Submitted:
25 August 2024
Posted:
27 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Key Physical Logic for Understanding the Unified Atomic Mass Unit
3. Key Physical Logic for Understanding the Avogadro Number
4. Reference Nuclear Binding Energy Formula
5. Strong and Electroweak Mass Formula
- 1)
- If Z is even and obtained is odd, then, .
- 2)
- If Z is even and obtained is even, then, .
- 3)
- If Z is odd and obtained is odd, then, .
- 4)
- If Z is odd and obtained is even, then, .
6. Comparative Results and Discussion
- 1)
- 2)
- Life time of super heavy proton numbers greater than 99 and mass numbers greater than 300 is too small to understand and estimate their ground state binding energies.
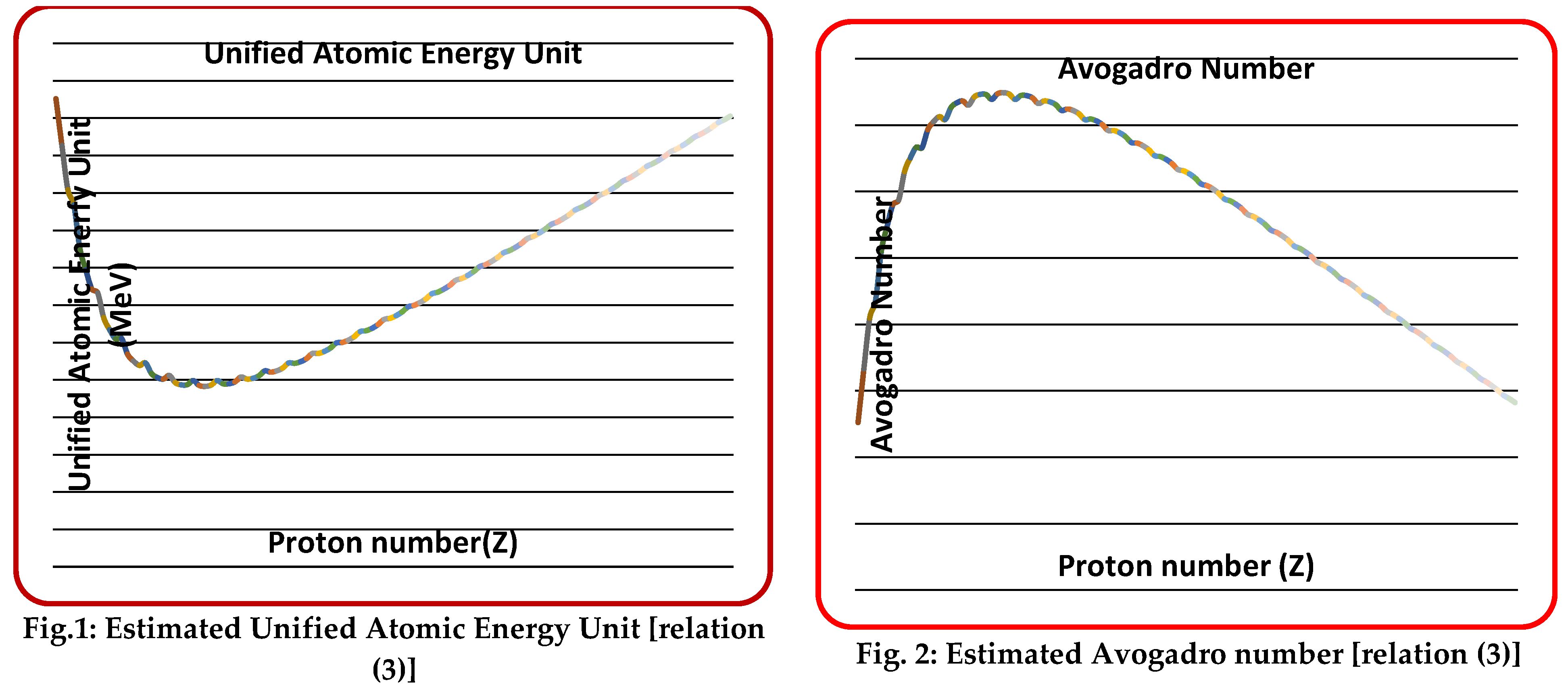
| Proceeding further, currently believed “mole” can be called as ‘gram mole’ and its corresponding SI unit can be called as ‘kg mole’. In CGS system of units, ‘Number of atoms per gram’ can be called as ‘per gram mole’ and in SI system of units, ‘Number of atoms per kg’ can be called as ‘per kg mole’. In this way, ‘mole’ concept can be understood clearly. Considering system of units, currently believed ‘molar mass’ can be called as ‘gram molar mass’ and ‘kg molar mass’. In CGS units, ‘gram molar mass’ of any substance can be assumed to have atoms and in SI units, ‘kg molar mass’ of any substance can be assumed to have atoms. Thinking in this way and by considering proton drip lines and neutron drip lines and by fixing the lower and upper mass numbers of Z=2 to 118, Avogadro number can be estimated with a better understanding and best accuracy | |||||||||||
| Table 1. Nuclear binding energy based estimation of unified atomic mass unit and Avogadro number. | |||||||||||
| Z | AL | AU | As | Relation (5) | Relation (3) | ||||||
| ABEPN (MeV) |
UAEU (MeV) |
UAMU (kg) |
NA No. of atoms/kg |
ABEPN (MeV) |
UAEU (MeV) |
UAMU (kg) |
NA No. of atoms/kg |
||||
| 6 | 12 | 21 | 12 | 6.779 | 932.651 | 1.66260E-27 | 6.01467E+26 | 6.527 | 932.903 | 1.66305E-27 | 6.01304E+26 |
| 7 | 14 | 24 | 15 | 7.411 | 932.019 | 1.66147E-27 | 6.01875E+26 | 6.777 | 932.653 | 1.66260E-27 | 6.01466E+26 |
| 8 | 16 | 28 | 16 | 7.097 | 932.333 | 1.66203E-27 | 6.01672E+26 | 7.014 | 932.416 | 1.66218E-27 | 6.01619E+26 |
| 9 | 18 | 32 | 19 | 7.454 | 931.975 | 1.66140E-27 | 6.01903E+26 | 7.095 | 932.335 | 1.66204E-27 | 6.01671E+26 |
| 10 | 20 | 35 | 20 | 7.283 | 932.147 | 1.66170E-27 | 6.01792E+26 | 7.326 | 932.104 | 1.66163E-27 | 6.01820E+26 |
| 11 | 22 | 38 | 23 | 7.656 | 931.774 | 1.66104E-27 | 6.02033E+26 | 7.442 | 931.988 | 1.66142E-27 | 6.01895E+26 |
| 12 | 24 | 42 | 24 | 7.398 | 932.032 | 1.66150E-27 | 6.01867E+26 | 7.544 | 931.886 | 1.66124E-27 | 6.01961E+26 |
| 13 | 26 | 46 | 27 | 7.619 | 931.811 | 1.66110E-27 | 6.02009E+26 | 7.566 | 931.864 | 1.66120E-27 | 6.01975E+26 |
| 14 | 28 | 49 | 28 | 7.471 | 931.959 | 1.66137E-27 | 6.01914E+26 | 7.698 | 931.732 | 1.66096E-27 | 6.02060E+26 |
| 15 | 30 | 52 | 31 | 7.732 | 931.698 | 1.66090E-27 | 6.02082E+26 | 7.762 | 931.668 | 1.66085E-27 | 6.02101E+26 |
| 16 | 32 | 56 | 32 | 7.517 | 931.913 | 1.66129E-27 | 6.01944E+26 | 7.811 | 931.619 | 1.66076E-27 | 6.02133E+26 |
| 17 | 34 | 60 | 35 | 7.672 | 931.758 | 1.66101E-27 | 6.02044E+26 | 7.809 | 931.621 | 1.66076E-27 | 6.02132E+26 |
| 18 | 36 | 63 | 38 | 7.856 | 931.574 | 1.66068E-27 | 6.02162E+26 | 7.893 | 931.537 | 1.66062E-27 | 6.02186E+26 |
| 19 | 38 | 66 | 39 | 7.742 | 931.688 | 1.66088E-27 | 6.02089E+26 | 7.930 | 931.500 | 1.66055E-27 | 6.02210E+26 |
| 20 | 40 | 70 | 42 | 7.842 | 931.588 | 1.66071E-27 | 6.02153E+26 | 7.953 | 931.477 | 1.66051E-27 | 6.02225E+26 |
| 21 | 42 | 74 | 43 | 7.675 | 931.755 | 1.66100E-27 | 6.02045E+26 | 7.940 | 931.490 | 1.66053E-27 | 6.02217E+26 |
| 22 | 44 | 77 | 46 | 7.822 | 931.608 | 1.66074E-27 | 6.02141E+26 | 7.996 | 931.434 | 1.66043E-27 | 6.02253E+26 |
| 23 | 46 | 80 | 49 | 7.942 | 931.488 | 1.66053E-27 | 6.02218E+26 | 8.018 | 931.412 | 1.66039E-27 | 6.02267E+26 |
| 24 | 48 | 84 | 50 | 7.798 | 931.632 | 1.66078E-27 | 6.02125E+26 | 8.026 | 931.404 | 1.66038E-27 | 6.02272E+26 |
| 25 | 50 | 88 | 53 | 7.863 | 931.567 | 1.66067E-27 | 6.02167E+26 | 8.007 | 931.423 | 1.66041E-27 | 6.02260E+26 |
| 26 | 52 | 91 | 56 | 7.958 | 931.472 | 1.66050E-27 | 6.02229E+26 | 8.045 | 931.385 | 1.66034E-27 | 6.02285E+26 |
| 27 | 54 | 94 | 57 | 7.873 | 931.557 | 1.66065E-27 | 6.02173E+26 | 8.057 | 931.373 | 1.66032E-27 | 6.02292E+26 |
| 28 | 56 | 98 | 60 | 7.918 | 931.512 | 1.66057E-27 | 6.02203E+26 | 8.056 | 931.374 | 1.66032E-27 | 6.02292E+26 |
| 29 | 58 | 102 | 63 | 7.956 | 931.474 | 1.66050E-27 | 6.02227E+26 | 8.034 | 931.396 | 1.66036E-27 | 6.02277E+26 |
| 30 | 60 | 105 | 66 | 8.020 | 931.410 | 1.66039E-27 | 6.02269E+26 | 8.060 | 931.370 | 1.66032E-27 | 6.02294E+26 |
| 31 | 62 | 108 | 67 | 7.946 | 931.484 | 1.66052E-27 | 6.02221E+26 | 8.065 | 931.365 | 1.66031E-27 | 6.02297E+26 |
| 32 | 64 | 112 | 70 | 7.972 | 931.458 | 1.66047E-27 | 6.02238E+26 | 8.058 | 931.372 | 1.66032E-27 | 6.02293E+26 |
| 33 | 66 | 116 | 73 | 7.993 | 931.437 | 1.66044E-27 | 6.02251E+26 | 8.034 | 931.396 | 1.66036E-27 | 6.02277E+26 |
| 34 | 68 | 119 | 74 | 7.924 | 931.506 | 1.66056E-27 | 6.02207E+26 | 8.051 | 931.379 | 1.66033E-27 | 6.02289E+26 |
| 35 | 70 | 122 | 77 | 7.973 | 931.457 | 1.66047E-27 | 6.02238E+26 | 8.051 | 931.379 | 1.66033E-27 | 6.02289E+26 |
| 36 | 72 | 126 | 80 | 7.986 | 931.444 | 1.66045E-27 | 6.02247E+26 | 8.041 | 931.389 | 1.66035E-27 | 6.02282E+26 |
| 37 | 74 | 130 | 83 | 7.996 | 931.434 | 1.66043E-27 | 6.02253E+26 | 8.015 | 931.415 | 1.66040E-27 | 6.02265E+26 |
| 38 | 76 | 133 | 84 | 7.935 | 931.495 | 1.66054E-27 | 6.02213E+26 | 8.026 | 931.404 | 1.66038E-27 | 6.02272E+26 |
| 39 | 78 | 136 | 87 | 7.969 | 931.461 | 1.66048E-27 | 6.02236E+26 | 8.022 | 931.408 | 1.66039E-27 | 6.02270E+26 |
| 40 | 80 | 140 | 90 | 7.975 | 931.455 | 1.66047E-27 | 6.02239E+26 | 8.009 | 931.421 | 1.66041E-27 | 6.02261E+26 |
| 41 | 82 | 144 | 93 | 7.977 | 931.453 | 1.66047E-27 | 6.02241E+26 | 7.982 | 931.448 | 1.66046E-27 | 6.02244E+26 |
| 42 | 84 | 147 | 94 | 7.921 | 931.509 | 1.66057E-27 | 6.02204E+26 | 7.989 | 931.441 | 1.66044E-27 | 6.02248E+26 |
| 43 | 86 | 150 | 97 | 7.945 | 931.485 | 1.66052E-27 | 6.02220E+26 | 7.982 | 931.448 | 1.66046E-27 | 6.02244E+26 |
| 44 | 88 | 154 | 100 | 7.945 | 931.485 | 1.66052E-27 | 6.02220E+26 | 7.966 | 931.463 | 1.66048E-27 | 6.02234E+26 |
| 45 | 90 | 158 | 103 | 7.942 | 931.488 | 1.66053E-27 | 6.02218E+26 | 7.940 | 931.490 | 1.66053E-27 | 6.02216E+26 |
| 46 | 92 | 161 | 106 | 7.957 | 931.472 | 1.66050E-27 | 6.02228E+26 | 7.942 | 931.488 | 1.66053E-27 | 6.02218E+26 |
| 47 | 94 | 164 | 107 | 7.907 | 931.523 | 1.66059E-27 | 6.02196E+26 | 7.933 | 931.497 | 1.66054E-27 | 6.02212E+26 |
| 48 | 96 | 168 | 110 | 7.902 | 931.528 | 1.66060E-27 | 6.02192E+26 | 7.916 | 931.514 | 1.66057E-27 | 6.02201E+26 |
| 49 | 98 | 172 | 113 | 7.895 | 931.535 | 1.66061E-27 | 6.02188E+26 | 7.889 | 931.541 | 1.66062E-27 | 6.02184E+26 |
| 50 | 100 | 175 | 116 | 7.906 | 931.524 | 1.66059E-27 | 6.02194E+26 | 7.889 | 931.541 | 1.66062E-27 | 6.02184E+26 |
| 51 | 102 | 178 | 119 | 7.913 | 931.517 | 1.66058E-27 | 6.02199E+26 | 7.878 | 931.552 | 1.66064E-27 | 6.02177E+26 |
| 52 | 104 | 182 | 122 | 7.902 | 931.528 | 1.66060E-27 | 6.02192E+26 | 7.860 | 931.570 | 1.66067E-27 | 6.02165E+26 |
| 53 | 106 | 186 | 123 | 7.840 | 931.590 | 1.66071E-27 | 6.02152E+26 | 7.833 | 931.597 | 1.66072E-27 | 6.02148E+26 |
| 54 | 108 | 189 | 126 | 7.846 | 931.584 | 1.66070E-27 | 6.02156E+26 | 7.830 | 931.600 | 1.66073E-27 | 6.02146E+26 |
| 55 | 110 | 192 | 129 | 7.850 | 931.580 | 1.66069E-27 | 6.02159E+26 | 7.818 | 931.612 | 1.66075E-27 | 6.02138E+26 |
| 56 | 112 | 196 | 132 | 7.837 | 931.593 | 1.66072E-27 | 6.02150E+26 | 7.799 | 931.631 | 1.66078E-27 | 6.02126E+26 |
| 57 | 114 | 200 | 135 | 7.823 | 931.607 | 1.66074E-27 | 6.02141E+26 | 7.772 | 931.658 | 1.66083E-27 | 6.02108E+26 |
| 58 | 116 | 203 | 138 | 7.823 | 931.607 | 1.66074E-27 | 6.02141E+26 | 7.767 | 931.663 | 1.66084E-27 | 6.02105E+26 |
| 59 | 118 | 206 | 141 | 7.821 | 931.609 | 1.66074E-27 | 6.02140E+26 | 7.754 | 931.676 | 1.66086E-27 | 6.02096E+26 |
| 60 | 120 | 210 | 142 | 7.767 | 931.663 | 1.66084E-27 | 6.02105E+26 | 7.734 | 931.696 | 1.66090E-27 | 6.02084E+26 |
| 61 | 122 | 214 | 145 | 7.751 | 931.679 | 1.66087E-27 | 6.02095E+26 | 7.707 | 931.723 | 1.66095E-27 | 6.02066E+26 |
| 62 | 124 | 217 | 148 | 7.749 | 931.681 | 1.66087E-27 | 6.02093E+26 | 7.701 | 931.729 | 1.66096E-27 | 6.02062E+26 |
| 63 | 126 | 220 | 151 | 7.745 | 931.685 | 1.66088E-27 | 6.02091E+26 | 7.687 | 931.743 | 1.66098E-27 | 6.02053E+26 |
| 64 | 128 | 224 | 154 | 7.727 | 931.703 | 1.66091E-27 | 6.02079E+26 | 7.667 | 931.763 | 1.66102E-27 | 6.02040E+26 |
| 65 | 130 | 228 | 157 | 7.709 | 931.721 | 1.66094E-27 | 6.02068E+26 | 7.640 | 931.790 | 1.66107E-27 | 6.02023E+26 |
| 66 | 132 | 231 | 160 | 7.703 | 931.727 | 1.66095E-27 | 6.02063E+26 | 7.632 | 931.798 | 1.66108E-27 | 6.02018E+26 |
| 67 | 134 | 234 | 163 | 7.695 | 931.735 | 1.66097E-27 | 6.02059E+26 | 7.617 | 931.813 | 1.66111E-27 | 6.02008E+26 |
| 68 | 136 | 238 | 166 | 7.676 | 931.754 | 1.66100E-27 | 6.02046E+26 | 7.597 | 931.833 | 1.66114E-27 | 6.01995E+26 |
| 69 | 138 | 242 | 167 | 7.629 | 931.801 | 1.66109E-27 | 6.02016E+26 | 7.570 | 931.860 | 1.66119E-27 | 6.01978E+26 |
| 70 | 140 | 245 | 170 | 7.621 | 931.809 | 1.66110E-27 | 6.02011E+26 | 7.561 | 931.869 | 1.66121E-27 | 6.01972E+26 |
| 71 | 142 | 248 | 173 | 7.612 | 931.818 | 1.66112E-27 | 6.02005E+26 | 7.545 | 931.885 | 1.66124E-27 | 6.01962E+26 |
| 72 | 144 | 252 | 176 | 7.592 | 931.838 | 1.66115E-27 | 6.01992E+26 | 7.525 | 931.905 | 1.66127E-27 | 6.01948E+26 |
| 73 | 146 | 256 | 179 | 7.571 | 931.859 | 1.66119E-27 | 6.01979E+26 | 7.498 | 931.932 | 1.66132E-27 | 6.01931E+26 |
| 74 | 148 | 259 | 182 | 7.561 | 931.869 | 1.66121E-27 | 6.01972E+26 | 7.488 | 931.942 | 1.66134E-27 | 6.01925E+26 |
| 75 | 150 | 262 | 185 | 7.550 | 931.880 | 1.66123E-27 | 6.01965E+26 | 7.472 | 931.958 | 1.66137E-27 | 6.01914E+26 |
| 76 | 152 | 266 | 188 | 7.528 | 931.902 | 1.66127E-27 | 6.01951E+26 | 7.451 | 931.979 | 1.66140E-27 | 6.01901E+26 |
| 77 | 154 | 270 | 191 | 7.506 | 931.924 | 1.66130E-27 | 6.01937E+26 | 7.425 | 932.005 | 1.66145E-27 | 6.01884E+26 |
| 78 | 156 | 273 | 194 | 7.494 | 931.936 | 1.66133E-27 | 6.01929E+26 | 7.414 | 932.016 | 1.66147E-27 | 6.01877E+26 |
| 79 | 158 | 276 | 197 | 7.481 | 931.949 | 1.66135E-27 | 6.01920E+26 | 7.397 | 932.033 | 1.66150E-27 | 6.01866E+26 |
| 80 | 160 | 280 | 200 | 7.458 | 931.972 | 1.66139E-27 | 6.01906E+26 | 7.376 | 932.054 | 1.66154E-27 | 6.01853E+26 |
| 81 | 162 | 284 | 203 | 7.436 | 931.994 | 1.66143E-27 | 6.01891E+26 | 7.350 | 932.079 | 1.66158E-27 | 6.01836E+26 |
| 82 | 164 | 287 | 206 | 7.422 | 932.008 | 1.66146E-27 | 6.01882E+26 | 7.339 | 932.091 | 1.66160E-27 | 6.01828E+26 |
| 83 | 166 | 290 | 209 | 7.407 | 932.023 | 1.66148E-27 | 6.01872E+26 | 7.322 | 932.108 | 1.66163E-27 | 6.01817E+26 |
| 84 | 168 | 294 | 212 | 7.384 | 932.046 | 1.66152E-27 | 6.01857E+26 | 7.301 | 932.129 | 1.66167E-27 | 6.01804E+26 |
| 85 | 170 | 298 | 215 | 7.360 | 932.070 | 1.66157E-27 | 6.01842E+26 | 7.275 | 932.155 | 1.66172E-27 | 6.01787E+26 |
| 86 | 172 | 301 | 218 | 7.345 | 932.085 | 1.66159E-27 | 6.01832E+26 | 7.263 | 932.167 | 1.66174E-27 | 6.01779E+26 |
| 87 | 174 | 304 | 219 | 7.316 | 932.114 | 1.66164E-27 | 6.01814E+26 | 7.245 | 932.185 | 1.66177E-27 | 6.01768E+26 |
| 88 | 176 | 308 | 222 | 7.293 | 932.137 | 1.66169E-27 | 6.01798E+26 | 7.224 | 932.206 | 1.66181E-27 | 6.01754E+26 |
| 89 | 178 | 312 | 225 | 7.269 | 932.161 | 1.66173E-27 | 6.01783E+26 | 7.199 | 932.231 | 1.66185E-27 | 6.01738E+26 |
| 90 | 180 | 315 | 228 | 7.253 | 932.177 | 1.66176E-27 | 6.01773E+26 | 7.186 | 932.244 | 1.66188E-27 | 6.01730E+26 |
| 91 | 182 | 318 | 231 | 7.237 | 932.193 | 1.66179E-27 | 6.01763E+26 | 7.168 | 932.262 | 1.66191E-27 | 6.01718E+26 |
| 92 | 184 | 322 | 234 | 7.213 | 932.217 | 1.66183E-27 | 6.01747E+26 | 7.147 | 932.283 | 1.66195E-27 | 6.01705E+26 |
| 93 | 186 | 326 | 237 | 7.189 | 932.241 | 1.66187E-27 | 6.01731E+26 | 7.122 | 932.308 | 1.66199E-27 | 6.01688E+26 |
| 94 | 188 | 329 | 240 | 7.172 | 932.258 | 1.66190E-27 | 6.01721E+26 | 7.108 | 932.322 | 1.66201E-27 | 6.01680E+26 |
| 95 | 190 | 332 | 243 | 7.155 | 932.275 | 1.66193E-27 | 6.01710E+26 | 7.091 | 932.339 | 1.66205E-27 | 6.01668E+26 |
| 96 | 192 | 336 | 246 | 7.130 | 932.300 | 1.66198E-27 | 6.01694E+26 | 7.070 | 932.360 | 1.66208E-27 | 6.01655E+26 |
| 97 | 194 | 340 | 249 | 7.106 | 932.324 | 1.66202E-27 | 6.01678E+26 | 7.045 | 932.385 | 1.66213E-27 | 6.01638E+26 |
| 98 | 196 | 343 | 252 | 7.088 | 932.342 | 1.66205E-27 | 6.01667E+26 | 7.031 | 932.399 | 1.66215E-27 | 6.01629E+26 |
| 99 | 198 | 346 | 255 | 7.070 | 932.360 | 1.66208E-27 | 6.01655E+26 | 7.013 | 932.417 | 1.66218E-27 | 6.01618E+26 |
| 100 | 200 | 350 | 258 | 7.045 | 932.385 | 1.66213E-27 | 6.01639E+26 | 6.992 | 932.438 | 1.66222E-27 | 6.01604E+26 |
| 101 | 202 | 354 | 261 | 7.021 | 932.409 | 1.66217E-27 | 6.01623E+26 | 6.967 | 932.463 | 1.66227E-27 | 6.01588E+26 |
| 102 | 204 | 357 | 264 | 7.002 | 932.428 | 1.66220E-27 | 6.01611E+26 | 6.953 | 932.477 | 1.66229E-27 | 6.01579E+26 |
| 103 | 206 | 360 | 269 | 6.990 | 932.440 | 1.66223E-27 | 6.01603E+26 | 6.934 | 932.496 | 1.66232E-27 | 6.01567E+26 |
| 104 | 208 | 364 | 272 | 6.965 | 932.465 | 1.66227E-27 | 6.01587E+26 | 6.913 | 932.517 | 1.66236E-27 | 6.01554E+26 |
| 105 | 210 | 368 | 275 | 6.939 | 932.491 | 1.66232E-27 | 6.01571E+26 | 6.889 | 932.541 | 1.66241E-27 | 6.01538E+26 |
| 106 | 212 | 371 | 278 | 6.920 | 932.510 | 1.66235E-27 | 6.01558E+26 | 6.874 | 932.556 | 1.66243E-27 | 6.01529E+26 |
| 107 | 214 | 374 | 281 | 6.901 | 932.529 | 1.66238E-27 | 6.01546E+26 | 6.856 | 932.574 | 1.66246E-27 | 6.01517E+26 |
| 108 | 216 | 378 | 284 | 6.875 | 932.555 | 1.66243E-27 | 6.01529E+26 | 6.835 | 932.595 | 1.66250E-27 | 6.01503E+26 |
| 109 | 218 | 382 | 287 | 6.850 | 932.580 | 1.66248E-27 | 6.01513E+26 | 6.811 | 932.619 | 1.66254E-27 | 6.01488E+26 |
| 110 | 220 | 385 | 290 | 6.830 | 932.600 | 1.66251E-27 | 6.01500E+26 | 6.796 | 932.634 | 1.66257E-27 | 6.01478E+26 |
| 111 | 222 | 388 | 293 | 6.810 | 932.620 | 1.66255E-27 | 6.01487E+26 | 6.777 | 932.653 | 1.66260E-27 | 6.01466E+26 |
| 112 | 224 | 392 | 296 | 6.784 | 932.646 | 1.66259E-27 | 6.01471E+26 | 6.756 | 932.674 | 1.66264E-27 | 6.01453E+26 |
| 113 | 226 | 396 | 299 | 6.759 | 932.671 | 1.66264E-27 | 6.01454E+26 | 6.732 | 932.698 | 1.66268E-27 | 6.01437E+26 |
| 114 | 228 | 399 | 302 | 6.739 | 932.691 | 1.66267E-27 | 6.01441E+26 | 6.717 | 932.713 | 1.66271E-27 | 6.01427E+26 |
| 115 | 230 | 402 | 305 | 6.718 | 932.712 | 1.66271E-27 | 6.01428E+26 | 6.698 | 932.731 | 1.66274E-27 | 6.01415E+26 |
| 116 | 232 | 406 | 308 | 6.692 | 932.738 | 1.66276E-27 | 6.01411E+26 | 6.678 | 932.752 | 1.66278E-27 | 6.01402E+26 |
| 117 | 234 | 410 | 311 | 6.667 | 932.763 | 1.66280E-27 | 6.01395E+26 | 6.654 | 932.776 | 1.66282E-27 | 6.01387E+26 |
| 118 | 236 | 413 | 314 | 6.646 | 932.784 | 1.66284E-27 | 6.01381E+26 | 6.638 | 932.791 | 1.66285E-27 | 6.01377E+26 |
7. Conclusions
Acknowledgments
References
- Tiesinga, E., Mohr, P. J., Newell, D. B. & Taylor, B. N. “CODATA recommended values of the fundamental physical constants: 2018”. Rev. Mod. Phys. 93, 025010, 2021. [CrossRef]
- Workman R.L. et al. (Particle Data Group), Prog. Theor. Exp. Phys. 2022, 083C01, 2022.
- Becker Peter and Bettin Horst. “The Avogadro constant: Determining the number of atoms in a single-crystal 28Si sphere”. Phil. Trans. R. Soc. A.3693925–3935, 2011. [CrossRef]
- K. Fujii, E. Massa, H. Bettin, N. Kuramoto and G. Mana. “Avogadro constant measurements using enriched 28Si monocrystals”. Bureau International des Poids et Mesures. Metrologia, 55, L1–L4, 2018. [CrossRef]
- Bengt Nordén. “The Mole, Avogadro’s Number and Albert Einstein”. Molecular Frontiers Journal. 5, 66-78, 2021. [CrossRef]
- Michalis Siafarikas, Georgios Stylos, Theodoros Chatzimitakos, Konstantinos Georgopoulos, Constantine Kosmidis and Konstantinos T Kotsis. “Experimental teaching of the Avogadro constant”. Phys. Educ. 58 (2023) 065026 (12pp). [CrossRef]
- Seshavatharam U.V.S and Lakshminarayana S. “A very brief review on strong and electroweak mass formula pertaining to 4G model of final unification”. Proceedings of the DAE Symp. on Nucl. Phys. 67,1173,2023.
- Seshavatharam U.V.S and Lakshminarayana S. “Exploring condensed matter physics with refined electroweak term of the strong and electroweak mass formula”. World Scientific News .193(2) 105-13, 2024.
- Seshavatharam U.V.S and Lakshminarayana S. “Inferring and confirming the rest mass of electron neutrino with neutron life time and strong coupling constant via 4G model of final unification”. World Scientific News 191, 127-156, 2024.
- Seshavatharam U.V.S and Lakshminarayana. “Understanding nuclear stability range with 4G model of nuclear charge”. World Scientific News. 177, 118-136, 2023.
- Seshavatharam U.V.S, S. Lakshminarayana, H. K. Cherop and K. M. Khanna, “Three Unified Nuclear Binding Energy Formulae. World Scientific News”. 163, 30-77, 2022.
- Seshavatharam U.V.S, Lakshminarayana, S., On the Combined Role of Strong and Electroweak Interactions in Understanding Nuclear Binding Energy Scheme. Mapana Journal of Sciences, 20(1), 1-18, 2021. [CrossRef]
- Seshavatharam U.V.S and Lakshminarayana S., “Strong and Weak Interactions in Ghahramany’s Integrated Nuclear Binding Energy Formula”. World Scientific News, 161, 111-129, 2021.
- Seshavatharam U. V. S., Gunavardhana Naidu T and Lakshminarayana S. “To confirm the existence of heavy weak fermion of rest energy 585 GeV”. AIP Conf. Proc. 2451 p 020003, 2022.
- Seshavatharam U.V.S and Lakshminarayana S. “4G model of final unification – A brief report”. Journal of Physics: Conference Series 2197 p 012029, 2022. [CrossRef]
- Myers W. D. and Swiatecki W. J. “Nuclear Properties According to the Thomas-Fermi Model”. LBL-36557 Rev. UC-413, 1995. [CrossRef]
- Myers W. D. and Swiatecki W. J. “Table of nuclear masses according to the 1994 Thomas-Fermi model”. United States: N. p., 1994. Web.
- X.W. Xia, Y. Lim, P.W. Zhao et al. “The limits of the nuclear landscape explored by the relativistic continuum Hartree–Bogoliubov theory”. Atomic Data and Nuclear Data Tables. 121–122, 1-215, 2018. [CrossRef]
- Djelloul Benzaid, Salaheddine Bentridi, Abdelkader Kerraci, Naima Amrani. “Bethe–Weizsa¨cker semiempirical mass formula coefficients 2019 update based on AME2016”. NUCL. SCI. TECH. 31:9, 2020. [CrossRef]
- Gao, Z.P., Wang, YJ., Lü, HL. et al., “Machine learning the nuclear mass”. NUCL. SCI. TECH. 32, 109, 2021. [CrossRef]
- J.M. Gates et al. “Towards the Discovery of New Elements: Production of Livermorium (Z=116) with 50Ti”. arXiv:2407.16079v1 [nucl-ex], 2024.
- Ghiorso Albert. “Einsteinium and Fermium. Chemical and Engineering News”. 81 (36): 174–175, 2003. [CrossRef]
- Kondev, F. G.; Wang, M.; Huang, W. J.; Naimi, S.; Audi, G. “The NUBASE2020 evaluation of nuclear properties (PDF)”. Chinese Physics C. 45 (3): 030001, 2021. [CrossRef]
- Peter Möller. “The limits of the nuclear chart set by fission and alpha decay”. EPJ Web Conf. 131, 03002, 2016. [CrossRef]
- Oganessian, Yu & Rykaczewski, Krzysztof. “A beachhead on the island of stability”. Physics Today. 68. 32-38, 2015.
- Hermann, J., Spencer, J., Choo, K. et al. “Ab initio quantum chemistry with neural-network wave functions”. Nat. Rev. Chem. 7, 692–709. 2023. [CrossRef]
- Kirkpatrick, J. et al. “Pushing the frontiers of density functionals by solving the fractional electron problem”. Science, 374(6573), 2021. [CrossRef]
- Unke, O. T. et al. “Machine learning force fields”. Chemical Reviews, 121(16), 10142–10186,2021. [CrossRef]
- Erler, J., Birge, N., Kortelainen, M. et al. “The limits of the nuclear landscape”. Nature 486, 509–512, 2012. [CrossRef]
- Tsunoda, N., Otsuka, T., Takayanagi, K. et al. “The impact of nuclear shape on the emergence of the neutron dripline”. Nature 587, 66–71, 2020. [CrossRef]
- Léo Neufcourt, Yuchen Cao, Samuel Giuliani, Witold Nazarewicz, Erik Olsen, Oleg B. Tarasov. “Beyond the proton drip line: Bayesian analysis of proton emitting nuclei”. Phys. Rev. C 101, 014319, 2020. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




