Submitted:
26 August 2024
Posted:
27 August 2024
You are already at the latest version
Abstract
Keywords:
Introduction
Methods
Design
Study Population
Dietary Patterns
Anthropometric Measurements and Biochemical Analysis
Markers of Oxidative Status
Determination of Glutathione (GSH) Concentration in Blood
Determination of Total Antioxidant Capacity (TAC) Concentrations in Blood
Determination of Thiobarbituric acid Reactive Substances (TBARS) Concentrations in Blood
Determination of GSH Concentration in Blood
Ethical Considerations
Statistical Analysis
Results
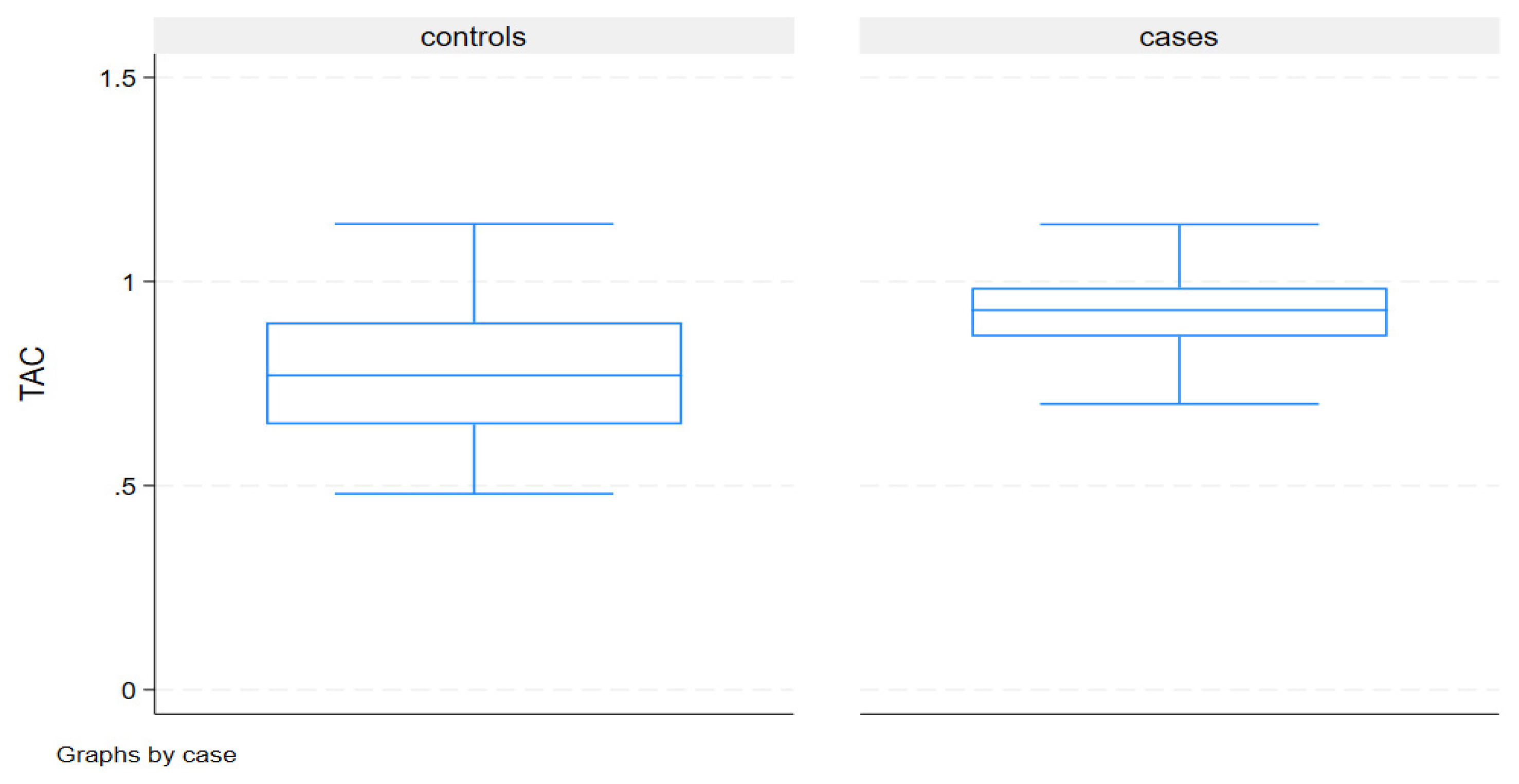
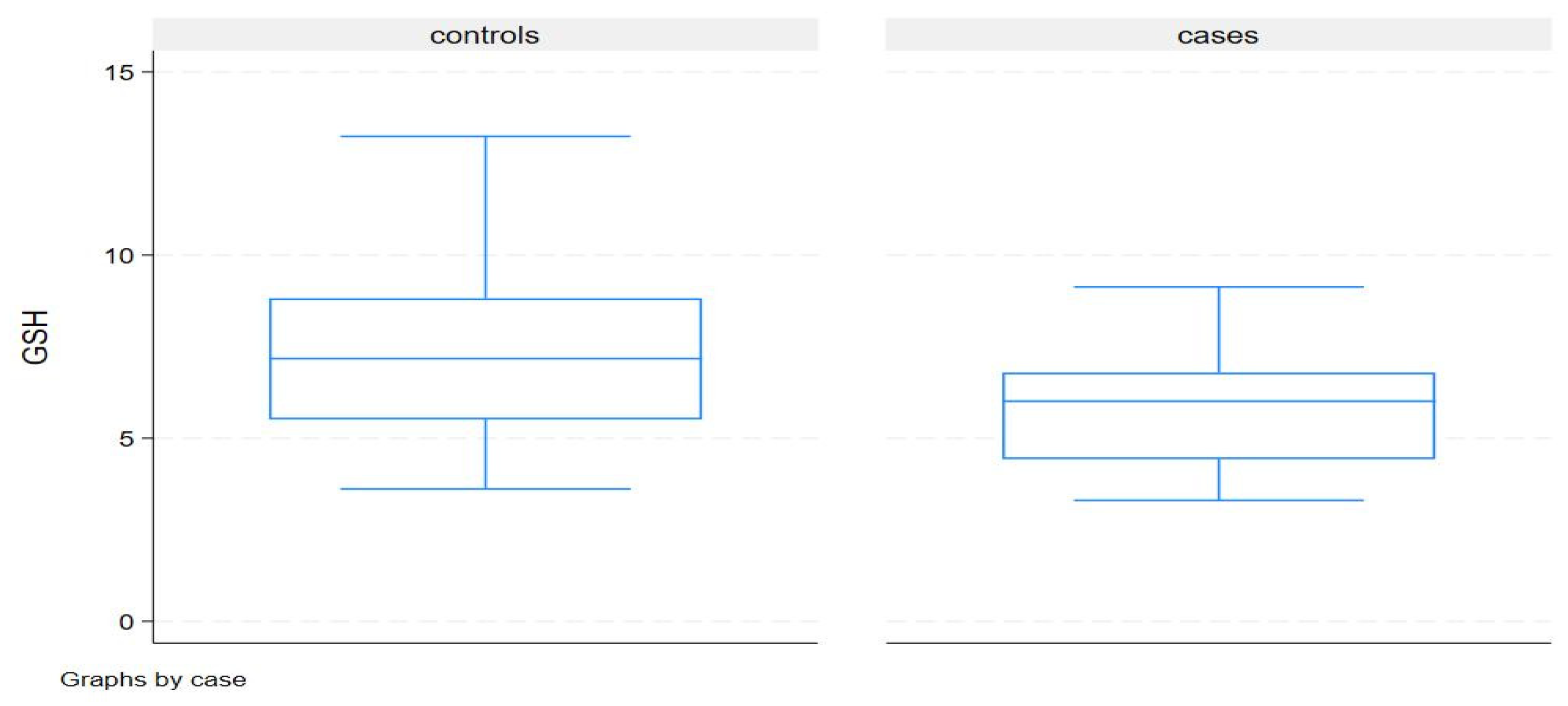
Discussion
References
- Trepanowski, J.F.; Bloomer, R.J. The impact of religious fasting on human health. Nutr. J. 2010, 9, 57. [Google Scholar] [CrossRef] [PubMed]
- Sarri, K.O.; Linardakis, M.K.; Bervanaki, F.N.; Tzanakis, N.E.; Kafatos, A.G. Greek Orthodox fasting rituals: A hidden characteristic of the Mediterranean diet of Crete. Br. J. Nutr. 2004, 92, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Sarri, K.O.; Tzanakis, N.E.; Linardakis, M.K.; Mamalakis, G.D.; Kafatos, A.G. Effects of Greek Orthodox Christian Church fasting on serum lipids and obesity. BMC Public Health 2003, 3, 16. [Google Scholar] [CrossRef] [PubMed]
- Sarri, K.; Linardakis, M.; Codrington, C.; Kafatos, A. Does the periodic vegetarianism of Greek Orthodox Christians benefit blood pressure? Prev. Med. 2007, 44, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Karras, S.N.; Koufakis, T.; Adamidou, L.; Antonopoulou, V.; Karalazou, P.; Thisiadou, K.; Mitrofanova, E.; Mulrooney, H.; Petróczi, A.; Zebekakis, P.; et al. Effects of orthodox religious fasting versus combined energy and time restricted eating on body weight, lipid concentrations and glycaemic profile. Int. J. Food Sci. Nutr. 2021, 72, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Karras, S.N.; Koufakis, T.; Adamidou, L.; Polyzos, S.A.; Karalazou, P.; Thisiadou, K.; Zebekakis, P.; Makedou, K.; Kotsa, K. Similar late effects of a 7-week orthodox religious fasting and a time restricted eating pattern on anthropometric and metabolic profiles of overweight adults. Int. J. Food Sci. Nutr. 2021, 72, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Karras, S.N.; Koufakis, T.; Adamidou, L.; Dimakopoulos, G.; Karalazou, P.; Thisiadou, K.; Makedou, K.; Kotsa, K. Effects of Christian Orthodox Fasting Versus Time-Restricted Eating on Plasma Irisin Concentrations among Overweight Metabolically Healthy Individuals. Nutrients 2021, 13, 1071. [Google Scholar] [CrossRef] [PubMed]
- Karras, S.N.; Koufakis, T.; Adamidou, L.; Dimakopoulos, G.; Karalazou, P.; Thisiadou, K.; Makedou, K.; Zebekakis, P.; Kotsa, K. Implementation of Christian Orthodox fasting improves plasma adiponectin concentrations compared with time-restricted eating in overweight premenopausal women. Int. J. Food Sci. Nutr. 2022, 73, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Azzeh, F.S.; Hasanain, D.M.; Qadhi, A.H.; Ghafouri, K.J.; Azhar, W.F.; Ghaith, M.M.; Aldairi, A.F.; Almasmoum, H.A.; Assaggaf, H.M.; Alhussain, M.H.; et al. Consumption of Food Components of the Mediterranean Diet Decreases the Risk of Breast Cancer in the Makkah Region, Saudi Arabia: A Case-Control Study. Front. Nutr. 2022, 9, 863029. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Vasilopoulou, E.; Georga, K. Macro- and micronutrients in a traditional Greek menu. Forum. Nutr. 2005, 57, 135–146. [Google Scholar]
- Karras, S.N.; Koufakis, T.; Petróczi, A.; Folkerts, D.; Kypraiou, M.; Mulrooney, H.; Naughton, D.P.; Persynaki, A.; Zebekakis, P.; Skoutas, D.; et al. Christian Orthodox fasting in practice: A comparative evaluation between Greek Orthodox general population fasters and Athonian monks. Nutrition 2019, 59, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Karras, S.N.; Persynaki, A.; Petróczi, A.; Barkans, E.; Mulrooney, H.; Kypraiou, M.; Tzotzas, T.; Tziomalos, K.; Kotsa, K.; Tsioudas, A.; et al. Health benefits and consequences of the Eastern Orthodox fasting in monks of Mount Athos: A cross-sectional study. Eur. J. Clin. Nutr. 2017, 71, 743–749. [Google Scholar] [CrossRef] [PubMed]
- 13 Karras, S.N.; Koufakis, T.; Adamidou, L.; Dimakopoulos, G.; Karalazou, P.; Thisiadou, K.; Zebekakis, P.; Makedou, K.; Kotsa, K. Different patterns of changes in free 25-hydroxyvitamin D concentrations during intermittent fasting among meat eaters and non-meat eaters and correlations with amino acid intake. Int. J. Food Sci. Nutr. 2023, 74, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Karras SN, Koufakis T, Popovic DS, Adamidou L, Karalazou P, Thisiadou K, Zebekakis P, Makedou K, KotsaK.Nutrients. 2023 Dec 9;15(24):5058. https://doi.org/10.3390/nu15245058 A Mediterranean Eating Pattern Combining Energy and Time-Restricted Eating Improves Vaspin and Omentin Concentrations Compared to Intermittent Fasting in Overweight Individuals.
- Karras SN, Koufakis T, Dimakopoulos G, Popovic DS, Kotsa K. Changes in dietary intake of aspartic acid during and after intermittent fasting correlate with an improvement in fasting glucose in overweight individuals. J Diabetes. 2023 Feb;15(2):181-184. doi: 10.1111/1753‐0407.13351. CrossRef].
- Karras SN, A Persynaki, A Petróczi, E Barkans, H Mulrooney, M Kypraiou 1, T Tzotzas, K Tziomalos, K Kotsa, A Tsioudas, C Pichard, D P Naughton Health benefits and consequences of the Eastern Orthodox fasting in monks of Mount Athos: a cross-sectional study Eur J Clin Nutr. 2017 Jun;71(6):743-749. doi: 10.1038/ejcn.2017.26. Epub 2017 Mar 22.
- Manoogian ENC, Laferrère B Time-restricted eating: What we know and where the field is going..Obesity (Silver Spring). 2023 Feb;31 Suppl 1(Suppl 1):7-8. doi: 10.1002/oby.23672.
- Koppold DA, Breinlinger C, Hanslian E, Kessler C, Cramer H, Khokhar AR, Peterson CM, Tinsley G, Vernieri C, Bloomer RJ, Boschmann M, Bragazzi NL, Brandhorst S, Gabel K, Goldhamer AC, Grajower MM, Harvie M, Heilbronn L, Horne BD, Karras SN, Langhorst J, Lischka E, Madeo F, Mitchell SJ, Papagiannopoulos-Vatopaidinos IE, Papagiannopoulou M, Pijl H, Ravussin E, Ritzmann-Widderich M, Varady K, Adamidou L, Chihaoui M, de Cabo R, Hassanein M, Lessan N, Longo V, Manoogian ENC, Mattson MP, Muhlestein JB, Panda S, Papadopoulou SK, Rodopaios NE, Stange R, Michalsen A.International consensus on fasting terminology. Cell Metab. 2024 Aug 6;36(8):1779‐1794.e4. doi: 10.1016/j.cmet.2024.06.013. Epub 2024 Jul 25.
- Wang X, He B.Endothelial dysfunction: molecular mechanisms and clinical implications. MedComm (2020). 2024 Jul 22;5(8):e651. doi: 10.1002/mco2.651. eCollection 2024 Aug.
- Sharebiani H, Mokaram M, Mirghani M, Fazeli B, Stanek A .The Effects of Antioxidant Supplementation on the Pathologic Mechanisms of Metabolic Syndrome and Cardiovascular Disease Development..Nutrients. 2024 May 27;16(11):1641. doi: 10.3390/nu16111641.
- Dobroslavska P, Silva ML, Vicente F, Pereira P Mediterranean Dietary Pattern for Healthy and Active Aging: A Narrative Review of an Integrative and Sustainable Approach.Nutrients. 2024 May 31;16(11):1725. doi: 10.3390/nu16111725.
- Rezig L, Ghzaiel I, Ksila M, Yammine A, Nury T, Zarrouk A, Samadi M, Chouaibi M, Vejux A, Lizard G Cytoprotective activities of representative nutrients from the Mediterranean diet and of Mediterranean oils against 7-ketocholesterol- and 7 beta-hydroxycholesterol-induced cytotoxicity: Application to age-related diseases and civilization diseases..Steroids. 2022 Nov;187:109093. doi: 10.1016/j.steroids.2022.109093. Epub 2022 Aug 24.
- Khalil M, Shanmugam H, Abdallah H, John Britto JS, Galerati I, Gómez-Ambrosi J, Frühbeck G, Portincasa P The Potential of the Mediterranean Diet to Improve Mitochondrial Function in Experimental Models of Obesity and Metabolic Syndrome..Nutrients. 2022 Jul 28;14(15):3112. doi: 10.3390/nu14153112.
- Oliveira JS, da Silva JA, de Freitas BVM, Alfenas RCG, Bressan J.A Mediterranean diet improves glycation markers in healthy people and in those with chronic diseases: a systematic review of clinical trials.Nutr Rev. 2024 May 8:nuae045. https://doi.org/10.1093/nutrit/nuae045. Online ahead of print.
- Greek National Dietary Guidelines for Adults. Available online: http://www.fao.org/nutrition/education/food- dietary- guidelines/regions/countries/greece/en/ (accessed on 25 July 2024).
- Jensen, M.D.; Ryan, D.H.; Apovian, C.M.; Ard, J.D.; Comuzzie, A.G.; Donato, K.A.; Hu, F.B.; Hubbard, V.S.; Jakicic, J.M.; Kushner, R.F.; et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation 2014, 129, S102–S138. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global database on body mass index. Accessed February 5, 2016. Available at: www.who.int/nutrition/databases/bmi/en/.
- Tanita Academy. Understanding your measurements. Available at: http:// tanita.eu/. Accessed May 25, 2018.
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: Insulin resistance and b-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–9.
- Reddy Y., Murthy S., Krishna D., Prabhakar M.C. Role of Free Radicals and Antioxidants in Tuberculosis Patients. Indian J. Tuberc. 2004;51:213–218.
- Veskoukis A.S., Kyparos A., Paschalis V., Nikolaidis M.G. Spectrophotometric Assays for Measuring Redox Biomarkers in Blood. Biomarkers. 2016;21:208–217. doi: 10.3109/1354750X.2015.1126648.
- Janaszewska A., Bartosz G. Assay of Total Antioxidant Capacity: Comparison of Four Methods as Applied to Human Blood Plasma. Scand. J. Clin. Lab. Invest. 2002;62:231–236. doi: 10.1080/003655102317475498.
- Keles M.S., Taysi S., Sen N., Aksoy H., Akçay F. Effect of Corticosteroid Therapy on Serum and CSF Malondialdehyde and Antioxidant Proteins in Multiple Sclerosis. Can. J. Neurol. Sci. 2001;28:141–43. doi: 10.1017/S0317167100052823.
- Calder PC, Ahluwalia N, Brouns F et al.. Dietary factors and low-grade inflammation in relation to overweight and obesity. Br J Nutr. 2011;106 (Suppl. 3):S5–78. doi:10.1017/S0007114511005460.
- Emilio Ros E. , Martínez-González M , Estruch R , Salas-Salvadó JS, Montserrat M Fitó M , Martínez JA , Corella D Mediterranean diet and cardiovascular health: Teachings of the PREDIMED study Adv Nutr. 2014 May 14;5(3):330S-6S.
- Dai J, Jones DP, Goldberg J, Ziegler TR, Bostick RM, Wilson PW, Manatunga AK, Shallenberger L, Jones L, Vaccarino V. Association between adherence to the Mediterranean diet and oxidative stress. Am J Clin Nutr. 2008 Nov;88(5):1364-70.
- Fitó M, Guxens M, Corella D et al.; PREDIMED Study Investigators. Effect of a traditional Mediterranean diet on lipoprotein oxidation: a randomized controlled trial. Arch Intern Med. 2007;167:1195–1203. https://doi.org/10.1001/archinte.167.11.1195 [Google Scholar].
- Tosti V, Bertozzi B, Fontana L. Health Benefits of the Mediterranean Diet: Metabolic and Molecular Mechanisms. J Gerontol A Biol Sci Med Sci. 2018 Mar 2;73(3):318-326.
- Calabrese CM, Valentini A, Calabrese G. Gut Microbiota and Type 1 Diabetes Mellitus: The Effect of Mediterranean Diet. Front Nutr. 2021 Jan 13;7:612773.
- Widmer, R.J.; Flammer, A.J.; Lerman, L.O.; Lerman, A. The Mediterranean diet, its components, and cardiovascular disease. Am.J. Med. 2015, 128, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Hooper, L.V.; Littman, D.R.; Macpherson, A.J. Interactions between the microbiota and the immune system. Science 2012, 336, 1268–1273MD. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Gu, X.; Zhuang, T.; Xu, Y.; Yang, L.; Zhou, M. Gut Microbiota: A Pivotal Hub for Polyphenols as Antidepressants. J. Agric. Food. Chem. 2020, 68, 6007–6020. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg T, Abdellatif M, Schroeder S et al. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat Med. 2016;22:1428–1438. doi:10.1038/nm.4222.
- Alex E. Mohr, Carissa McEvoy, Dorothy D. Sears, Paul J. Arciero, Karen L. Sweazea,Impact of intermittent fasting regimens on circulating markers of oxidative stress in overweight and obese humans: A systematic review of randomized controlled trials,Advances in Redox Research, Volume 3, 2021, 100026.
- McAllister, MJ, Gonzalez, AE, and Waldman, HS. Impact of time restricted feeding on markers of cardiometabolic health and oxidative stress in resistance-trained firefighters. J Strength Cond Res 36(9): 2515-2522, 2022.
- Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E, Peterson CM. Early Time-Restricted Feeding Improves Insulin Sensitivity, Blood Pressure, and Oxidative Stress Even without Weight Loss in Men with Prediabetes. Cell Metab. 2018 Jun 5;27(6):1212-1221.e3.
- Cienfuegos S, Gabel K, Kalam F, Ezpeleta M, Wiseman E, Pavlou V, Lin S, Oliveira ML, Varady KA. Effects of 4- and 6-h Time-Restricted Feeding on Weight and Cardiometabolic Health: A Randomized Controlled Trial in Adults with Obesity. Cell Metab. 2020 Sep 1;32(3):366-378.
- Wimalawansa SJ. Vitamin D Deficiency: Effects on Oxidative Stress, Epigenetics, Gene Regulation, and Aging. Biology (Basel). 2019 May 11;8(2):30.
| Orthodox nuns (n=50) | Lay women(n=50) | p | |
|---|---|---|---|
| Demographics | |||
| Age (years) | 42(36-50) | 38 (34-42) | 0.03 |
| Weight (kg) | 71.5(64-82) | 66(60-87) | 0.31 |
| BMI (kg/m2) | 27.0(24.2-29.0) | 26.8(22.0-32.0) | 0.19 |
| Body fat (%) | 24.5 ± 9.4 | 22.1 ± 8.1 | 0.23 |
| Lean body mass (%) | 39.9 ± 6.3 | 41.2 ± 7.1 | 0.15 |
| Waist circumference (cm) | 92.4 | 89.1 | 0.11 |
| Physical activity | |||
| Light | N = 9 | N = 7 | 0.31 |
| Moderate | N = 27 | N = 25 | 0.48 |
| Intense | N = 14 | N = 18 | 0.03 |
| Years of monasticism | 10.5 ± 9.8 - - | ||
| Deaconship of Orthodox Nuns | Baker(3);Botanist(2);Cook(5);Cooking assistant (5);Dining assistant (5); Ecclesiastical chanter (6); Gardener (3); Housekeeper (3); Iconographer (6); Laundry assistant (4); Pharmacist (2); | ||
| Energy (kcal) | 1565.9 ± 64.5 | 1890.0 ± 71.0 | <0.01 |
| Carbohydrates (g) | 159.6 ± 21.8 | 194.3 ± 23.4 | 0.03 |
| Protein (g) | 89.2 ± 1.3 | 72.3 ± 1.3 | 0.04 |
| Daily fat intake (g) | 21.0 ± 0.1 | 24.4 ± 0.6 | 0.02 |
| Daily saturated fat intake (g) | 12.7 ± 0.0 | 16.4 ± 0.0 | 0.01 |
| Total fibre intake (g) | 36.1 ± 0.8 | 24.2 ± 0.8 | 0.02 |
| 25-hydroxy-vitamin D3 (ng/Ml) | 15.7 (11.4-19.8) | 26.1 (18.2-31.9) | 0.02 |
| PTH (pg/ml) | 45.6(39.6-54.7) | 19.4(13.1-28.5) | <0.001 |
| Calcium (mg/dl) | 9.4 (9.1-9.7) | 9.1(8.8-9.3) | 0.15 |
| Insulin (IU/L) | 5.3 (3.4-6.7) | 7.1(4.7-11) | 0.02 |
| Fasting glucose (mg/dl) | 84.4 ± 10.1 | 89.2±9.7 | 0.43 |
| HOMA-IR | 1.02 ± 0.4 | 1.26 ± 0.7 | 0.21 |
| Oxidative status | |||
| TAC | 0.93(0.87-0.99) | 0.77 (0.65-0.90) | <.001 |
| GSH | 6.0(4.4-6.8) | 7.2(5.5-8.8) | 0.04 |
| TBARS | 7.3(5.8-8.3) | 7.6(6.9-8.4) | 0.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





