1. Introduction
Total global energy demand increases by 0.7% until 2030 in the stated policies scenario (STEPS), in the announced commitments scenario (APS), total energy demand decreases by an average of 0.1% per year until 2030 due to the implementation faster renewable sources, increased energy efficiency, and in the scenario with net zero emissions until 2050 (NZE), decreases by 1.2% per year until 2030, [
1]. Population is a major factor in energy demand. On average, the population growth rate is expected to slow over time, but the global population is approaching 9.7 billion by 2050. At the same time, it is estimated that the likely increase by 2050 of 1.7 million people will be in cities.
The predictions of the increase in the average temperature until 2100 are, in degrees Celsius, 2.4 in the STEP scenario, 1.7 in the APC and 1.4 in the NZE, which led to the adoption of
emission regulations, through the Paris agreement, [
2], and at the EU level, [
3].
As the proportion of energy generated from renewable sources, particularly solar and wind, continues to rise, a clear divergence emerges between these sustainable alternatives and traditional fossil fuel-based energy production.
Renewable solar energy captured by PV photovoltaic panels is continuously increasing in any scenario, and that from panels mounted on the building is estimated to be half of the total, mainly due to the continuously decreasing costs, [
1].
The total capacity installed in renewable sources has doubled in the last 10 years, reaching over 3,370 GW in 2022, of which hydro over 1,390 GW, wind over 890 GW, solar energy over 1,050 GW, bioenergy almost 150 GW, geothermal energy almost 15 GW. These capacities are connected to the electrical network, [
4].
This growing gap reflects not only a shift in the sources of energy but also fundamental differences in their environmental impact, sustainability, and long-term viability. Renewable sources offer clean, sustainable energy with minimal greenhouse gas emissions and reduced environmental degradation. In contrast, fossil fuel-based energy production remains inherently linked to carbon emissions, air and water pollution, and finite resource depletion.
Conventional fuels are predicted to decrease continuously in the APS and NZE scenarios, but the liquid and gaseous co-fuels still remain almost constant for the STEPS scenario, [
1].
The outlook for coal depends heavily on the strength of the world's resolve to tackle climate change. In STEPS, coal demand falls gradually, in APS, it falls by 25% below current levels by 2030 and by 75% by 2050, and in the NZE scenario, global demand falls by about 45% by 2030 and by 90 % by 2050.
Moreover, the transition towards renewable energy signifies a broader societal and economic shift towards cleaner, more resilient energy systems. Investments in renewable energy infrastructure spur innovation, create jobs, and enhance energy security while reducing dependency on volatile fossil fuel markets.
Electricity, being an ordered form of energy, is more versatile in use than other types of energy, being able to be efficiently converted into other forms. For example, electricity can be converted into mechanical form with close to 100% efficiency or into heat with 100% efficiency. Thermal energy, on the other hand, cannot be converted to electricity with such high efficiency because it is a disordered form of energy, the overall thermal to electrical conversion efficiency is less than 50%. A disadvantage of electricity is that it cannot be easily stored on a large scale. Almost all electricity used today is consumed as it is generated. This presents no difficulty in conventional power plants, where the fuel consumption is continuously varied according to the load requirement. Wind and photovoltaics (PV), both being intermittent sources of energy, cannot meet the load demand at all times, 24 hours a day, 365 days a year, [
6]. Therefore, energy storage is a desirable feature to incorporate with such power systems to significantly improve load availability, a key requirement for any power system. Current and future energy storage technologies that may be considered for stand-alone wind or photovoltaic power systems fall into the following broad categories: batteries; fly-wheel; compressed air; superconducting coil, pumped hydro plants, thermal energy storage, gravity storage, [
7].
In 2022,
emissions were over 37 Gt of
worldwide, of which the EU over 3 Gt, [
8].
Thus, as renewable energy continues to gain momentum, it not only reshapes the energy landscape but also represents a crucial step towards a more sustainable and climate-resilient future.
The intermittent nature of renewable sources imposes many technical and infrastructural challenges [
9].
Classic fossil fuel power plants, even the most modern ones, emit carbon dioxide (
) into the atmosphere, requiring the capture, storage and/or use of
from flue gases to achieve carbon neutrality [
10].
capture and storage (CCS) technologies offer a practical solution for reducing
emissions, playing a crucial role in mitigating climate change and achieving sustainable environmental goals [
11].
There are three methods for capturing
from power plants: pre-combustion carbon capture, oxy-fuel combustion and post-combustion carbon capture (PCC) [
12]. PCC is considered a viable and adoptable option due to its potential to retrofit existing fossil fuel power plants with minimal modifications, enabling low, near-zero
emissions [
13]. Technologies available for capturing
from thermal power plants considered as point sources are: absorption of
in amine-type solvents, such as mono-ethanolamine (MEA) [
14], diethanolamine (DEA) [
15], piperazine (PZ) [16 ] and amine mixtures [
17]. The costs of
capture through technologies that use amines vary between
$30-50/(ton) of
and become attractive at a
emission tax of over
$125/(t)
, [
18]. On the other hand, the capture of
from the air, distributed over it, is estimated between
$160/(t)
and
$270/(t)
, [
19].
storage costs depend a lot on the structure and capacity of the geological storage reservoir, [
20].
For the above reasons, alternative solutions for energy reuse of
were analyzed by using green hydrogen to transform, through methanization,
into other energy vectors such as methane,
, or methanol,
, [
21,
22].
To ensure climate neutrality, it is necessary to reduce carbon emissions including in all sectors, industrial, transport and buildings, which includes residential, commercial and public buildings. In 2018, buildings consumed 122 EJ (about a third of total final energy consumption), of which 30% for heating, 24% for cooking, 20% for water heating, 18% for lighting and other appliances and 8% for cooling. Thermal energy represents over two thirds and comes mainly from fossil fuels, mainly natural gas, which leads to about 3 Gt of
emissions. By 2050, the share of renewable sources in buildings will increase from 34% in 2018 to 89%, and the total energy consumption in buildings in 2050 would decrease by 14% compared to 2018, [
23].
It should be mentioned that, in the technologies for the production of different renewable energy vectors such as hydrogen through water electrolysis, [
24,
25], electricity through fuel cells, [
26,
27], or methane through methanization, [28, 29], generate energy thermal, [
30,
31].
Green hydrogen serves as a link between the renewable energy sector and the fossil fuel sector, playing a crucial role not only in the generation of synthetic fuels (methane and methanol) but also in energy storage, competing with other forms of storage. In addition, it will serve as an environmentally friendly feedstock for hydrogen-consuming industries, as well as for transportation and residential uses that are carbon neutral, [
31,
32,
33].
The process of storing energy to counterbalance the intermittent nature of renewable sources involves a spectrum of techniques and technologies, each with its own advantages and limitations.
Energy storage and its impact on the grid and transportation sectors have expanded globally in recent years as storage costs continue to fall and new opportunities are defined in a variety of industry sectors and applications. Grid-scale energy storage does not have strict power constraints, allowing a multitude of storage technologies to compete to provide current and future emerging grid flexibility services. Commercially available energy storage technologies, in different energy-power ratios, are: batteries, compressed air, hydropower with pumped storage, thermal energy storage, gravity storage, bidirectional hydrogen energy storage, [
4,
5] . Bidirectional hydrogen storage technology consists of three components, namely: 1) the charging system that includes electrolyzer modules, controllers, water treatment units, mass flow regulators, electrolyzer management system, compressor and rectifier; 2) the discharge system consists of stationary fuel cell modules, controllers, gas handling units, blowers, mass flow regulators, fuel cell management system and inverter; 3) the storage system usually includes pipes, tanks or a cavern, [5, 34, 35]. The main cost categories are: investment or capital costs, operation or functioning costs and decommissioning or decommissioning costs.
While green hydrogen storage stands out as a promising option, it's just one piece of the puzzle in the quest for reliable energy storage solutions.
The journey towards effective energy storage is not without its challenges. Significant capital investments are needed for infrastructure development, alongside ongoing operational costs. These investments drive innovation and research into more efficient storage methods, ensuring that the energy transition is not only sustainable but also economically viable in the long run.
As the research unfolds, it will delve into the complexities of energy storage, considering factors such as scalability, reliability, and environmental impact. By comprehensively analyzing these aspects, the study aims to provide insights into the development of a robust energy storage infrastructure capable of supporting a carbon-neutral energy system.
Regarding the transportation of hydrogen through pipelines, global achievements as of 2022 are modest, with a total length of about km, mostly for industrial use. For the use of hydrogen in the energy and residential sectors, the length of the pipelines, even if initially only a mix is used, will need to increase considerably, primarily due to the dispersion of consumers (for natural gas pipelines worldwide, the length is about );
Ultimately, the goal is to chart a course towards a future where energy storage solutions seamlessly integrate with renewable energy sources, facilitating a transition to a carbon-neutral energy landscape. Through careful examination and nuanced exploration, the research will contribute to shaping the roadmap for achieving absolute neutrality in emissions within the energy sector.
2. The carbon-neutral thermal energy sector
Although in 2022 there were over 2.4 billion people without access to clean food preparation and over 770 million people without access to electricity, [
36] total
emissions increased by 1% in 2022, reaching a record of 41 .5 gigatons of carbon dioxide (
) equivalent, a slower growth compared to 2021, [37, 38].
The percentage of thermal energy from renewable sources, excluding traditional biomass, increased to 11.5% in 2020, [10-15]. Renewable fuels continued to be biofuels that contributed 3.6% of the total fuel supply in 2020, [
12].
Renewable hydrogen was seen as a savior solution for decarbonisation, so in 2022 the number of electrolysis plants reached a capacity of about 1 gigawatt (GW). However, more than 95% of current hydrogen production still relies on fossil fuels [
39].
The solution of including carbon dioxide in other fuels is the most viable and future.
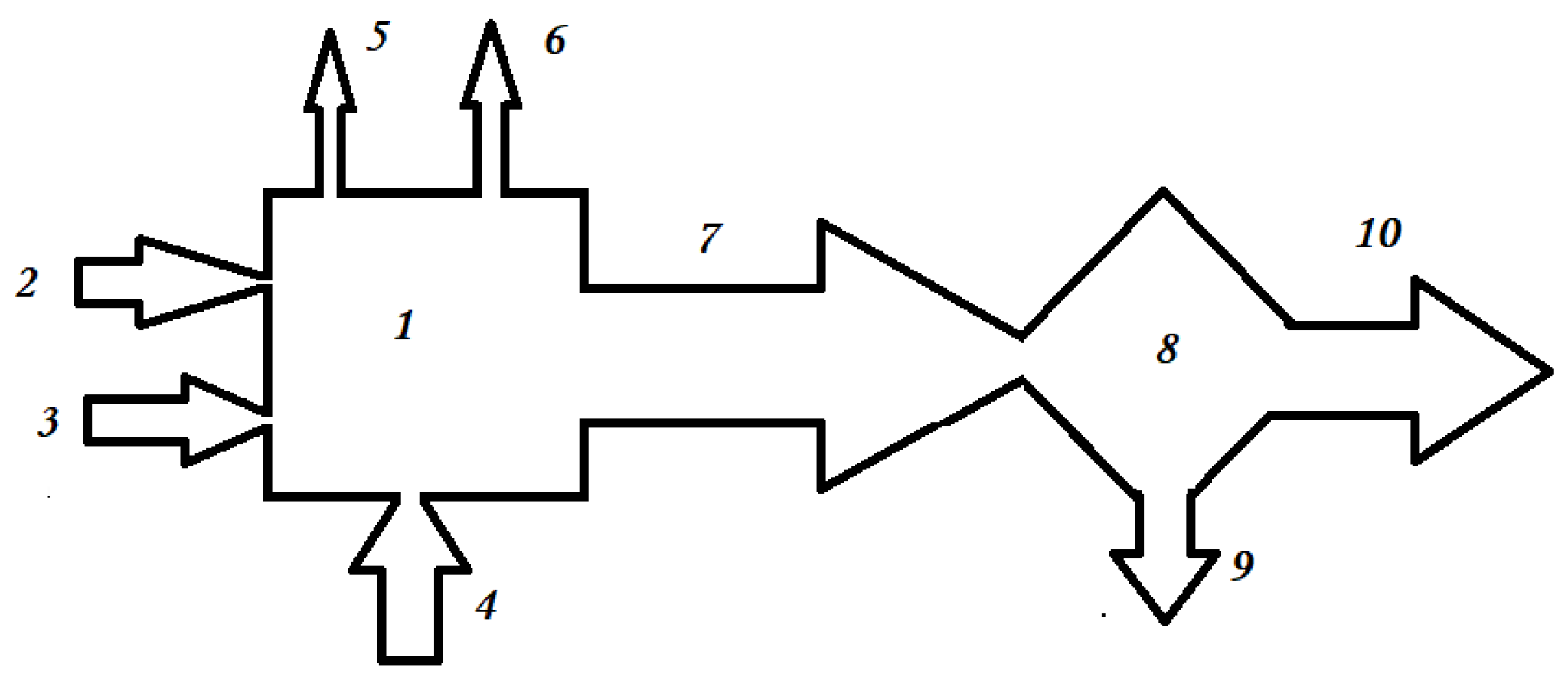
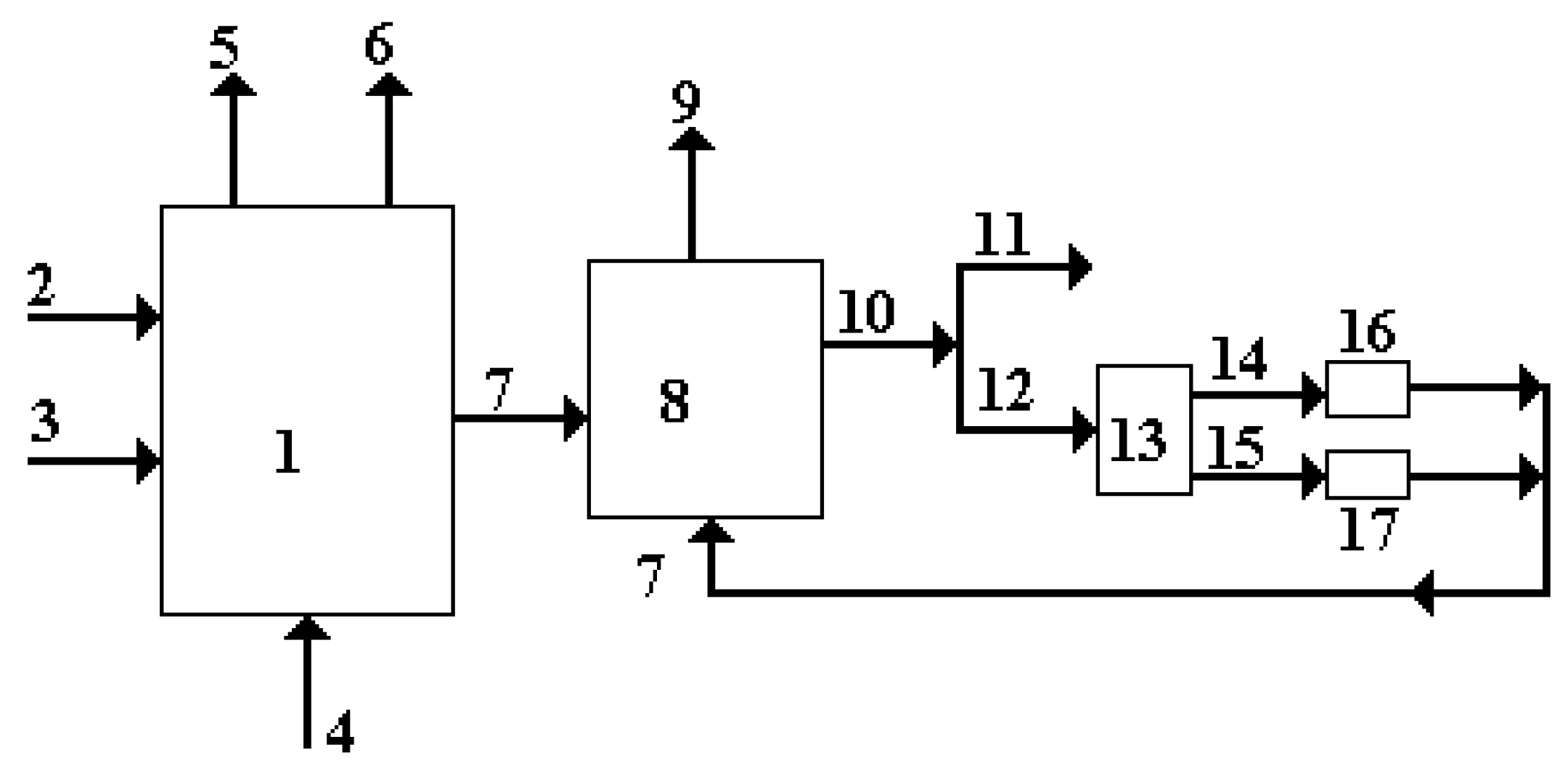
Technological developments for methane and methanol reactions already allow high yields and competitive prices. The capture of carbon dioxide, which can be carried out directly from the air or from combustion gases, is schematically represented in figure 1.
Figure 1.
Capturing carbon dioxide from combustion gases.
Figure 1.
Capturing carbon dioxide from combustion gases.
The notations are: 1 – Thermal energy plant with fossil fuel, usually natural gas; 2 – natural gas (GN) supply; 3 – air supply necessary for combustion; 4 – hydrogen supply, usually impure with other combustible components; 5 – electricity produced; 6 – thermal energy produced; 7 – combustion gases resulting from combustion; 8 – installation for capturing carbon dioxide from combustion gases; 9 – captured carbon dioxide with high purity (over 90%); 10 – combustion gases without , concentrations of the order of ppm.
As is known, the concentration in the atmosphere is continuously increasing, being about 425 ppm in March 2024 compared to about 421 ppm in March 2023 [
36]. The concept of direct
capture consists of capturing
directly from the air by circulating air through regenerative filters and has been used since the 1930s for submarines with cryogenic separation facilities. As a method to decrease atmospheric
concentration, DAC was first introduced by Lackner in 1999 [
40].
It should be noted that the methods of capturing from the air do not differ essentially from the methods of capturing it from the combustion gases [
41]. Solid solvents and strongly interacting adsorbents, including basic solvents, supported amine and ammonium materials, and metal-organic frameworks (MOFs), are described as primary classes of chemical adsorbents [42, 43]. What differs greatly are the concentrations in the flue gases and the impurities of other compounds in these gases [
44].
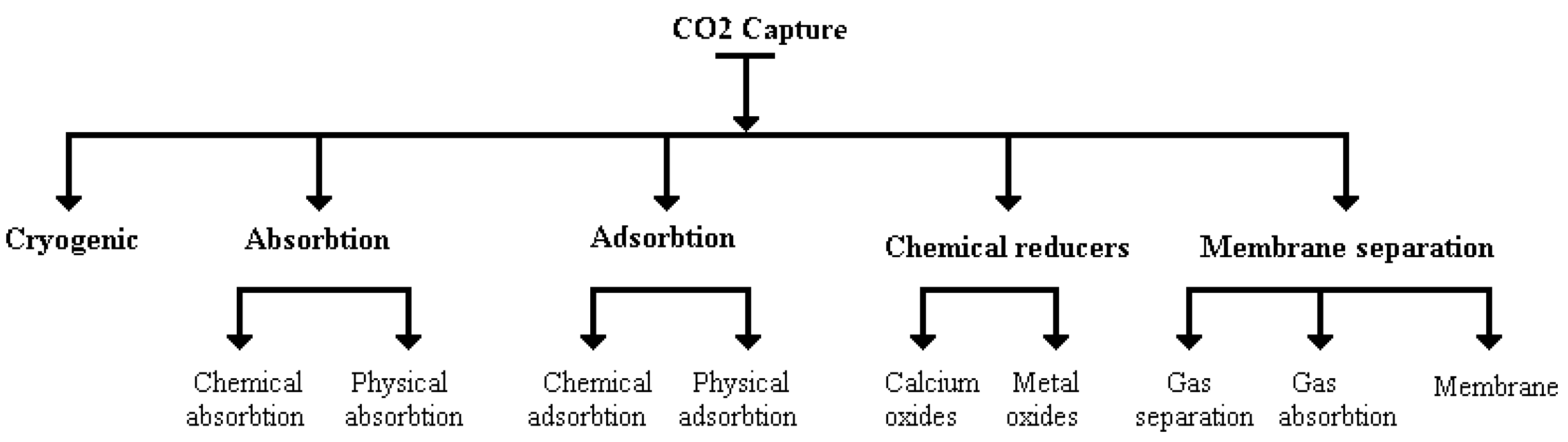
A comprehensive overview of
capture technologies is presented in
Figure 2, showing various methods and techniques used to capture
emissions [45, 46].
In general, the cryogenic separation of a gas mixture consists of fractional condensation and distillation at low temperatures and allows the capture of
with purity levels of 99.99% [
47]. The advantage of this process is that the recovery of
in pure form allows for liquid storage, which can be easily transported [
48]. Additionally, the process requires no chemical absorbent and can operate at atmospheric pressure, but the energy consumption is very high. The energy required for the separation of liquefied carbon dioxide is 0.125 kWh/kg
(449 kJ/kg
) [
49].
Since
is an acidic gas, the chemisorption of
from gas streams typically relies on acid-base neutralization [
50]. Chemical solvents for absorbing
from natural gas were developed more than 60 years ago. Chemisorption is effective for low
partial pressures, and carbonates or amine solutions are commonly used as solvents [
51,
52].
Among the most used solvents at present are amines, aqueous ammonia, alkalis, and calcium oxides or other metals. The absorbent based on amines binds
forming a chemical complex which, in the desorption unit, is removed from the solvent by countercurrent steam at 100-200 degrees Celsius and atmospheric pressure. Then, the adsorbent is cooled to 40-65 degrees Celsius and recycled in the absorption column [
53]. The capture efficiency is almost 98%, and the energy consumption is 3.6–4 GJ per ton of captured
.
Amine-based chemical absorption (MEA) could be replaced by the Aqueous Ammonia Process (AAP) to capture acid gases such as
,
,
,
, and
. Unlike amine scrubbing, AAP does not exhibit absorbent degradation or equipment corrosion by
and
. The energy requirement for absorbent regeneration is much lower than in the MEA process [
54]. Additionally, the main by-products from AAP are ammonium bicarbonate
ammonium sulfate
and ammonium nitrate (
, which are well-known fertilizers [
55].
Chemical absorption with alkali is based on the Solvay process from 1860, which is the chemical synthesis process of sodium carbonate (washing soda) [
54].
Chemical absorption with metal oxides is based on capturing in reaction with to form , which releases carbon dioxide through calcination. The composite materials used are lithium zirconate and lithium silicate [56, 57, 58, 59, 60].
and other acid gases such as can be physically absorbed in various solvents. Physical absorption involves no chemical reaction, so solvent regeneration is generally easier. The solubility of in the solvent increases with pressure and decreases at higher temperatures, and the trapped is released after depressurization. Commercially, physical absorption is used to remove acid gases from natural gas and to capture
from syngas during the production of ammonia, methanol, and hydrogen. Some
capture methodologies combine physical and chemical solvents. The most commonly applied examples are Amisol, a mixture of methanol and secondary amines, and Sulfinol, a mixture of amines such as diisopropylamine or methyl diethanolamine (MDEA) and the physical solvent sulfolane.
Pressure water washing (PWS) is one of the most desirable technologies for capturing from biogas because it can also remove and has low methane losses [61, 62, 63]. The process requires a large amount of water and generates a lot of wastewater and has low flexibility.
The organic solvents most used in physical absorption are Selexol (dimethyl ether polyethylene glycol), Rectisol (chilled methanol), and FLUOR (propylene carbonate) [64-67].
The use of physical adsorbents (zeolite or carbon) for
capture has received significant attention in recent years [
30]. The adsorption process depends on the thermodynamic properties that must be changed for the combustion gases to attach to a solid material. This adsorption can be either chemical (chemisorption) or physical (physisorption) [
54].
Activated carbon has been applied in a wide range of industrial processes [
68]. The surface of activated carbon contains heteroatoms that exist as acidic, basic, or neutral organic functional groups [
69]. The surface of activated carbon can be modified by incorporating heteroatoms such as nitrogen to enhance the specific adsorbate–adsorbent interaction. Nitrogen increases the number of primary groups, thereby altering the charge in the graphene layer. Therefore, the ability of activated carbon to adsorb
can be increased by introducing nitrogen functional groups into its structure [
70,
71].
Physical adsorption utilizes a molecular sieve designed to separate molecules based on their molecular size. The molecular sieve is a material with small pores of uniform size, and thus molecules larger than the pore diameter cannot enter the porous structure to be adsorbed. Molecular sieve technology is expensive, but it can be adapted to almost every carbon capture process [
53].
Membranes function as semi-permeable barriers that can separate components in a gas stream by concentration, electrical potential, or pressure gradients [
72]. Membranes are available in different materials (inorganic or polymeric) and can be either porous or dense (non-porous). Porous membranes act somewhat similarly to molecular sieves. There are three types of membrane materials: polymeric (organic) membranes, ceramic (inorganic) membranes, and hybrid membranes [
73].
Chemical reduction technology utilizes two reactors, an air reactor, and a fuel reactor. These reactors typically have fluidized beds that are coupled for carrier transport. In the air reactor, the oxidation of an "oxygen carrier," usually metal particles such as iron, manganese, or copper, occurs with the oxygen in the air. As a result of the reaction, metal oxides are formed. These compounds are transported to a second reactor, where they react with the fuel. Metal oxides are reduced during combustion, producing energy and flue gases in the form of and streams. Flue gases can be condensed to obtain pure [74, 75].
The costs associated with capture from industrial processes are around 60-100 EUR per ton, with the lowest costs observed for oxy-combustion processes [
42]. Due to the low concentration of
in the air, the price of direct capture is much higher, reaching over 1000 EUR per ton. A cost reduction is estimated until 2030 to be 30-50 EUR per ton of
[
76].
The following section employs an analytical methodology to analyze the costs of producing green hydrogen.
For the analysis of the efficiency and costs of
capture, a series of costs are defined in the specialized literature, the most significant of which are as follows [
77]:
The levelized cost of electricity,
este:
where
is expressed
is the levelized cost of electricity generation,
is the total capital cost,
is the fixed charge factor, FOM €/(yr) is the fixed operating and maintenance (O&M) cost,
€/(MWh) is the variable non-fuel O&M cost,
MJ/(MWh) is the net power plant heat rate,
€/(MJ) is the unit fuel cost, CF (fraction) is the plant capacity factor,
(MW) is the net plant capacity, and 8760 h/(yr) accounts for the total hours in an average year. All of the parameters in Equation represent their levelized values and it is assumed to remain constant over all the plant lifetime.
• Avoided
cost.
where
is the cost of
avoided €/
),
is the mass emission rate of
to the atmosphere based on the net capacities of each power plant, CCS and NCCS refer to plants with and without CCS, respectively.
• The cost of
capture
where
is the
capture cost €/
),
is the total mass of
captured per net MWh for the installation with Carbon Capture and Storage
(equal to
produced minus emissions). The cost of captured
excludes storage and transport. Capturing
from combustion gases becomes much easier and efficient due to its increased concentration, typically exceeding 5%, compared to the air where the concentration increase.
where:
the volume of
emitted through combustion is measured in cubic meters per cubic meter
for gaseous fuels or in cubic meters per kilogram
for liquid fuels (solid fuels are not considered);
– the volume of combustion gases released by stoichiometric (theoretical) combustion, or ;
the volume of air required for stoichiometric combustion.
Stoichiometric combustion is characterized by a unity excess air factor,. In this study, we will approach a statistical calculation of combustion, according to the lower calorific value, Hi. Based on our own research, we estimate the volumes of and the volumes of air and gases for stoichiometric combustion depending on the lower calorific value and the percentage moisture content, W [%].
For gaseous fuels: ; ; The composition of combustion gases was presented based on the quality of hydrocarbons through their calorific value.
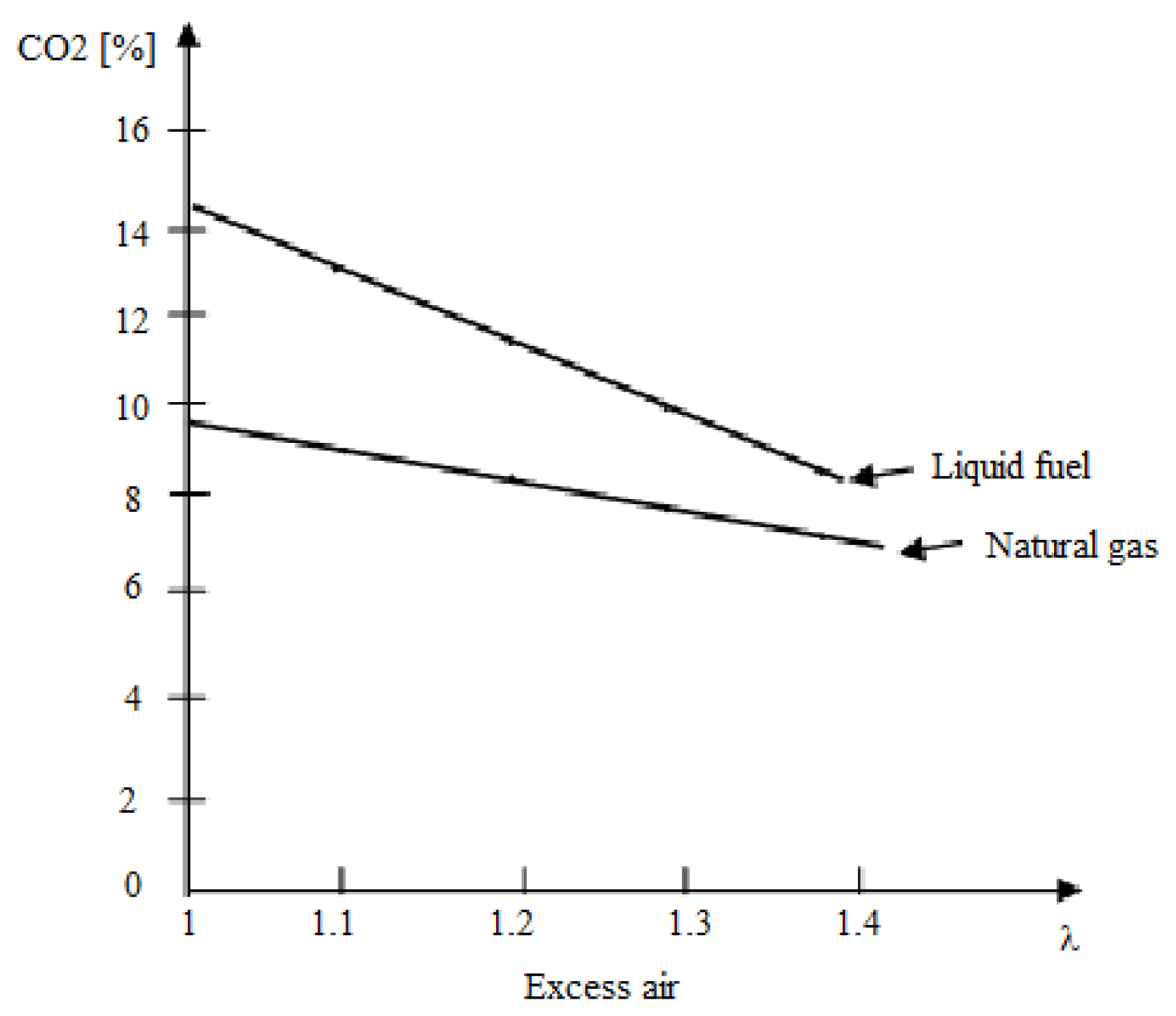
; ; where: , is expressed in , ; ; and W represents the fuel moisture content in percentages. An applied calculation for natural gas and diesel, depending on the excess air at the stack exit, indicates the carbon dioxide concentrations within the limits presented in figure 3.
Figure 3.
The variation of the content depending on the excess of air and fuel.
Figure 3.
The variation of the content depending on the excess of air and fuel.
For gas turbine plants, including combined cycle, flue gas concentrations can be reduced by about 30%. This decrease is often due to the use of high values for excess air in the combustion process. However, even with this decrease, concentrations remain significantly higher than those found in ambient air, making the capture process still economically viable. Thus, capture costs can experience a significant decrease, as the previously presented data also shows. The concentration of nitrogen, moisture and oxygen in the combustion gases also results from the ratio of their volumes to the total volume of the combustion gases: ; ; .
An increase in excess air leads to a decrease in concentration and flue gas (a decrease shown for the component in figure 3) and an increase in the component, which comes exclusively from excess air.
In order to more clearly characterize the disruptive effect of residual gases and on the methane conversion process, they are also presented by relating them to the active component or to the dominant component, thus appearing the criteria:
,
sau
,
. The pilot-scale plant experiments included the ranges shown in
Table 1 for flue gas residuals.
These aspects related to the presence alongside the component intended for capture and other gases, among which nitrogen () is dominant, complicate the methanation process compared to when it is reported only on the basis of the concentration at 100%.
Energy installations for hydrocarbons have low values for excess air (the reference being
, corresponding to a λ value of 1.16). On the other hand, gas turbine plants with and without a recovery boiler have a very high excess air, with typical values of
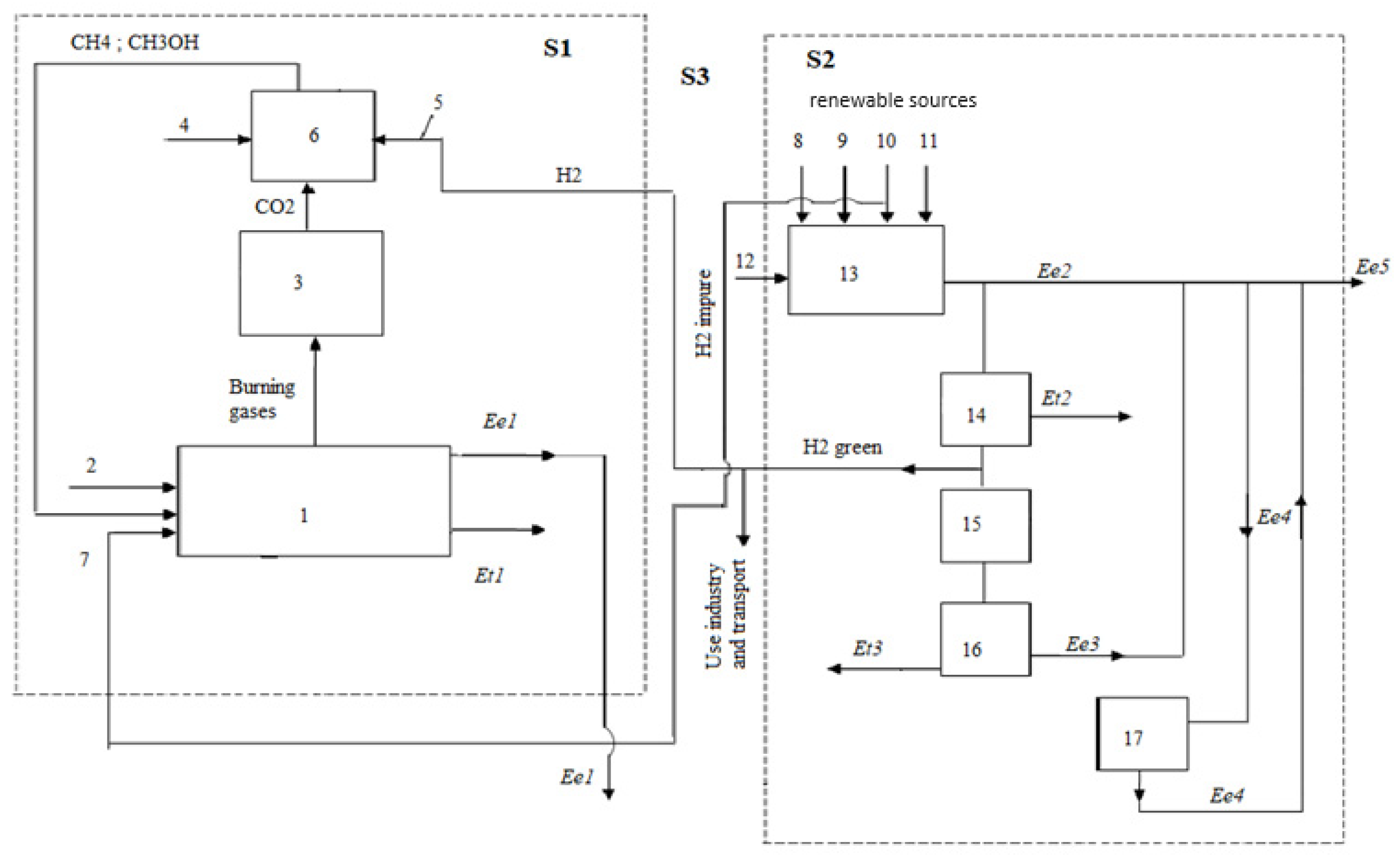
. Carbon dioxide capture targets the following environmental solutions, shown in
Figure 4.
For the possibility of using syngas in the scheme shown in
Figure 4, the coupling of the synthesis plant with potential residential, industrial, or energy consumers was presented. This approach is a consequence of the reduced calorific value of syngas due to the massive presence of nitrogen, as shown in
Table 1.
The notations used are: 1 – thermoelectric plant with fossil fuel, usually natural gas; 2 – natural gas (NG); 3 – air required for combustion; 4 – impure hydrogen obtained by thermochemical methods; 5 – electricity produced; 6 – thermal energy produced; 7 – burnt gases with content; 8 – capture facility; 9 – gases without or very low content of the order of ppm; 10 – concentrated ; 11 – storage by sequestration in the soil; 12 – for synthesis; 13 – the plant for transforming into energy vectors through synthesis processes; 14 – methane, ; 15 – methyl, ; 16 – thermal power plant with methane; 17 – energy installation on methyl.
The transformation of captured into fuels eliminates external storage, giving a recycling aspect to the or pair.
Currently, methanol production is mostly for industry, being used in various chemical and industrial processes. However, there is a growing interest in expanding methanol use in other areas, such as the energy sector and transportation. This paradigm shift is based on the need to find sustainable and less polluting alternatives to traditional fossil fuels. One promising solution is the direct methanation of combustion gases, which involves the direct conversion of from combustion gases into methane (), a gas with great potential for use as fuel or as a raw material in industry. This method has the advantage that it can be integrated into existing gas flows and can be used to reduce carbon emissions. In this technology, is captured from combustion gases, which mainly contain nitrogen, but also water vapor and oxygen from the excess air used in the combustion process. Thus, through methanation, is transformed into methane, thereby reducing the impact on the environment by reducing greenhouse gas emissions and transforming into a useful fuel.
This approach not only offers a way to reduce carbon emissions but also to utilize as a resource, turning it into a useful fuel or raw material. Furthermore, the use of methanol or methane produced can contribute to diversifying energy sources and reducing dependence on fossil fuels.
Although this technology is still in the testing and development phase, progress in capture and utilization offers promising prospects for the future energy and ecological sustainability of our society.
The methanation reaction, known as the "Sabatier reaction," must adhere to the following molar steps:
The temperature ranges between
, while the pressure varies from 1 to 30 bar, [
78].
The team of authors conducted experimental research on the conversion of pure (captured) as well as found in combustion gases, using a pilot installation that was later patented. The methodology included:
-development of a calculation model;
-construction of the experimental pilot installation;
-experimental determinations and their validation.
The syngas technology via the Sabatier reaction between and , not with pure but directly in the combustion gases, represents an original achievement led by the authors up to the experimental pilot phase.
The efficiency (degree) of methanation is also influenced by the catalysts used. The degree of methanation is expressed through a relationship that refers only to the
gas component, which is the easiest and most accurate to measure:
The experimental tests conducted on the pilot plant demonstrated a methanation efficiency between and . In which represents the initial content, and represents the carbon dioxide content at the end of the reaction. The degree of methanation for pure is over 95%. For present in combustion gases, the degree of methanation ranges from 77-92% (water vapor, but especially oxygen in combustion gases, represent a negative effect on methanation).
The pilot installation included a reactor with an internal diameter of 120 mm and a height of 500 mm, with an active section having a diameter of 53 mm. For initial heating, the reactor was equipped with a 2 KW heating element. The active body was filled with alumina powder, and the catalyst used was of the Ni Raney type. The experimental methodology involved heating to , removing air from the circuit, and finally introducing hydrogen at the stoichiometric ratio for the Sabatier reaction, achieved by the partial pressure ratio of these two gases. The working temperature varied in the range of . The efficiency of the process was determined by measuring the concentration of in the synthesis gas, which should approach zero.
The pressure in the pilot methanizer for capturing the component from combustion gases was around 1.01 – 1.1 bar, similar to the value of the combustion gases expelled from an energy installation.
The water vapor content in combustion gases is significantly reduced in power plants with water vapor condensation. Additionally, even in traditional power plants, condensation of water vapor post-power generation can be considered through additional cooling of the combustion gases. The importance of achieving a high degree of methanation is twofold. Firstly, it affects energy efficiency, and secondly, it impacts
pollution, which is represented by
, [
79].
For electricity generated from wind energy, the price of methane produced through methanation has varied between 3.5 and 3.9 Euros per kilogram of
for
captured from a power plant and approximately 5.5 Euros per kilogram of
for
captured from the air (DAC). For electricity generated from solar energy and new water dissociation technologies, the price of methane produced by capturing carbon dioxide from combustion gases can decrease to approximately 1.0 Euro per kilogram of
. The price of methane is further reduced, at around 0.6 Euros per kilogram of
for
captured at a power plant, [
80,
81].
In conclusion, the cost of capture, along with the price of methane obtained through chemical synthesis processes, is still very high. The data presented, with high prices associated with the capture reaction with green and the reuse of hydrocarbons in energy production, lead to the hypothesis that this energy sector will need to gradually decrease over time in favor of renewable sources.
Impure green hydrogen defines the hydrogen-rich gaseous fuel obtained from biomass through processes such as gasification, pyrolysis, wastewater purification, and fermentation. Such an anhydrous fuel is defined by the composition:
where xi is the respective percentage content:
- of molecular hydrogen;
- of methane;
- molecular nitrogen;
- carbon dioxide;
- carbon dioxide;
- molecular oxygen, [
40].
The team of authors investigated hydrogen production through the gasification of agricultural biomass. For this purpose, a 4 kW pilot installation was used, representing a fixed-bed gasifier with downward flow. The comprehensive research included:
-development of an analytical calculation model;
-sizing of the 4 kW experimental pilot installation;
-experiments aimed at achieving the maximum H2 content in the synthesis gas (the maximum value was in the range of 24-30%);
-validation of experimental results.
The maximization of hydrogen in the synthesis gas was achieved by maintaining a specific temperature in the fixed bed (above ), a process regulated by the excess air introduced above the bed. The measurements regarding the quality of the synthesis gas were also validated by igniting it at the end of the exhaust section into the atmosphere. Following combustion, the components and transform into , a gas that adds to the existing amount of . The direct combustion of these gases is similar in terms of emissions to the technology of burning a mixture of hydrogen and methane. However, the use of capture facilities radically alters the composition of the combustion gases by removing carbon dioxide, operating similarly to facilities in energy plants that use hydrocarbons. The calorific value of the presented synthetic fuels is significantly lower than that of hydrocarbon fuels, typically being below . Nevertheless, these fuels must be taken into account, as they represent the biomass contribution to the energy balance.
4. The structure of the renewable energy sector
At the current stage, the following renewable sources are used [4, 5, 43,44 ]: Solar energy; Wind power; Hydropower; Geothermal energy; Energy from biomass; Nuclear energy.
Relief and climate naturally influence the share of these renewable sources in energy production. Energy and environmental policies in EU countries also leave their mark on the structure of energy production.
Terrain and climate play a crucial role in determining the efficiency and availability of different renewable energy sources in a given region. For example, regions with significant sun exposure are more suitable for solar energy development, while areas with strong winds are favorable for wind energy. Lands with rivers and waterfalls can be exploited for hydroelectric power, while geothermal areas offer potential for geothermal power. In addition, the energy and environmental policies of the European Union countries have a significant impact on the direction in which renewable energy sources are developed and used. The EU has set itself the goal of becoming carbon neutral by 2050 and significantly increasing the share of renewable energy in the energy mix. This involves setting clear targets for increasing renewable energy capacities, incentives for investment and research in the field, as well as promoting policies that encourage the transition to clean energy sources. Therefore, by combining the natural influence of terrain and climate with energy and environmental policy, the structure of renewable energy production in EU countries is shaped in a way that supports sustainability goals and reduces environmental impact. Considering that 9 EU countries achieved electricity production with a share of more than 50% from renewable sources and another 10 countries achieved a share between 25% and 50%, a significant trend towards the use of clean energy sources in Europe. These figures reflect a serious commitment to the transition to a greener and more sustainable economy.
The exclusion of nuclear energy from this analysis reflects a certain conceptual perception, which can be seen as outdated in the current context of energy debates. However, nuclear energy remains an important component of the energy mix in many EU countries due to its ability to produce energy consistently and with low carbon emissions. Countries that have managed to reach or approach the 50% and 25-50% renewables thresholds show that there is a variety of solutions and approaches to the energy transition. Moreover, these figures highlight that some countries have made significant progress in promoting and adopting renewable energy sources.
However, it is important to note that hydropower remains a significant component in the energy mix of many European countries, including Romania, where it represents approximately 34% of total electricity production. This indicates the rich potential that some countries have in terms of hydropower resources and the continued importance of this type of energy in ensuring energy security and reducing carbon emissions.
For the current structure of the energy market, characterized by a significant increase in the prices of gaseous fuels, solar and wind sources have become more and more profitable. However, as solar and wind farms continue to be developed on a larger scale, a competent assessment of their environmental impact will be required [
45].
While there is relative predictability in terms of diurnal and seasonal variation for electricity produced from solar sources, forecasts for wind power are based solely on wind sources. Figure 6 illustrates the annual variation trend of the electricity produced from solar sources, which depends on the interaction between the radiative power and the period of its manifestation for the surface covered by solar panels.
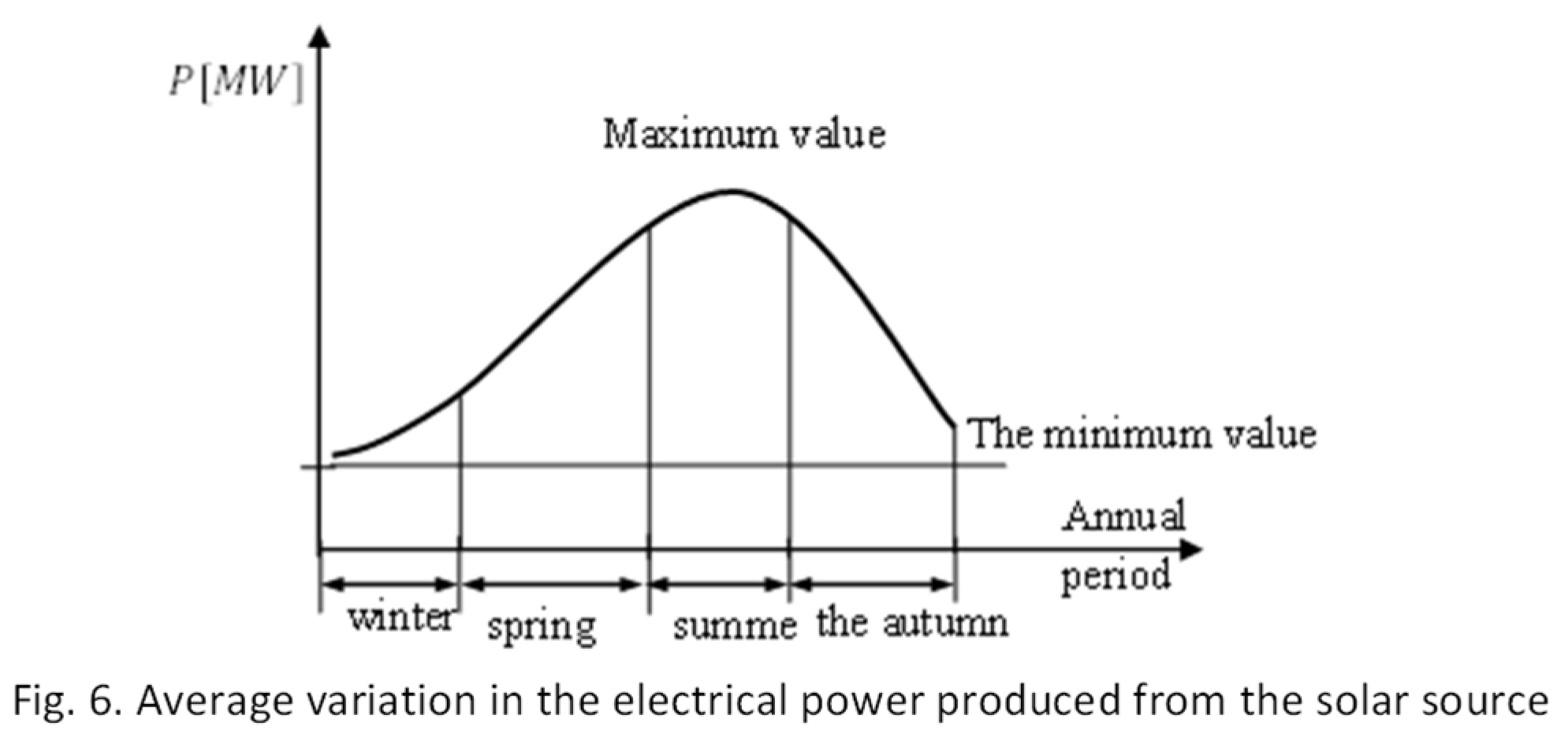
For Europe, the winter and late autumn periods will not represent significant electricity production given its latitude, even if the energy produced from wind sources is more significant during these periods, which are deficient for solar energy.
For the production of solar sources, the average effective power is about 15% of the installed power (.
Consequently, the average annual effective energy from solar sources becomes lower than the average annual effective energy from solar sources. For example, statistical data from Romania, a country with higher investments in renewable energy as previously presented, is provided, with reference to enel.ro/electricpedia from February 2024. Thus, at the national level, the structure of electricity production comes from the following primary sources, shown in the table below, [
46].
There is a high share represented by renewable energy of 63.05% (including nuclear energy and biomass, of which sources with high temporal variation represented by solar and wind energy represent 16.03% of the total energy produced and 25.42% of the total renewable energy The annual production of energy from solar sources compared to wind energy represents 25.62% (this ratio indicating a high uncertainty in the forecast of renewable energy), [
46].
Table 2 provides a detailed overview of the contribution of each source to Romania's energy mix in 2022, highlighting the role and share of each source in the total electricity production.
Regarding the price of electricity, it is worth noting that it is still distorted by the health crisis induced by the Covid-19 virus and the energy crisis that followed. This results in a double objective for the energy transition period, which includes both the replacement of conventional
-generating sources and the achievement of a minimum price for energy through the application of particularly efficient technologies, [
47].
Grid connection for small and medium-sized power parks, typically below 20 MW, depends on the existence of the nearby grid, with interconnection with a distribution system operator and transmission operator.
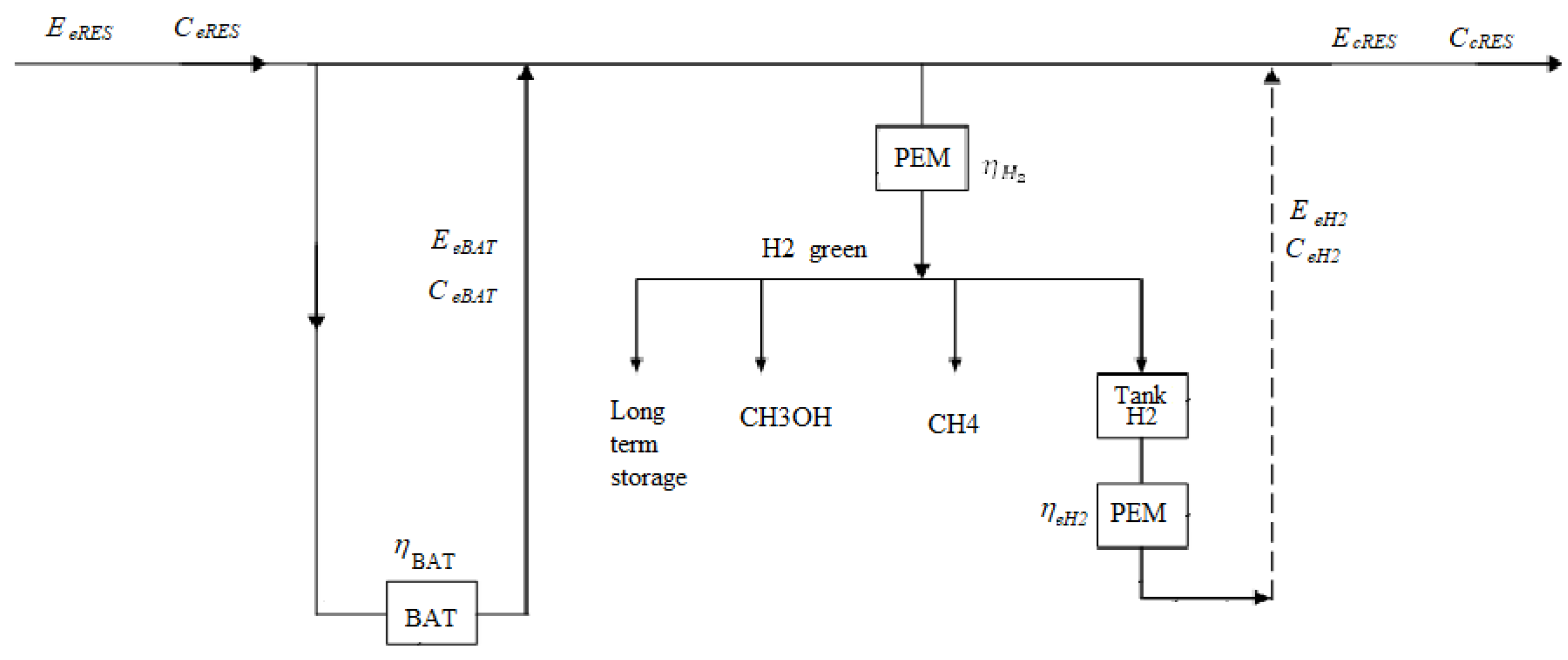
As mentioned earlier, the variation in electricity for solar and wind renewables will need to be compensated by energy storage. Other renewable sources are not subject to these variations. If the price of using fossil fuels is very high due to the costs of climate neutrality, the weight will shift to the renewable energy production sector where prices are reduced for all resources up to the level of green hydrogen generation and energy storage - figure 7.
Figure 7.
The full utilization of electricity produced from renewable sources.
Figure 7.
The full utilization of electricity produced from renewable sources.
Renewable sources charge the electricity produced instantaneously, expressed in kWh to the common electricity grid, 1, at a weighted average cost expressed in Eur/(kWh). The electrical energy demanded instantaneously by the local or general network is, expressed in kWh.
If the two instantaneous energies, which obviously in this case are the electric powers themselves, are equal, respectively , then the consumers will be supplied from renewable sources with the average cost of Eur/(kWh).
If the two instantaneous energies are not equal, respectively , then two situations can occur:
• The surplus of energy produced is accumulated in storage batteries that have the efficiency , and when consumers will be supplied from renewable sources and from batteries with the average cost, which takes into account the energy in the batteries, and the cost of this energy, expressed in Eur/(kWh).
• The surplus energy produced is transformed into hydrogen with the efficiency of , generally in a fuel cell with a proton exchange membrane (PEM), or by electrolysis, into green hydrogen. This green hydrogen can then be used in various industrial or even energy applications by reconverting it in a fuel cell into electrical energy, for example a quantity of [kWh], with an average cost of expressed in Eur/(kWh).
The average cost of hydrogen production, according to the IEA, [104] depends on the primary energy source being within quite wide limits, as evidence that many of the technologies are under development. Thus, the hydrogen production cost in
is for: natural gas 1.4-6.5; wind 3.40-11.1; PV: 3.5—10.4. , [
82]
The increase in the share of solar energy decided at COP 28 is based on this observation regarding the price of generated energy. If we denote the costs of electricity produced in the fossil fuel sector as [Eur/kWh] and the costs of energy from renewable sources as [Eur/kWh], the result is: CEc1 > CEc2 .
Compared to the electricity that feeds the network, the production of green hydrogen with electrolyzers will introduce a cost associated with an average efficiency of about 85%. The unit price of hydrogen mass versus the unit price of consumed energy expressed in kWh for alkaline electrolysis is 40-46, while for PME electrolysis it is 46-55. If we consider the efficiency for the production of green hydrogen by electrolysis:.
For the production of electricity from green hydrogen, through the compression and storage chain, an efficiency of 50% is achieved, so the yield of electricity production becomes: . This requires comparing the price of energy produced from fossil fuels with the price of energy produced from hydrogen [Eur/kWh], the decision to maintain or suspend the energy sector will be determined .
Regarding off-circuit storage of green hydrogen denoted by 17, it is stated that the yield varies between 70-90% (
). The price differences for the energy produced in the hydrogen green line will require operation within the following matrix:
Following the economic analysis, it is concluded that hydrogen electricity production should be limited to long-term energy storage operation only (an advantage in this regard over battery storage systems).
Another problem arising from the simultaneous use of the two energy sectors results from the different degree of amortization of the installations, not knowing how to operate with fully amortized ones compared to the newly implemented ones. The cost of depreciation will further increase the differences in energy prices produced for certain channels. There is a clear advantage for primary sources of hydro and nuclear energy, which usually have their depreciation quota exhausted in all UE countries. However, new investments in nuclear facilities, given their very high cost, will require economic and impact studies to justify their necessity compared to other sources of energy production.
Storing energy in sources other than green hydrogen leads to a wide dispersion of energy return efficiency depending on its constructive-functional variant. If only the efficiency of electricity production is used, the selection criterion will be represented by reaching the maximum yield for the operating consumption. However, the investment and maintenance criteria cannot be removed.
The most efficient storage is represented by hydraulic energy. The pumped hydropower plants are mentioned, as well as the coupling of wind turbine installations with storage in hydraulic potential. Similar to hydraulic potential storage is pneumatic potential storage, but for this system, all the installations are more complex and more expensive as a whole (storage becomes similar to natural gas storage in geological structures, and the gradual reduction of natural gas energy could represent a path to pneumatic storage ).
Mechanical storage can be achieved by the potential energy of masses of certain weights, shapes and displacement heights, even by spring compression. All these solutions depend not only on investment costs and operating costs, but also on the amount of energy needed to be stored.
Regarding the interaction between the electrical system and the thermal energy system, a disjunction is observed because the thermal energy will be predominantly created within the S1 sector, regressing to the S2 sector, where the thermal energies and are residual (fig. 5).
For the transition stage to a energy-free system, thermal energy can also be generated within the S3 system by burning a mixture of with .
The combustion of the mixture represents an initial stage, with the proportion being below 15%. In Romania, ROMGAZ has conducted tests on the use of a 10% and 90% mixture for residential applications, involving 101 households. The tests aimed at successfully using the current installations. At the University of Civil Engineering Bucharest, experimental research was carried out on the combustion of the mixture in wall-mounted boilers for residential heating. At the Politehnica University of Bucharest, the concept of combustion in coaxial air-hydrogen jets was developed to prevent flame return, for concentrations ranging from 70% to 100%.
However, burning only green hydrogen for heat production, both for industrial and residential purposes, cannot be considered due to safety concerns and associated costs [
48], [
49], [50 ].
Impure hydrogen, obtained by gasification, pyrolysis, biomass fermentation, as well as water purification, can be used for direct combustion.
To avoid emissions, the installations will have to be equipped with capture, thus being attached to the SI sector. For the transition stage, direct combustion shows a behavior similar to direct combustion of the mixture and can be used in the S3 sector.
5. Conclusions
The energy sector in the countries within the European Union is undergoing a rapid transformation towards a carbon-neutral one.
For the current phase of the transition to climate neutrality, the original model developed in
Figure 5 includes the parallel existence of conventional
emitting sources and renewable sources. Climate neutrality will involve capturing
from conventional energy sources, as well as utilizing it in synthetic fuels to avoid storage, which is considered technologically complex and costly. Support policies, coming from European or national sources, will play a decisive role in the dynamics and performance of achieving climate neutrality.
The development of carbon dioxide () capture mechanisms, from emission facilities in the atmosphere for fossil fuel combustion to direct air capture, has a significant impact on the fossil fuel energy sector. These technologies enable the reduction of emissions into the atmosphere, thereby contributing to mitigating climate change and maintaining the operation of the fossil fuel energy sector in a more sustainable manner.
Capturing from fossil fuel burning facilities, such as coal-fired power plants or thermoelectric plants, is an efficient method for reducing greenhouse gas emissions. This capture can be achieved through various technologies, such as solvent absorption, amine absorption, or solid sorbent capture. The captured can then be stored underground in deep geological formations, a process known as geological storage.
In addition to capturing from emission sources, there are also technologies that allow for direct capture of from the air. While these technologies are still in early stages of development, they have the potential to be used to reduce atmospheric concentrations and offset emissions from other sources.
Moreover, green hydrogen, produced from renewable energy sources such as solar and wind energy, plays an important role in the energy sector transformation. Green hydrogen can be used to produce synthetic fuels, such as methane () and methanol (), through chemical synthesis processes. These synthetic fuels can be used as energy sources in various applications, such as electricity generation or as fuels for road or maritime transport.
The team of authors conducted experimental research in this field, confirming the respective option.
The development of capture mechanisms and the use of green hydrogen and synthetic fuels are key elements in transitioning to a cleaner and more sustainable energy sector. These technologies enable the integration of the fossil fuel energy sector into a circular economy, contributing to the reduction of emissions and the protection of the environment.
Therefore, the role of green hydrogen as a complex energy vector becomes evident, having a decisive impact both in the renewable energy sector and in the fossil fuel energy sector, as well as in the intermediary sector represented by industry, transportation, and residential economy. The paper analyzes the complex interactions between these energy sectors, aiming to highlight several important aspects:
Neutrality for emissions: green hydrogen provides a solution for achieving neutrality in emissions, contributing to reducing the impact on climate change;
Production of synthetic fuels and their recirculation in the -neutral energy system green hydrogen can be used for the production of synthetic fuels, which can then be recirculated in a -neutral energy system;
The paper includes the authors' results in this field, specifying the technical limits achieved.
Production of green hydrogen from renewable sources:the use of renewable sources for green hydrogen production is essential for reducing dependence on fossil fuels and increasing the sustainability of the energy system;
Transformation of green hydrogen into a complex energy vector, optimizing the integration of energy sectors: green hydrogen can be integrated into a complex energy system, contributing to optimizing interactions between different energy sectors;
Development of a competitive economic and technical system for energy storage:green hydrogen can play an important role in developing an efficient energy storage system, helping manage fluctuations in energy production and consumption.
However, the high cost of green hydrogen makes it less recommended for direct use in energy production at the current stage, remaining primarily a storage vector. The analysis highlights the complexity of the energy systems in countries where classical sectors coexist with important sectors for renewable energy, emphasizing the need for an integrated approach and appropriate policies to manage the transition to a more sustainable and resource-efficient energy system.
Other aspects for future studies include the pollution factor associated with the use of renewable energy sources and green hydrogen production, as well as hydrogen storage. Comparative technical and economic studies will be needed between transport using fuel cells and direct combustion.
It can be considered that the data presented in the paper contribute to defining the current energy stage, which is still filled with apparent contradictions. There is an emphasis on replacing
storage with its conversion into synthetic fuel gas. The approach of reacting
with
directly in the combustion gases represents a technological and scientific novelty that the authors have addressed up to the experimental pilot level. For the period of the energy transition to a climate-neutral society, the technological ensemble described in the energy system depicted in
Figure 5 is necessary.