.2%) | 73.3 | -1.1 | -0.9 | ||||||
| DMSO (0.1%) | 73.2 | -1.2 | -1.0 | ||||||
| DMSO (0.04%) | 73.0 | -1.5 | -1.3 | ||||||
| DMSO (0.02%) | 72.0 | -2.9 | -2.6 | ||||||
| WYE-28 | 0.14 | ||||||||
| WYE-28 (1 µM) | 74.6 | +0.7 | +0.9 | ||||||
| WYE-28 (100 nM) | 72.4 | -2.3 | -2.1 | ||||||
| WYE-28 (10 nM) | 74.0 | -0.1 | +0.1 | ||||||
| WYE-28 (1 nM) | 71.8 | -3.0 | -2.8 | ||||||
| WYE-28 (100 pM) | 74.2 | +0.1 | +0.4 | ||||||
| WYE-28 (10 pM) | 76.6 | +3.4 | +3.6 | ||||||
| Control (Regular) | 76.2 | ||||||||
| Control (H2O) | 75.7 | -0.6 | |||||||
| WYE-132 | <0.0005 | ||||||||
| WYE-132 (1 µM) | 0.083 | 73.5 | -3.0 | -3.6 | |||||
| WYE-132 (100 nM) | 0.53 | 76.1 | +0.4 | -0.2 | |||||
| WYE-132 (10 nM) | 0.57 | 76.6 | +1.1 | +0.5 | |||||
| WYE-132 (1 nM) | 0.60 | 76.5 | +1.0 | +0.4 | |||||
| WYE-132 (100 pM) | 0.31 | 77.3 | +2.0 | +1.4 | |||||
| WYE-132 (10 pM) | 0.001 | 80.3 | +6.0 | (<0.0005) | +5.3 | (0.012) | |||
| LY294002 | 0.12 | ||||||||
| LY294002 (10 µM) | 75.6 | -0.2 | -0.8 | ||||||
| LY294002 (5 µM) | 75.3 | -0.5 | -1.1 | ||||||
| LY294002 (1 µM) | 76.9 | +1.5 | +0.9 | ||||||
| LY294002 (100 nM) | 74.2 | -2.0 | -2.6 | ||||||
| Control (Regular) | 73.3 | ||||||||
| Control (H2O) | 75.3 | +2.7 | |||||||
| AZD8055 | 0.036 | ||||||||
| AZD8055 (10 µM) | 0.009 | 78.1 | +3.7 | (0.006) | +6.6 | (0.006) | |||
| AZD8055 (1 µM) | 0.29 | 76.0 | +0.9 | +3.7 | |||||
| AZD8055 (100 nM) | 0.013 | 78.1 | +3.7 | (0.011) | +6.5 | (0.008) | |||
| AZD8055 (10 nM) | 0.38 | 75.4 | +0.1 | +2.8 | |||||
| AZD8055 (1 nM) | 0.078 | 76.7 | +1.8 | +4.6 | |||||
| AZD8055 (100 pM) | 0.45 | 75.1 | -0.3 | +2.4 | |||||
| Rapamycin | <0.0005 | ||||||||
| Rapamycin (400 µM) | <0.0005 | 41.8 | -44.5 | (<0.0005) | -42.9 | (<0.0005) | |||
| Rapamycin (200 µM) | <0.0005 | 47.7 | -36.7 | (<0.0005) | -34.9 | (<0.0005) | |||
| Rapamycin (100 µM) | <0.0005 | 54.2 | -28.0 | (<0.0005) | -26.1 | (<0.0005) | |||
| Control (Regular) | 73.4 | ||||||||
| Control (H2O) | 75.7 | +3.2 | |||||||
| Rapamycin | 0.22 | ||||||||
| Rapamycin (10 µM) | 74.2 | -2.0 | +1.1 | ||||||
| Rapamycin (1 µM) | 72.5 | -4.3 | -1.3 | ||||||
| Rapamycin (500 nM) | 73.1 | -3.4 | -0.3 | ||||||
| Rapamycin (100 nM) | 73.5 | -3.0 | +0.1 | ||||||
| Ku-0063794 | 0.062 | ||||||||
| Ku-0063794 (10 µM) | 75.8 | +0.1 | +3.3 | ||||||
| Ku-0063794 (1 µM) | 77.2 | +1.9 | +5.1 | ||||||
| Ku-0063794 (100 nM) | 75.6 | -0.1 | +3.0 | ||||||
| Ku-0063794 (10 nM) | 77.3 | +2.1 | +5.3 | ||||||
| Ku-0063794 (1 nM) | 74.7 | -1.4 | +1.8 | ||||||
| Wortmannin | <0.0005 | ||||||||
| Wortmannin (10 µM) | <0.0005 | 22.6 | -70.2 | (<0.0005) | -69.2 | (<0.0005) | |||
| Wortmannin (1 µM) | 0.21 | 72.6 | -4.1 | -1.0 | |||||
| Wortmannin (500 nM) | 0.27 | 74.7 | -1.4 | +1.8 | |||||
| Wortmannin (100 nM) | 0.11 | 73.9 | -2.4 | +0.7 | |||||
| Wortmannin (10 nM) | 0.11 | 74.4 | -1.7 | +1.4 | |||||
| Wortmannin (1 nM) | 0.092 | 76.2 | +0.7 | +3.8 | |||||
| Control (Regular) | 75.5 | ||||||||
| Control (H2O) | 75.4 | -0.1 | |||||||
| PX-866-17OH | 0.26 | ||||||||
| PX-866-17OH (10 µM) | 78.0 | +3.5 | +3.4 | ||||||
| PX-866-17OH (1 µM) | 74.2 | -1.6 | -1.7 | ||||||
| PX-866-17OH (500 nM) | 76.7 | +1.7 | +1.6 | ||||||
| PX-866-17OH (100 nM) | 75.6 | +0.2 | +0.1 | ||||||
| PX-866-17OH (10 nM) | 76.7 | +1.7 | +1.6 | ||||||
| PX-866-17OH (1 nM) | 74.5 | -1.2 | -1.3 | ||||||
| Control (H2O) | 75.4 | ||||||||
| PI-103 HCl | 0.029 | ||||||||
| PI-103 HCl (10 µM) | 0.010 | 78.4 | +4.0 | ||||||
| PI-103 HCl (1 µM) | 0.95 | 75.2 | -0.3 | ||||||
| PI-103 HCl (100 nM) | 0.41 | 76.6 | +1.5 | ||||||
| PI-103 HCl (10 nM) | 0.36 | 76.4 | +1.3 | ||||||
| PI-103 HCl (1 nM) | 0.91 | 75.6 | +0.3 | ||||||
| PI-103 HCl (100 pM) | 0.75 | 74.4 | -1.3 | ||||||
| Torin2 | 0.40 | ||||||||
| Torin2 (10 µM) | 75.3 | -0.1 | |||||||
| Torin2 (1 µM) | 76.3 | +1.2 | |||||||
| Torin2 (100 nM) | 75.4 | -0.1 | |||||||
| Torin2 (10 nM) | 74.9 | -0.7 | |||||||
| Torin2 (1 nM) | 75.0 | -0.6 | |||||||
| Torin2 (100 pM) | 75.3 | -0.1 | |||||||
| Supplement (Concentration) 1 | p 2 | Life Span (Days) | % vs. H2O (p) | % vs. Regular (p) | % vs. DMSO (p) 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control (Regular) | 72.5 | ||||||||||
| Control (H2O) | 66.8 | -7.8 | (0.065) | ||||||||
| DMSO | <0.0005 | ||||||||||
| DMSO (0.2%) | <0.0005 | 76.5 | +14.5 | (<0.0005) | +5.6 | (<0.0005) | |||||
| DMSO (0.04%) | 0.20 | 65.6 | -1.9 | -9.5 | |||||||
| DMSO (0.0004%) | 0.022 | 66.6 | -0.3 | (0.45) | -8.1 | (0.005) | |||||
| DMSO (0.0000004%) | 0.12 | 71.8 | +7.4 | -1.0 | |||||||
| AZD8055 | 0.001 | ||||||||||
| AZD8055 (50 µM) | 0.16 | 66.6 | -0.4 | -8.1 | -13.0 | ||||||
| AZD8055 (10 µM) | 0.030 | 69.9 | +4.6 | (0.020) | -3.6 | (0.39) | +6.6 | (0.12) | |||
| AZD8055 (100 nM) | 0.001 | 73.4 | +9.8 | (<0.0005) | +1.3 | (0.030) | +10.2 | (<0.0005) | |||
| PI-103 HCl | 0.10 | ||||||||||
| PI-103 HCl (50 µM) | 71.8 | +7.5 | -0.9 | -6.1 | |||||||
| PI-103 HCl (10 µM) | 72.2 | +8.1 | -0.3 | +10.2 | |||||||
| Rapamycin | 0.003 | ||||||||||
| Rapamycin (50 µM) | 0.12 | 72.2 | +8.0 | -0.4 | -5.6 | ||||||
| Rapamycin (10 µM) | 0.002 | 71.4 | +6.9 | (0.002) | -1.4 | (0.069) | +9.0 | (0.007) | |||
| WYE-132 (10 pM) | 0.033 | 66.0 | -1.2 | (0.59) | -8.9 | (0.008) | -8.0 | (0.016) | |||
| Supplement (Concentration) 1 | p 2 | Survival (Days) | % vs. H2O (p) | Medium (Figure) | Supplement Onset (Days) | |
|---|---|---|---|---|---|---|
| Control (H2O) | 0.079 | 20.8 | Standard | 50 | ||
| 2-HP-β-CD (0.14 g/L) | 22.1 | +6.5 | (5A) | |||
| 2-HP-β-CD (0.56 g/L) | 22.3 | +7.1 | ||||
| 2-HP-β-CD (2.80 g/L) | 20.5 | -1.6 | ||||
| Control (H2O) | 0.002 | 24.2 | Standard | 50 | ||
| 2-HP-β-CD (0.14 g/L) | 23.1 | -4.8 | (0.12) | (5B) | ||
| 2-HP-β-CD (0.56 g/L) | 21.8 | -10.2 | (<0.0005) | |||
| 2-HP-β-CD (2.80 g/L) | 22.6 | -6.6 | (0.017) | |||
| Control (H2O) | 0.31 | 71.1 | Standard | 1 | ||
| 2-HP-β-CD (0.14 g/L) | 70.9 | -0.2 | (5C) | |||
| 2-HP-β-CD (0.56 g/L) | 72.0 | +1.3 | ||||
| 2-HP-β-CD (2.80 g/L) | 71.0 | -0.0 | ||||
| Control (H2O) | 0.040 | 71.0 | High | 1 | ||
| 2-HP-β-CD (0.14 g/L) | 74.6 | +5.1 | (0.27) | Sugar/ | ||
| 2-HP-β-CD (0.56 g/L) | 74.3 | +4.6 | (0.032) | Yeast | ||
| 2-HP-β-CD (2.80 g/L) | 72.7 | +2.3 | (0.65) | (5D) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





