Submitted:
25 August 2024
Posted:
27 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
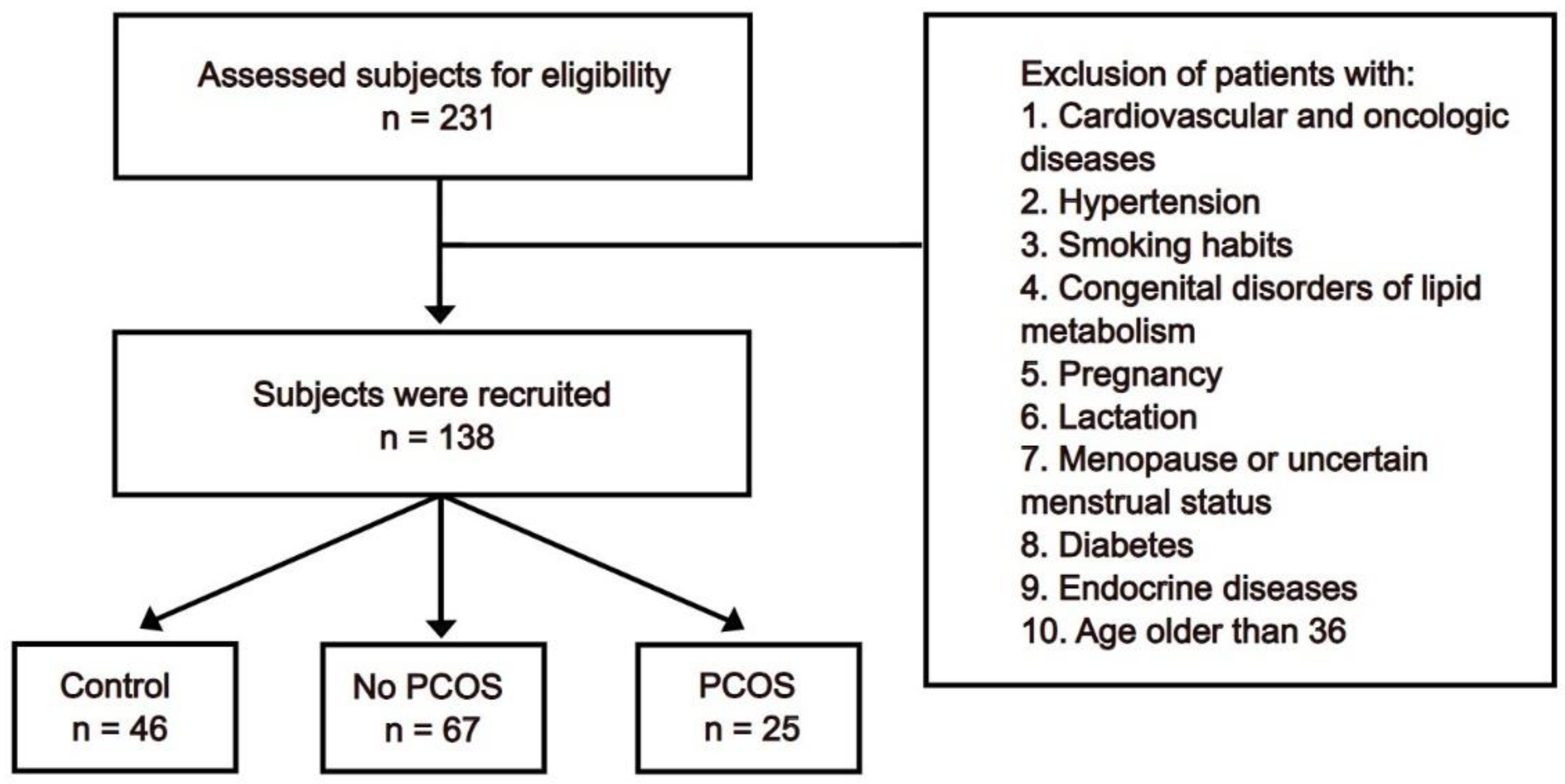
2. Materials and Methods
- 1)
- Survey of patients: this was conducted to collect anamnestic data to assess health and heredity.
- 2)
- Anthropometric data: measurements included height, weight, waist circumference, hip circumference, and blood pressure. The body mass index (BMI) was calculated using Quetelet’s index formula: weight (kg) / height (m2). Obesity was considered abdominal when the ratio of waist to hip measurement (WM/HM) in women was more than 0.8.
- 3)
-
Laboratory indicators:
- a.
- Lipid profile: total cholesterol and triglycerides were measured by the enzymatic colorimetric method using the COBAS INTEGRA analyzer (Roche Diagnostics, Basel, Switzerland). Low-density lipoproteins (LDL) and high-density lipoproteins (HDL) were measured by the homogeneous enzymatic colorimetric method using the Roche/Hitachi cobas c analyzer (Roche Diagnostics, Basel, Switzerland).
- b.
- Fasting glucose level: measured by the standard enzymatic method using the Roche/Hitachi cobas c analyzer (Roche Diagnostics, Basel, Switzerland).
- c.
- Glycated hemoglobin (HbA1c): measured by turbidimetric inhibition immunoassay using a Roche analyzer (Roche Diagnostics, Basel, Switzerland).
- d.
- Hormone levels: levels of thyrotropic hormone, prolactin, estrogens, progesterone, follicle-stimulating hormone (FSH), luteinizing hormone (LH), testosterone, and insulin were measured by Eclia electrochemiluminescence immunoassay using the Elecsys and cobas e analyzer (Beckman Coulter, Inc., Brea, CA, USA). The level of anti-Müllerian hormone (AMH) was measured by ELISA. Levels of adiponectin and resistin were measured by the ELISA method using specific monoclonal antibodies.
- 4)
- Ultrasound investigations (U/S): these were carried out on the seventh day of the menstrual cycle using the Logiq E9 (GE HealthCare, Chicago, IL, USA) with ML6-15 Mhz linear array transducer and IС 5-9 Mhz transvaginal curvilinear transducer. The investigations included 250 pelvic ultrasounds, 250 thyroid ultrasounds, and 250 breast ultrasounds.
3. Results
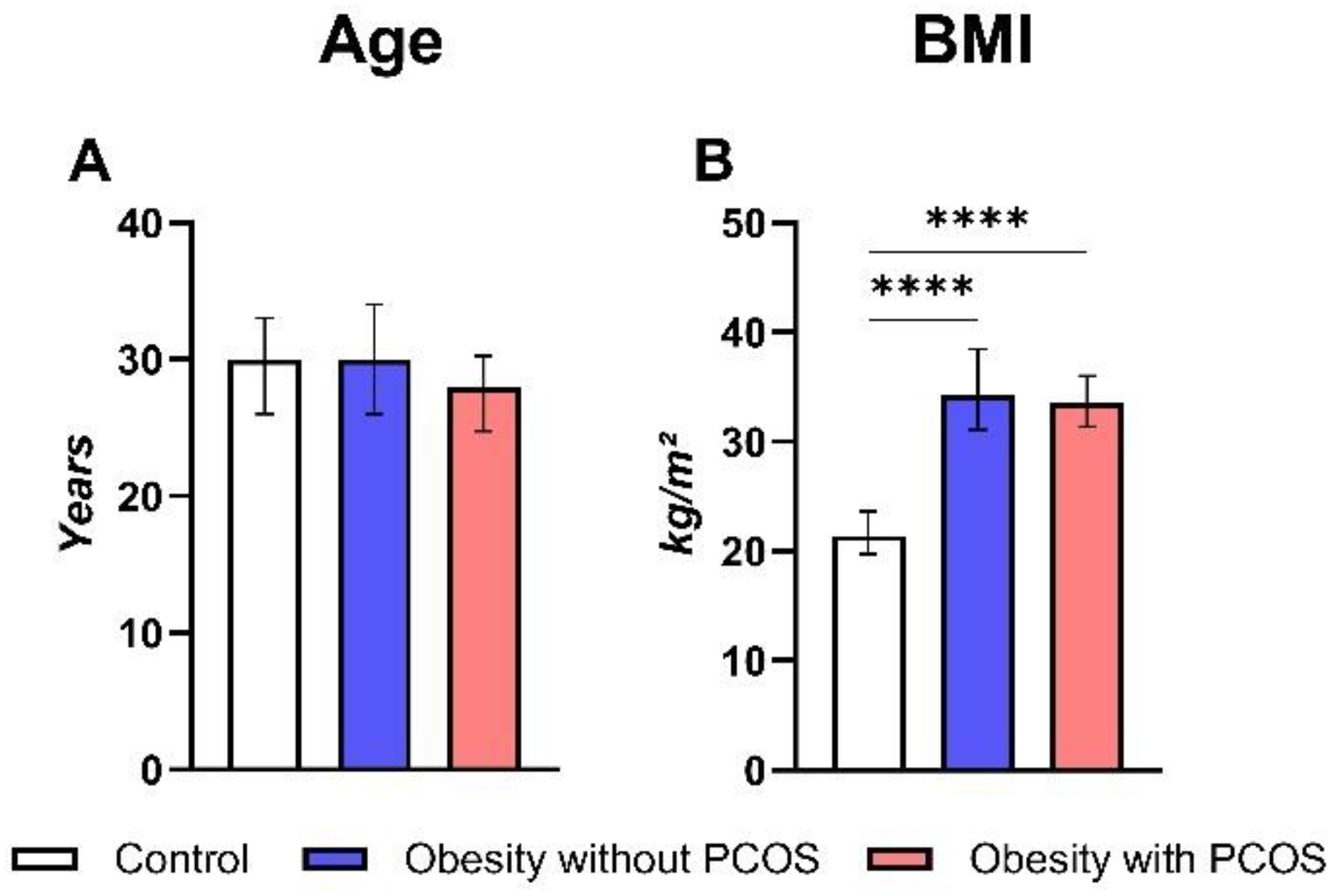
3.1. General Parameters
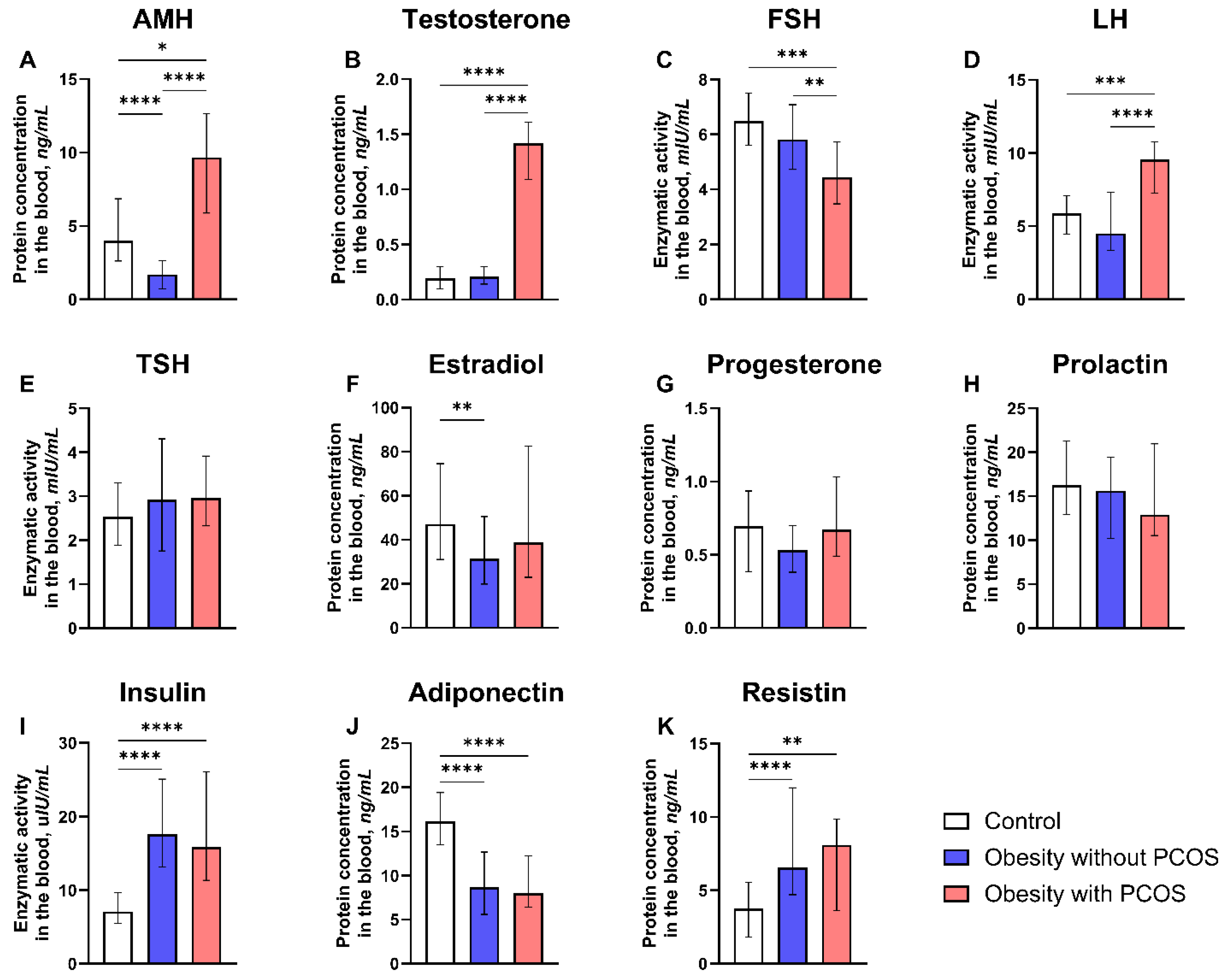
3.2. Hormonal Parameters
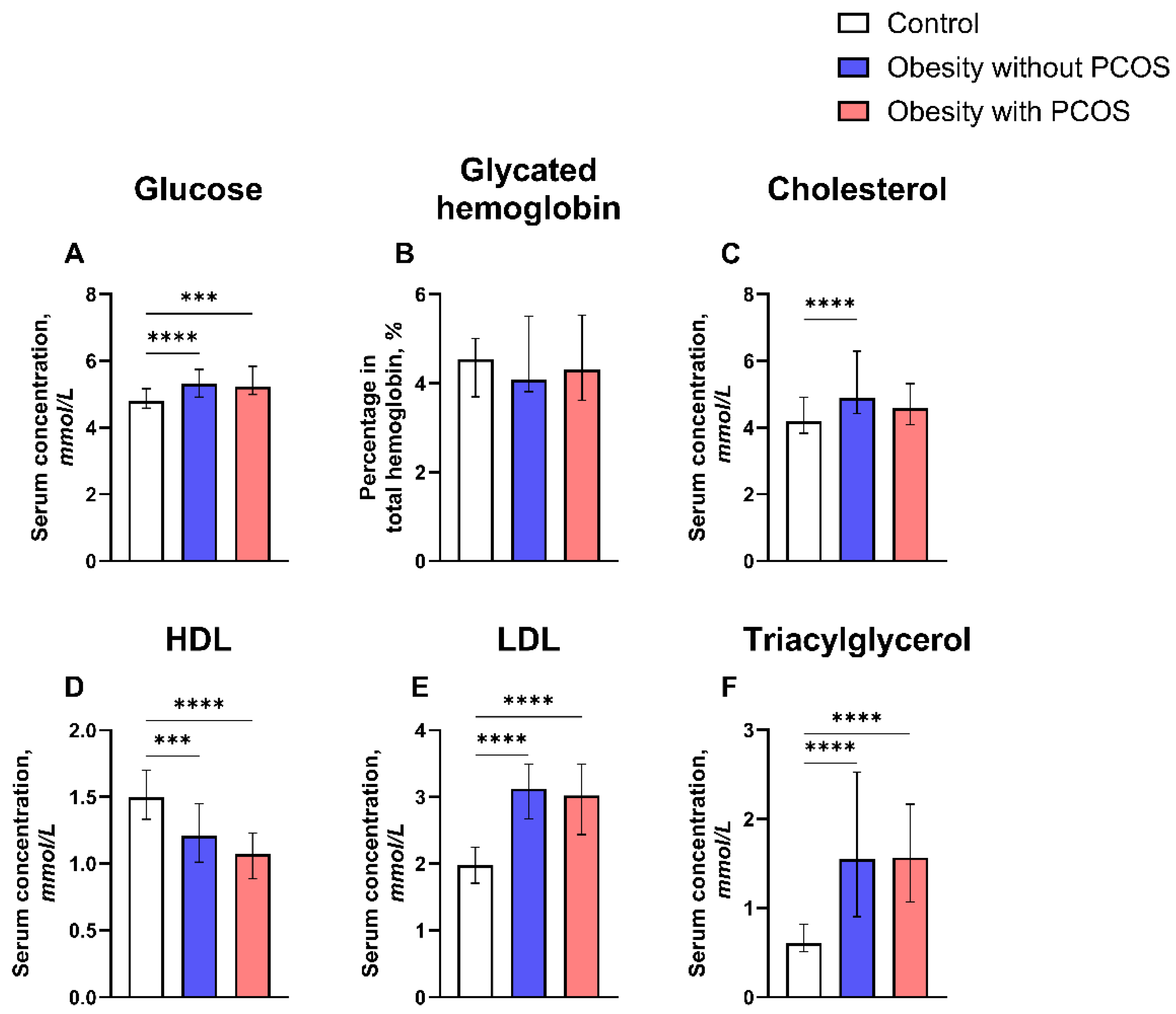
3.3. Metabolic Parameters
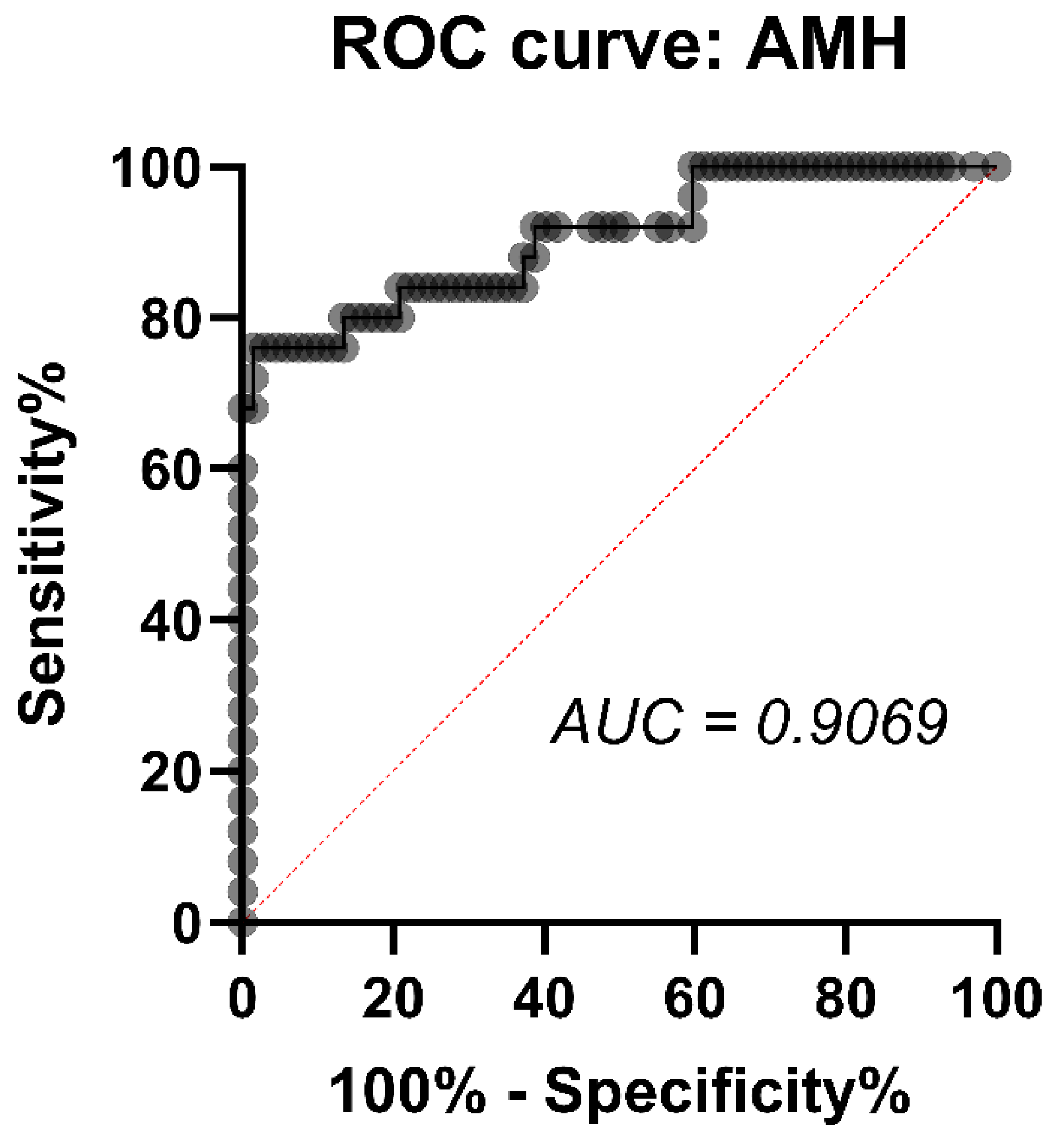
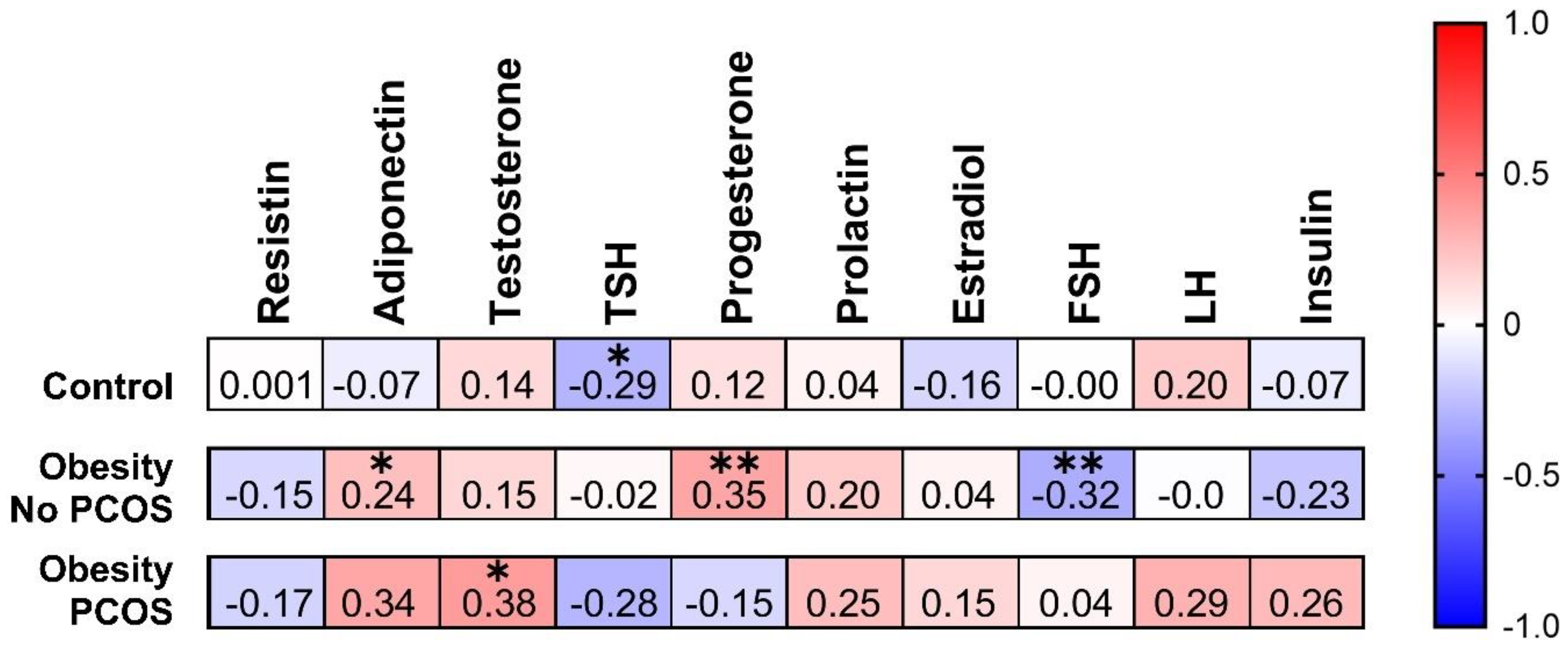
3.4. Correlation between AMH and Some Hormonal Parameters
4. Discussion
Supplementary Materials
Author Contributions
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Silvestris, E.; de Pergola, G.; Rosania, R.; Loverro, G. Obesity as disruptor of the female fertility. Reprod. Biol. Endocrinol. 2018, 16, 22. [Google Scholar] [CrossRef]
- Miazgowski, T.; Martopullo, I.; Widecka, J.; Miazgowski, B.; Brodowska, A. National and regional trends in the prevalence of polycystic ovary syndrome since 1990 within Europe: the modeled estimates from the Global Burden of Disease Study 2016. Arch. Med. Sci. 2021, 17, 343–351. [Google Scholar] [CrossRef]
- Barber, T.M.; Dimitriadis, G.K.; Andreou, A.; Franks, S. Polycystic ovary syndrome: insight into pathogenesis and a common association with insulin resistance. Clin. Med. 2016, 16, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Christ, J.P.; Cedars, M.I. Current guidelines for diagnosing PCOS. Diagnostics (Basel) 2023, 13. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wu, Q.; Hao, Y.; Jiao, M.; Wang, X.; Jiang, S.; Han, L. Measuring the global disease burden of polycystic ovary syndrome in 194 countries: Global Burden of Disease Study 2017. Hum. Reprod. 2021, 36, 1108–1119. [Google Scholar] [CrossRef] [PubMed]
- De Leo, V.; Musacchio, M.C.; Cappelli, V.; Massaro, M.G.; Morgante, G.; Petraglia, F. Genetic, hormonal and metabolic aspects of PCOS: an update. Reprod. Biol. Endocrinol. 2016, 14, 38. [Google Scholar] [CrossRef]
- Pankhurst, M.W.; McLennan, I.S. Human blood contains both the uncleaved precursor of anti-Mullerian hormone and a complex of the NH2- and COOH-terminal peptides. Am. J. Physiol. Endocrinol. Metab. 2013, 305, E1241–E1247. [Google Scholar] [CrossRef]
- Moolhuijsen, L.M.E.; Visser, J.A. Anti-Müllerian Hormone and Ovarian Reserve: Update on Assessing Ovarian Function. J. Clin. Endocrinol. Metab. 2020, 105, 3361–3373. [Google Scholar] [CrossRef]
- di Clemente, N.; Racine, C.; Pierre, A.; Taieb, J. Anti-Müllerian hormone in female reproduction. Endocr. Rev. 2021. [Google Scholar] [CrossRef] [PubMed]
- La Marca, A.; Giulini, S.; Tirelli, A.; Bertucci, E.; Marsella, T.; Xella, S.; Volpe, A. Anti-Müllerian hormone measurement on any day of the menstrual cycle strongly predicts ovarian response in assisted reproductive technology. Hum. Reprod. 2007, 22, 766–771. [Google Scholar] [CrossRef] [PubMed]
- Pigny, P.; Merlen, E.; Robert, Y.; Cortet-Rudelli, C.; Decanter, C.; Jonard, S.; Dewailly, D. Elevated serum level of anti-mullerian hormone in patients with polycystic ovary syndrome: relationship to the ovarian follicle excess and to the follicular arrest. J. Clin. Endocrinol. Metab. 2003, 88, 5957–5962. [Google Scholar] [CrossRef] [PubMed]
- Cook, C.L.; Siow, Y.; Brenner, A.G.; Fallat, M.E. Relationship between serum müllerian-inhibiting substance and other reproductive hormones in untreated women with polycystic ovary syndrome and normal women. Fertil. Steril. 2002, 77, 141–146. [Google Scholar] [CrossRef]
- Wagner, I.V.; Savchuk, I.; Sahlin, L.; Kulle, A.; Klöting, N.; Dietrich, A.; Holterhus, P.-M.; Dötsch, J.; Blüher, M.; Söder, O. De Novo and Depot-Specific Androgen Production in Human Adipose Tissue: A Source of Hyperandrogenism in Women with Obesity. Obes. Facts 2022, 15, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.J.; Perfetti, T.A.; Hayes, A.W.; Berry, S.C. Obesity as a source of endogenous compounds associated with chronic disease: A review. Toxicol. Sci. 2020, 175, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, N.; Mahey, R.; Cheluvaraju, R.; Rajasekaran, K.; Patkar, D.; Prabhakar, P.; Rajput, M.; Upadhyay, A. Serum Anti-Mullerian Hormone (AMH) Levels Among Different PCOS Phenotypes and Its Correlation with Clinical, Endocrine, and Metabolic Markers of PCOS. Reprod. Sci. 2023, 30, 2554–2562. [Google Scholar] [CrossRef]
- Aalpona, F.Z.; Ananya, K.F.; Kamrul-Hasan, A.B. Correlation of Serum Anti-Mullerian Hormone with Clinical, Metabolic and Hormonal Parameters in Bangladeshi Women with Polycystic Ovary Syndrome: A Cross-sectional Study. Mymensingh Med. J. 2023, 32, 606–612. [Google Scholar]
- Nguyen, M.T.; Krishnan, S.; Phatak, S.V.; Karakas, S.E. Anti-Mullerian Hormone-Based Phenotyping Identifies Subgroups of Women with Polycystic Ovary Syndrome with Differing Clinical and Biochemical Characteristics. Diagnostics (Basel) 2023, 13. [Google Scholar] [CrossRef]
- Dilaver, N.; Pellatt, L.; Jameson, E.; Ogunjimi, M.; Bano, G.; Homburg, R.; D Mason, H.; Rice, S. The regulation and signalling of anti-Müllerian hormone in human granulosa cells: relevance to polycystic ovary syndrome. Hum. Reprod. 2019, 34, 2467–2479. [Google Scholar] [CrossRef]
- Dewailly, D.; Robin, G.; Peigne, M.; Decanter, C.; Pigny, P.; Catteau-Jonard, S. Interactions between androgens, FSH, anti-Müllerian hormone and estradiol during folliculogenesis in the human normal and polycystic ovary. Hum. Reprod. Update 2016, 22, 709–724. [Google Scholar] [CrossRef]
- Devillers, M.M.; Petit, F.; Cluzet, V.; François, C.M.; Giton, F.; Garrel, G.; Cohen-Tannoudji, J.; Guigon, C.J. FSH inhibits AMH to support ovarian estradiol synthesis in infantile mice. J. Endocrinol. 2019, 240, 215–228. [Google Scholar] [CrossRef]
- Roy, S.; Gandra, D.; Seger, C.; Biswas, A.; Kushnir, V.A.; Gleicher, N.; Kumar, T.R.; Sen, A. Oocyte-Derived Factors (GDF9 and BMP15) and FSH Regulate AMH Expression Via Modulation of H3K27AC in Granulosa Cells. Endocrinology 2018, 159, 3433–3445. [Google Scholar] [CrossRef]
- Lv, P.-P.; Jin, M.; Rao, J.-P.; Chen, J.; Wang, L.-Q.; Huang, C.-C.; Yang, S.-Q.; Yao, Q.-P.; Feng, L.; Shen, J.-M.; Feng, C. Role of anti-Müllerian hormone and testosterone in follicular growth: a cross-sectional study. BMC Endocr. Disord. 2020, 20, 101. [Google Scholar] [CrossRef] [PubMed]
- Masjedi, F.; Keshtgar, S.; Agah, F.; Karbalaei, N. Association Between Sex Steroids and Oxidative Status with Vitamin D Levels in Follicular Fluid of Non-obese PCOS and Healthy Women. J. Reprod. Infertil. 2019, 20, 132–142. [Google Scholar] [PubMed]
- Seow, K.-M.; Juan, C.-C.; Wu, L.-Y.; Hsu, Y.-P.; Yang, W.-M.; Tsai, Y.-L.; Hwang, J.-L.; Ho, L.-T. Serum and adipocyte resistin in polycystic ovary syndrome with insulin resistance. Hum. Reprod. 2004, 19, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Hu, E.; Liang, P.; Spiegelman, B.M. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J. Biol. Chem. 1996, 271, 10697–10703. [Google Scholar] [CrossRef]
- Nambiar, V.; Vijesh, V.V.; Lakshmanan, P.; Sukumaran, S.; Suganthi, R. Association of adiponectin and resistin gene polymorphisms in South Indian women with polycystic ovary syndrome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 200, 82–88. [Google Scholar] [CrossRef]
- Oldfield, A.L.; Kazemi, M.; Lujan, M.E. Impact of Obesity on Anti-Mullerian Hormone (AMH) Levels in Women of Reproductive Age. J. Clin. Med. 2021, 10. [Google Scholar] [CrossRef]
- Estienne, A.; Mellouk, N.; Bongrani, A.; Plotton, I.; Langer, I.; Ramé, C.; Petit, C.; Guérif, F.; Froment, P.; Dupont, J. Involvement of chemerin and CMKLR1 in the progesterone decrease by PCOS granulosa cells. Reproduction 2021, 162, 427–436. [Google Scholar] [CrossRef]
- Daghestani, M.H.; Daghestani, M.H.; Warsy, A.; El-Ansary, A.; Omair, M.A.; Omair, M.A.; Hassen, L.M.; Alhumaidhi, E.M.; Al Qahtani, B.; Harrath, A.H. Adverse effects of selected markers on the metabolic and endocrine profiles of obese women with and without PCOS. Front Endocrinol (Lausanne) 2021, 12, 665446. [Google Scholar] [CrossRef]
- Liang, Z.; Xu, Z.; Liu, J. Mendelian randomization study of thyroid function and anti-Müllerian hormone levels. Front Endocrinol (Lausanne) 2023, 14, 1188284. [Google Scholar] [CrossRef]
- Jeppesen, J.V.; Anderson, R.A.; Kelsey, T.W.; Christiansen, S.L.; Kristensen, S.G.; Jayaprakasan, K.; Raine-Fenning, N.; Campbell, B.K.; Yding Andersen, C. Which follicles make the most anti-Mullerian hormone in humans? Evidence for an abrupt decline in AMH production at the time of follicle selection. Mol. Hum. Reprod. 2013, 19, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Ambiger, S.; B. Patil, S.; M., R.; Dhananjaya, S. Role of leutenising hormone LH and insulin resistance in polycystic ovarian syndrome. Int. J. Reprod. Contracept. Obstet. Gynecol. 2017, 6, 3892. [Google Scholar] [CrossRef]
- Johansson, J.; Stener-Victorin, E. Polycystic ovary syndrome: effect and mechanisms of acupuncture for ovulation induction. Evid. Based Complement. Alternat. Med. 2013, 2013, 762615. [Google Scholar] [CrossRef]
- Shrivastava, S.; Conigliaro, R.L. Polycystic Ovarian Syndrome. Med. Clin. North Am. 2023, 107, 227–234. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




